Abstract
Background:
Methylphenidate (MPH) is the most commonly used medication for Attention-Deficit/Hyperactivity Disorder (ADHD), but to date, there are neither consistent nor sufficient findings on conditions differentiating responsiveness to MPH response in ADHD.
Objective:
To develop a predictive model of MPH response, using a longitudinal and naturalistic follow-up study, in a Spanish sample of children and adolescents with ADHD.
Methods:
We included all children and adolescents with ADHD treated with MPH in our outpatient Clinic (2005 to 2015), evaluated with the K-SADS interview. We collected ADHD-RS-IV.es and CGI-S scores at baseline and at follow up, and neuropsychological testing (WISC-IV, Continuous Performance Test (CPT-II) & Stroop). Clinical response was defined as >30% reduction from baseline of total ADHD-RS-IV.es score and CGI-S final score of 1 or 2 maintained for the previous 3 months.
Results:
We included 518 children and adolescents with ADHD, mean (SD) age of patients was 11.4 (3.3) years old; 79% male; 51.7% had no comorbidities; and 75.31% had clinical response to a mean MPH dose of 1.2 mg/kg/day. Lower ADHD-RS-IV.es scores, absence of comorbidities (oppositional-defiant symptoms, depressive symptoms and alcohol/cannabis use), fewer altered neuropsychological tests, higher total IQ and low commission errors in CPT-II, were significantly associated with a complete clinical response to methylphenidate treatment.
Conclusion:
Oppositional-defiant symptoms, depressive symptoms, and a higher number of impaired neuropsychological tests are associated with worse clinical response to methylphenidate. Other stimulants or non-stimulants treatment may be considered when these clinical and neuropsychological variables converged in the first clinical interview.
Keywords: ADHD, Predictive factors, Neuropsychological variables, Methylphenidate, Treatment, Spanish sample
1. INTRODUCTION
Attention-Deficit/Hyperactivity Disorder (ADHD) is a heterogeneous neurodevelopmental disorder defined as developmentally inappropriate levels of hyperactivity, impulsivity and/or inattention [1, 2]. Prevalence is stable internationally, with rates in children and adolescents of 7.2% (95% CI 6.7-7.8) [3, 4]. Boys: girls’ ratio is 3-4:1 in epidemiological samples [3] and 7-8:1 in clinical samples, suggesting referral bias against girls [5]. A gold standard assessment for diagnoses should include structured clinical interviews with the patient and parents, information from teachers’ questionnaires or interviews [6] and neuropsychological evaluation of intellectual function and Executive Function (EF) [7].
ADHD may be associated with multiple neuropsychological deficits [8-12], particularly in the measures of EF [13-17]. These studies conclude that the differences between ADHD and controls are focused on inhibition [18], working memory [19, 20], attentional set-shifting and planning [21, 22], reaction time variability [23, 24], and emotional dysregulation [25]. However, not all children with ADHD have EF deficits [8, 26], some patients display a single deficit, while others suffer multiple deficits [27, 28].
Available psychopharmacological treatments with good to moderate effect sizes include stimulants (methylphenidate and lisdexamfetamine), and non-stimulants medications (atomoxetine and alfa-agonist agents guanfacine and clonidine) [29]. About 66% of studies, showed positive cognitive effects with MPH treatment [30]. However, not all patients have an optimal clinical response to treatment [31]. Whilst around 65% of children diagnosed with ADHD tolerate and respond to Methylphenidate (MPH), 35% will not [32, 33].
One source of suboptimal response could be comorbidity. Given that ADHD is highly comorbid [34], investigations have studied the potential role of comorbidity in the individual variation of MPH response, but findings were inconclusive. Typically, the best pharmacotherapy response has been obtained in patients with ADHD without comorbidities [35]. Few studies have found that ADHD children with or without the presence of anxiety, Conduct Disorder (CD) or Oppositional Defiant Disorders (ODD) responded equally well to MPH [36-39]. However, other studies found that the presence of comorbid anxiety, ODD, or CD is associated with a worse MPH response [40-42]. Other comorbidities, such as substance use disorder (SUD), present in up to 50% of ADHD patients [43], are also associated with worse MPH response in most studies [35, 44].
In the last decades, the interest in finding predictive factors of response to different treatments has increased. Coghill and colleagues, found in a randomized placebo-controlled trial that poor performance on a “delayed matching to sample” (DMtS) task at baseline was the only pre-treatment factor that correlated with clinical response to MPH [19]. However, they concluded that their model required a wide range of measures [19, 45]. Denney and Rapport also evaluated several MPH response predictive models (empirical, homeostatic, attentional and disinhibition), but none of these models predicted MPH response in children with ADHD. They suggested that a comprehensive model of MPH response would need to include both biological and behavioral components [45].
To date, there are no objective, clinical or biological markers that can robustly predict MPH treatment in patients with ADHD [36, 46]. Given the importance of clinical clues that may help physicians to accurately choose the optimal treatment from each individual patient, our goal is to develop a predictive model of response to MPH based on neuropsychological and clinical variables in a Spanish sample of children and adolescents with ADHD.
2. MATERIALS AND METHODS
2.1. Patients
We included consecutively all drug-naïve patients aged 6 to < 18 years old diagnosed with ADHD treated with methylphenidate for at least three months. We follow them up from January 2000 to April 2015. ADHD was diagnosed according to DSM-IV-TR criteria [47] using a Kiddie-Schedule for Affective Disorders and Schizophrenia-Present and Lifetime Version (KSADS-PL) interview template [48] by experienced child and adolescent psychiatrists. All clinical data and neuropsychological tests from the routine assessment were collected by unblinded Child & Adolescent Psychiatrists at baseline, defined as the first visit, even if the diagnosis was previously made by other specialists. Patients with commonly comorbid conditions; ODD, CD, mood or anxiety disorder were not excluded from the study. Exclusion criteria included patients with an IQ <80 or with a comorbid neurological disease, or patients with ADHD taking other lisdexamfetamine, atomoxetine or an alfa-agonist agent.
2.2. Materials
2.2.1. Clinical and Neuropsychological Variables
All patients were evaluated twice, at baseline (T1) and in the last follow-up visit, defined as the last recorded visit (T2), with these assessments:
-
Clinical variables
The Spanish version of the ADHD-Rating Scale (ADHD-RS-IV.es) [49, 50], is an 18-item scale designed to reflect the presence of ADHD-related symptoms of inattention and hyperactivity based on DSM-IV-TR criteria. ADHD-RS-IV.es scores range from 0 to 54, with higher scores indicating more severe symptoms.
The SNAP-IV [51], is a 26-item scale which includes the 18 ADHD symptoms (9 for inattentive, 9 for hyperactive/impulsive) and 8 Oppositional and Defiant Disorder symptoms specified in the DSM-IV-TR. In our study, we only used the ODD-subscale score (SNAP-IV ODD-s) to evaluate ODD symptoms.
CGI-S (Clinical Global Impressions Severity scale) [52], provides an overall clinician-determined summary measure that takes into account all available information and the impact of the symptoms on the patient’s ability to function. It is scored on a scale of 1 (normal, not at all ill) to 7 (severely ill).
-
Neuropsychological variables: IQ, based on:
Wechsler Intelligence Scale Revised (WISC-IV) [53, 54], T-IQ scores were classified in the following seven levels: ≥130, very superior; 120-129, superior; 110-119, normal-high; 90-109, normal; 80-89, normal-low; 70-79, borderline; and ≤69, intellectual dysfunction/disability.
Stroop color word Test (SCWT) [55] evaluates the cognitive function of inhibitory control (Interference) in the clinical setting and requires the inhibition of competing responses [56-59].
The Conners’ Continuous Performance Test II (CPT-II) [60] that measures the ability to maintain focused attention over longer periods of time (usually 5-20 minutes) while responding to a target stimuli and inhibiting responses to non-target stimuli [61] and produces multiple dependent variables: Omission, Commission, Response Time, Variability of Standard Error, and Detectability.
To evaluate the tasks described above, we use a total predictive value (TPV) of ≥ 60% as a general criterion for diagnostic efficiency [62]. The altered neuropsychological test was defined by the criterion of Doyle and Biederman [28] that found that the cut-off of 1.0 SD below the controls' mean (1 SD cut-off rule) provided the most efficient diagnostic profile across different tests. We used this cut-off to define which test was altered or not in our patients with ADHD, and we also evaluated how many neuropsychological tests were altered to see if there was a correlation with MPH response.
2.2.2. Clinical Response to Treatment
We used the definition of treatment response most used in randomized clinical trials which are based on data from scales filled by parents [29, 63-68]. “Complete Response” was defined as the presence of a ≥30% reduction in ADHD-RS-IV.es total score from baseline and a final CGI-S score of 1 or 2 points that lasted at least three months. We defined “Partial Response” as a reduction of <30% in ADHD-RS-IV.es total score from baseline and/or a CGI-S reduction of ≥2 points from baseline but no more than 3 points. “Non-response” was defined as no variations or deterioration in ADHD-RS-IV.es and/or CGI-S scores.
We also divided our sample into a two-variable clinical response to treatment based on a CGI-S score filled by the physician in each visit. A “complete response” to MPH was defined by 1 or 2 points at T2 that correspond to an ADHD-RS-IV.es total score reduction from baseline of 30-50% [69, 70].
2.3. Statistical Analyses
Differences in ADHD-RS-IV.es and CGI-S over time were analyzed using two-tailed Τ-test. Correlations between different neuropsychological variables were performed, correlation coefficients Cohen [71], were interpreted as small (r=0.10), medium (r=0.30) or large (r=0.50). All these variables were examined in relation to inattention and hyperactivity symptoms. Correlations, multiple regressions, and logistic regressions analyses were used to investigate socio-demographic, clinical, neuropsychological and psychopharmacological predictors of clinical response. Two-tailed p values <.05 were considered statistically significant. A statistician supervised all analyses and they were conducted using statistical package SPSS (SPSS Inc., Chicago, Illinois) for Windows (v.20.0).
3. RESULTS
3.1. Sample Characteristics
A total of 518 children and adolescents (79.7% boys; mean age (SD): 11.40 (3.30) years old) were included in the study. There were no statistical differences between boys and girls neither in the age of onset (p>.1) nor in diagnostic presentation (DSM-IV subtype). However, the mean age-of-onset in the inattentive subtype (or current presentation on DSM-5) was significantly higher than in the combined group (p=.027). Patients had a mean (SD) follow-up of 33 (22.8) months. According to the DSM-IV-TR, we classified ADHD into two groups: 1. Inattentive subtype (31.7%) and 2. Hyperactive-impulsive and Combined presentations (68.3%). The mean (SD) MPH dose during the dose-maintenance period was 1.21 (0.41) mg/kg/day (Table 1).
Table 1. Sample characteristics in 518 children and adolescents with ADHD.
| Total Sample | Sex | ADHD subtype | Age of onset* | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Boys | Girls | p | IA | C | p | Children | Adolescents | p | ||
| Age mean (SD) years | 11.40 (3.3) | 11.38 (3.2) | 11.5 (3.69) | > .1 | 11.9 (3.4) | 11.2 (3.2) | .027* | 8.9 (1.7) | 14.6 (1.8) | <.001 |
| Follow-up, months | 33 (22.7) | 32.4 (23.2) | 35.3 (20.7) | > .1 | 31.4 (20.8) | 33.7 (23.6) | > .1 | 37.7 (25.1) | 27 (17.7) | <.001 |
| ADHD-RS-IV.es | ||||||||||
| T1 ADHD-RS-IV (SD) | 29.9 (10.42) | 30.71 (10.5 | 26.7 (9.6) | <.001 | 28.82 (10.6) | 30.4 (10.3) | 31.7 (10) | 27.6 (10.3) | ||
| Inattention score | 17.85 (5.47) | 18.2 (5.4) | 16.6 (5.4) | <.001 | 17.4 (5.8) | 18 (5.3) | >.1 | 17.9 (5.4) | 17.8 (5.5) | > .1 |
| Hyperactive score | 8.76 (6.0) | 12.5 (7.1) | 10.1 (6.4) | <.001 | 11.4 (7.2) | 12.3 (6.9) | >.1 | 13.8 (6.8) | 9.8 (6.7) | <.001 |
| T2 ADHD-RS-IV (SD) | 23.33 (14.19) | 24.2 (14.5) | 20 (12.6) | <.001 | 23.6 (15.7) | 23.2 (13.4) | 24.3 (14.1) | 22 (14.2) | ||
| Inattention score | 14.60 (8.7) | 15.2 (8.9) | 12.3 (7.6) | <.001 | 14.8 (9.9) | 14.5 (8.2) | >.1 | 15.1 (8.6) | 14 (8.7) | > .1 |
| Hyperactive score | 8.76 (6.0) | 9 (6.1) | 7.7 (5.6) | <.001 | 8.9 (6.5) | 8.7 (5.8) | >.1 | 9.2 (6) | 8 .1 (6) | .049 |
| T1-T2 ADHD-RS-IV | <.001 | <.001 | ||||||||
| SNAP-IV | ||||||||||
| T1 ODD score | 10.08 (4.47) | 10.1 (4.5) | 8.7 (4.7) | <.001 | 9.3 (4.7) | 10.1 (4.5) | >.1 | 9.9 (4.7) | 9.9 (4.7) | > .1 |
| T2 ODD score | 6.36 (5.0) | 6.7 (5) | 4.8 (4.5 | <.001 | 6.2 (4.9) | 6.4 (4.9) | >.1 | 6.7 (5) | 5.9 (5) | > .1 |
| T1-T2 ODD score | <.001 | <.001 | <.001 | |||||||
| CGI-S | ||||||||||
| T1 CGI-S | 3.85 (0.77) | 3.9 (0.8) | 3.7 (0.8) | .09 | 3.9 (0.7) | 3.8 (0.8) | >.1 | 3.8 (0.8) | 3.9 (0.8) | >.1 |
| T2 CGI-S | 2.71 (0.94) | 2.7 (0.9) | 2.6 (0.9) | .06 | 2.7 (0.9) | 2.7 (0.9) | >.1 | 2.8 (0.9) | 2.8 (0.9) | .009 |
| T1-T2 CGI-S | <.001 | <.001 | <.001 | |||||||
Note: ADHD = Attention-deficit/hyperactivity disorder. ADHD subtype presentation: IA (inattentive); C (Combined). ADHD-RS-IV.es: ADHD rating scale-IV (Spanish version). ODD: oppositional defiant disorder CGI-S: Clinical Global Impressions Severity Scale. T1: baseline; T2: end point-last follow-up visit. p<0.005: statistically significant *Children (<11.9y), Adolescents (12-18y)
We analyzed the prevalence of perinatal risk factors in our sample following what is described in the literature (Park et al., 2014). In our sample 8.3% of children were adopted, 10.6% had low weight at birth (<2,500 gr), 15.3% had prenatal nicotine exposure and 10.6% had prenatal alcohol exposure. Among adolescents, only 12% reported occasional alcohol consumption; 7.3% smoked cigarettes and 7.3% had used cannabis (THC) occasionally. Almost half of the patients (48.7%) had co-occurring comorbidities: 23.6% had Oppositional Defiant Disorder (ODD), 21.4% had anxiety symptoms, 10.8% had comorbid depressive symptoms, 2.1% had Bipolar Disorder (BD) and 0.4% had Obsessive-Compulsive Disorder (OCD).
3.2. Neuropsychological Profile
We found that 48.3% of patients showed <1 SD from the mean in at least one neuropsychological test that evaluates EF, 23% showed >1 SD from the mean in one test, and 14.5% showed > 1 SD in two tests (Table 2, Figs. 1 and 2).
Table 2. Neuropsychological profile in 518 children and adolescents with ADHD.
| Total Sample | Sex | ADHD subtype | Age of onset | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Boys | Girls | p | IA | C | p | Children | Adolescents | p | |||
| WISC-IV | |||||||||||
| Total IQ | 99.30 | 100.06 | 96.38 | <.001 | 99.08 | 99.08 | >.1 | 99.37 | 99.23 | >.1 | |
| V-IQ | 101.16 | 102.58 | 94.89 | <.001 | 101.13 | 101.22 | >.1 | 100.09 | 102.50 | >.1 | |
| M-IQ | 99.59 | 99.89 | 98.24 | >.1 | 99.43 | 99.94 | >.1 | 98.78 | 100.60 | >.1 | |
| VC | 98.13 | 99.23 | 94.71 | <.001 | 98.25 | 97.83 | >.1 | 100.50 | 95.51 | <.001 | |
| PR | 97.14 | 99.70 | 99.76 | >.1 | 99.21 | 100.88 | >.1 | 102.64 | 96.47 | <.001 | |
| WM | 99.71 | 97.62 | 95.63 | >.1 | 96.60 | 98.39 | >.1 | 95.39 | 98.97 | >.1 | |
| PS | 96.59 | 95.53 | 99.64 | >.1 | 96.78 | 96.13 | >.1 | 95.49 | 97.76 | >.1 | |
| CPT-II | |||||||||||
| CE | 47.11 | 47.23 | 46.62 | >.1 | 46.95 | 47.45 | >.1 | 47.67 | 46.45 | >.1 | |
| OE | 50.79 | 50.42 | 52.26 | >.1 | 51.07 | 50.18 | >.1 | 57.84 | 42.65 | <.001 | |
| Hit RT | 54.37 | 54.56 | 56.36 | >.1 | 55.16 | 54.40 | >.1 | 60.37 | 48.66 | <.001 | |
| V’ | 52.17 | 52.03 | 52.72 | >.1 | 52.52 | 51.40 | >.1 | 59.69 | 43.48 | <.001 | |
| P’ | 54.93 | 55.15 | 54.09 | >.1 | 55.62 | 53.40 | >.1 | 62.17 | 46.64 | <.001 | |
| Hit RT BC | 51.19 | 52.05 | 47.83 | >.1 | 51.62 | 50.25 | >.1 | 53.95 | 48.01 | <.001 | |
| SCWT | |||||||||||
| Interference | 51.63 | 52.01 | 50.30 | .032 | 51.37 | 52.18 | >.1 | 50.57 | 52.77 | <.001 | |
Note. ADHD = Attention-deficit/hyperactivity disorder; IA= Inattentive subtype, C= Combined Subtype; WISC= Wechsler Intelligence Scale ( IQ= intelligence quotient, T-IQ: Total IQ, V-IQ: Verbal IQ, M-IQ: Manipulative IQ, VC: Verbal Comprehension, PR: Perceptual Reasoning, WM: working memory, PS: perceptual reasoning) CPT-II: Conners’ Continuous Performance Test II (CE: commission errors, OE: omission errors, Hit RT: Reaction time, V’: variability, P’: perseverance, HitRTBC: response style). SCWT: Stroop COLOR WORD Test *Children (<11.9y), Adolescents (12-18y)
Fig. (1).
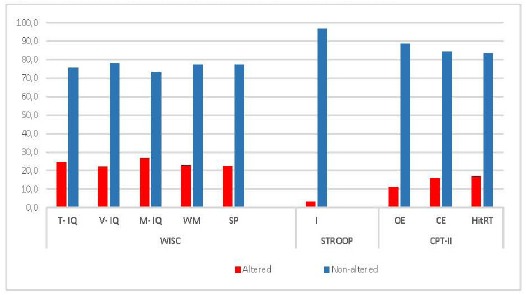
Proportion of ADHD patients with Altered Neuropsychological Tests.
Considering that the presence of a 1SD away from the average value of the healthy population in any of the test to evaluate the Executive Function (EF) of ADHD patients, we found that a most patients showed no alteration in the different neuropsychological tests. These data suggest that not all children with ADHD suffer from neuropsychological dysfunction.
Note : WISC= Wechsler Intelligence Scale (IQ: intelligence quotient, T-IQ : Total IQ, V-IQ: Verbal IQ, M-IQ: Manipulative IQ, VC: Verbal Comprehension, PR: Perceptual Reasoning, WM: Working Memory, PS: Perceptual Reasoning) CPT-II: Conners’ Continuous Performance Test II (CE: Commission Errors, OE: Omission Errors, Hit RT: Reaction Time,). STROOP: Stroop COLOR WORD Test (I= interference).
Fig. (2).
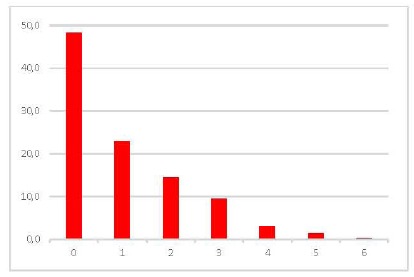
Number of Proportion Altered Neuropsychological Tests in ADHD patients.
Neuropsychological Tests evaluated are detailed and described in Fig. (1). We consider “altered test”, according to 1SD cut-off rule (Biederman et al., 1993). In our sample, 48.3% of ADHD patients had no neuropsychological impairment, and only 23% had impairment in one neuropsychological test, and 14.5% had two-test impairment.
WISC-IV: Total IQ (mean (SD): 99.31 (12.91)) did not differ significantly (t-test, p>.1) neither between ADHD presentations (inattentive vs. combined) nor in children vs. adolescents. The 79.7% of children had a Total IQ score between 81 and 110, and 19.3% had a Total IQ >111.
CPT-II: In the CPT-II test, we found no baseline differences neither between boys vs. girls nor between inattentive vs. combined ADHD presentations, (t-test, p>.1). We found that children were more likely to show higher scores than adolescents in all CPT-II measures (t-scores) (p<.05) with the exception of the Commission Errors.
Interference, Stroop Task: The interference effect revealed a significant main effect for sex and age of the onset but not for ADHD presentations. Boys showed higher interference (t=2.16, p=.032) than girls; and this nterference appears to decrease with age (adolescents vs. children) (t=-3.36, p<.001).
3.3. Treatment Response Rates
Efficacy was assessed using the investigator rated ADHD-RS-IV.es and CGI-S scores.
ADHD-RS-IV.es and SNAP-IV: At baseline ADHD-RS-IV.es mean severity (SD) was: ADHD-RS-IV.es total score= 29.9 (10.42), inattention subscale score= 17.85 (5.47) and hyperactivity subscale score=12.05 (7.05), and SNAP-IV ODD symptoms´ score was 10.08 (4.47). Patients had a statistically significant improvement in ADHD-RS-IV.es total score from baseline (treatment-naïve) to end point (t=10.67, p<.001), both in girls (t=5.66, p<.001) and in boys (t=9.18, p<.001). We found a significant improvement in both adolescents (t=5.89, p<.001) and children (t=9.05, p<.001). We also found significant improvement in SNAP-IV ODD symptoms from baseline to end point (t=-15.88, p<.001), both in boys (t=-13.66, p<.001) and in girls (t=-8.29, p<.001), in children (t=-11.58, p<.001) and in adolescents (t=-5.89, p<.001) (Table 1).
CGI-S: The mean (SD) CGI-S (clinician scored) baseline was 3.85 (0.77). Patients had statistically significant improvement in CGI-S scores from baseline to end point (t=26.13, p<.001), both in girls (t=11.79, p<.001) and in boys (t=23.29, p<.001). We also found a significant improvement in both adolescents (t=16.0, p<.001) and children (t=20.75 p<.001).
According to the definition of response, 37.60% of patients had a “complete response” with a mean MPH dose of 1.23 mg/kg/day, and 35.80% of patients had a “partial response” with a mean MPH dose of 1.18 mg/kg/day. Finally, 26.60% of patients had a “non-response” with a mean MPH dose of 1.25 mg/kg/day. There was no difference in MPH dose in responders vs. non-responders (t-test, p > .1) (ANOVA, p > .1) (Table 3).
Table 3. MPH (mg/kg/day) according to CGI-S response.
| Complete response | Partial response | No-response | ANOVA | |
|---|---|---|---|---|
| Total Sample | 1.21 (0.39) | 1.17 (0.45) | 1.24 (0.39) | p > .1 |
| Sex | ||||
| Boys | 1.29 (0.40) | 1.13 (0.39) | 1.26 (0.35) | p > .1 |
| Girls | 1.19 (0.39) | 1.18 (0.46) | 1.24 (0.41) | p > .1 |
| ADHD presentation | ||||
| Inattentive | 1.12 (0.33) | 1.12 (0.42) | 1.11 (0.37) | p > .1 |
| Combined | 1.26 (0.39) | 1.20 (0.46) | 1.28 (0.39) | p > .1 |
| Age of onset | ||||
| Children | 1.24 (0.41) | 1.24 (0.45) | 1.29 (0.37) | p > .1 |
| Adolescents | 1.17 (0.36) | 1.09 (0.43) | 1.19 (0.41) | p > .1 |
3.4. Predictors of Treatment Response
A prediction model was designed considering treatment response according to CGI-S, given than a 30% reduction from baseline in ADHD-RS-IV.es total score corresponds to a CGI-S score of 1 or 2 points [69, 70] (Table 4).
Table 4. Predictors of MPH complete response based on CGI-S.
| B | Wald | p | |
|---|---|---|---|
| Age of onset | -0.380 | 3.16 | .076 |
| Follow-up, months | -0.002 | 0.250 | .617 |
| Gender | 0.142 | 0.338 | .561 |
| MPH (mg/kg/day) | 0.165 | 0.309 | .578 |
| ADHD subtype | 0.066 | 0.088 | .766 |
| T1 ADHD-RS Total | -0.020 | 5.35 | .021 |
| Inattention score | -0.032 | 2.98 | .084 |
| “forgets” | -0.38 | 7.65 | .006 |
| Hyperactivity-score | 0.002 | 0.019 | .891 |
| “fidgets” | 0.245 | 3.9 | .048 |
| T1 SNAP-IV ODD score | -0.553 | 4.58 | .032 |
| “rejects rules” | -0.376 | 4.58 | .032 |
| “angry” | -0.421 | 3.94 | .047 |
| WISC-IV | |||
| Total IQ | 0.021 | 4.62 | .032 |
| CPT-II | |||
| CE | -0.13 | 3.87 | .049 |
| SCWT | |||
| Interference | -0.778 | 4.61 | .032 |
| Comorbidities | |||
| OH use | -1.30 | 6.93 | .008 |
| THC use | -0.054 | 5.69 | .017 |
| Depressive symptoms | -0.732 | 4.81 | .026 |
Note. ADHD = Attention-deficit/hyperactivity disorder; MPH= methylphenidate; ADHD-RS: ADHD rating scale; WISC= Wechsler Intelligence Scale; CPT-II:Conners’ Continuous Performance Test II (CE: commission errors); OH= alcohol use; THC: cannabis use.
3.4.1. Neuropsychological Predictors
According to the number of altered neuropsychological tests, we observed a gradual decline in the response rate, as the number of neuropsychological altered tests (>1 SD) increased. Treatment response rate (Complete and Partial) was 76.4% in patients with 0 altered tests, 73.1% in patients with 1 altered test, and 62.7% in patients with 2 altered tests. So, in our sample, there was an association between more altered EF-tests, and a worse MPH response (Fig. 3).
Fig. (3).
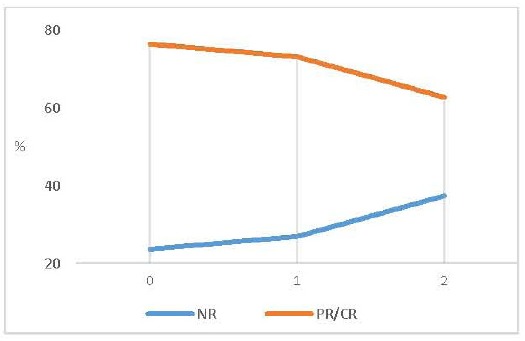
Treatment response (%) reached at end-point (last follow-up visit) according to number of altered neuropshychological tests.
We observed a gradual decline in the response rate, as the number of altered tests increased. The response rate (CGI) in patients with normal executive function (EF) evaluation was 76.4%, while the response rate for patients with 1 or 2 altered tests was 73.1% and 62.7% respectively.
Note : NR: “no-response” PR= “partial response” CR = “complete response”.
When analyzing each test separately, we found that higher baseline Total IQ scores (WISC-IV) and fewer commission errors in the CPT-II predicted a Complete Response (B=0.21, p=.032 B=-0.13, p=.049). None of the other cognitive variables significantly predicted treatment response.
3.4.2. Clinical and Physiological Predictors
When analyzing the role of initial ADHD symptom severity, we found that higher baseline ADHD-RS-IV.es total scores predicted accurately non-response to MPH (B=-0.020, p=.021). More specifically, we found that higher scores in several items significantly predicted (p<.05) a non-response to MPH (“forgets”, “fidgets”, “angry/resentful” and “challenge rules”).
When analyzing the role of the comorbid conditions, we found that comorbid alcohol (B=-1.30, p=.008) and THC use (B=-0.054, p=.017) in adolescence significantly predicted non-response to MPH. Moreover, the presence of comorbid ODD symptoms reported in the SNAP-IV (B=-0.553, p=.032) and depressive symptoms (B=-0.732, p=.026) were predictors of non-response to MPH treatment.
None of the sociodemographic, physiological variables (sex, age of diagnosis, weight, and height), peri-/pre-/postnatal variables (SUD in pregnancy, low birth weight, prematurity, and adoption) significantly predicted treatment response. ADHD subtype presentation (inattentive vs. combined) did not predict treatment response.
Our predictive model indicates that approximately between 5.1% and 6.9% (Cox-Snell R2= .051; Nagelkerke R2 = .069) of the variation in the dependent variable (complete treatment response) is explained by these variables, indicating a small relationship of 5.1% to 6.9% between the predictors and the treatment response.
4. DISCUSSION
Children with ADHD do not all equally respond to MPH treatment [72, 73], and only about 60-70% have an adequate response [23, 25, 32, 33, 74, 75]. According to ADHD-RS-IV.es scores, in our sample, 56.8% of patients had a positive response (complete or partial), and 75.31% obtained a positive response (partial and complete) on CGI-S scores, that was maintained at the follow-up after 33 (22.8) months. These results correlate with the main findings in the literature [32, 33]. According to the multimodal treatment study of ADHD (MTA) [76], we found that children with the most severe levels of ADHD before MPH treatment showed less chance of response than children with less severe ADHD.
As shown in previous studies that examined differences between ADHD subtypes’ response to pharmacological treatment [61, 77-79], we did not find a discriminating validity of the effectiveness of MPH by ADHD sub-grouping by DSM-IV-TR (Inattentive vs. Combined subtypes). However, despite not been statistically significant, we found that among all the patients that reached a complete clinical response the doses required by the combined-subgroup of ADHD patients required higher MPH dose than the inattentive subtype group (1.25 [0.42] mg/kg/day) vs. 1.12 [0.37] mg/kg/day respectively). Similar findings were reported by Stein et al. (2003) examining the possibility that inattentive children could respond optimally to lower MPH doses, while combined presentation children could respond best to higher MPH doses [80].
When comparing clinical response between sex and age, there was no difference in response in boys vs. girls nor in children vs. adolescents, However, although it was not statistically significant, we found that children received higher MPH doses to get a complete response (1.26 [0.42] mg/kg/day) than adolescents (1.15 [0.40] mg/kg/day). Moreover, there were differences in MPH dose in boys vs. girls to reach a complete response. Therefore, the significance of age and sex as predictors of treatment response in ADHD is unclear.
The differential response to MPH in ADHD has been linked to a number of comorbidities [42]. Unlike the findings of some studies [38, 40, 41], we found that ADHD children with comorbid depression showed worse MPH response [33, 36]. Moreover, we found that the presence of ODD symptoms decreased the probability of positive MPH response in our sample. As noted by Jensen et al. (2001) in a MTA sub-analysis, our results lead to support the evidence that ADHD children with ODD/CD symptoms (but without anxiety disorders) would respond best to pharmacological treatment (with or without behavioral treatments), while children with ODD/CD and anxiety would respond optimally to combined (medication+behavioral) treatments [81]. Most investigations show a negative influence of comorbid SUD in MPH response [35, 44]. Supporting these previous findings, we found that more severe ADHD at baseline and comorbid THC or alcohol use predicted worse response to MPH treatment.
We found that children and adolescents with a higher number of altered neuropsychological tests in the EF assessments had a significantly worse response to MPH treatment (Fig. 3). Total IQ measured by WISC-IV, Commission and Omission errors in the CPT-II and the Interference in the Stroop test has often been related to changes in the MPH treatment in ADHD [82]. Regarding how the dysfunction in EF may affect the treatment response in youth with ADHD, Coghill et al (2007) investigated the relationship between MPH response and several neuropsychological measures, in a randomized placebo-controlled trial design [19]. They identified 13 variables, as being the most likely to distinguish MPH responders from non-responders: three demographic variables, three clinical variables and various measures from four neuropsychological tasks. They found that poor performance on a ‘delayed matching to sample’ (DMtS) task at baseline was the only pre-treatment correlate of clinical MPH-response. Their data supports previous findings [32] that a comprehensive model of the MPH response would likely rely on a wide range of measures, rather than a single measure. However, to date, there is no objective, biological makers that can robustly predict MPH response in patients with ADHD [46]. Whilst Hinshaw (2007) and Coghill (2007) identified that age, IQ and symptom severity were possible predictors of MPH response, we found that both baseline ADHD-RS-IV.es symptom severity score (and some specific symptoms), Total-IQ, comorbid disorders (alcohol, THC use, depressive symptoms and ODD symptoms) and the Commission errors could be considered as predictive factors of MPH response [19, 76].
At baseline, patients in our sample who had a higher number of altered neuropsychological tests, most severe ADHD symptoms’ score and comorbid depression or alcohol/THC use, showed worse MPH response. On the other hand, patients with higher Total IQ scores and lower Commission errors showed better clinical response to MPH. Further investigations are needed to clarify whether the treatment of comorbid conditions could play a positive role in the MPH response.
5. LIMITATIONS
This study has some important limitations that should be considered for a correct interpretation and generalization of its findings. First of all, the present study did not include any measure related to motivational aspects such as delay aversion and emotional functioning that have also been shown to be predictive of later ADHD symptoms and, as a result, potential predictors of treatment response. Although teacher’s assesments are not essential for diagnosing ADHD or monitoring treatment response [83], the use of this assessment must be considered in future studies. We also did not include behavioral measures of executive functioning, other than the parameters collected from neuropsychological testing (CPT-II, IQ) based on the fact that literature shows poor correlation between EF impairment in neuropsychological testing and EF assessments using questionnaires [84, 85]. In this study we did not include a normal control group nor blinded raters, thus we could not control placebo effects or rater bias in responders vs. non-responders. However, as we are trying to compare responders vs. non-responders, those two groups could act as controls for each other. We could not find any demographic differences between responders and non-responders. In order to focus mostly on impairment due to ADHD, we excluded children with Total IQ < 80. This is a limitation because maybe these children with lower IQ may have higher neuropsychological deficits and lower rates of response. However, if we had included IQ < 80, the limitation would be that the response or neuropsychological deficits possible differences could be due not to the ADHD but to the low Total IQ.
CONCLUSION
Our findings show that factors related to response inhibition or low impulsivity, and the absence of comorbidity, and lower baseline severity of ADHD may be associated with a better MPH response. On the other hand, the presence of at least one or more than one altered neuropsychological test is associated with worse treatment response. The presence of comorbidities (ODD symptoms, depressive symptoms and alcohol or cannabis use) impact negatively in the treatment response. Furthermore, from the initial interview, the clinician could estimate and adjust the expectations to pharmacological treatment response and have a more frequent follow-up to those patients with a potential “non-responder profile”: children and adolescents with ADHD who have lower Total IQ, ODD symptoms, depressive symptoms and/or substance use, and or altered neuropsychological tests. Comorbidities should be treated, as well as the ADHD, in order to optimize and for a better outcome.
Therefore, there some possible strands of evidence suggesting that MPH response may be, at least partially, predictable using clinical and neuropsychological and clinical data that is often collected within routine clinical practice.
ACKNOWLEDGEMENTS
We would like to thank Marta García-Granero for her statistical advice.
AUTHORS' CONTRIBUTIONS
All authors have made substantial contributions to the conception and design of the work; and the analysis and interpretation of data; and have drafted the work or substantively revised it to have approved this submitted version. All authors agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
Not applicable.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
FUNDING
María Vallejo-Valdivielso, MD has received research funds for her department (non-personal) from Caja Navarra Foundation (CAN), Vasco-Navarra Society of Psychiatry (SVNP), Government of Navarra and Spanish Society of Child and Adolescent Psychiatry (AEPNYA). She has received financial support on continuous scientific education from Shire, Janssen and Lundbeck.
Pilar de Castro-Manglano, MD, PhD has received research funds for her department (non-personal) from Caja Navarra Foundation (CAN), Eli Lilly, Lundbeck and Shire. She has served as Consultant / Advisory Board for: Alicia Koplowitz Foundation, Editorial Médica Panamericana and Eli Lilly. She has served in the Speaker's Bureau / has given talks on Continuous Medical Education (not about a product) for Shire.
Azucena Díez-Suárez, MD, PhD has received research funds for her department (non-personal) from Caja Navarra Foundation (CAN), Otsuka Pharmaceutics, Lundbeck and Shire. She has served as Consultant / Advisory Board for: Alicia Koplowitz Foundation and Editorial Médica Panamericana. She has served in the Speaker's Bureau and has given talks on Continuous Medical Education (not about a product) for Shire.
Juan J. Marín-Méndez, BsC, PhD has received research funds for his department (non-personal) from Caja Navarra Foundation (CAN), Carlos III Health Institute, Government of Navarra, Qpea Foundation and Shire. He has received financial support for continuous scientific education from Shire, Lilly, Rovi, Roche and Pfizer.
César A. Soutullo, MD, PhD has received research funds for his department (non-personal) from Caja Navarra Foundation (CAN), Eli Lilly, Lundbeck, Shire and TEVA. He has served as Consultant / Advisory Board for: Alicia Koplowitz Foundation, Editorial Médica Panamericana, Eli Lilly, EUNETHYDIS (European Network on Hyperkinetic Disorder), Instituto de Salud Carlos III (FIS), NeuroTech Solutions Ltd, Spanish Health Ministry Quality Plan (Clinical Practice Guidelines on TDAH and Clinical Practice Guidelines on Depression), Rubió and Shire. He has served in the Speaker's Bureau / has given talks on Continuous Medical Education (not about a product) for Eli Lilly, Shire, Universidad Internacional Menéndez Pelayo and Universidad Internacional de La Rioja (UNIR). He has received Royalties from: DOYMA, Editorial Médica Panamericana, EUNSA and Mayo Ediciones.
CONFLICT OF INTEREST
The author declares no conflict of interest, financial or otherwise.
REFERENCES
- 1.American Psychiatric Association (APA) Diagnostic and statistical manual of mental disorders. 2013. [Google Scholar]
- 2.Faraone S.V., Asherson P., Banaschewski T., Biederman J., Buitelaar J.K., Ramos-Quiroga J.A., Rohde L.A., Sonuga-Barke E.J., Tannock R., Franke B. Attention-deficit/hyperactivity disorder. Nat. Rev. Dis. Primers. 2015;1:15020–15020. doi: 10.1038/nrdp.2015.20. [DOI] [PubMed] [Google Scholar]
- 3.Polanczyk G.V., Salum G.A., Sugaya L.S., Caye A., Rohde L.A. Annual research review: A meta-analysis of the worldwide prevalence of mental disorders in children and adolescents. J. Child Psychol. Psychiatry. 2015;56(3):345–365. doi: 10.1111/jcpp.12381. [DOI] [PubMed] [Google Scholar]
- 4.Thomas R., Sanders S., Doust J., Beller E., Glasziou P. Prevalence of attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Pediatrics. 2015;135(4):e994–e1001. doi: 10.1542/peds.2014-3482. [DOI] [PubMed] [Google Scholar]
- 5.Thapar A., Cooper M. Attention deficit hyperactivity disorder. Lancet. 2016;387(10024):1240–1250. doi: 10.1016/S0140-6736(15)00238-X. [DOI] [PubMed] [Google Scholar]
- 6.Biederman J., Faraone S.V., Monuteaux M.C., Grossbard J.R. How informative are parent reports of attention-deficit/hyperactivity disorder symptoms for assessing outcome in clinical trials of long-acting treatments? A pooled analysis of parents’ and teachers’ reports. Pediatrics. 2004;113(6):1667–1671. doi: 10.1542/peds.113.6.1667. [DOI] [PubMed] [Google Scholar]
- 7.Bledsoe J.C., Xiao D., Chaovalitwongse A., Mehta S., Grabowski T.J., Semrud-Clikeman M., Pliszka S., Breiger D. Diagnostic Classification of ADHD Versus Control: Support Vector Machine Classification Using Brief Neuropsychological Assessment. J. Atten. Disord. 2016:1087054716649666. doi: 10.1177/1087054716649666. [DOI] [PubMed] [Google Scholar]
- 8.Coghill D.R., Seth S., Matthews K. A comprehensive assessment of memory, delay aversion, timing, inhibition, decision making and variability in attention deficit hyperactivity disorder: advancing beyond the three-pathway models. Psychol. Med. 2014;44(9):1989–2001. doi: 10.1017/S0033291713002547. [DOI] [PubMed] [Google Scholar]
- 9.Sonuga-Barke E., Bitsakou P., Thompson M. Beyond the dual pathway model: evidence for the dissociation of timing, inhibitory, and delay-related impairments in attention-deficit/hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2010;49(4):345–355. doi: 10.1097/00004583-201004000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Willcutt E.G., Doyle A.E., Nigg J.T., Faraone S.V., Pennington B.F. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biol. Psychiatry. 2005;57(11):1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 11.Sergeant J.A., Geurts H., Oosterlaan J. How specific is a deficit of executive functioning for attention-deficit/hyperactivity disorder? Behav. Brain Res. 2002;130(1-2):3–28. doi: 10.1016/S0166-4328(01)00430-2. [DOI] [PubMed] [Google Scholar]
- 12.Douglas V.I. Stop, look and listen: The problem of sustained attention and impulse control in hyperactive and normal children. Can. J. Behav. Sci. 1972;4:259–282. doi: 10.1037/h0082313. [DOI] [Google Scholar]
- 13.Schoemaker K., Mulder H., Deković M., Matthys W. Executive functions in preschool children with externalizing behavior problems: a meta-analysis. J. Abnorm. Child Psychol. 2013;41(3):457–471. doi: 10.1007/s10802-012-9684-x. [DOI] [PubMed] [Google Scholar]
- 14.Fair D.A., Bathula D., Nikolas M.A., Nigg J.T. Distinct neuropsychological subgroups in typically developing youth inform heterogeneity in children with ADHD. Proc. Natl. Acad. Sci. USA. 2012;109(17):6769–6774. doi: 10.1073/pnas.1115365109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frazier T.W., Demaree H.A., Youngstrom E.A. Meta-analysis of intellectual and neuropsychological test performance in attention-deficit/hyperactivity disorder. Neuropsychology. 2004;18(3):543–555. doi: 10.1037/0894-4105.18.3.543. [DOI] [PubMed] [Google Scholar]
- 16.Seidman L.J. Neuropsychological functioning in people with ADHD across the lifespan. Clin. Psychol. Rev. 2006;26(4):466–485. doi: 10.1016/j.cpr.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 17.Miyake A., Friedman N.P., Emerson M.J., Witzki A.H., Howerter A., Wager T.D. The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: a latent variable analysis. Cognit. Psychol. 2000;41(1):49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- 18.Barkley R.A. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol. Bull. 1997;121(1):65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- 19.Coghill D.R., Rhodes S.M., Matthews K. The neuropsychological effects of chronic methylphenidate on drug-naive boys with attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2007;62(9):954–962. doi: 10.1016/j.biopsych.2006.12.030. [DOI] [PubMed] [Google Scholar]
- 20.Rhodes S.M., Coghill D.R., Matthews K. Methylphenidate restores visual memory, but not working memory function in attention deficit-hyperkinetic disorder. Psychopharmacology (Berl.) 2004;175(3):319–330. doi: 10.1007/s00213-004-1833-7. [DOI] [PubMed] [Google Scholar]
- 21.Rhodes S.M., Coghill D.R., Matthews K. Neuropsychological functioning in stimulant-naive boys with hyperkinetic disorder. Psychol. Med. 2005;35(8):1109–1120. doi: 10.1017/S0033291705004599. [DOI] [PubMed] [Google Scholar]
- 22.Kempton S., Vance A., Maruff P., Luk E., Costin J., Pantelis C. Executive function and attention deficit hyperactivity disorder: stimulant medication and better executive function performance in children. Psychol. Med. 1999;29(3):527–538. doi: 10.1017/S0033291799008338. [DOI] [PubMed] [Google Scholar]
- 23.Tamm L., Narad M.E., Antonini T.N., O’Brien K.M., Hawk L.W., Jr, Epstein J.N. Reaction time variability in ADHD: A review. Neurotherapeutics. 2012;9(3):500–508. doi: 10.1007/s13311-012-0138-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Castellanos F.X., Sonuga-Barke E.J.S., Milham M.P., Tannock R. Characterizing cognition in ADHD: beyond executive dysfunction. Trends Cogn. Sci. (Regul. Ed.) 2006;10(3):117–123. doi: 10.1016/j.tics.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 25.Shaw P., Stringaris A., Nigg J., Leibenluft E. Emotion dysregulation in attention deficit hyperactivity disorder. Am. J. Psychiatry. 2014;171(3):276–293. doi: 10.1176/appi.ajp.2013.13070966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sjöwall D., Bohlin G., Rydell A.M., Thorell L.B. Neuropsychological deficits in preschool as predictors of ADHD symptoms and academic achievement in late adolescence. Child Neuropsychol. 2015:1–18. doi: 10.1080/09297049.2015.1063595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nigg J.T., Willcutt E.G., Doyle A.E., Sonuga-Barke E.J.S. Causal heterogeneity in attention-deficit/hyperactivity disorder: do we need neuropsychologically impaired subtypes? Biol. Psychiatry. 2005;57(11):1224–1230. doi: 10.1016/j.biopsych.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 28.Doyle A.E., Biederman J., Seidman L.J., Weber W., Faraone S.V. Diagnostic efficiency of neuropsychological test scores for discriminating boys with and without attention deficit-hyperactivity disorder. J. Consult. Clin. Psychol. 2000;68(3):477–488. doi: 10.1037/0022-006X.68.3.477. [DOI] [PubMed] [Google Scholar]
- 29.Coghill D.R., Banaschewski T., Soutullo C., Cottingham M.G., Zuddas A. Systematic review of quality of life and functional outcomes in randomized placebo-controlled studies of medications for attention-deficit/hyperactivity disorder. Eur. Child Adolesc. Psychiatry. 2017;26(11):1283–1307. doi: 10.1007/s00787-017-0986-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pietrzak R.H., Mollica C.M., Maruff P., Snyder P.J. Cognitive effects of immediate-release methylphenidate in children with attention-deficit/hyperactivity disorder. Neurosci. Biobehav. Rev. 2006;30(8):1225–1245. doi: 10.1016/j.neubiorev.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 31.van der Schaaf M.E., Fallon S.J., Ter Huurne N., Buitelaar J., Cools R. Working memory capacity predicts effects of methylphenidate on reversal learning. Neuropsychopharmacology. 2013;38(10):2011–2018. doi: 10.1038/npp.2013.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnston B.A., Coghill D., Matthews K., Steele J.D. Predicting methylphenidate response in attention deficit hyperactivity disorder: a preliminary study. J. Psychopharmacol. (Oxford) 2015;29(1):24–30. doi: 10.1177/0269881114548438. [DOI] [PubMed] [Google Scholar]
- 33.Hodgkins P., Shaw M., Coghill D., Hechtman L. Amfetamine and methylphenidate medications for attention-deficit/hyperactivity disorder: complementary treatment options. Eur. Child Adolesc. Psychiatry. 2012;21(9):477–492. doi: 10.1007/s00787-012-0286-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anastopoulos A.D., DuPaul G.J., Weyandt L.L., Morrissey-Kane E., Sommer J.L., Rhoads L.H., Murphy K.R., Gormley M.J., Gudmundsdottir B.G. Rates and Patterns of Comorbidity Among First-Year College Students With ADHD. 2016. [DOI] [PMC free article] [PubMed]
- 35.Carpentier P.J., Levin F.R. Pharmacological Treatment of ADHD in Addicted Patients: What Does the Literature Tell Us? Harvard review of psychiatry. 2017;25(2):50–64. doi: 10.1097/HRP.0000000000000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chan M.H., Leung P.W., Ho T.P., Hung S.F., Lee C.C., Tang C.P., Cheung K.C., Ching F.Y., Chan F.H., Chen L.H., Garcia-Barcelo M., Sham P.C. Neuropsychiatric Disease and Treatment. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abikoff H., McGough J., Vitiello B., McCracken J., Davies M., Walkup J., Riddle M., Oatis M., Greenhill L., Skrobala A., March J., Gammon P., Robinson J., Lazell R., McMahon D.J., Ritz L., RUPP ADHD/Anxiety Study Group Sequential pharmacotherapy for children with comorbid attention-deficit/hyperactivity and anxiety disorders. J. Am. Acad. Child Adolesc. Psychiatry. 2005;44(5):418–427. doi: 10.1097/01.chi.0000155320.52322.37. [DOI] [PubMed] [Google Scholar]
- 38.Gadow K.D., Nolan E.E., Sverd J., Sprafkin J., Schwartz J. Anxiety and depression symptoms and response to methylphenidate in children with attention-deficit hyperactivity disorder and tic disorder. J. Clin. Psychopharmacol. 2002;22(3):267–274. doi: 10.1097/00004714-200206000-00007. [DOI] [PubMed] [Google Scholar]
- 39.Pliszka S.R. Effect of anxiety on cognition, behavior, and stimulant response in ADHD. J. Am. Acad. Child Adolesc. Psychiatry. 1989;28(6):882–887. doi: 10.1097/00004583-198911000-00012. [DOI] [PubMed] [Google Scholar]
- 40.Ter-Stepanian M., Grizenko N., Zappitelli M., Joober R. Clinical response to methylphenidate in children diagnosed with attention-deficit hyperactivity disorder and comorbid psychiatric disorders. Can. J. Psychiatry. 2010;55(5):305–312. doi: 10.1177/070674371005500506. [DOI] [PubMed] [Google Scholar]
- 41.Moshe K., Karni A., Tirosh E. Anxiety and methylphenidate in attention deficit hyperactivity disorder: a double-blind placebo-drug trial. Atten. Defic. Hyperact. Disord. 2012;4(3):153–158. doi: 10.1007/s12402-012-0078-2. [DOI] [PubMed] [Google Scholar]
- 42.Gray J.R., Kagan J. The Challenge of Predicting Which Children with Attention Deficit-Hyperactivity Disorder Will Respond Positively to Methylphenidate. J. Appl. Dev. Psychol. 2000;21(5):471–489. doi: 10.1016/S0193-3973(00)00050-2. [DOI] [Google Scholar]
- 43.Sullivan M.A., Rudnik-Levin F. Attention deficit/hyperactivity disorder and substance abuse. Diagnostic and therapeutic considerations. Ann. N. Y. Acad. Sci. 2001;931:251–270. doi: 10.1111/j.1749-6632.2001.tb05783.x. [DOI] [PubMed] [Google Scholar]
- 44.Tamm L., Trello-Rishel K., Riggs P., Nakonezny P.A., Acosta M., Bailey G., Winhusen T. Predictors of treatment response in adolescents with comorbid substance use disorder and attention-deficit/hyperactivity disorder. J. Subst. Abuse Treat. 2013;44(2):224–230. doi: 10.1016/j.jsat.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Denney C.B., Rapport M.D. Predicting methylphenidate response in children with ADHD: theoretical, empirical, and conceptual models. J. Am. Acad. Child Adolesc. Psychiatry. 1999;38(4):393–401. doi: 10.1097/00004583-199904000-00011. [DOI] [PubMed] [Google Scholar]
- 46.Kim J.W., Sharma V., Ryan N.D. Predicting Methylphenidate Response in ADHD Using Machine Learning Approaches. Int. J. Neuropsychopharmacol. 2015;18(11):pyv052. doi: 10.1093/ijnp/pyv052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.American Psychiatric Association (APA) Diagnostic and statistical manual of mental disorders. fourth edition, text revision. Washington, DC: DSMIV-TR; 2000. [Google Scholar]
- 48.Kaufman J., Birmaher B., Brent D., Rao U.R.N. Schedule for Affective Disorders and Schizophrenia for School Aged Children- Present and Lifetime Version (K-SADS-PL). Pittsburgh: University of Pittsburgh Press; 1996. [DOI] [PubMed] [Google Scholar]
- 49.DuPaul G.J., Power T.J., Anastopoulos A.D., Reid R. ADHD Rating Scale-IV: Checklists, norms, and clinical interpretation. New York, NY: Guilford Press; 1998. [Google Scholar]
- 50.Vallejo-Valdivielso M., Soutullo C.A., de Castro-Manglano P., Marín-Méndez J.J., Díez-Suárez A. Validation of a Spanish-language version of the ADHD Rating Scale IV in a Spanish sample. Neurologia. 2017;2017 doi: 10.1016/j.nrl.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 51.Swanson J., Nolan W., Pelham W. The SNAP-IV Rating Scale. 1992. [Google Scholar]
- 52.Guy W. ECDEU assessment manual for psychopharmacology, revised. Rockvill. National Institute of Mental Health Psychopharmacology Research Branch; 1976. [Google Scholar]
- 53.Wechsler D. Wechsler Intelligence Scale for Children. 2003. [Google Scholar]
- 54.Wechsler D. Wechsler Intelligence Scale for Children. 1991. [Google Scholar]
- 55.Stroop J. Studies of interference in serial verbal reaction. J. Exp. Psychol. 1935;18:643–662. doi: 10.1037/h0054651. [DOI] [Google Scholar]
- 56.Adleman N.E., Menon V., Blasey C.M., White C.D., Warsofsky I.S., Glover G.H., Reiss A.L. A developmental fMRI study of the Stroop color-word task. Neuroimage. 2002;16(1):61–75. doi: 10.1006/nimg.2001.1046. [DOI] [PubMed] [Google Scholar]
- 57.Zysset S., Müller K., Lohmann G., von Cramon D.Y. Color-word matching stroop task: separating interference and response conflict. Neuroimage. 2001;13(1):29–36. doi: 10.1006/nimg.2000.0665. [DOI] [PubMed] [Google Scholar]
- 58.MacLeod C.M. Half a century of research on the Stroop effect: an integrative review. Psychol. Bull. 1991;109(2):163–203. doi: 10.1037/0033-2909.109.2.163. [DOI] [PubMed] [Google Scholar]
- 59.Golden C. Stroop Color and Word Test: A Manual for Clinical and experimental uses. Chicago: Stoelting Co.; 1978. [Google Scholar]
- 60.Conners C.K. Conners’ continuous performance test. Toronto: Multi-Health Systems; 1995. [Google Scholar]
- 61.Wang L.J., Huang Y.S., Chiang Y.L., Hsiao C.C., Shang Z.Y., Chen C.K. Clinical symptoms and performance on the Continuous Performance Test in children with attention deficit hyperactivity disorder between subtypes: a natural follow-up study for 6 months. BMC Psychiatry. 2011;11:65. doi: 10.1186/1471-244X-11-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Biederman J., Faraone S.V., Doyle A., Lehman B.K., Kraus I., Perrin J., Tsuang M.T. Convergence of the Child Behavior Checklist with structured interview-based psychiatric diagnoses of ADHD children with and without comorbidity. J. Child Psychol. Psychiatry. 1993;34(7):1241–1251. doi: 10.1111/j.1469-7610.1993.tb01785.x. [DOI] [PubMed] [Google Scholar]
- 63.Huss M., Sikirica V., Hervas A., Newcorn J.H., Harpin V., Robertson B. Guanfacine extended release for children and adolescents with attention-deficit/hyperactivity disorder: efficacy following prior methylphenidate treatment. Neuropsychiatr. Dis. Treat. 2016;112(112):1085–1101. doi: 10.2147/NDT.S94158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Newcorn J.H., Harpin V., Huss M., Johnson M., Ramos-Quiroga J.A. Long-term maintenance of efficacy of extended-release guanfacine hydrochloride in children and adolescents with attention-deficit/hyperactivity disorder: double-blind, placebo-controlled, multicentre, Phase 3 randomized withdrawal study. 3rd EUNETHYDIS International Conference on ADHD; Istanbul, Turkey. 2014. [Google Scholar]
- 65.Coghill D., Banaschewski T., Lecendreux M., Soutullo C., Johnson M., Zuddas A., Anderson C., Civil R., Higgins N., Lyne A., Squires L. European, randomized, phase 3 study of lisdexamfetamine dimesylate in children and adolescents with attention-deficit/hyperactivity disorder. Eur. Neuropsychopharmacol. 2013;23(10):1208–1218. doi: 10.1016/j.euroneuro.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 66.Soutullo C., Banaschewski T., Lecendreux M., Johnson M., Zuddas A., Anderson C., Civil R., Higgins N., Bloomfield R., Squires L.A., Coghill D.R. A post hoc comparison of the effects of lisdexamfetamine dimesylate and osmotic-release oral system methylphenidate on symptoms of attention-deficit hyperactivity disorder in children and adolescents. CNS Drugs. 2013;27(9):743–751. doi: 10.1007/s40263-013-0086-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Biederman J., Mick E., Surman C., Doyle R., Hammerness P., Kotarski M., Spencer T. A randomized, 3-phase, 34-week, double-blind, long-term efficacy study of osmotic-release oral system-methylphenidate in adults with attention-deficit/hyperactivity disorder. J. Clin. Psychopharmacol. 2010;30(5):549–553. doi: 10.1097/JCP.0b013e3181ee84a7. [DOI] [PubMed] [Google Scholar]
- 68.Findling R.L., Adeyi B., Chen G., Dirks B., Babcock T., Scheckner B., Lasser R., Pucci M.L., Abdullah H.I., McGough J.J. Clinical Response and Symptomatic Remission in Children Treated With Lisdexamfetamine Dimesylate for Attention-Deficit/Hyperactivity Disorder. CNS Spectr. 2010;15(9):559–568. doi: 10.1017/S1092852900000535. [DOI] [Google Scholar]
- 69.Gao H., Zhao Y., Levine L., Allen A. Determining cut-points for clinically meaningful improvement: a receiver operating characteristic characteristic approach. Scientific Proceedings of the 53rd Annual Meeting of the American Academy of Child and Adolescent Psychiatry. Washington, DC. J. Am. Acad. Child Adolesc. Psychiatry. 2006;2006:201. [Google Scholar]
- 70.Goodman D., Faraone S.V., Adler L.A., Dirks B., Hamdani M., Weisler R. Interpreting ADHD rating scale scores: linking ADHD rating scale scores and CGI levels in two randomized controlled trials of lisdexamfetamine dimesylate in ADHD. Prim. Psychiatry. 2010;17(3):44–52. [Google Scholar]
- 71.Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale: Erlbaum; 1988. [Google Scholar]
- 72.Dittmann R.W., Cardo E., Nagy P., Anderson C.S., Adeyi B., Caballero B., Hodgkins P., Civil R., Coghill D.R. Treatment response and remission in a double-blind, randomized, head-to-head study of lisdexamfetamine dimesylate and atomoxetine in children and adolescents with attention-deficit hyperactivity disorder. CNS Drugs. 2014;28(11):1059–1069. doi: 10.1007/s40263-014-0188-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hautmann C., Rothenberger A., Döpfner M. Daily Symptom Profiles of Children With ADHD Treated With Modified-Release Methylphenidate: An Observational Study. Journal of Attention Disorders. 2013;21(2):120–128. doi: 10.1177/1087054713502233. [DOI] [PubMed] [Google Scholar]
- 74.Ikeda Y., Okuzumi H., Kokubun M. Stroop/reverse-Stroop interference in typical development and its relation to symptoms of ADHD. Res. Dev. Disabil. 2013;34(8):2391–2398. doi: 10.1016/j.ridd.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 75.Newcorn J.H., Halperin J.M., Jensen P.S., Abikoff H.B., Arnold L.E., Cantwell D.P., Conners C.K., Elliott G.R., Epstein J.N., Greenhill L.L., Hechtman L., Hinshaw S.P., Hoza B., Kraemer H.C., Pelham W.E., Severe J.B., Swanson J.M., Wells K.C., Wigal T., Vitiello B. Symptom profiles in children with ADHD: effects of comorbidity and gender. J. Am. Acad. Child Adolesc. Psychiatry. 2001;40(2):137–146. doi: 10.1097/00004583-200102000-00008. [DOI] [PubMed] [Google Scholar]
- 76.Hinshaw S.P., Carte E.T., Sami N., Treuting J.J., Zupan B.A. Preadolescent girls with attention-deficit/hyperactivity disorder: II. Neuropsychological performance in relation to subtypes and individual classification. J. Consult. Clin. Psychol. 2002;70(5):1099–1111. doi: 10.1037/0022-006X.70.5.1099. [DOI] [PubMed] [Google Scholar]
- 77.Beery S.H., Quay H.C., Pelham W.E., Jr Differential response to methylphenidate in inattentive and combined subtype ADHD. J. Atten. Disord. 2013 doi: 10.1177/1087054712469256. [DOI] [PubMed] [Google Scholar]
- 78.Gorman E.B., Klorman R., Thatcher J.E., Borgstedt A.D. Effects of methylphenidate on subtypes of attention-deficit/hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2006;45(7):808–816. doi: 10.1097/01.chi.0000214191.57993.dd. [DOI] [PubMed] [Google Scholar]
- 79.Barkley R.A., DuPaul G.J., McMurray M.B. Attention deficit disorder with and without hyperactivity: clinical response to three dose levels of methylphenidate. Pediatrics. 1991;87(4):519–531. [PubMed] [Google Scholar]
- 80.Stein M.A., Sarampote C.S., Waldman I.D., Robb A.S., Conlon C., Pearl P.L., Black D.O., Seymour K.E., Newcorn J.H. A dose-response study of OROS methylphenidate in children with attention-deficit/hyperactivity disorder. Pediatrics. 2003;112(5):e404–e413. doi: 10.1542/peds.112.5.e404. [DOI] [PubMed] [Google Scholar]
- 81.Jensen P.S., Hinshaw S.P., Kraemer H.C., Lenora N., Newcorn J.H., Abikoff H.B., March J.S., Arnold L.E., Cantwell D.P., Conners C.K., Elliott G.R., Greenhill L.L., Hechtman L., Hoza B., Pelham W.E., Severe J.B., Swanson J.M., Wells K.C., Wigal T., Vitiello B. ADHD comorbidity findings from the MTA study: comparing comorbid subgroups. J. Am. Acad. Child Adolesc. Psychiatry. 2001;40(2):147–158. doi: 10.1097/00004583-200102000-00009. [DOI] [PubMed] [Google Scholar]
- 82.Matsumoto A., Yuji H., Watanabe M. [Life-span development of stroop and reverse-Stroop interference measured using matching responses]. Shinrigaku Kenkyu. 2012;83(4):337–346. doi: 10.4992/jjpsy.83.337. [DOI] [PubMed] [Google Scholar]
- 83.Bied A., Biederman J., Faraone S. Parent-based diagnosis of ADHD is as accurate as a teacher-based diagnosis of ADHD. Postgrad. Med. 2017;129(3):375–381. doi: 10.1080/00325481.2017.1288064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Toplak M.E., Bucciarelli S.M., Jain U., Tannock R. Executive functions: performance-based measures and the behavior rating inventory of executive function (BRIEF) in adolescents with attention deficit/hyperactivity disorder (ADHD). Child Neuropsychol. 2009;15(1):53–72. doi: 10.1080/09297040802070929. [DOI] [PubMed] [Google Scholar]
- 85.Gioia G.A., Isquith P.K., Guy S., Kenworthy L. BRIEF: Behavior Rating Inventory of Executive Function professional manual. Lutz, FL: Psychological Assessment Resources; 2000. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.


