Abstract
Low back pain is a prevalent socio-economic burden and is often associated with damaged or degenerated intervertebral discs (IVDs). When conservative therapy fails, removal of the IVD (discectomy), followed by intersomatic spinal fusion, is currently the standard practice in clinics. The remaining space is filled with an intersomatic device (cage) and with bone substitutes to achieve disc height compensation and bone fusion. As a complication, in up to 30% of cases, spinal non-fusions result in a painful pseudoarthrosis. Bone morphogenetic proteins (BMPs) have been clinically applied with varied outcomes. Several members of the BMP family, such as BMP2, BMP4, BMP6, BMP7, and BMP9, are known to induce osteogenesis. Questions remain on why hyper-physiological doses of BMPs do not show beneficial effects in certain patients. In this respect, BMP antagonists secreted by mesenchymal cells, which might interfere with or block the action of BMPs, have drawn research attention as possible targets for the enhancement of spinal fusion or the prevention of non-unions. Examples of these antagonists are noggin, gremlin1 and 2, chordin, follistatin, BMP3, and twisted gastrulation. In this review, we discuss current evidence of the osteogenic effects of several members of the BMP family on osteoblasts, IVD cells, and mesenchymal stromal cells. We consider in vitro and in vivo studies performed in human, mouse, rat, and rabbit related to BMP and BMP antagonists in the last two decades. We give insights into the effects that BMP have on the ossification of the spine. Furthermore, the benefits, pitfalls, and possible safety concerns using these cytokines for the improvement of spinal fusion are discussed.
Keywords: Spinal fusion, intervertebral discs, mesenchymal stromal cells, osteogenesis, bone morphogenetic proteins, antagonists of bone morphogenetic proteins
1. INTRODUCTION
Spinal fusion is currently a widely applied surgical intervention to treat painful discs, but its use involves intricacies, like non-union, in some cases. Low back pain (LBP) is a significant health problem in today’s society. It is estimated that up to 80% of the population suffers from LBP symptoms at least once during their lifetime [1, 2]. When LBP fails to be treated with first-line conservative treatments, such as pharmacological and physical therapy, invasive procedures are required [3]. One of the current surgical interventions to
treat degenerated or damaged discs is intersomatic spinal fusion. After removal of the intervertebral disc (IVD), a cage is implanted into the created space. The cage is usually filled with bone graft or bone substitutes. For further mechanical stability, the involved segments are frequently fixed with additional pedicle screws.
The primary source of autologous bone grafts (ABGs) is the iliac crest; other sources are the proximal tibia, the fibula, the ribs, and the vertebral body [4]. In case no ABGs are available, bone formation is promoted by non-autologous materials, such as allograft, cancellous chips, demineralized bone matrix, ceramics, tricalcium phosphate, and hydroxyapatite [5]. Although this surgical procedure is widely used, it can lead to failure in bone formation and/or [6] pseudoarthrosis, which occurs in 5% to 35% of treated patients [7-10].
Bone morphogenetic proteins (BMPs) are used in clinics as osteoinductive factors, but they cause side effects. In the last decades, BMP and BMP antagonists have been found to promote fracture healing and bone regeneration [11-15].
Bone morphogenetic protein 2 (BMP2) and bone morphogenetic protein 7 (BMP7) are clinically approved to treat fracture non-unions [16-18]. In 2002, the US Food and Drug Administration (FDA) approved the use of recombinant BMP2 in clinics for the promotion of bone fusion in anterior lumbar interbody fusion [19, 20]. However, the use of recombinant BMP2 has been questioned: it is not cost-effective, and safety concerns have also been raised with both lumbar and cervical fusion procedures [21, 22]. Moreover, the application of BMPs did not result in better outcomes compared with the use of ABGs [23]. Supraphysiological doses of BMPs in the order of milligram are required to induce sufficient bone formation. This may potentially cause adverse events, such as overgrowth and abnormal bone formation, osteoclast activity and vertebral osteolysis, and local problems (e.g., inflammation, edema, and wound problems). Negative effects on the exposed dura and nerves, graft subsidence, graft migration, formation of neutralizing antibodies against BMPs, and carcinogenicity have also been reported [24-28]. In the field of spinal applications, several meta-analyses have been performed to quantify the success of BMP2 administration in spinal fusion. Carragee et al. (2011) [29] and Simmonds et al. (2013) [30] reported on the outcomes of industrial-sponsored BMP2 studies that claim no BMP2 side effects. Furthermore, Carragee et al. [29] compared conclusions about safety and related efficacy in industry-sponsored BMP2 studies with subsequently available FDA data summaries. They suggested the occurrence of adverse effects associated with rhBMP2 after spinal fusion surgery, and these ranged from 10% to 50% depending on the approach used. These studies questioned whether BMP2 had any positive effects on pain relief, and they explored whether cases of cancer could be connected to its application [30]. In light of these neutral and adverse outcomes, it seems evident that the biology and underlying pathways of BMPs are not yet understood, resulting in its low efficacy and poor results in clinics. To date, however, no systemic effects caused by the local application of BMP2 have been reported. Recombinant BMP7 is the second member of the BMP family, which is FDA approved and available for clinical use. An essential property of BMPs is their nature of distribution. When administered in buffer only, BMP2 has a half-life of 7 minutes in non-human primates [31]; BMP4 also has a rapid initial clearance rate. Conversely, BMP7 possesses an extended terminal half-life, which results in low and more permanent circulating levels of the protein. As an important fact for clinical use, it also has to be considered, that BMPs are pleiotropic proteins. In the case of BMP7, the pleiotropic nature seems to play a role, as it was found that when administered systemically, BMP7 protects the kidney by preventing tubulointerstitial fibrosis and preserving renal function [32].
Currently, only little is known about the expression pattern of BMP antagonists during spinal fusion. However, the physiological imbalance between BMP and BMP antagonists may be the reason for spinal non-union [6, 13]. The question on whether an insufficient bone formation is caused by a suboptimal BMP expression, an increase in local levels of BMP antagonists, or both remains unanswered [33]. This BMP imbalance and the failure of bone formation could further be discussed for IVDs. Clinical observations indicate that partial IVD removal often leads to spinal non-union [34, 35]. The central question is whether IVD cells can influence the BMP signaling pathway by expressing BMP antagonists. Recent studies already indicated the expression of BMP antagonists in IVD cells [6, 35]. Another question is how IVD cells react upon stimulation with BMPs. Earlier studies showed the anabolic effect of BMP2 stimulation of IVD cells. In a newer report, it is even hypothesized that IVD cells might shift toward an osteogenic phenotype [6].
In this review, we summarize current knowledge on the molecular pathways of BMP signaling with relevance to the bone and the spine. The effects of BMPs and BMP antagonists in spinal fusion and bone healing for in vitro and in vivo studies are discussed. We also present an overview of the latest research on BMP2 in bone healing or spinal fusion, which was the primary focus in the past, as well as new directions (i.e., involving BMP antagonists and other members of the TGF-β pathway). The main focus is on how these cytokines affect the respective target cells, namely osteoblasts (OBs), mesenchymal stem cells (MSCs), and IVD cells, which could play a role in spinal fusion. The expression levels of BMPs and BMP antagonists in spinal fusion models are also described.
2. DISCOVERY OF BMPs
The bone matrix consists of organic and inorganic components. BMPs were actually discovered through the study of these components. Approximately after two weeks when the osteoid (uncalcified matrix) is formed, the osteoid starts to mineralize; 70% of the final amount is reached very quickly, whereas the remaining 30% needs several months until deposition is completed. Seventy percent of the final bone-dry weight consists of hydroxyapatite mineral; the remaining 30% consists of organic materials, such as collagens, glycoproteins, proteoglycans, and sialoproteins [36]. In 1965, the orthopedic surgeon Marshall R. Urist studied the concept of induced bone formation for the first time by transplanting a specifically prepared allogenic bone matrix, called the demineralized bone matrix (DMBM), into muscle tissue and found the material to induce ectopic bone formation [37]. This discovery was a breakthrough in biologic bone graft substitute technology. DMBM is an osteoinductive scaffold produced by acidic treatment of allograft bone [38]. It is a mixture of non-collagenous proteins, osteoinductive growth factors, and collagen. Primarily, collagen type 1 (COL1) is present, but other types of collagen are also present, albeit at a lower proportion than that in soft tissue connections. Because of the enhanced bioavailability of growth factors and allografts, DMBM showed better osteoinductive potential than allograft [39]. Urist (1965) [37] discovered in his studies that the extracellular matrix of the bone has materials that can induce bone formation; he called these substances BMPs. Although Urist spent the next decades isolating and purifying BMPs, they were first cloned only in 1988 by John Wozney [40]. The research team of Wozney and Rosen (1988) [41] proved that BMPs are members of the transforming growth factor beta (TGF-β) superfamily. In 1988, the first studies on the effect of BMPs on spinal fusion were conducted by Johnson et al. (1988) [42]. Since then, many studies have shown the ability of BMPs to induce differentiation from MSCs into bone.
3. BMPs IN DEVELOPMENTAL PROCESSES
As described above, BMPs were initially known to induce bone formation. They play an important role in adult tissue homeostasis, such as sustaining joint integrity, initiating fracture repair, and remodeling the vasculature [43-45]. Today, it is well known that BMPs also have key functions in the development of all organ systems. BMP signaling is involved in cell growth, differentiation, survival, activation, and apoptosis during developmental processes [46, 47]. Besides embryogenesis and its function in the skeletal system, BMP signaling is important in the muscle [48], gastrointestinal [49], cardiovascular and pulmonary [50], urinary [51], neurological and ophthalmic [52], and reproductive systems [53], as well as in adipogenesis [54]. In embryogenesis, BMPs are especially essential for mesoderm and cardiac formation [55]. Both BMP2 and BMP4 knock-out mice experience embryonic lethality during early gastrulation because of the failure of mesoderm induction [50, 56]. BMP2-deficient mice have malformation of the amnion/chorion, which is caused by failure of proamniotic canal closing. They also have defects in cardiac development. With BMP4 deficiency, mice lack mesoderm differentiation [57]. Conversely, the knockout of BMP1, BMP7 (also called osteogenic protein 1 or OP-1), or BMP11 leads to death after birth [58-60]. A lack of BMP1 leads to failure in ventral body closure [58]. Furthermore, mice with BMP7 null mutations die shortly after birth because of renal failure. They are also characterized by eye defects and mild skeletal changes [61].
4. THE BMP SIGNALING PATHWAY
Two major pathways have particularly strong effects on bone homeostasis, and these are i) BMP signaling and ii) Wnt/β-catenin signaling cascade [62, 63]. A crosstalk has also been identified in several cell types between these two signaling pathways. One example is the activation of Wnt3a or the overexpression of β-catenin/TCF4, which both activate BMP2 expression in OBs [64].
BMPs are members of the TGF-β superfamily and play an essential role in skeletal tissue formation, as they induce the commitment of MSCs toward OBs. Hence, BMPs are involved in cartilage and bone formation during embryonic development, postnatal bone metabolism, and fracture healing [65].
Today, more than 22 members of the BMP family have been identified [14]. Several BMPs, such as BMP2, BMP4, BMP6, BMP7, and BMP9, have shown their potential for the induction of the osteogenic differentiation of MSCs toward osteoblastic lineage cells in vitro and in vivo [66]. BMPs bind as dimers to BMP type I (BMPRI) and type II (BMPRII) serine/threonine kinase receptors. Type I receptors are divided into the following three subtypes: BMPRIA (aka activin receptor-like kinase 3 (ALK3)), BMPRIB (ALK6), and activin receptor type-1 ActRI (ALK2)) [67]. BMP receptors are localized as heterodimers or homodimers in a caveolar structure on the cell surface [68]. The heterotetrameric signaling complex can vary, depending on which BMP binds to the receptors. BMP6 and BMP7 interact with type II receptors and activate type I receptors, whereas BMP2 and BMP4 mainly bind to BMP type I receptors and activate BMP type II receptors [69]. Through binding BMPs to their cognate receptors, BMPRII form a heterodimer with BMPRI. The kinase activity of BMPRII then activates BMPRI by phosphorylation and mediates signal cascade by initiating single mothers against decapentaplegic homolog (Smad) signaling. The signaling, however, can also proceed through the mitogen-activated protein (MAP) kinase pathway or possibly also other pathways [68]. The following three classes of Smad have been described: i) receptor-regulated Smad (R-Smad), which can be activated by BMP (Smad1/5/8) or TGF-β (Smad2 and 3), ii) common BMP- and TGF-β-mediated Smad (co-Smad: Smad3 and 4), and iii) inhibitory Smad (I-Smad: Smad 6 and 7). After BMP-activated BMP receptors, Smad1/5/8 are carboxy-terminally phosphorylated and build heteromers with Smad4, the whole complex translocates into the nucleus, where transcription is activated or inhibited [67, 70] (Fig. 1). To be more specific, the complex binds in the nucleus DNA sequences or interacts with transcription factors. The three key transcription factors for bone formation, stimulated by the BMP signaling pathway, are distal-less homeobox protein 5 (DLX5), Osterix (SP7), and the runt-related transcription factor 2 (RUNX2) [67, 71]. SP7 and RUNX2 are regulators for many OB-specific genes, including osteopontin (SPP1), osteocalcin (BGLAP), COL1, and bone sialoprotein (BSP). SP7 acts in conjunction with RUNX2; however, the levels of RUNX2 remain unaffected by SP7 gene absence, which indicates that SP7 may act downstream or independently of RUNX2 [72]. Furthermore, it was reported that the absence of RUNX2 (but with BMP2 stimulation) had no effect on SP7 expression, which indicates further SP7 independence. However, the gene DLX5 seems to play an important role in SP7 expression, as the inactivation of DLX5 leads to the suppression of SP7 [73]. Through these factors, OBs undergo terminal differentiation; the matrix mineralizes, and, in the last step, it undergoes apoptosis [74].
Fig. (1).
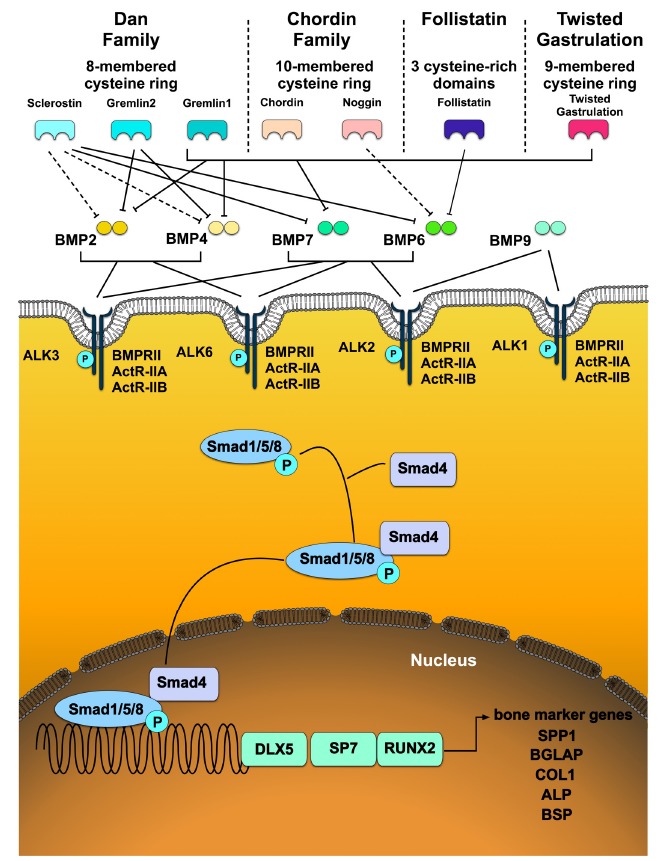
Schematic overview of bone morphogenetic protein (BMP) signaling pathways. BMPs, such as BMP2, BMP4, BMP6, BMP7, and BMP9, which are discussed in this review and known for their potential to induce osteogenic differentiation, are presented. BMPs bind as dimers to BMP type I (BMPRI) and type II (BMPRII) serine/threonine receptors. BMPRs are localized in caveolar structures on the cell surface. Through the binding of BMPs with the receptor, Smad signaling is initiated. BMPs activate Smad1/5/8 and build heteromers with Smad4. After translocation into the nucleus, transcription is either activated or inhibited. The complex binds to DNA sequences or interacts with transcription factors as runt-related transcription factor 2 (RUNX2) directly. This plays an important role in the regulation of various osteoblast (OB)-specific genes, such as osteocalcin (BGLAP), osteopontin (SPP1), collagen type 1 (COL1), bone sialoprotein (BSP), and alkaline phosphatase (ALP). Furthermore, Osterix (SP7) acts as a master regulator for multiple OB-specific genes, including SSP1, COL1, and BSP. The gene distal-less homeobox protein 5 (DLX5) seems to play an important role in SP7 expression. ALK2 = ActR-I, ALK3 = BMPR1A, ALK6 = BMPR1B. Dotted line: weak binding of BMP antagonists, continuous line: strong binding of BMP antagonists to their respective BMP [12, 63, 79-92]. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Most BMP expressions in OBs are regulated by transcriptional auto-regulation, which can act as a negative feedback loop. BMP signaling can be regulated at different levels in the cell, for example, at the intracellular level by inhibitory Smads, miRNA, and methylation, as well as at the extracellular level by pseudoreceptors or BMP antagonists [75]. The BMP signaling pathway is influenced by BMP antagonists, which block BMP signal transduction at multiple levels. The members of BMP antagonists, including noggin (NOG), chordin (CHRD), gremlin (GREM1 and GREM2), twisted gastrulation (TWSG), and sclerostin (SOST), negatively regulate BMP signal transduction by competing with BMP ligands [76-78].
5. BMPs AND THEIR ANTAGONISTS
Bone-inducing BMPs can be categorized into three different groups based on the homology of their amino acid
sequences [93]. BMP2 and BMP4 comprise the first subgroup; BMP5, BMP6, BMP7, and BMP8 comprise the second group; and BMP9 and BMP10, comprise the third group [94]. BMP1, which is not a member of the TGF-β superfamily, is a metalloprotease; it plays a role in collagen maturation as a procollagen C-proteinase and also induces bone and cartilage development [95]. But BMPs are not only inducers of bone formation but are also inhibitors, such as BMP3, which reduces bone density, and BMP13, which inhibits bone formation [96]. In bone, BMPs are mainly produced by osteoprogenitor cells, OBs, chondrocytes, and platelets; upon secretion, they are integrated in the extracellular matrix [97, 98].
Previous studies showed that BMP2 turns out to be the most osteoinductive member of the BMP family because of its biological activity throughout most stages of bone healing [20]. BMP2 could induce the differentiation of MSCs toward cells of osteoblastic lineage and enhance the differentiated function of OBs [99, 100]. BMP2 interacts primarily with ALK6 [82] and also has a higher binding affinity for ALK3. As already mentioned above it has lower binding affinity to BMPRII, ActR-IIB and ActR-IIB [101]. BMP2 can be up- and downregulated by other BMPs. Interestingly, promoters of BMP2 and BMP4 contain binding sequences for RUNX2, which may indicate regulation by a positive feedback loop [102].
Another BMP often discussed is BMP4, which is structurally highly related to BMP2 and interacts mostly with ALK3 and very likely also with ALK6 in some cell types [82]. Furthermore, BMP6 forms another subclass together with BMP5, BMP7, and BMP8, as BMP6 has a high degree of homology at the amino acid sequence level to BMP7. Both proteins BMP6 and BMP7 can bind to ALK2 and to the same receptors as BMP2 and BMP4 but conversely with higher affinity to type II than type I receptor [82, 103]. BMP9 is most likely inducing osteogenic differentiation by interacting with ALK1 and ALK2 [104].
The following selection of BMP antagonists belongs to extracellular BMP antagonists and includes secreted proteins, binding with different affinities to BMPs and thus preventing their interaction with BMP receptors. They can be grouped into the following three subfamilies based on the size of their cysteine knot: i) differential screening-selected gene aberrative in neuroblastoma (DAN) family (which includes GREM1 and 2 and cerberus) (an eight-membered cysteine ring), ii) TWSG (a nine-membered cysteine ring), and iii) CHRD family (in which NOG is included) (a 10-membered cysteine ring) [105]. Follistatin (FLST), which contains three cysteine-rich homologous domains, forms another group.
NOG plays a major role in the inhibition of osteogenesis, particularly in bone and joint formation [106]. NOG is secreted as a glycosylated homodimer of 64 kilodaltons (kDa) and is an antagonist to BMPs. It antagonizes through direct binding to BMP2, BMP4, BMP6, and BMP7, with a higher affinity to BMP2 and BMP4. Furthermore, NOG gene expression is upregulated in osteogenic cells upon exposure to BMPs, and it acts as a negative regulator to suppress BMP-induced bone formation [107]. In OBs, for example, NOG is upregulated in response to BMP2, BMP4, or BMP6, which suggests negative feedback to limit the excessive exposure of cells to BMP signaling [108]. These studies indicate that the effect of NOG varies between mouse and human. Rifas et al. (2007) [109] suggested that in the case of human MSCs (hMSCs), NOG might either balance the impact of BMPs or act, in their absence, as a ligand for BMP receptors to induce differentiation. Another BMP antagonist is CHRD, which is secreted as a glycosylated homodimer of 120 kDa. The expression of CHRD in OB lineage cells is limited, but it is highly expressed in undifferentiated chondrocytes [108, 110]. GREM1 and 2 are also antagonists of BMP. GREM1, also called downregulated by v-mos (DRM), is structurally characterized by an eight-cysteine carboxy-terminal ring domain [105]; it was first isolated from Xenopus embryos [111]. It has a molecular weight of 27 kDa and belongs to the DAN family; GREM1 is primarily present on the external surface of expressing cells, but it can also be found in small amounts in the endoplasmic reticulum-Golgi complex [112]. It exists in both secreted and cell-associated forms [113]. Generally, GREM1 shows the highest affinity for BMP2, followed by BMP4 and then BMP7 [78]. GREM2, which is also called the protein related to DAN and cerberus (PRDC), was initially found in embryonic stem cells and also belongs to the DAN family [81, 114]. Whereas GREM1 is expressed in the cartilage and bone of the skeleton, GREM2 is expressed in OBs during in vivo skeletogenesis [115, 116]. Another less-investigated BMP antagonist is FLST. Null mutation of the antagonist in mice leads to deficiencies in multiple tissues, including the skeleton; they are also unable to breathe, and they die just some hours after birth [117]. FLST expression is downregulated by BMPs and induced by TGF-β [118]. Another BMP antagonist that has been studied less is TWSG, with a molecular weight of 23.5 kDa and was initially identified in Drosophila sp. [119].
6. L51P AS A GENERAL INHIBITOR
L51P is a BMP2 analog that has one amino acid substituted at position 51 with leucine to proline. The proline variant loses one central hydrogen bond because of this substitution. With this, the dissociation constant between L51P and BMPRI goes about 8,000 times up compared with wild-type BMP2 binding; this leads to a lower affinity of L51P to the ligand binding domain of BMPRI [120, 121]. However, the binding affinity of L51P to BMPRII is comparable to that of the wild type. While the affinity for BMPRI is lower, L51P retains the wild-type affinity to BMP antagonists, such as NOG and GREM [121].
It was found that the application of L51P in combination with BMP2 induces improved bone formation in a rat animal large bone defect model and rat calvaria cells [122-124]. However, if L51P was applied alone, no osteoinduction could be observed in primary murine OBs, MC3T3-E1, ATCDC5, and pro-myoblasts [122, 123, 125]. These experimental data further indicate that L51P seemed to act as a general inhibitor of BMP antagonists and does not solely block the NOG-specific pathway. In a recent study by Hauser et al. (2018) [126] ovariectomized Wistar Crl:WI (Han) rats (female, retired breeders, 8–10 months) were treated with alendronate (at eight weeks postsurgery), which belongs to the group of bisphosphonates. At 14 weeks postsurgery, diaphyseal femoral defects were applied, followed by stabilization with a rigid osteosynthesis system. The defects were then filled with β-tricalcium phosphate ceramics, which were loaded with BMP2, L51P, or a combination of the two cytokines. In this study, Hauser et al. (2018) [126] confirmed improved bone formation through the combined application of BMP2 and L51P by using µCT and histology.
The application of L51P to improve spinal fusion could be a promising approach, as the inhibition of NOG seems to result in a more efficient and physiological bone formation than the application of BMP [121].
7. BMPS AND BMP ANTAGONISTS AND THEIR IMPORTANCE IN SPINAL FUSION
Bone formation is driven by two different types of ossification - the direct intramembranous process, in which bone is formed directly into the primitive connective tissue, or the indirect endochondral process, in which cartilage is formed as a precursor before bone formation. As already discussed BMPs act, depending on their concentration gradient as differentiating factors, but they can also attract various cell types and function further as chemotactic and mitogenic agents [127]. Besides affecting the proliferation of cartilage- and bone-forming cells, they induce, as already described above, the differentiation of MSC toward chondroblasts and OBs. Therefore, BMPs most probably influence both direct and indirect bone formation [128].
Several studies in the last two decades have been conducted to investigate the effect of various BMPs or BMP antagonists in spinal fusion models. These studies showed the feasibility of using growth factors for the improvement of fusion rates. Table 1 provides an overview of studies focusing on spinal fusion, bone healing, or, in general, OB and osteoblastic cell line culture with the application of BMPs or BMP antagonists.
Table 1. Application of BMP and BMP antagonists in spinal fusion and bone healing.
| Author, Year | Study Type, Species | Cytokine | Study | Conclusion | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Boden, 2002 [141] | In vivo, human | BMP2 | Lumbar arthrodesis of patients (N = 25) by autograft/Texas Scottish Rite Hospital (TSRH) pedicle screw instrumentation (N = 5), rhBMP2/TSRH (N = 11) or rhBMP2 only without internal fixation (N = 9) (20 mg/mL of rgBMP2 in hydroxyapatite/tricalcium phosphate carrier) | Radiographic fusion rate of the TSRH group was 40%, both groups treated with rhBMP2 showed 100% fusion rate | ||||||||
| Govender, 2002 [25] | In vivo, human | BMP2 | Open tibial fractures patients (N = 450) received standard treatment with an implant containing 0.75 mg/mL (total 6 mg) of rhBMP2 or an implant of 1.5 mg/mL (total 12 mg) | Significantly faster fracture healing with 1.5 mg/mL over the current standard of care | ||||||||
| Friedlaender, 2001 [142] | In vivo, human | BMP7 | Tibial non-union patients (N = 124) were treated by an intramedullary rod, accompanied by BMP7 in a COL1 carrier or by fresh bone autograft | After 9 months, 75% of patients in the BMP7 treated group had healed fractures (evaluated by radiographic criteria) | ||||||||
| Klineberg, 2014 [136] | In vivo, rabbit | NOG | SiRNA against NOG was electroporated in paraspinal muscle of bilateral, posterolateral intertransverse lumbar fusion in skeletally mature New Zealand White rabbits (L5-L6) | NOG protein was knocked down in vivo for seven days and detectable by six weeks No significantly improvement of overall fusion rates compared to controls |
||||||||
| Minamide, 2001 [132] | In vivo, rabbit | BMP2 | Japanese white rabbits, underwent single-level bilateral posterolateral intertransverse process fusion (L4-L5) Animals was implanted sintered bovine bone TBC coated with COL1 infiltrated ± 100 µg of rhBMP2 or COL1 sheet ± 100 µg of rhBMP2 |
TBC showed to be a more efficient carrier for rhBMP2 compared to collagen sheet, the process of spinal arthrosis showed a faster and stronger fusion The use of rhBMP2 resulted in a higher fusion rate (inTBC and collagen group) |
||||||||
| Koerner, 2018 [131] | In vivo, rat | BMP2 | Adult Wistar rats (age approximately 8 weeks) underwent postlateral intraverse fusion with DBM (L4-L5) 10 or 100 µg of rhBMP2 were added on an allograft collagen sponge. Animals were sacrificed at time points up to four weeks |
Enhanced inflammatory reaction and expression of inflammatory cytokines in the early time points (1 hour, 6 hours) because of rhBMP2 Growth factor (VEGF, IGF1, PDGF, TGF-β) expression appears first suppressed followed by a peak at 24 hours and 7 days TNFα showed a lower expression in rhBMP2 treated groups at days 1, 2 and 4. |
||||||||
| Zhu, 2017 [129] | In vivo, rat | BMP2 | Mature male Sprague Dawley rats (8 weeks) undergoing posterolateral spinal fusion (L4-L5) were implanted with (A) demineralised bone matrix (DBM), (B) with a combination of DBM and BMP2 or (C) with DBM and a combination of collagen binding bone morphogenetic protein 2 (CBD-BMP2) | CBD-BMP2 showed a higher affinity to the scaffold than commercial BMP2. Bone formation in group C was observed to be earlier and larger, compared to the other groups Better trabecular bone microarchitecture assessment and statistically larger bone mineral density. |
||||||||
| Song, 2010 [27] | In vitro, rat osteosarcoma cell line, C2C12 myoblasts | BMP2, BMP4, BMP5, BMP6, BMP7, GDF5, GDF6, NOG | Stimulation of cells with different BMP in the presence or absence of NOG Induction of NOG knockdown in C2C12 cells and exogenous stimulation of BMP6 and BMP7 (each 50 ng/mL) |
Shallower slopes of dose-response curves for ALP activity for BMP2 and BMP4 compared to BMP5, BMP6, and BMP7 (suggesting a more negative regulatory mechanism for BMP2 and BMP4) More NOG induction by BMP7 than BMP6 But BMP6 is more resistant to NOG inhibition than BMP7 |
||||||||
| Author, Year | Study Type, Species | Cytokine | Study | Conclusion | ||||||||
| Helm, 2000 [133] | In vivo, rat | BMP9 | Injection of BMP9 adenoviral vextor in 16-week old athymic male rats in the lumbar paraspinal musculature (sacrificed after 16 weeks) | Induction of massive bone at the injection sites, leading to solid spinal arthrodesis No evidence of pseudarthroses, nerve root compression, or systemic side effects |
||||||||
| Alden, 1999 [130] | In vivo, rat | BMP2 | Recombinant, replication-defective type 5 adenovirus with cytomegalovirus (CMV) promoter and BMP2 gene injection bilaterally or on the right side, percutaneously and paraspinally at the lumbosacral junction in athymic nude rats | Expression of BMP2 leads to endochondral bone formation in the paraspinal region Detection of cartilaginous tissue after three months postinjection at the injection site |
||||||||
| Suzuki, 2012 [140] | In vitro, C2C12 | BMP2, GREM1, GREM2 | Performance of microarray analysis on mRNA extracted from C2C12 cells, stimulated with different concentrations of BMP2 (0-400 ng/mL). siRNA was used to down-regulate GREM1 and GREM2 |
GREM1 and GREM2 were differently regulated by BMP2; GREM1 was downregulated, whereas GREM2 was upregulated after stimulation in a dose- and time-dependent manner Groups treated with siRNA and stimulated with BMP2 showed a significantly enhanced ALP activity compared to control groups |
||||||||
| Ideno, 2009 [115] | In vitro, mouse | GREM2 | GREM2 expression was upregulated by adenovirus or downregulated by siRNA in pre-osteoblasts of embryonic day 18.5 mouse calvariae | Upregulated expression suppressed exogenous BMP activity and endogenous levels of phosphorylated Smad1/5/8 (pSmad1/5/8) protein Downregulation elevated ALP activity, increased endogenous levels of pSmad1/5/8 protein and induced matrix mineralisation. |
||||||||
| Takayama, 2009 [107] |
In vitro, C2C12 (myoblastic cell line), In vivo, mouse |
NOG | NOG-siRNA silencing in C2C12 in rhBMP2 (0-300 ng/mL) stimulated cells NOG silencing in sites of exogenous rhBMP2-induced (5 µg/mL) ectopic ossicles in the muscle of six week old male ICR mice, by electroporation |
NOG mRNA expression was upregulated in response to rhBMP2 in C2C12 cells, in a dose- and time-dependent way Silencing of NOG expression by transfection of NOG siRNA, suppressed BMP2-stimulated NOG expression, resulting in acceleration of BMP2-induced osteoblastic differentiation. No enhancement of BMP2 induced new bone formation, in sites where NOG expression was silenced But increase of radiological density of NOG-targeted siRNA transfected and rhBMP2 stimulated ossicles. |
||||||||
| Tsuji, 2008 [139] | In vivo, mouse | BMP4 | Mice with floxed BMP4 alleles were bred with Prx1-cre transgenic mice to establish limb-specific removal of BMP4 | Limb skeletogenesis usually occurs in absence of BMP4, so postnatal skeletal growth was unaffected with removal of BMP4 Mice lacking BMP4 were able to mount a successful healing response |
||||||||
| Wan, 2007 [135] | In vitro, in vivo mouse | NOG | NOG was downregulated in MC3T3-E1 preosteoblast and primary mouse calvarial osteoblasts, from 5-day-old CD-1 mice, by using siRNA or by adeno-CMV-Cre infection of floxed NOG osteoblasts. Treatment of critical-size calvarial defects by osteoblasts expressing NOG-specific siRNA constructs |
Both cell types expressed enhanced osteogenic differentiation markers and showed more bone nodule deposition The removal of NOG leading to an increased signalling activity of endogenously produced BMP (enhanced levels of pSmads). Acceleration of early reossification of defects two or four weeks following injury |
||||||||
| Author, Year | Study Type, Species | Cytokine | Study | Conclusion | ||||||||
| Okamoto, 2006 [138] | In vivo, mouse | BMP4, NOG | Mice overexpressing BMP4 or NOG in osteoblasts under the control of the COL1 alpha 1 (COL1A1) promoter sequence were generated by microinjecting of the respective insert into the pronuclei of fertilized eggs from F1 hybrid mice (C57BL/6 x DBA) | Mice overexpressing BMP4 developed severe osteopenia, associated with increased numbers of osteoclasts Mice overexpressing NOG showed an increase in bone volume but decreased bone formation rate and reduced osteoclast number. |
||||||||
| Devlin 2003 [137] | In vivo, mouse | NOG | Fertilized oocytes were taken from CD-1 outbred albino mice and transfer of microinjected embryos into pseudopregnant mice Transgenic mice overexpressing NOG under the control of the osteoblastic specific osteocalcin promoter |
Mice overexpressing NOG developed decreased bone volume and osteopenia Bone mineral content, osteopenia and poor healing of fractures were persistent for 6 months OB activity seemed to be reduced, osteoclast (OC) number was not increased, and neither was bone resorption affected |
||||||||
| Abe, 2000 [134] | In vitro, mouse | BMP2, BMP6, NOG | C2C12 were cultured with 100 ng/mL BMP2, BMP6 and/or 10-600 ng/mL NOG for three days. | BMP2 induced ALP activity was inhibited by human recombinant NOG in a dose-dependent manner The effect of BMP6 was not affected |
||||||||
Previous studies that investigated BMP2 particularly focused on spinal fusion models. Several in vivo rat studies have demonstrated the beneficial effect of BMP2 on bone healing and spinal fusion [129-131]. Zhu et al. (2017) [129] showed early and large bone formation with transplanted DMBM and collagen binding BMP2 in a posterolateral rat spinal fusion model. The study of Alden et al. (1999) [130], which is based on the classic work of Urist et al. (1965) [37], focused on viral gene therapy and observed paraspinal endochondral ossification after injections in the paraspinal musculature of gene constructs. However, in a recent study by Koerner et al. (2018) [131], the application of BMP2 (10 or 100 µg) in a spinal fusion rat model demonstrated enhanced inflammatory reactions and inflammatory cytokine expression. Furthermore, growth factors, such as vascular endothelial growth factor, insulin-like growth factor 1, platelet-derived growth factor, and TGF-β, appeared to be repressed in the early stage of BMP2 treatment. This study showed that BMP2 leads to inflammatory reactions, but it may also contribute to enhancing the fusion process [131]. As in the study of Zhu et al. (2017) [129], rats were approximately eight weeks in age, and spinal fusion was processed at the same level (L4–L5), but the rat subspecies was not the same. Besides rat, rabbit and mouse studies have been conducted to investigate the effect of cytokines on spinal fusion. There are, of course, species-specific differences in the metabolism and age of the animals. In a study by Minamide et al. (2001) [132], rabbits underwent single-level process fusion (L4-L5). The animals were implanted with sintered bovine bone true bone ceramics, coated with a type 1 collagen sheet supplemented with BMP2 (rhBMP2) or just the collagen sheet with rhBMP2. Again, the use of rhBMP2 resulted in a higher fusion rate [132].
Besides BMP2, other BMPs have been investigated in relation to spinal fusion. Previously, we discussed the study of Alden et al. (1999) [130], which showed that BMP2 has the ability to induce bone formation in a tight musculature. In a similar study, Helm et al. (2000) [133] applied BMPs in the paraspinal region of 16-week-old rats and showed the ability of BMP9 to induce bone formation in rodents by gene therapy.
Not only BMPs but also BMP antagonists have gradually been a part of spinal fusion studies in recent years. Furthermore, studies that focused not only on BMPs but also on BMPs in combination with their antagonists or on antagonists alone have been conducted. Research on the BMP antagonist NOG were particularly of interest, as NOG seems to play an important role in bone and cartilage formation. For example, Abe et al. (2000) [134] examined the effect of NOG with BMP6. Although the BMP antagonist NOG can bind to BMP6, Abe et al. (2000) [134] showed that NOG does not antagonize BMP6 activity, as the ALP activity measured from BMP6 and NOG stimulated mouse C2C12 cells. Furthermore, in more recent studies by Song et al. (2010) [27] in which different cell lines were stimulated with different BMPs in the presence or absence of NOG, BMP7 has been shown to be more potent than BMP6 as a negative regulator. NOG expression was also more potently induced by BMP7 than by BMP6. BMP6 was more potent for OB differentiation promotion in vitro and bone regeneration in vivo [27]. Furthermore, Takayama et al. (2009) [107] showed that NOG mRNA was upregulated in C2C12 in response to rhBMP2. In their study, the silencing of NOG resulted in the acceleration of BMP-induced osteoblastic differentiation [107]. Wan et al. (2007) [135] conducted an in vitro and in vivo mouse study of the effect of NOG suppression in OBs. The inhibition of NOG was found to results in enhanced BMP signaling and in vivo bone formation [135]. However, Klineberg et al. (2014) [136] could not find a significant improvement in overall fusion rates compared with that of the controls when NOG was downregulated by electroporation of siRNA targeting NOG in the paraspinal muscle of a posterolateral intertransverse rabbit lumbar fusion model. When NOG is overexpressed, such as in the in vivo mouse study of Devlin et al., (2003) [137] the animals developed decreased bone volume and osteopenia.
In a mouse study by Okamoto et al. (2006) [138], the overexpression of BMP4 and NOG was investigated in bone. The animals overexpressing BMP4 developed severe osteopenia, and the animals overexpressing NOG showed an increased bone volume associated with decreased bone formation [138]. In a study by Tsuji et al. (2008) [139], it was revealed that BMP4 is not necessary for successful skeletal growth and bone healing, which was investigated in mice with floxed BMP4 alleles bred with Prx1-cre transgenic mice to establish the limb-specific removal of BMP4.
Moreover, a study by Suzuki et al. (2012) [140] showed that both BMP antagonists GREM1 and 2 were differentially regulated by BMP2 in the C2C12 cell line. GREM1 expression was found to be downregulated by BMP2, while expression levels of GREM2 were dose-dependently increased upon treatment with BMP2 [140]. Furthermore, Ideno et al. (2009) [115] investigated GREM2 in the pre-OBs of embryonic day 18.5 mouse calvariae, and they confirmed its negative feedback behavior on BMP signaling.
Finally, BMP2 and BMP7 were also investigated in clinical studies. Boden et al. (2002) [141] and Govender et al. (2002) [25] demonstrated the beneficial effect of BMP2 on bone formation in an open tibial fracture or spinal fusion in clinical studies. Friedlaender et al. (2001) [142] conducted a study in 2001, in which the effect of BMP7 was investigated in tibial non-unions. BMP7 was found to be a safe and effective treatment for tibial non-unions, and its output its comparable to that which could be achieved with bone autografts.
Besides this application, the expression level of BMP family members has been the focus of several past studies, such as those listed in Table 2. In a posterolateral intertransverse fusion model in rabbit, Tang et al. (2001) [143] investigated the expression of BMP2, BMP4, BMP7, NOG, and CHRD. They distinguished between the outer (over transverse processes) and inner (between transverse processes) parts, as well as the surrounding muscles. Interestingly, the outer part showed the highest expression of BMP and early bone maturation. During the reparative and remodeling phase, NOG activity was decreased, whereas BMP expression was significantly increased in the outer and inner parts. However, CHRD expression in these two zones increased [143]. This finding suggests that these two BMP antagonists regulate BMP activity through a different mechanism. Furthermore, in the surrounding muscles, BMP expression could be detected, which may indicate that muscle contributes to spinal fusion.
Table 2. Expression of BMP and BMP antagonists in spinal fusion and bone healing.
| Author, Year | Study Type, Species | Cytokine | Study | Conclusion | ||
|---|---|---|---|---|---|---|
| Kloen, 2012 [33] | In vitro, human | BMP2, BMP3, BMP7, pSmad1/5/8, NOG, GREM1, CHRD, BMABI | Expression of endogenous BMP ligands and BMP inhibitors in non-union compared to normal fracture healing | Decreased BMP2 and almost absent BMP7 expression in chondrocytes in non-unions Expression of BMP antagonists, i.e., NOG and GREM1 nearly the same in osteoblasts, chondrocytes, and fibroblasts of both fracture callus and non-unions Generally, expression of BMP antagonists was stronger than BMP |
||
| Fajardo, 2009 [145] | In vitro, human | BMP2, BMP4, BMP5, BMP7, BMP8, CHRD, NOG, GREM1, FLST | Two tissue samples from non-union patients: fibrous tissue from the non-union site and healing bone from the surrounding region | BMP4 and investigated BMP antagonists were upregulated in non-union compared to fracture callus Healing bone showed upregulation of BMP7, and in non-union tissue, an increased expression of BMP4, GREM1, FLST, and NOG but not of CHRD |
||
| Kwong, 2009a [144] | In vitro, human | NOG, CHRD, BMP2, GDF5 | Determination of regional distribution of NOG, CHRD, BMP2, and GDF5 in tissue samples of patients undergoing surgery for failure of conservative management, or failure of the original surgical fixation to maintain alignment of their fracture and who were subsequently found to have fracture union on follow-up by immunohistochemistry Fracture biopsies were taken from extra-articular sites of the humerus, clavicle, femur, tibia, fibula, and acetabulum |
Expression of NOG and CHRD in areas of cartilage formation Detection of NOG in active osteoblasts in areas of bone formation, in endothelial cells, and pericytes of the newly formed blood vessels of the fracture callus GDF5 staining revealed the strongest in fractures, expression, in parts of cartilage formation, it was detected in chondrocytes, osteoblasts, and fibroblastic cells BMP2 expression was the strongest in areas of endochondral ossification in hypertrophic chondrocytes and also in lower extent in osteoblasts, osteocytes, and osteoclasts |
||
| Kwong, 2009b [148] | In vitro, human | NOG, CHRD, BMP2, GDF5 | Investigation of expression of NOG, CHRD, BMP2, and GDF5 in human biopsy samples from fractures, which heal normally or became non-unions | Biopsies from patients with non-union turned out to have a reduction in BMP and GDF5 expression No differences in expression level for NOG and CHRD between non-union and control group |
||
| Tang, 2011 [143] | In vivo, rabbit | BMP2, BMP4, BMP7, NOG, CHRD | Posterolateral intertransverse spinal fusion with autogenous bone graft Investigation of BMP2, BMP4, BMP7, NOG, CHRD, sex determining region Y-box 9 (SOX9), and RUNX2 from specimens collected from the outer zone over the transverse process, in the inner zone between the transverse process, the muscle surrounding bone grafts and the transverse process |
BMP2, BMP4, and BMP7, NOG, and CHRD were co-localized in outer osteoblasts, osteoclasts, and chondrocytes The muscle around bone grafts showed significantly higher BMP expression and RUNX2 activity. |
||
| Dudarić, 2013 [77] | In vitro, rat | BMP2, BMP4, BMP7, CHRD, NOG, FLST | Investigation of expression levels of several BMP and BMP antagonists in induced ectopic bone formation in rats | Increased level of BMP2, BMP4, NOG, and FLST at day 14 of osteogenesis Increased level of GREM1 and CHRD in the later phase, which indicates their role in the regulation of the osteogenesis initiation |
||
| Author, Year | Study Type, Species | Cytokine | Study | Conclusion | ||
| Niikura, 2006 [146] | In vitro, rat | BMP2, BMP3, BMP3B, BMP4, BMP6, BMP7, GDF5, GDF7, NOG, GREM, SOST, BAMBI | Creation of atrophic non-unions in rat femurs, by periosteal cauterisation at the fracture site Measurement of BMP and BMP antagonist’s expression in RNA extracted from the callus of standard healing fracture and fibrosus tissue of non-union |
Gene expression of BMP2, BMP3B, BMP4, BMP6, BMP7, GDF5, GDF7, NOG, DRM, SOST, and BMP and activin membrane-bound inhibitor (BAMBI) were significantly lowered in non-unions compared to normal healing fractures at multiple time points | ||
| Dean, 2010 [147] | In vivo, mouse | BMP2, BMP4, BMP7, BMPRIA, BMPRII, PRDC, SOST, Smad7, GREM1 | Controlled femoral fractures of 40 mice, the tissue samples at the fracture sites were harvested at days 1, 3, 7, 14, and 21 after the intervention and quantified for the expression of BMP and BMP antagonists. | Upregulation of BMP2, BMP4 and BMP7 during frature healing, whereas expressions of GREM2, SOST, Smad7, GREM1, and CER were generally downregulated Significantly upregulation of NOG in the first week after fracture Seven days after the fracture other BMP antagonists such as DAN, CHRD, Smad6, and BAMBI showed increased expression. |
||
| Yoshimura, 2001 [149] | In vivo, mouse | NOG, BMP4 | Temporal and spatial expression of NOG and BMP4 in a repair model of fracture in adult mice | Localisation of BMP4 and NOG were similar in cells within the proliferating periosteal layer, cells lining the newly formed bone (osteoblasts), cartilage tissue including differentiating chondrocytes, and hypertrophic chondrocytes | ||
Kwong et al. (2009) [144] determined the regional and cellular distribution in human healing bone fractures. All four proteins investigated, BMP2, the growth and differentiation factor (GDF) 5, CHRD, and NOG, were expressed stronger in cartilage formation and to a lesser extent in areas of bone formation. In a similar study, the same group compared the same cytokines in biopsies from human fractures that either healed normally or resulted in non-unions. BMP2 and GDF5 were found found to be expressed at lower levels in the non-union group compared with the case in which fractures normally healed. These findings of Kwong et al. (2009) [144] correspond with those of Kloen et al. (2012) [33], who conducted a similar study three years later on comparisons between regular healing fractures and non-unions. Similar to Kwong et al. (2009) [144], they detected decreased BMP2 and an almost absent BMP7 expression in the chondrocytes of non-unions. They agreed with the presence of a different balance between BMP and BMP antagonists [144]. In another highly similar study by Fajardo et al. (2009) [145], gene expressions in non-union and healing bone specimens were compared by qPCR. These authors observed an upregulation of BMP7 in the healing bone, whereas NOG, CHRD, and FLST were upregulated in non-union tissue [145]. Furthermore, Niikura et al. (2006) [146] investigated gene expression from several members of the BMP family in callus standard healing fractures and fibrous tissues of non-union. Similar to Kwong et al. (2009) [144], these authors detected a significantly lower gene expression of several BMPs (BMP2, BMP4, BMP6, BMP7, GDF5, and GDF7) and, surprisingly, even BMP antagonists [146]. Dean et al. (2010) [147] made different observations regarding the
time point of BMP antagonist expression levels during bone healing. They suggested that BMP antagonists should be divided into functional groups - those that are suppressed for the initiation of osteogenesis and those that are upregulated to induce bone remodeling [147].
8. APPLICATION OF BMPS AND BMP ANTAGONISTS IN SPINAL FUSION AND BONE HEALING BY USING MSCs
BMPs exhibit broad spectra of biological activities not only during embryogenesis but also throughout life by influencing various tissues, such as bone, cartilage, blood vessels, heart, kidneys, neurons, liver, and lungs. As previously mentioned, BMPs were first identified for their ability to induce bone formation and hence their coined name. Later, it was also discovered that they act as pleiotropic players [150].
Proper bone formation requires the process of BMP signaling, which leads MSCs to differentiate toward OBs. Besides the studies conducted on spinal fusion models and OB cultures, much research has investigated the effect of BMP family cytokines in MSCs. Because of the plasticity of MSCs, they are useful tools for tissue engineering, gene therapy, or a combination of these two approaches. In this section, the different effects of BMP family cytokines on MSCs are discussed. An overview of the studies belonging to this can be found in Table 3.
Table 3. Application of BMP and BMP antagonists in spinal fusion and bone healing by using mesenchymal stromal cells.
| Author, Year | Study Type, Species | Cytokine | Study | Conclusion | ||||
|---|---|---|---|---|---|---|---|---|
| Wang, 2018 [153] | In vitro, human | NOG, GREM1, CHRD | Knock down of CHRD, NOG and GREM in hBMSCs from patient with normal bone healing and with nonunion Measurement of expression of BMPs and BMP antagonists were measured in hBMSCs of those patients |
hBMSCs, treated with CHRD siRNA had a higher expression of SP7, BGLAP and COLIA1 than hBMSCs, treated with GREM1 siRNA Knock down of NOG decreased the expression of the before mentioned genes Higher expression of NOG CHRD and in hBMSCs from patients with nonunion than from patients with normal fracture healing |
||||
| Hu, 2017 [164] | In vitro, human | BMP2, GREM1 | GREM1 was downregulated in hMSCs by using siRNA | GREM1 suppression significantly increased DNA content, cell metabolism, and enzymatic ALP activity | ||||
| Chen, 2012 [151] | In vitro, human | NOG, BMP2 | Expression of NOG in hMSCs, when stimulated with BMP2, in a dose- and time-depended manner NOG expression was knocked down in MSCs by using siRNA and stimulated with osteogenic medium supplemented with 0.1 µg/mL BMP2 |
NOG induction was enhanced by BMP2 at concentrations from 0.01 to 1 µg/mL, the induction decreased at higher concentrations (1 to 50 µg/mL) Osteoblastic genes (ALP, BSP2, Msh homeobox 2 (MSX2), BGLAP, SPP1, and RUNX2) were significantly decreased in MSC with NOG knockdown |
||||
| Ramasubramanian, 2011 [165] | In vitro, human | BMP2, NOG | Human adipose-derived stromal cells (hADSC) were treated with varying doses of BMP2 DNA and/or siRNA of guanine nucleotide binding protein alpha stimulating activity polypeptide (GNAS) and NOG | No increase in matrix mineralization in hADSC treated with BMP2, while co-delivery of BMP2 with siGNAS or siNOG led to more intense mineralisation Groups treated with BMP2 showed a decrease in calcium deposits and ALP activity compared to siRNA groups Increase in BMP2 expression level in hADSC suppressing NOG Co-delivery of siNOG and BMP2 DNA reduced NOG knockdown and accelerated the differentiation towards osteogenic phenotype marked with increase bone marker expression and mineralisation |
||||
| Kwong, 2008 [152] | In vitro, human | BMP2, CHRD | Measurement of BMP2 and CHRD expression in hMSCs during stimulation with osteogenic medium CHRD knock down was induced using RNA interference during osteogenic stimulation |
Osteogenic differentiation was associated with an increase in BMP2 expression CHRD expression was not detectable with conventional qPCR Knock down of CHRD resulted in a significant increase of ALP activity and deposition of extracellular mineral |
||||
| Rifas, 2007 [109] | In vitro, human | NOG | hMSCs were stimulated with NOG in addition to DEX, BMP (BMP2, BMP6 or BMP7) or inflammatory cytokines. | NOG induced an anabolic effect and induced hMSCs into a committed osteoblast lineage NOG showed no inhibition of DEX-induced ALP activity but rather acting in an additive manner. NOG does not lead to an inhibition of mineralisation induced by BMP; it even synergised with DEX to increase mineralisation NOG induced BMP2 and BGLAP but not RUNX2 |
||||
| Author, Year | Study Type, Species | Cytokine | Study | Conclusion | ||||
| Friedman, 2006 [161] | In vitro, human | BMP2, BMP4, BMP6, BMP7, GDF5 | Osteoinductive effects of hMSCs were examined when stimulated with different BMP | BMP6 showed the most potent, donor-independent osteoinductive effects BMP6 was upregulated by DEX treatment and additional exogenous BMP6 induced hMSCs to differentiate towards an osteoblast phenotype When investigating BMP combinations, only co-treatment with BMP6 lead to robust mineralisation BMP6 leads to expression of SP7, DLX5, BGLAP, SPP1 and BSP but no change in RUNX2 and COL1 expression. |
||||
| Dragoo, 2003 [158] | In vitro, human | BMP2 | Stimulation of pluripotent mesenchymal progenitor cells from liposuction aspirates, and bone marrow aspirate with BMP2 or exposition to adenovirus containing BMP2 Comparison to hOBs or cells cultured in osteogenic media |
Pluripotent mesenchymal progenitor cells were positively transduced with BMP2 gene and transform towards an osteogenic phenotype, comparable when cells were stimulated with exogenous rhBMP or hOBs. | ||||
| Wang, 2016 [81] |
In vitro, human In vivo, mouse |
GREM2, BMP2 | Bone-marrow-derived hMSC were stimulated with BMP2 and GREM2 mRNA expression was investigated in dose-response (0-50 µg/mL BMP2) and time-course studies (0.1 µg/mL BMP2) hMSC were transfected with siRNA targeting GREM2 or transfection with GREM2 expression plasmid Creation of 0.8 mm in diameter segmental bone defect of the left femur in male BALB/C nude mice followed by transplantation of hMSCs resuspended in medium and Matrigel (BD Bioscience) Partial were MSCs infected before with siGREM2 or Lentivirus (LV)-GREM2. |
Higher concentration of BMP2 increased GREM2 expression GREM2 siRNA MSC showed significant suppression of GREM2 expression and a significant increase in COL1A1, BGLAP, SPP1, and ALP Increase of ALP activity after 14 days of induction and measurement of more calcium deposits BMP2/Smad/RUNX2 was activated, as levels of BMPRII, RUNX2 and pSmad1/5/8 were higher in the siGREM2 group compared to control group GREM2 expression in LV-GREM2 transfected cells was significantly increased, as well as protein level Osteoblastic genes, ALP activity and calcium deposits were significantly decreased Callus size in mice femoral bone defect model was considerably larger in siGREM2 groups |
||||
| Hasharoni, 2005 [154] |
In vitro, human In vivo, mouse |
BMP2 | Genetically engineered MSCs, expressing rhBMP2 were implanted into the paraspinal muscles of mice | At 4 weeks postinjection genetically engineered MSCs induce active osteogenesis at the site of implantation Seven days of BMP2 induction was sufficient to form new bone tissue |
||||
| Fan, 2013 [166] | In vitro, mouse | NOG, BMP2 | Downregulation of NOG in adipose-derived stem cells using short hairpin technology (shRNA) Seeding of cells in chitosan or chondroitin sulphate scaffolds loaded with 15 µg/mL BMP2 and culturing in osteogenic medium |
Osteogenic differentiation was significantly higher in NOG shRNA treated cells compared to control cells (both stimulated with BMP2) | ||||
| Author, Year | Study Type, Species | Cytokine | Study | Conclusion | ||||
| Hannallah, 2004 [162] | In vivo, mouse | BMP4, NOG | Implantation of muscle-derived stromal cells (MDSC) transduced with BMP4 into both hind limbs of SCID mice with 0.1, 0.5 or 1 Mio of NOG expressing MDSC (mice were sacrificed after 4 weeks) Human DMBM was implanted into the hind limbs with 0.1, 0.5 or 1 Mio MDSC (mice were sacrificed after 8 weeks) |
Varying doses of NOG expressing MDSC induced a reduction in heterotopic ossification in a dose-dependent manner Each of the three varying doses of NOG expressing MDSC significantly inhibited the heterotopic ossification |
||||
| Sheyn, 2008 [160] |
In vitro, porcine In vivo, mouse |
BMP6 | Primary porcine adipose-tissue-derived stem cells were nucleofected ex vivo with a plasmid containing rhBMP6. Cells were then injected into the lumbar paravertebral muscle in immunodeficient mice |
Cells induced functional bone tissue formation and efficient spinal fusion. | ||||
| Wang, 2010 [159] |
In vitro, canine In vivo, rat |
BMP2 | Stimulation of beagle MSC with different concentrations of BMP2 (0, 25, 50, 100, or 200 ng/mL) or a combination of BMP2 with basic fibroblast growth factor (bFGF) in different ratios Calcium phosphate cement (CPC) was seeded with BMSCs in medium containing 100 ng/mL BMP2, 50 ng/mL bFGF or a combination of BMP2 and bFGF, subsequently cells were subcutaneously implanted in four sites in nude rats (rats were sacrificed four or 12 weeks postoperatively) |
rhBMP2 was a more potent stimulator of BMSC differentiation than bFGF, proliferation was more stimulated with bFGF than with BMP2 CPC demonstrated to be a good bone scaffold, it induces rapid deposition of new bone at the cement surface interface In vitro was the bone formation the highest in groups stimulated with BMP2/bFGF treatment. |
||||
| Wang, 2003 [155] | In vivo, rat | BMP2 | Intertransverse spinal arthrodesis (L4 - L5) was attempted in Lewis rats with BMP2-producing rat bone marrow cells (Ad-BMP2 cells), created through adenoviral gene transfer with guanidine hydrochloride-extracted DBM as a carrier or Ad-BMP2 cells on a collagen sponge carrier Ten µg of recombinant BMP2 (rhBMP2) in a guanidine hydrochloride-extracted DBM carrier or 10 µg of rhBMP2 in a collagen sponge carrier. |
Spines were fused four weeks postoperatively Spines that had received BMP2-producing bone marrow cells were filled with coarse trabecular bone postoperatively Spines receiving rhBMP2 were filled with thin, lace-like trabecular bone |
||||
| Cheng, 2001 [157] | In vitro, in vivo, rabbit | BMP2 | Transfuction of rabbit MSCs with an adenoviral vector carrying human BMP2 gene Transduced MSCs were implanted autologously into the intertransverse process space (L5 and L6 of donor rabbits) |
Cells differentiated into an osteoprogenitor line, bone formation in vitro was induced by increased ALP activity and expression of COL1, SPP1, and BGLAP and induction of matrix mineralisation After four weeks, new bone formation could be demonstrated in vivo |
||||
| Riew, 1998 [156] | In vitro, in vivo, rabbit | BMP2 | MSCs derived from rabbits were transduced with an adenoviral vector carrying the human BMP2 Transduced cells were then autologously reimplanted into donor rabbits |
MSCs transduced with adenovirus carrying the BMP2 gene, overproduce the BMP2 protein Only one of five rabbits, where the transduce MSCs were reimplanted produced radiographically and histologically evident bone |
||||
Several studies have described the upregulation of NOG expression when MSCs are stimulated with BMP2 [151]. Surprisingly, Chen et al. (2012) [151] detected a decrease in osteoblastic genes when NOG was downregulated in hMSCs. Rifas et al. (2007) [109] made a similar observation when they stimulated hMSCs with NOG. hMSCs underwent differentiation toward the osteoblastic lineage induced by NOG. Furthermore, NOG did not inhibit matrix mineralization as expected, but it rather synergized with dexamethasone (DEX) to increase calcium deposition. However, Kwong et al. (2008) [152] showed the upregulation of ALP activity when CHRD was downregulated in hMSCs. They also investigated the expression of BMP2 and CHRD when MSCs were stimulated with standard osteogenic differentiation media, in which an upregulation of BMP2 with the induction of osteogenic differentiation was confirmed; surprisingly, however, no CHRD expression could be detected [152]. Wang et al. (2017) [81] investigated the knock-down of GREM2 in hMSCs and an upregulation in bone-specific markers, such as BGLAP, SPP1, and ALP; higher calcium deposition was detected. The reverse was true when cells were treated with a GREM2 expression plasmid, in which a decrease in osteoblastic genes, ALP activity, and calcium deposits was observed [81]. Recently Wang et al. (2018) [153] performed NOG, GREM1 and CHRD knockdown by using siRNA in human bone mesenchymal stromal cells (hBMSCs). They detected that the knockdown of CHRD induced a stronger osteogenic response than did the knockdown of GREM1 and NOG [153].
Lately, gene therapy studies with MSCs have been conducted to induce bone formation. Hasharoni et al. (2005) [154] implanted hMSCs overexpressing BMP2 in the
paraspinal muscle of mice and induced this is the hallmark of BMP activity, induction of ectopic bone formation osteogenesis at the site of implantation. Wang et al. (2017) [81] compared the different effects of rat MSCs expressing BMP2 through adenoviral transfer with those of BMP2. Both were applied either with DMBM or with a collagen sponge in a rat spinal fusion model (intertransverse spinal arthrodesis between L4 and L5). Here, as in comparable studies, all spines from all groups were fused. However, differences in the manner of bone production could be observed. Spines that received BMP2-producing bone marrow cells were filled with a coarse trabecular bone, whereas spines that received rhBMP2 were filled with a thin, lace-like trabecular bone [155]. An early but similar study by Riew et al. (1998) [156] investigated the effect of MSCs in an in vivo rabbit model. They reimplanted MSCs into donor rabbits and transduced them with an adenovirus, leading to BMP2 overexpression. Surprisingly, compared with the results of other studies, no significant effects were observed, as only one of the five rabbits produced an evident bone because of the reimplantation of transduced MSC. In another study by the same group of Cheng et al. (2001) [157], rabbit MSCs were transduced with an adenoviral vector carrying the human BMP2 gene and were implanted autologously into the intertransverse process space between L5 and L6 of the donor rabbits. This study could confirm bone formation in vitro and in vivo. Another human study by Dragoo et al. (2003) [158] demonstrated successful gene therapy with pluripotent mesenchymal progenitor cells, which were isolated from liposuction aspirates. Here, the cells were transfected with an adenovirus containing BMP2, which led to BMP2 overexpression and, finally, to a shift in mesenchymal progenitor cells toward an osteogenic phenotype [158]. Dragoo et al. (2010) [158] and Wang et al. (2010) [159] also demonstrated the beneficial effects of MSCs stimulated exogenously with BMP2.
In another gene therapy study by Sheyn et al. (2008) [160], MSCs overexpressing BMP6 were injected into the lumbar paravertebral muscle in immune-deficient mice. An induction of spinal fusion was observed here [160]. Friedman et al. (2006) [161] examined the same by testing the effect of several BMPs on hMSCs. The results clearly showed that BMP6 had the most potent osteoinductive effects. In the case of BMP4, the in vivo mouse study of Hannallah et al. (2004) [162] demonstrated that NOG inhibits heterotopic ossification caused by BMP4. However, BMP stimulation had adverse effects. A study by Diefenderfer et al. (2003) [163] showed that stimulation with BMP2, BMP4, or BMP7 on hMSCs after six days does not lead to an upregulation of ALP activity but to an upregulation of the NOG mRNA level. These results may suggest that MSCs use more than one system for transcriptional activation. Several in vitro studies have investigated if silencing of GREM1 or NOG could induce a stronger ossification using hMSCs [164-167], see Table 3.
9. BMPs AND BMP ANTAGONISTS AND THE IVD
LBP, which redundancy is currently one of the most prevalent health problems worldwide, is often associated with damaged or degenerated discs [2]. Because of the avascular nature of IVD, its regeneration capacity is only limited. So far, no therapies are available for IVD regeneration. Painful discs, which cannot be treated anymore with painkillers or physical therapies, are removed, followed by the placement of a structural spacer, internal fixation, and fusion of the degenerated segment through natural bone growth, which is often supported by the application of osteoinductive growth factors. However, different treatment strategies to regenerate a damaged IVD are under investigation, with one approach being the application of growth factors. The most widely used therapy during spinal fusion, particularly in clinics, is the application of BMP2. BMP2 has been examined not only in multiple in vivo studies on spinal fusion but also in several ex vivo and in vivo IVD models, and promising results for IVD regeneration have been obtained. In recent studies, a new aspect that might influence the field in the future was investigated. Whether IVD can undergo ossification because of the cytokines of the BMP family and whether this might be an alternative treatment for spinal fusion patients have been discussed.
For the most widely used therapies - those that involve BMP2 and BMP7 - anabolic effects have been found in different studies (Table 4). An upregulation of aggrecan (ACAN) and COL2 in IVD cells because of BMP2 or BMP7 administration could be confirmed in different species, such as canine, rabbit, rat, bovine, and human [167-171]. However, the effect on COL1 and BGLAP expression remains inconclusive. No change in COL1 expression was observed in the studies of Li et al. (2004) [169] after the stimulation of rat AFC in vitro or after BMP2/BMP7 stimulation of bovine nucleus pulposus cells (NPCs) in a 3D fibrin-hyaluronan culture (2017) [170], whereas Kim et al. (2003) [171] showed an upregulation of COL1 in human IVD cells in 3D alginate beads stimulated with BMP2. Besides COL1, Kim et al. (2003) [171] also showed an upregulation of ACAN and COL2 expression. However, their study found no change in BGLAP, whereas Brown et al. (2018) [6] was able to observe an upregulation of BGLAP in a human NPC monolayer stimulated with BMP2. Furthermore, Lee et al. (2012) [172] could not detect an osteogenic effect on rabbit NPCs after stimulation with exogenous BMP2, as they did not observe any BGLAP expression. In the study of Kim et al. (2003) [171], cells cultured in a monolayer showed no osteogenic marker gene expression, ALP activity, or calcium deposition at the given dose of BMP2 [171].
Table 4. Application of BMP and BMP antagonists in intervertebral disc regeneration or ossification.
| Author, Year | Study Type, Species | Cytokine | Study | Conclusion | ||||
|---|---|---|---|---|---|---|---|---|
| Brown, 2018 [6] |
In vitro, in vivo, human |
BMP2, BMP7 | Stimulation of NPCs from hIVDs in monolayer with osteogenic medium containing 1.25-dihydroxyvitamin D3 (VitD3), parathyroid hormone (PTH) and BMP2/BMP7 Explant cultures of IVDs in osteogenic medium ± prior exposure to VitD3 and BMP2 |
Upregulation of RUNX2, BGLAP, and SPP1 No osteogenic differentiation after application of BMP. One culture explant showed regions of calcification after stimulation with VitD3 and BMP2, another one after the stimulation with osteogenic medium |
||||
| Imai, 2007 [178] | In vitro, human | BMP7 | NPCs and AFCs from four cadaveric discs and one surgical specimen were cultured in 3D alginate beads for 21 days and stimulated with 0, 100 or 200 ng/mL BMP7 and 10% fetal bovine serum | Significant upregulation of proteoglycan synthesis in BMP7 treated NPC and AFC beads compared to control beads Cell proliferation was stimulated because of BMP7 |
||||
| Wei, 2008 [173] | In vitro, human | BMP7 | hNPC were stimulated with rhBMP7 and inducers of apoptosis (tumour necrosis factor-alpha (TNF-α) or hydrogen peroxide (H2O2)) | BMP7 had a positive effect on extracellular matrix production, which was reduced because of TNF-α and H2O2 stimulation BMP7 showed antiapoptotic effects, but no evidence of bone formation induction |
||||
| Kim, 2003 [171] | In vitro, human | BMP2 | hIVD cells were cultured in 3D alginate beads and stimulated with different concentrations of BMP2 (0-2000 ng/mL) for 21 days. | ACAN, COL1 and COL2 expression, as well as proteoglycan synthesis, were upregulated in stimulated cells, BGLAP showed no expression upon stimulation | ||||
| Li, 2017 [170] | In vitro, bovine | BMP2/7 | Regenerative effect of BMP2/7 heterodimer was investigated in bovine in vitro and in organ culture NPCs were cultured in a fibrin-hyaluronan hydrogel for 14 days. For in organ culture, a BMP2/7 heterodimer was delivered into the nucleotomized region |
COL2 and ACAN expression and glycosaminoglycan (GAG) content were upregulated in NPC Remaining NP tissue showed an increase in proteoglycan synthesis after stimulation with BMP2/7, COL1expression and ALP activity were not affected No observation of fibroblastic or osteogenic effects in the disc tissue |
||||
| Willems, 2015 [167] |
In vitro, in vivo, canine |
BMP7 | Stimulation of canine NPC in vitro with 100 ng/mL rhBMP7 to assess anabolic effects Injection of different dosages of rhBMP7 (2.5 µg, 25 µg, 250 µg) into early degenerated IVDs of canines (evaluation after six months or post-mortem) |
Gene expression of ACAN and COL2A1 was upregulated after the stimulation of canine NPC in vitro. In vivo, no regenerative effects, but extensive extradiscal bone formation after the intradiscal injection with 25 µg and 250 µg of rhBMP7 |
||||
| Haschtmann, 2012 [168] | In vitro, rabbit | BMP2, TGF-β3 | Stimulation of rabbit IVD explants with 1 µg/mL BMP2 or TGF-β3 for 21 days (NP and AF were analysed separately) | Upregulation of COL1, COL2, and ACAN because of stimulation with BMP2 and TGF-β3 in AF, but matrix metallopeptidase genes were inhibited In NP, BMP2 stimulation leads to decreased COL2 expression Induction of ossification in AF tissue (shown by histology) |
||||
| Lee, 2012 [172] | In vitro, rabbit | BMP2, TGF-β1 | Rabbit NPCs were cultured in antelocollagen type 1 and 2 scaffolds and stimulated with exogenous BMP2 (100 ng/mL) and TGF-β1 (10 ng/mL). | Significant increase in proteoglycan production in cells in antelocollagen 2 scaffold and TGF-β1 stimulation or co-treatment Groups of both scaffolds with BMP2 or/and TGF-β1 had increased COL1, COL2, and ACAN expression, but no expression of BGLAP Treatment groups exhibited a significantly increased cellular proliferation, but no additive or synergistic effects could be detected for the two cytokines, neither in proliferation nor matrix synthesis. |
||||
| Author, Year | Study Type, Species | Cytokine | Study | Conclusion | ||||
| Leckie, 2012 [179] | In vitro, rabbit | BMP2 | Rabbit discs (L2-L3, L3-L4, and L4-L5) were punctured and then treated with adeno-associated virus serotype 2 carrying BMP2 gene (analysis after 0, 6 and 12 weeks, rabbits were sacrificed after 12 weeks) | Delay of degenerative changes of the disc confirmed by MRI, histology, serum biochemical, and biomechanical criteria analysis | ||||
| Huang, 2007 [180] | In vitro, rabbit | BMP2 | Injection of 1 mg/ rhBMP2 ± coral grafts (L2-L3, L3-L4, and L4-L5) in rabbits after receiving annular tears | More degeneration in groups treated with BMP2 than control groups (treated with a physiological saline solution onl). BMP2 induced hypervascularity and fibroblast proliferation after an annular tear |
||||
| Masuda, 2006 [181] | In vivo, rabbit | BMP7 | Injection of BMP7 into the NP of rabbits, which received an annular puncture with an 18-gauge needle four weeks prior (after 2, 4, 8, 12 and 24 weeks, rabbits were sacrificed) | Single injection of BMP7 into the NP of the punctured rabbit discs lead to a restoring effect of the IVDs Disc height was sustained for up to 24 weeks. |
||||
| An, 2005 [182] | In vivo, rabbit | BMP7 | Injection in consecutive rabbit discs, 2 µg BMP7 in the NP by using a 28-gauge needle (radiographically analysis after 2, 4 and 8 weeks) in control group, discs were treated with physiological saline solution | Mean disc height was greater and proteoglycan content higher in groups treated with BMP7 compared to control group Higher DNA content in AF in BMP7 treated groups, no differences in NP |
||||
| Masuda, 2003 [183] | In vitro, rabbit | BMP7 | Rabbit NPCs and AFCs were cultured in 3D alginate beads and stimulated with 0, 50, 100 or 200 ng/mL BMP7 | Proteoglycans and collagens were upregulated in a dose-related manner (increase in COL2 and ACAN mRNA level) DNA content was increased after BMP7 stimulation compared to control groups. |
||||
| Li, 2004 [169] | In vitro, rat | BMP2 | Rat AFCs and cells from the transition zone were stimulated with 200 ng/mL BMP2 | Increased production and expression of COL2 and ACAN after three days of stimulation with BMP2 but COL1 stayed unaffected Significant increase of BMP7 expression by BMP2 stimulation |
||||
| Yoon, 2003 [184] | In vitro, rat | BMP2 | Rat AFC and cells of the transition zones of lumbar IVDs were treated with different concentrations of rhBMP2 (0, 10, 100, 1000 ng/mL). | Upregulation in GAG content in dose depending manner after seven days Upregulation of COL2, ACAN, SOX9, and BGLAP in cells treated with a higher dosage of rhBMP2 (100 and 1000 ng/mL) but no change in COL1 expression Overall rhBMP2 treatment increased disc cell proliferation. |
||||
However, studies concerning the osteogenic potential of IVD contradict themselves. While some authors were able to detect ossification in the outer AF [6, 168], other investigations did not show any evidence of osteogenic effects in the tissue [170, 173]. Interestingly, in a study by Willems et al. [167], an extensive ventral extradiscal bone formation after the injection of rhBMP7 in the NP (T13-S1) of canine discs could be observed. Furthermore, in some diseases, an ossification of the outer AF could be detected, as in diffuse idiopathic skeletal hyperostosis or ankylosing spondylitis [174, 175].
Moreover, the studies of Karamouzian et al. (2010) [176] and Brown et al. (2018) [6] showed that herniated discs possess a higher ability to calcify compared with normal or degenerated discs. One possible reason for this inequality in calcification ability among IVDs may be the difference in expression of BMP antagonists, such as NOG, GREM1, or CHRD in IVDs, which has already been shown in several studies [6, 35]. This endogenous expression of BMP antagonists might also be a possible reason for spinal fusion failure [34, 35, 177].
Lately, Chan et al. (2015) [34] found evidence of the inhibitory effects of IVD cells if they are co-cultured with bone-marrow-derived MSCs undergoing ossification. These effects could also be found in direct contact culture of fresh human disc tissue samples on MSC monolayers, as detected histologically with alizarin red staining (Fig. 2). For this, either NP tissue (NPT), annulus fibrosus (AFT), or cartilaginous endplate tissue (CEPT) were placed on an hMSC monolayer and stimulated for 21 days with an osteogenic medium. After 21 days, a lower deposition of the mineralized matrix was detected in areas surrounding the NPT, AFT, or CEPT compared with the remaining MSC monolayer. In another experiment, these results could also be statistically confirmed by a higher donor number. Furthermore, some of the co-cultures were additionally supplemented with L51P [177]. In these supplemented co-cultures, osteogenic differentiation was significantly less affected by the presence of IVD cells. These in vitro results are promising, because the action of L51P seemed to unblock the inhibitory effects of the secretome of IVD cells.
Fig. (2).
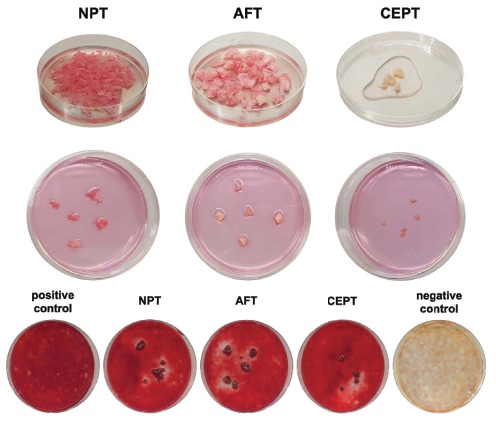
Human mesenchymal stromal cells (hMSCs) were seeded in 100 mm Petri dishes and cultured until they reached 90% confluency. Human intervertebral disc (IVD) explants (tissue of the nucleus pulposus (NPT), annulus fibrosus (AFT), or cartilaginous endplate (CEPT), 2 - 5 mm3) were cultured in direct contact with the hMSCs in an osteogenic medium (lacking bone morphogenetic protein 2). Top row: Preparation of the tissue. Middle row: Contribution of human NPT, AFT, and CEPT on top of the hMSC monolayer. Bottom row: Alizarin red staining of direct culture after stimulation for 21 days with an osteogenic medium (except negative control) and co-cultured with NPT, AFT, and CEPT. Proof-of-concept of inhibitory effects (N = 1). (A higher resolution / colour version of this figure is available in the electronic copy of the article).
However, when the experiment is repeated but adult OBs isolated from human femur instead of bone-marrow-derived MSCs are the focus, the inhibitory effects could not be observed to the same extent [35].
CONCLUSION
The aim of this review is to provide an overview on the specific roles of various BMP and BMP antagonists in orthopedic surgeries. We discussed studies from the last two decades, in which BMP and BMP antagonists were investigated in spinal fusion and bone healing models and in MSC or IVD cells.
In clinics, BMPs are leading to robust bone formation but frequently also to adverse effects. In pre-clinical testing, however, Zara et al. (2011) [185] described the side effects of a high dose of BMP2 (up to 45 µg in the rat femoral segmental defect model) by observing tissue inflammatory infiltrates and cyst-like bone formation after application. Furthermore, there are other problems in the application of growth factors, such as their short-term bioavailability when they are directly applied, the need for a complex biological carrier, or the control of growth factor release over time; these issues need to be addressed [133]. The BMP variant L51P could be a possible solution to decrease the high amounts of exogenous BMP2 that have been used [29]. Some in vitro studies have already investigated the effects of L51P. However, in vivo studies are still needed.
In general, a higher fusion potential was concluded in studies investigating any of the above-mentioned BMPs as summarised in Table 1. BMP antagonists, such as GREM1 and GREM2, showed inhibitory effects on bone formation, whereas the effect of NOG remains inconlusive. The same was true regarding the expression pattern during bone formation; the expression, particularly of BMP antagonists, remains uncertain. Tang et al. (2011) [143] detected an increased NOG but a decreased CHRD expression during the reparative phase after posterolateral spine fusion in rabbit. It has been proven that BMP expressions differ between non-unions and regular healing fractures.
In multiple mouse and rat studies, it has been shown that BMPs promote OB differentiation [134], whereas the effect of BMPs in hMSCs has not been conclusive [163, 186, 187]. hMSCs seem to show differences in the regulation of BMPs. Friedmann et al. (2006) [161] did not find a significant increase in ALP activity in hMSCs with the application of BMP2 under serum-free conditions. However, they demonstrated a beneficial synergistic effect when different BMPs were applied together (BMP6 and BMP7). One possible explanation for the lack of hMSC response to BMP2 and BMP4 could be the high affinity of BMP antagonists for BMP2 and BMP4, whereas BMP6 and BMP7 have a much lower affinity [161]. Fan et al. (2006) [166] discovered that adipose-derived stem cells with an NOG knock-down and stimulated with BMP2 showed enhanced osteogenic differentiation compared with control cells, which indicated that NOG suppression can enhance the activity of exogenous BMP. Co-delivery of siNOG and BMP2 DNA in human ADSC reduced NOG knockdown. Overexpression of BMP2 might stimulate the expression of NOG through a negative feedback mechanism (Fig. 3) [165].
Fig. (3).
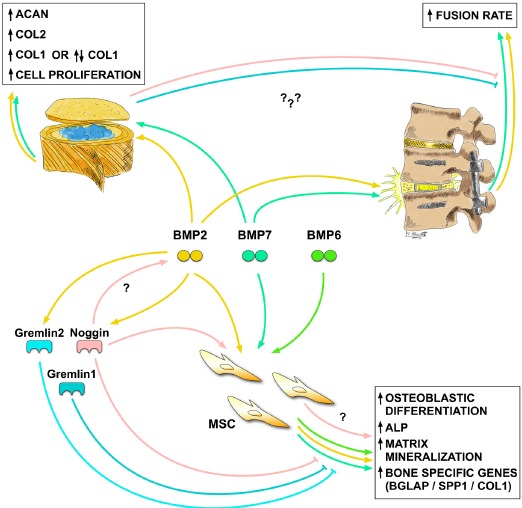
Summary of the different effects of the main BMPs and BMP antagonists discussed in this review. This overview provides the main findings and how BMPs and BMP antagonists affect the bone in spinal fusion studies, the intervertebral disc (IVD), and mesenchymal stromal cells (MSCs). Effects that have already been found but thus far not confirmed are labeled with a question mark. BMP2 and BMP7 have a beneficial effect on spinal fusion, whereas BMP antagonists (probably also expressed in IVD) have been shown to inhibit bone formation after spinal fusion. In IVD, BMP2 and BMP7 induce upregulation of aggrecan (ACAN) and collagen type 2 (COL2) and in some studies, as well, collagen type 1 (COL1); however, COL1 also seems to be unaffected in some cases. Regarding the effect on MSCs, it could be shown in several studies that BMPs, as well as noggin (NOG) in some cases, increase osteoblastic differentiation by up-regulating alkaline phosphatase (ALP) activity, matrix mineralization, and bone-specific genes as osteopontin (SPP1), osteocalcin (BGLAP), and COL1. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
With regard to how BMP antagonists influence MSCs, there is no consensus in the literature. Dean et al. (2010) [147] explained that BMP antagonists related to bone healing can be divided into two functional groups: one that is downregulated and is essential for the initiation of osteogenesis and another that is upregulated and thus functions in the remodeling of newly formed bones [147].
Current studies also disagree on the interaction of BMPs in IVD cells. Overall, the conclusion is that whether BMP has a chondrogenic or an osteogenic effect on IVD cells remains unclear. In most of the studies in which IVD cells were stimulated with BMPs, an upregulation of ACAN and COL2 was observed, whereas the exact effect on COL1 seems to be inconclusive. In several studies, however, even an ossification of the AF could be detected.
The clinical application of cytokines seems to be a good option to improve fusion rates, but the correct dose, the ideal conditions, and the exact mechanism involved remain unclear. Despite the many studies investigating the effect of BMPs and their antagonists, additional research is necessary to shed light on their effects on degenerated IVDs.
ACKNOWLEDGEMENTS
Declared none.
LIST OF ABBREVIATIONS
- ABG
Autologous Bone Graft
- ActRI
Activin Receptor Type-1
- ADSC
Human Adipose-Derived Stem Cell
- AF
Annulus Fibrosus
- AFC
AF Cell
- AFT
AF Tissue
- ACAN
Aggrecan
- ALK3
Activin Receptor-Like Kinase 3
- ALK6
Activin Receptor-Like Kinase 6
- ALP
Alkaline Phosphatase
- BAMBI
BMP and Activin Membrane-Bound Inhibitor
- bFGF
Basic Fibroblast Growth Factor
- BGLAP
Osteocalcin
- bMSC
Bone Mesenchymal Stromal Cell
- BMP
Bone Morphogenetic Protein
- BMP2
Bone Morphogenetic Protein 2
- BMPRI
BMP Receptor I
- BMPRII
BMP Receptor II
- BSP
Bone Sialoprotein
- CBD-BMP2
Collagen Binding BMP2
- CEPT
Cartilaginous end Plate Tissue
- CHRD
Chordin
- CMV
Cytomegalovirus
- COL1
Collagen Type 1
- COL2
Collagen Type 2
- CPC
Calcium Phosphate Cement
- DAN
Differential Screening-Selected Gene Aberrative in Neuroblastoma
- DMBM
Demineralized Bone Matrix
- DLX5
Distal-Less Homeobox Protein 5
- DRM
Downregulated by V-Mos
- FLST
Follistatin
- GAG
Glycosaminoglycan
- GDF
Growth and Differentiation Factor
- GNAS
Guanine Nucleotide Binding Protein Alpha Stimulating Activity Polypeptide
- GREM1
Gremlin 1
- GREM2
Gremlin 2
- H2O2
Hydrogen Peroxide
- hMSC
Human MSC
- IGF1
Insulin-Like Growth Factor 1
- IVD
Intervertebral Disc
- kDA
Kilodalton
- LBP
Low Back Pain
- MDSC
Muscle-Derived Stromal Cell
- MSC
Mesenchymal Stromal Cell
- MSX2
Msh Homeobox 2
- NOG
Noggin
- NP
Nucleus Pulposus
- NPC
NP Cell
- NPT
NP Tissue
- PDGF
Platelet-Derived Growth Factor
- PRDC
Protein related to DAN and Cerberus
- pSmad
Phosphorylated Smad
- PTH
Parathyroid Hormone
- rhBMP2
Recombinant Human BMP2
- RUNX2
Runt-Related Transcription Factor 2
- shRNA
Small Hairpin RNA
- siRNA
Small Interfiering RNA
- Smad
Single Mothers Against Decapentaplegic Homolog
- SOST
Sclerostin
- SOX9
Sex-Determining Region Y-box 9
- SP7
Osterix
- SPP1
Osteopontin
- TBC
True Bone Ceramics
- TGF-β
Transforming Growth Factor Beta
- TGF-β1
Transforming Growth Factor Beta 1
- TGF-β3
Transforming Growth Factor Beta 3
- TNF-α
Tumor Necrosis Factor-Alpha
- TSRH
Texas Scottish Rite Hospital
- TWSG
Twisted Gastrulation
- VEGF
Vascular Endothelial Growth Factor
- VitD3
1.25-dihydroxyvitamin D3
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
This work was supported by direct funds from Hansjörg Wyss and from the Hansjörg Wyss Medical foundation. Christoph E. Albers was supported by grants from Swiss Orthopaedics and the clinical trial unit of the Insel hospital Bern.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
SELECTION CRITERIA FOR INCLUDED STUDIES
For this review, we performed a search on PubMed using the keywords “spinal fusion” or “osteoblast,” “intervertebral disc,” “mesenchymal stem cell,” AND “BMP” or “BMP antagonist.”
We included studies published in 1998 to 2019 that were considered relevant to the subject. The search exceptions are important historical publications on BMPs.
REFERENCES
- 1.Rubin D.I. Epidemiology and risk factors for spine pain. Neurol. Clin. 2007;25(2):353–371. doi: 10.1016/j.ncl.2007.01.004. [http://dx.doi.org/10.1016/j.ncl.2007.01.004]. [PMID: 17445733]. [DOI] [PubMed] [Google Scholar]
- 2.Hoy D., March L., Brooks P., et al. The global burden of low back pain: estimates from the Global Burden of Disease 2010 study. Ann. Rheum. Dis. 2014;73(6):968–974. doi: 10.1136/annrheumdis-2013-204428. [http://dx.doi.org/10.1136/annrheumdis-2013-204428]. [PMID: 24665116]. [DOI] [PubMed] [Google Scholar]
- 3.Knezevic N.N., Mandalia S., Raasch J., Knezevic I., Candido K.D. Treatment of chronic low back pain - new approaches on the horizon. J. Pain Res. 2017;10:1111–1123. doi: 10.2147/JPR.S132769. [http://dx.doi.org/10.2147/JPR.S132769]. [PMID: 28546769]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaz K., Verma K., Protopsaltis T., Schwab F., Lonner B., Errico T. Bone grafting options for lumbar spine surgery: A review examining clinical efficacy and complications. SAS J. 2010;4(3):75–86. doi: 10.1016/j.esas.2010.01.004. [http://dx.doi.org/10.1016/j.esas.2010.01.004]. [PMID: 25802654]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bodalia P.N., Balaji V., Kaila R., Wilson L. Effectiveness and safety of recombinant human bone morphogenetic protein-2 for adults with lumbar spine pseudarthrosis following spinal fusion surgery: A systematic review. Bone Joint Res. 2016;5(4):145–152. doi: 10.1302/2046-3758.54.2000418. [http://dx.doi.org/10.1302/2046-3758.54.2000418]. [PMID: 27121215]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown S.J., Turner S.A., Balain B.S., Davidson N.T., Roberts S. Is osteogenic differentiation of human nucleus pulposus cells a possibility for biological spinal fusion? Cartilage. 2018:1947603518754628. doi: 10.1177/1947603518754628. [http://dx.doi.org/10.1177/1947603518754628]. [PMID: 29361851]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berjano P., Langella F., Damilano M., et al. Fusion rate following extreme lateral lumbar interbody fusion. Eur. Spine J. 2015;24(Suppl. 3):369–371. doi: 10.1007/s00586-015-3929-7. [http://dx.doi.org/10.1007/s00586-015-3929-7]. [PMID: 25893332]. [DOI] [PubMed] [Google Scholar]
- 8.Chun D.S., Baker K.C., Hsu W.K. Lumbar pseudarthrosis: A review of current diagnosis and treatment. Neurosurg. Focus. 2015;39(4):E10. doi: 10.3171/2015.7.FOCUS15292. [http://dx.doi.org/10.3171/2015.7.FOCUS15292]. [PMID: 26424334]. [DOI] [PubMed] [Google Scholar]
- 9.DePalma A.F., Rothman R.H. The nature of pseudoarthrosis. 1968. Clin. Orthop. Relat. Res. 1992;(284):3–9. [PMID: 1395309]. [PubMed] [Google Scholar]
- 10.Osterman H., Sund R., Seitsalo S., Keskimäki I. Risk of multiple reoperations after lumbar discectomy: a population-based study. Spine. 2003;28(6):621–627. doi: 10.1097/01.BRS.0000049908.15854.ED. [http://dx.doi.org/10.1097/01.BRS.0000049908.15854.ED]. [PMID: 12642772]. [DOI] [PubMed] [Google Scholar]
- 11.Walsh D.W., Godson C., Brazil D.P., Martin F. Extracellular BMP-antagonist regulation in development and disease: Tied up in knots. Trends Cell Biol. 2010;20(5):244–256. doi: 10.1016/j.tcb.2010.01.008. [http://dx.doi.org/10.1016/j.tcb.2010.01.008]. [PMID: 20188563]. [DOI] [PubMed] [Google Scholar]
- 12.Wu M., Chen G., Li Y-P. TGF-β and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res. 2016;4:16009. doi: 10.1038/boneres.2016.9. [http://dx.doi.org/10.1038/boneres.2016.9]. [PMID: 27563484]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nolan K., Thompson T.B. The DAN family: Modulators of TGF-β signaling and beyond. Protein Sci. 2014;23(8):999–1012. doi: 10.1002/pro.2485. [http://dx.doi.org/10.1002/pro.2485]. [PMID: 24810382]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brazil D.P., Church R.H., Surae S., Godson C., Martin F. BMP signalling: agony and antagony in the family. Trends Cell Biol. 2015;25(5):249–264. doi: 10.1016/j.tcb.2014.12.004. [http://dx.doi.org/10.1016/j.tcb.2014.12.004]. [PMID: 25592806]. [DOI] [PubMed] [Google Scholar]
- 15.Worthley D.L., Churchill M., Compton J.T., et al. Gremlin 1 identifies a skeletal stem cell with bone, cartilage, and reticular stromal potential. Cell. 2015;160(1-2):269–284. doi: 10.1016/j.cell.2014.11.042. [http://dx.doi.org/10.1016/j.cell.2014.11.042]. [PMID: 25594183]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vaibhav B., Nilesh P., Vikram S., Anshul C. Bone morphogenic protein and its application in trauma cases: A current concept update. Injury. 2007;38(11):1227–1235. doi: 10.1016/j.injury.2006.12.012. [http://dx.doi.org/10.1016/j.injury.2006.12.012]. [PMID: 17307180]. [DOI] [PubMed] [Google Scholar]
- 17.Axelrad T.W., Einhorn T.A. Bone morphogenetic proteins in orthopaedic surgery. Cytokine Growth Factor Rev. 2009;20(5-6):481–488. doi: 10.1016/j.cytogfr.2009.10.003. [http://dx.doi.org/10.1016/j.cytogfr.2009.10.003]. [PMID: 19892584]. [DOI] [PubMed] [Google Scholar]
- 18.Argintar E., Edwards S., Delahay J. Bone morphogenetic proteins in orthopaedic trauma surgery. Injury. 2011;42(8):730–734. doi: 10.1016/j.injury.2010.11.016. [http://dx.doi.org/10.1016/j.injury.2010.11.016]. [PMID: 21145058]. [DOI] [PubMed] [Google Scholar]
- 19.Burkus J.K., Gornet M.F., Dickman C.A., Zdeblick T.A. Anterior lumbar interbody fusion using rhBMP-2 with tapered interbody cages. J. Spinal Disord. Tech. 2002;15(5):337–349. doi: 10.1097/00024720-200210000-00001. [http://dx.doi.org/10.1097/00024720-200210000-00001]. [PMID: 12394656]. [DOI] [PubMed] [Google Scholar]
- 20.Cheng H., Jiang W., Phillips F.M., et al. Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs). J. Bone Joint Surg. Am. 2003;85(8):1544–1552. doi: 10.2106/00004623-200308000-00017. [http://dx.doi.org/10.2106/00004623-200308000-00017]. [PMID: 12925636]. [DOI] [PubMed] [Google Scholar]
- 21.McClellan J.W., Mulconrey D.S., Forbes R.J., Fullmer N. Vertebral bone resorption after transforaminal lumbar interbody fusion with bone morphogenetic protein (rhBMP-2). J. Spinal Disord. Tech. 2006;19(7):483–486. doi: 10.1097/01.bsd.0000211231.83716.4b. [http://dx.doi.org/10.1097/01.bsd.0000211231.83716.4b]. [PMID: 17021411]. [DOI] [PubMed] [Google Scholar]
- 22.Buttermann G.R. Prospective nonrandomized comparison of an allograft with bone morphogenic protein versus an iliac-crest autograft in anterior cervical discectomy and fusion. Spine J. 2008;8(3):426–435. doi: 10.1016/j.spinee.2006.12.006. [http://dx.doi.org/10.1016/j.spinee.2006.12.006]. [PMID: 17977799]. [DOI] [PubMed] [Google Scholar]
- 23.Garrison K.R., Shemilt I., Donell S., et al. Bone morphogenetic protein (BMP) for fracture healing in adults. Cochrane Database Syst. Rev. 2010;(6):CD006950. doi: 10.1002/14651858.CD006950.pub2. [http://dx.doi.org/10.1002/14651858.CD006950.pub2]. [PMID: 20556771]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poynton A.R., Lane J.M. Safety profile for the clinical use of bone morphogenetic proteins in the spine. Spine. 2002;27(16) Suppl. 1:S40–S48. doi: 10.1097/00007632-200208151-00010. [http://dx.doi.org/10.1097/00007632-200208151-00010]. [PMID: 12205419]. [DOI] [PubMed] [Google Scholar]
- 25.Govender S., Csimma C., Genant H.K., et al. Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: a prospective, controlled, randomized study of four hundred and fifty patients. J. Bone Joint Surg. Am. 2002;84(12):2123–2134. doi: 10.2106/00004623-200212000-00001. [http://dx.doi.org/10.2106/00004623-200212000-00001]. [PMID: 12473698]. [DOI] [PubMed] [Google Scholar]
- 26.Boden S.D., Labropoulos P.A., Ragsdale B.D., Gullino P.M., Gerber L.H. Retinyl acetate-induced arthritis in C3H-A(vy) mice. Arthritis Rheum. 1989;32(5):625–633. doi: 10.1002/anr.1780320517. [http://dx.doi.org/10.1002/anr.1780320517]. [PMID: 2719733]. [DOI] [PubMed] [Google Scholar]
- 27.Song K., Krause C., Shi S., et al. Identification of a key residue mediating bone morphogenetic protein (BMP)-6 resistance to noggin inhibition allows for engineered BMPs with superior agonist activity. J. Biol. Chem. 2010;285(16):12169–12180. doi: 10.1074/jbc.M109.087197. [http://dx.doi.org/10.1074/jbc.M109.087197]. [PMID: 20048150]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cahill K.S., Chi J.H., Day A., Claus E.B. Prevalence, complications, and hospital charges associated with use of bone-morphogenetic proteins in spinal fusion procedures. JAMA. 2009;302(1):58–66. doi: 10.1001/jama.2009.956. [http://dx.doi.org/10.1001/jama.2009.956]. [PMID: 19567440]. [DOI] [PubMed] [Google Scholar]
- 29.Carragee E.J., Hurwitz E.L., Weiner B.K. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J. 2011;11(6):471–491. doi: 10.1016/j.spinee.2011.04.023. [http://dx.doi.org/10.1016/j.spinee.2011.04.023]. [PMID: 21729796]. [DOI] [PubMed] [Google Scholar]
- 30.Simmonds M.C., Brown J.V., Heirs M.K., et al. Safety and effectiveness of recombinant human bone morphogenetic protein-2 for spinal fusion: a meta-analysis of individual-participant data. Ann. Intern. Med. 2013;158(12):877–889. doi: 10.7326/0003-4819-158-12-201306180-00005. [http://dx.doi.org/10.7326/0003-4819-158-12-201306180-00005]. [PMID: 23778905]. [DOI] [PubMed] [Google Scholar]
- 31.Lo K.W., Ulery B.D., Ashe K.M., Laurencin C.T. Studies of bone morphogenetic protein-based surgical repair. Adv. Drug Deliv. Rev. 2012;64(12):1277–1291. doi: 10.1016/j.addr.2012.03.014. [http://dx.doi.org/10.1016/j.addr.2012.03.014]. [PMID: 22512928]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hruska K.A., Guo G., Wozniak M., et al. Osteogenic protein-1 prevents renal fibrogenesis associated with ureteral obstruction. Am. J. Physiol. Renal Physiol. 2000;279(1):F130–F143. doi: 10.1152/ajprenal.2000.279.1.F130. [http://dx.doi.org/10.1152/ajprenal.2000.279.1.F130]. [PMID: 10894795]. [DOI] [PubMed] [Google Scholar]
- 33.Kloen P., Lauzier D., Hamdy R.C. Co-expression of BMPs and BMP-inhibitors in human fractures and non-unions. Bone. 2012;51(1):59–68. doi: 10.1016/j.bone.2012.03.032. [http://dx.doi.org/10.1016/j.bone.2012.03.032]. [PMID: 22521262]. [DOI] [PubMed] [Google Scholar]
- 34.Chan S.C., Tekari A., Benneker L.M., Heini P.F., Gantenbein B. Osteogenic differentiation of bone marrow stromal cells is hindered by the presence of intervertebral disc cells. Arthritis Res. Ther. 2015;18:29. doi: 10.1186/s13075-015-0900-2. [http://dx.doi.org/10.1186/s13075-015-0900-2]. [PMID: 26809343]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.May R.D., Frauchiger D.A., Albers C.E., Benneker L.M., Kohl S., Gantenbein B. Inhibitory Effects of Human Primary Intervertebral Disc Cells on Human Primary Osteoblasts in a Co-Culture System. Int. J. Mol. Sci. 2018;19(4):19. doi: 10.3390/ijms19041195. [http://dx.doi.org/10.3390/ijms19041195]. [PMID: 29652862]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salgado A.J., Coutinho O.P., Reis R.L. Bone tissue engineering: State of the art and future trends. Macromol. Biosci. 2004;4(8):743–765. doi: 10.1002/mabi.200400026. [http://dx.doi.org/10.1002/mabi.200400026]. [PMID: 15468269]. [DOI] [PubMed] [Google Scholar]
- 37.Urist M.R. Bone: Formation by autoinduction. Science. 1965;150(3698):893–899. doi: 10.1126/science.150.3698.893. [http://dx.doi.org/10.1126/science.150.3698.893]. [PMID: 5319761]. [DOI] [PubMed] [Google Scholar]
- 38.Shehadi J.A., Elzein S.M. Review of commercially available demineralized bone matrix products for spinal fusions: A selection paradigm. Surg. Neurol. Int. 2017;8:203. doi: 10.4103/sni.sni_155_17. [http://dx.doi.org/10.4103/sni.sni_155_17]. [PMID: 28904830]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gamradt S.C., Lieberman J.R. Bone graft for revision hip arthroplasty: biology and future applications. Clin. Orthop. Relat. Res. 2003;(417):183–194. doi: 10.1097/01.blo.0000096814.78689.77. [PMID: 14646716]. [DOI] [PubMed] [Google Scholar]
- 40.Grgurevic L., Pecina M., Vukicevic S., Marshall R., Marshall R. Urist and the discovery of bone morphogenetic proteins. Int. Orthop. 2017;41(5):1065–1069. doi: 10.1007/s00264-017-3402-9. [http://dx.doi.org/10.1007/s00264-017-3402-9]. [PMID: 28188395]. [DOI] [PubMed] [Google Scholar]
- 41.Wozney J.M., Rosen V., Celeste A.J., et al. Novel regulators of bone formation: molecular clones and activities. Science. 1988;242(4885):1528–1534. doi: 10.1126/science.3201241. [http://dx.doi.org/10.1126/science.3201241]. [PMID: 3201241]. [DOI] [PubMed] [Google Scholar]
- 42.Johnson E.E., Urist M.R., Finerman G.A. Bone morphogenetic protein augmentation grafting of resistant femoral nonunions. A preliminary report. Clin. Orthop. Relat. Res. 1988;(230):257–265. [PMID: 3284678]. [PubMed] [Google Scholar]
- 43.Kobayashi T., Lyons K.M., McMahon A.P., Kronenberg H.M. BMP signaling stimulates cellular differentiation at multiple steps during cartilage development. Proc. Natl. Acad. Sci. USA. 2005;102(50):18023–18027. doi: 10.1073/pnas.0503617102. [http://dx.doi.org/10.1073/pnas.0503617102]. [PMID: 16322106]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsuji K., Bandyopadhyay A., Harfe B.D., et al. BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nat. Genet. 2006;38(12):1424–1429. doi: 10.1038/ng1916. [http://dx.doi.org/10.1038/ng1916]. [PMID: 17099713]. [DOI] [PubMed] [Google Scholar]
- 45.Huang Z., Wang D., Ihida-Stansbury K., Jones P.L., Martin J.F. Defective pulmonary vascular remodeling in Smad8 mutant mice. Hum. Mol. Genet. 2009;18(15):2791–2801. doi: 10.1093/hmg/ddp214. [http://dx.doi.org/10.1093/hmg/ddp214]. [PMID: 19419974]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hemmati-Brivanlou A., Thomsen G.H. Ventral mesodermal patterning in Xenopus embryos: expression patterns and activities of BMP-2 and BMP-4. Dev. Genet. 1995;17(1):78–89. doi: 10.1002/dvg.1020170109. [http://dx.doi.org/10.1002/dvg.1020170109]. [PMID: 7554498]. [DOI] [PubMed] [Google Scholar]
- 47.Zou H., Niswander L. Requirement for BMP signaling in interdigital apoptosis and scale formation. Science. 1996;272(5262):738–741. doi: 10.1126/science.272.5262.738. [http://dx.doi.org/10.1126/science.272.5262.738]. [PMID: 8614838]. [DOI] [PubMed] [Google Scholar]
- 48.Winbanks C.E., Chen J.L., Qian H., et al. The bone morphogenetic protein axis is a positive regulator of skeletal muscle mass. J. Cell Biol. 2013;203(2):345–357. doi: 10.1083/jcb.201211134. [http://dx.doi.org/10.1083/jcb.201211134]. [PMID: 24145169]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Torihashi S., Hattori T., Hasegawa H., Kurahashi M., Ogaeri T., Fujimoto T. The expression and crucial roles of BMP signaling in development of smooth muscle progenitor cells in the mouse embryonic gut. Differentiation. 2009;77(3):277–289. doi: 10.1016/j.diff.2008.10.003. [http://dx.doi.org/10.1016/j.diff.2008.10.003]. [PMID: 19272526]. [DOI] [PubMed] [Google Scholar]
- 50.Zhang H., Bradley A. Mice deficient for BMP2 are nonviable and have defects in amnion/chorion and cardiac development. Development. 1996;122(10):2977–2986. doi: 10.1242/dev.122.10.2977. [PMID: 8898212]. [DOI] [PubMed] [Google Scholar]
- 51.Esquela A.F., Lee S.J. Regulation of metanephric kidney development by growth/differentiation factor 11. Dev. Biol. 2003;257(2):356–370. doi: 10.1016/s0012-1606(03)00100-3. [http://dx.doi.org/10.1016/S0012-1606(03)00100-3]. [PMID: 12729564]. [DOI] [PubMed] [Google Scholar]
- 52.Gram J. Eye development. Curr Top Dev Biol. 2010;90:343–386. doi: 10.1016/S0070-2153(10)90010-0. [http://dx.doi.org/10.1016/S0070-2153(10)90010-0]. [PMID: 20691855]. [DOI] [PubMed] [Google Scholar]
- 53.Settle S., Marker P., Gurley K., et al. The BMP family member Gdf7 is required for seminal vesicle growth, branching morphogenesis, and cytodifferentiation. Dev. Biol. 2001;234(1):138–150. doi: 10.1006/dbio.2001.0244. [http://dx.doi.org/10.1006/dbio.2001.0244]. [PMID: 11356025]. [DOI] [PubMed] [Google Scholar]
- 54.Huang H., Song T.J., Li X., et al. BMP signaling pathway is required for commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc. Natl. Acad. Sci. USA. 2009;106(31):12670–12675. doi: 10.1073/pnas.0906266106. [http://dx.doi.org/10.1073/pnas.0906266106]. [PMID: 19620713]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang R.N., Green J., Wang Z., et al. Bone Morphogenetic Protein (BMP) signaling in development and human diseases. Genes Dis. 2014;1(1):87–105. doi: 10.1016/j.gendis.2014.07.005. [http://dx.doi.org/10.1016/j.gendis.2014.07.005]. [PMID: 25401122]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Winnier G., Blessing M., Labosky P.A., Hogan B.L. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 1995;9(17):2105–2116. doi: 10.1101/gad.9.17.2105. [http://dx.doi.org/10.1101/gad.9.17.2105]. [PMID: 7657163]. [DOI] [PubMed] [Google Scholar]
- 57.Dunn N.R., Winnier G.E., Hargett L.K., Schrick J.J., Fogo A.B., Hogan B.L. Haploinsufficient phenotypes in Bmp4 heterozygous null mice and modification by mutations in Gli3 and Alx4. Dev. Biol. 1997;188(2):235–247. doi: 10.1006/dbio.1997.8664. [http://dx.doi.org/10.1006/dbio.1997.8664]. [PMID: 9268572]. [DOI] [PubMed] [Google Scholar]
- 58.Suzuki N., Labosky P.A., Furuta Y., et al. Failure of ventral body wall closure in mouse embryos lacking a procollagen C-proteinase encoded by Bmp1, a mammalian gene related to Drosophila tolloid. Development. 1996;122(11):3587–3595. doi: 10.1242/dev.122.11.3587. [PMID: 8951074]. [DOI] [PubMed] [Google Scholar]
- 59.McPherron A.C., Lawler A.M., Lee S.J. Regulation of anterior/posterior patterning of the axial skeleton by growth/ differentiation factor 11. Nat. Genet. 1999;22(3):260–264. doi: 10.1038/10320. [http://dx.doi.org/10.1038/10320]. [PMID: 10391213]. [DOI] [PubMed] [Google Scholar]
- 60.Dudley A.T., Lyons K.M., Robertson E.J. A requirement for bone morphogenetic protein-7 during development of the mammalian kidney and eye. Genes Dev. 1995;9(22):2795–2807. doi: 10.1101/gad.9.22.2795. [http://dx.doi.org/10.1101/gad.9.22.2795]. [PMID: 7590254]. [DOI] [PubMed] [Google Scholar]
- 61.Luo G., Hofmann C., Bronckers A.L., Sohocki M., Bradley A., Karsenty G. BMP-7 is an inducer of nephrogenesis, and is also required for eye development and skeletal patterning. Genes Dev. 1995;9(22):2808–2820. doi: 10.1101/gad.9.22.2808. [http://dx.doi.org/10.1101/gad.9.22.2808]. [PMID: 7590255]. [DOI] [PubMed] [Google Scholar]
- 62.Rosen V. BMP and BMP inhibitors in bone. Ann. N. Y. Acad. Sci. 2006;1068:19–25. doi: 10.1196/annals.1346.005. [http://dx.doi.org/10.1196/annals.1346.005]. [PMID: 16831902]. [DOI] [PubMed] [Google Scholar]
- 63.Chen G., Deng C., Li Y.P. TGF-β and BMP signaling in osteoblast differentiation and bone formation. Int. J. Biol. Sci. 2012;8(2):272–288. doi: 10.7150/ijbs.2929. [http://dx.doi.org/10.7150/ijbs.2929]. [PMID: 22298955]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang R., Oyajobi B.O., Harris S.E., et al. Wnt/β-catenin signaling activates bone morphogenetic protein 2 expression in osteoblasts. Bone. 2013;52(1):145–156. doi: 10.1016/j.bone.2012.09.029. [http://dx.doi.org/10.1016/j.bone.2012.09.029]. [PMID: 23032104]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Canalis E., Economides A.N., Gazzerro E. Bone morphogenetic proteins, their antagonists, and the skeleton. Endocr. Rev. 2003;24(2):218–235. doi: 10.1210/er.2002-0023. [http://dx.doi.org/10.1210/er.2002-0023]. [PMID: 12700180]. [DOI] [PubMed] [Google Scholar]
- 66.He X., Liu Y., Yuan X., Lu L. Enhanced healing of rat calvarial defects with MSCs loaded on BMP-2 releasing chitosan/alginate/hydroxyapatite scaffolds. PLoS One. 2014;9(8):e104061. doi: 10.1371/journal.pone.0104061. [http://dx.doi.org/10.1371/journal.pone.0104061]. [PMID: 25084008]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miyazono K., Maeda S., Imamura T. BMP receptor signaling: transcriptional targets, regulation of signals, and signaling cross-talk. Cytokine Growth Factor Rev. 2005;16(3):251–263. doi: 10.1016/j.cytogfr.2005.01.009. [http://dx.doi.org/10.1016/j.cytogfr.2005.01.009]. [PMID: 15871923]. [DOI] [PubMed] [Google Scholar]
- 68.Nohe A., Keating E., Underhill T.M., Knaus P., Petersen N.O. Dynamics and interaction of caveolin-1 isoforms with BMP-receptors. J. Cell Sci. 2005;118(Pt 3):643–650. doi: 10.1242/jcs.01402. [http://dx.doi.org/10.1242/jcs.01402]. [PMID: 15657086]. [DOI] [PubMed] [Google Scholar]
- 69.de Caestecker M. The transforming growth factor-beta superfamily of receptors. Cytokine Growth Factor Rev. 2004;15(1):1–11. doi: 10.1016/j.cytogfr.2003.10.004. [http://dx.doi.org/10.1016/j.cytogfr.2003.10.004]. [PMID: 14746809]. [DOI] [PubMed] [Google Scholar]
- 70.Balemans W., Van Hul W. Extracellular regulation of BMP signaling in vertebrates: a cocktail of modulators. Dev. Biol. 2002;250(2):231–250. [http://dx.doi.org/10.1006/dbio.2002.0779]. [PMID: 12376100]. [PubMed] [Google Scholar]
- 71.Ducy P., Starbuck M., Priemel M., et al. A Cbfa1-dependent genetic pathway controls bone formation beyond embryonic development. Genes Dev. 1999;13(8):1025–1036. doi: 10.1101/gad.13.8.1025. [http://dx.doi.org/10.1101/gad.13.8.1025]. [PMID: 10215629]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Harada H., Tagashira S., Fujiwara M., et al. Cbfa1 isoforms exert functional differences in osteoblast differentiation. J. Biol. Chem. 1999;274(11):6972–6978. doi: 10.1074/jbc.274.11.6972. [http://dx.doi.org/10.1074/jbc.274.11.6972]. [PMID: 10066751]. [DOI] [PubMed] [Google Scholar]
- 73.Lee M.H., Kwon T.G., Park H.S., Wozney J.M., Ryoo H.M. BMP-2-induced Osterix expression is mediated by Dlx5 but is independent of Runx2. Biochem. Biophys. Res. Commun. 2003;309(3):689–694. doi: 10.1016/j.bbrc.2003.08.058. [http://dx.doi.org/10.1016/j.bbrc.2003.08.058]. [PMID: 12963046]. [DOI] [PubMed] [Google Scholar]
- 74.Pereira R.M., Delany A.M., Canalis E. Cortisol inhibits the differentiation and apoptosis of osteoblasts in culture. Bone. 2001;28(5):484–490. doi: 10.1016/s8756-3282(01)00422-7. [http://dx.doi.org/10.1016/S8756-3282(01)00422-7]. [PMID: 11344047]. [DOI] [PubMed] [Google Scholar]
- 75.Rider C.C., Mulloy B. Bone morphogenetic protein and growth differentiation factor cytokine families and their protein antagonists. Biochem. J. 2010;429(1):1–12. doi: 10.1042/BJ20100305. [http://dx.doi.org/10.1042/BJ20100305]. [PMID: 20545624]. [DOI] [PubMed] [Google Scholar]
- 76.Merino R., Rodriguez-Leon J., Macias D., Gañan Y., Economides A.N., Hurle J.M. The BMP antagonist Gremlin regulates outgrowth, chondrogenesis and programmed cell death in the developing limb. Development. 1999;126(23):5515–5522. doi: 10.1242/dev.126.23.5515. [PMID: 10556075]. [DOI] [PubMed] [Google Scholar]
- 77.Dudarić L., Cvek S.Z., Cvijanović O., et al. Expression of the BMP-2, -4 and -7 and their antagonists gremlin, chordin, noggin and follistatin during ectopic osteogenesis. Coll. Antropol. 2013;37(4):1291–1298. [PMID: 24611347]. [PubMed] [Google Scholar]
- 78.Church R.H., Krishnakumar A., Urbanek A., et al. Gremlin1 preferentially binds to bone morphogenetic protein-2 (BMP-2) and BMP-4 over BMP-7. Biochem. J. 2015;466(1):55–68. doi: 10.1042/BJ20140771. [http://dx.doi.org/10.1042/BJ20140771]. [PMID: 25378054]. [DOI] [PubMed] [Google Scholar]
- 79.Iemura S., Yamamoto T.S., Takagi C., et al. Direct binding of follistatin to a complex of bone-morphogenetic protein and its receptor inhibits ventral and epidermal cell fates in early Xenopus embryo. Proc. Natl. Acad. Sci. USA. 1998;95(16):9337–9342. doi: 10.1073/pnas.95.16.9337. [http://dx.doi.org/10.1073/pnas.95.16.9337]. [PMID: 9689081]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yeung C.Y., Gossan N., Lu Y., et al. Gremlin-2 is a BMP antagonist that is regulated by the circadian clock. Sci. Rep. 2014;4:5183. doi: 10.1038/srep05183. [http://dx.doi.org/10.1038/srep05183]. [PMID: 24897937]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang C.L., Xiao F., Wang C.D., et al. Gremlin2 Suppression Increases the BMP-2-Induced Osteogenesis of Human Bone Marrow-Derived Mesenchymal Stem Cells Via the BMP-2/Smad/Runx2 Signaling Pathway. J. Cell. Biochem. 2017;118(2):286–297. doi: 10.1002/jcb.25635. [http://dx.doi.org/10.1002/jcb.25635]. [PMID: 27335248]. [DOI] [PubMed] [Google Scholar]
- 82.Aoki H., Fujii M., Imamura T., et al. Synergistic effects of different bone morphogenetic protein type I receptors on alkaline phosphatase induction. J. Cell Sci. 2001;114(Pt 8):1483–1489. doi: 10.1242/jcs.114.8.1483. [PMID: 11282024]. [DOI] [PubMed] [Google Scholar]
- 83.Schmidl M., Adam N., Surmann-Schmitt C., et al. Twisted gastrulation modulates bone morphogenetic protein-induced collagen II and X expression in chondrocytes in vitro and in vivo. J. Biol. Chem. 2006;281(42):31790–31800. doi: 10.1074/jbc.M603419200. [http://dx.doi.org/10.1074/jbc.M603419200]. [PMID: 16905550]. [DOI] [PubMed] [Google Scholar]
- 84.Chang C., Holtzman D.A., Chau S., et al. Twisted gastrulation can function as a BMP antagonist. Nature. 2001;410(6827):483–487. doi: 10.1038/35068583. [http://dx.doi.org/10.1038/35068583]. [PMID: 11260717]. [DOI] [PubMed] [Google Scholar]
- 85.Larraín J., Bachiller D., Lu B., Agius E., Piccolo S., De Robertis E.M. BMP-binding modules in chordin: a model for signalling regulation in the extracellular space. Development. 2000;127(4):821–830. doi: 10.1242/dev.127.4.821. [PMID: 10648240]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Thouverey C., Caverzasio J. Sclerostin inhibits osteoblast differentiation without affecting BMP2/SMAD1/5 or Wnt3a/β-catenin signaling but through activation of platelet-derived growth factor receptor signaling in vitro. Bonekey Rep. 2015;4:757. doi: 10.1038/bonekey.2015.126. [http://dx.doi.org/10.1038/bonekey.2015.126]. [PMID: 26587226]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Song B., Estrada K.D., Lyons K.M. Smad signaling in skeletal development and regeneration. Cytokine Growth Factor Rev. 2009;20(5-6):379–388. doi: 10.1016/j.cytogfr.2009.10.010. [http://dx.doi.org/10.1016/j.cytogfr.2009.10.010]. [PMID: 19926329]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lamplot J.D., Qin J., Nan G., et al. BMP9 signaling in stem cell differentiation and osteogenesis. Am. J. Stem Cells. 2013;2(1):1–21. [PMID: 23671813]. [PMC free article] [PubMed] [Google Scholar]
- 89.Rahman M.S., Akhtar N., Jamil H.M., Banik R.S., Asaduzzaman S.M. TGF-β/BMP signaling and other molecular events: Regulation of osteoblastogenesis and bone formation. Bone Res. 2015;3:15005. doi: 10.1038/boneres.2015.5. [http://dx.doi.org/10.1038/boneres.2015.5]. [PMID: 26273537]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.García de Vinuesa A., Abdelilah-Seyfried S., Knaus P., Zwijsen A., Bailly S. BMP signaling in vascular biology and dysfunction. Cytokine Growth Factor Rev. 2016;27:65–79. doi: 10.1016/j.cytogfr.2015.12.005. [http://dx.doi.org/10.1016/j.cytogfr.2015.12.005]. [PMID: 26823333]. [DOI] [PubMed] [Google Scholar]
- 91.Pierre A., Pisselet C., Monget P., Monniaux D., Fabre S. Testing the antagonistic effect of follistatin on BMP family members in ovine granulosa cells. Reprod. Nutr. Dev. 2005;45(4):419–425. doi: 10.1051/rnd:2005031. [http://dx.doi.org/10.1051/rnd:2005031]. [PMID: 16045890]. [DOI] [PubMed] [Google Scholar]
- 92.Kusu N., Laurikkala J., Imanishi M., et al. Sclerostin is a novel secreted osteoclast-derived bone morphogenetic protein antagonist with unique ligand specificity. J. Biol. Chem. 2003;278(26):24113–24117. doi: 10.1074/jbc.M301716200. [http://dx.doi.org/10.1074/jbc.M301716200]. [PMID: 12702725]. [DOI] [PubMed] [Google Scholar]
- 93.Tsiridis E., Upadhyay N., Giannoudis P. Molecular aspects of fracture healing: which are the important molecules? Injury. 2007;38(Suppl. 1):S11–S25. doi: 10.1016/j.injury.2007.02.006. [http://dx.doi.org/10.1016/j.injury.2007.02.006]. [PMID: 17383481]. [DOI] [PubMed] [Google Scholar]
- 94.Miyazono K., Kamiya Y., Morikawa M. Bone morphogenetic protein receptors and signal transduction. J. Biochem. 2010;147(1):35–51. doi: 10.1093/jb/mvp148. [http://dx.doi.org/10.1093/jb/mvp148]. [PMID: 19762341]. [DOI] [PubMed] [Google Scholar]
- 95.Kessler E., Takahara K., Biniaminov L., Brusel M., Greenspan D.S. Bone morphogenetic protein-1: The type I procollagen C-proteinase. Science. 1996;271(5247):360–362. doi: 10.1126/science.271.5247.360. [http://dx.doi.org/10.1126/science.271.5247.360]. [PMID: 8553073]. [DOI] [PubMed] [Google Scholar]
- 96.Shen B., Bhargav D., Wei A., et al. BMP-13 emerges as a potential inhibitor of bone formation. Int. J. Biol. Sci. 2009;5(2):192–200. doi: 10.7150/ijbs.5.192. [http://dx.doi.org/10.7150/ijbs.5.192]. [PMID: 19240811]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pecina M., Vukicevic S. Biological aspects of bone, cartilage and tendon regeneration. Int. Orthop. 2007;31(6):719–720. doi: 10.1007/s00264-007-0425-7. [http://dx.doi.org/10.1007/s00264-007-0425-7]. [PMID: 17704918]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Urist M.R., Strates B.S. The classic: Bone morphogenetic protein. Clin. Orthop. Relat. Res. 2009;467(12):3051–3062. doi: 10.1007/s11999-009-1068-3. [http://dx.doi.org/10.1007/s11999-009-1068-3]. [PMID: 19727989]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jäger M., Fischer J., Dohrn W., et al. Dexamethasone modulates BMP-2 effects on mesenchymal stem cells in vitro. J. Orthop. Res. 2008;26(11):1440–1448. doi: 10.1002/jor.20565. [http://dx.doi.org/10.1002/jor.20565]. [PMID: 18404732]. [DOI] [PubMed] [Google Scholar]
- 100.Schwartz Z., Simon B.J., Duran M.A., Barabino G., Chaudhri R., Boyan B.D. Pulsed electromagnetic fields enhance BMP-2 dependent osteoblastic differentiation of human mesenchymal stem cells. J. Orthop. Res. 2008;26(9):1250–1255. doi: 10.1002/jor.20591. [http://dx.doi.org/10.1002/jor.20591]. [PMID: 18404656]. [DOI] [PubMed] [Google Scholar]
- 101.Berasi S.P., Varadarajan U., Archambault J., et al. Divergent activities of osteogenic BMP2, and tenogenic BMP12 and BMP13 independent of receptor binding affinities. Growth Factors. 2011;29(4):128–139. doi: 10.3109/08977194.2011.593178. [http://dx.doi.org/10.3109/08977194.2011.593178]. [PMID: 21702718]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Helvering L.M., Sharp R.L., Ou X., Geiser A.G. Regulation of the promoters for the human bone morphogenetic protein 2 and 4 genes. Gene. 2000;256(1-2):123–138. doi: 10.1016/s0378-1119(00)00364-4. [http://dx.doi.org/10.1016/S0378-1119(00)00364-4]. [PMID: 11054542]. [DOI] [PubMed] [Google Scholar]
- 103.Heldin C.H., Miyazono K., ten Dijke P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- 104.Luo J., Tang M., Huang J., et al. TGFbeta/BMP type I receptors ALK1 and ALK2 are essential for BMP9-induced osteogenic signaling in mesenchymal stem cells. J. Biol. Chem. 2010;285(38):29588–29598. doi: 10.1074/jbc.M110.130518. [http://dx.doi.org/10.1074/jbc.M110.130518]. [PMID: 20628059]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Avsian-Kretchmer O., Hsueh A.J. Comparative genomic analysis of the eight-membered ring cystine knot-containing bone morphogenetic protein antagonists. Mol. Endocrinol. 2004;18(1):1–12. doi: 10.1210/me.2003-0227. [http://dx.doi.org/10.1210/me.2003-0227]. [PMID: 14525956]. [DOI] [PubMed] [Google Scholar]
- 106.Brunet L.J., McMahon J.A., McMahon A.P., Harland R.M. Noggin, cartilage morphogenesis, and joint formation in the mammalian skeleton. Science. 1998;280(5368):1455–1457. doi: 10.1126/science.280.5368.1455. [http://dx.doi.org/10.1126/science.280.5368.1455]. [PMID: 9603738]. [DOI] [PubMed] [Google Scholar]
- 107.Takayama K., Suzuki A., Manaka T., et al. RNA interference for noggin enhances the biological activity of bone morphogenetic proteins in vivo and in vitro. J. Bone Miner. Metab. 2009;27(4):402–411. doi: 10.1007/s00774-009-0054-x. [http://dx.doi.org/10.1007/s00774-009-0054-x]. [PMID: 19252814]. [DOI] [PubMed] [Google Scholar]
- 108.Gazzerro E., Gangji V., Canalis E. Bone morphogenetic proteins induce the expression of noggin, which limits their activity in cultured rat osteoblasts. J. Clin. Invest. 1998;102(12):2106–2114. doi: 10.1172/JCI3459. [http://dx.doi.org/10.1172/JCI3459]. [PMID: 9854046]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rifas L. The role of noggin in human mesenchymal stem cell differentiation. J. Cell. Biochem. 2007;100(4):824–834. doi: 10.1002/jcb.21132. [http://dx.doi.org/10.1002/jcb.21132]. [PMID: 17133353]. [DOI] [PubMed] [Google Scholar]
- 110.Zhang D., Ferguson C.M., O’Keefe R.J., Puzas J.E., Rosier R.N., Reynolds P.R. A role for the BMP antagonist chordin in endochondral ossification. J. Bone Miner. Res. 2002;17(2):293–300. doi: 10.1359/jbmr.2002.17.2.293. [http://dx.doi.org/10.1359/jbmr.2002.17.2.293]. [PMID: 11811560]. [DOI] [PubMed] [Google Scholar]
- 111.Hsu D.R., Economides A.N., Wang X., Eimon P.M., Harland R.M. The Xenopus dorsalizing factor Gremlin identifies a novel family of secreted proteins that antagonize BMP activities. Mol. Cell. 1998;1(5):673–683. doi: 10.1016/s1097-2765(00)80067-2. [http://dx.doi.org/10.1016/S1097-2765(00)80067-2]. [PMID: 9660951]. [DOI] [PubMed] [Google Scholar]
- 112.Topol L.Z., Bardot B., Zhang Q., et al. Biosynthesis, post-translation modification, and functional characterization of Drm/Gremlin. J. Biol. Chem. 2000;275(12):8785–8793. doi: 10.1074/jbc.275.12.8785. [http://dx.doi.org/10.1074/jbc.275.12.8785]. [PMID: 10722723]. [DOI] [PubMed] [Google Scholar]
- 113.Wordinger R.J., Zode G., Clark A.F. Focus on molecules: gremlin. Exp. Eye Res. 2008;87(2):78–79. doi: 10.1016/j.exer.2007.11.016. [http://dx.doi.org/10.1016/j.exer.2007.11.016]. [PMID: 18201700]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Leijten J.C., Bos S.D., Landman E.B., et al. GREM1, FRZB and DKK1 mRNA levels correlate with osteoarthritis and are regulated by osteoarthritis-associated factors. Arthritis Res. Ther. 2013;15:R126. doi: 10.1186/ar4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ideno H., Takanabe R., Shimada A., et al. Protein related to DAN and cerberus (PRDC) inhibits osteoblastic differentiation and its suppression promotes osteogenesis in vitro. Exp. Cell Res. 2009;315(3):474–484. doi: 10.1016/j.yexcr.2008.11.019. [http://dx.doi.org/10.1016/j.yexcr.2008.11.019]. [PMID: 19073177]. [DOI] [PubMed] [Google Scholar]
- 116.Nilsson O., Parker E.A., Hegde A., Chau M., Barnes K.M., Baron J. Gradients in bone morphogenetic protein-related gene expression across the growth plate. J. Endocrinol. 2007;193(1):75–84. doi: 10.1677/joe.1.07099. [http://dx.doi.org/10.1677/joe.1.07099]. [PMID: 17400805]. [DOI] [PubMed] [Google Scholar]
- 117.Matzuk M.M., Lu N., Vogel H., Sellheyer K., Roop D.R., Bradley A. Multiple defects and perinatal death in mice deficient in follistatin. Nature. 1995;374(6520):360–363. doi: 10.1038/374360a0. [http://dx.doi.org/10.1038/374360a0]. [PMID: 7885475]. [DOI] [PubMed] [Google Scholar]
- 118.Shibanuma M., Mashimo J., Mita A., Kuroki T., Nose K. Cloning from a mouse osteoblastic cell line of a set of transforming-growth-factor-beta 1-regulated genes, one of which seems to encode a follistatin-related polypeptide. Eur. J. Biochem. 1993;217(1):13–19. doi: 10.1111/j.1432-1033.1993.tb18212.x. [http://dx.doi.org/10.1111/j.1432-1033.1993.tb18212.x]. [PMID: 7901004]. [DOI] [PubMed] [Google Scholar]
- 119.Mason E.D., Konrad K.D., Webb C.D., Marsh J.L. Dorsal midline fate in Drosophila embryos requires twisted gastrulation, a gene encoding a secreted protein related to human connective tissue growth factor. Genes Dev. 1994;8(13):1489–1501. doi: 10.1101/gad.8.13.1489. [http://dx.doi.org/10.1101/gad.8.13.1489]. [PMID: 7958834]. [DOI] [PubMed] [Google Scholar]
- 120.Khattab H.M., Kubota S., Takigawa M., et al. The BMP-2 mutant L51P: A BMP receptor IA binding-deficient inhibitor of noggin. J. Bone Miner. Metab. 2019;37:199–205. doi: 10.1007/s00774-018-0925-0. [PMID: 29667005]. [DOI] [PubMed] [Google Scholar]
- 121.Keller S., Nickel J., Zhang J-L., Sebald W., Mueller T.D. Molecular recognition of BMP-2 and BMP receptor IA. Nat. Struct. Mol. Biol. 2004;11(5):481–488. doi: 10.1038/nsmb756. [http://dx.doi.org/10.1038/nsmb756]. [PMID: 15064755]. [DOI] [PubMed] [Google Scholar]
- 122.Sebald H.J., Klenke F.M., Siegrist M., Albers C.E., Sebald W., Hofstetter W. Inhibition of endogenous antagonists with an engineered BMP-2 variant increases BMP-2 efficacy in rat femoral defect healing. Acta Biomater. 2012;8(10):3816–3820. doi: 10.1016/j.actbio.2012.06.036. [http://dx.doi.org/10.1016/j.actbio.2012.06.036]. [PMID: 22750247]. [DOI] [PubMed] [Google Scholar]
- 123.Khattab H.M., Ono M., Sonoyama W., et al. The BMP2 antagonist inhibitor L51P enhances the osteogenic potential of BMP2 by simultaneous and delayed synergism. Bone. 2014;69:165–173. doi: 10.1016/j.bone.2014.09.011. [http://dx.doi.org/10.1016/j.bone.2014.09.011]. [PMID: 25240457]. [DOI] [PubMed] [Google Scholar]
- 124.Hauser M., Siegrist M., Denzer A., et al. Bisphosphonates reduce biomaterial turnover in healing of critical-size rat femoral defects. J. Orthop. Surg. (Hong Kong) 2018;26:2309499018802487. doi: 10.1177/2309499018802487. [DOI] [PubMed] [Google Scholar]
- 125.Albers C.E., Hofstetter W., Sebald H.J., Sebald W., Siebenrock K.A., Klenke F.M. L51P - A BMP2 variant with osteoinductive activity via inhibition of Noggin. Bone. 2012;51(3):401–406. doi: 10.1016/j.bone.2012.06.020. [http://dx.doi.org/10.1016/j.bone.2012.06.020]. [PMID: 22750402]. [DOI] [PubMed] [Google Scholar]
- 126.Hauser M., Siegrist M., Keller I., Hofstetter W. Healing of fractures in osteoporotic bones in mice treated with bisphosphonates - A transcriptome analysis. Bone. 2018;112:107–119. doi: 10.1016/j.bone.2018.04.017. [http://dx.doi.org/10.1016/j.bone.2018.04.017]. [PMID: 29680263]. [DOI] [PubMed] [Google Scholar]
- 127.Reddi A.H., Cunningham N.S. Initiation and promotion of bone differentiation by bone morphogenetic proteins. J. Bone Miner. Res. 1993;8(Suppl. 2):S499–S502. doi: 10.1002/jbmr.5650081313. [http://dx.doi.org/10.1002/jbmr.5650081313]. [PMID: 8122519]. [DOI] [PubMed] [Google Scholar]
- 128.Sykaras N., Opperman L.A. Bone morphogenetic proteins (BMPs): how do they function and what can they offer the clinician? J. Oral Sci. 2003;45(2):57–73. doi: 10.2334/josnusd.45.57. [http://dx.doi.org/10.2334/josnusd.45.57]. [PMID: 12930129]. [DOI] [PubMed] [Google Scholar]
- 129.Zhu W., Qiu Y., Sheng F., et al. An effective delivery vehicle of demineralized bone matrix incorporated with engineered collagen-binding human bone morphogenetic protein-2 to accelerate spinal fusion at low dose. J. Mater. Sci. Mater. Med. 2017;29(1):2. doi: 10.1007/s10856-017-6007-3. [http://dx.doi.org/10.1007/s10856-017-6007-3]. [PMID: 29196819]. [DOI] [PubMed] [Google Scholar]
- 130.Alden T.D., Pittman D.D., Beres E.J., et al. Percutaneous spinal fusion using bone morphogenetic protein-2 gene therapy. J. Neurosurg. 1999;90(1) Suppl.:109–114. doi: 10.3171/spi.1999.90.1.0109. [PMID: 10413134]. [DOI] [PubMed] [Google Scholar]
- 131.Koerner J.D., Markova D.Z., Schroeder G.D., et al. The local cytokine and growth factor response to rhBMP-2 after spinal fusion. Spine J. 2018;18:1424–1433. doi: 10.1016/j.spinee.2018.03.006. [http://dx.doi.org/10.1016/j.spinee.2018.03.006]. [PMID: 29550606]. [DOI] [PubMed] [Google Scholar]
- 132.Minamide A., Kawakami M., Hashizume H., Sakata R., Tamaki T. Evaluation of carriers of bone morphogenetic protein for spinal fusion. Spine. 2001;26(8):933–939. doi: 10.1097/00007632-200104150-00017. [http://dx.doi.org/10.1097/00007632-200104150-00017]. [PMID: 11317116]. [DOI] [PubMed] [Google Scholar]
- 133.Helm G.A., Alden T.D., Beres E.J., et al. Use of bone morphogenetic protein-9 gene therapy to induce spinal arthrodesis in the rodent. J. Neurosurg. 2000;92(2) Suppl.:191–196. doi: 10.3171/spi.2000.92.2.0191. [PMID: 10763690]. [DOI] [PubMed] [Google Scholar]
- 134.Abe E., Yamamoto M., Taguchi Y., et al. Essential requirement of BMPs-2/4 for both osteoblast and osteoclast formation in murine bone marrow cultures from adult mice: antagonism by noggin. J. Bone Miner. Res. 2000;15(4):663–673. doi: 10.1359/jbmr.2000.15.4.663. [http://dx.doi.org/10.1359/jbmr.2000.15.4.663]. [PMID: 10780858]. [DOI] [PubMed] [Google Scholar]
- 135.Wan D.C., Pomerantz J.H., Brunet L.J., et al. Noggin suppression enhances in vitro osteogenesis and accelerates in vivo bone formation. J. Biol. Chem. 2007;282(36):26450–26459. doi: 10.1074/jbc.M703282200. [http://dx.doi.org/10.1074/jbc.M703282200]. [PMID: 17609215]. [DOI] [PubMed] [Google Scholar]
- 136.Klineberg E., Haudenschild D.R., Snow K.D., et al. The effect of noggin interference in a rabbit posterolateral spinal fusion model. Eur. Spine J. 2014;23(11):2385–2392. doi: 10.1007/s00586-014-3252-8. [http://dx.doi.org/10.1007/s00586-014-3252-8]. [PMID: 24740279]. [DOI] [PubMed] [Google Scholar]
- 137.Devlin R.D., Du Z., Pereira R.C., et al. Skeletal overexpression of noggin results in osteopenia and reduced bone formation. Endocrinology. 2003;144(5):1972–1978. doi: 10.1210/en.2002-220918. [http://dx.doi.org/10.1210/en.2002-220918]. [PMID: 12697704]. [DOI] [PubMed] [Google Scholar]
- 138.Okamoto M., Murai J., Yoshikawa H., Tsumaki N. Bone morphogenetic proteins in bone stimulate osteoclasts and osteoblasts during bone development. J. Bone Miner. Res. 2006;21(7):1022–1033. doi: 10.1359/jbmr.060411. [http://dx.doi.org/10.1359/jbmr.060411]. [PMID: 16813523]. [DOI] [PubMed] [Google Scholar]
- 139.Tsuji K., Cox K., Bandyopadhyay A., Harfe B.D., Tabin C.J., Rosen V. BMP4 is dispensable for skeletogenesis and fracture-healing in the limb. J. Bone Joint Surg. Am. 2008;90(Suppl. 1):14–18. doi: 10.2106/JBJS.G.01109. [http://dx.doi.org/10.2106/JBJS.G.01109]. [PMID: 18292351]. [DOI] [PubMed] [Google Scholar]
- 140.Suzuki D., Yamada A., Aizawa R., et al. BMP2 differentially regulates the expression of Gremlin1 and Gremlin2, the negative regulators of BMP function, during osteoblast differentiation. Calcif. Tissue Int. 2012;91(1):88–96. doi: 10.1007/s00223-012-9614-5. [http://dx.doi.org/10.1007/s00223-012-9614-5]. [PMID: 22644325]. [DOI] [PubMed] [Google Scholar]
- 141.Boden S.D., Kang J., Sandhu H., Heller J.G. Use of recombinant human bone morphogenetic protein-2 to achieve posterolateral lumbar spine fusion in humans: a prospective, randomized clinical pilot trial: 2002 Volvo Award in clinical studies. Spine. 2002;27(23):2662–2673. doi: 10.1097/00007632-200212010-00005. [http://dx.doi.org/10.1097/00007632-200212010-00005]. [PMID: 12461392]. [DOI] [PubMed] [Google Scholar]
- 142.Friedlaender GE, Perry CR, Cole JD, et al. Osteogenic protein-1(bone morphogenetic protein-7) in the treatment of tibial nonunions. J Bone Joint Surg Am. 2001;83-A(Pt 2) Suppl. 1:S151–S158. doi: 10.2106/00004623-200100002-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tang Y., Ye X., Klineberg E.O., Curtiss S., Maitra S., Gupta M.C. Temporal and spatial expression of BMPs and BMP antagonists during posterolateral lumbar fusion. Spine. 2011;36(4):E237–E244. doi: 10.1097/BRS.0b013e3181d73541. [PMID: 21099737]. [DOI] [PubMed] [Google Scholar]
- 144.Kwong F.N., Hoyland J.A., Evans C.H., Freemont A.J. Regional and cellular localisation of BMPs and their inhibitors’ expression in human fractures. Int. Orthop. 2009;33(1):281–288. doi: 10.1007/s00264-008-0691-z. [http://dx.doi.org/10.1007/s00264-008-0691-z]. [PMID: 19023570]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Fajardo M., Liu C.J., Egol K. Levels of expression for BMP-7 and several BMP antagonists may play an integral role in a fracture nonunion: a pilot study. Clin. Orthop. Relat. Res. 2009;467(12):3071–3078. doi: 10.1007/s11999-009-0981-9. [http://dx.doi.org/10.1007/s11999-009-0981-9]. [PMID: 19597895]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Niikura T., Hak D.J., Reddi A.H. Global gene profiling reveals a downregulation of BMP gene expression in experimental atrophic nonunions compared to standard healing fractures. J. Orthop. Res. 2006;24(7):1463–1471. doi: 10.1002/jor.20182. [http://dx.doi.org/10.1002/jor.20182]. [PMID: 16705710]. [DOI] [PubMed] [Google Scholar]
- 147.Dean D.B., Watson J.T., Jin W., et al. Distinct functionalities of bone morphogenetic protein antagonists during fracture healing in mice. J. Anat. 2010;216(5):625–630. doi: 10.1111/j.1469-7580.2010.01214.x. [http://dx.doi.org/10.1111/j.1469-7580.2010.01214.x]. [PMID: 20298438]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kwong F.N., Hoyland J.A., Freemont A.J., Evans C.H. Altered relative expression of BMPs and BMP inhibitors in cartilaginous areas of human fractures progressing towards nonunion. J. Orthop. Res. 2009;27(6):752–757. doi: 10.1002/jor.20794. [http://dx.doi.org/10.1002/jor.20794]. [PMID: 19058174]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yoshimura Y., Nomura S., Kawasaki S., Tsutsumimoto T., Shimizu T., Takaoka K. Colocalization of noggin and bone morphogenetic protein-4 during fracture healing. J. Bone Miner. Res. 2001;16(5):876–884. doi: 10.1359/jbmr.2001.16.5.876. [http://dx.doi.org/10.1359/jbmr.2001.16.5.876]. [PMID: 11341332]. [DOI] [PubMed] [Google Scholar]
- 150.Kishigami S., Mishina Y. BMP signaling and early embryonic patterning. Cytokine Growth Factor Rev. 2005;16(3):265–278. doi: 10.1016/j.cytogfr.2005.04.002. [http://dx.doi.org/10.1016/j.cytogfr.2005.04.002]. [PMID: 15871922]. [DOI] [PubMed] [Google Scholar]
- 151.Chen C., Uludağ H., Wang Z., Jiang H. Noggin suppression decreases BMP-2-induced osteogenesis of human bone marrow-derived mesenchymal stem cells in vitro. J. Cell. Biochem. 2012;113(12):3672–3680. doi: 10.1002/jcb.24240. [http://dx.doi.org/10.1002/jcb.24240]. [PMID: 22740073]. [DOI] [PubMed] [Google Scholar]
- 152.Kwong F.N.K., Richardson S.M., Evans C.H. Chordin knockdown enhances the osteogenic differentiation of human mesenchymal stem cells. Arthritis Res. Ther. 2008;10(3):R65. doi: 10.1186/ar2436. [http://dx.doi.org/10.1186/ar2436]. [PMID: 18533030]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wang C., Xiao F., Gan Y., et al. Improving Bone Regeneration Using Chordin siRNA Delivered by pH-Responsive and Non-Toxic Polyspermine Imidazole-4,5-Imine. Cell. Physiol. Biochem. 2018;46(1):133–147. doi: 10.1159/000488416. [http://dx.doi.org/10.1159/000488416]. [PMID: 29587276]. [DOI] [PubMed] [Google Scholar]
- 154.Hasharoni A., Zilberman Y., Turgeman G., Helm G.A., Liebergall M., Gazit D. Murine spinal fusion induced by engineered mesenchymal stem cells that conditionally express bone morphogenetic protein-2. J. Neurosurg. Spine. 2005;3(1):47–52. doi: 10.3171/spi.2005.3.1.0047. [http://dx.doi.org/10.3171/spi.2005.3.1.0047]. [PMID: 16122022]. [DOI] [PubMed] [Google Scholar]
- 155.Wang J.C., Kanim L.E., Yoo S., Campbell P.A., Berk A.J., Lieberman J.R. Effect of regional gene therapy with bone morphogenetic protein-2-producing bone marrow cells on spinal fusion in rats. J. Bone Joint Surg. Am. 2003;85(5):905–911. doi: 10.2106/00004623-200305000-00020. [http://dx.doi.org/10.2106/00004623-200305000-00020]. [PMID: 12728043]. [DOI] [PubMed] [Google Scholar]
- 156.Riew K.D., Wright N.M., Cheng S., Avioli L.V., Lou J. Induction of bone formation using a recombinant adenoviral vector carrying the human BMP-2 gene in a rabbit spinal fusion model. Calcif. Tissue Int. 1998;63(4):357–360. doi: 10.1007/s002239900540. [http://dx.doi.org/10.1007/s002239900540]. [PMID: 9744997]. [DOI] [PubMed] [Google Scholar]
- 157.Cheng S.L., Lou J., Wright N.M., et al. In vitro and in vivo induction of bone formation using a recombinant adenoviral vector carrying the human BMP-2 gene. Calcif. Tissue Int. 2001;68:87–94. [http://dx.doi.org/10.1007/BF02678146]. [PubMed] [Google Scholar]
- 158.Dragoo J.L., Choi J.Y., Lieberman J.R., et al. Bone induction by BMP-2 transduced stem cells derived from human fat. J. Orthop. Res. 2003;21(4):622–629. doi: 10.1016/S0736-0266(02)00238-3. [http://dx.doi.org/10.1016/S0736-0266(02)00238-3]. [PMID: 12798061]. [DOI] [PubMed] [Google Scholar]
- 159.Wang L., Huang Y., Pan K., Jiang X., Liu C. Osteogenic responses to different concentrations/ratios of BMP-2 and bFGF in bone formation. Ann. Biomed. Eng. 2010;38(1):77–87. doi: 10.1007/s10439-009-9841-8. [http://dx.doi.org/10.1007/s10439-009-9841-8]. [PMID: 19921434]. [DOI] [PubMed] [Google Scholar]
- 160.Sheyn D., Pelled G., Zilberman Y., et al. Nonvirally engineered porcine adipose tissue-derived stem cells: use in posterior spinal fusion. Stem Cells. 2008;26(4):1056–1064. doi: 10.1634/stemcells.2007-0858. [http://dx.doi.org/10.1634/stemcells.2007-0858]. [PMID: 18218819]. [DOI] [PubMed] [Google Scholar]
- 161.Friedman M.S., Long M.W., Hankenson K.D. Osteogenic differentiation of human mesenchymal stem cells is regulated by bone morphogenetic protein-6. J. Cell. Biochem. 2006;98(3):538–554. doi: 10.1002/jcb.20719. [http://dx.doi.org/10.1002/jcb.20719]. [PMID: 16317727]. [DOI] [PubMed] [Google Scholar]
- 162.Hannallah D., Peng H., Young B., Usas A., Gearhart B., Huard J. Retroviral delivery of Noggin inhibits the formation of heterotopic ossification induced by BMP-4, demineralized bone matrix, and trauma in an animal model. J. Bone Joint Surg. Am. 2004;86(1):80–91. doi: 10.2106/00004623-200401000-00013. [http://dx.doi.org/10.2106/00004623-200401000-00013]. [PMID: 14711949]. [DOI] [PubMed] [Google Scholar]
- 163.Diefenderfer D.L., Osyczka A.M., Reilly G.C., Leboy P.S. BMP responsiveness in human mesenchymal stem cells. Connect. Tissue Res. 2003;44(Suppl. 1):305–311. [http://dx.doi.org/10.1080/03008200390181825]. [PMID: 12952214]. [PubMed] [Google Scholar]
- 164.Hu K., Sun H., Gui B., et al. Gremlin-1 suppression increases BMP-2-induced osteogenesis of human mesenchymal stem cells. Mol. Med. Rep. 2017;15(4):2186–2194. doi: 10.3892/mmr.2017.6253. [http://dx.doi.org/10.3892/mmr.2017.6253]. [PMID: 28260028]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ramasubramanian A., Shiigi S., Lee G.K., et al. Non-viral delivery of inductive and suppressive genes to adipose-derived stem cells for osteogenic differentiation. Pharm. Res. 2011;28(6):1328–1337. doi: 10.1007/s11095-011-0406-9. [http://dx.doi.org/10.1007/s11095-011-0406-9]. [PMID: 21424160]. [DOI] [PubMed] [Google Scholar]
- 166.Fan J., Park H., Tan S., et al. Enhanced osteogenesis of adipose derived stem cells with Noggin suppression and delivery of BMP-2. PLoS One. 2013;8(8):e72474. doi: 10.1371/journal.pone.0072474. [http://dx.doi.org/10.1371/journal.pone.0072474]. [PMID: 23977305]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Willems N., Bach F.C., Plomp S.G., et al. Intradiscal application of rhBMP-7 does not induce regeneration in a canine model of spontaneous intervertebral disc degeneration. Arthritis Res. Ther. 2015;17:137. doi: 10.1186/s13075-015-0625-2. [http://dx.doi.org/10.1186/s13075-015-0625-2]. [PMID: 26013758]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Haschtmann D., Ferguson S.J., Stoyanov J.V. BMP-2 and TGF-β3 do not prevent spontaneous degeneration in rabbit disc explants but induce ossification of the annulus fibrosus. Eur. Spine J. 2012;21(9):1724–1733. doi: 10.1007/s00586-012-2371-3. [http://dx.doi.org/10.1007/s00586-012-2371-3]. [PMID: 22639297]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Li J., Yoon S.T., Hutton W.C. Effect of bone morphogenetic protein-2 (BMP-2) on matrix production, other BMPs, and BMP receptors in rat intervertebral disc cells. J. Spinal Disord. Tech. 2004;17(5):423–428. doi: 10.1097/01.bsd.0000112084.85112.5d. [http://dx.doi.org/10.1097/01.bsd.0000112084.85112.5d]. [PMID: 15385883]. [DOI] [PubMed] [Google Scholar]
- 170.Li Z., Lang G., Karfeld-Sulzer L.S., et al. Heterodimeric BMP-2/7 for nucleus pulposus regeneration-In vitro and ex vivo studies. J. Orthop. Res. 2017;35(1):51–60. doi: 10.1002/jor.23351. [http://dx.doi.org/10.1002/jor.23351]. [PMID: 27340938]. [DOI] [PubMed] [Google Scholar]
- 171.Kim D.J., Moon S.H., Kim H., et al. Bone morphogenetic protein-2 facilitates expression of chondrogenic, not osteogenic, phenotype of human intervertebral disc cells. Spine. 2003;28(24):2679–2684. doi: 10.1097/01.BRS.0000101445.46487.16. [http://dx.doi.org/10.1097/01.BRS.0000101445.46487.16]. [PMID: 14673369]. [DOI] [PubMed] [Google Scholar]
- 172.Lee K.I., Moon S.H., Kim H., et al. Tissue engineering of the intervertebral disc with cultured nucleus pulposus cells using atelocollagen scaffold and growth factors. Spine. 2012;37(6):452–458. doi: 10.1097/BRS.0b013e31823c8603. [http://dx.doi.org/10.1097/BRS.0b013e31823c8603]. [PMID: 22037529]. [DOI] [PubMed] [Google Scholar]
- 173.Wei A., Brisby H., Chung S.A., Diwan A.D. Bone morphogenetic protein-7 protects human intervertebral disc cells in vitro from apoptosis. Spine J. 2008;8(3):466–474. doi: 10.1016/j.spinee.2007.04.021. [http://dx.doi.org/10.1016/j.spinee.2007.04.021]. [PMID: 18082466]. [DOI] [PubMed] [Google Scholar]
- 174.Resnick D., Guerra J., Jr, Robinson C.A., Vint V.C. Association of diffuse idiopathic skeletal hyperostosis (DISH) and calcification and ossification of the posterior longitudinal ligament. AJR Am. J. Roentgenol. 1978;131(6):1049–1053. doi: 10.2214/ajr.131.6.1049. [http://dx.doi.org/10.2214/ajr.131.6.1049]. [PMID: 104572]. [DOI] [PubMed] [Google Scholar]
- 175.Kuperus J.S., Westerveld L.A., Rutges J.P., et al. Histological characteristics of diffuse idiopathic skeletal hyperostosis. J. Orthop. Res. 2017;35(1):140–146. doi: 10.1002/jor.23267. [http://dx.doi.org/10.1002/jor.23267]. [PMID: 27101345]. [DOI] [PubMed] [Google Scholar]
- 176.Karamouzian S., Eskandary H., Faramarzee M., et al. Frequency of lumbar intervertebral disc calcification and angiogenesis, and their correlation with clinical, surgical, and magnetic resonance imaging findings. Spine. 2010;35(8):881–886. doi: 10.1097/BRS.0b013e3181b9c986. [http://dx.doi.org/10.1097/BRS.0b013e3181b9c986]. [PMID: 20354479]. [DOI] [PubMed] [Google Scholar]
- 177.Tekari A., May R.D., Frauchiger D.A., Chan S.C., Benneker L.M., Gantenbein B. The BMP2 variant L51P restores the osteogenic differentiation of human mesenchymal stromal cells in the presence of intervertebral disc cells. Eur. Cell. Mater. 2017;33:197–210. doi: 10.22203/eCM.v033a15. [http://dx.doi.org/10.22203/eCM.v033a15]. [PMID: 28266688]. [DOI] [PubMed] [Google Scholar]
- 178.Imai Y., Miyamoto K., An H.S., Thonar E.J., Andersson G.B., Masuda K. Recombinant human osteogenic protein-1 upregulates proteoglycan metabolism of human anulus fibrosus and nucleus pulposus cells. Spine. 2007;32(12):1303–1309. doi: 10.1097/BRS.0b013e3180593238. [http://dx.doi.org/10.1097/BRS.0b013e3180593238]. [PMID: 17515818]. [DOI] [PubMed] [Google Scholar]
- 179.Leckie S.K., Bechara B.P., Hartman R.A., et al. Injection of AAV2-BMP2 and AAV2-TIMP1 into the nucleus pulposus slows the course of intervertebral disc degeneration in an in vivo rabbit model. Spine J. 2012;12(1):7–20. doi: 10.1016/j.spinee.2011.09.011. [http://dx.doi.org/10.1016/j.spinee.2011.09.011]. [PMID: 22023960]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Huang K.Y., Yan J.J., Hsieh C.C., Chang M.S., Lin R.M. The in vivo biological effects of intradiscal recombinant human bone morphogenetic protein-2 on the injured intervertebral disc: an animal experiment. Spine. 2007;32(11):1174–1180. doi: 10.1097/01.brs.0000263369.95182.19. [http://dx.doi.org/10.1097/01.brs.0000263369.95182.19]. [PMID: 17495773]. [DOI] [PubMed] [Google Scholar]
- 181.Masuda K., Imai Y., Okuma M., et al. Osteogenic protein-1 injection into a degenerated disc induces the restoration of disc height and structural changes in the rabbit anular puncture model. Spine. 2006;31(7):742–754. doi: 10.1097/01.brs.0000206358.66412.7b. [http://dx.doi.org/10.1097/01.brs.0000206358.66412.7b]. [PMID: 16582847]. [DOI] [PubMed] [Google Scholar]
- 182.An H.S., Takegami K., Kamada H., et al. Intradiscal administration of osteogenic protein-1 increases intervertebral disc height and proteoglycan content in the nucleus pulposus in normal adolescent rabbits. Spine. 2005;30(1):25–31. doi: 10.1097/01.brs.0000148002.68656.4d. [http://dx.doi.org/10.1097/01.brs.0000148002.68656.4d]. [PMID: 15626976]. [DOI] [PubMed] [Google Scholar]
- 183.Masuda K., Takegami K., An H., et al. Recombinant osteogenic protein-1 upregulates extracellular matrix metabolism by rabbit annulus fibrosus and nucleus pulposus cells cultured in alginate beads. J. Orthop. Res. 2003;21(5):922–930. doi: 10.1016/S0736-0266(03)00037-8. [http://dx.doi.org/10.1016/S0736-0266(03)00037-8]. [PMID: 12919882]. [DOI] [PubMed] [Google Scholar]
- 184.Tim Yoon S., Su Kim K., Li J., et al. The effect of bone morphogenetic protein-2 on rat intervertebral disc cells in vitro. Spine. 2003;28(16):1773–1780. doi: 10.1097/01.BRS.0000083204.44190.34. [http://dx.doi.org/10.1097/01.BRS.0000083204.44190.34]. [PMID: 12923462]. [DOI] [PubMed] [Google Scholar]
- 185.Zara J.N., Siu R.K., Zhang X., et al. High doses of bone morphogenetic protein 2 induce structurally abnormal bone and inflammation in vivo. Tissue Eng. Part A. 2011;17(9-10):1389–1399. doi: 10.1089/ten.tea.2010.0555. [http://dx.doi.org/10.1089/ten.tea.2010.0555]. [PMID: 21247344]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Osyczka A.M., Diefenderfer D.L., Bhargave G., Leboy P.S. Different effects of BMP-2 on marrow stromal cells from human and rat bone. Cells Tissues Organs (Print) 2004;176(1-3):109–119. doi: 10.1159/000075032. [http://dx.doi.org/10.1159/000075032]. [PMID: 14745240]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Gruber R., Graninger W., Bobacz K., Watzek G., Erlacher L. BMP-6-induced osteogenic differentiation of mesenchymal cell lines is not modulated by sex steroids and resveratrol. Cytokine. 2003;23(4-5):133–137. doi: 10.1016/s1043-4666(03)00223-0. [http://dx.doi.org/10.1016/S1043-4666(03)00223-0]. [PMID: 12967649]. [DOI] [PubMed] [Google Scholar]


