Abstract
Neurodegenerative diseases such as Parkinson’s disease (PD), dementia with Lewy bodies (DLB), and Alzheimer’s disease (AD) involve loss of cholinergic neurons in the basal forebrain. Here, we investigate how cholinergic dysfunction impacts the frontal cortex during interval timing, a process that can be impaired in PD and AD patients. Interval timing requires participants to estimate an interval of several seconds by making a motor response, and depends on the medial frontal cortex (MFC), which is richly innervated by basal forebrain cholinergic projections. Past work has shown that scopolamine, a muscarinic cholinergic receptor antagonist, reliably impairs interval timing. We tested the hypothesis that scopolamine would attenuate time-related ramping, a key form of temporal processing in the MFC. We recorded neuronal ensembles from 8 mice during performance of a 12-s fixed-interval timing task, which was impaired by the administration of scopolamine. Consistent with past work, scopolamine impaired timing. To our surprise, we found that time-related ramping was unchanged, but stimulus-related activity was enhanced in the MFC. Principal component analyses revealed no consistent changes in time-related ramping components, but did reveal changes in higher components. Taken together, these data indicate that scopolamine changes stimulus-processing rather than temporal processing in the MFC. These data could help understand how cholinergic dysfunction affects cortical circuits in diseases such as PD, DLB, and AD.
Keywords: Scopolamine, interval timing, medial frontal cortex, neuronal ensemble recording, cholinergic circuit dysfunction, Parkinson’s disease, dementia with Lewy Bodies, Alzheimer’s disease
Introduction
Cholinergic dysfunction is a major feature of Alzheimer’s disease (AD), Parkinson’s disease (PD), and dementia with Lewy bodies (DLB)(Arendt et al., 1983; Tiraboschi et al., 2000; Bohnen et al., 2003, 2006; Bohnen and Albin, 2011). In particular, cholinergic neurons are located in the basal forebrain, which suffers marked neurodegeneration in AD, PD, and DLB (Johnston et al., 1979; Bigl et al., 1982; Arendt et al., 1983; Whitehouse et al., 1983). Furthermore, drugs that block acetylcholine breakdown, such as donepezil and rivastigmine, can improve cognitive function in PD, DLB, and AD. Basal forebrain cholinergic neurons project broadly to the cortex; however, it is unknown how cholinergic dysfunction affects cortical circuits.
One cognitive process that depends on cholinergic circuits and is consistently impaired in AD and PD is interval timing, which requires subjects to estimate an interval of several seconds by making a motor response (Malapani et al., 1998; Caselli et al., 2009; Parker et al., 2015; Kim et al., 2017). Interval timing is ideal for investigating cortical cholinergic function because 1) it depends on the medial frontal cortex (MFC), which is disrupted in AD, PD, and DLB (Kim et al., 2009; Coull et al., 2011; Parker et al., 2013, 2014; Emmons et al., 2017), 2) it is highly conserved across mammalian species and thus can be readily investigated in rodent models (Buhusi and Meck, 2005), and 3) interval timing in rodents is reliably impaired when they are given scopolamine, a cholinergic inhibitor of muscarinic receptors (Meck, 1996; Abner et al., 2001; Balci et al., 2008). Our recent work has indicated that a key form of temporal processing in the rodent MFC is time-related ramping activity; in other words, monotonic increases or decreases in firing rate across a temporal interval (Simen et al., 2011; Narayanan, 2016; Emmons et al., 2017). Based on these data, interval timing is ideally suited to cortical consequences of cholinergic deficits in AD and PD. Specifcally, we hypothesized that scopolamine would impair time-related ramping by MFC neurons.
We tested this hypothesis by recording neuronal ensembles from mice performing a 12-s fixed-interval-timing task, and administering intraperitoneal saline or scopolamine. We found that while scopolamine impaired interval timing, it did not affect time-related ramping in the MFC. Surprisingly, it increased stimulus-related processing in this brain structure. We interpret these data in the context of cholinergic functions and circuits relevant for AD, PD, and DLB.
Experimental Procedures
Mice:
This study used 8 wild-type C57/BL6J male mice purchased from Jackson Laboratories (000664) at 3 months of age. Mice consumed 1–1.5 g of sucrose pellets during each behavioral session, and additional food was provided 1–2 hr after each behavioral session in the home cage. Single housing and a 12-hr light/dark cycle were used; all experiments took place during the light cycle. Mice were maintained at 80–85% of their baseline body weight during the course of these experiments for motivation. All procedures were approved by the Animal Care and Use Committee (#707239) at the University of Iowa, in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Mouse fixed-interval timing task:
Mice were trained to perform an interval-timing task with a 12-sec interval (Kim et al., 2017; Kim and Narayanan, 2018). Operant chambers (MedAssociates) were equipped with a nose poke hole with a yellow LED stimulus light (ENV-313W), a pellet dispenser (ENV-203–20), and a house light (ENV-315W). Behavioral chambers were housed in sound-attenuating chambers (MedAssociates). All behavioral responses including nose pokes and access to pellet receptacles were recorded with infra-red sensors. First, animals learned to make operant nose pokes to receive rewards (20-mg rodent purified pellets, F0071, Bio-Serv). After fixed-ratio training (FR1), animals were trained in a 12-sec fixed-interval timing task in which rewards were delivered for responses after a 12-sec interval (Figure 1A). The house light was turned on to signal the start of the 12-sec interval. Early responses were not rewarded. Responses after 12 sec resulted in trial termination with reward delivery. Rewarded nose pokes were signaled by the house light turning off. Each trial was followed by a 30± 6-sec pseudorandom inter-trial interval that concluded with the house light turning on, signaling the beginning of the next trial. All sessions were 60 min long. Responses were summed into time-response histograms with 1-sec bins from 0 to 18 sec after trial start. For plotting, we used the MATLAB function ksdensity.m to estimate the probability density function time-response histograms with a bandwidth of 1, normalized to maximum response rate, and averaged across animals. We quantified timing using a measure of the curvature of time-response histograms. This metric is based on the cumulative distribution function’s deviation from a straight line; it is 0 when the time-response curve is flat during the interval but closer to 1 when more responses are at 12 s and time-response histograms are more curved. We and others have used this metric extensively to quantify timing because curvature is resistant to differences in overall response rate(Fry et al., 1960; Narayanan et al., 2012; Kim et al., 2017; Kim and Narayanan, 2018). Finally, to investigate sources of variance contributing to interval timing, we used gaussian-mixture models (fitgmdist.m) to fit time-response histograms with 1 or 2 parameters to compare unimodal vs. bimodal distributions (Matell and Portugal, 2007). Fits were compared via Bayes Information Criteria (BIC).
Figure 1. Scopolamine impairs interval timing.
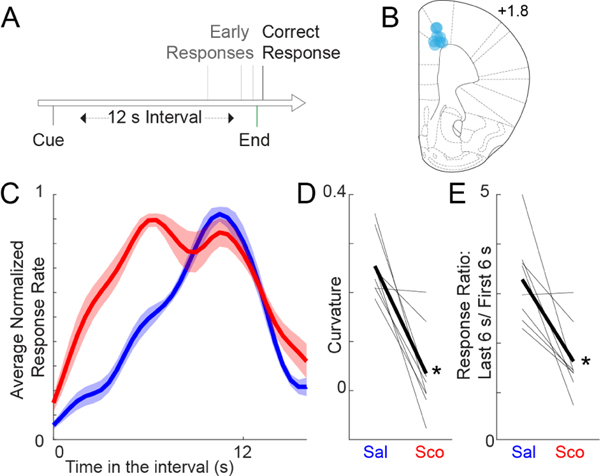
A) Interval timing task: Mice were trained to perform a fixed-interval timing task with a 12 s interval. The first response after 12 s led to a food reward; early responses were unreinforced. B) Recording electrode locations (blue dots) in the medial frontal cortex. C) Time-response histograms during fixed-interval timing. Data for mice administered saline are plotted in blue, and those for mice administered scopolamine are plotted in red. D) Timing can be quantified by computing a curvature index of time-response histograms; scopolamine decreases the curvature index, indicating flatter time-response histograms. E) We also noticed that animals with scopolamine responded more during the earlier portion of the interval. The ratio of responses in the last 6 vs. first 6 s closer to one, unlike in sessions with saline injected. Data from 8 mice; * = Signrank p<0.05.
Surgical procedures:
Mice trained in the 12-sec fixed-interval-timing task were implanted with recording microelectrode arrays (Microprobes) targeting the MFC prior to neurophysiology recordings. Briefly, mice were anesthetized using ketamine (100 mg/kg) and xylazine (10 mg/kg). A surgical level of anesthesia was maintained, with ketamine supplements (10 mg/kg) given hourly (or as needed) and regular monitoring for stable respiratory rate and absent toe pinch response. Mice were placed in the stereotactic equipment with non-rupturing ear bars. A heating pad was used to prevent hypothermia. Under aseptic surgical conditions, the skull was leveled between the bregma and lambda. A single craniotomy was drilled over the area above the MFC and three holes were drilled for skull screws. For recording experiments, animals were implanted (coordinates from the bregma: AP: +1.8, ML + 0.5, DV −1.8) with a microelectrode array configured as a 4×4 array of 50 μm stainless steel wires (200 μm between wires and rows; impedance measured in vitro at 400–600 kΩ; Microprobes). Electrode ground wires were wrapped around the skull screws. The electrode array was inserted while concurrently recording neuronal activity. The craniotomy was sealed with cyanoacrylate (“SloZap”, Pacer Technologies) accelerated by “ZipKicker” (Pacer Technologies), and methyl methacrylate (“dental cement”; AM Systems). Following implantation, animals were allowed to recover for two weeks before being reacclimatized to behavioral and recording procedures.
Neuronal ensemble recordings:
Freely moving electrophysiological recordings were performed as described in detail previously (Kim et al., 2017; Kim and Narayanan, 2018). Following training in a 12-sec interval timing task and MFC implantation with 16-channel microelectrodes, mice were subjected to intraperitoneal injection with normal saline or scopolamine (1mg/kg, Sigma-Aldrich, S0929) while being recorded during the 12-sec interval timing task. Mice were connected to the recording head stage and a cable without anesthesia. Neuronal ensemble recordings in the MFC were made using a multi-electrode recording system (Open Ephys). Raw wideband signal was high-pass filtered at 0.05Hz with total gain of 5000, and recorded with 16bit digitization at 30k Hz sampling rate. To detect spikes, raw signals were rereferenced using common median referencing to minimize potential non-neural electrical noise, and band-pass filtered between 300 and 6000 Hz offline. Spikes were detected with a threshold of 5 median absolute deviations. A Plexon Offline Sorter was used to sort single units and to remove artifacts. PCA and waveform shape were used for spike sorting. Single units were identified as having 1) consistent waveform shape, 2) separable clusters in PCA space, and 3) a consistent refractory period of at least 1 ms in inter-spike-interval histograms. Unique neurons were verified by constructing two-dimensional cumulative distribution probabilities from Pearson’s correlation coefficients of pair-wise waveform and inter-spike-interval comparisons, and using a one-tailed threshold of p<0.05. Spike activity was analyzed for all cells that fired at rates above 0.1 Hz. Local field potential (LFP) was recorded with bandpass filters between 0.05 and 1000 Hz. Statistical summaries were based on all recorded neurons. No subpopulations were selected or filtered out of the neuron database. Analysis of neuronal activity and quantitative analysis of basic firing properties were carried out with custom routines for MATLAB (all raw data and MATLAB scripts are available at our lab website: https://narayanan.lab.uiowa.edu/article/datasets). All behavioral events were recorded simultaneously using TTL inputs at 30k Hz. Peri-event rasters and average histograms were constructed around trial start.
We analyzed our neuronal data according to procedures described at length previously (Kim et al., 2017; Kim and Narayanan, 2018). For each neuron, we constructed peri-event spike data from −2 sec to 12 sec after trial start. As in the past, we defined time-related ramping neurons as those with a significant fit via linear regression of time vs. firing rate over the interval binned at 1 sec. Finally, we defined stimulus-modulated and response-modulated neurons as those with trial-by-trial changes in firing rate 0–200 msec after event onset compared to −500 to - 300 msec prior to stimulus onset with a p<0.05 via a paired t-test. Pearson’s chi-squared test was used to compare the number of modulated neurons between saline and scopolamine sessions.
We analyzed neuronal patterns using PCA, which we have used to identify patterns of neuronal activity in an unbiased, data-driven manner (Chapin and Nicolelis, 1999; Narayanan and Laubach, 2009; Parker et al., 2014; Kim et al., 2017). PCA was constructed from z-transformed peri-event time histograms over the entire interval binned at 0.1 sec and smoothed with a gaussian kernel over 5 bins. All neurons from 8 mice from sessions with saline or scopolamine infusions were included in PCA. We then used a t-test to compare PCs between saline and scopolamine sessions.
MFC LFP power was calculated in defined frequency bands (delta: 1–4 Hz, theta: 5–8 Hz; alpha: 9–12 Hz; beta: 13–30 Hz) during the interval (0–12 s) using wavelet-based time-frequency analyses.
Histology:
When experiments were complete, mice were euthanized by injections of 100 mg/kg sodium pentobarbital. All mice were intracardially perfused with 4% paraformaldehyde. The brain was removed and post-fixed in paraformaldehyde overnight and immersed in 30% sucrose until the brains sank. Sections (40 μm) were made on a cryostat (Leica) and stored in cryoprotectant (50% PBS, 30% ethylene glycol, 20% glycerol) at −20°C, before being mounted onto slides with mounting media containing DAPI (Invitrogen P36962). Images were captured on an Olympus VS120 Microscope.
Statistics:
We used nonparametric tests to account for the possibility of non-normal data. Wilcoxon sign rank (signrank.m in MATLAB) was used to compare paired data, while Wilcoxon rank sum (ranksum.m in MATLAB) was used to compare non-paired data, and chi-squared tests were used to compare categorical variables. All tests were preplanned to match prior work (Parker et al., 2014; Kim et al., 2017; Kim and Narayanan, 2018); accordingly, multiple comparisons corrections were not performed. In neuronal data, animal-specific variance was accounted for by running a generalized linear mixed-effects model (fitglme.m in MATLAB) and each animal was included as a random effect. Statistical approaches were reviewed by an independent statistician at the Institute for Clinical and Translational Sciences are the University of Iowa.
Results
Scopolamine impairs fixed-interval timing
Past studies in rodents have demonstrated that scopolamine impairs interval timing (Meck, 1996; Abner et al., 2001; Balci et al., 2008). During neuronal recording sessions, we found that scopolamine infusion markedly changed time-response histograms during fixed-interval timing (Fig 1C). We calculated the curvature of time-response histograms to measure timing, a metric based on cumulative distribution functions that we and others have used in the past (Fry et al., 1960; Narayanan et al., 2012; Kim et al., 2017). We found that scopolamine significantly decreased the curvature of time-response histograms (0.25 +/− 0.02 with saline vs. 0.03 +/− 0.03 with scopolamine; Signrank p=0.008; Fig 1D). Furthermore, we noticed that there were more responses early in the interval and that scopolamine decreased the ratio of responses late in the interval vs. early in the interval (last 6 sec divided by first 6 sec; 3.27 +/− 0.31 with saline vs. 1.60 +/− 0.26 with scopolamine, Signrank p = 0.02; Fig 1E). Time-response histograms with scopolamine appeared to be bimodal; accordingly, BIC values were lower (implying a better fit) for bimodal distributions relative to unimodal distributions for scopolamine sessions (99.4%) vs. saline sessions (99.8%). Scopolamine did not change the number of overall responses between 0 and 12 sec (86.2 +/− 23.0 with saline vs. 98.4 +/− 28.5 with scopolamine) or the number of rewards (66.6 +/− 5.3 with saline vs. 63.8 +/− 5.3 with scopolamine). Taken together, our findings are consistent with past work demonstrating that scopolamine impairs interval timing (Meck, 1996; Abner et al., 2001; Balci et al., 2008).
Scopolamine does not change MFC ramping but increases stimulus-related processing.
Prior work by our group and others has demonstrated that time-related ramping activity by MFC neurons is a key correlate of temporal processing (Narayanan, 2016; Emmons et al., 2017). As scopolamine impairs interval timing, we hypothesized that this drug would impair time-related ramping. We tested this idea by identifying time-related ramping MFC neurons by linear regression (Fig 2A), and comparing the number of MFC ramping neurons in sessions with saline and scopolamine. In 8 mice, of 117 MFC neurons recorded during saline sessions, 41 exhibited time-related ramping (35%). Critically, a similar fraction of MFC neurons exhibited ramping with scopolamine (36 of 108, or 33%); this did not support our hypothesis.
Figure 2. Scopolamine does not change MFC time-related ramping, but increases MFC stimulus-related activity.
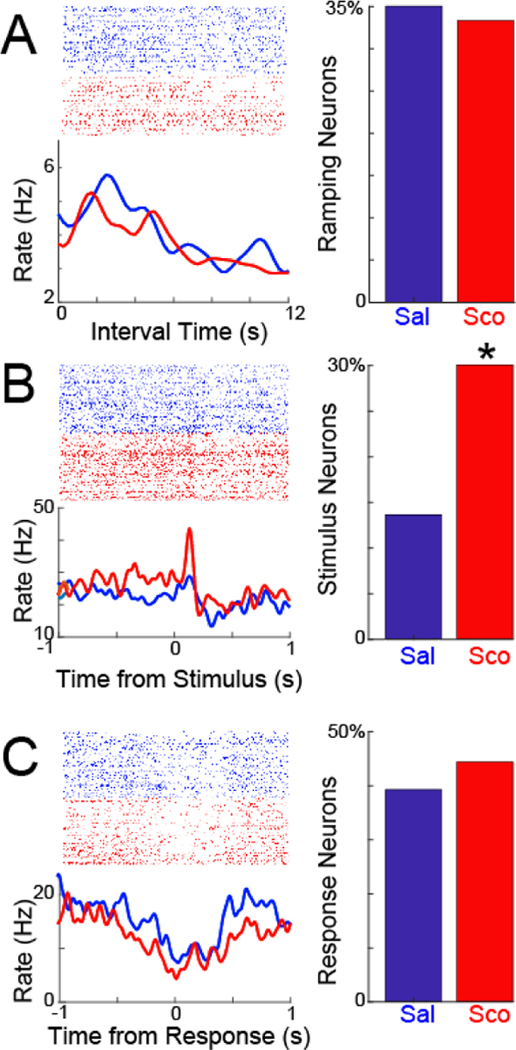
A) We identified MFC neurons with time-related ramping activity by linear regression. Crucially, scopolamine did not change the number of MFC ramping neurons. B) By contrast, scopolamine dramatically increased the number of MFC neurons with stimulus-related modulation. C) Scopolamine did not change MFC neurons with response-related modulation. Rasters are from the same putative neurons in saline and scopolamine sessions. Data from 8 mice; 117 MFC neurons recorded in saline sessions and 108 neurons recorded in scopolamine sessions; * =p<0.05 via χ2 test.
Next, we looked at other modulation patterns in MFC. Prefrontal regions can powerfully affect stimulus-processing (Passetti et al., 2002; Zanto et al., 2011; Liebe et al., 2012). During fixed-interval timing, this stimulus is a light that goes on at trial start (Fig 2B). Surprisingly, there were twice as many stimulus-modulated MFC neurons in scopolamine sessions (32 of 108, or 30%) compared to saline sessions (16 of 117, or 14%; X2=7.6, p=0.006; linear mixed-effects model accounting for animal-specific variance p<0.002). There were no differences in the number of response-modulated neurons (Figure 2C; 39% with saline vs. 44% with scopolamine). These data provide evidence that neither MFC ramping nor response-related activities are changed by scopolamine; by contrast, scopolamine increased MFC stimulus-related modulation.
When comparing average MFC neuronal ensemble activity in saline and scopolamine sessions, we noticed subtle differences late in the interval (increased activity at black arrow in Fig 3A vs. decreased activity at black arrow in Fig 3B). These resulted in different average activities of MFC neuronal ensembles with saline vs. scopolamine (Fig 3C). To capture these differences with data-driven techniques, we turned to principal component analysis, which have been used extensively to identify patterns in complex neuronal data (Chapin and Nicolelis, 1999; Narayanan and Laubach, 2009; Parker et al., 2014; Kim et al., 2017). We found three common patterns. PC1, which explained 29% of variance, exhibited time-related ramping activity, consistent with extensive past work from our group (Fig 3D–E). Of note, this component did not change with scopolamine, consistent with our results above and contrary to our hypothesis. PC2, which explained 19% of variance, was broadly modulated across the interval, and also did not change with scopolamine. By contrast, PC3, which explained 12% of variance, had a more complex pattern, with a peak close to 7 sec in the interval. PC3 was more negative compared to saline sessions (Ranksum p = 0.01; linear mixed-effects model accounting for animal-specific variance p<0.02). These data provide further evidence that scopolamine did not change MFC ramping but could change more complex aspects of MFC neuronal ensemble activity.
Figure 3. Neuronal ensemble effects of scopolamine:
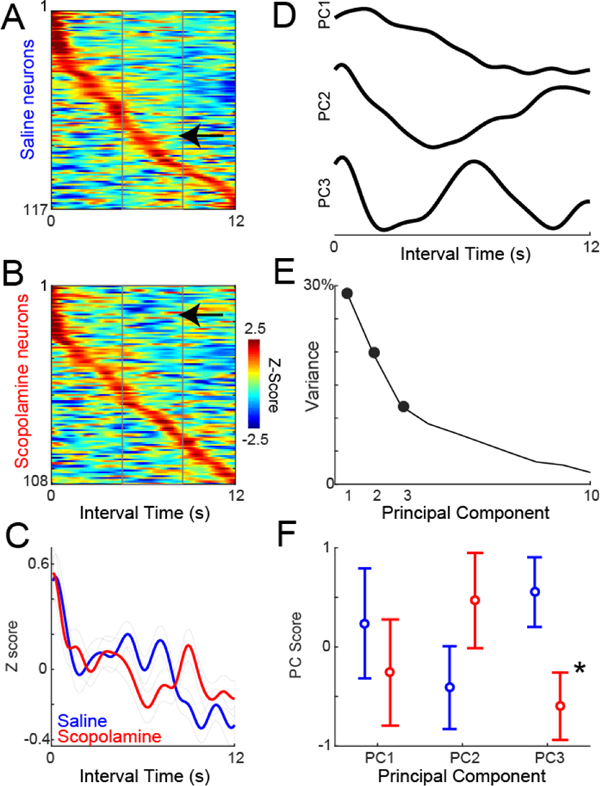
A) Average Z-scored neuronal activity during the interval shown for all MFC neurons treated with saline, and B) scopolamine, sorted by peak activity. We noticed subtle differences in activity, with more activity in saline (arrow in A), vs. less activity in scopolamine (arrow in B) late in the interval. Activity binned at 10 ms and smoothed over 5 bins. C) There were differences during the interval between average MFC neuronal activities in saline vs. scopolamine sessions. D) To quantify these differences using data-driven techniques, we turned to principal component analysis, which identified 3 major patterns. PC1 had ramping patterns, PC2 was modulated during the interval, and PC3 had more complex modulation. E) Fraction of variance explained by each component. F) Only PC3 was different between saline and scopolamine sessions. Data from 8 mice; 117 MFC neurons recorded in saline sessions and 108 neurons recorded in scopolamine sessions; * = Ranksum p<0.05.
Finally, we examined MFC LFP oscillatory power during the interval (0–12 s; Figure 4). We found no consistent changes in delta (1–4 Hz), theta (5–8 Hz), alpha (9–12 Hz), or beta activity (13–30 Hz). In summary, our results suggest that scopolamine impaired interval timing and enhances stimulus-related processing in MFC without changing MFC temporal processing or MFC LFPs.
Figure 4. Scopolamine does not change MFC LFP activity:
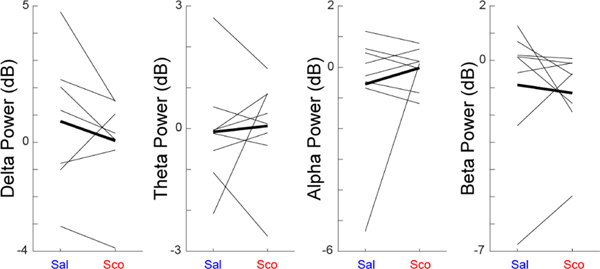
We measured MFC LFPs from 8 mice in delta (1–4 Hz), theta (5–8 Hz), alpha (9–12 Hz), and beta (13–30 Hz) power in saline and scopolamine sessions. We did not observe consistent differences between saline and scopolamine sessions. Data from 8 mice.
Discussion
We tested the hypothesis that scopolamine would attenuate time-related ramping activity in the MFC. We found no evidence that scopolamine changed MFC ramping by linear regression or PCA. To our surprise, we found that stimulus-related processing was increased in the MFC with scopolamine. These data provide insight into how scopolamine might influence cortical circuits during interval timing.
Many cognitive behaviors are impaired by the muscarinic cholinergic inhibitor scopolamine, often through attentional and stimulus-processing deficits (Sarter and Bruno, 1997; Klinkenberg and Blokland, 2010). Scopolamine reliably causes timing deficits in rodents and here we report similar deficits during fixed-interval timing(Meck, 1996; Abner et al., 2001; Balci et al., 2008). Surprisingly, MFC ramping is intact with scopolamine administration. There are two possibilities that might account for this result. First, we and others have identified neuronal ramping as a key mechanism of temporal processing in the MFC (Simen et al., 2011; Narayanan, 2016; Emmons et al., 2017; Wang et al., 2018), but it is possible that ramping is not explicitly related to timing. In this case, others have proposed temporal computations based on oscillatory activity (Matell and Meck, 2004), and we note that PC3 has oscillatory features, although the period appeared to be longer than 1 second. Scopolamine may affect neuronal oscillatory activity that was not detected by our analyses. A second possibility is that scopolamine affects stimulus-processing mechanisms in the MFC and this triggers animals to respond despite intact MFC temporal processing (Daffner et al., 2000; Passetti et al., 2002; Liebe et al., 2012). The bimodal shape of scopolamine time-response (Fig 1C) and the increase in responses early in the interval (Fig 1E) and the histograms is supportive of this idea.
Previous studies have shown that acetylcholine is crucial for stimulus processing. Microdialysis has revealed that acetylcholine is increased during tasks involving sustained attention, changes in ambient light, food anticipation, motor activity, and handling, while amperometry has indicated that MFC acetylcholine can increase for reward-predictive cues (Day et al., 1991; Inglis and Fibiger, 1995; Himmelheber et al., 1997, 1998; Sarter and Bruno, 1997; Bruno et al., 2006; Parikh and Sarter, 2008). Acetylcholine can also be increased during attentionally demanding tasks such as 5-choice serial reaction-time tasks (Passetti et al., 2000, 2002). Lesioning of MFC cholinergic projections impairs the processing of fast but not slow stimuli, as well as causing marked deficits in visual attentional performance (Winters et al., 2004). To our knowledge MFC cholinergic projections have never been studied in interval timing, although cholinergic projections to the visual cortex affect learning of temporal intervals but not interval-timing performance (Chubykin et al., 2013). Lesioning of MFC cholinergic projections decreased stimulus-related activity and the performance of a visual attention task, contrary to our results here with scopolamine, a muscarinic antagonist (Gill et al., 2000). In primates, cholinergic deafferentation can affect working memory, and cholinergic effects on working memory and attention occur as a result of the direct effects on stimulus processing (Furey et al., 2007; Croxson et al., 2011) . These studies broadly support a role for cortical cholinergic circuits in task-related stimulus processing.
Of note, scopolamine may produce effects beyond stimulus processing. For instance, scopolamine can increase premature responding and omissions during 5-choice serial reaction-time performance in rats (Bruinsma et al., 2019) . During differential reinforcement of low rates of responding tasks, scopolamine increased impulsivity (Jayarajan et al., 2013). It is unclear these features contribute to behavioral deficits in our version of the fixed-interval task. Indeed, interval-timing lends itself to detailed computational modeling and inferences about component processing (Luzardo et al., 2017). Unfortunately, because our focus was on neuronal activity, we used a fixed-interval task with a single interval and no peak trials, severely constraining computational insights from our task.
Patients with AD and PD have deficits in timing (Nichelli et al., 1993; Parker et al., 2013; Zhang et al., 2015). Patients with AD show increased variability with both overestimation and underestimation of temporal intervals (Nichelli et al., 1993; Carrasco et al., 2000; Papagno et al., 2004; Caselli et al., 2009; Rueda and Schmitter-Edgecombe, 2009). In one animal models of AD (5xFAD), mice responded earlier in the interval, consistent with encoding-related distortions of temporal intervals, although this was not consistent with other AD models (Gür et al., 2019a, 2019b).
PD patients can have both shifts in timing and increased variability, with dopamine powerfully modulating temporal performance (Malapani et al., 1998, 2002; Buhusi and Meck, 2005; Merchant et al., 2008). PD patients suffer neurodegeneration in dopaminergic as well as cholinergic neurons (Bohnen et al., 2003, 2006; Fahn, 2008; Bohnen and Albin, 2011; Narayanan et al., 2013). Dopaminergic deficits have been associated with clock-speed and temporal memories in PD (Malapani et al., 2002; Malapani and Rakitin, 2003; Buhusi and Meck, 2005) which can be modeled in animals (Meck, 2006; Narayanan et al., 2012; Soares et al., 2016). Cholinergic deficits in PD correlate with decreased attentional and executive function (Bohnen et al., 2006), as well as other gait and non-motor symptoms (Bohnen and Albin, 2011). In this study, we find that blocking muscarinic acetylcholine receptors is quite different from dopaminergic manipulations in the frontal cortex or striatum (Parker et al., 2014, 2015a; Emmons et al., 2017; Kim et al., 2017). While dopaminergic manipulations also reliably affect interval timing, our work indicates that manipulating prefrontal dopamine via D1-type dopamine receptors affects both time-related ramping activity and ~4 Hz rhythms (Parker et al., 2014, 2015b, 2017). By contrast, scopolamine did not affect MFC ramping or MFC LFPs, but enhanced stimulus-related activity. These data suggest that cholinergic and dopaminergic manipulations have distinct and specific effects on cortical circuits. Future studies with cell-type specific resolution might be able to further resolve themes of cortical cholinergic vs. dopaminergic circuits. AD, PD, and DLB patients have prominent cholinergic deficits (Arendt et al., 1983; Tiraboschi et al., 2000), and cholinergic drugs can improve cognition in these patients (Emre et al., 2004; Zhang et al., 2015). However, little is known about the relevant cholinergic circuit mechanisms of these effects, and future work will precisely delineate cholinergic contributions to interval timing. Cholinergic dysfunction likely contributes to timing deficits in both AD and PD through compromised stimulus processing, while other neurotransmitter systems such as dopamine might impair other aspects of temporal processing.
Our study has several limitations. First, scopolamine was administered systemically and is a poor model of cognitive dysfunction in dementia, as it can act on all muscarinic acetylcholine receptors in the brain and other brain systems (Klinkenberg and Blokland, 2010; Falsafi et al., 2012). In addition, it is possible that there are dose-dependent effects. Furthermore, it can have non-specific locomotor and autonomic effects, although we did not observe an increased response rate and observed highly specific neuronal effects on stimulus-processing in this study. Cholinergic interneurons in the striatum or the MFC may also be critical mediators of cognitive processing (Witten et al., 2010). Finally, we are unsure if scopolamine directly modulates MFC stimulus-related neurons or modulates other brain areas. Nevertheless, our results lay important groundwork for highly specific investigation of cholinergic circuits using cell-type specific methods in future work.
In conclusion, we performed neuronal ensemble recording from the MFC of freely moving rodents, and found that muscarinic cholinergic inhibition caused timing deficits and hyper-stimulus-modulation during interval timing. MFC temporal processing was not significantly affected. These results are broadly in line with a role acetylcholine plays in attention, working memory and stimulus processing, while the dopaminergic system is crucial for the neuronal “ramping” activities and an internal pacemaker. Neuromodulation therapies targeting the cholinergic circuits have potential as treatments for AD as well as DLB (Zhang et al., 2015). Data from this study will help us understand the cholinergic circuits and may have relevance for diseases involving cholinergic deficits, such as AD and DLB.
Highlights.
The cholinergic muscarinic inhibitor scopolamine impairs interval timing behavior.
Scopolamine does not change time-related ramping activity in the medial frontal cortex.
Medial prefrontal stimulus-related modulation increased
Acknowledgements:
NN is supported by R01MH116043A1. QZ is supported by the NINDS R25 grant, a pilot project grant from the Aging Mind and Brain Initiative at University of Iowa, and the physician scientist training program at University of Iowa. QZ is a trainee of the University of Iowa Clinical Neuroscientist Training Program (CNS-TP).
Footnotes
Conflicts of interests:
There are no conflicts of interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abner RT, Edwards T, Douglas A, Brunner D (2001) Pharmacology of temporal cognition in two mouse strains. Int J Comp Psychol 14:189–210. [Google Scholar]
- Arendt T, Bigl V, Arendt A, Tennstedt A (1983) Loss of neurons in the nucleus basalis of Meynert in Alzheimer’s disease, paralysis agitans and Korsakoff’s Disease. Acta Neuropathol (Berl) 61:101–108. [DOI] [PubMed] [Google Scholar]
- Balci F, Ludvig EA, Gibson JM, Allen BD, Frank KM, Kapustinski BJ, Fedolak TE, Brunner D (2008) Pharmacological manipulations of interval timing using the peak procedure in male C3H mice. Psychopharmacology (Berl) 201:67–80. [DOI] [PubMed] [Google Scholar]
- Bigl V, Woolf NJ, Butcher LL (1982) Cholinergic projections from the basal forebrain to frontal, parietal, temporal, occipital, and cingulate cortices: A combined fluorescent tracer and acetylcholinesterase analysis. Brain Res Bull 8:727–749. [DOI] [PubMed] [Google Scholar]
- Bohnen NI, Albin RL (2011) The cholinergic system and Parkinson disease. Behav Brain Res 221:564–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnen NI, Kaufer DI, Hendrickson R, Ivanco LS, Lopresti BJ, Constantine GM, Mathis CA, Davis JG, Moore RY, Dekosky ST (2006) Cognitive correlates of cortical cholinergic denervation in Parkinson’s disease and parkinsonian dementia. J Neurol 253:242–247. [DOI] [PubMed] [Google Scholar]
- Bohnen NI, Kaufer DI, Ivanco LS, Lopresti B, Koeppe RA, Davis JG, Mathis CA, Moore RY, DeKosky ST (2003) Cortical cholinergic function is more severely affected in parkinsonian dementia than in Alzheimer disease: an in vivo positron emission tomographic study. Arch Neurol 60:1745–1748. [DOI] [PubMed] [Google Scholar]
- Bruinsma B, Terra H, de Kloet SF, Luchicchi A, Timmerman AJ, Remmelink E, Loos M, Pattij T, Mansvelder HD (2019) An automated home-cage-based 5-choice serial reaction time task for rapid assessment of attention and impulsivity in rats. Psychopharmacology (Berl). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno JP, Gash C, Martin B, Zmarowski A, Pomerleau F, Burmeister J, Huettl P, Gerhardt GA (2006) Second-by-second measurement of acetylcholine release in prefrontal cortex. Eur J Neurosci 24:2749–2757. [DOI] [PubMed] [Google Scholar]
- Buhusi CV, Meck WH (2005) What makes us tick? Functional and neural mechanisms of interval timing. Nat Rev Neurosci 6:755–765. [DOI] [PubMed] [Google Scholar]
- Carrasco MC, Guillem MJ, Redolat R (2000) Estimation of short temporal intervals in Alzheimer’s disease. Exp Aging Res 26:139–151. [DOI] [PubMed] [Google Scholar]
- Caselli L, Iaboli L, Nichelli P (2009) Time estimation in mild Alzheimer’s disease patients. Behav Brain Funct BBF 5:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapin JK, Nicolelis MA (1999) Principal component analysis of neuronal ensemble activity reveals multidimensional somatosensory representations. J Neurosci Methods 94:121–140. [DOI] [PubMed] [Google Scholar]
- Chubykin AA, Roach EB, Bear MF, Shuler MGH (2013) A cholinergic mechanism for reward timing within primary visual cortex. Neuron 77:723–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull JT, Cheng R-K, Meck WH (2011) Neuroanatomical and neurochemical substrates of timing. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol 36:3–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croxson PL, Kyriazis DA, Baxter MG (2011) Cholinergic modulation of a specific memory function of prefrontal cortex. Nat Neurosci 14:1510–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daffner KR, Mesulam MM, Scinto LFM, Acar D, Calvo V, Faust R, Chabrerie A, Kennedy B, Holcomb P (2000) The central role of the prefrontal cortex in directing attention to novel events. Brain 123:927–939. [DOI] [PubMed] [Google Scholar]
- Day J, Damsma G, Fibiger HC (1991) Cholinergic activity in the rat hippocampus, cortex and striatum correlates with locomotor activity: an in vivo microdialysis study. Pharmacol Biochem Behav 38:723–729. [DOI] [PubMed] [Google Scholar]
- Emmons EB, Corte BJD, Kim Y, Parker KL, Matell MS, Narayanan NS (2017) Rodent Medial Frontal Control of Temporal Processing in the Dorsomedial Striatum. J Neurosci 37:8718–8733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emre M, Aarsland D, Albanese A, Byrne EJ, Deuschl G, De Deyn PP, Durif F, Kulisevsky J, van Laar T, Lees A, Poewe W, Robillard A, Rosa MM, Wolters E, Quarg P, Tekin S, Lane R (2004) Rivastigmine for dementia associated with Parkinson’s disease. N Engl J Med 351:2509–2518. [DOI] [PubMed] [Google Scholar]
- Fahn S (2008) The history of dopamine and levodopa in the treatment of Parkinson’s disease. Mov Disord Off J Mov Disord Soc 23 Suppl 3:S497–508. [DOI] [PubMed] [Google Scholar]
- Falsafi SK, Deli A, Höger H, Pollak A, Lubec G (2012) Scopolamine Administration Modulates Muscarinic, Nicotinic and NMDA Receptor Systems. PLOS ONE 7:e32082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry W, Kelleher RT, Cook L (1960) A mathematical index of performance on fixed-interval schedules of reinforcement. J Exp Anal Behav 3:193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furey ML, Pietrini P, Haxby JV, Drevets WC (2007) Selective Effects of Cholinergic Modulation on Task Performance during Selective Attention. Neuropsychopharmacology 33:913–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill TM, Sarter M, Givens B (2000) Sustained visual attention performance-associated prefrontal neuronal activity: evidence for cholinergic modulation. J Neurosci Off J Soc Neurosci 20:4745–4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gür E, Fertan E, Alkins K, Wong AA, Brown RE, Balcı F (2019a) Interval timing is disrupted in female 5xFAD mice: An indication of altered memory processes. J Neurosci Res 97:817–827. [DOI] [PubMed] [Google Scholar]
- Gür E, Fertan E, Kosel F, Wong AA, Balcı F, Brown RE (2019b) Sex differences in the timing behavior performance of 3xTg-AD and wild-type mice in the peak interval procedure. Behav Brain Res 360:235–243. [DOI] [PubMed] [Google Scholar]
- Himmelheber AM, Fadel J, Sarter M, Bruno JP (1998) Effects of local cholinesterase inhibition on acetylcholine release assessed simultaneously in prefrontal and frontoparietal cortex. Neuroscience 86:949–957. [DOI] [PubMed] [Google Scholar]
- Himmelheber AM, Sarter M, Bruno JP (1997) Operant performance and cortical acetylcholine release: role of response rate, reward density, and non-contingent stimuli. Brain Res Cogn Brain Res 6:23–36. [DOI] [PubMed] [Google Scholar]
- Inglis FM, Fibiger HC (1995) Increases in hippocampal and frontal cortical acetylcholine release associated with presentation of sensory stimuli. Neuroscience 66:81–86. [DOI] [PubMed] [Google Scholar]
- Jayarajan P, Nirogi R, Shinde A (2013) Effect of olanzapine on scopolamine induced deficits in differential reinforcement of low rate 72s (DRL-72s) schedule in rats: involvement of the serotonergic receptors in restoring the deficits. Eur J Pharmacol 720:344–354. [DOI] [PubMed] [Google Scholar]
- Johnston MV, McKinney M, Coyle JT (1979) Evidence for a cholinergic projection to neocortex from neurons in basal forebrain. Proc Natl Acad Sci U S A 76:5392–5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Jung AH, Byun J, Jo S, Jung MW (2009) Inactivation of medial prefrontal cortex impairs time interval discrimination in rats. Front Behav Neurosci 3:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y-C, Han S- W, Alberico SL, Ruggiero RN, De Corte B, Chen K- H, Narayanan NS (2017) Optogenetic Stimulation of Frontal D1 Neurons Compensates for Impaired Temporal Control of Action in Dopamine-Depleted Mice. Curr Biol CB 27:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y-C, Narayanan NS (2018) Prefrontal D1 Dopamine-Receptor Neurons and Delta Resonance in Interval Timing. Cereb Cortex N Y N 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinkenberg I, Blokland A (2010) The validity of scopolamine as a pharmacological model for cognitive impairment: a review of animal behavioral studies. Neurosci Biobehav Rev 34:1307–1350. [DOI] [PubMed] [Google Scholar]
- Liebe S, Hoerzer GM, Logothetis NK, Rainer G (2012) Theta coupling between V4 and prefrontal cortex predicts visual short-term memory performance. Nat Neurosci 15:456–462, S1–2. [DOI] [PubMed] [Google Scholar]
- Luzardo A, Rivest F, Alonso E, Ludvig EA (2017) A drift–diffusion model of interval timing in the peak procedure. J Math Psychol 77:111–123. [Google Scholar]
- Malapani C, Deweer B, Gibbon J (2002) Separating storage from retrieval dysfunction of temporal memory in Parkinson’s disease. J Cogn Neurosci 14:311–322. [DOI] [PubMed] [Google Scholar]
- Malapani C, Rakitin B, Levy R, Meck WH, Deweer B, Dubois B, Gibbon J (1998) Coupled temporal memories in Parkinson’s disease: a dopamine-related dysfunction. J Cogn Neurosci 10:316–331. [DOI] [PubMed] [Google Scholar]
- Malapani C, Rakitin BC (2003) Interval timing in the dopamine-depleted basal ganglia: From empirical data to timing theory In: Functional and neural mechanisms of interval timing, pp 485–514. Boca Raton, FL, US: CRC Press. [Google Scholar]
- Matell MS, Meck WH (2004) Cortico-striatal circuits and interval timing: coincidence detection of oscillatory processes. Brain Res Cogn Brain Res 21:139–170. [DOI] [PubMed] [Google Scholar]
- Matell MS, Portugal GS (2007) Impulsive responding on the peak-interval procedure. Behav Processes 74:198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meck WH (1996) Neuropharmacology of timing and time perception. Brain Res Cogn Brain Res 3:227–242. [DOI] [PubMed] [Google Scholar]
- Meck WH (2006) Neuroanatomical localization of an internal clock: A functional link between mesolimbic, nigrostriatal, and mesocortical dopaminergic systems. Brain Res 1109:93–107. [DOI] [PubMed] [Google Scholar]
- Merchant H, Luciana M, Hooper C, Majestic S, Tuite P (2008) Interval timing and Parkinson’s disease: heterogeneity in temporal performance. Exp Brain Res Exp Hirnforsch Expérimentation Cérébrale 184:233–248. [DOI] [PubMed] [Google Scholar]
- Narayanan NS (2016) Ramping activity is a cortical mechanism of temporal control of action. Curr Opin Behav Sci 8:226–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan NS, Land BB, Solder JE, Deisseroth K, DiLeone RJ (2012) Prefrontal D1 dopamine signaling is required for temporal control. Proc Natl Acad Sci U S A 109:20726–20731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan NS, Laubach M (2009) Delay activity in rodent frontal cortex during a simple reaction time task. J Neurophysiol 101:2859–2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan NS, Rodnitzky RL, Uc EY (2013) Prefrontal dopamine signaling and cognitive symptoms of Parkinson’s disease. Rev Neurosci 24:267–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichelli P, Venneri A, Molinari M, Tavani F, Grafman J (1993) Precision and accuracy of subjective time estimation in different memory disorders. Brain Res Cogn Brain Res 1:87–93. [DOI] [PubMed] [Google Scholar]
- Papagno C, Allegra A, Cardaci M (2004) Time estimation in Alzheimer’s disease and the role of the central executive. Brain Cogn 54:18–23. [DOI] [PubMed] [Google Scholar]
- Parikh V, Sarter M (2008) Cholinergic mediation of attention: contributions of phasic and tonic increases in prefrontal cholinergic activity. Ann N Y Acad Sci 1129:225–235. [DOI] [PubMed] [Google Scholar]
- Parker KL, Chen K-H, Kingyon JR, Cavanagh JF, Narayanan NS (2014) D1-Dependent 4 Hz Oscillations and Ramping Activity in Rodent Medial Frontal Cortex during Interval Timing. J Neurosci 34:16774–16783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KL, Chen K-H, Kingyon JR, Cavanagh JF, Naryanan NS (2015a) Medial frontal ~4 Hz activity in humans and rodents is attenuated in PD patients and in rodents with cortical dopamine depletion. J Neurophysiol:jn.00412.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KL, Kim YC, Kelley RM, Nessler AJ, Chen K-H, Muller-Ewald VA, Andreasen NC, Narayanan NS (2017) Delta-frequency stimulation of cerebellar projections can compensate for schizophrenia-related medial frontal dysfunction. Mol Psychiatry 22:647–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KL, Lamichhane D, Caetano MS, Narayanan NS (2013) Executive dysfunction in Parkinson’s disease and timing deficits. Front Integr Neurosci 7:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KL, Ruggiero RN, Narayanan NS (2015b) Infusion of D1 Dopamine Receptor Agonist into Medial Frontal Cortex Disrupts Neural Correlates of Interval Timing. Front Behav Neurosci 9:294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passetti F, Chudasama Y, Robbins TW (2002) The frontal cortex of the rat and visual attentional performance: dissociable functions of distinct medial prefrontal subregions. Cereb Cortex 12:1254–1268. [DOI] [PubMed] [Google Scholar]
- Passetti F, Dalley JW, O’Connell MT, Everitt BJ, Robbins TW (2000) Increased acetylcholine release in the rat medial prefrontal cortex during performance of a visual attentional task. Eur J Neurosci 12:3051–3058. [DOI] [PubMed] [Google Scholar]
- Rueda AD, Schmitter-Edgecombe M (2009) Time estimation abilities in mild cognitive impairment and Alzheimer’s disease. Neuropsychology 23:178–188. [DOI] [PubMed] [Google Scholar]
- Sarter M, Bruno JP (1997) Cognitive functions of cortical acetylcholine: toward a unifying hypothesis. Brain Res Brain Res Rev 23:28–46. [DOI] [PubMed] [Google Scholar]
- Simen P, Balci F, de Souza L, Cohen JD, Holmes P (2011) A model of interval timing by neural integration. J Neurosci Off J Soc Neurosci 31:9238–9253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares S, Atallah BV, Paton JJ (2016) Midbrain dopamine neurons control judgment of time. Science 354:1273–1277. [DOI] [PubMed] [Google Scholar]
- Tiraboschi P, Hansen LA, Alford M, Sabbagh MN, Schoos B, Masliah E, Thal LJ, Corey-Bloom J (2000) Cholinergic dysfunction in diseases with Lewy bodies. Neurology 54:407–411. [DOI] [PubMed] [Google Scholar]
- Wang J, Narain D, Hosseini EA, Jazayeri M (2018) Flexible timing by temporal scaling of cortical responses. Nat Neurosci 21:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehouse PJ, Hedreen JC, White CL, Price DL (1983) Basal forebrain neurons in the dementia of Parkinson disease. Ann Neurol 13:243–248. [DOI] [PubMed] [Google Scholar]
- Winters BD, Robbins TW, Everitt BJ (2004) Selective cholinergic denervation of the cingulate cortex impairs the acquisition and performance of a conditional visual discrimination in rats. Eur J Neurosci 19:490–496. [DOI] [PubMed] [Google Scholar]
- Witten IB, Lin S-C, Brodsky M, Prakash R, Diester I, Anikeeva P, Gradinaru V, Ramakrishnan C, Deisseroth K (2010) Cholinergic Interneurons Control Local Circuit Activity and Cocaine Conditioning. Science 330:1677–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanto TP, Rubens MT, Thangavel A, Gazzaley A (2011) Causal role of the prefrontal cortex in top-down modulation of visual processing and working memory. Nat Neurosci 14:656–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Kim Y-C, Narayanan NS (2015) Disease-modifying therapeutic directions for Lewy-Body dementias. Front Neurosci 9:293. [DOI] [PMC free article] [PubMed] [Google Scholar]


