Abstract
Backgroud:
The present study aimed to investigate the association between immune cells and gestational diabetes mellitus (GDM) and identify a reasonable predictor of insulin resistance in women with GDM.
Objective:
The clinical and biochemical characteristics of 124 women with GDM and 168 healthy pregnant women were compared.
Methods:
The percentage of immune cells in the blood of the subjects was analyzed by flow cytometry. Pearson’s correlation analysis revealed the correlation between the percentage of B lymphocytes and insulin resistance. A cutoff point was determined for the percentage of B lymphocytes, based on insulin resistance, using receiver operating characteristic (ROC) curves.
Results:
Compared to the healthy pregnant women, the percentages of B lymphocytes and IgA produced by B-cells were significantly different in women with GDM. The percentage of B lymphocytes was positively related to insulin resistance.The number of 14.05% of B lymphocytes was an optimal cutoff point that predicted the insulin resistance in women with GDM.
Conclusion:
The percentage of B lymphocytes was positively associated with insulin resistance, and hence, might serve as an appropriate predictor of insulin resistance in women with GDM.
Keywords: B lymphocytes, insulin resistance, inflammation, GDM, immune, IgA, flow cytometry
1. INTRODUCTION
Gestational Diabetes Mellitus (GDM) is regarded as a pregnancy complication that is characterized by hyperglycemia. Due to differences in geographic regions, ethnic groups, and diagnostic criteria, the incidence of GDM varies greatly from 0.6-15% [1]. The risk factors for GDM include a high pre-gestational Body Mass Index (BMI), a sedentary lifestyle, older age during pregnancy, and heredity [2]. GDM threatens the health of pregnant women and their offspring. Pregnant women who present GDM are prone to hypertension, pre-eclampsia, preterm delivery and Type 2 Diabetes Mellitus (T2DM) after delivery [3]. In addition, the risk of pregnant women with GDM suffering from the cardiovascular disease is much higher than that of non-diabetic women. On the other hand, due to hyperglycemiea, the incidence of macrosomia and the consequent neonatal injury and the rate of cesarean section become higher. The fetus is at a high risk of hypoglycemia, pulmonary hypoplasia, and neonatal respiratory distress syndrome. Furthermore, it enhances the risk of having offspring who suffer from T2DM. Accordingly, several studies have shown that GDM is an early phase of T2DM and some groups even regard GDM as T2DM during the period of pregnancy [4]. However, little is known about the etiology of GDM, which has been a widely discussed topic since a prolonged duration. During the earlier months of pregnancy, the fasting blood glucose is usually low. With the increase of gestational age, the level of insulin resistance grows higher as a result of hormones produced by placenta [5]. Some people even suffer from the dysfunction of β-cells in pancreatic island. Although the exact pathogenesis of GDM is unclear presently, it has been established that GDM results from insulin resistance and a progressive β-cell dysfunction [6].
Rencently, several pieces of evidence suggest that chronic low-grade inflammation is a major factor in the etiology of insulin resistance, and immune system disorders play a key role during the development of inflammation. T-cells, B-cells, macrophages, and eosinophils are involved in abnormal immune regulation [7]. Much attention has been paid on the role of T lymphocytes and macrophages in immune regulation of insulin resistance. Studies have demonstrated that some CD8+ and CD4+ T cells can infiltrate inflamed visceral adipose tissue (VAT) and activate M1 macrophages, resulting in the production of a series of cytokines, such as TNF-αand IL-6,which contribute to both local and systemic insulin resistance [8, 9].
In fact, apart from T cells and macrophages, B lymphocytes also take an important part in many chronic inflammatory and autoimmune diseases. Previous studies have suggested that B-cells infiltrate the visceral adipose tissue (VAT) before T-cells and regulate insulin resistance, together with neutrophils, granulocytes, and M1 macrophages. In mice fed high-fat diet, elevated B-cells and group transformation were detected, which are critical signs of the activation of an immune reaction [10]. The adaptive immune reaction involving B-cells regulates the development of obesity and insulin resistance. B-cells affect the activities of T-cells and the level of insulin resistance not only via the modulation of cytokines, including IL-6, IL-10, and leptin but also through the production of antibodies [11].
The production of antibodies is the fundamental function of B lymphocytes. The antibodies produced by B-cells promote the formation and development of inflammatory diseases, such as rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE) [11]. Furthermore, several studies demonstrated that obesity and hyperglycemia could directly affect the production of antibodies and group transformation. The production of antibodies modulates the level of insulin resistance and vice versa [12]. Some studies suggested that IgG antibody levels in obese mice are much higher than those in lean mice. These IgG antibodies might regulate the obesity-related insulin resistance by modulating the function of macrophages in VAT [10, 13].
Hitherto, a majority of the data describing the impact of immune cells on the regulation of insulin resistance have been obtained from animal experiments. Only a few reports have stated the role of immune cells in the modulation of insulin resistance in human organs and tissue. Herein, we selected the cells from the blood of pregnant women with and without GDM by flow cytometry and analyzed the data. A significant difference in the percentage of B-cells was observed between women with GDM and normal controls. Among the antibodies produced by B-cells, only IgA differed between the two groups. Pearson’s correlation analysis demonstrated that the percentage of B-cells was positively correlated with the level of insulin resistance. Receiver operating characteristic (ROC) curve analysis suggested that a cutoff point of 14.05% for B cells could accurately and sensitively distinguish the biochemical indicators of women with GDM from those with normal glucose tolerance. In summary, the percentage of B lymphocytes was positively associated with insulin resistance, and this value might serve as a reasonable predictor of insulin resistance in pregnant women with GDM. Our elementary study has disclosed the correlation between the percentage of B lymphocytes and GDM, thus laid the ground work for the further research on the immunological pathogenesis involving B lymphocytes, which is believed to be meaningful in reducing adverse pregnancy outcomes and promoting the health of both pregant women and their offspring.
2. MATERIAL AND METHODS
2.1. Object
A total of 124 pregnant women with GDM and 168 non-diabetic pregnant women from the Department of Obstetrics, Shanghai General Hospital, were randomly enrolled in the current study from January to March 2016. The diagnositic criteria of IADPSG (International Association of Diabetes and Pregnancy Study Group) was used in our study. The cutoff values of 75g oral glucose tolerance test was 5.1mmol/l for fasting plasma glucose, 10.0mmol/l for 1h plasma glucose and 8.5mmol/l for 2h plasma glucose. And the patients with PGDM (pregestational diabetes mellitus) were excluded from this study, including women who have been diagnosed with DM (T1DMor T2DM) before pregnancy or whose FPG (fasting plasma glucose)≥7.0mmol/l or 2h PBG (2-hour postprandial blood glucose)≥11.1mmol/l or RBG (random blood glucose)≥11.1mmol/l or HbA1c (glycated hemoglobin) ≥6.5% during pregnancy.
2.2. Blood Test Indicators
All blood tests were performed by trained practitioners in a clinical laboratory at the Shanghai General Hospital. After the participants had fasted for 8 h, venous blood was withdrawn, and indicators including white blood cells, blood platelets, triglycerides, sterol, HbA1c, glycated albumin (GA), and lactate dehydrogenase (LDH) were evaluated.
2.3. Flow Cytometry for In vitro Diagnosis (IVD)
2.3.1. For Lymphocytes Subsets
A 50 µL aliquot of whole blood was collected in each of the two tubes: one for T-cell and the other for B- and NK-cells, followed by the addition of 20 µL four-color reagent CD3-FITC/CD8-PE/CD45-PerCP/CD4-APC (2014060100 for IVD, Mindray Bio-Medical Electronics Co. Ltd., Shenzhen, China) to the T-cells and 20 µL CD3-FITC/ CD16+56-PE/CD45-PerCP/CD19-APC (2014060121 for IVD, Mindray) to B- and NK-cells. The antibodies and the whole blood were incubated for 15 min in the dark, with gentle agitation at room temperature, followed by addition of 450 µL 1× Hemolysins (M-30PCFL, Mindray) to both tubes and incubated for 5 min at room temperature with the soft vortex. Finally, these tubes were placed on a Bricyte E6 (Mindray) after calibration by SpheroTM Supra Rainbow Midrange Fluorescent Particles (Spherotec, No. SRCP-35-2A, USA). Figures and analyses were performed using the FACSDIVA 6.1 software (BD Bioscience, San Joes, CA, USA) [14, 15].
2.3.2. For Immunoglobulin Detection
Immunoglobulins (IgG, IgE, IgA, and IgM) were detected by the Immunoglobulin Quantification Kit (Mindray China) according to the manufacturer’s instructions using SAL 8000 equipment (Mindray) [16].
2.4. Statistical Analysis
Data were analyzed using SPSS 20.0. A two-sided test with p<0.05 was considered as statistically significant. The differences between the GDM and non-GDM groups were calculated using a chi-squared test. Pearson’s correlation analysis revealed the correlations between the percentage of B lymphocytes and the biochemical measurements. The cutoff point for the percentage of B cells, based on insulin resistance, was estimated by the ROC curve. In the present study, we evaluated the level of insulin resistance using the homeostatic model assessment (HOMA-IR). The following equation was used to calculate the insulin resistance: HOMA-IR= [insulin (mU/L)] × [fasting plasma glucose (FPG) (mmol/L)]/22.5. The homeostatic model assessment of insulin sensitivity (HOMA-IS) was calculated to determine the function of β-cells based on the following equation: HOMA-IS=1/HOMA-IR.
3. RESULTS
3.1. Percentage of B Lymphocytes in the GDM Group was Higher than that in the Non-GDM Group
The PBMCs primarily included lymphocytes (T-cells, B-cells, and NK cells), monocytes, and dendritic cells. Moreover, lymphocytes constituted 70-90% of PBMCs and the frequencies of cell types within the lymphocyte population were as follows: 70-85% CD3+ T-cells (45-70% of PBMCs), 5-20% B-cells (up to 15% of PBMCs), and 5-20% NK cells (up to 15% of PBMCs). Using flow cytometry, we detected CD3+ T-cells, CD3-CD19+ B-cells, and CD3-CD16+CD56 NK cells in PBMCs in both GDM and non-GDM groups.
As shown in Fig. (1), the percentage of B-cells was significantly higher in the GDM group than that in the non-GDM group (13.52±0.36% vs. 10.54±0.42%, p<0.0001) and the percentage of NK cells was significantly lower (2.06±0.05% vs. 2.54±0.06%, p<0.0001). However, no differences were observed in the abundance of CD3+ T between the GDM and non-GDM groups (74.11±0.87% vs. 75.43%±0.42, p=0.13) (Fig. 1). The biochemical measurements and composition of blood cells were compared between the GDM and non-GDM groups, and the percentage of B lymphocytes in the GDM group was significantly higher than that in the non-GDM group (Table 1).
Fig. (1).
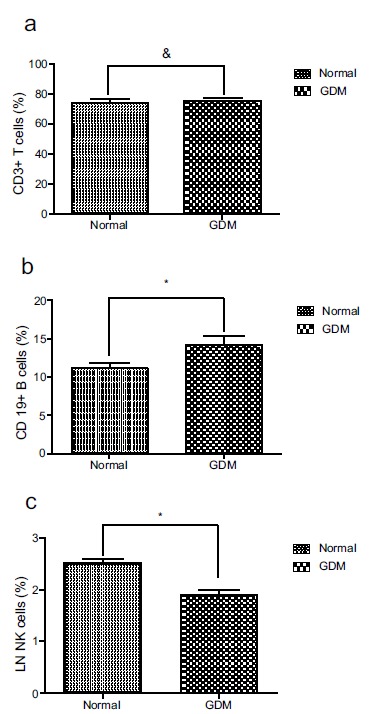
The percentage of CD19+ B cells and NK cells in peripheral blood are different in GDM women and normal pregnant women. The percentage of T cells, B cells and the LN-transferring of the percentage of NK cells fitted normal standard distribution. The differences between Normal and GDM were analyzed by unpaired t test (a). There is no difference of the percentage of CD3+ T cells between GDM and normal pregnant women. & p=0.13 (b). The percentage of CD19+ B cells is higher in GDM than that in normal pregnant women. * p<0.05 (c). The percentage of LN NK cells is lower in GDM than that in normal pregnant women. *p<0.0001.
Table 1. Demographic and biochemical features of the study subjects.
| Variable | Normal (n=168) | GDM (n=124) | p |
|---|---|---|---|
| Age (years) | 28.11±3.75 | 31.31±5.12 | 0.00a |
| HbA1c (%) | 4.97±0.27 | 5.82±0.79 | 0.00b |
| GA (%) | 12.77±1.23 | 13.56±2.15 | 0.02c |
| WBC (×109/L) | 10.02±2.11 | 8.41±2.07 | 0.00 |
| Neutrophils | 7.33±1.79 | 5.71±1.66 | 0.00 |
| Lymphocytes | 2.08±1.78 | 1.95±0.48 | 0.77d |
| Monocytes | 0.62±0.17 | 0.59±0.18 | 0.38e |
| Eosinophils | 0.11±0.09 | 0.12±0.09 | 0.48f |
| Basophils | 0.03±0.01 | 0.02±0.01 | 0.00g |
| Lymphocytes ratio | 19.72±4.73 | 23.85±5.40 | 0.00 |
| PLT (×1012/L) | 216.14±52.14 | 210.65±54.99 | 0.28 |
| Triglyceride (mmol/L) | 2.04±0.71 | 2.67±1.18 | 0.00h |
| Sterol (mmol/L) | 5.67±1.12 | 5.09±0.98 | 0.00 |
| LDH (U/L)i | 141.00±26.42 | 155.96±33.41 | 0.00 |
a. Logarithmic processing to the base 10 of data was used to correct the heterogeneity of this variance.
b. Cubic reciprocal values were performed to correct the inconsistent variance
c, h. Inverse square root was used to correct the inconsistent variance .
d, f, g.Natural logarithm was used to correct the heterogeneity of this variance.
e. Square root was used to correct the inconsistent variance.
HbA1c: Hemoglobin A1c ; GA: Glycated Albumin.
i. lactate dehydrogenase.
3.2. Level of IgA Antibodies Produced by B-cells in the GDM Group was Higher than that in the Non-GDM Group
As shown in Table 1, the clinical data indicated that the percentage of B cells in the GDM group was higher than that in the non-GDM group. Previous studies have shown that B-cells promote insulin resistance via modulation of other lymphocytes and the production of pathogenic antibodies. To further explore the differences and roles of B-cells in the two groups, we detected the level of immunoglobulins, such as IgG, IgE, IgA, and IgM, produced by B-cells; only the level of IgA differed between the two groups. The level of IgA was higher in the blood of the GDM group than that in the non-GDM group (Fig. 2).
Fig. (2).
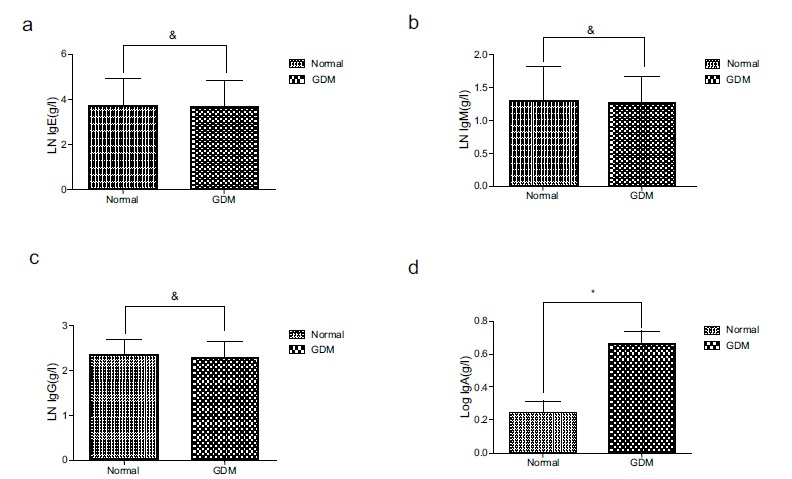
The level of IgA in peripheral blood in GDM is higher than that in normal pregnant women. For analyzation, these data were transformed into their natural logarithmic form (IgE, IgM and IgG) or common logarithmic form (IgA). (a). There is no statistical difference of the level of IgE between normal pregnant women and women presenting GDM. & p>0.05 (b). There is no statistical difference of the level of IgM between normal pregnant women and women presenting GDM. & p>0.05 (c). There is no statistical difference of the level of IgG between normal pregnant women and women presenting GDM. & p>0.05 (d). The level of IgA is higher in GDM women than that in normal pregnant women. * p<0.05.
3.3. B-cells and IgA were Independent Predictors of GDM
Since the B-cells and NK percentages, as well as the levels of IgA, differed between the GDM and non-GDM groups, we sought to determine whether the B-cell percentage and the IgA level could predict GDM by binary logistic
regression. Thus, we found that the B-cell percentage [OR=2.30, 95% confidence interval (CI): 1.42-3.71] and the IgA level (OR=0.02, 95% CI: 0.00-0.22) were independent predictors or risk factors of GDM (Table 2). Also, NK cells were another predictors or risk factors for GDM.
Table 2. Independent predictors of GDM in the binary logistic regression analysis.
| βa | pb | ORc | 95% CId(OR) | |
|---|---|---|---|---|
| T cell | 0.064 | 0.412 | 1.070 | 0.910-1.240 |
| NK | -0.731 | 0.002 | 0.480 | 0.300-0.770 |
| B cell | 0.832 | 0.001 | 2.300 | 1.420-3.710 |
| IgA | -6.279 | 0.010 | 0.002 | 0.000-0.220 |
| IgG | -0.693 | 0.706 | 0.500 | 0.010-18.390 |
| IgE | 0.012 | 0.966 | 1.010 | 0.570-1.800 |
| IgMe | 0.140 | .858 | 1.150 | 0.250-5.230 |
a. Regression coefficient.
b. Probability.
c. Odds rate.
d. Confidence interval.
e. Immunoglobin M.
Next, we performed a ROC curve analysis to determine an optimal cutoff point to distinguish the characteristics of
GDM from the non-GDM group. In the ROC analysis, the area under the curve (AUC) was 0.76 (95% CI: 0.69-0.83). In this study cohort, a cutoff value of 14.05% achieved a 58% sensitivity and 91% specificity, which suggested that the B-cell percentage could be a useful tool for GDM diagnosis (Fig. 3).
Fig. (3).
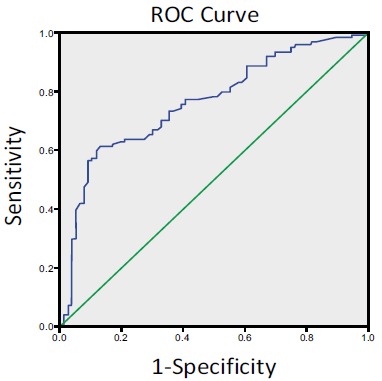
ROC analysis showing the percentage of B lymphocytes =14.05% as an optimal cutoff point for insulin resistance in women with GDM.
3.4. Percentage of B-cells was Positively Correlated with Insulin Resistance
HOMA is a method for assessing β-cell function, insulin resistance, and insulin sensitivity (IS) from basal (fasting) glucose and insulin or C-peptide concentrations. Pearson’s correlation analysis of B lymphocytes based on HOMA-IR, HOMA-IS, fasting insulin, triglyceride, and sterol values revealed that the percentage of B-cells was positively related to HOMA-IR, and the levels of fasting insulin, HbA1c, GA, and triglycerides but negatively correlated with HOMA-IS and sterol levels (Table 3, Fig. 4).
Table 3. Pearson correlation analysis of the percentage of B lymphocytes and the related biochemical measurements.
|
1/(Cubic
HbA1c) |
1/(Sqrt GA) |
Sqrt
HOMAIR |
1/(Sqrt HOMAIS) | 1/(Square FPG) |
Sqrt
Finsulin |
Sqrt FCT | Sterol | 1/(Triglycerid e) | |
|---|---|---|---|---|---|---|---|---|---|
| Pearson correlation | -.194* | -0.128 | 0.277 | 0.303 | -0.162 | .306** | 0.135 | -0.111 | 0.08 |
| Sig.(2- tailed) | .031 | 0.039 | 0.002 | 0.001 | 0.072 | .001 | 0.135 | 0.222 | 0.374 |
| N | 124 | 124 | 124 | 124 | 124 | 124 | 124 | 124 | 124 |
1/Cubic HbA1c: the cubic reciprocal value of HbA1c.
1/Sqrt GA: the inverse square root of GA.
Sqrt HOMAIR: the square root of HOMAIR.
1/(Sqrt HOMAIS): the inverse square root of of HOMAIS.
1/(Square FPG): the inverse square root of fasting plasma glucose.
Sqrt Finsulin: the square root of fasting insulin.
Sqrt FCT: the square root of fasting C-peptide.
Fig. (4).
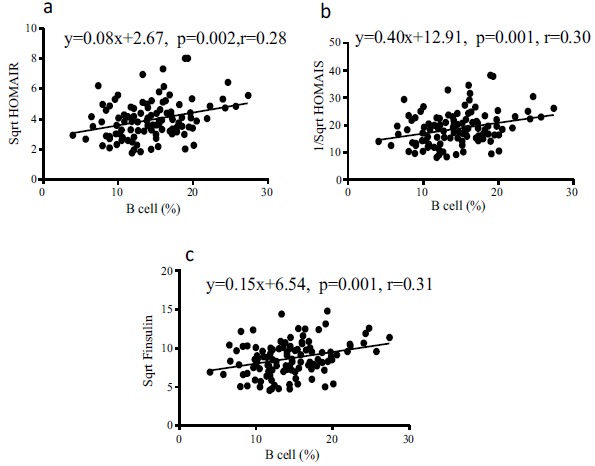
The percentage of B lymphocytes is positively correlated with insulin resistance and fasting insulin level but negatively correlated with the function of β cells of pancreatic islets. (a). The percentage of B lymphocytes is positively correlated with HOMAIR and the correlation coefficient is 0.28. (b). The percentage of B lymphocytes is positively correlated with the inverse square root of HOMAIS and the correlation coefficient is 0.30. In other words, the percentage of B cells is negatively correlated with HOMAIS itself. (c). The percentage of B lymphocytes is positively correlated with the square root of fasting insulin and the correlation coefficient is 0.31, which means the percentage of B cells is positively related with fasting insulin level itself.
3.5. Optimal Cutoff Point for the Percentage of B Lymphocytes Based on Insulin Resistance was 14.05%
Since the percentage of B-cells was positively correlated with insulin resistance, we used the ROC curve to define the optimal cutoff point for the percentage of B lymphocytes. The optimal cutoff value of the B-cell percentage was 14.05% (Fig. 3), which could be utilized to specifically and sensitively group the clinical and biochemical indicators, including the fasting insulin level, HbA1c, GA, insulin resistance, the function of insulin, and neonatal weight in the GDM group (Table 4). The B-cell-low and -high groups within the GDM group included 58 (46.78%) and 66 (53.22%) patients, respectively. To evaluate the relevance of B-cells to GDM-related characteristics, we assessed the differences in the baseline characteristics of the patients, based on different B-cell categories. Significant differences were observed between the B-cell-low and -high groups were with respect to the following continuous variables: fasting insulin (p=0.019), HbA1c% (p=0.024), GA% (p=0.024), HOMA-IR (p=0.036), HOMA-IS (p=0.022), and neonatal weight (p=0.045) (Table 4).
Table 4. The cutoff point of the percentage of B lymphocytes might be a good predictor for the level of insulin resistance in patients with GDM.
| Lower (<14.05) | Higher (>=14.05) | p | |
|---|---|---|---|
| N | 58 | 66 | |
| FPG | 4.49±1.02 | 4.70±1.02 | 0.314 |
| Finsulin | 70.73±36.45 | 87.12±39.21 | 0.019 c |
| FCT | 738.69±297.92 | 761.85±312.47 | 0.675 c |
| HbA1c | 5.65±0.63 | 5.97±0.87 | 0.024 a |
| GA | 13.08±1.80 | 13.96±2.39 | 0.024 b |
| HOMAIR | 14.24±8.99 | 18.71±12.57 | 0.036 c |
| HOMAIS | 0.0043±0.0029 | 0.0033±0.002 | 0.022b |
| Neonatal Weight (g) | 3321±386.50 | 3673±456.80 | 0.045 |
Finsulin:fasting insulin
aCubic reciprocal values were performed to correct the inconsistent variance;
bInverse square root was used to correct the inconsistent variance;
cSquare root was used to correct the inconsistent variance.
4. DISCUSSION
GDM is a common metabolic disorder that occurs during pregnancy due to which, pregnant women and their offspring experience adverse effects [17-19]. Hence, GDM has attracted increasing attention. Although the cause of this disorder is not yet well understood, the onset of GDM is known to be associated with insulin resistance and β-cell dysfunction. Nonetheless, physiological pregnancy is a process of development of insulin resistance [20]. From the gestational age of 12 weeks, the level of insulin resistance increases gradually, until full-term, when insulin resistance closely resembles T2DM. To balance the abnormal insulin secretion resulting from insulin resistance, β-cells produce an increased level of insulin to maintain the blood glucose at a stable level during pregnancy. However, in GDM pregnancies, the high level of insulin resistance, decreased the sensitivity of insulin, and impairment of β-cell secretion highly increase the level of blood glucose [2, 21].
Immune dysfunction is speculated to play a critical role in insulin resistance and β-cell impairment. Several studies have demonstrated that T lymphocytes could infiltrate into the VAT and produce a series of pro-inflammatory cytokines, leading to the formation and development of insulin resistance. In animal experiments, IFN-γ in obese mice stimulated the production of CD8+ T- and Th1 CD4+ T-cells. Both cell types infiltrated into the VAT and overcame the anti-inflammatory effects of Th2 and Treg cells in order to trigger the classical pro-inflammatory factors, resulting in the production of cytokines, such as TNF-α, IL-1β, and IL-6, and the development of local insulin resistance. Furthermore, these cytokines could also escape the VAT, enter the peripheral blood circulation, and downregulate the level of insulin in the organs, such as the liver and muscles, eventually leading to systemic insulin resistance [22-24]. In the current study, our data showed a non-significant difference between normal and GDM patients, indicating that T-cells may not be involved in GDM or the non-significant difference might be limited by the sample number.
In recent years, the role of B-cells in insulin resistance has gained increasing attention. B-cells modulate insulin resistance differentially. First, B-cells can infiltrate into the VAT and participate in the process of active immunity and phenotype transformation, which regulate the function of T-cells and macrophages [10, 23]. Second, B-cells can induce T-cells to produce pro-inflammatory cytokines such as IFN-γ, which enhances the level of insulin resistance [25, 26]. Third, B-cells regulate insulin resistance by producing other types of cytokines. For example, B-cells reduce the production of IL-10, an acknowledged anti-inflammatory cytokine; however, the levels of pro-inflammatory cytokines, such as IL-8 and leptin, are increased to promote resistance [27, 28]. Finally, B-cells not only present antibodies to T-cells and secrete inflammatory cytokines but also produce pathogenic antibodies, which can directly promote insulin resistance [10, 12].
In this study, we analyzed the biochemical parameters and composition of blood cells of 124 GDM pregnancies and 168 non-diabetic pregnancies at the Department of Obstetrics. The flow cytometry results showed that the percentage of B lymphocytes in the GDM group was significantly higher than that in the non-GDM group. Next, we selected antibodies, such as IgA, IgM, IgG, and IgE, produced by B-cells, and the result demonstrated that only the level of IgA antibodies differed between the two groups. These results led us to examine whether B-cell-related immune reactions are involved in the pathogenesis of GDM and whether B cells participate in the process of insulin resistance and the impairment of β-cells. Furthermore, Pearson’s correlation analysis demonstrated the relationship between the percentage of B-cells and the level of insulin resistance as well as other biochemical indicators. Consequently, the B-cell percentage was positively correlated with insulin resistance, indicating it as an adequate predictor of insulin resistance. Hence, we used ROC curve analysis to determine that the optimal cutoff point for the B-cell percentage, based on insulin resistance, was 14.05%.
The current study was the first report described the correlation between B lymphocytes and insulin resistance in GDM patients. The determination of the percentage of B-cells as a predictor of insulin resistance would provide a basis for the future study on the pathogenesis of GDM. Although the etiology of GDM is complicated, we deduced that the NK percentage was significantly different between normal and GDM patients, and it was also an independent risk factor for GDM, which suggested that NK cells play a critical role in GDM.
Additional issues will be addressed by future research, including the mechanism underlying B-cells-modulated insulin resistance in GDM, the antigens of antibodies produced by B-cells in the regulation of insulin resistance, and the role of obesity in B-cell-related immune activity in GDM.
CONCLUSION
Our study demonstrated that the percentage of B lymphocytes is positively related to fasting plasma glucose, fasting insulin, HbA1c and insulin resistance, but negatively correlated with the function of βcells in pancreatic islets. What we’ve found indicated B lymphocytes might be a good predictor of GDM. The mechanism of how B cells modulate the insulin resistance of GDM requires further investigation.
ACKNOWLEDGEMENTS
The study was sponsored by Shanghai Natural Science Foundation of China (16ZR1427900 to Y.Y.H.) and Shanghai Talent Development Foundation of China (2016014 to Y.Y.H.).
Yan Zhuang has designed and performed the research,collected and analyzed data as well as wrote the paper.
Jin Zhang has performed the study and collected data.
Yiwei Li has collected data and written the paper.
Hongqin Gu has analyzed data and wrote the paper.
Jinyan Zhao has contributed important reagents and performed the study.
Ya Sun has collected and analyzed data.
Rencheng Wang has performed the research and wrote the paper.
Chunyan Zhang has collected and analyzed data.
Wen Chen has collected data.
Jianrong Weng has contributed important reagents and worte the paper.
Lan Qi has analyzed data and wrote the paper.
Huifang Lu has collected data.
Jiarong Zhang has designed the study and analyzed data.
Qin Liu has contributed important reagents and analyze data.
Yinyan He has designed the research and analyzed data.
Xianming Xu has contributed important reagent and analyzed data.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the Ethics Committee of Shanghai Jiao Tong University School of Medicine, Shanghai, China (Predictive value study of the percentage of B lymphocytes for insulin resistance in gestational diabetes mellitus, 2018KY240).
HUMAN AND ANIMAL RIGHTS
No animals were used in this study. The research was performed in human in accordance with the standard set forth in the Declaration of Helsinki principle of 1975, as revised in 2008 (http://www.wma.net/en/20activities/10ethics/10helsinki/.
CONSENT FOR PUBLICATON
A written informed consent was obtained from all patients prior to the publication of the study.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Zhu Y., Zhang C. Prevalence of gestational diabetes and risk of progression to Type 2 diabetes: A global perspective. Curr. Diab. Rep. 2016;1:7. doi: 10.1007/s11892-015-0699-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chiefari E., Arcidiacono B., Foti D., Brunetti A. Gestational diabetes mellitus: An updated overview. J. Endocrinol. Invest. 2017;40:899–909. doi: 10.1007/s40618-016-0607-5. [DOI] [PubMed] [Google Scholar]
- 3.Basri N.I., Mahdy Z.A., Ahmad S., Abdul Karim A.K., Shan L.P., Abdul Manaf M.R., Ismail N.A.M. The World Health Organization (WHO) versus The International Association of Diabetes and Pregnancy Study Group (IADPSG) diagnostic criteria of gestational diabetes mellitus (GDM) and their associated maternal and neonatal outcomes. Horm. Mol. Biol. Clin. Investig. 2018;34(1):pii:/j/hmbci.2018.34.issue-1/hmbci-2017-0077/hmbci-2017-0077.xml.. doi: 10.1515/hmbci-2017-0077.. [DOI] [PubMed] [Google Scholar]
- 4.Buchanan T.A., Xiang A.H., Page K.A. Gestational diabetes mellitus: Risks and management during and after pregnancy. Nat. Rev. Endocrinol. 2012;8:639–649. doi: 10.1038/nrendo.2012.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kendrick J.M. Screening and diagnosing gestational diabetes mellitus revisited: Implications from HAPO. J. Perinat. Neonatal Nurs. 2011;25(3):226–232. doi: 10.1097/JPN.0b013e318222dded. [DOI] [PubMed] [Google Scholar]
- 6.Weinert L.S. International Association of Diabetes and Pregnancy Study Groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy: Comment to the International Association of Diabetes and Pregnancy Study Groups Consensus Panel. Diabetes Care. 2010;33(7):e9. doi: 10.2337/dc09-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Talukdar S., Oh D.Y., Bandyopadhyay G., Li D., Xu J., McNelis J. Lu, Min.; Li, P.; Yan, Q.; Zhu, Y.; Ofrencio.; Lin, M.; Brenner, M.B.; Olefsky, J.M. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat. Med. 2012;18:1407–1412. doi: 10.1038/nm.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Justin I.O., Ajay C. Pleiotropic actions of insulin resistance and inflammation in metabolic homeostasis. Science. 2013;339(6116):172–177. doi: 10.1126/science.1230721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Satoshi N., Ichiro M., Mika N., Koji E., Hiroshi Y., Mitsuru O., Makoto O., Kazuo H., Kohjiro U., Seiryo S., Kotaro Y., Takashi K., Ryozo N. CD8 + effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat. Med. 2009;15(8):914–920. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 10.Winer D.A., Winer S., Shen L., Wadia P.P., Yantha J., Paltser G., Tsui H., Wu P., Davidson M.G., Alonso M.N., Leong H., Glassford A., Caimol M., Kenkel J.A., Tedder T.F., McLaughlin T., Miklos D.B., Dosch H.M., Engleman E.G. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat. Med. 2011;17:610–617. doi: 10.1038/nm.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Winer D.A., Winer S., Chng M.H., Shen L., Engleman E.G.B. Lymphocytes in obesity-related adipose tissue inflammation and insulin resistance. Cell. Mol. Life Sci. 2014;71:1033–1043. doi: 10.1007/s00018-013-1486-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nikolajczyk B.S., Jagannathan-Bogdan M., Shin H., Gyurko R. State of the union between metabolism and the immune system in type 2 diabetes. Genes Immun. 2011;12:239–250. doi: 10.1038/gene.2011.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palming J., Gabrielsson B.G., Jennische E., Smith U., Carlsson B., Carlsson L.M., Lonn M. Plasma cells and Fc receptors in human adipose tissue--lipogenic and anti-inflammatory effects of immunoglobulins on adipocytes. Biochem. Biophys. Res. Commun. 2006;343:43–48. doi: 10.1016/j.bbrc.2006.02.114. [DOI] [PubMed] [Google Scholar]
- 14.Bhatia D., Sinha A., Hari P., Sopory S., Saini S., Puraswani M., Saini H., Mitra D.K., Bagga A. Rituximab modulates T- and B-lymphocyte subsets and urinary CD80 excretion in patients with steroid-dependent nephrotic syndrome. Pediatr. Res. 2018;84(4):520–526. doi: 10.1038/s41390-018-0088-7. [DOI] [PubMed] [Google Scholar]
- 15.Adamo L., Staloch L., Rocha-Resende C., Matkovich S.J., Jiang W., Bajpai G., Weinheimer C.J., Kovacs A., Schilling J.D., Barger P.M., Bhattacharya D., Mann D.L. Modulation of subsets of cardiac B lymphocytes improves cardiac function after acute injury. JCI Insight. 2018;3(11):e120137. doi: 10.1172/jci.insight.120137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gelsinger S.L., Smith A.M., Jones C.M., Heinrichs A.J. Technical note: Comparison of radial immunodiffusion and ELISA for quantification of bovine immunoglobulin G in colostrum and plasma. J. Dairy Sci. 2015;98(6):4084–4089. doi: 10.3168/jds.2014-8491. [DOI] [PubMed] [Google Scholar]
- 17.Aune D., Sen A., Henriksen T., Saugstad O.D., Tonstad S. Physical activity and the risk of gestational diabetes mellitus: A systematic review and dose-response meta-analysis of epidemiological studies. Eur. J. Epidemiol. 2016;31:967–997. doi: 10.1007/s10654-016-0176-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.West N.A., Crume T.L., Maligie M.A., Dabelea D. Cardiovascular risk factors in children exposed to maternal diabetes in utero. Diabetologia. 2011;54:504–507. doi: 10.1007/s00125-010-2008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tam W.H., Ma R.C., Yang X., Ko G.T., Tong P.C., Cockram C.S., Sahota D.S., Rogers M.S., Chan J.C.N. Glucose intolerance and cardiometabolic risk in children exposed to maternal gestational diabetes mellitus in utero. Pediatrics. 2008;122:1229–1234. doi: 10.1542/peds.2008-0158. [DOI] [PubMed] [Google Scholar]
- 20.DiCianni G., Miccoli R., Volpe L., Lencioni C., Del Prato S. Intermediate metabolism in normal pregnancy and in gestational diabetes. Diabetes Metab. Res. Rev. 2003;19:259–270. doi: 10.1002/dmrr.390. [DOI] [PubMed] [Google Scholar]
- 21.Harlev A., Wiznitzer A. New insights on glucose pathophysiology in gestational diabetes and insulin resistance. Curr. Diab. Rep. 2010;10:242–247. doi: 10.1007/s11892-010-0113-7. [DOI] [PubMed] [Google Scholar]
- 22.Olefsky J.M., Glass C.K. Macrophages, inflammation, and insulin resistance. Annu. Rev. Physiol. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 23.Odegaard J.I., Chawla A. Pleiotropic actions of insulin resistance and inflammation in metabolic homeostasis. Science. 2013;339:172–177. doi: 10.1126/science.1230721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schober L., Radnai D., Spratte J., Kisielewicz A., Schmitt E., Mahnke K., Fluhr H., Uhlmann L., Sohn C., Steinborn A. The role of regulatory T cell (Treg) subsets in gestational diabetes mellitus. Clin. Exp. Immunol. 2014;177:76–85. doi: 10.1111/cei.12300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lund F.E. Cytokine-producing B lymphocytes - key regulators of immunity. Curr. Opin. Immunol. 2008;20(3):332–338. doi: 10.1016/j.coi.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeFuria J., Belkina A.C., Jagannathan-Bogdan M., Snyder-Cappione J., Carr J.D., Nersesova Y.R., Markham D., Strissel K.J., Watkins A.A., Zhu M., Allen J., Bouchard J., Toraldo G., Jasuja R., Obin M.S., McDonnell M.E., Apovian C., Denis G.V., Nykolajczyk B. B cells promote inflammation in obesity and type 2 diabetes through regulation of T-cell function and an inflammatory cytokine profile. Proc. Natl. Acad. Sci. USA. 2013;110:5133–5138. doi: 10.1073/pnas.1215840110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jagannathan M., McDonnell M., Liang Y., Hasturk H., Hetzel J., Rubin D., Kantarci A., Van Dyke T.E., Ganley-Leal L.M., Nykolajczyk B.S. Toll-like receptors regulate B cell cytokine production in patients with diabetes. Diabetologia. 2010;53:1461–1471. doi: 10.1007/s00125-010-1730-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hong E.G., Ko H.J., Cho Y.R., Kim H.J., Ma Z., Yu T.Y., Friedlien R.H., Kurt-Jones E., Finberg R., Fischer M.A., Granger E.L., Norbury C.C., Hauschka S.D., Philbrick W.M., Lee C.G., Elias J.K., Kim J.K. Interleukin-10 prevents diet-induced insulin resistance by attenuating macrophage and cytokine response in skeletal muscle. Diabetes. 2009;58:2525–2535. doi: 10.2337/db08-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]


