Abstract
Background and Objective:
To identify the optimal epoch length for power spectral analysis of cardiac beat-to-beat intervals (BBi) in critically ill newborns.
Materials and Method:
BBi of 49 term newborns undergoing therapeutic hypothermia for hypoxic-ischemic encephalopathy with well-defined outcomes (good outcome (n=28): no or mild brain injury and adverse outcome (n=21): moderate or severe brain injury or death) served as test population. A power spectrum of BBi was calculated with an autoregressive model in three different epoch lengths: two minutes, five minutes, and ten minutes. Spectral power was quantified in three different frequency bands: very low-frequency (0.016 – 0.04 Hz), low-frequency (0.05 – 0.25 Hz), and high-frequency (0.3 – 1 Hz). In each frequency band, the absolute power and the normalized power were calculated. Furthermore, standard deviation (SDNN) of BBi was calculated. These metrics were compared between the outcome groups with a receiver operator characteristic (ROC) analysis in three-hour windows. The ROC curve area >0.7 was regarded as a significant separation.
Results:
The absolute spectral powers in all three epoch lengths in all three frequency bands and SDNN distinguished the two outcome groups consistently for most time points. The spectral metrics calculated with a two-minute epoch length performed as well as the five- and ten-minute epoch lengths (paired t – test P<0.05).
Conclusion:
Spectral analysis of BBi in two-minute epoch shows a similar discriminatory power as longer epoch lengths. A shorter epoch also has clinical advantages for translation into a continuous real-time bedside monitor of heart rate variability in the intensive care unit.
Keywords: Spectral analysis, newborn cardiac beat-to-beat intervals, autoregressive model, hypoxic-ischemic encephalopathy
1. Introduction
The clinical significance of fetal electrocardiogram (ECG) was recognized when Hon and Hess demonstrated the relation between intrapartum fetal heart rate variability (HRV) and fetal distress [1]. Newborn HRV has been shown to be inversely related to respiratory distress [2]. Impaired parasympathetic tone during preterm infants’ first week of life has been shown to be related to the development of infection [3]. Furthermore, an altered parasympathetic tone on the first day of life has been shown to be associated with the development of intraventricular hemorrhage [4].
The central autonomic network refers to connected brain regions that modulate autonomic tone. These brain regions have been established using animal model, humans undergoing brain surgery [5, 6] and functional image analysis [7]. In our earlier studies, we have used brain injury as a model and identified the relationship between the region of brain injury and heart rate variability. In term hypoxic-ischemic encephalopathic (HIE) infants, injury in the right cerebral hemisphere has been associated with a decrease in the low-frequency power whereas injury in the left cerebral hemisphere has been associated with a decrease in the high-frequency power [8, 9]. Furthermore, injuries in the deep brain regions were associated with higher depression of heart rate variability compared to absence of injury [10]. These studies highlight the importance of monitoring HRV of newborns, which can be performed easily at the bedside.
Several methods have been used to characterize the normal beat-to-beat interval (BBi) [11–14]. Among all methods, power spectral analysis is commonly used since it allows for characterization of slow and fast changes in the BBi in selected frequency bands. Initial studies focused on calculating the spectrum using the square of the magnitude of the Fourier coefficients; whereas later studies used estimation techniques [11, 15]. In general, estimation techniques such as Welch periodogram or autoregressive model (AR), attenuate stochastic (statistical) fluctuations in the BBi and allow reliable characterization of the changes in the autonomic tone. For short datasets, the AR approach yields reliable results compared to the Welch periodogram approach [16].
The Task Force recommends using five-minute epoch length of heart rate for HRV analysis. Yet different periods are currently used to examine critically ill infants’ HRV. In our previous studies, we have used ten-minute epoch length of BBi [17]. Others have used five-minute epoch lengths [18–20] or 20-minute epoch length of BBi [21]. This difference in choice of epoch length is dependent on the technique used to characterize heart rate. The heart rate during early development is high and it decreases with maturation [12]. This reflects the increase in parasympathetic tone, and the consequent decrease in the sympathovagal tone ratio, with maturation [12]. The number of beats sampled in a given period is greater when the heart rate is high compared to when the heart rate is low. When the dynamics of the system is changing quickly it can be more effectively characterized using short measurements. In addition to these inherent dynamics, disease dynamics (e.g., evolving encephalopathy) may change the course of the physiology from its natural path. A system that exhibits fast change in dynamics can be more effectively characterized using a short epoch length of data as the essential features of the dynamics may be diluted when a long period of data is used. This higher heart rate in newborns may allow characterization of the HRV in shorter epoch lengths compared to older children and adults. Thus, the goal of this manuscript is to test the feasibility of characterizing the autonomic tone using two-minute epoch length of heart rate in term newborns. To test this hypothesis, we consider term newborns undergoing therapeutic hypothermia for hypoxic-ischemic encephalopathy (HIE) with well-defined clinical outcome groups (good versus adverse outcomes [10]) based on MRI findings. We hypothesize that the HRV characterized using a two-minute epoch will distinguish the two outcome groups of cooled HIE newborns to the same degree or better than the HRV of longer (five-minute and ten-minute) epoch lengths.
2. Materials and Methods
In this prospective study, acutely ill HIE newborns referred to our level 4 neonatal intensive care unit for therapeutic hypothermia were examined. The newborns were undergoing whole-body therapeutic hypothermia based on a well-established National Institute of Child Health and Human Development protocol [22]. Briefly, the protocol includes maintaining the newborn’s core temperature at 33.5 ℃ for 72 hours and gradually rewarming to normothermia at a rate ≤ 0.5 ℃/hour over six hours. The Children’s National Institutional Review Board approved the study and informed consent was obtained.
We have abstracted the clinical variables from the clinical server. The clinical variables are shown in Table 1. The mean gestational age at birth is 38.65 weeks and 51% of the infants were male. The average weight of the infants was 3.2 kilogram. The medians of APGAR scores at 1, 5, and 10 minutes were 1, 3, and 5, respectively. The mean base deficit was 19.79.
Table 1.
Clinical variables (n=49)
| Clinical variable | Mean ± standard deviation or median (minimum, maximum) |
|---|---|
| GA | 38.65 ± 1.54 |
| Weight | 3.2 ± 0.78 |
| APGAR1* | 1 (0, 9) |
| APGAR5** | 3 (0, 9) |
| APGAR10*** | 5 (0, 9) |
| BD**** | 19.79 ± 5.41 |
| Male (n %) † | 25 (51%) |
indicate that their statistics were calculated only from 48, 47, 42 and 38 subjects, respectively.
Expressed as number (percentage).
Their electrocardiograph (ECG) and vital signs were monitored with a cardiorespiratory monitor (Intellivue MP70, Philips, MA, USA). The ECG waveform was retrieved and digitized at a rate of 1000 Hz using custom software developed in Labview (National Instruments, TX, USA). Brain magnetic resonance (MR) imaging (Discovery MR750, GE Healthcare, WI, USA) was performed in surviving infants at a median age of 10 days (range 3 to 18 days). The MR images were read and scored [23] by an experienced neuroradiologist (G.V.), who was blinded to the clinical course of the infant. Outcomes were classified as good (basal ganglia score < 3, or watershed score <4) or adverse (for details refer [10]).
ECG was bandpass filtered between 0.5–70 Hz using the 4th order Butterworth filter with zero phase distortion. The QRS complexes were identified using a combination of Hilbert transform and an adaptive threshold detection approach [24], and the BBi was calculated. The spikes in the BBi were corrected using a computer-based approach [25]. For spectral analysis, the BBi was converted into evenly sampled data with a cubic spline interpolation scheme with a sample period of 0.25 seconds.
The BBi was partitioned into two-minute, non-overlapping epochs. A spectral analysis was performed for BBi in each epoch using an autoregressive (AR) model. The AR coefficients were solved with the modified Yule-Walker approach [26, 27]. The model order was systematically increased from 1 to 20. The spectrum was calculated as the linear combination of the product of AR coefficients and Fourier coefficients. The spectrum was estimated for every model order. The order of the AR model was determined with an intraclass correlation coefficient. If the intraclass correlation coefficient between the spectra calculated from two successive model orders was greater than 0.95, the spectrum was assumed to have converged and the calculation was terminated. Usually, the spectrum converged around the model order 6 or 7. Spectral power was calculated from the power spectrum for three frequency bands of very low-frequency (VLF) (0.016 – 0.04 Hz), low-frequency (LF) (0.05–0.25 Hz) and high-frequency (HF) (0.3 – 1 Hz). In each frequency band, the absolute and normalized power were calculated. The normalized spectral power was obtained by calculating the sum of spectral powers in that band divided by the sum of powers in the frequencies below 2 Hz for each frequency band and denoted nVLF, nLF, nHF, for very low-frequency, low-frequency, and high-frequency, respectively. The original power values were not normally distributed (Jarque-Bera test P<10−3) and hence the absolute power was calculated as the median of the logarithm of power in that frequency band and denoted VLF, LF, HF, respectively. Of note, the direct component which reflects the square of the mean BBi at zero frequency is not included in the calculation. In addition to spectral metrics, an asymmetric index was calculated for each two-minute epoch as follows: Let the median BBi be µ. Let the sequence of BBi less or equal to µ be S2 and the sequence of BBi greater than µ be S1. R1 was calculated as the mean of the square of the deviations of 1 from µ. Similarly, R2 was calculated as the mean of the square of the deviations of S2 from µ. To this end, R was calculated as the ratio of R1 to R2. The metrics calculated for the BBi in a two-minute epoch was considered for comparison if R was within 0.8 and 2.
The very low-frequency (0.016 – 0.04 Hz) and low-frequency power (0.05 – 0.25 Hz) quantify the sympathetic arm of the autonomic nervous system (ANS) whereas the high-frequency power quantifies the parasympathetic arm of the ANS [10, 11]. However, low-frequency power has been regarded as the interaction between sympathetic and parasympathetic nervous systems [28]. In addition to spectral metrics, we also calculated the standard deviation of the beat-to-beat intervals (SDNN) in every epoch. SDNN is also regarded as a measure of total power of a signal.
The asymmetric index is not a commonly used approach. It is being used in the Heart Rate Observation (HeRO) monitor [29]. The premise of this metric is that a stationary beat-to-beat interval will yield a distribution that will be symmetric around the median value. The presence of any outliers (bradycardia, tachyarrhythmia, missed beats or extra beats) will make the distribution of BBi asymmetric around the median value. There are several approaches to quantify any asymmetry in the BBi distribution (e.g., kurtosis); however, Kovatchev et al. have shown that asymmetric index calculated with the approach explained in this manuscript characterizes the asymmetry in the BBi distribution better than the traditional approaches [29, 30]. We used this approach to identify spurious beats that escaped our spike-correction approach. It merely serves as a beat-to-beat interval quality gauging metric.
Statistical Analysis
We checked the spectral metrics for Normality condition using the Jarque-Bera test before the receiver operating characteristic (ROC) analysis. The normalized and absolute spectral powers for each frequency band were compared between the good and adverse outcome groups using a ROC analysis. For each newborn, the median of the normalized or absolute spectral power was calculated for every three-hour period of time until 93 hours of life. The median spectral power findings for the outcome groups were compared using a ROC analysis. Since the infants in the good outcome group were expected to show increased variability, this group was treated as a positive group. An area under the ROC curve (AUC) greater than 0.7 was regarded as a clinically meaningful separation [31–33].
The above analysis and comparison were repeated for the BBi partitioned into five-minute and ten-minute epoch lengths. For every 3-hour period, for every metric, there were three AUCs calculated for two-, five-, and ten-minute epoch lengths. To identify the epoch length that best distinguished the outcome groups, the three AUCs from every three-hour epoch were compared using a paired t-test. In this exploratory study, the P-values were not adjusted for multiple comparisons. All analyses were performed offline in MATLAB 2018a (Mathworks Inc, MA, USA).
3. Results
A total of 49 newborns were included in this study. The median (minimum, maximum) duration of ECG monitoring was 74.03 (4.633, 91.8) hours. The median hours of life at study onset was 13.97 (5.81, 25.24) hours and the median hours of life at study completion was 89.15 (14.05, 111.61) hours. Twenty-eight newborns had a good outcome, and 21 newborns had an adverse outcome (11 had moderate to severe brain injury and 10 died).
The number of infants analyzed in every three-hour period for the good and adverse outcome groups is shown in Figure 1. Except for the first (9–12 hours), and the last 3-hour period of life (90 – 93 hours), all epoch lengths had at least ten infants in each group.
Figure 1.
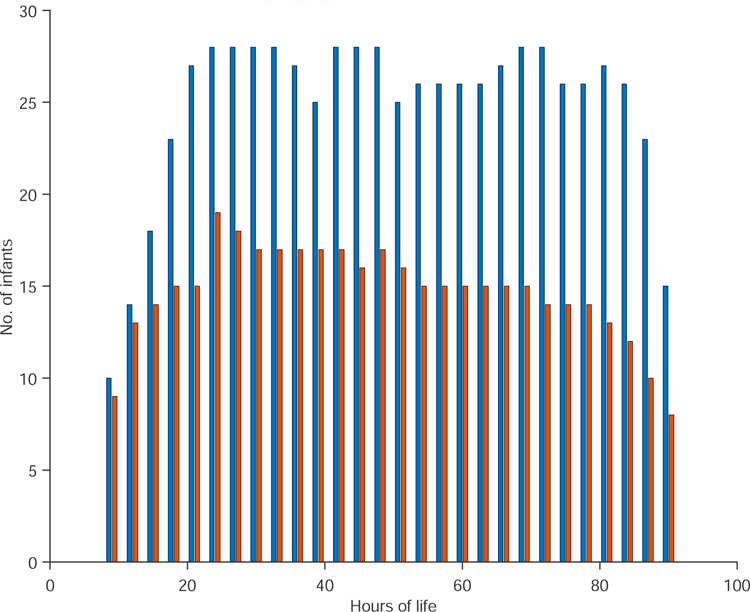
(Color online) Histogram of the number of infants compared in every 3-hour epoch. The good outcome group is shown in blue bar and the adverse outcome group in red bar. This distribution is the same for all three epoch lengths.
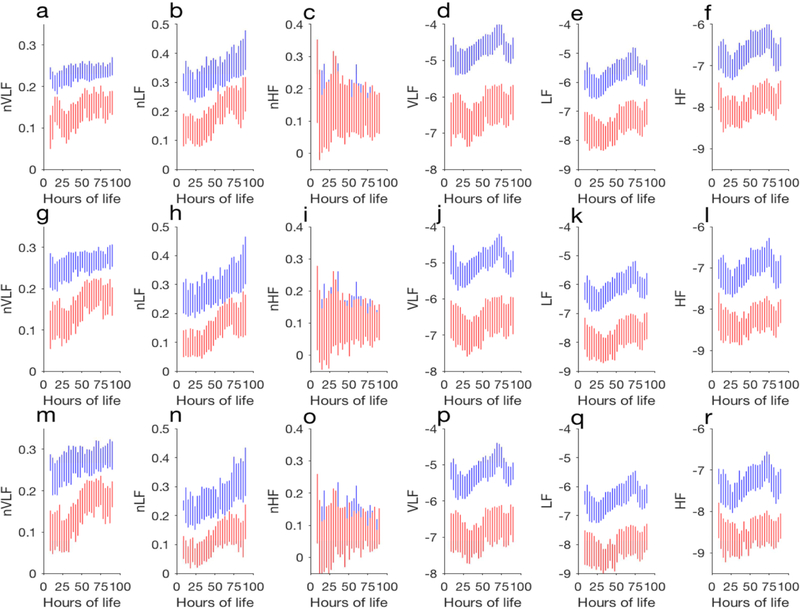
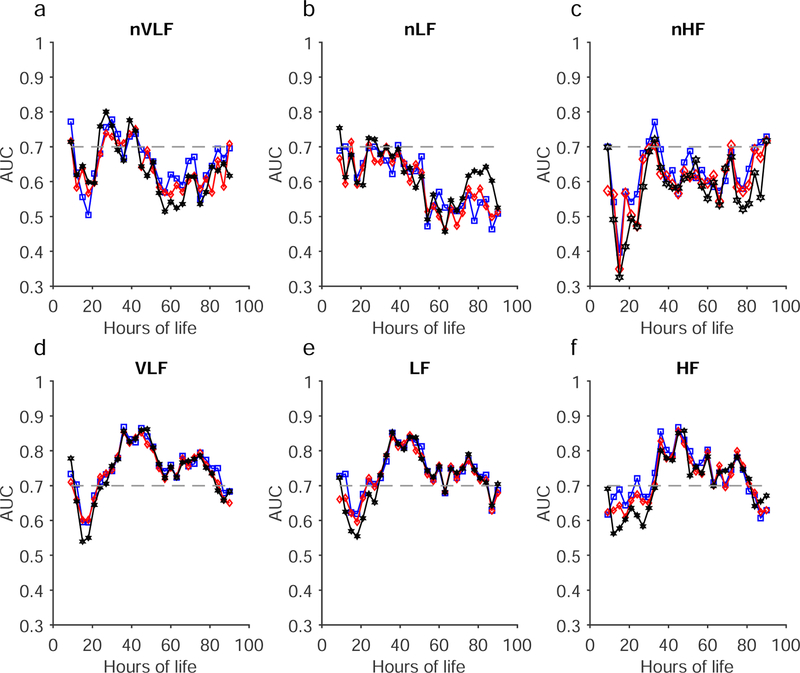
The mean and standard deviation of the spectral power in each band are shown in Figure 2. For clarity, we have shown mean plus standard deviation of the spectral metrics of the good outcome infants and mean minus standard deviation of the spectral metrics of the adverse outcome infants. Except for nHF, almost all metrics are different between the two outcome groups. The differences in the metrics are quantitated using a receiver operating characteristic (ROC) analysis and the ROC AUCs are shown in Figure 3.
Figure 2.
(color online) Summary of HRV metrics in every 3 hours. In each plot, mean plus standard deviation of the spectral power of the good outcome group and mean minus standard deviation of the spectral power of the adverse outcome group are shown. The spectral metrics from two-minute analysis are shown in a) nVLF, b) nLF, c) nHF, d)VLF, e) LF, and f) HF. The results shown in g-i, and m-r are the same as shown in a-f but obtained for five-minute and ten-minute analyses, respectively.
Figure 3.
(Color online) The area under the ROC curve (AUC) obtained from the six spectral metrics are shown as a function of hours of life. a) normalized very low-frequency (nVLF), b) normalized low-frequency (nLF), c) normalized high-frequency (nHF), d) very low-frequency (VLF), e) low-frequency (LF), and f) high-frequency (HF). The results from two-, five-, and ten-minute analyses are shown in blue, red, and black, respectively. The horizontal dashed line at AUC=0.7 is a reference line.
The Jarque-Bera test showed that all of the metrics (nVLF, nLF, nHF, VLF, LF, HF, and SDNN) were Normally distributed (P<0.001). The comparison AUC values for normalized and absolute spectral powers for each frequency band calculated for 2, 5, and ten-minute epoch lengths are shown in Figure 3a–f. The normalized spectral power distinguished the good versus adverse outcome groups only at narrow time points. In the nVLF, all three epoch lengths distinguished the outcome groups at approximately 21 – 42 hours of life although there is a drop in the AUCs around 36 hours for the two-minute results and drops at 36 and 38 hours. In the nLF, the two groups were distinguished at 20–30 hours of life. In the nHF, the two groups were distinguishable at 30–33 hours of life and again after 80 hours of life.
The absolute spectral power for all three frequency bands was superior to the normalized spectral power in distinguishing the outcome groups in each frequency band between 20–80 hours of life (Figure 3 d–f). This was true whether the BBi was partitions at two, five, or ten-minute epoch lengths.
The p-values comparing the AUCs of the spectral power analysis at two, five and ten-minute partition epoch lengths are shown in Table 2. Only the normalized spectral powers for BBi in the very low-frequency band showed a distinction between two, five, and ten-minute partition epochs lengths. Although nLF and nHF exhibited P<0.00001, the AUC values obtained for these metrics were less than 0.7 for majority of the time points and hence they are not discussed further. The magnitude and direction of separation can be inferred from the t-scores shown in Table 2. For absolute spectral powers, the two-minute epoch partition yielded higher AUC values each in all three frequency bands (see the t-score values reported for two-minute vs five-minute and two-minute vs ten-minute) compared to five and ten-minute epoch lengths.
Table 2.
Comparison of the area under the ROC obtained for every spectral metric for each length. P-values obtained from a paired t-test are given. The significant P values are shown in bold face. The t-values calculated for every pair are shown also in this table.
| two-minute vs five-minute | two-minute vs ten-minute | five-minute vs ten-minute | ||||
|---|---|---|---|---|---|---|
| t-score | P-value | t-score | P-value | t-score | P-value | |
| nVLF | 3.034 | 0.005 | 4.942 | 0.000 | 4.140 | 0.000 |
| nLF | 0.891 | 0.381 | −1.376 | 0.180 | −2.675 | 0.013 |
| nHF | −3.526 | 0.002 | −5.130 | 0.000 | −2.304 | 0.029 |
| VLF | 2.480 | 0.020 | 2.758 | 0.010 | 1.478 | 0.151 |
| LF | 2.185 | 0.038 | 2.034 | 0.052 | 0.719 | 0.478 |
| HF | 3.147 | 0.004 | 2.167 | 0.039 | 1.184 | 0.247 |
The AUCs obtained from the comparison of the SDNN of BBi of the two groups in each epoch length are shown in Figure 4. Similar to the absolute power in the VLF, the SDNN also distinguished the two groups better in all three epoch lengths. The paired t-test comparison of AUCs obtained for SDNN and those obtained for very low-frequency (VLF) showed the spectral powers yielded higher AUCs in all three epoch lengths (two-minute: P=0.0025; five-minute: P = 0.0455; ten-minute = 0.0088). Furthermore, the paired t-test analysis of AUCs obtained using SDNN comparison showed they were not different from each other; that is AUC-two-minute versus AUC-five-minute, AUC-five-minute versus AUC-ten-minute as well as AUC-five-minute versus AUC-ten-minute showed they were not different from each other (P>0.05).
Figure 4.
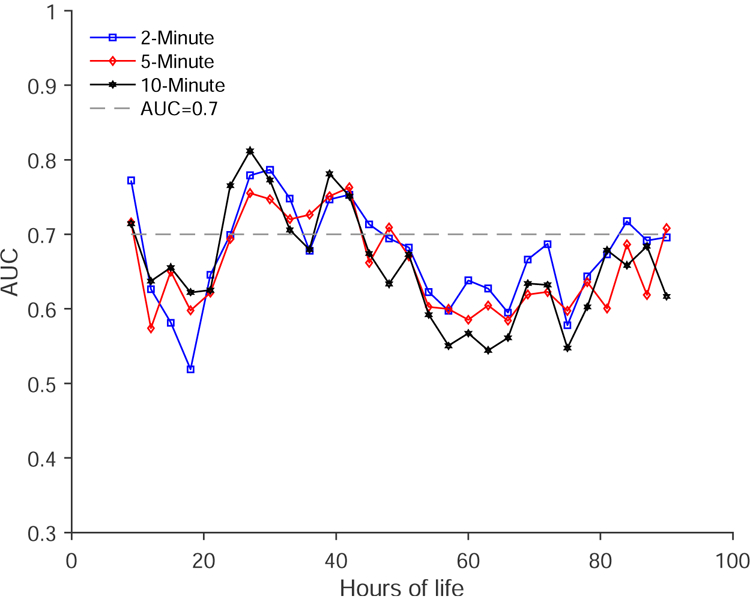
AUC obtained for the comparison of standard deviation of BBi in two-minute, five-minute, and ten-minute between good outcome and adverse outcome groups.
4. Discussion
The AR model allows characterizing spectral power of short BBi data. The HRV metrics obtained from two-minute analysis performed as well as those obtained from 5 and 10 minutes. The absolute spectral power in the different frequency bands distinguished the two outcome groups of HIE infants rather than their normalized spectral power counter parts. Both SDNN and spectral power showed the variability in the BBi was higher in good outcome group infants compared to adverse outcome group infants.
The sympathetic and parasympathetic components of the autonomic nervous system mature at different rates during early human development [34]. Several fetal electro/magnetocardiogram studies have shown an increase in the high-frequency spectral power after 31 weeks of gestational age [35–37]. The rapid maturation of the parasympathetic component in the third trimester is regarded as an indication of increased respiratory activity and increased presence of the active behavioral state [35, 38]. Also, newborn studies have shown an increase in the high-frequency spectral power and a decrease in the ratio of the low-frequency to high-frequency spectral powers [12, 39] with maturation. These studies indicate the maturation of the parasympathetic component and a relative decrease in the ratio of sympathovagal tone during development.
The Task Force recommendations for BBi analysis are made for adult studies [11]. The current epoch length for BBi analysis of newborns and frequency bands used to define low-frequency and high-frequency spectral power of BBi are taken from adult studies. These definitions may not apply to newborns. For example, the probability that a normal-to-normal interval to be greater than x milliseconds from its previous interval denoted as (pNNx, x=50) metric that distinguished BBi of normal volunteers from individuals with congestive heart failure did not work for fetal data. For fetal BBi, owing to the shorter interbeat intervals, pNN10 has been shown to distinguish low-risk and high-risk fetuses [40]. Since the heart rate of newborns is faster than that of adults, BBi from short time epochs should be sufficient to distinguish the heart rate variability of two groups of HIE infants. In particular, in HF band the AUC was higher in the two-minute epoch than that were obtained with five- and ten-minute epoch lengths.
We and others have shown that infants with HIE who had adverse neurological outcomes had depressed autonomic tone [41–44]. Earlier studies have shown a correlation between HRV and qualitative and quantitative electroencephalographic characteristics of encephalopathy [43, 44]. In our earlier study [42], we demonstrated a high predictive ability of nLF and nHF to discriminate neurodevelopmental outcomes in infants with HIE. Our results indicate that the higher heart rate during early human development enables two minutes of heart rate to be sufficient to quantify HRV, exhibiting the same discriminatory power as those of five-minute and ten-minute epochs.
Currently, there are no gold standard indices for characterizing autonomic tone. While the AUC obtained from two-minute analysis is, on an average, higher than the AUC values obtained from the five- and ten- minute analyses, we can only safely conclude that two minutes of BBis are sufficient to distinguish the two groups of HIE infants. Further studies are needed to validate if two minutes of BBi are sufficient, or most optimal, to characterize autonomic tones in other populations of high-risk newborns.
In the current study, we observed poor predictive abilities for nLF and nHF, whereas absolute LF and HF power demonstrated better discrimination of outcome groups. The differences between the two studies may be attributable to several factors. First, in our earlier study, the spectral powers from individual ten-minute epochs were used in the comparison whereas in the current study the spectral powers of individual epochs were averaged over three-hour period. Additionally, we confined our analyses to the comparison of epochs with a specified asymmetry index as an additional quality assurance metric, which was not used in the prior study. We did not consider the impact of clinical variables on the predictive value of the various HRV for discriminating outcome groups. As the goal of this work was to evaluate the comparability of varying time windows on the estimation of HRV, evaluating whether clinical variables mediated relationships observed between HRV and outcome was beyond the scope of this project. We have previously demonstrated that clinical factors, including encephalopathy grade, gestational age, and temperature, can impact the relationships between HRV and outcomes [10]. Finally, we evaluated neurological outcomes defined by developmental assessments in the earlier study whereas the current study defined outcomes based on severity of brain injury by MRI. Side by side comparison of these methods will be considered in detail in our future studies.
Early studies of HIE infants have shown an evolution of sleep-wake cycles in infants that had good outcome whereas this evolution was not observed in infants with adverse outcomes [45, 46]. The higher spectral power in good outcome infants might represent an evolution of sleep-wake cycling and the depressed spectral power in the adverse outcome infants might represent a lack of the sleep-wake cycling in these newborns. Furthermore, the altered spectral power in adverse outcome group infants might be due to impairment of central autonomic network, brain centers that orchestrate autonomic modulation – caused by brain injury, however further studies are needed to confirm these inferences.
We also compared the spectral powers of the two groups of infants in LF calculated between 0.05 – 0.3 Hz and high-frequency calculated between 0.3 – 1.5 Hz. We found the LF frequency comparisons were not affected when extending the frequency band from 0.05 – 0.25 Hz to 0.05 – 0.3 Hz. However, in the HF power defined between 0.3 – 1.5 Hz the discriminatory power was compromised. These results clearly justify the choice of our frequency bands.
The absolute power in the very low-frequency can be regarded as a quantification of slow changes in the beat-to-beat intervals, which can also be quantified with SDNN. This relationship between very low-frequency power and SDNN shows the similarity between these measures in distinguishing the two groups of infants. However, the failure of SDNN to distinguish among three epoch lengths might indicate that total power (as it includes power below 0.04 Hz as well above 1 Hz) is not as robust as the power in the very low-frequency.
The strengths of this study include the evaluation of long recordings of BBi from a well-characterized cohort and the use of advanced signal processing techniques with robust approaches for artifact rejection and quality assurance. This study also has certain limitations. As a free-standing children’s hospital without an attached labor and delivery unit, we do not have access to low-risk newborns. Hence, we have included only acutely ill HIE newborns in this study. While examining a healthy term cohort would be of interest, we feel that the application of this work would be most clinically relevant to distinguish acutely ill newborns (i.e., normal and pathological tracings). Because the AR modeling has been shown to yield reliable spectral estimation from short dataset, we chose this approach in this study [16]. The model order of the AR process was fixed based on the convergence of the spectrum which is different from the traditional way wherein the model order is determined by the Akaike Information Criterion (AIC). Although the spectral convergence and AIC are closely related, this needs further investigation. We would also like to mention that the commonly used Akaike Information Criterion (AIC) is not reliable to identify the model order for short datasets. In this study, only the epochs with asymmetric index in a heuristically selected range were used. This might have confined the analysis to a certain behavioral (quiet) state of the infant. Further studies are needed to relate the AI to behavioral states. The characterization of sleep-wake cycles can help to interpret the spectral power differences between the two groups however, this has not been done in our cohort. We have also not corrected for any influence of behavioral states on variability analysis. Because low-frequency to high-frequency ratio has been shown to be an unreliable measure of sympathovagal balance [47], we did not use this metric in this study. Finally, although this method is demonstrated using HIE population with a well-characterized outcome, this method could be generalized to other types of critically-ill infants.
5. Conclusion
Analysis of BBi of newborns in two-minute epoch length is capable of distinguishing clinical groups. Extension of this approach to a larger cohort of HIE infant is needed and is currently underway with plans to translate this approach to real-time analysis for bedside application.
AR model allows characterization of spectral power of heart rate in short durations
An application is discussed for critically-ill infants with well-defined outcome groups
Spectral power of 2-minute of heart rate distinguished the two outcome groups reliably
Acknowledgment
This work was supported by the Clinical and Translational Science Institute at Children’s National (UL1TR000075 and 1KL2RR031987–01) and the Intellectual and Developmental Disabilities Research Consortium (NIH U54HD090257). The sponsors had no role in the design and conduct of the study; in the collection, management, analysis and interpretation of data; or in the preparation or review of the manuscript. We would like to thank Ms. Camarin King for her editorial assistant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare no conflict of interest.
References
- [1].Hon EH, Hess OW, The clinical value of fetal electrocardiography, Am J Obstet Gynecol, 79 (1960) 1012–1023. [DOI] [PubMed] [Google Scholar]
- [2].Cabal LA, Siassi B, Zanini B, Hodgman JE, Hon EE, Factors affecting heart rate variability in preterm infants, Pediatrics, 65 (1980) 50–56. [PubMed] [Google Scholar]
- [3].Doheny KK, Palmer C, Browning KN, Jairath P, Liao D, He F, Travagli RA, Diminished vagal tone is a predictive biomarker of necrotizing enterocolitis-risk in preterm infants, Neurogastroenterol Motil, 26 (2014) 832–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Tuzcu V, Nas S, Ulusar U, Ugur A, Kaiser JR, Altered heart rhythm dynamics in very low birth weight infants with impending intraventricular hemorrhage, Pediatrics, 123 (2009) 810–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Janig W, The Integrative Action of the Autonomic Nervous System Neurobiology of Homeostasis, Cambridge University Press, New York, NY, 2008. [Google Scholar]
- [6].Janig W, Habler HJ, Neurophysiological analysis of target-related sympathetic pathways--from animal to human: similarities and differences, Acta Physiol Scand, 177 (2003) 255–274. [DOI] [PubMed] [Google Scholar]
- [7].Barbieri R, Triedman JK, Saul JP, Heart rate control and mechanical cardiopulmonary coupling to assess central volume: a systems analysis, Am J Physiol Regul Integr Comp Physiol, 283 (2002) R1210–1220. [DOI] [PubMed] [Google Scholar]
- [8].Schneebaum Sender N, Govindan RB, Sulemanji M, Al-Shargabi T, Lenin RB, Eksioglu YZ, du Plessis AJ, Effects of regional brain injury on the newborn autonomic nervous system, Early Hum Dev, 90 (2014) 893–896. [DOI] [PubMed] [Google Scholar]
- [9].Schneebaum Sender N, Govindan RB, Whitehead MT, Massaro AN, Metzler M, Wang J, Cheng YI, du Plessis AJ, Cerebral modulation of the autonomic nervous system in term infants, J Perinatol, 37 (2017) 558–562. [DOI] [PubMed] [Google Scholar]
- [10].Metzler M, Govindan R, Al-Shargabi T, Vezina G, Andescavage N, Wang Y, du Plessis A, Massaro AN, Pattern of brain injury and depressed heart rate variability in newborns with hypoxic ischemic encephalopathy, Pediatr Res, 82 (2017) 438–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Task Force of the European Society of Cardiology the North American Society of Pacing Electrophysiology, Heart rate variability: standards of measurement, physiological interpretation, and clinical use, Circulation, 93 (1996) 1043–1065. [PubMed] [Google Scholar]
- [12].Chatow U, Davidson S, Reichman BL, Akselrod S, Development and maturation of the autonomic nervous system in premature and full-term infants using spectral analysis of heart rate fluctuations, Pediatr Res, 37 (1995) 294–302. [DOI] [PubMed] [Google Scholar]
- [13].Lake DE, Richman JS, Griffin MP, Moorman JR, Sample entropy analysis of neonatal heart rate variability, Am J Physiol Regul Integr Comp Physiol, 283 (2002) R789–797. [DOI] [PubMed] [Google Scholar]
- [14].Peng CK, Havlin S, Stanley HE, Goldberger AL, Quantification of scaling exponents and crossover phenomena in nonstationary heartbeat time series, Chaos, 5 (1995) 82–87. [DOI] [PubMed] [Google Scholar]
- [15].Akselrod S, Gordon D, Ubel FA, Shannon DC, Berger AC, Cohen RJ, Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control, Science, 213 (1981) 220–222. [DOI] [PubMed] [Google Scholar]
- [16].Kay SM, Modern Spectral Estimation Theory and Application, Prentice Hall, New Jersey, 1998. [Google Scholar]
- [17].Govindan RB, Massaro AN, Niforatos N, du Plessis A, Mitigating the effect of non-stationarity in spectral analysis-an application to neonate heart rate analysis, Comput Biol Med, 43 (2013) 2001–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Yiallourou SR, Poole H, Prathivadi P, Odoi A, Wong FY, Horne RS, The effects of dummy/pacifier use on infant blood pressure and autonomic activity during sleep, Sleep Med, 15 (2014) 1508–1516. [DOI] [PubMed] [Google Scholar]
- [19].Fyfe KL, Yiallourou SR, Wong FY, Odoi A, Walker AM, Horne RS, The Effect of Gestational Age at Birth on Post-Term Maturation of Heart Rate Variability, Sleep, 38 (2015) 1635–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Takatani T, Takahashi Y, Yoshida R, Imai R, Uchiike T, Yamazaki M, Shima M, Nishikubo T, Ikada Y, Fujimoto S, Relationship between frequency spectrum of heart rate variability and autonomic nervous activities during sleep in newborns, Brain Dev, 40 (2018) 165–171. [DOI] [PubMed] [Google Scholar]
- [21].Moorman JR, Lake DE, Griffin MP, Heart rate characteristics monitoring for neonatal sepsis, IEEE Trans Biomed Eng, 53 (2006) 126–132. [DOI] [PubMed] [Google Scholar]
- [22].Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, Fanaroff AA, Poole WK, Wright LL, Higgins RD, Finer NN, Carlo WA, Duara S, Oh W, Cotten CM, Stevenson DK, Stoll BJ, Lemons JA, Guillet R, Jobe AH, Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy, N Engl J Med, 353 (2005) 1574–1584. [DOI] [PubMed] [Google Scholar]
- [23].Barkovich AJ, Hajnal BL, Vigneron D, Sola A, Partridge JC, Allen F, Ferriero DM, Prediction of neuromotor outcome in perinatal asphyxia: evaluation of MR scoring systems, AJNR Am J Neuroradiol, 19 (1998) 143–149. [PMC free article] [PubMed] [Google Scholar]
- [24].Ulusar UD, Govindan RB, Wilson JD, Lowery CL, Preissl H, Eswaran H, Adaptive rule based fetal QRS complex detection using hilbert transform, Conf Proc IEEE Eng Med Biol Soc, 1 (2009) 4666–4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Govindan RB, Al-Shargabi T, Metzler M, Andescavage NN, Joshi R, Du Plessis A, A spike correction approach for variability analysis of heart rate in sick infants, Physica A, 444 (2016) 35–42. [Google Scholar]
- [26].Brillinger D, Time series-Data Analysis and Theory, 2nd edn ed., Holden Day, San Francisco, 1981. [Google Scholar]
- [27].Friedlander B, Porat B, The modified Yule-Walker method of ARMA spectral estimation, IEEE Transactions on Aerospace and Electronic Systems, (1984) 158–173.
- [28].Houle MS, Billman GE, Low-frequency component of the heart rate variability spectrum: a poor marker of sympathetic activity, The American journal of physiology, 276 (1999) H215–223. [DOI] [PubMed] [Google Scholar]
- [29].Fairchild KD, Aschner JL, HeRO monitoring to reduce mortality in NICU patients, Research and Report in Neonatology, 2 (2012) 65–76. [Google Scholar]
- [30].Kovatchev BP, Farhy LS, Cao H, Griffin MP, Lake DE, Moorman JR, Sample asymmetry analysis of heart rate characteristics with application to neonatal sepsis and systemic inflammatory response syndrome, Pediatr Res, 54 (2003) 892–898. [DOI] [PubMed] [Google Scholar]
- [31].Al-Shargabi T, Reich D, Govindan RB, Shankar S, Metzler M, Cristante C, McCarter R, Sandler AD, Said M, Plessis A.d., Changes in Autonomic Tone in Premature Infants Developing Necrotizing Enterocolitis, Amer J Perinatol, 35 (2018) 1079–1086. [DOI] [PubMed] [Google Scholar]
- [32].Vanagas G, Receiver operating characteristic curves and comparison of cardiac surgery risk stratification systems, Interactive CardioVascular and Thoracic Surgery, 3 (2004) 319–322. [DOI] [PubMed] [Google Scholar]
- [33].Greiner M, Pfeiffer D, Smith R, Principles and practical application of the receiver-operating characteristic analysis for diagnostic tests, Preventive veterinary medicine, 45 (2000) 23–41. [DOI] [PubMed] [Google Scholar]
- [34].Assali NS, Brinkman CR 3rd, Woods JR Jr., Dandavino A, Nuwayhid B, Development of neurohumoral control of fetal, neonatal, and adult cardiovascular functions, Am J Obstet Gynecol, 129 (1977) 748–759. [DOI] [PubMed] [Google Scholar]
- [35].Van Leeuwen P, Geue D, Lange S, Hatzmann W, Gronemeyer D, Changes in the frequency power spectrum of fetal heart rate in the course of pregnancy, Prenat Diagn, 23 (2003) 909–916. [DOI] [PubMed] [Google Scholar]
- [36].Groome LJ, Mooney DM, Bentz LS, Singh KP, Spectral analysis of heart rate variability during quiet sleep in normal human fetuses between 36 and 40 weeks of gestation, Early Hum Dev, 38 (1994) 1–9. [DOI] [PubMed] [Google Scholar]
- [37].Ferrazzi E, Pardi G, Setti PL, Rodolfi M, Civardi S, Cerutti S, Power spectral analysis of the heart rate of the human fetus at 26 and 36 weeks of gestation, Clin Phys Physiol Meas, 10 Suppl B (1989) 57–60. [DOI] [PubMed] [Google Scholar]
- [38].Nijhuis JG, Prechtl HF, Martin CB Jr., Bots RS, Are there behavioural states in the human fetus?, Early Hum Dev, 6 (1982) 177–195. [DOI] [PubMed] [Google Scholar]
- [39].Yiallourou SR, Witcombe NB, Sands SA, Walker AM, Horne RS, The development of autonomic cardiovascular control is altered by preterm birth, Early Hum Dev, 89 (2012) 145–152. [DOI] [PubMed] [Google Scholar]
- [40].Govindan RB, Lowery CL, Campbell JQ, Best TH, Murphy P, Preissl HT, Eswaran H, Early maturation of sinus rhythm dynamics in high-risk fetuses, Am J Obstet Gynecol, 196 (2007) 572 e571–577; discussion 572 e577. [DOI] [PubMed] [Google Scholar]
- [41].Govindan RB, Massaro AN, Al-Shargabi T, Andescavage NN, Chang T, Glass P, Du Plessis A, Detrended fluctuation analysis of non-stationary cardiac beat-to-beat interval of sick infants, EPL (Europhysics Letters), 108 (2014) 40005-p40001–p40006. [Google Scholar]
- [42].Massaro AN, Govindan RB, Al-Shargabi T, Andescavage NN, Metzler M, Chang T, Glass P, du Plessis AJ, Heart rate variability in encephalopathic newborns during and after therapeutic hypothermia, J Perinatol, 34 (2013) 836–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Vergales BD, Zanelli SA, Matsumoto JA, Goodkin HP, Lake DE, Moorman JR, Fairchild KD, Depressed heart rate variability is associated with abnormal EEG, MRI, and death in neonates with hypoxic ischemic encephalopathy, Am J Perinatol, 31 (2013) 855–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Goulding RM, Stevenson NJ, Murray DM, Livingstone V, Filan PM, Boylan GB, Heart rate variability in hypoxic ischaemic encephalopathy: correlation with EEG grade and two-year neurodevelopmental outcome, Pediatr Res, 77 (2015) 681–687. [DOI] [PubMed] [Google Scholar]
- [45].Sewell EK, Vezina G, Chang T, Tsuchida T, Harris K, Ridore M, Glass P, Massaro AN, Evolution of Amplitude-Integrated Electroencephalogram as a Predictor of Outcome in Term Encephalopathic Neonates Receiving Therapeutic Hypothermia, Am J Perinatol, 35 (2018) 277–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Massaro AN, Tsuchida T, Kadom N, El-Dib M, Glass P, Baumgart S, Chang T, aEEG evolution during therapeutic hypothermia and prediction of NICU outcome in encephalopathic neonates, Neonatology, 102 (2012) 197–202. [DOI] [PubMed] [Google Scholar]
- [47].Billman GE, The LF/HF ratio does not accurately measure cardiac sympatho-vagal balance, Front Physiol, 4 (2013) 26. [DOI] [PMC free article] [PubMed] [Google Scholar]