Abstract
Abstract: Background:
As2O3 and resveratrol have been widely considered to be effective in anti-cancer therapies and the underlying mechanisms have been reported extensively. However, the combined treatment effect and potential target of As2O3 and resveratrol in the treatment of tumors remains elusive. The purpose of this study was to investigate the benefits and efficacy of As2O3 in combination with resveratrol in the treatment of colon cancer, as well as looking for new targets that could provide alternative explanation of the efficacy of drugs.
Methods:
The proliferation of cancer cells was measured by the MTT and EdU staining assay, while the apoptosis of cancer cells was determined by the flow cytometry. Western blot and immunoprecipitation were performed to measure the expression levels of proteins and the interaction between hERG and integrin β1, respectively.
Results:
In this study, we found that both As2O3 and resveratrol can effectively inhibit cell proliferation and promote cell apoptosis in colon cancer, and the combined effect of the two drugs on colon cancer cells is more preeminent. The combination of As2O3 with resveratrol, on the one hand reduced the expression of hERG channels on the membrane, and on the other hand weaken the binding between hERG and integrin β 1, which may be the main cause of downstream signaling pathways alterations, including the activation of the apoptotic pathway.
Conclusion:
Taken together, hERG, as a subunit of potassium ion channel on the cell membrane, is highly likely to be involved in the As2O3 and resveratrol induced intracellular signaling cascade disorder, and this novel signaling pathway that sustains the progression of colon cancer may be a promising therapeutic target for human colon cancer treatment in the future.
Keywords: hERG, β1 integrin, proliferation, apoptosis, colon cancer, resveratrol
1. INTRODUCTION
Cancer is a heterogeneous disease with an accumulation of intracellular signaling pathway disorders. A range of molecular alterations ultimately determine the phenotypes and tumor progression [1]. Pathological examinations such as immunohistochemical (IHC) analyses have uncovered the molecular and morphological diversity within tumors, providing a basis for the treatment of cancers and a guideline for studying human cancers. The proteins associated with the malignant phenotype of tumor cells are the ideal targets for molecular targeted therapy. Unlike traditional cytotoxic chemotherapy, molecular targeted therapy has specific and efficacious antitumor effects, and the toxicity is significantly reduced, creating a new area of cancer chemotherapy [2]. Therefore, the discovery of new cancer therapeutic targets may identify novel treatment strategies for molecular targeted therapy in clinical practice.
Potassium channels are the most widely distributed type of ion channels on cell membranes. Evidence is increasing to support the hypothesis that, by triggering and modulating intracellular signaling cascades, potassium ion channels control biological activities in various types of human cancers [3-5]. In particular, the α-subunits of IKr encoded by the human ether-a-go-go-related gene (hERG) are often aberrantly expressed in human cancers [6, 7]. However, the roles of hERG and its potential downstream signaling cascades in the progression of tumors are not well established.
It has been reported that the inhibition of Kv11.1/hERG1 currents contributes to overcoming cisplatin resistance in colorectal cancer cells, which means that targeting hERG channels could increase the sensitivity and efficacy of certain anticancer drugs [8]. Fortunato et al. found that hERG expression levels are associated with cell proliferative and tumorigenic abilities [9]. The documented involvement of hERG in biological processes in cancers suggests that targeting hERG may be a putative strategy for the treatment of cancers [10-12]. According to the screening of our previous experiments and the previous literature [8, 9, 13], the colon cancer cell line HCT116 was selected as the research target herein due to its high expression level of hERG channels.
Traditional Chinese medicines have been proven by a large number of documents to have effective antitumor effects, either alone or in combination with other anticancer medications [14, 15]. As2O3 (ATO) and resveratrol (RES) are two common drugs studied by our research group, especially their roles in arrhythmia [16, 17]. As the main ingredient of arsenic, As2O3 is an effective drug for the treatment of acute promyelocytic leukemia (APL) and has been proven to be an effective drug for the treatment of certain solid tumors [18]. Resveratrol shows a broad spectrum of anti-inflammatory effects and even has an anticancer effect [19]. Previous studies have shown that the effects of As2O3 and resveratrol are closely associated with the function and expression of hERG channels in hERG-HEK293 cells and neonatal rat cardiomyocytes [16, 17]. Thus, in the present study, we aimed to explore the possible effects of As2O3 and resveratrol, alone or in combination, on the phenotypes of the colon cancer cell line HCT116, especially with respect to cell proliferation and apoptosis. In addition, the potential underlying mechanisms, particularly the role of hERG1-mediated signaling cascades in tumor progression, are further elucidated in our present study.
2. MATERIALS AND METHODS
2.1. Cells Culture
The human colon carcinoma cell line HCT116 was purchased from the Shanghai Institute of Cell Biology, Chinese Academy of Sciences (Shanghai, China). The cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS, HyClone, Logan, UT, United States of America) at 37°C and exposed to an atmosphere of 5% CO2.
2.2. Protein Extraction and Western Blotting
Briefly, cells were lysed in radioimmunoprecipitation assay (RIPA, Beyotime, Shanghai, PRC) buffer, followed by centrifugation at 4°C for 15 min at 13,500 rpm to remove debris. The total protein concentrations were determined by a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). After loading, the buffer was added (Beyotime, Shanghai, China), and the samples were boiled and separated using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Then, the proteins were transferred to a polyvinylidene fluoride (PVDF) membrane, which was subsequently incubated with 5% nonfat milk (Becton Dickinson Co. USA) for 2 h at room temperature and then probed with antibodies against hERG1 (Santa Cruz, USA), integrin β1 (Abcam, Cambridge, United Kingdom), Bcl-2 (Abcam, Cambridge, United Kingdom), and Bax (Abcam, Cambridge, United Kingdom) overnight at 4°C on a shaker. The membranes were washed thrice with 0.05% PBS plus Tween (PBST) and incubated with secondary antibodies (Molecular Probes) for 1 h in the dark at room temperature. Finally, the bands in the membranes were detected with an Odyssey instrument (Li-COR, American Gene Corp.), and Image Studio (Li-COR, American Gene Corp.) was used to analyze and quantify the density of the bands.
2.3. Immunoprecipitation
Cells in different groups were lysed in RIPA buffer, followed by centrifugation at 4°C for 15 min at 13,500 rpm to remove debris. After the protein concentrations were measured, 1.0 mg of protein in each group was incubated with 2.0 μg of the appropriate primary antibody overnight at 4°C with slight shaking. Then, 30 μL of protein A/G PLUS agarose beads (Santa Cruz, USA) was added to the mixtures of proteins and antibodies and incubated overnight at 4°C with slight shaking. Subsequently, the beads were washed 2-3 times with ice-cold TBST buffer. Finally, 150 μl of 1×loading buffer was added, and the immunoprecipitation samples were boiled for 8 min, followed by centrifugation at 4°C for 5 min at 1500 rpm. The supernatants were collected for western blot analysis.
2.4. MTT Assay
HCT116 and A549 cells were cultured to 80% confluence. Then, the cells were digested with trypsin, and a single cell suspension was prepared. Cells were inoculated into a 96-well plate (5×103 cells per well) and incubated overnight in RPMI 1640 medium supplemented with 10% FBS. The next day, the cells were treated with different drugs. After 24 h, the proliferation of cells was determined using MTT (Beyotime, Shanghai, China) according to the manufacturer’s instructions. Briefly, 10 μl of MTT (5 mg/ml) was added to each well and incubated for 4 h at 37°C. Then, the crystals in the test well were dissolved in 100% DMSO (20 μl/well). The absorbance was measured at a wavelength of 570 nm.
2.5. EdU (5-Ethynyl-2'-Deoxyuridine) Staining
Cell proliferation was also determined by using a Cell-LightTM EdU Apollo 488 In Vitro Imaging kit (C10310-3, RiboBio Co., Ltd, Guangzhou, China) according to the manufacturer's instructions. Briefly, HCT116 cells were seeded in 96-well plates (3,000 cells/well) one day prior to treatment. Following drug treatment, HCT116 cells were incubated with 50 µM EdU for 2 h at room temperature after fixation and permeabilization. Afterward, the cell nuclei were stained with Hoechst 33342 for 15 min. The images were obtained with a fluorescence microscope (Olympus, Japan).
2.6. Flow Cytometry
HCT116 cells in the logarithmic growth phase were trypsinized using EDTA-free trypsin (Beyotime, Shanghai, China) and centrifuged at 1500 rpm for 5 min. Then, 1×106 cells were stained with 10 μl of annexin V/FITC according to the manufacturer’s protocol. Finally, the cells were incubated in 150 μl of binding buffer containing 10 μl PI at room temperature for 10 min. Apoptosis was detected using a BD FACS Calibur flow cytometer (BD Biosciences, USA). The percentage of apoptotic cells was calculated using FlowJo software.
2.7. In vivo Tumor Xenograft Study
All animal experiments were conducted in accordance with the ethical standards and national guidelines and approved by the Ethics Committee of Harbin Medical University (Harbin, China). Four-week-old BALB/c-nu/nu nude mice with randomized gender were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). A total of 24 mice weighing 20-25 g each were maintained under standard conditions (a 12-h light/dark cycle at 20.0 to 27.0°C and 30% to 60% humidity). HCT116 cells (2x106) were subcutaneously injected into the flank of each mouse. Following the outgrowth of palpable tumors, 24 mice were randomly divided into four groups (n=6 per group) and received saline, ATO (1.5 mg/kg), RES (25 mg/kg) or ATO (1.5 mg/kg) plus RES (25 mg/kg) every 2 days. Tumor volumes were calculated using the following formula: Volume= L x(W)2/2 (L refers to the longest diameter, and W refers to the shorter diameter). At the end of the study, mice were euthanized, and tumor tissues were excised for further research.
2.8. Statistical Analysis
All statistical analyses were performed with GraphPad Prism 5.0 software. All data were expressed as the mean ± standard error of the mean (SEM). One-way analysis of variance (ANOVA) followed by Student-Newman-Keuls post hoc test was used for comparisons among multiple groups. Pairwise comparisons between two groups were conducted with Student’s t-test. A P value < 0.05 was considered to indicate statistical significance.
3. RESULTS
3.1. The Combination of ATO with RES Enhances the Inhibition of Cancer Cell Proliferation
To determine the effect of ATO in combination with RES on the proliferation of HCT-116 colon cancer cells and A549 lung adenocarcinoma cells, HCT-116 and A549 cells were first treated with various concentrations of ATO (1, 3, and 5 μM/L) or RES (25, 50, and 100 μM/L). The results of the MTT assay (Fig. 1) illustrated that ATO could inhibit the proliferation of both HCT-116 (Fig. 1A) and A549 cells (Fig. 1D) in a dose-dependent manner. RES exerted a dual effect on HCT-116 cells, promoting proliferation at lower concentrations (25, 50 μM/L) but inhibiting proliferation at high concentrations (100 μM/L). In addition, RES reduced the growth capacity of A549 cells in a non-concentration-dependent manner (Fig. 1E). In contrast, following administration of ATO (3 μM/L) in combination with RES (100 μM/L), the viability of both HCT-116 and A549 cells was significantly reduced compared with the application of ATO or RES alone. These results collectively indicate that ATO and RES combination chemotherapy may be more effective in treating cancer than ATO or RES individually. Given that HCT-116 cells were more sensitive than A549 cells to the combination therapy, we chose HCT116 cells as the research model for the subsequent experiments.
Fig. (1).
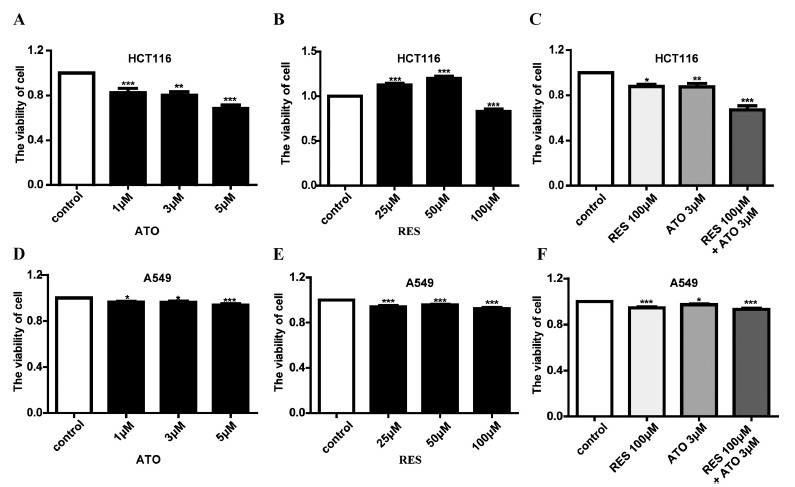
ATO in combination with RES suppresses HCT116 and A549 cell proliferation. (A) ATO inhibited HCT116 cell viability in a dose-dependent manner. (B) RES exhibits a dual effect on the viability of HCT116 cells, depending on the drug concentrations. (C) ATO in combination with RES suppresses HCT116 cell proliferation. (D) The viability of A549 cells inhibited by different concentrations of ATO. (E) The viability of A549 cells inhibited by different concentrations of RES. (F) ATO in combination with RES suppresses A549 cell proliferation. Data are shown as the mean ± SEM of three independent experiments; *P<0.05, **P<0.01, and ***P<0.001 vs. control.
The inhibitory effect of the combination of ATO with RES on the proliferation of HCT116 was further confirmed by the EdU experiment. The results (Fig. 2) revealed that the proliferation of HCT116 cells was significantly decreased in the presence of ATO or RES, and this reduction was more pronounced in the presence of ATO and RES, as indicated by the reduced green staining. These observed phenomena are consistent with the results of cell proliferation as measured by MTT.
Fig. (2).
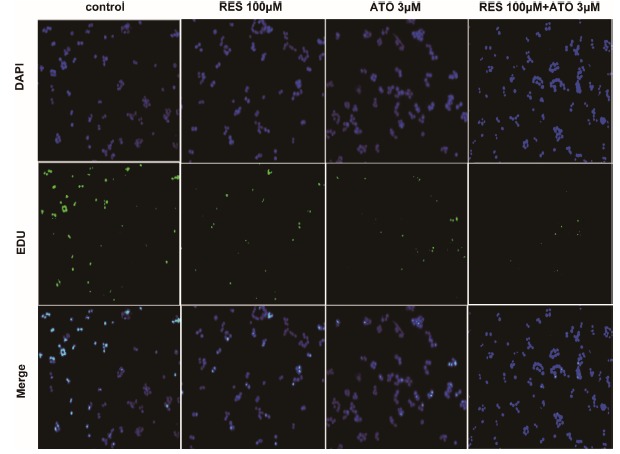
HCT116 proliferation determined by EDU assay. Representative images of HCT116 cell proliferation detected by EDU assay.
3.2. ATO in Combination with RES Increases Apoptosis
Dysregulated apoptosis is one of the prominent hallmarks of tumor cells; therefore, we performed flow cytometry with annexin V/PI to examine the apoptosis rate of HCT116 cells in the presence of different drugs. As shown in Figs. 3A-D, treatment with ATO (3 μM/L) or RES (100 μ M/L) resulted in a significantly increased apoptotic rate in HCT116 cells compared with the blank control cells, while the effect of the combination of ATO with RES on apoptosis of HCT116 cells was more pronounced than the application of ATO or RES alone.
Fig. (3).
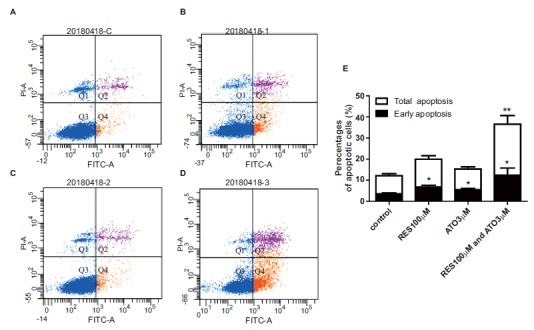
HCT116 apoptosis determined by flow cytometry assay. (A-D) Representative images of HCT116 apoptosis detected by flow cytometry assay. (E) Corresponding statistical results of the apoptosis assay. Data are shown as the mean ± SEM of three independent experiments, *P<0.05 vs. control.
3.3. Involvement of hERG/integrin β1 in the Survival and Apoptosis of HCT116 Cells
hERG is known to be involved in the progression of various tumors. To obtain further insight into the underlying mechanisms in regard to the treatment of cancers with chemotherapy drugs, we examined the changes in hERG expression in the presence of ATO, RES or ATO combined with RES. Notably, the expression of hERG was positively correlated with the proliferation rate of HCT116 cells (Figs. 4A-C), which is consistent with previous reports that hERG promotes tumorigenesis in colon cancer [20]. The protein levels of hERG were significantly reduced by ATO (1, 3, and 5 μM/L) or RES (100 μM/L), and was further decreased by coadministration of ATO (3 μM/L) with RES (100 μM/L).
Fig. (4).
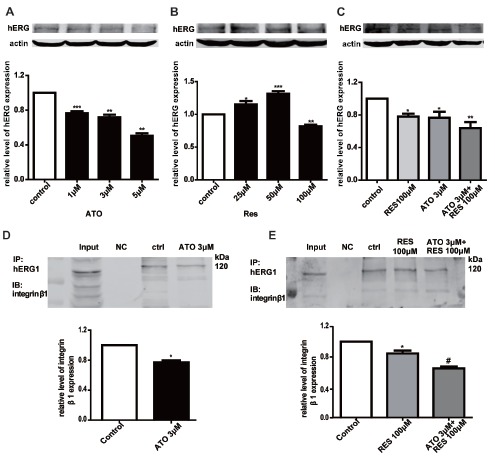
The alteration of the hERG/integrin β1 complex signaling pathway. (A-C) Effect of different concentrations of ATO, RES and their combination on hERG protein expression. (D) The effects of ATO on the interactions of hERG proteins with integrin β1 detected by immunoprecipitation. Representative immunoprecipitation bands are shown. (E) The effects of RES and the combination of RES with ATO on the interactions of hERG proteins with integrin β1 detected by immunoprecipitation. Representative immunoprecipitation bands are shown.
Considering that the hERG channel is mainly distributed on the surface of the cell membrane, we asked whether signals produced by extracellular stimuli, such as the application of drugs, could be transmitted into the cell through the hERG channel-dependent cascade. Additionally, it has been reported that hERG and integrin β1 can both coprecipitate with caveolin-1, suggesting that hERG and integrin β1 may colocalize near the cell membrane [21]. Thus, we conducted an immunoprecipitation assay to explore the effects of ATO and RES on the interaction between hERG and integrin β1 in hERG/HEK-293 cells, which is the cell model commonly used by our research group. As depicted in Figs. 4D-E, the hERG/integrin β1 complex was significantly reduced by ATO (3 μ M/L) or RES (100 μM/L), while the reduction became more apparent in the presence of ATO (3 μ M/L) with RES (100 μ M/L). These results collectively indicate that the alterations in HCT116 tumor cell phenotypes caused by ATO and RES may be mediated through the hERG/integrin β1 complex signaling pathway.
3.4. The Apoptotic Mechanism of ATO and RES in HCT116 Cells
To further determine the possible downstream targets of the hERG/integrin β1 complex that mediate the apoptosis of HCT116 cells, we examined alterations in the expression of the apoptosis-associated proteins Bcl-2 and Bax, which have been reported to be downstream targets of integrin β1 signaling. The expression levels of the antiapoptotic protein Bcl-2 and the proapoptotic protein Bax in HCT116 cells were analyzed by western blot analysis. ATO (3 μM/L) or RES (100 μM/L) decreased the abundance of Bcl-2 compared with the control (Figs. 5A-B) and enhanced the expression of Bax. The alterations in the ratio of Bcl-2/Bax (Fig. 5C) were more
Fig. (5).
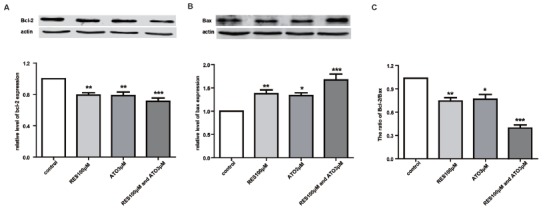
ATO in combination with RES activates the apoptotic pathway. (A-B) Effect of ATO, RES and their combination on Bcl-2 and Bax protein expression. (C) The statistical column chart represents the ratio of Bcl-2/Bax in different groups. *P<0.05, **P<0.01, and ***P<0.001 vs. control.
pronounced when ATO (3 μM/L) was coapplied with RES (100 μM/L).
3.5. The Combination of ATO with RES Enhances the Inhibition of Tumor Growth In vivo
To validate the therapeutic effect of ATO combined with RES on colon cancer cell growth in vivo, we established a xenograft tumor mouse model. Following the outgrowth of palpable tumors, we intraperitoneally injected tumor-bearing mice with ATO (1.5 mg/kg), RES (25 mg/kg) or ATO (1.5 mg/kg) combined with RES (25 mg/kg). During the four weeks of treatment, the volume of the tumors was measured twice a week, and the weight of the tumors was obtained after the mice were euthanized. Fig. 6A shows the tumor tissues pictures stripped from tumor-bearing mice treated with different drugs. As depicted in Fig. 6, the volume (Fig. 6B) and weight (Fig. 6C) of the tumors were significantly reduced by ATO (1.5 mg/kg) or RES (25 mg/kg), while the combination of ATO (1.5 mg/kg) with RES (25 mg/kg) further enhanced the inhibitory effect on colon tumor growth.
Fig. (6).
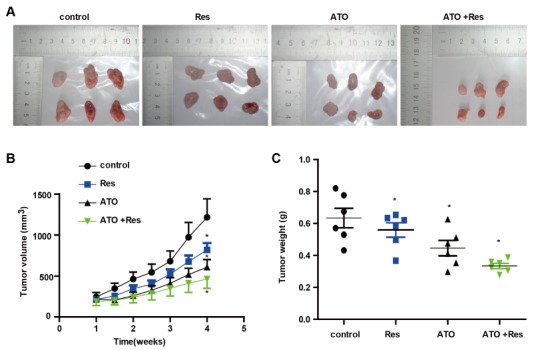
ATO in combination with RES increases the inhibition of tumor growth in vivo. (A) Representative images of tumors formed by HCT116 cells in the tumor xenograft model. (B) Tumor volume measured at different time points in different groups. (C) Tumor weight was measured after the mice were sacrificed. *P<0.05 vs. control.
4. DISCUSSION
Currently, the main cancer treatments include surgery, chemotherapy, radiotherapy, and combinations of these methods. Traditional Chinese medicines or their ingredients have also shown strong antitumor efficacy. If the underlying mechanisms of these agents in the treatment of cancers are explored more extensively, the application of these agents for targeted therapy may become a possibility in clinical practice. Therefore, it would be extremely beneficial for chemotherapeutic treatment to explore the molecular mechanisms of reliable alternative agents, either alone or in combination.
As2O3 (ATO) and resveratrol (RES) are two common drugs widely studied in terms of antitumor activity [18, 19]. Although reports have demonstrated the antitumor effect and the corresponding molecular alterations of these two agents, we sought to identify a novel signaling pathway that could provide an alternative explanation for the antitumor effect of ATO and RES by investigating the ion channel-mediated pathway. Previous studies have shown that targeting hERG potassium channels could increase the sensitivity of anticancer drugs [8], and hERG is closely related to cell proliferation and tumorigenic abilities [9]. In the present study, we demonstrated that the proliferation inhibition of HCT116 colon cells elicited by ATO or RES was enhanced by the coadministration of ATO with RES. For the investigation of apoptosis, we confirmed that both ATO and RES could promote the apoptosis of HCT116 colon cells, which was more prominent in the presence of both ATO and RES. To explore the underlying mechanisms, we examined the alterations in the expression of hERG channels and found that ATO or RES reduces the protein level of hERG channels, which was further reduced by the application of both ATO and RES. In addition, the combination of ATO and RES greatly reduced the binding between hERG and integrin β 1, which possibly eventually leading to alterations in the levels of apoptosis-related proteins. In vivo experiments showed that the combined use of ATO with RES effectively suppressed the growth of colon cancer cells, which was consistent with the in vitro results.
CONCLUSION
In conclusion, the present study demonstrates that the treatment of cancer cells with the combination of ATO with RES is significantly more effective than the application of ATO or RES alone. This finding is partially attributable to the downregulation of the potassium ion channel hERG. Consequently, the binding between the hERG channel and integrin β 1 is reduced, and eventually the balance of the intracellular signaling pathway is disrupted, leading to the activation of an abnormal apoptotic signaling pathway.
Our studies suggest an alternative approach to understanding the role of potassium ion channels in cancer development and drug efficacy, which may provide a novel strategy for colon cancer chemotherapy. Further studies are needed to determine the clinical relevance of ATO in combination with RES in the future.
ACKNOWLEDGEMENTS
Declared none.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
All the experiments were approved by the Ethics Committee of Harbin Medical University, Harbin, China.
HUMAN and Animal RIGHTS
No humans were used. All animal experiments were conducted in accordance with the ethical standards and national guidelines.
CONSENT for PUBLICATION
Not applicable.
AVAILABILITY of Data and MATERIALS
Not applicable.
FUNDING
This work was supported by grants from the National Natural Science Foundation of China (81673636, 81173050) and UNPYSCT-2016186.
CONFLICT of INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Eguether T., Hahne M. Mixed signals from the cell’s antennae: primary cilia in cancer. EMBO Rep. 2018;19(11):e46589. doi: 10.15252/embr.201846589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babiker H.M., McBride A., Newton M., et al. Cardiotoxic effects of chemotherapy: A review of both cytotoxic and molecular targeted oncology therapies and their effect on the cardiovascular system. Crit. Rev. Oncol. Hematol. 2018;126:186–200. doi: 10.1016/j.critrevonc.2018.03.014. [DOI] [PubMed] [Google Scholar]
- 3.Xia J., Wang H., Li S., et al. Ion channels or aquaporins as novel molecular targets in gastric cancer. Mol. Cancer. 2017;16(1):54. doi: 10.1186/s12943-017-0622-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pardo L.A., Stühmer W. The roles of K(+) channels in cancer. Nat. Rev. Cancer. 2014;14(1):39–48. doi: 10.1038/nrc3635. [DOI] [PubMed] [Google Scholar]
- 5.D’Amico M., Gasparoli L., Arcangeli A. Potassium channels: novel emerging biomarkers and targets for therapy in cancer. Recent Patents Anticancer Drug Discov. 2013;8(1):53–65. doi: 10.2174/1574892811308010053. [DOI] [PubMed] [Google Scholar]
- 6.Arcangeli A., Becchetti A. hERG Channels: From antitargets to novel targets for cancer therapy. Clin. Cancer Res. 2017;23(1):3–5. doi: 10.1158/1078-0432.CCR-16-2322. [DOI] [PubMed] [Google Scholar]
- 7.Jehle J, Schweizer PA, Katus HA, Thomas D. Novel roles for hERG K(+) channels in cell proliferation and apoptosis. Cell Death Dis. 2011:2e193.. doi: 10.1038/cddis.2011.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pillozzi S., D’Amico M., Bartoli G., et al. The combined activation of KCa3.1 and inhibition of Kv11.1/hERG1 currents contribute to overcome Cisplatin resistance in colorectal cancer cells. Br. J. Cancer. 2018;118(2):200–212. doi: 10.1038/bjc.2017.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fortunato A. The role of hERG1 ion channels in epithelial-mesenchymal transition and the capacity of riluzole to reduce cisplatin resistance in colorectal cancer cells. Cell Oncol. (Dordr.) 2017;40(4):367–378. doi: 10.1007/s13402-017-0328-6. [DOI] [PubMed] [Google Scholar]
- 10.Lastraioli E., Lottini T., Bencini L., Bernini M., Arcangeli A. hERG1 Potassium Channels: Novel Biomarkers in Human Solid Cancers. BioMed Res. Int. 2015;2015:896432. doi: 10.1155/2015/896432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patanè S. HERG-targeted therapy in both cancer and cardiovascular system with cardiovascular drugs. Int. J. Cardiol. 2014;176(3):1082–1085. doi: 10.1016/j.ijcard.2014.07.129. [DOI] [PubMed] [Google Scholar]
- 12.Babcock J.J., Li M. hERG channel function: beyond long QT. Acta Pharmacol. Sin. 2013;34(3):329–335. doi: 10.1038/aps.2013.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gong J.H., Liu X.J., Shang B.Y., Chen S.Z., Zhen Y.S. HERG K+ channel related chemosensitivity to sparfloxacin in colon cancer cells. Oncol. Rep. 2010;23(6):1747–1756. doi: 10.3892/or_00000820. [DOI] [PubMed] [Google Scholar]
- 14.Liao Y.H., Li C.I., Lin C.C., Lin J.G., Chiang J.H., Li T.C. Traditional Chinese medicine as adjunctive therapy improves the long-term survival of lung cancer patients. J. Cancer Res. Clin. Oncol. 2017;143(12):2425–2435. doi: 10.1007/s00432-017-2491-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong R., Sagar C.M., Sagar S.M. Integration of Chinese medicine into supportive cancer care: a modern role for an ancient tradition. Cancer Treat. Rev. 2001;27(4):235–246. doi: 10.1053/ctrv.2001.0227. [DOI] [PubMed] [Google Scholar]
- 16.Chu W., Li C., Qu X., et al. Arsenic-induced interstitial myocardial fibrosis reveals a new insight into drug-induced long QT syndrome. Cardiovasc. Res. 2012;96(1):90–98. doi: 10.1093/cvr/cvs230. [DOI] [PubMed] [Google Scholar]
- 17.Yan M., Feng L., Shi Y., et al. Mechanism of As2O3-induced action potential prolongation and using hiPS-CMs to evaluate the rescue efficacy of drugs with different rescue mechanism. Toxicol. Sci. 2017;158(2):379–390. doi: 10.1093/toxsci/kfx098. [DOI] [PubMed] [Google Scholar]
- 18.Kayser S., Schlenk R.F., Platzbecker U. Management of patients with acute promyelocytic leukemia. Leukemia. 2018;32(6):1277–1294. doi: 10.1038/s41375-018-0139-4. [DOI] [PubMed] [Google Scholar]
- 19.Huminiecki L., Horbańczuk J. The functional genomic studies of resveratrol in respect to its anti-cancer effects. Biotechnol. Adv. 2018;36(6):1699–1708. doi: 10.1016/j.biotechadv.2018.02.011. [DOI] [PubMed] [Google Scholar]
- 20.Lastraioli E., Guasti L., Crociani O., et al. herg1 gene and HERG1 protein are overexpressed in colorectal cancers and regulate cell invasion of tumor cells. Cancer Res. 2004;64(2):606–611. doi: 10.1158/0008-5472.CAN-03-2360. [DOI] [PubMed] [Google Scholar]
- 21.Cherubini A., Hofmann G., Pillozzi S., et al. Human ether-a-go-go-related gene 1 channels are physically linked to beta1 integrins and modulate adhesion-dependent signaling. Mol. Biol. Cell. 2005;16(6):2972–2983. doi: 10.1091/mbc.e04-10-0940. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


