Abstract
The past few decades have witnessed the booming field of cancer immunotherapy. Cancer therapeutic vaccines, either alone or in combination with other immunotherapies such as adoptive cell therapy or immune checkpoint blockade therapy, are an attractive class of cancer immunotherapeutics. However, cancer vaccines have thus far shown suboptimal efficacy in the clinic. Nanomedicines offer unique opportunities to improve the efficacy of these vaccines. A variety of nanoplatforms have been investigated to deliver molecular or cellular or subcellular vaccines to target lymphoid tissues and cells, thereby promoting the potency and durability of anti-tumor immunity while reducing adverse side effects. In this article, we reviewed the key parameters and features of nanovaccines for cancer immunotherapy; we highlighted recent advances in the development of cancer nanovaccines based on synthetic nanocarriers, biogenic nanocarriers, as well as semi-biogenic nanocarriers; and we summarized newly emerging types of nanovaccines, such as those based on stimulator of interferon genes agonists, cancer neoantigens, mRNA vaccines, as well as artificial antigen-presenting cells.
This article is categorized under:
Therapeutic Approaches and Drug Discovery > Nanomedicine for Oncologic Disease
Keywords: cancer immunotherapy, co-delivery, mRNA vaccines, nanocarriers, nanovaccine, neoantigen vaccines, STING agonists
1 |. INTRODUCTION
Cancer therapeutic vaccines are an attractive alternative or complement to conventional cancer treatments (Goforth et al., 2009; Krishnamachari & Salem, 2009). Cancer vaccines work by manipulating the patients’ own immune system to recognize and destroy cancer cells. Cancer vaccines can stimulate specific antitumor immune responses, induce specific killing of tumor cells with minimal damage to healthy cells, and elicit immune memory that provides long-term protection against tumor recurrence (Blattman & Greenberg, 2004; Diebold, Kaisho, Hemmi, Akira, & Sousa, 2004; Melief, 2007; Williams, 1996). Chemically defined subunit vaccines, which are easy to manufacture and typically safe to administer, have been attractive for drug development. However, subunit vaccines often have weak immunogenicity and only induce short-term immune responses. To address this challenge, pharmaceutical engineering approaches have been employed to formulate subunit vaccines with delivery vehicles (e.g., micro/nanoparticles [NPs]) in order to promote antigen delivery and presentation by antigen-presenting cells (APCs). Nanovaccines have multiple advantages over subunit vaccines. (a) Encapsulating antigens in nanocarriers can prevent antigenic degradation and improve antigen stability. (b) Co-encapsulation of antigens and adjuvants in nanovaccines can co-deliver antigens and adjuvants, thereby enhancing the immunogenicity and therapeutic efficacy of vaccines. (c) Nanovaccines can be easily phagocytized and processed by APCs. (d) NPs designed for cytoplasmic antigen delivery can enhance cross-presentation and major histocompatibility complex I (MHC-I) presentation of antigens, thereby promoting adaptive immune responses including cytotoxic T-cell lymphocyte (CTL) responses that are crucial for cancer immunotherapy. (e) Surface modifications of NPs with targeting ligands permits specific targeting to lymphoid tissues and APCs for precise immunomodulation. Finally, the polyvalent presentation of antigens on the surface of nanovaccines allows B cell receptors to be cross-linked for enhanced humoral immune responses (Cai, Wang, Wang, & Li, 2019).
In the present review article, we focus on advances in anticancer nanovaccines and discuss the challenges and opportunities in nanovaccine development for cancer immunotherapy. We will discuss nanovaccines based on different types of nanocarriers—synthetic nanocarriers, semi-biogenic nanocarriers, and biogenic nanocarriers, all of which can deliver subunit vaccine components ranging from tumor-associated antigens (TAAs) or neoantigens in the form of peptides or mRNA, to a variety of immunostimulatory adjuvants (Figure 1). Furthermore, we will discuss how such nanovaccines enhance T cell responses for cancer immunotherapy.
FIGURE 1.
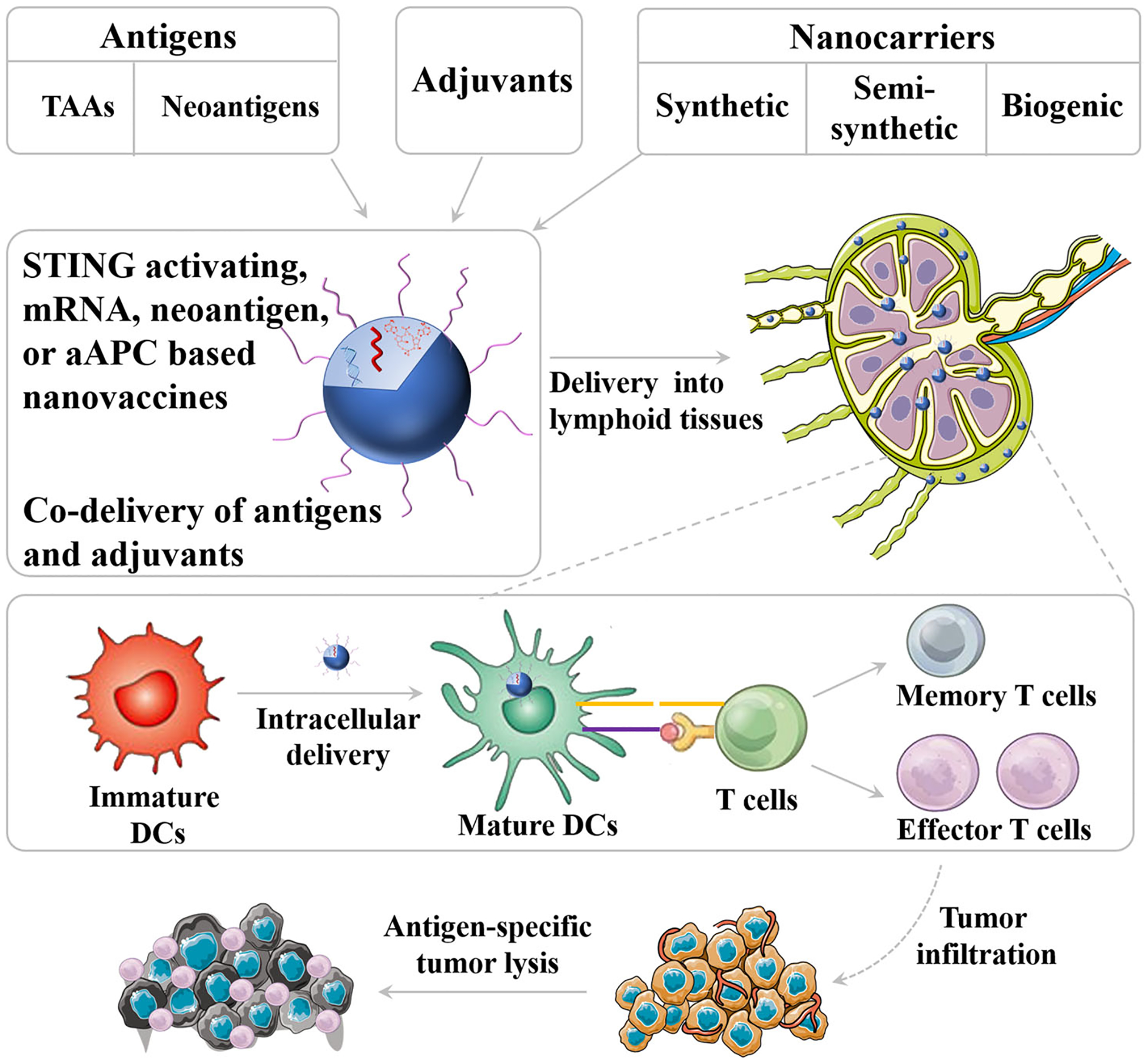
Schematic depiction of nanovaccine. Nanovaccines can be loaded with both adjuvant and antigens on the surface of nanocarriers or inside nanocarriers. Nanovaccines can efficiently co-deliver adjuvant and tumor antigen to lymphoid tissues and intracellular delivery into antigen-presenting cells (APCs) for efficient induction of antitumor T cell response
2 |. WHAT MAKES CANCER NANOVACCINES?
Nanomaterials have offered unique opportunities to improve the therapeutic efficacy of cancer vaccines. Nanovaccines are typically composed of antigens, molecular or nanoadjuvants and/or nanocarriers. Many nanomaterials per se have appeared to be immunomodulatory, enabling them to function as nanoadjuvants (Temizoz, Kuroda, & Ishii, 2016). In this article, we will focus on using nonimmunomodulatory nanocarriers to delivery molecular antigens and molecular adjuvants. A variety of nanomaterials have been studied as nanocarriers in nanovaccines. Here, we categorize these nanocarriers into three classes by the way of their manufacture—synthetic nanocarriers, semisynthetic nanocarriers, and biogenic nanocarriers. In this section, we will discuss the components of nanovaccines and the functions of each component.
2.1 |. Antigens
Tumor antigens are favorably (TAAs) or exclusively expressed (neoantigens) in tumor cells and presented on the MHC molecules on tumor cells. Due to mechanisms such as immunosuppression and immune evasion, endogenous cancer antigens are unable to elicit therapeutically significant immune responses. By introducing exogenous tumor-relevant antigens, cancer vaccines potentiate antigen-specific anticancer immune responses which mediate cancer immunotherapy (Mellman, Coukos, & Dranoff, 2011). To date, a large number of TAAs have been identified, some of which have been investigated in the clinic as cancer therapeutic vaccines (Fioretti, Iurescia, Fazio, & Rinaldi, 2010).
2.1.1 |. Tumor-associated antigens
TAAs or shared antigens can be expressed by both cancer cells and healthy tissues, and they may be expressed by more than one type of cancer cells (Fioretti et al., 2010). For example, cancer-testis (CT) antigens are a class of TAAs that are expressed only in specific cancer cells and some immunologically privileged germline tissues, but not in normal adult cells. Though the role of CT antigens such as, melanoma-associated antigens-A (MAGE-A), the gene encoding New York’s esophageal squamous cell carcinoma 1 (NY-ESO-1), LAGE-1, and TTK protein kinase (TTK), in cancer development remains to be fully understood (Zhang, Zhang, & Zhang, 2019), CT antigens have been investigated as candidates for cancer vaccines (Bruggen et al., 2002). Another type of TAA-based cancer vaccine candidates is overexpressed self-proteins (Lowe, Shearer, Jumper, & Kennedy, 2007). A classic example of such TAAs is HER-2/neu, an oncoprotein commonly associated with breast cancer and ovarian cancer (Ross & Fletcher, 1998).
2.1.2 |. Neoantigens
Unlike TAAs, tumor-specific unique antigens or neoantigens are derived from genetic events such as random somatic mutations or abnormal gene expression in tumor cells (Coulie et al., 1995). These antigens are expressed solely in tumor cells, but not in any normal cells (Parmiani, De Filippo, Novellino, & Castelli, 2007). As such, these neoantigens can be recognized as non-self and not be subjected to central immune tolerance, providing an opportunity to generate tumor-selective antitumor immune responses by using cancer neoantigen vaccines.
2.2 |. Immunostimulatory adjuvants
Immune adjuvants for immunostimulatory cancer vaccines can stimulate the immune system, potentiate the immune responses elicited by antigens (Montomoli et al., 2011), and guide the type of immune responses (Copland, Rades, Davies, & Baird, 2005). All these properties of adjuvants are crucial for subunit antigens which per se are typically weakly immunogenic. Based on their principal mechanisms of action, adjuvants can be generally divided into two classes (Schijns, 2003)—(a) vaccine delivery systems, such as mineral salts, emulsions, liposomes, and virosomes (Felnerova, Viret, Gluck, & Moser, 2004; Schwaninger et al., 2004); vaccine delivery systems can help present antigens to the immune system in a more efficient way and control the release and storage of the antigens; (b) immunostimulatory molecular adjuvants, including toll-like receptor (TLR) agonists (e.g., CpG oligonucleotides, polyI:C, R848, monophosphoryl lipid A), STING agonists (e.g., c-di-AMP), costimulatory ligands (e.g., anti-CD40), and cytokines (Adams, 2009; Gnjatic, Sawhney, & Bhardwaj, 2010); immunostimulatory adjuvants activate immune cells such as APCs, leading to the potentiation of the antigen-specific immune responses.
2.3 |. Nanocarriers
NPs are an excellent platform for the delivery of subunit vaccines. Various nanomaterials have been studied for vaccine delivery in cancer immunotherapy. Here, we will discuss recent progresses in the development of nanovaccines based on biogenic, semisynthetic, or synthetic nanocarriers for cancer immunotherapy.
2.3.1 |. Biogenic nanocarriers
Biogenic nanocarriers are the nanomaterials derived from biological entities, such as biological cells. Biogenic nanocarriers are considered as the “self” and have the potential of great biodegradability and biocompatibility as well as low toxicity. Examples of biogenic nanocarriers include exosomes and outer membrane vesicles (OMVs) (Figure 2).
FIGURE 2.
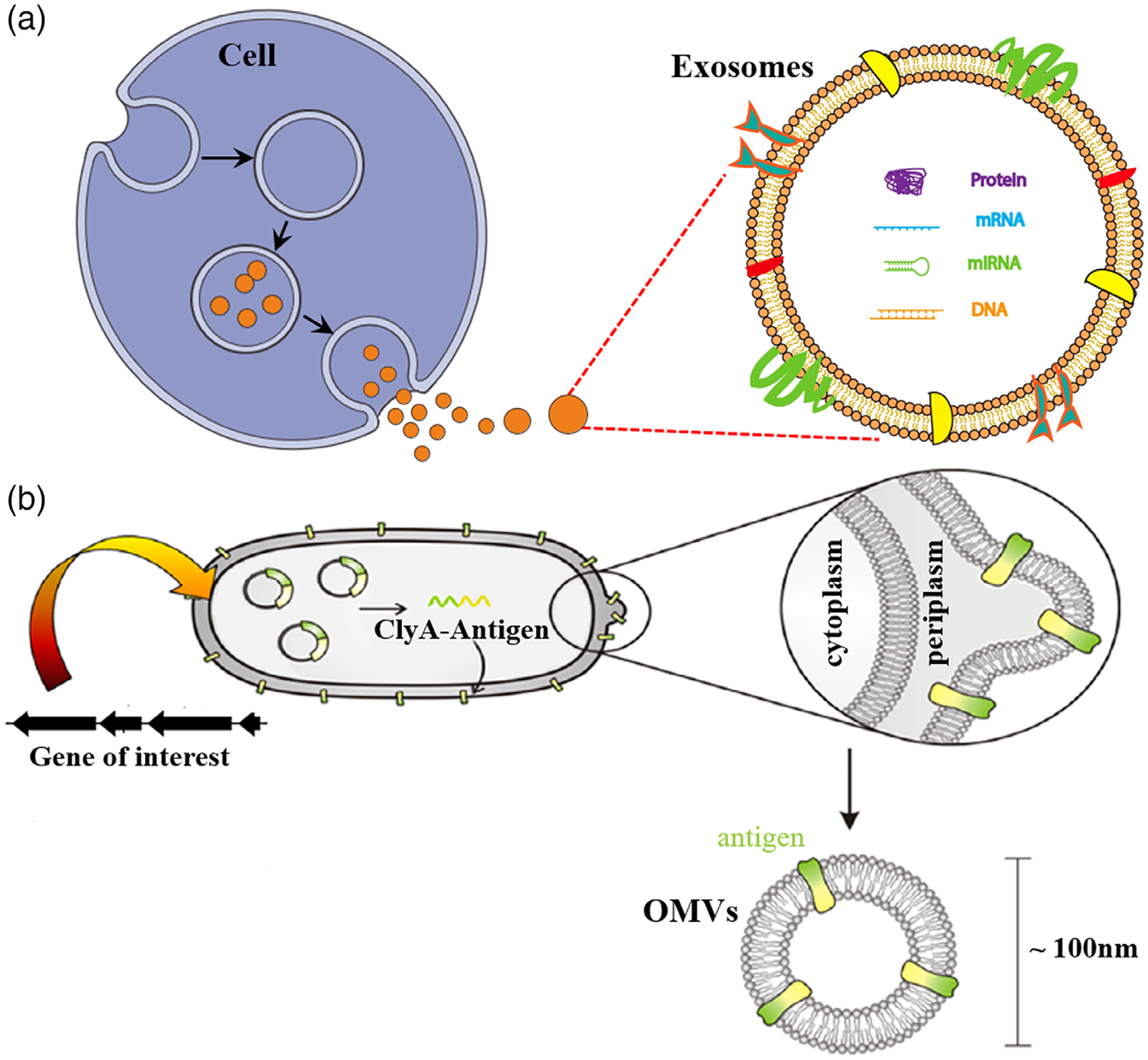
Biogenic nanocarriers of vaccines. (a) Schematic representation of the generation of exosomes. (Reprinted with permission from Jella et al. (2018). Copyright 2018 MDPI Publishing Group) (b) Schematic representation of the generation of engineered antigen-loaded OMVs. (Reprinted with permission from Rosenthal et al. (2014). Copyright 2014 PLOS Publishing Group)
With the sizes typically in 30–150 nm, exosomes have the potential as carriers for efficient vaccine delivery. Exosomes have been found to be secreted by a variety of cells including T cells, B cells, tumor cells, and APCs (Thery et al., 2002). Depending on cell origins, exosomes can be immunostimulatory or immunosuppressive, which indicate potential applications in the immunotherapy of tumor or autoimmune diseases, respectively (A. Tan, De La Pena, & Seifalian, 2010). For example, for cancer immunotherapy, tumor-derived exosomes that contain MHC/epitope molecular complexes for recognition by T cell receptors for T cell activation have been demonstrated to have immunotherapeutic efficacy (Lee, Kim, Cho, & Kim, 2011). Moreover, DC-derived exosomes are enriched with receptors and molecules that are important for antigen presentation and T cell activation (Syn, Wang, Chow, Lim, & Goh, 2017). Further, their ability to deliver exogenous vaccines is also valuable for cancer immunotherapy. The challenges for exosome-based nanomedicines include the cost and time it takes to manufacture exosomes especially at clinically significant large scales.
OMVs are another class of biogenic nanocarriers that are attractive for cancer immunotherapy. Naturally, OMVs are formed from the outer membranes of Gram-negative bacteria to communicate among themselves or with other microorganisms in their environment (Wang, Gao, & Wang, 2018). Specifically, OMVs are involved in trafficking bacterial cell signaling biochemicals, which may include DNA, RNA, proteins, endotoxins, and allied virulence molecules. Interestingly, the presence of immunostimulatory “danger signals” in bacteria-derived OMVs make these vesicles attractive immunostimulatory adjuvants. Furthermore, OMVs are 50–250 nm in diameters, making OMVs great carriers for efficient lymph node homing and intracellular delivery into APCs (Lin, Chattopadhyay, Lin, & Hu, 2018). Up to now, OMVs have been studied to develop bacterial vaccines (Lin et al., 2018) and more recently cancer therapeutic vaccines (Kim et al., 2017; Wang et al., 2017). For instance, OMVs were loaded with antitumor cytokines CXCL10 and interferon-γ as therapeutic agents (Kim et al., 2017), which showed remarkable efficacy to induce long-term antitumor immune responses that eradicated established tumors without notable adverse effects. Further, engineered OMVs have been engineered to display Human Papillomavirus 16 (HPV16) E7 protein to induce E7-specific cellular immunity. These OMVs showed significant therapeutic efficacy, thus demonstrating the potential of OMVs as cancer nanovaccines (Wang et al., 2017).
2.3.2 |. Semi-biogenic nanocarriers
Semisynthetic nanocarriers are formed from partially biogenic components together with synthetic components. When properly engineered, semisynthetic nanocarriers may inherit some features of biogenic nanocarriers, such as biocompatibility and low toxicity, but can also integrate synthetic NPs’ capabilities such as relatively easy and reproducible large-scale manufacture. Here, we will discuss three types of semisynthetic nanocarriers—cell membrane-coated nanocarriers, virus-like particles (VLPs), and endogenous protein-based nanocarriers.
Recently, cell membrane camouflage-based nanocarriers have become a biomimetic platform for drug delivery (Gou et al., 2014; Luk & Zhang, 2015). The cell membrane of interest can be extracted and coated onto the nanoparticle surfaces or formed as building blocks to form nanocarriers. Cancer cell membrane-coated NPs can carry the full array of cancer cell membrane antigens, which offer a platform of cancer nanovaccines (Figure 3; Fang et al., 2014). Further, a triple combination of an adjuvant, cell membrane antigens, and a targeted ligand can produce a powerful anti-cancer immune response similar to the levels under the situation of bacterial infection. Using patients’ own cancer cells as source, cell membrane camouflage-based nanovaccines can have a broad applicability in the clinic, and further allow straightforward engineering of adjuvants and cell membrane materials. Taken together, these studies have demonstrated that cell membrane-coated NPs have significant potential as nanovaccines.
FIGURE 3.
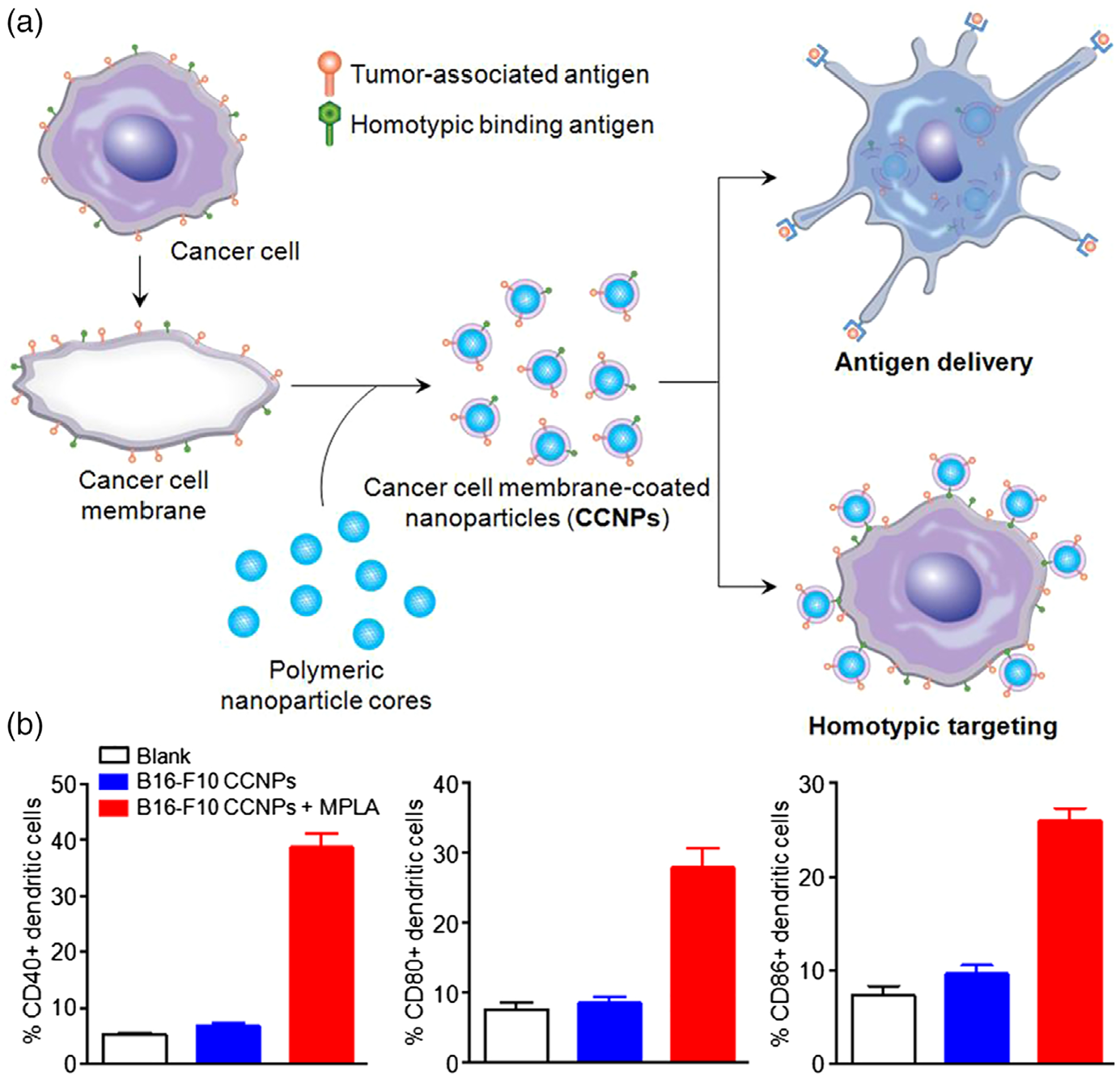
Cell membrane camouflage-based nanocarriers of vaccines. (a) Schematic representation of the fabrication of cancer cell membrane-coated nanoparticle (CCNP) and the potential applications. (b) CCNPs for the delivery of tumor-associated antigens, and maturation of dendritic cells. (Reprinted with permission from Fang et al. (2014). Copyright 2014 American Chemical Society Publishing Group)
VLPs are another type of semisynthetic nanocarriers with potential as cancer nanovaccines. VLPs structurally resemble viruses but are noninfectious because they contain no virus genetic material. VLPs are self-assembled from virus structural proteins that are expressed in vitro. VLPs hold great potential as nanovaccines because they are amenable for tailor-designed engineering of VLPs and antigens on VLPs. Further, repetitive antigenic structures can be engineered for efficient immuno-activation. VLPs are readily taken up into APCs and thus, if antigens are engineered on VLP-based nanovaccines, are able to prime long-lasting adaptive immune responses (Roy & Noad, 2008). In one example, Luján showed that multiple antigens can be expressed at the surface of VLPs by using the G protein of the vesicular stomatitis virus (VSV-G) as a paradigm (Bellier et al., 2009; Garrone et al., 2011). Another strategy of antigen modification on VLPs is chemical conjugation post-VLP assembly via bifunctional crosslinkers (Basle, Joubert, & Pucheault, 2010). For example, Buchholz’s group has demonstrated that VLPs displaying tumor antigens elicited a strong immune response and effectively treated cancer (Schneider et al., 2018). Taken together, these studies demonstrated the potential of VLPs as cancer nanovaccines.
Endogenous protein, such as albumin, have been studied as endogenous drug carriers since 1970s for the delivery of drugs, including antiviral drugs (Kamps et al., 1996), anticancer drugs (Peng et al., 2017), radionucleotide (Tian et al., 2017), and more recently molecular vaccines (Zhu, Lynn, et al., 2017). Specifically for vaccine delivery, the as-assembled protein/drug nanocomplexes often fall into the “sweet spot” size range that permit efficient lymphatic draining and intracellular uptake, both of which are highly desired for the delivery of vaccines to lymphoid tissues and APCs. In a seminal study by Darrell Irvine group, an albumin-binding lipid was conjugated with subunit vaccines, resulting in efficient accumulation in lymph nodes and dramatic enhancement of immunostimulation (Liu et al., 2014). Such immunomodulation efficacy was translated to remarkable therapeutic efficacy for cancer as well as viral diseases. In another example, by conjugating molecular vaccines with an albumin-binding Evans blue derivative to synthesize albumin-binding vaccines (AlbiVax), albumin/AlbiVax nanocomplexes were self-assembled in vivo from AlbiVax and endogenous albumin for efficient vaccine delivery and potent cancer immunotherapy (Figure 4; Zhu, Lynn, et al., 2017). Compared with conventional synthetic nanomaterials or biogenic nanomaterials, these endogenous albumin-based nanovaccines, which are formed from endogenous protein carriers and exogenous chemically defined molecular vaccines, may have some unique advantages: (a) the chemically defined molecular vaccines can have facile manufacturing and quality control, and may take advantage of established manufacturing pipelines at good manufacturing practice (cGMP) grade; (b) albumin has a long half-life in vivo (>20 days in humans) (Zhu, Zhang, Ni, Niu, & Chen, 2017); and (c) one of albumin receptors, neonatal Fc receptor (FcRn) is highly expressed in APCs such as monocyte, DCs, and macrophages, which likely facilitates the intracellular delivery of albumin/vaccine nanocomplexes through active endocytosis of albumin.
FIGURE 4.
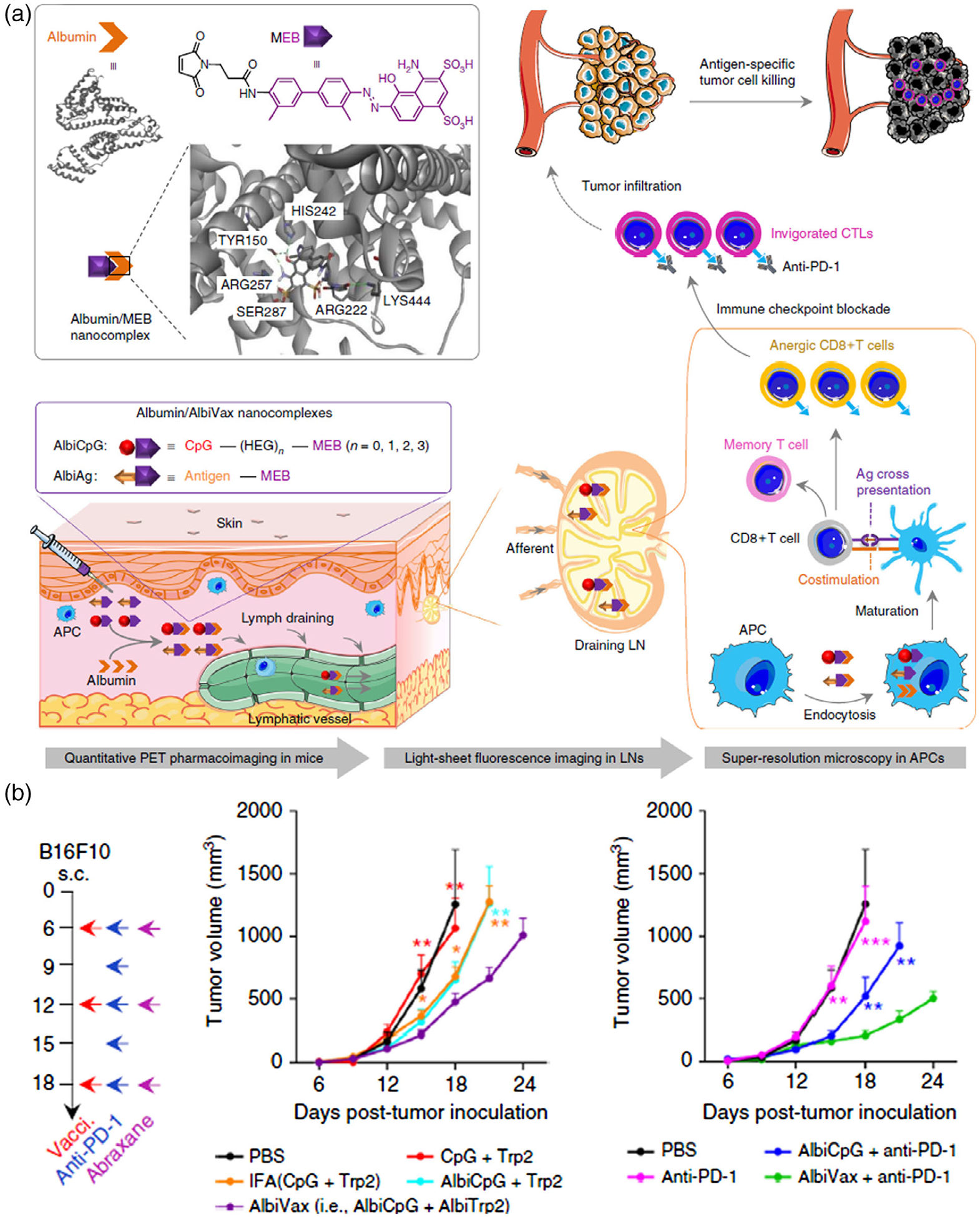
Endogenous protein-based vaccine carriers. (a) Schematic depiction of albumin/AlbiVax nanocomplexes for efficient vaccine delivery and combination cancer immunotherapy. (b) Albumin/AlbiVax nanocomplexes for melanoma combination immunotherapy. (Reprinted with permission from Zhu, Lynn, et al. (2017). Copyright 2017 Nature Publishing Group)
2.3.3 |. Synthetic nanocarriers
A wide array of synthetic nanomaterials has been studied as carriers for vaccine delivery in cancer immunotherapy. Here, we will discuss some representative examples of nanovaccines based on polymer NPs, liposomes, and inorganic NPs.
Polymer NPs have been extensively investigated for vaccine delivery in the immunotherapy of various diseases, including cancer. Polylactide-co-glycolide (PLGA) polymer-based nanoparticle is one example. PLGA is biodegradable, and its ester bonds can be eventually cleaved in vivo into metabolizable monomers lactic acid and glycolic acid. The size, solubility and stability of PLGA NPs can be fine-tuned. Further, PLGA can be coupled with polyethylene glycol (PEG) or polyetherimide to form block copolymers, which can self-assemble into a polymeric micelle, and the resulting micelles can encapsulate hydrophobic payloads such as some of hydrophobic peptide antigens (Sah, Thoma, Desu, Sah, & Wood, 2013). Antigen-loaded polymer nanovaccines have proven more effective in increasing T cell responses than the corresponding molecular antigens (Rietscher et al., 2016).
Liposomes are another widely studied platform of nanovaccines. Liposomes are composed of phospholipid bilayer and have good biodegradability. Compared to antigens alone, antigens that are either conjugated to liposomes (Taneichi et al., 2006) or encapsulated in liposomes (Ignatius et al., 2000) have shown increased proliferation of antigen-specific CTLs. For example, ovalbumin (OVA)-encoding plasmid DNA delivered by liposomes elicited a CTL response higher than plasmid DNA alone (Bacon, Caparros-Wanderley, Zadi, & Gregoriadis, 2002). In an example of mRNA-encapsulated liposome vaccines (Geall et al., 2012), in which the antigen-encoding synthetic mRNAs showed greater safety than plasmid DNA, the mRNA can also be encapsulated in liposomes and delivered via liposome, which substantially increased the immunogenicity of antigens relative to unformulated mRNA. These studies suggest that the great potential for liposome as carriers for the delivery of a variety of vaccines.
Inorganic materials have also been investigated for nanovaccine development. Inorganic nanocarriers can be easily functionalized and ingested by immune cells. A series of inorganic NPs conjugated to TAAs have been shown to suppress tumor growth in an antigen-specific manner in murine tumor models (Ahn et al., 2014; Meng et al., 2008). For example, spherical nucleic acids (SNAs) have been implemented for the delivery of cancer molecular vaccines. SNAs are formed with gold nanoparticle (AuNP) cores and nucleic acids on the surfaces (Radovic-Moreno et al., 2015). The AuNP core allows SNAs to enter cells without auxiliary delivery vehicles or transfection reagents. Compared with the corresponding molecular vaccines, the nanovaccines, also called immunostimulatory SNAs (IS-SNA), exhibited up to 80-fold increase in the potency of immunomodulatory activity, 700-fold higher antibody titers, and 400-fold higher cellular immune responses to a model antigen, and improved treatment of murine lymphomas (Figure 5).
FIGURE 5.
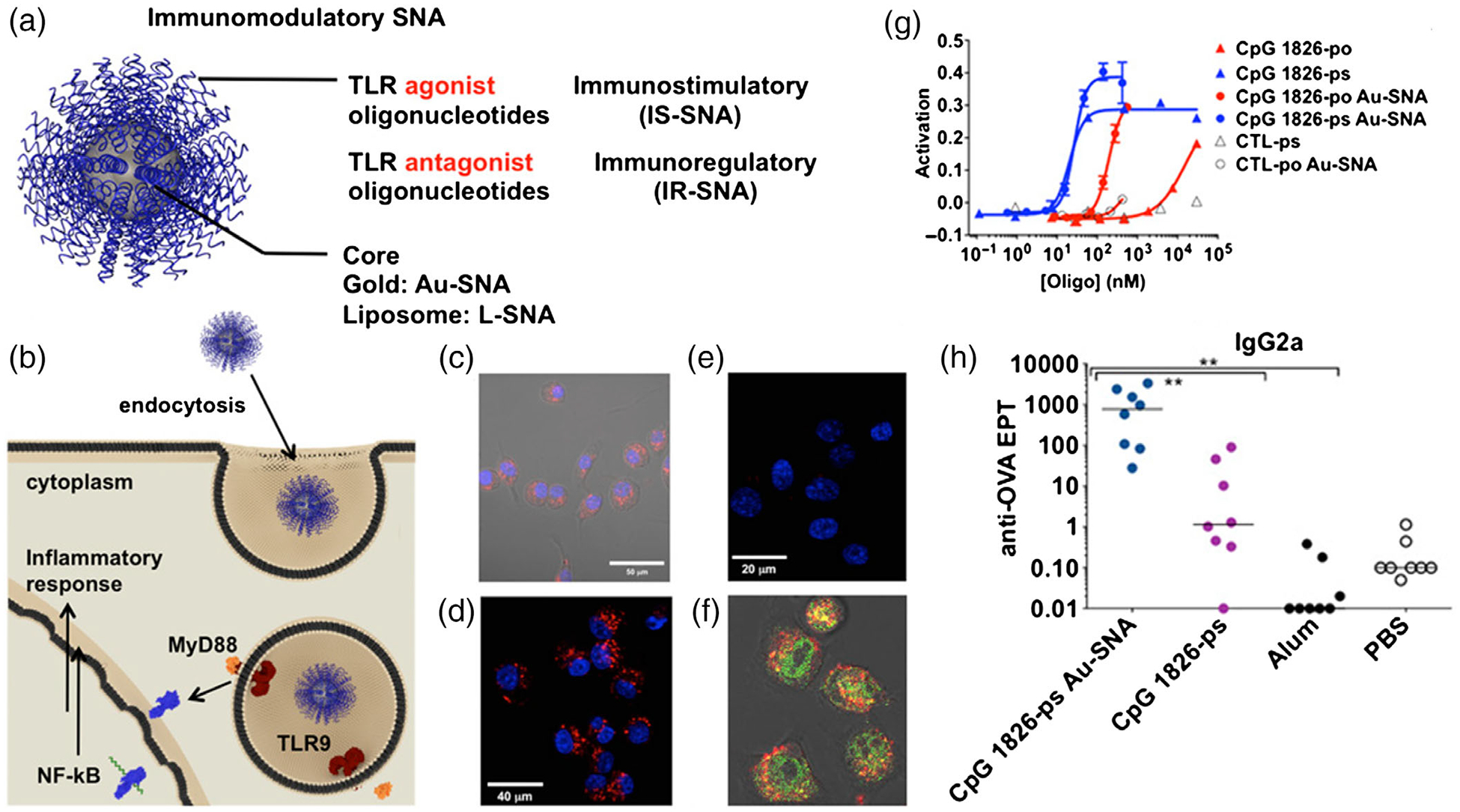
Synthetic SNA-based nanovaccines for cancer immunotherapy. (a) Schematic diagram of an SNA platform, which contain a shell of TLR agonist or TLR antagonist. (b) Schematic depiction of proposed mechanism for uptake and TLR interaction between APCs and SNAs. (c–f) Confocal microscopy image showing the Au-SNAs have been internalized. (g) IS-SNAs were tested for their ability to induce NF-κB following incubation with RAW-Blue macrophages overnight. (h) IS-SNAs enhance humoral and cellular immune responses to antigen, independent of core template. (Reprinted with permission from Radovic-Moreno et al. (2015). Copyright 2015 National Academy of Sciences Publishing Group)
3 |. WHY NANOCARRIERS FOR VACCINE DELIVERY?
Nanotechnology is playing an increasingly important role in vaccine development. As the development of vaccines moves toward “minimal” compositions that, however, have low immunogenicity, there is a growing need for formulations that improve the effectiveness of antigens and adjuvants. Using nanocarriers in vaccine delivery can not only promote tissue and cell delivery of vaccines, but also improve the stability and immunogenicity of antigens. In this section, we will discuss a few key functionalities of nanocarriers for the delivery of cancer vaccines.
3.1 |. Nanocarriers can enable efficient tissue and cell delivery of vaccines
NPs can be designed to be favorably delivered to immune-rich organs such as lymph nodes or spleens. For instance, nanovaccines can be efficiently drained into the lymphatic system after local administration, enabling abundant accumulation in lymph nodes where high densities of a series of immune cells are located and a variety of immunomodulation events are coordinated (Bachmann & Jennings, 2010; Irvine, Hanson, Rakhra, & Tokatlian, 2015; Reddy et al., 2007). In addition to nanocarrier-based passive delivery, nanocarriers can be further modified with targeting ligands for actively targeted delivery to specific subtypes of immune cells (Chen et al., 2016; Shannahan, Bai, & Brown, 2015). In one example, the coating erythrocyte membrane of nanovaccines was inserted with mannose for active targeting of APCs in the lymphatic tissues (Guo et al., 2015). Moreover, nanovaccines can also be designed to promote cytoplasm localization by penetration through cell membranes and endosomal escaping that can maximize the immunostimulatory activity of the payloads (Behzadi et al., 2017).
3.2 |. Nanocarriers permit co-delivery of antigens and adjuvants
Antigen and adjuvant molecules can be co-delivered via one nanocarrier, owing to the amenability of many nanocarriers to be loaded with multiple copies of different molecules such as antigens and adjuvants. Since a single copy of antigens can elicit CD4 or CD8 T cell responses (Huang et al., 2013; Sykulev, Joo, Vturina, Tsomides, & Eisen, 1996), effective delivery of one single nanovaccine that co-deliver antigens and adjuvants into APCs can effectively elicit T cell responses, which are pivotal for cancer immunotherapy. Specifically, co-delivery of antigens and adjuvants to APCs by nanocarriers can maximize the chance of APC maturation, antigen cross-presentation, and then activation of anti-cancer T cell responses (Petersen, Dickgreber, & Hermans, 2010).
3.3 |. Nanocarriers allow multivalent antigens and/or adjuvants to potentiate immunomodulation
The large surface areas and/or spacy cores of nanocarriers entail nanovaccines to be loaded with multiple copies of antigen and/or adjuvant molecules. This proves valuable for the immunomodulation in scenarios such as cancer immunotherapy. Lipid-coated NPs were developed to deliver multiple tumor peptides (S. Tan et al., 2014), which showed more significantly immunotherapy efficacy than single peptide-loaded NPs. In another example, Lim developed PLGA NPs loaded with OVA as a model antigen, the immunomodulatory components STAT3 small interfering RNA (siRNA), and an immune response modifier (imiquimod, R837) for the activation of DCs through the toll-like receptor 7 (TLR7; Heo & Lim, 2014). And the results of this research demonstrated that this nanovaccine loaded with multivalent antigens and adjuvants displayed excellent effective increasing cytokine levels and decreasing tumor volume.
3.4 |. Self-adjuvanted nanocarriers
In addition to serving as vaccine carriers, nanomaterials per se may also function as adjuvants. For example, chitosan NPs have great potential to enhance cellular and humoral immune responses and elicited a balanced Th1/Th2 response (Wen, Xu, Zou, & Xu, 2011). Similarly, polymethylmethacrylate NPs induce long-term antibody titers in mouse HIV2 whole-virus vaccine 100 times more than that by conventional aluminum adjuvants (Stieneker, Kreuter, & Lower, 1991). Furthermore, Al2O3 NPs act as effective immunoadjuvants to stimulate the T-cell response (H. Li, Li, Jiao, & Hu, 2011). Worth noting, due to their own immunostimulatory efficacy, some NPs may exacerbate adverse allergic reactions.
4 |. NANOVACCINE STRATEGIES ON HORIZON
Nanotechnology has the potential to significantly improve the therapeutic efficacy compared with traditional formulations and alter the landscape of cancer therapeutic vaccines. Here, we will discuss very recent strategies of nanovaccine development for cancer immunotherapy. We will specifically discuss STING activating nanovaccines, neoantigen nanovaccines, mRNA nanovaccines, aAPCs, and combination therapy involving nanovaccines.
4.1 |. STING agonist-based nanovaccines
STING is a signaling molecule associated with the endoplasmic reticulum and regulates the transcription of a number of host defense genes. Upon sensing cytosolic aberrant DNA species or cyclic dinucleotides (CDNs), STING triggers the expression of type I interferons (IFNs) and pro-inflammatory cytokines (Burdette et al., 2011; Ishikawa & Barber, 2008; Ishikawa, Ma, & Barber, 2009). By this basic mechanism, STING has discovered to be involved in a wide variety of pathological and biological activities. Specifically, STING plays a critical role in the innate immune responses to many bacterial pathogens (Jones et al., 2010; Watson, Manzanillo, & Cox, 2012), viral pathogens (Holm et al., 2012), and eukaryotic pathogens (Sharma et al., 2011); STING also appears to be involved in certain autoimmune diseases initiated by inappropriate recognition of self-DNA (Gall et al., 2012); STING-dependent signaling has been shown to induce adaptive immunity in response to DNA vaccines (Ishikawa et al., 2009); and STING has also been proposed to sense membrane-fusion events associated with viral entry, in a manner independent of the sensing of nucleic acids (Holm et al., 2012). STING can mediate type I IFN production by CD8α + DCs that can active CD8+ T cell through antigen cross-presentation and T cell priming for cancer therapy (Ng, Marshall, Bell, & Lam, 2018). Further, tumor-derived STING-activating components can be recognized by leukocytes such as CD11b + and B cells, and subsequently activate STING to induce type I IFN production by leukocytes to prime NK cells for cytotoxic killing of tumor cells (Sundararaman & Barbie, 2018). Moreover, recent studies have shown that the STING pathway is essential for radiation-induced as well as spontaneous natural antitumor T cell responses (Woo et al., 2014). These studies clearly suggest that STING is a central role player in a variety of innate and adaptive immune responses, which can be leveraged for cancer immunotherapy.
Early on, a STING agonist 5,6-dimethylxanthenone-4-acetic acid was developed and tested for cancer therapy, but was unfortunately found to be selectively active for murine STING rather than human STING, which led to its setback in clinical testing (Roberts et al., 2007). CDNs, another class of STING agonist, have been enthusiastically studied for cancer immunotherapy. Upon binding to STING, CDNs trigger structural reconfiguration of STING, which subsequently leads to the activation of STING signaling pathway (Burdette & Vance, 2013). CDNs have been investigated as part of a vaccine for cancer therapy by intralesional vaccination. This vaccine has shown remarkable therapeutic efficacy in mouse tumor models. Though the first clinical testing of this vaccine did not show significant therapeutic advantage relative to a control arm, it is expected that, by optimization in terms of formulations or clinic trial design, CDN-based vaccines still have great potential for cancer immunotherapy. One approach to formulation improvement might be through nanocarriers (An et al., 2018; Hanson et al., 2015; Junkins et al., 2018; Koshy, Cheung, Gu, Graveline, & Mooney, 2017; Nakamura et al., 2015; Shae et al., 2019; Wilson et al., 2018). Indeed, encapsulation of CDNs into NPs has been explored to improve cytosolic CDN delivery and promote immune responses. In one study, cyclic di-GMP (cdGMP) was encapsulated into PEGylated lipid NPs and used to redirect this adjuvant to draining lymph nodes (Figure 6; Hanson et al., 2015). After vaccination, both CD8+ and CD4+ T cell responses were significantly increased relative to free CDNs. Though this particular nanovaccine was not directly tested for cancer immunotherapy in this study, it proves the principle of STING-adjuvanted nanovaccine which may also be applicable for cancer immunotherapy. More recently, CDNs have also been incorporated into NPs consisting of endosomolytic polymersomes assembled from pH-responsive diblock copolymers for enhanced cytosolic delivery of CDNs (Shae et al., 2019). Luo et al. (2017) developed a pH-responsive polymer nanovaccine that is STING-activating per se, presumably by disrupting subcellular organelles and then exposing nucleic acids in the cytosol. These results demonstrate that STING agonist-based nanovaccines can improve the bioavailability and therapeutic efficacy of CDNs, enhance STING signaling, and potentiate immune responses for the immunotherapy of cancer.
FIGURE 6.

STING agonist-based nanovaccines for cancer immunotherapy. (a) Schematic diagram of NP-MPER and NP-cdGMP (cyclic di-GMP) vaccine. (b) Measurement of the NP-cdGMP vaccine in the blood of mice. (c) Representative flow cytometry plots of CDN fluorescence in APCs 24 hours following s.c. injection. (d) The stability of the NP-cdGMP vaccine in serum. (Reprinted with permission from Hanson et al. (2015). Copyright 2015 American Society for Clinical Investigation Publishing Group)
4.2 |. Neoantigen nanovaccines
Tumor neoantigens are generated from genetic events such as somatic mutations in tumor cells, but not healthy cells. Such cell-selective presence makes neoantigens attractive targets for cancer therapeutic vaccines, because immunization against tumor-specific neoantigens has the potential to bypass central and peripheral immune tolerance mechanism and to minimize risk of inducing autoimmunity. mRNA/DNA or synthetic long peptides have been employed to develop neoantigen-based cancer vaccines (L. Li, Goedegebuure, & Gillanders, 2017). In current clinical testing, neoantigens have been identified using a standard workflow of genomic sequencing of cancer cells and normal cells and/or MS proteomic analysis, followed by bioinformatic analysis to identify somatic mutations and predicting MHC-I/II binding with neoepitopes, then validating neoantigen immunogenicity in vitro and manufacturing validated neoantigens, and finally formulating neoantigen vaccines for administration back into the corresponding patients (Ott et al., 2017; Sahin et al., 2017). Though these technologies are powerful to identify scarce neoantigens, this process also present technique barriers and will take months to complete. The majority of somatic mutation products have undetectable immunogenicity, which may refrain them from broad adoption in the clinic.
Nanovaccines may at least partially address these challenges by promoting the vaccine delivery and thus promoting the immunogenicity of neoantigens, which can expand the fraction of somatic mutation products that qualify neoantigen vaccines and may eventually expand the population of patients who can benefit from neoantigen vaccine-based immunotherapy. In one example, synthetic high-density lipoprotein nanodiscs, a clinically safe and scalable material, were used to facilitate the delivery of peptide neoantigens through disulfide conjugation and cholesteryl-modified adjuvant to draining lymph nodes (Figure 7; Kuai et al., 2017). The nanodisc-based neoantigen vaccines induce extremely high levels (~30%) of antigen-specific CTL response, which, especially when combined with immune checkpoint blockade, showed remarkable tumor therapeutic efficacy in mouse tumor models. In addition, Zhu et al. reported self-assembled intertwining DNA–RNA nanocapsules that can co-deliver DNA CpG, short hairpin RNA adjuvants, and tumor-specific peptide neoantigens into APCs for cancer immunotherapy (Zhu, Mei, et al., 2017). This nanovaccine elicit highly frequent neoantigen-specific peripheral CD8+ T cells and significantly inhibit the progression of neoantigen-specific tumors. Likewise, neoantigen delivery has been demonstrated based on albumin/vaccine nanocomplexes (Zhu, Lynn, et al., 2017) as well as STING-activating ultra-pH-sensitive polymers, as discussed above (Luo et al., 2017). These studies suggest that properly designed nanovaccines are particularly useful in facilitating the development of neoantigen vaccines. In addition to peptides, mRNA that encodes neoantigens has also shown tremendous potential in preclinical and clinical testings, which will be discussed in detail in a later section (Ott et al., 2017; Sahin et al., 2017).
FIGURE 7.
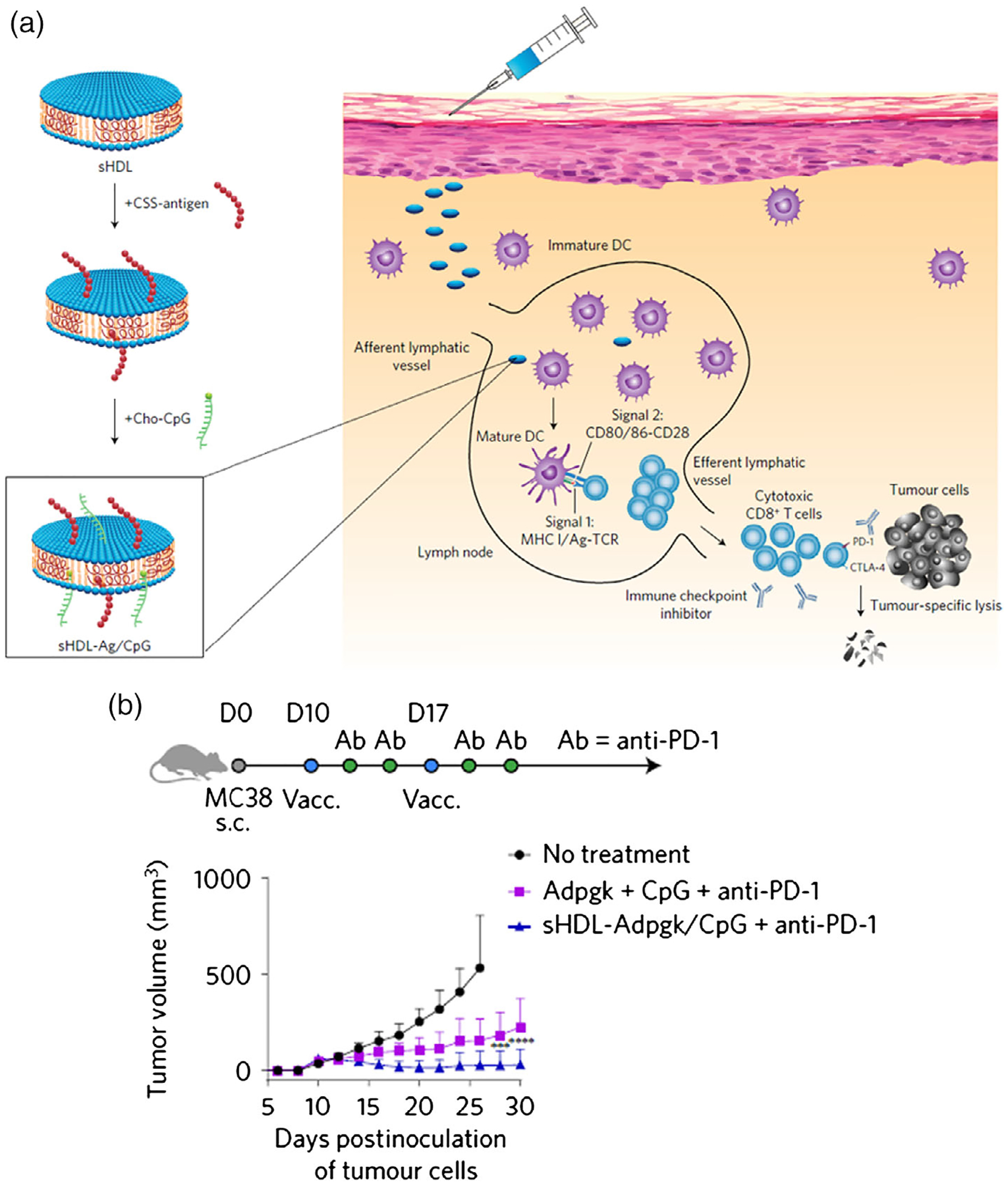
Synthetic high-density lipoprotein (sHDL) nanodisc-based personalized cancer therapeutic vaccines. (a) Design of sHDL nanodisc platform for personalized cancer vaccines. (b) Treatment of MC-38 tumor-bearing C57BL/6 mice with sHDL-Ag/CpG vaccines. (Reprinted with permission from Kuai, Ochyl, Bahjat, Schwendeman, & Moon (2017). Copyright 2017 Nature Publishing Group)
4.3 |. mRNA-based nanovaccines
mRNA has been investigated as an attractive vector to deliver tumor antigens to DCs. mRNA vaccines combine desirable immunostimulatory properties of mRNA by itself with an outstanding safety profile as well as the flexibility of genetic vaccines. mRNA can induce short but long enough protein expression without the risk of integration into the host genome. The interest in using mRNA as a means to load tumor antigens onto DCs began to rise in the late 1990s, thanks to the pioneering work of Gilboa and collaborators at the Duke University (Boczkowski, 1996; Galluzzi et al., 2012). Their mRNA-based approach was based on passive pulses of DCs, in other words relying on DCs to absorb mRNA, convert it into protein, which is then processed into peptides and presented to CD8+ T cells (Boczkowski, 1996).
However, there are several challenges about mRNA-based vaccines. First, antigen-encoding mRNAs need to be ingested by APCs before degradation by extracellular ribonucleases. Second, in order for antigen translation in the cytosol, the mRNA must escape from the acidic endolysosomes post-ingestion. It is thus crucial to develop delivery systems, such as nanocarriers, that protect mRNAs from degradation and promote intracellular delivery into APCs. The encapsulation of mRNA vaccine in nanocarriers for immunotherapy has recently experienced an exponential increase. For instance, liposomes-based mRNA nanovaccines significantly enhanced their delivery efficiency into spleen and DCs (Figure 8; Kranz et al., 2016). This study employed liposomes to be complexed with mRNA and form RNA-lipoplexes (RNA-LPX), which enabled effective targeting and major transfection of DCs after systemic administration. Moreover, the LPX protects RNA from degradation by extracellular ribonucleases and mediates its efficient uptake and expression of the encoded antigen by DCs and macrophages in various lymphoid compartments. Another study reported the development of a lipid nanoparticle for the delivery of mRNA vaccines to induce a cytotoxic CD8 T cell response (Oberli et al., 2017). Their ionizable lipid is positively charged at low pH to allow complexation with the negatively charged mRNA and may also help with cellular uptake and endosomal escape. These studies demonstrated the principle of mRNA nanovaccines for cancer immunotherapy.
FIGURE 8.
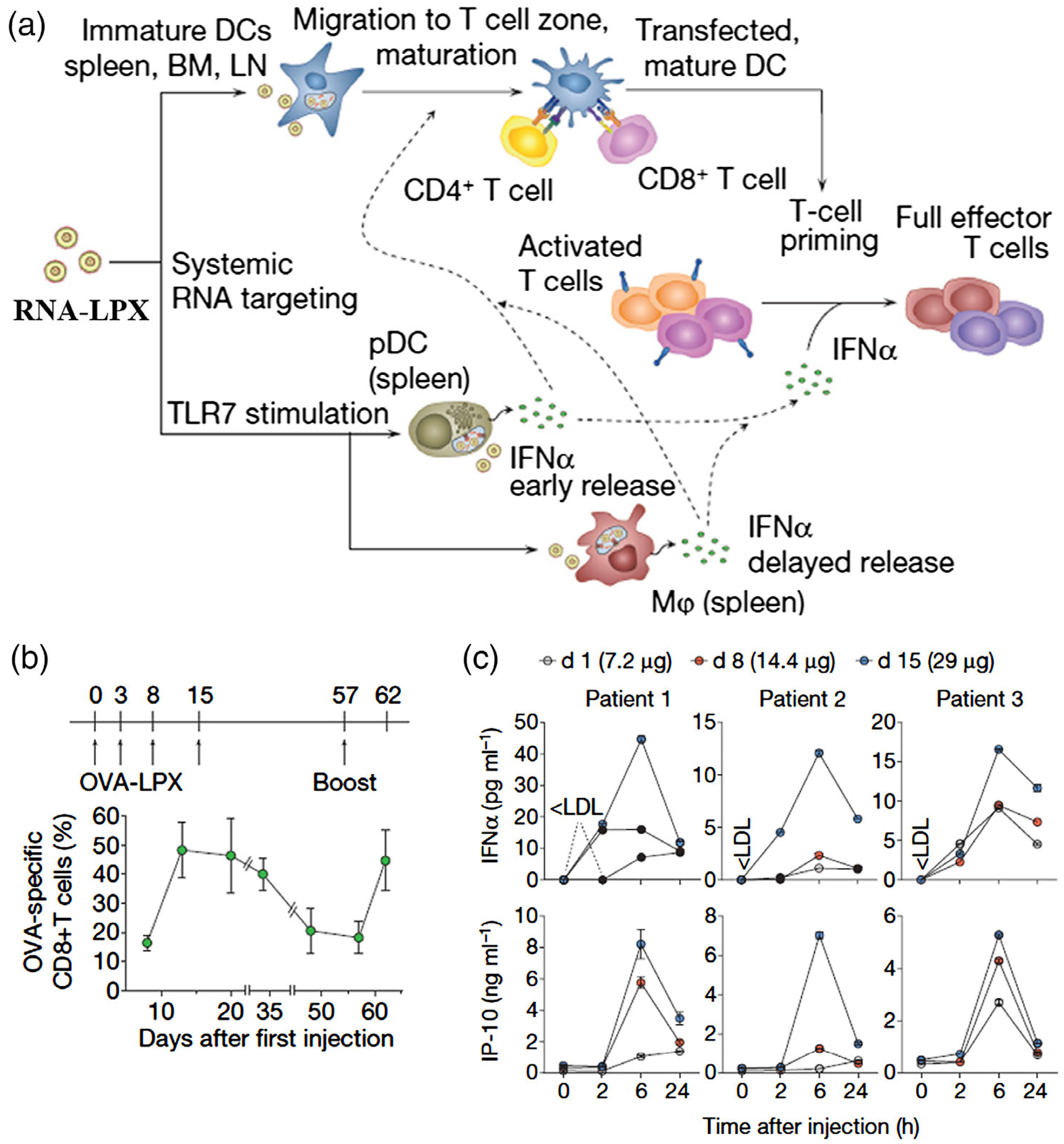
Liposome nanocarrier-based mRNA vaccine delivery for cancer immunotherapy. (a) Mechanism of action for RNALPX. (b) Kinetics of OVA specific CD8+ T cell frequencies within CD8+ T cells in blood after intravenous OVA-LPX vaccination. (c) Serum cytokines were monitored after injection RNA-LPX in phase I clinical trial. (Reprinted with permission from Kranz et al. (2016). Copyright 2016 Nature Publishing Group)
4.4 |. aAPCs
APCs, such as DCs, can convey information from vaccines to T cells or B cells, and mediate T cells or B cells to generate adaptive immune responses that are key to cancer immunotherapy. Given such key role of DCs in cancer immunotherapy, autologous DC-based cancer vaccines have been extensively investigated by isolating DCs from patients and then exposing DCs to cancer antigens to generate activated antigen-specific DCs. The resulting specifically tasked DC vaccines can be injected back into the corresponding patients to elicit immune responses for cancer therapy. This approach, however, is time consuming and expensive, which greatly limits their clinical adoption. To address this challenge, an alternative approach is to develop aAPCs. aAPCs are the artificial synthetic APCs, which include two parts: a cognate antigenic peptide presented in the context of MHC and co-stimulatory molecules, which can respectively bind to TCRs and co-stimulatory receptors to activate T cells (Zang et al., 2017). Compared with natural DCs, aAPCs have relatively defined composition as well as controllable and uniform signal representation; aAPCs could be manufactured at a large scale, and can be developed as off-the-shelf vaccines (Eggermont, Paulis, Tel, & Figdor, 2014). A number of different strategies of aAPCs have thus far been investigated. One strategy is genetically modified cellular aAPC, such as K562 human leukemic cells (Fisher et al., 2014) and NIH/3T3 murine fibroblasts (Hasan et al., 2016) that were transduced to co-express CD86, CD137L, and IL-15 to activate cytotoxic CD8+ T cells. Another approach to synthetic aAPCs is by incorporating bioengineering principles (Hickey, Vicente, Howard, Mao, & Schneck, 2017; Siefert, Fahmy, & Kim, 2017). Here, we will mainly discuss the second strategy for cancer therapy.
The physicochemical parameters (e.g., size, shape) of aAPCs may impact T cell activation, and thus aAPCs can be engineered by tuning these parameters for optimal immunomodulation and therapy efficacy. For instance, nanomaterials with high aspect ratio may enhance the interactions with T cells (Fadel et al., 2014). For example, in order to mimic the physiological functions of APCs to stimulate T cells, a fluid lipid bilayer supported by mesoporous silica microrods were engineered, and this lipid bilayer presents membrane-bound cues for T cell receptor stimulation and co-stimulation (Figure 9; Cheung et al., 2018). In addition to in vitro T cell activation and expansion, aAPC can also be directly used for in vivo T cell activation in cancer immunotherapy (Kosmides et al., 2017).
FIGURE 9.
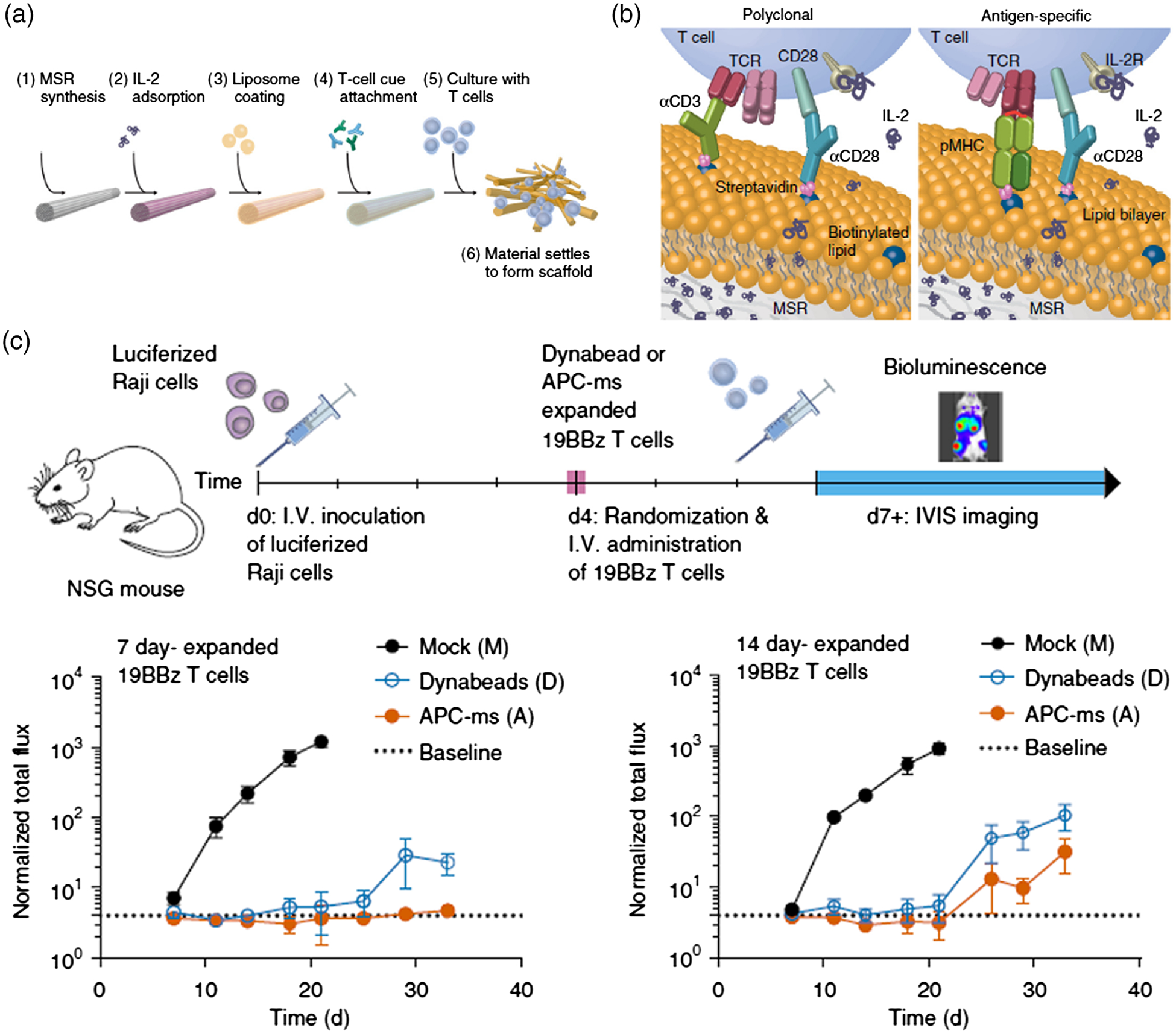
aAPCs for cancer immunotherapy. (a) Process for preparing APC mimetic scaffolds (APC-ms) from mesoporous silica microrods (MSRs). (b) Schematic depiction of polyclonal and antigen-specific T cell expansion. (c) in vivo efficacy of restimulated 19BBz CAR-T cells in a disseminated lymphoma xenograft model. (Reprinted with permission from Cheung, Zhang, Koshy, & Mooney (2018). Copyright 2018 Nature Publishing Group)
4.5 |. Nanovaccines for combination therapy
Given the heterogeneity of cancer in multiple levels, combination cancer therapy has been and will continue to be instrumental for cancer therapy. Nanovaccines have great potential for synergistic combination therapy. For example, Andrew Wang’s group has developed antigen-capturing NPs (AC-NPs) that significantly improved the abscopal effect in tumor radiotherapy (Figure 10; Min et al., 2017). These AC-NPs can bind with antigens released from tumor during radiotherapy via nanoparticle surfaces that are functionalized with reactive chemical groups, and these antigen-bound AC-NPs were successfully transported to nearby tumor draining lymph nodes through APC-mediated transportation. Combination of nanovaccines with immune checkpoint inhibitors and/or immunostimulatory cytokines has also been extensively studied. For example, combination of albumin-binding vaccines with immune checkpoint inhibitors as well as a long-acting interleukin 2 have successfully eradicated large tumors in multiple murine tumor models.
FIGURE 10.
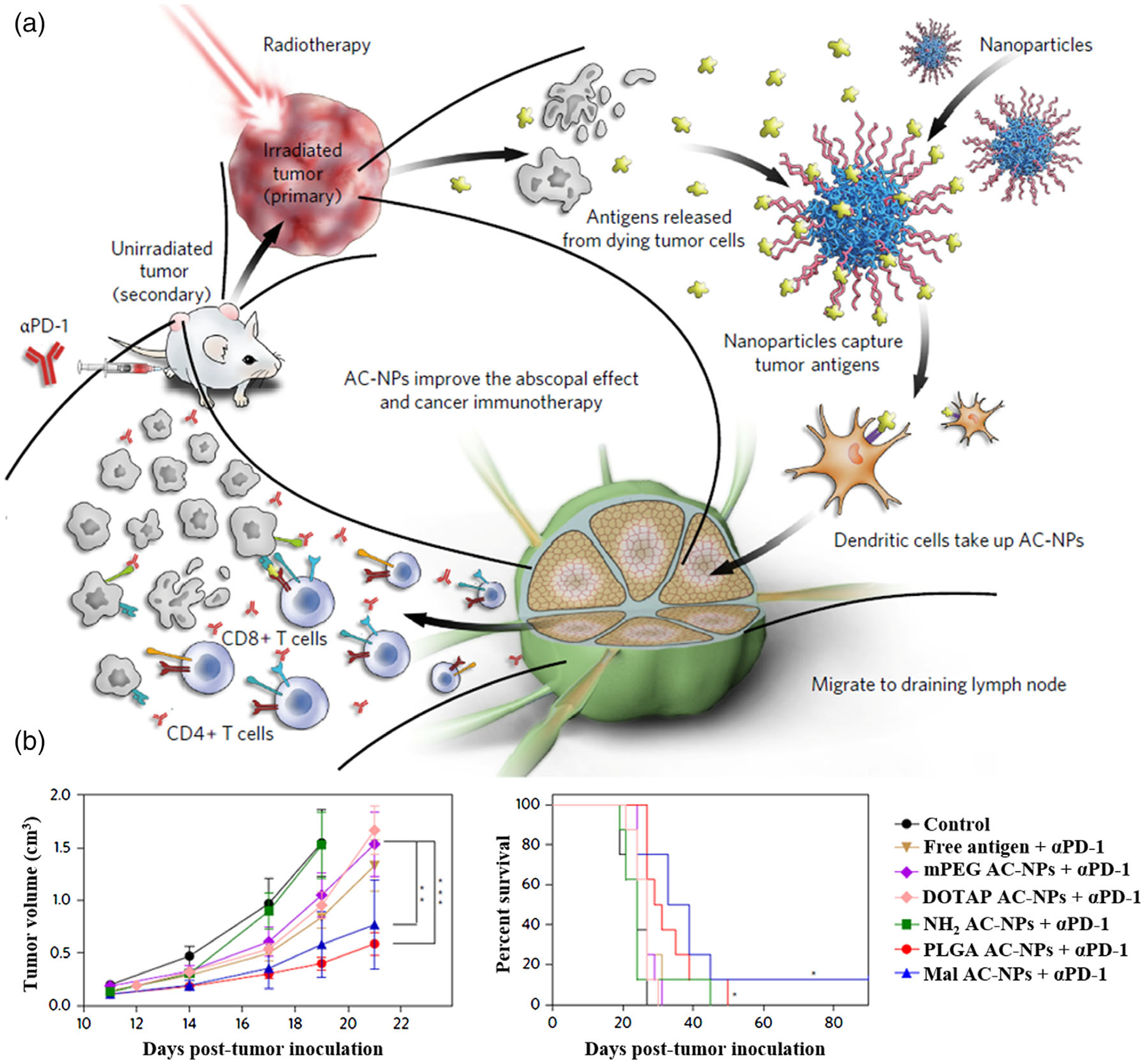
Nanovaccines for combination therapy. (a) Schematic depiction of utilizing AC-NPs to improve cancer immunotherapy. (b) TDPA-coated AC-NPs enhance the efficacy of immunotherapy based on cancer vaccination. (Reprinted with permission from Min et al. (2017). Copyright 2017 Nature Publishing Group)
5 |. CONCLUSION AND OUTLOOK
By treating the immune system to treat diseases, immunotherapy is likely going to be another instrumental pillar for cancer treatment. Cancer immunotherapy has been investigated in a variety of approaches including adoptive cell transfer therapy, immune checkpoint blockade, cancer therapeutic vaccines, as well as oncolytic virotherapy. Cancer therapeutic vaccines hold tremendous potential to elicit cancer-specific immune responses with minimal adverse autoimmunity, ameliorate local and/or immunosuppression, and synergize with other immunotherapeutic approaches. These features empower cancer vaccines to overcome challenges, such as low response rates and immune-related adverse events, which have been faced by current immunotherapies. Nanovaccines are promising to maximize the potential of cancer therapeutic vaccines. By efficient co-delivery of multivalent molecular antigens and adjuvants into lymphoid tissues and immune cells, nanovaccines can dramatically increase the immunogenicity of molecular vaccines, and hence potentiate antigen-specific adaptive immune responses for cancer therapy. By integrating pharmaceutical engineering principles, nanotechnology, and immuno-oncology, a variety of nanovaccines have been developed and tested in preclinical models and occasionally in human for cancer immunotherapy. The multi-disciplinary field of nanovaccines has continued benefiting by the advancement in each relevant field of science and technology. We have discussed some recent examples of newly emerging types of nanovaccines, including STING-activating nanovaccines, cancer neoantigen nanovaccines, and mRNA nanovaccines. These newly emerging nanovaccine strategies could help the field to revolutionize or supplement the landscape of cancer immunotherapy.
Funding information
National Center for Advancing Translational Sciences, Grant/Award Number: UL1TR002649; National Natural Science Foundation of China, Grant/Award Number: 81772999; American Cancer Society Institutional Research Grants (ACS-IRG), Grant/Award Number: IRG-18-159-43; Wright Center for Clinical and Translational Research Endowment Fund, Grant/Award Number: UL1TR002649; Pilot Research Grant, VCU Massey Cancer Center, Grant/Award Number: P30 CA106059
Footnotes
CONFLICT OF INTEREST
G.Z. was listed as an inventor for the application of a patent associated with immunomodulatory materials.
REFERENCES
- Adams S (2009). Toll-like receptor agonists in cancer therapy. Immunotherapy, 1(6), 949–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn S, Lee IH, Kang S, Kim D, Choi M, Saw PE, … Jon S (2014). Gold nanoparticles displaying tumor-associated self-antigens as a potential vaccine for cancer immunotherapy. Advanced Healthcare Materials, 3(8), 1194–1199. 10.1002/adhm.201300597 [DOI] [PubMed] [Google Scholar]
- An M, Yu C, Xi J, Reyes J, Mao G, Wei WZ, & Liu H (2018). Induction of necrotic cell death and activation of STING in the tumor microenvironment via cationic silica nanoparticles leading to enhanced antitumor immunity. Nanoscale, 10(19), 9311–9319. 10.1039/c8nr01376d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann MF, & Jennings GT (2010). Vaccine delivery: A matter of size, geometry, kinetics and molecular patterns. Nature Reviews Immunology, 10(11), 787–796. 10.1038/nri2868 [DOI] [PubMed] [Google Scholar]
- Bacon A, Caparros-Wanderley W, Zadi B, & Gregoriadis G (2002). Induction of a cytotoxic T lymphocyte (CTL) response to plasmid DNA delivered via Lipodine liposomes. Journal of Liposome Research, 12, 173–183. [DOI] [PubMed] [Google Scholar]
- Basle E, Joubert N, & Pucheault M (2010). Protein chemical modification on endogenous amino acids. Chemistry & Biology, 17(3), 213–227. 10.1016/j.chembiol.2010.02.008 [DOI] [PubMed] [Google Scholar]
- Behzadi S, Serpooshan V, Tao W, Hamaly MA, Alkawareek MY, Dreaden EC, … Mahmoudi M (2017). Cellular uptake of nanoparticles: Journey inside the cell. Chemical Society Reviews, 46(14), 4218–4244. 10.1039/c6cs00636a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellier B, Huret C, Miyalou M, Desjardins D, Frenkiel MP, Despres P, … Klatzmann D (2009). DNA vaccines expressing retrovirus-like particles are efficient immunogens to induce neutralizing antibodies. Vaccine, 27(42), 5772–5780. 10.1016/j.vaccine.2009.07.059 [DOI] [PubMed] [Google Scholar]
- Blattman JN, & Greenberg PD (2004). Cancer immunotherapy: A treatment for the masses. Science, 305, 200–205. [DOI] [PubMed] [Google Scholar]
- Boczkowski D (1996). Dendritic cells pulsed with RNA are potent antigen-presenting cells in vitro and in vivo. Journal of Experimental Medicine, 184(2), 465–472. 10.1084/jem.184.2.465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruggen P. v. d., Zhang Y, Chaux P, Stroobant V, Panichelli C, Schultz ES, … Boon T. (2002). Tumor-specific shared antigenic peptides recognized by human T cells. Immunological Reviews, 188, 51–64. [DOI] [PubMed] [Google Scholar]
- Burdette DL, Monroe KM, Sotelo-Troha K, Iwig JS, Eckert B, Hyodo M, … Vance RE (2011). STING is a direct innate immune sensor of cyclic di-GMP. Nature, 478(7370), 515–518. 10.1038/nature10429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdette DL, & Vance RE (2013). STING and the innate immune response to nucleic acids in the cytosol. Nature Immunology, 14(1), 19–26. 10.1038/ni.2491 [DOI] [PubMed] [Google Scholar]
- Cai J, Wang H, Wang D, & Li Y (2019). Improving cancer vaccine efficiency by nanomedicine. Advanced Biosystems, 3, 1800287 10.1002/adbi.201800287 [DOI] [PubMed] [Google Scholar]
- Chen P, Liu X, Sun Y, Zhou P, Wang Y, & Zhang Y (2016). Dendritic cell targeted vaccines: Recent progresses and challenges. Human Vaccines & Immunotherapeutics, 12(3), 612–622. 10.1080/21645515.2015.1105415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung AS, Zhang DKY, Koshy ST, & Mooney DJ (2018). Scaffolds that mimic antigen-presenting cells enable ex vivo expansion of primary T cells. Nature Biotechnology, 36(2), 160–169. 10.1038/nbt.4047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copland MJ, Rades T, Davies NM, & Baird MA (2005). Lipid based particulate formulations for the delivery of antigen. Immunology & Cell Biology, 83(2), 97–105. 10.1111/j.1440-1711.2005.01315.x [DOI] [PubMed] [Google Scholar]
- Coulie PG, Lehmann F, Lethe B, Herman J, Lurquin C, Andrawiss M, & Boon T (1995). A mutated intron sequence codes for an antigenic peptide recognized by cytolytic T lymphocytes on a human melanoma. Proceedings of the National Academy of Sciences of the United States of America, 92, 7976–7980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diebold SS, Kaisho T, Hemmi H, Akira S, & Sousa CRE (2004). Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science, 303, 1529–1531. [DOI] [PubMed] [Google Scholar]
- Eggermont LJ, Paulis LE, Tel J, & Figdor CG (2014). Towards efficient cancer immunotherapy: Advances in developing artificial antigen-presenting cells. Trends in Biotechnology, 32(9), 456–465. 10.1016/j.tibtech.2014.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadel TR, Sharp FA, Vudattu N, Ragheb R, Garyu J, Kim D, … Fahmy TM (2014). A carbon nanotube-polymer composite for T-cell therapy. Nature Biotechnology, 9(8), 639–647. 10.1038/nnano.2014.154 [DOI] [PubMed] [Google Scholar]
- Fang RH, Hu CM, Luk BT, Gao W, Copp JA, Tai Y, … Zhang L (2014). Cancer cell membrane-coated nanoparticles for anticancer vaccination and drug delivery. Nano Letters, 14(4), 2181–2188. 10.1021/nl500618u [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felnerova D, Viret JF, Gluck R, & Moser C (2004). Liposomes and virosomes as delivery systems for antigens, nucleic acids and drugs. Current Opinion in Biotechnology, 15(6), 518–529. 10.1016/j.copbio.2004.10.005 [DOI] [PubMed] [Google Scholar]
- Fioretti D, Iurescia S, Fazio VM, & Rinaldi M (2010). DNA vaccines: Developing new strategies against cancer. Journal of Biomedicine and Biotechnology, 2010, 1–16. 10.1155/2010/174378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JP, Yan M, Heuijerjans J, Carter L, Abolhassani A, Frosch J, … Anderson J (2014). Neuroblastoma killing properties of Vdelta2 and Vdelta2-negative gammadeltaT cells following expansion by artificial antigen-presenting cells. Clinical Cancer Research, 20(22), 5720–5732. 10.1158/1078-0432.CCR-13-3464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall A, Treuting P, Elkon KB, Loo YM, Gale M Jr., Barber GN, & Stetson DB (2012). Autoimmunity initiates in nonhematopoietic cells and progresses via lymphocytes in an interferon-dependent autoimmune disease. Immunity, 36(1), 120–131. 10.1016/j.immuni.2011.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L, Senovilla L, Vacchelli E, Eggermont A, Fridman WH, Galon J, … Kroemer G (2012). Trial watch: Dendritic cell-based interventions for cancer therapy. Oncoimmunology, 1(7), 1111–1134. 10.4161/onci.21494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrone P, Fluckiger A-C, Mangeot PE, Gauthier E, Dupeyrot-Lacas P, Mancip J, … Dalba C (2011). A prime-boost strategy using virus-like particles pseudotyped for HCV proteins triggers broadly neutralizing antibodies in macaques. Science Translational Medicine, 3(94), 94ra71. [DOI] [PubMed] [Google Scholar]
- Geall AJ, Verma A, Otten GR, Shaw CA, Hekele A, Banerjee K, … Mandl CW (2012). Nonviral delivery of self-amplifying RNA vaccines. Proceedings of the National Academy of Sciences of the United States of America, 109(36), 14604–14609. 10.1073/pnas.1209367109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnjatic S, Sawhney NB, & Bhardwaj N (2010). Toll-like receptor agonists: Are they good adjuvants? Cancer Journal, 16(4), 382–391. 10.1097/PPO.0b013e3181eaca65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goforth R, Salem AK, Zhu X, Miles S, Zhang XQ, Lee JH, & Sandler AD (2009). Immune stimulatory antigen loaded particles combined with depletion of regulatory T-cells induce potent tumor specific immunity in a mouse model of melanoma. Cancer Immunology, Immunotherapy, 58(4), 517–530. 10.1007/s00262-008-0574-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gou M, Qu X, Zhu W, Xiang M, Yang J, Zhang K, … Chen S (2014). Bio-inspired detoxification using 3D-printed hydrogel nanocomposites. Nature Communications, 5, 3774 10.1038/ncomms4774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Wang D, Song Q, Wu T, Zhuang X, Bao Y, … Zhang Z (2015). Erythrocyte membrane-enveloped polymeric nanoparticles as nanovaccine for induction of antitumor immunity against melanoma. ACS Nano, 9(7), 6918–6933. [DOI] [PubMed] [Google Scholar]
- Hanson MC, Crespo MP, Abraham W, Moynihan KD, Szeto GL, Chen SH, … Irvine DJ (2015). Nanoparticulate STING agonists are potent lymph node-targeted vaccine adjuvants. Journal of Clinical Investigation, 125(6), 2532–2546. 10.1172/JCI79915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan AN, Selvakumar A, Shabrova E, Liu XR, Afridi F, Heller G, … O’Reilly RJ (2016). Soluble and membrane-bound interleukin (IL)-15 Ralpha/IL-15 complexes mediate proliferation of high-avidity central memory CD8(+) T cells for adoptive immunotherapy of cancer and infections. Clinical and Experimental Immunology, 186(2), 249–265. 10.1111/cei.12816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo MB, & Lim YT (2014). Programmed nanoparticles for combined immunomodulation, antigen presentation and tracking of immunotherapeutic cells. Biomaterials, 35(1), 590–600. 10.1016/j.biomaterials.2013.10.009 [DOI] [PubMed] [Google Scholar]
- Hickey JW, Vicente FP, Howard GP, Mao HQ, & Schneck JP (2017). Biologically inspired design of nanoparticle artificial antigen-presenting cells for immunomodulation. Nano Letters, 17(11), 7045–7054. 10.1021/acs.nanolett.7b03734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm CK, Jensen SB, Jakobsen MR, Cheshenko N, Horan KA, Moeller HB, … Paludan SR (2012). Virus-cell fusion as a trigger of innate immunity dependent on the adaptor STING. Nature Immunology, 13(8), 737–743. 10.1038/ni.2350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Brameshuber M, Zeng X, Xie J, Li QJ, Chien YH, … Davis MM (2013). A single peptide-major histocompatibility complex ligand triggers digital cytokine secretion in CD4(+) T cells. Immunity, 39(5), 846–857. 10.1016/j.immuni.2013.08.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignatius R, Mahnke K, Rivera M, Hong K, Isdell F, Steinman RM, … Stamatatos L (2000). Presentation of proteins encapsulated in sterically stabilized liposomes by dendritic cells initiates CD8+ T-cell responses in vivo. Blood, 96, 3505–3513. [PubMed] [Google Scholar]
- Irvine DJ, Hanson MC, Rakhra K, & Tokatlian T (2015). Synthetic nanoparticles for vaccines and immunotherapy. Chemical Reviews, 115(19), 11109–11146. 10.1021/acs.chemrev.5b00109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H, & Barber GN (2008). STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature, 455(7213), 674–678. 10.1038/nature07317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H, Ma Z, & Barber GN (2009). STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature, 461(7265), 788–792. 10.1038/nature08476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jella KK, Nasti TH, Li Z, Malla SR, Buchwald ZS, & Khan MK (2018). Exosomes, their biogenesis and role in inter-cellular communication, tumor microenvironment and cancer immunotherapy. Vaccines (Basel), 6(4), 69–84. 10.3390/vaccines6040069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JW, Kayagaki N, Broz P, Henry T, Newton K, O’Rourke K, … Monack DM (2010). Absent in melanoma 2 is required for innate immune recognition of Francisella tularensis. Proceedings of the National Academy of Sciences of the United States of America, 107(21), 9771–9776. 10.1073/pnas.1003738107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junkins RD, Gallovic MD, Johnson BM, Collier MA, Watkins-Schulz R, Cheng N, … Ting JP (2018). A robust microparticle platform for a STING-targeted adjuvant that enhances both humoral and cellular immunity during vaccination. Journal of Controlled Release, 270, 1–13. 10.1016/j.jconrel.2017.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamps JAAM, Swart PJ, Morselt H. t. W. M., Pauwels R, Behune M-PD, Clercq ED, … Scherphof GL. (1996). Preparation and characterization of conjugates of (modified) human serum albumin and liposomes: Drug carriers with an intrinsic anti-HIV activity. Biochimica et Biophysica Acta, 1278, 183–190. [DOI] [PubMed] [Google Scholar]
- Kim OY, Park HT, Dinh NTH, Choi SJ, Lee J, Kim JH, … Gho YS (2017). Bacterial outer membrane vesicles suppress tumor by interferon-gamma-mediated antitumor response. Nature Communications, 8(1), 626 10.1038/s41467-017-00729-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshy ST, Cheung AS, Gu L, Graveline AR, & Mooney DJ (2017). Liposomal delivery enhances immune activation by STING agonists for cancer immunotherapy. Advanced Biosystems, 1(1–2), 1600013 10.1002/adbi.201600013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosmides AK, Meyer RA, Hickey JW, Aje K, Cheung KN, Green JJ, & Schneck JP (2017). Biomimetic biodegradable artificial antigen presenting cells synergize with PD-1 blockade to treat melanoma. Biomaterials, 118, 16–26. 10.1016/j.biomaterials.2016.11.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranz LM, Diken M, Haas H, Kreiter S, Loquai C, Reuter KC, … Sahin U (2016). Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature, 534(7607), 396–401. 10.1038/nature18300 [DOI] [PubMed] [Google Scholar]
- Krishnamachari Y, & Salem AK (2009). Innovative strategies for co-delivering antigens and CpG oligonucleotides. Advanced Drug Delivery Reviews, 61(3), 205–217. 10.1016/j.addr.2008.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuai R, Ochyl LJ, Bahjat KS, Schwendeman A, & Moon JJ (2017). Designer vaccine nanodiscs for personalized cancer immunotherapy. Nature Materials, 16(4), 489–496. 10.1038/nmat4822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, Kim SH, Cho JA, & Kim CW (2011). Introduction of the CIITA gene into tumor cells produces exosomes with enhanced anti-tumor effects. Experimental & Molecular Medicine, 43(5), 281–290. 10.3858/emm.2011.43.5.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Li Y, Jiao J, & Hu HM (2011). Alpha-alumina nanoparticles induce efficient autophagy-dependent cross-presentation and potent anti-tumour response. Nature Nanotechnology, 6(10), 645–650. 10.1038/nnano.2011.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Goedegebuure SP, & Gillanders WE (2017). Preclinical and clinical development of neoantigen vaccines. Annals of Oncology, 28(Suppl. 12), xii11–xii17. 10.1093/annonc/mdx681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin LC, Chattopadhyay S, Lin JC, & Hu CJ (2018). Advances and opportunities in nanoparticle- and nanomaterial-based vaccines against bacterial infections. Advanced Healthcare Materials, 7(13), e1701395 10.1002/adhm.201701395 [DOI] [PubMed] [Google Scholar]
- Liu H, Moynihan KD, Zheng Y, Szeto GL, Li AV, Huang B, … Irvine DJ (2014). Structure-based programming of lymph-node targeting in molecular vaccines. Nature, 507(7493), 519–522. 10.1038/nature12978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe DB, Shearer MH, Jumper CA, & Kennedy RC (2007). Towards progress on DNA vaccines for cancer. Cellular and Molecular Life Sciences, 64(18), 2391–2403. 10.1007/s00018-007-7165-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk BT, & Zhang L (2015). Cell membrane-camouflaged nanoparticles for drug delivery. Journal of Controlled Release, 220(Pt. B, 600–607. 10.1016/j.jconrel.2015.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo M, Wang H, Wang Z, Cai H, Lu Z, Li Y, … Gao J (2017). A STING-activating nanovaccine for cancer immunotherapy. Nature Nanotechnology, 12(7), 648–654. 10.1038/nnano.2017.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melief CJM (2007). Immune pact with the enemy. Nature, 450, 803–804. [DOI] [PubMed] [Google Scholar]
- Mellman I, Coukos G, & Dranoff G (2011). Cancer immunotherapy comes of age. Nature, 480(7378), 480–489. 10.1038/nature10673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng J, Meng J, Duan J, Kong H, Li L, Wang C, … Yang XD (2008). Carbon nanotubes conjugated to tumor lysate protein enhance the efficacy of an antitumor immunotherapy. Small, 4(9), 1364–1370. 10.1002/smll.200701059 [DOI] [PubMed] [Google Scholar]
- Min Y, Roche KC, Tian S, Eblan MJ, McKinnon KP, Caster JM, … Wang AZ (2017). Antigen-capturing nanoparticles improve the abscopal effect and cancer immunotherapy. Nature Nanotechnology, 12(9), 877–882. 10.1038/nnano.2017.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montomoli E, Piccirella S, Khadang B, Mennitto E, Camerini R, & De Rosa A (2011). Current adjuvants and new perspectives in vaccine formulation. Expert Review of Vaccines, 10(7), 1053–1061. 10.1586/erv.11.48 [DOI] [PubMed] [Google Scholar]
- Nakamura T, Miyabe H, Hyodo M, Sato Y, Hayakawa Y, & Harashima H (2015). Liposomes loaded with a STING pathway ligand, cyclic di-GMP, enhance cancer immunotherapy against metastatic melanoma. Journal of Controlled Release, 216, 149–157. 10.1016/j.jconrel.2015.08.026 [DOI] [PubMed] [Google Scholar]
- Ng KW, Marshall EA, Bell JC, & Lam WL (2018). cGAS-STING and cancer: Dichotomous roles in tumor immunity and development. Trends in Immunology, 39(1), 44–54. 10.1016/j.it.2017.07.013 [DOI] [PubMed] [Google Scholar]
- Oberli MA, Reichmuth AM, Dorkin JR, Mitchell MJ, Fenton OS, Jaklenec A, … Blankschtein D (2017). Lipid nanoparticle assisted mRNA delivery for potent cancer immunotherapy. Nano Letters, 17(3), 1326–1335. 10.1021/acs.nanolett.6b03329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott PA, Hu Z, Keskin DB, Shukla SA, Sun J, Bozym DJ, … Wu CJ (2017). An immunogenic personal neoantigen vaccine for patients with melanoma. Nature, 547(7662), 217–221. 10.1038/nature22991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmiani G, De Filippo A, Novellino L, & Castelli C (2007). Unique human tumor antigens: Immunobiology and use in clinical trials. Journal of Immunology, 178(4), 1975–1979. 10.4049/jimmunol.178.4.1975 [DOI] [PubMed] [Google Scholar]
- Peng Y, Zhao Z, Liu T, Li X, Hu X, Wei X, … Tan W (2017). Smart human-serum-albumin-As2 O3 nanodrug with self-amplified folate receptor-targeting ability for chronic myeloid leukemia treatment. Angewandte Chemie International Edition, 56(36), 10845–10849. 10.1002/anie.201701366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen TR, Dickgreber N, & Hermans IF (2010). Tumor antigen presentation by dendritic cells. Critical Reviews in Immunology, 30(4), 345–386. [DOI] [PubMed] [Google Scholar]
- Radovic-Moreno AF, Chernyak N, Mader CC, Nallagatla S, Kang RS, Hao L, … Gryaznov SM (2015). Immunomodulatory spherical nucleic acids. Proceedings of the National Academy of Sciences of the United States of America, 112(13), 3892–3897. 10.1073/pnas.1502850112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy ST, van der Vlies AJ, Simeoni E, Angeli V, Randolph GJ, O’Neil CP, … Hubbell JA (2007). Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nature Biotechnology, 25(10), 1159–1164. 10.1038/nbt1332 [DOI] [PubMed] [Google Scholar]
- Rietscher R, Schroder M, Janke J, Czaplewska J, Gottschaldt M, Scherliess R, … Lehr CM (2016). Antigen delivery via hydrophilic PEG-b-PAGE-b-PLGA nanoparticles boosts vaccination induced T cell immunity. European Journal of Pharmaceutics and Biopharmaceutics, 102, 20–31. 10.1016/j.ejpb.2016.02.014 [DOI] [PubMed] [Google Scholar]
- Roberts ZJ, Goutagny N, Perera PY, Kato H, Kumar H, Kawai T, … Vogel SN (2007). The chemotherapeutic agent DMXAA potently and specifically activates the TBK1-IRF-3 signaling axis. Journal of Experimental Medicine, 204(7), 1559–1569. 10.1084/jem.20061845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal JA, Huang CJ, Doody AM, Leung T, Mineta K, Feng DD, … Putnam D (2014). Mechanistic insight into the TH1-biased immune response to recombinant subunit vaccines delivered by probiotic bacteria-derived outer membrane vesicles. PLoS One, 9(11), e112802 10.1371/journal.pone.0112802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross JS, & Fletcher JA (1998). The HER-2/neu oncogene in breast cancer: Prognostic factor, predictive factor, and target for therapy. Stem Cells, 16, 413–428. [DOI] [PubMed] [Google Scholar]
- Roy P, & Noad R (2008). Virus-like particles as a vaccine delivery system: Myths and facts. Human Vaccines, 4(1), 5–12. 10.4161/hv.4.1.5559 [DOI] [PubMed] [Google Scholar]
- Sah H, Thoma LA, Desu HR, Sah E, & Wood GC (2013). Concepts and practices used to develop functional PLGA-based nanoparticulate systems. International Journal of Nanomedicine, 8, 747–765. 10.2147/IJN.S40579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin U, Derhovanessian E, Miller M, Kloke BP, Simon P, Lower M, … Tureci O (2017). Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature, 547(7662), 222–226. 10.1038/nature23003 [DOI] [PubMed] [Google Scholar]
- Schijns VEJC (2003). Mechanisms of vaccine adjuvant activity: Initiation and regulation of immune responses by vaccine adjuvants. Vaccine, 21, 829–831. [DOI] [PubMed] [Google Scholar]
- Schneider IC, Hartmann J, Braun G, Stitz J, Klamp T, Bihi M, … Buchholz CJ (2018). Displaying tetra-membrane spanning Claudins on enveloped virus-like particles for cancer immunotherapy. Biotechnology Journal, 13(3), e1700345 10.1002/biot.201700345 [DOI] [PubMed] [Google Scholar]
- Schwaninger R, Waelti E, Zajac P, Wetterwald A, Mueller D, & Gimmi CD (2004). Virosomes as new carrier system for cancer vaccines. Cancer Immunology, Immunotherapy, 53(11), 1005–1017. 10.1007/s00262-004-0545-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shae D, Becker KW, Christov P, Yun DS, Lytton-Jean AKR, Sevimli S, … Wilson JT (2019). Endosomolytic polymersomes increase the activity of cyclic dinucleotide STING agonists to enhance cancer immunotherapy. Nature Nanotechnology, 14, 269–278. 10.1038/s41565-018-0342-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannahan JH, Bai W, & Brown JM (2015). Implications of scavenger receptors in the safe development of nanotherapeutics. Receptors & Clinical Investigation, 2(3), e811 10.14800/rci.811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, DeOliveira RB, Kalantari P, Parroche P, Goutagny N, Jiang Z, … Golenbock DT (2011). Innate immune recognition of an AT-rich stem-loop DNA motif in the Plasmodium falciparum genome. Immunity, 35(2), 194–207. 10.1016/j.immuni.2011.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siefert AL, Fahmy TM, & Kim D (2017). Artificial antigen-presenting cells for immunotherapies. Methods in Molecular Biology, 1530, 343–353. 10.1007/978-1-4939-6646-2_21 [DOI] [PubMed] [Google Scholar]
- Stieneker F, Kreuter J, & Lower J (1991). High antibody titers in mice with polymethylmethacrylate nanoparticles as adjuvant for HIV vaccines. AIDS, 5, 431–435. [DOI] [PubMed] [Google Scholar]
- Sundararaman SK, & Barbie DA (2018). Tumor cGAMP awakens the natural killers. Immunity, 49(4), 585–587. 10.1016/j.immuni.2018.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykulev Y, Joo M, Vturina I, Tsomides TJ, & Eisen HN (1996). Evidence that a single peptide–MHC complex on a target cell can elicit a cytolytic T cell response. Immunity, 4, 565–571. [DOI] [PubMed] [Google Scholar]
- Syn NL, Wang L, Chow EK, Lim CT, & Goh BC (2017). Exosomes in cancer nanomedicine and immunotherapy: Prospects and challenges. Trends in Biotechnology, 35(7), 665–676. 10.1016/j.tibtech.2017.03.004 [DOI] [PubMed] [Google Scholar]
- Tan A, De La Pena H, & Seifalian AM (2010). The application of exosomes as a nanoscale cancer vaccine. International Journal of Nanomedicine, 5, 889–900. 10.2147/IJN.S13402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan S, Sasada T, Bershteyn A, Yang K, Ioji T, & Zhang Z (2014). Combinational delivery of lipid-enveloped polymeric nanoparticles carrying different peptides for anti-tumor immunotherapy. Nanomedicine (London, England), 9(5), 635–647. 10.2217/nnm.13.67 [DOI] [PubMed] [Google Scholar]
- Taneichi M, Ishida H, Kajino K, Ogasawara K, Tanaka Y, Kasai M, … Uchida T (2006). Antigen chemically coupled to the surface of liposomes are cross-presented to CD8+ T cells and induce potent antitumor immunity. Journal of Immunology, 177(4), 2324–2330. 10.4049/jimmunol.177.4.2324 [DOI] [PubMed] [Google Scholar]
- Temizoz B, Kuroda E, & Ishii KJ (2016). Vaccine adjuvants as potential cancer immunotherapeutics. International Immunology, 28(7), 329–338. 10.1093/intimm/dxw015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thery C, Duban L, Segura E, Veron P, Lantz O, & Amigorena S (2002). Indirect activation of naive CD4+ T cells by dendritic cell-derived exosomes. Nature Immunology, 3(12), 1156–1162. 10.1038/ni854 [DOI] [PubMed] [Google Scholar]
- Tian L, Chen Q, Yi X, Wang G, Chen J, Ning P, … Liu Z (2017). Radionuclide I-131 labeled albumin-paclitaxel nanoparticles for synergistic combined chemo-radioisotope therapy of cancer. Theranostics, 7(3), 614–623. 10.7150/thno.17381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Gao J, & Wang Z (2018). Outer membrane vesicles for vaccination and targeted drug delivery. WIREs Nanomedicine and Nanobiotechnology, 11(2), e1523 10.1002/wnan.1523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Huang W, Li K, Yao Y, Yang X, Bai H, … Ma Y (2017). Engineered outer membrane vesicle is potent to elicit HPV16E7-specific cellular immunity in a mouse model of TC-1 graft tumor. International Journal of Nanomedicine, 12, 6813–6825. 10.2147/IJN.S143264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson RO, Manzanillo PS, & Cox JS (2012). Extracellular M. tuberculosis DNA targets bacteria for autophagy by activating the host DNA-sensing pathway. Cell, 150(4), 803–815. 10.1016/j.cell.2012.06.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen ZS, Xu YL, Zou XT, & Xu ZR (2011). Chitosan nanoparticles act as an adjuvant to promote both Th1 and Th2 immune responses induced by ovalbumin in mice. Marine Drugs, 9(6), 1038–1055. 10.3390/md9061038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams N (1996). An immune boost to the war on cancer. Science, 272, 28–30. [DOI] [PubMed] [Google Scholar]
- Wilson DR, Sen R, Sunshine JC, Pardoll DM, Green JJ, & Kim YJ (2018). Biodegradable STING agonist nanoparticles for enhanced cancer immunotherapy. Nanomedicine, 14(2), 237–246. 10.1016/j.nano.2017.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo SR, Fuertes MB, Corrales L, Spranger S, Furdyna MJ, Leung MY, … Gajewski TF (2014). STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity, 41(5), 830–842. 10.1016/j.immuni.2014.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang X, Zhao X, Hu H, Qiao M, Deng Y, & Chen D (2017). Nanoparticles for tumor immunotherapy. European Journal of Pharmaceutics and Biopharmaceutics, 115, 243–256. 10.1016/j.ejpb.2017.03.013 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhang Y, & Zhang L (2019). Expression of cancer-testis antigens in esophageal cancer and their progress in immunotherapy. Journal of Cancer Research and Clinical Oncology, 145, 281–291. 10.1007/s00432-019-02840-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu G, Lynn GM, Jacobson O, Chen K, Liu Y, Zhang H, … Chen X (2017). Albumin/vaccine nanocomplexes that assemble in vivo for combination cancer immunotherapy. Nature Communications, 8(1), 1954 10.1038/s41467-017-02191-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu G, Mei L, Vishwasrao HD, Jacobson O, Wang Z, Liu Y, … Chen X (2017). Intertwining DNA–RNA nanocapsules loaded with tumor neoantigens as synergistic nanovaccines for cancer immunotherapy. Nature Communications, 8(1), 1482 10.1038/s41467-017-01386-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu G, Zhang F, Ni Q, Niu G, & Chen X (2017). Efficient nanovaccine delivery in cancer immunotherapy. ACS Nano, 11(3), 2387–2392. 10.1021/acsnano.7b00978 [DOI] [PubMed] [Google Scholar]


