Abstract
Background
Prostate cancer is the sixth leading cause of death, among all cancer deaths By 2030, this burden is expected to increase with 1.7 million new cases and 499,000 new deaths. We aimed to evaluate the efficacy and safety of Nilutamide in metastatic prostate cancer (mPCa) patients who underwent orchiectomy.
Methods
A comprehensive search was conducted in the Medline/PubMed and Cochrane Library. References from included studies and studies from clinicaltrials.gov were explored without language and date restrictions. We included only randomized controlled trials, comparing the safety and efficacy of Nilutamide in Metastatic Prostate Cancer (mPCa) patients who underwent orchiectomy with placebo. The outcomes of concerns were survival and the response of drug and safety.. Quality of the included studies was assessed using the Cochrane Risk of Bias Tool. Two authors were independently involved in the study selection, data extraction and quality assessment. Disagreements between the two reviewers were resolved by consulting a third reviewer.
Results
A total of five out of 244 studies were included in meta-analysis involving1637 participants. Nilutamide group showed improved response rate (RR=1.77, 95%CI 1.46-2.14, p<0.00001), disease progression (RR=0.59, 95%CI 0.47-0.73, p<0.00001), complete response (RR=2.13, 95%CI 1.40-3.23, p=0.003) and clinical benefit (RR=1.23, 95%CI 1.13-1.34, p<0.00001) when compared to placebo; however, stable disease favored the control group (RR=0.80, 95%CI 0.68-0.94, p=0.007). In addition, patients on Nilutamide showed prolonged progression-free survival and overall survival. Nausea and vomiting were the most common adverse events reported in Nilutamide group.
Conclusion
Evidence suggests that patients with mPCa who underwent orchiectomy receiving Nilutamide showed significant improvement in progression-free survival and overall survival response rate and clinical benefits in comparison with the placebo group.
Keywords: Nilutamide, metastatic prostate cancer, anti-androgen, orchiectomy, safety, efficacy
1. INTRODUCTION
Prostate Cancer (PCa) is the sixth primary cause of cancer death, the second most common cancer in men across the world. By 2030, PCa burden is expected to be 1.7 million new cases and 499000 new deaths and it is due to the increase in worldwide population and ageing of people. According to the population-based cancer registries (PBCRs) of India, Delhi, Kolkata, Pune and Thiruvananthapuram are the cities where PCa is the second leading cancer, whereas in Bangalore and Mumbai, it is the third leading cancer. There is a significant increase in annual percentage changes of mPCa, which are estimated as 3.4%, 4.2%, 3.3%, 0.9% and 11.6% in Bangalore, Chennai, Delhi, Mumbai and Kamrup Urban district in Assam, respectively [1].
The surgical or medical castration is the first-line of choice for newly diagnosed patients with Prostate Cancer (PCa). Hormone naive, mPCa Androgen deprivation therapy (ADT) by bilateral orchiectomy (surgical castration) or by control of testicular androgen synthesis (medical castration) using luteinizing hormone-releasing hormone agonists or antagonists remains as the keystone for initial treatment. The ADT by both methods results in symptomatic improvement, reduction of serum testosterone to a level of less than 50 ng/dL along with reduced Prostate-specific antigen (PSA) and/or radiographic response in most of the patients. Whereas, combined androgen blockade, CAB (addition of first-generation anti-androgens to ADT) is less effective, toxic and has high cost as compared to ADT alone [2, 3].
Nilutamide is a non-steroidal anti-androgen with high-affinity, which focuses on the androgen receptors (AR) ligand binding and blocks the transcription of androgen response elements (AREs). Nilutamide can attain a significantly constant PSA response with a favourable toxicity profile when it is used as a second-line agent. The most common side effects observed in patients receiving Nilutamide as monotherapy are libido, potency, nausea, vomiting, abdominal discomfort and elevation in liver function tests [4, 5].
A study conducted in Mexico involving 104 patients showed the higher safety and efficacy of Nilutamide, when in combination with Buserelin [6]. Another study, with a follow up of 8.5 revealed that when Nilutamide was used after orchiectomy, it proved effective in improving survival and progression interval of the patient in comparison with the combination of orchiectomy and placebo [7].
A collaborative meta-analysis of 27 Randomized Controlled trials (RCTs) involving 8275 patients, observed no significant difference in the 5-year survival rate between the groups. One group of patients received a CAB with a non-steroidal anti-androgen (flutamide or Nilutamide), while the other group received castration alone [8]. This signifies that safety and efficacy of Nilutamide are still questionable. In this systematic review, we aimed to evaluate the efficacy and safety of Nilutamide in metastatic PCa (mPCa) patients who underwent orchiectomy.
2. METHODOLOGY
2.1. Criteria for Considering Studies for this Review
2.1.1. Types of Studies and Participants
We reviewed only randomized controlled trials (RCTs) comparing Nilutamide versus placebo in metastatic stages of PCa patients who underwent orchiectomy. Studies recruiting men at advanced stages of PCa who underwent orchiectomy were eligible for final analysis. We included studies evaluating men with PCa, that had spread locally outside the prostate gland (locally advanced, T3-4, N0, M0, D0), to regional lymph nodes (local to regionally advanced, T1-4, N1, M0 or stage D1), to the bones or to other areas (advanced, T1-4, N0-1, M1 or D2), or those who had recurrent disease after local therapy. We did not exclude studies based on age and ethnicity.
2.1.2. Types of Interventions
We focused on the interventional group as the patients who received the non-steroidal anti-androgen drug, Nilutamide and the placebo as the control group those who underwent orchiectomy and did not receive any drug.
2.1.3. Types of Outcome Measures
We evaluated both efficacy and safety outcomes of Nilutamide followed by orchiectomy. Response rate, including Objective Response (OR), Disease Progression (DP), Stable Disease (SD), Partial Response (PR) and Clinical benefit or disease control rate (DCR) were our primary outcome. Overall Survival (OS) Progression-Free Survival (PFS), time to distant metastasis or death, Tolerability and treatment discontinuation (TTD) and Safety including total adverse events, any grade 3/4 adverse event were considered as the secondary outcomes.
2.2. Search Methods for Identification of Studies
An extensive literature search was performed using all the possible terms on 11 November 2017 in the PubMed, and the Cochrane Library without any restriction to language, date or publication format. A detailed search strategy in PubMed is provided in Appendix 1. The reference lists of all identified articles were screened to identify additional potentially relevant citations. Additionally, we searched the clinical trials registry (www.clinicaltrials.gov) for the other relevant studies.
Appendix 1. Detailed search strategy in PubMed.
| Search | Query | Items Found | Facet |
|---|---|---|---|
| 1 | Search prostate neoplasms OR neoplasms,prostate OR neoplasm,prostate OR prostate neoplasm OR neoplasms,prostatic OR neoplasm,prostatic OR prostatic neoplasm OR prostate cancer OR cancer,prostate OR cancers, prostate OR prostate cancers OR cancer of the prostate OR prostatic cancer OR cancer, prostatic OR cancers,prostatic OR prostatic cancers OR cancer of prostate. | 153098 | Disease |
| 2 | Search prostate cancer. | 149845 | |
| 3 | Search (#1 or #2). | 154135 | |
| 4 | Search (Nilutamide[Supplementary Concept]) OR Nilutamide. | 293 | Intervention |
| 5 | Search ((((((5,5-dimethyl-3-(4-nitro-3-(trifluoromethyl)phenyl)- 2,4 imidazolidinedione) OR Nilandron) OR Nilutamide) OR RU 23908-10) OR Anandron) OR RU 23908) OR RU-23908. | 311 | |
| 6 | Search (#4 OR #5). | 24046 | |
| 7 | Search (#3 AND #6). | 1092 | D+I |
| 8 | Search (#3 and #6) Filters: Humans. | 244 | Filters |
2.3. Selection of Studies
All titles and abstracts retrieved from the searches were screened for eligibility. Eligible studies were retrieved in full and assessed for inclusion by two reviewers (MR, SK) independently based on the inclusion criteria outlined above. Any disagreements between the reviewers were resolved through discussion with the third reviewer. We included only the studies published from 1990.
2.4. Data Extraction
Two independent reviewers did the data extraction to the standardized data extraction sheet. Identification of studies was done with the aid of name of the first author and year of publication were used to identify the study. All data were extracted in the form of the text. Data extraction for each study consisted of a summary of all the included studies, publication details, study design details, treatment details, patient characteristics, details of outcomes relevant to this review including methods of measurements and timelines of assessment. Disagreements in data extraction were resolved by consulting a third reviewer.
2.5. Assessment of Risk of Bias
Two review authors independently assessed the methodological quality of included studies using The Cochrane Collaboration’s tool for assessing the risk of bias in accordance with the following criteria: Random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other sources of bias.
2.6. Statistical Analysis
Review Manager Software (RevMan, version 5.3 for Windows; The Cochrane Collaboration, Oxford, UK) was used for conducting the meta-analysis. Risk ratio (RR) and 95% confidence interval (95% CI) values were calculated. Statistical heterogeneity of data was assessed using the I2 statistic. The fixed-effects model was used for studies without significant heterogeneity (I2≤50% or P≥0.10), whereas the random-effects model was used for studies with significant heterogeneity (I2>50% or P≤0.10). Publication bias was detected using funnel plots, generated with the help of RevMan.
3. RESULT
3.1. Eligible Studies and Data Summary
A total of 296 studies were first identified for evaluation. Based on the criteria described in the methods, 25 publications were eligible for full-text evaluation. Finally, a total of six RCTs out of 296 studies including 1855 randomized patients were included in this meta-analysis. A total of 912 Patients received Nilutamide followed by orchiectomy, and a total of 943 Patients received placebo.
The search process is illustrated well inFig. (1). All the included used placebo as a comparator followed by the orchiectomy and treatment of Nilutamide as first-line therapy. Only 3 of 6 studies used intention to treat (ITT) analysis in efficacy population. None of the studies provided information regarding the male and female distribution in the trial. Table 1 describes the characteristics of the eligible studies in detail.
Fig. (1).
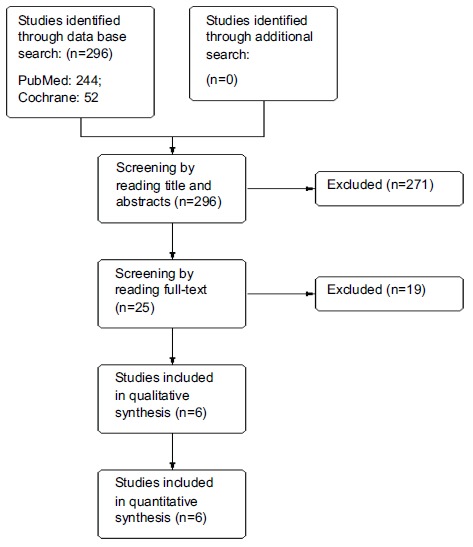
Flow-chart of the literature search process (PRISMA).
Table 1. Characteristics of included studies.
| First Author, Year |
Total
Patients, N |
Stage | Age Median or Mean (Range) | Follow up Time | Nilutamide Dose | Comparator | Efficacy Population |
|---|---|---|---|---|---|---|---|
| Beland 1990 [9] | 194 | stage D2 | 69 (42-93) | Up to 48 months and for a minimum of 18 months |
100 mg TID† | Placebo | NR* |
| Beland 1991 [10] | 174 | stage D2 | NR | NR | 100 mg TID | Placebo | NR |
| Dijkman 1997 [7] | 457 | stage D2 | NR | 8.5 years | 300 mg for 1 month and then 150 mg OD‡ | Placebo | ITT§ |
| Janknegt 1993 (1) [11] | 457 | stage (M+) | NR | 5 years | 300 mg for 1 month and then 150 mg OD | Placebo | ITT |
| Janknegt 1993 (2) [12] | 423 | stage (M+) | 71 (46-86) | 35 month | 300 mg for 1 month and then 150 mg OD | Placebo | ITT |
| Namer 1990 [13] | 150 | Stage D1 and D2 | NR | NR | 300 mg | Placebo | NR |
*NR: Not reported; †TID: Three times a day; ‡OD: Once a day; §ITT: Intent to treat.
3.2. Objective Response Patients with Nilutamide and Placebo
A total of 4 studies with 1110 participants were reported OR with Nilutamide and placebo in mPCa patients who underwent orchiectomy. The OR rates in the patients with Nilutamide were significantly higher than those in the placebo group (RR, 1.68; 95%CI 1.42-1.99; Fig. (2). There was no heterogeneity between the results of different studies (I2=25%, P=0.26), so the fixed-effects model was applied for data analysis.
Fig. (2).
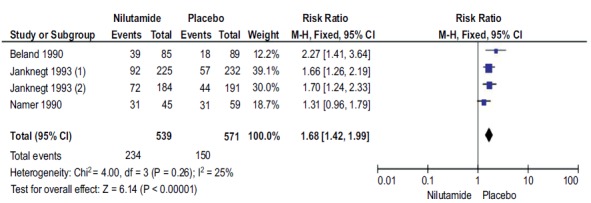
Forest plot: Objective response with nilutamide and placebo in patients with mPCa.
3.3. Complete Response Patients with Nilutamide and Placebo
A total of 3 studies with 653 participants reported CR with Nilutamide and placebo in mPCa who underwent orchiectomy. The CR rates in the patients with Nilutamide were significantly higher than those in the placebo group (RR, 2.03; 95% CI, 1.35-3.06; Fig. (3). There was no heterogeneity between the results of different studies (I2=0%, P=0.44), so the fixed-effects model was applied for data analysis.
Fig. (3).
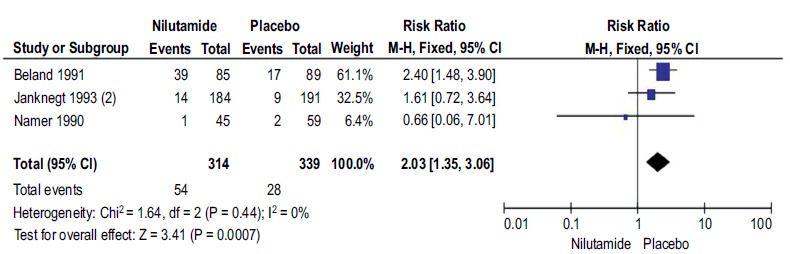
Forest plot: Complete response with nilutamide and placebo in patients with mPCa.
3.4. Disease Progression in Patients with Nilutamide and Placebo
A total of 4 studies with 1110 participants reported DP with Nilutamide and placebo in mPCa who underwent orchiectomy. The DP rates in the patients with Nilutamide were significantly lower than those in the placebo group (RR, 0.59; 95% CI, 0.47-0.73; Fig. (4). There was no heterogeneity between the results of different studies (I2=0%, P=0.97), so the fixed-effects model was applied for data analysis.
Fig. (4).
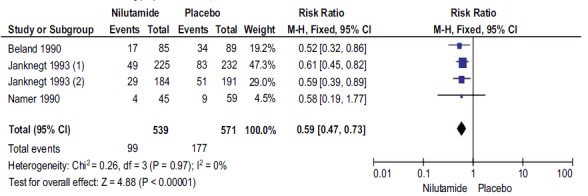
Forest plot: Disease progression with nilutamide and placebo in patients with mPCa.
3.5. Stable Disease in Patients with Nilutamide and Placebo
A total of 4 studies with 827 participants reported SD with Nilutamide and placebo in mPCa who underwent orchiectomy. SD rates in patients with Nilutamide were 21% lower than those in the placebo group (RR, 0.79; 95% CI, 0.67-0.93; Fig. (5). There was low heterogeneity between the results of different studies (I2=20%, P=0.29), so the fixed-effects model was applied for data analysis.
Fig. (5).
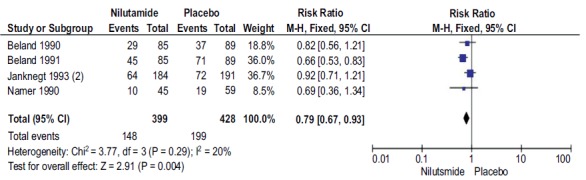
Forest plot: Stable disease rate with nilutamide and placebo in patients with mPCa.
3.6. Disease Control Rate in Patients with Nilutamide and Placebo
A total of 4 studies with 1110 participants reported DCR with Nilutamide and placebo in mPCa patients who underwent orchiectomy. The DCR rates in patients with Nilutamide were significantly higher than those in the placebo group (RR, 1.21; 95% CI, 1.12-1.31; Fig. (6). There was low heterogeneity between the results of different studies (I2=7%, P=0.36), so the fixed-effects model was applied for data analysis.
Fig. (6).
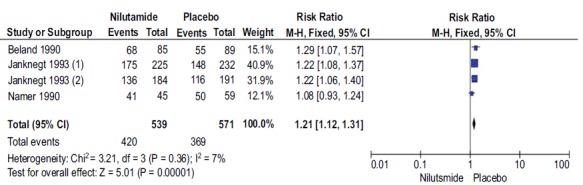
Forest plot: Disease control rate with nilutamide and placebo in patients with mPCa.
3.7. Overall Survival
A total of 3 studies with 1037 participants reported the overall survival. The OS rates in patients with Nilutamide were significantly higher than those in the placebo group. It ranged from 24.3 to 27.3 months in Nilutamide group and 18.9 to 24.2 months in the placebo group, respectively.
Beland et al. reported an increased survival rate of 5.4 months in the Nilutamide group as compared to the placebo, but this difference was non-significant (24.3 months vs 18.9
months; p=0.28). The survival rate of Nilutamide and placebo at 6, 12, 18 and 24 month was 98% vs 96%, 12 month: 75% vs 67%, 49% vs 36% and 27% vs 22% respectively [9].
Dijkman et al. reported a significant increase in the survival rate of 3.7 months in the Nilutamide group compared to the placebo (27.3 months vs 23.6 months; p= 0.0326). The survival rate of Nilutamide and placebo at 6 years was 32% vs 21% [7]. Janknegt et al., also reported a significant increase in the survival rate of 3.1 months in the Nilutamide group compared to the placebo (27.3 months vs 24.2 months; p= 0.041) [12].
3.8. Progression-free Survival
Two studies with 867 participants reported progression-free survival. PFS rates in patients with Nilutamide were significantly higher than those in the placebo group.
Dijkman et al. reported a significant increase in the PFS rate of 6.5 months in the Nilutamide group compared to the placebo (21.2 months vs 14.7 months; p= 0.002). The PFS rate of Nilutamide and placebo at 5 years was 20% vs 12% [7]. Janknegt et al., reported a significant increase in the survival rate of 6.1 months in the Nilutamide group compared to the placebo (20.8 months vs 14.7 months; p= 0.0041) [11].
Beland et al., reported that PFS rate of Nilutamide and placebo at 6, 12, 18 and 24 month was 72% vs 59%, 45% vs 42%, 32% vs 22% and 17% vs 16%, respectively [10].
3.9. Time to Distant Metastasis or Death
Two studies with 867 participants reported time to distant metastasis or death. The TTD in the patients with Nilutamide were significantly longer than those in the placebo group.
Dijkman et al., reported a significant increase in TTD of 7.2 months in the Nilutamide group compared to the placebo (37.0 months vs 29.8 months; p= 0.013) [7]. Similarly, Janknegt et al. reported a significant increase in TTD of 7.3 months in the Nilutamide group compared to the placebo (37.1 months vs 29.8 months; p= 0.041) [11].
Beland et al., reported that DDT rate of Nilutamide and placebo at 3, 6, 12, 18, 24, 30 and 36 months were 94% vs 95, 86% vs 85%, 73% vs 66%, 52% vs 39%, 32% vs 21%, 10% vs 10% and 2% vs 4%, respectively [10].
3.10. Tolerability and Treatment Discontinuation
All six studies discussed the treatment discontinuation due to various reasons such as efficacy or safety. Only Janknegt et al. described treatment discontinuation due to disease progression. A total of 1842 (Nilutamide: 907; placebo: 935) participants were assessed for tolerability. Out of that, overall 49.40% (910; Nilutamide: 462; placebo: 448) of patients were discontinued. Across five studies a total of 8.30% (153; Nilutamide: 98; placebo: 55) patients were discontinued due to safety reason. Janknegt et al. recorded that a total of 252 (Nilutamide: 112; placebo: 140) were discontinued. It also revealed that a 388 (Nilutamide: 190; placebo: 198) patients were discharged from the study [12].
3.11. Meta-analysis
A total of 6 studies with 1842 participants reported the treatment discontinuation with Nilutamide and placebo in mPCa patients who underwent orchiectomy. The difference in discontinuation rates between the groups was not statistically significant. (RR, 1.08; 95% CI, 0.91-1.30; Fig. (7). There was high heterogeneity found between the results of different studies (I2=89%, P<0.00001), so the random-effects model was applied for data analysis.
Fig. (7).
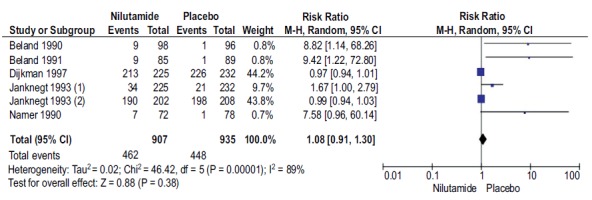
Forest plot: Discontinuation rate with nilutamide and placebo in patients with mPCa.
3.12. Safety
Namer et al. reported regarding the treatment-related adverse events. 15 (21%) of Nilutamide exposed patients and 14 (18%) of the placebo group experienced the various treatment-related adverse events across the body. The common adverse effects reported followed by the use of Nilutamide include delayed adaptability to darkness or blurred vision, alcohol intolerance, hot flash, nausea, respiratory disorders, and elevation in the liver enzyme [13].
3.13. Publication Bias
It was not appropriate to test the publication bias using the funnel plot since the number of studies included in each comparison was less than ten. Hence, we did not perform the statistical estimation using Egger’s or Begg's test.
4. DISCUSSION
PCa is the second most deadly cancer in the USA and European male patients [14]. In spite of this, PCa, as well as mPCa, usually responding to the treatment and sometimes it is curable when it is localized. All these treatments are aimed to increase the overall and progression-free survival along with the high quality of life [15].
An initial search of various databases retrieved 296 studies, out of that 5 studies with 1734 randomized patients were found to be eligible to include in our analysis. The overall quality of the included studies was low to moderate. We included the RCTs evaluating the efficacy and safety of Nilutamide in mPCa patients followed by the orchiectomy. It was mandatory that the patients had to undergo orchiectomy. All the studies used placebo as the comparator.
Our review revealed that the combined androgen blockade with Nilutamide and orchiectomy was effective and beneficial in terms of objective response (RR, 1.68; 95%CI 1.42-1.99), complete response (RR, 2.03; 95% CI, 1.35-3.06), disease progression (RR, 0.59; 95% CI, 0.47-0.73), and clinical benefit (RR, 1.21; 95% CI, 1.12-1.31). Whereas, stable disease was found to be lower with the placebo group (RR, 0.79; 95% CI, 0.67-0.93) when compared to the orchiectomy with placebo respectively.
Interestingly, when we carried out the meta-analysis of treatment discontinuation (tolerability) and found that there was no significant difference between Nilutamide and the placebo group when we considered the confidence interval (RR, 1.08; 95% CI, 0.91-1.30).
A meta-analysis in 2002 assessed randomized trials comparing monotherapy (orchiectomy or luteinizing hormone-releasing hormone [LHRH] agonists) with combination therapy (CAB) using orchiectomy or an LHRH agonist plus a nonsteroidal or steroidal anti-androgen. The study demonstrated that there was no statistically significant difference between the groups in terms of survival at 2 years, whereas the survival rate at 5 years was better with the CAB group than the monotherapy [16].
Another review also gave the similar kind of results, which reported the improved efficacy of Nilutamide with orchidectomy in terms of objective response of disease and the time to disease progression [17]. Kunath et al. compared the treatment with medical or surgical castration using non-steroidal anti-androgens, which was associated with overall survival reduction and increased clinical progression when compared [18].
In a recent PCa Trialist’s Collaborative Group (PCTCG) meta-analysis of 27 MAB (Maximum Androgen Blockage) trials (trail began before 1991 and which used Flutamide, Nilutamide or Cyproterone acetate as the anti-androgen component), they found that the magnitude of the 5-year survival benefit for MAB appeared to be influenced by the anti-androgen component, with non-steroidal anti-androgens, having an advantage over the steroidal compound [19].
The methodological quality according to the Cochrane risk of bias assessment tool was found to be low bias (High quality) in 4 studies and moderate in other 2 studies. We were limited to find the publication bias using the funnel plot, as the number of studies included in each comparison was less than ten.
4.1. Limitation
We could not retrieve grey literature of published studies before the year 1990, because of this reason we included a study conducted from 1990 onwards.
CONCLUSION
Evidence suggests that Nilutamide shows significant improvement in OS, PFS, response rate and clinical benefit when compared to placebo in patients with mPCa who underwent orchiectomy. The review can be extended further to all drugs under the non-steroidal anti-androgen in mPCa patients.
ACKNOWLEDGEMENTS
The authors would like to thank all the colleagues, friends and staffs from JSS College of Pharmacy, ISF College of Pharmacy and SAC College of Pharmacy for their support and encouragement.
List of Abbreviations
- ADT
Androgen deprivation therapy
- AR
Androgen receptors
- AREs
Androgen response elements
- CAB
Combined androgen blockade
- CR
Complete Response
- DCR
Disease Control Rate
- DP
Disease Progression
- ITT
Intention to treat
- LHRH
Leutinizaing Hormone Releasing Hormone
- mPCa
Metastatic Prostate Cancer
- NR
Not reported
- OD
Once a day
- OR
Objective Response
- OS
Overall Survival
- PBCRs
Population-based cancer registries
- PCa
Prostate Cancer
- PFS
Progression Free Survival
- PSA
Prostate-specific antigen
- RCTs
Randomized Controlled Trials
- RR
Risk ratio
- SD
Stable Disease
- TID
Three times a day
- TTD
Time to distant Metastasis
CONSENT FOR PUBLICATION
Not applicable.
STANDARDS OF REPORTING
The present study was designed and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCEs
- 1.Jain S., Saxena S., Kumar A. Epidemiology of prostate cancer in India. Meta Gene. 2014;2:596–605. doi: 10.1016/j.mgene.2014.07.007. [http://dx.doi.org/10.1016/j.mgene.2014.07.007]. [PMID: 25606442]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park J.C., Eisenberger M.A. Proceedings of the InMayo Clinic. Elsevier; 2015. Advances in the treatment of meta-static prostate cancer. pp. 1719–33. [DOI] [PubMed] [Google Scholar]
- 3.Yoo S., Choi S.Y., You D., Kim C.S. New drugs in prostate cancer. Prostate Int. 2016;4(2):37–42. doi: 10.1016/j.prnil.2016.05.001. [http://dx.doi.org/10.1016/j.prnil.2016.05.001]. [PMID: 27358841]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ricci F., Buzzatti G., Rubagotti A., Boccardo F. Safety of antiandrogen therapy for treating prostate cancer. Expert Opin. Drug Saf. 2014;13(11):1483–1499. doi: 10.1517/14740338.2014.966686. [http://dx.doi.org/10.1517/14740338.2014.966686]. [PMID: 25270521]. [DOI] [PubMed] [Google Scholar]
- 5.Kassouf W., Tanguay S., Aprikian A.G. Nilutamide as second line hormone therapy for prostate cancer after androgen ablation fails. J. Urol. 2003;169(5):1742–1744. doi: 10.1097/01.ju.0000057795.97626.66. [http://dx.doi.org/10.1097/01.ju.0000057795.97626.66]. [PMID: 12686822]. [DOI] [PubMed] [Google Scholar]
- 6.Velázquez-Macías R.F., Aguilar-Patiño S., Cortez-Betancourt R., Rojas-Esquivel I., Fonseca-Reyes G., Contreras-González N. Evaluation of efficacy of buserelin plus Nilutamide in Mexi-can Male patients with advanced prostate cancer. Rev. Mex. Urol. 2016;76:346–351. [Google Scholar]
- 7.Dijkman G.A., Janknegt R.A., De Reijke T.M., Debruyne F.M. Long-term efficacy and safety of nilutamide plus castration in advanced prostate cancer, and the significance of early prostate specific antigen normalization. J. Urol. 1997;158(1):160–163. doi: 10.1097/00005392-199707000-00051. [http://dx.doi.org/10.1097/00005392-199707000-00051]. [PMID: 9186345]. [DOI] [PubMed] [Google Scholar]
- 8.Group P.C. Maximum androgen blockade in advanced prostate cancer: an overview of the randomised trials. Prostate Cancer Trialists’ Collaborative Group. Lancet. 2000;355(9214):1491–1498. [http://dx.doi.org/10.1016/S0140-6736(00)02163-2]. [PMID: 10801170]. [PubMed] [Google Scholar]
- 9.Béland G., Elhilali M., Fradet Y., et al. A controlled trial of castration with and without nilutamide in metastatic prostatic carcinoma. Cancer. 1990;66(5) Suppl.:1074–1079. doi: 10.1002/cncr.1990.66.s5.1074. [http://dx.doi.org/10.1002/cncr.1990.66.s5.1074]. [PMID: 2203517]. [DOI] [PubMed] [Google Scholar]
- 10.Beland G. Combination of Anandron with orchiectomy in treatment of metastatic prostate cancer. Results of a double-blind study. Urology. 1991;37(2) Suppl.:25–29. doi: 10.1016/0090-4295(91)80098-r. [http://dx.doi.org/10.1016/0090-4295(91)80098-R]. [PMID: 1992600]. [DOI] [PubMed] [Google Scholar]
- 11.Janknegt R.A. Total androgen blockade with the use of orchiectomy and nilutamide (Anandron) or placebo as treatment of metastatic prostate cancer. Cancer. 1993;72(12) Suppl.:3874–3877. doi: 10.1002/1097-0142(19931215)72:12+<3874::aid-cncr2820721722>3.0.co;2-#. [http://dx.doi.org/10.1002/1097-0142(19931215)72:12+<3874:AID-CNCR2820721722>3.0.CO;2-#]. [PMID: 8252507]. [DOI] [PubMed] [Google Scholar]
- 12.Janknegt R.A., Abbou C.C., Bartoletti R., et al. Orchiectomy and nilutamide or placebo as treatment of metastatic prostatic cancer in a multinational double-blind randomized trial. J. Urol. 1993;149(1):77–82. doi: 10.1016/s0022-5347(17)36003-2. [http://dx.doi.org/10.1016/S0022-5347(17)36003-2]. [PMID: 7678043]. [DOI] [PubMed] [Google Scholar]
- 13.Namer M., Toubol J., Caty A., et al. A randomized double-blind study evaluating Anandron associated with orchiectomy in stage D prostate cancer. J. Steroid Biochem. Mol. Biol. 1990;37(6):909–915. doi: 10.1016/0960-0760(90)90442-n. [http://dx.doi.org/10.1016/0960-0760(90)90442-N]. [PMID: 2285605]. [DOI] [PubMed] [Google Scholar]
- 14.Wallis C.J.D., Klaassen Z., Bhindi B., et al. Comparison of abiraterone acetate and docet-axel with androgen deprivation therapy in high-risk and meta-static hormone-naïve prostate cancer: a systematic review and network meta-analysis. Eur. Urol. 2018;73(6):834–844. doi: 10.1016/j.eururo.2017.10.002. [http://dx.doi.org/10.1016/j.eururo.2017.10.002]. [PMID: 29037513]. [DOI] [PubMed] [Google Scholar]
- 15.Botrel T.E., Clark O., dos Reis R.B., et al. Intermittent versus continuous androgen deprivation for locally advanced, recurrent or metastatic prostate cancer: a systematic review and meta-analysis. BMC Urol. 2014;14:9. doi: 10.1186/1471-2490-14-9. [http://dx.doi.org/10.1186/1471-2490-14-9]. [PMID: 24460605]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Samson D.J., Seidenfeld J., Schmitt B., et al. Systematic review and meta-analysis of monotherapy compared with combined androgen blockade for patients with advanced prostate carcinoma. Cancer. 2002;95(2):361–376. doi: 10.1002/cncr.10647. [http://dx.doi.org/10.1002/cncr.10647]. [PMID: 12124837]. [DOI] [PubMed] [Google Scholar]
- 17.Bertagna C., De Géry A., Hucher M., François J.P., Zanirato J. Efficacy of the combination of nilutamide plus orchidectomy in patients with metastatic prostatic cancer. A meta-analysis of seven randomized double-blind trials (1056 patients). Br. J. Urol. 1994;73(4):396–402. doi: 10.1111/j.1464-410x.1994.tb07603.x. [http://dx.doi.org/10.1111/j.1464-410X.1994.tb07603.x]. [PMID: 8199827]. [DOI] [PubMed] [Google Scholar]
- 18.Kunath F., Grobe H.R., Ruecker G., et al. Non‐steroidal anti-androgen monotherapy compared with luteinising hormone–releasing hormone agonists or surgical castration monotherapy for ad-vanced prostate cancer. Cochrane Libr. 2014 doi: 10.1002/14651858.CD009266.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akaza H., Yamaguchi A., Matsuda T., et al. Superior anti-tumor efficacy of bicalutamide 80 mg in combination with a luteinizing hormone-releasing hormone (LHRH) agonist versus LHRH agonist monotherapy as first-line treatment for advanced prostate cancer: interim results of a randomized study in Japanese patients. Jpn. J. Clin. Oncol. 2004;34(1):20–28. doi: 10.1093/jjco/hyh001. [http://dx.doi.org/10.1093/jjco/hyh001]. [PMID: 15020659]. [DOI] [PubMed] [Google Scholar]


