Abstract
A large number of children in the autism spectrum disorder suffer from gastrointestinal (GI) conditions, such as constipation and diarrhea. Clostridium bolteae is a part of a set of pathogens being regularly detected in the stool samples of hosts affected by GI and autism symptoms. Accompanying studies have pointed out the possibility that such microbes affect behaviour through the production of neurotoxic metabolites in a so-called, gut-brain connection. As an extension of our Clostridium difficile polysaccharide (PS)-based vaccine research, we engaged in the discovery of C. bolteae surface carbohydrates. So far, studies revealed that C. bolteae produces a specific immunogenic PS capsule comprised of disaccharide repeating blocks of mannose (Manp) and rhamnose (Rhap) units: α-D-Manp-(1→[-4)-β-D-Rhap-(1→3)-α-D-Manp-(1→]n. For vaccinology and further immunogenic experiments, a method to produce C. bolteae PS conjugates has been developed, along with the chemical syntheses of the PS non-reducing end linkage, with D-Rha or L-Rha, α-D-Manp-(1→4)-α-D-Rhap-(1→O(CH2)5NH2 and α-D-Manp-(1→4)-α-L-Rhap-(1→O(CH2)5NH2, equipped with an aminopentyl linker at the reducing end for conjugation purposes. The discovery of C. bolteae PS immunogen opens the door to the creation of non-evasive diagnostic tools to evaluate the frequency and role of this microbe in autistic subjects and to a vaccine to reduce colonization levels in the GI tract, thus impeding the concentration of neurotoxins.
Keywords: Clostridium bolteae, polysaccharide, synthesis, conjugate, TEMPO, autism, diagnostic, vaccine, gastrointestinal disorders, gut-brain axis
1. INTRODUCTION
Autism is a neurological disorder clinically defined by social and communication impairments with repetitive and restricted patterns of interests and behaviours [1]. It is estimated that the occurrence of autism spectrum disorders (ASD) ranges from 0.72 - 1.57% in child populations [1] with the cost for each person, over a lifetime, believed to be around $3.2 million [2]. Associated with ASDs is a high incidence of medical conditions that include epilepsy, sleep deprivation and gastrointestinal (GI) problems [1]. Indeed, data points to the fact that approximately 90% of ASD patients suffer from GI problems [3]. A number of GI complications are observed in ASD patients, such as diarrhea, excess wind, abdominal pain, constipation and abnor-
mal feces [3]. Parents and care-givers of ASD patients regularly claim that the GI problems and behavioral symptoms are exhibited in parallel [3].
It has been speculated that there is a connection between the administration of antibiotics and the onset of GI disorders. On average, children who present/develop ASD suffered from a higher instance of ear infections, than developmentally normal children, and therefore were administered significantly more antibiotics [2]. It has been shown that the use of antibiotics has the ability to disrupt the GI commensal flora and create an ecological imbalance [4]. This shift in microbial equilibrium then allows for the overgrowth of bacterial species that have the potential of being pathogenic [4].
Several chronic diseases have been associated with a GI tract limited in microorganism diversity [5], but it is difficult to determine whether the decreased biotic diversity is due to the disease or vice-versa. Along these lines, it has been observed that ASD patients also have abnormal GI bacterial flora compared to developmentally normal children [6]. It has been demonstrated that switching the gut microbiota of a timid mouse line with a more aggressive mouse line resulted in a concurrent switch of the behavioral profiles of the mice [5, 7].
In 2005, Parracho et al. reported that approximately 90% of ASD patients suffered from GI disorders, including constipation and diarrhea [3]. Adding to this data, in 2006, Valicenti-McDermott et al. evaluated patients with ASD and with a history of the family auto-immune disease, and found that 70% of children with ASD also had GI disorders, compared to 42% of children with other developmental disorders [8, 9]. A 2002 study showed that children with ASD had a higher abundance of Clostridia species in their GI flora than control children [8, 10]. One Clostridia pathogen that is now regularly affiliated in this relationship, is Clostridium bolteae [11].
Clostridia species, when fed a diet of sugar and refined carbohydrates, produce short-chain fatty acids (SCFAs), such as propionic acid (PPA) [12, 13]. Due to its existence in both ionized and non-ionized form at physiological pH, PPA is able to readily cross the gut-blood barrier [14]. PPA is able to travel even further in the body through its ability to cross the blood-brain barrier and ultimately enter the central nervous system (CNS) [14]. When SCFAs are able to reach the CNS they are often taken up by the glia and, less frequently, by the neurons [14]. The SCFAs have an effect on an array of physiological processes and excessive concentration of PPA may lead to negative effects on health and behaviour. There are a number of conditions, either inherited or acquired, that are developed at varying stages of life due to PPA [14], and are often associated with symptoms such as developmental delay or regression, seizures, metabolic acidosis and GI problems [14]. Symptoms associated with elevated levels of PPA are somewhat reminiscent of those associated with ASDs, and recent studies have begun to explore the possibility of PPA playing a role in behavioral and health symptoms associated with ASDs. The brain and behavioural abnormalities instigated by PPA are similar to the symptoms observed in humans with ASD [15]. This connection strongly points out a direct influence of a bacterial metabolite on human behaviour [8].
In 1998, Ms. Bolte approached Dr. Finegold in a quest to find answers about persistent GI ailments in ASD patients [16]; their initial research ultimately pointing to Clostridium species being a possible culprit, specifically C. tetani [16]. The involvement of C. tetani was particularly interesting due to the fact that this anaerobic bacillus produces a potent neurotoxin. Indeed, the Clostridia microbe family is thought to be involved in the initiation of many illnesses [16]. As aforementioned, colonization of the GI ecosystem by opportunistic bacteria, such as Clostridia species (e.g. Clostridium difficile), is made easier by the repeated use of general antibiotics. It has also been noted that prior to a child regressing into late-onset autism, they have been subjected to multiple doses of broad-spectrum antibiotics [16]. The new hypothesis formulated by Bolte and Finegold led to an increased interest in the GI microbial content found in ASD patients, and indeed, subsequent in-depth studies have revealed possible roles of microorganisms in the onset of ASD symptoms [17-19].
Nowadays, stool samples from ASD patients are the target of analyses looking for abnormalities in flora diversity, including differences in bacterial colony counts. In a 2004 study, it was noticed that another Clostridia specie, C. clostridioforme, was present in ASD patients (with late-onset autism) but was not observed in stool samples from the control group [17, 20]. Additional examinations of C. clostridioforme uncovered that it was comprised of 3 principle species; C. clostridioforme, C. hatheway and C. bolteae [20]. Of these, C. bolteae was present in the stool of the majority of children involved in the study [17, 20], and with the concentration of C. bolteae being significantly higher in ASD children than the control group [20].
Clostridia are usually eliminated through the use of broad-spectrum antibiotics, such as vancomycin. Given that Clostridia species, such as C. bolteae, may be associated with ASDs, a vancomycin treatment was attempted in a small sampling of children presenting regressive late-onset autism [21]. During the 8 week study, the children were observed for changes in behavior and communication [21]. The majority of the children showed an improvement in these psychological markers over the 8 weeks, but parents reported that regression to their baseline began about 2 weeks after the vancomycin treatment was ceased [21]. Two to eight months after the treatment ended, all children, except one, were reported to have deteriorated back to baseline behavior or worse [21]. Vancomycin is a ‘last-resort’ antibiotic that is utilized to treat Gram-positive bacterial infections. While it is an effective treatment to fight C. difficile infections, its use causes a delay in the recovery of the native fecal microflora [22], causing reoccurrences of Gram-positive pathogens. In addition to the reoccurrence of infection, the chance of developing antibiotic resistance also increases with repetitive use of this strong antibiotic. Indeed, Enterococci, such as staphylococci, have begun to show vancomycin resistance, a major problem now in the US and Europe [23, 24]. The increasing threat of antibiotic resistance means that prolonged use of vancomycin is not a good option for ASD treatment. Increasing our knowledge base about microbes putatively involved in GI illnesses, and associated behavioural traits, perhaps antibiotic-free treatment options can be advanced.
Microbes, such as bacteria, expose complex carbohydrates, polysaccharides (PS), as the outer-most decoration on their cell wall. These specie-specific PSs can form the basis of microbial serotyping systems, and used as diagnostic and vaccine targets. As microbiologists begin to establish which bacterial species may be associated with GI disorders in ASD patients, their surface carbohydrates can be explored for clinical purposes. For example, a C. difficile PS-based vaccine has shown the potential to control colonization and disease burden [25]. Hence, it may be feasible to generate PS-based products to help detect and control C. bolteae, thus preventing the accumulation of high levels of neurotoxic metabolites.
2. THE CLOSTRIDIUM BOLTEAE POLYSACCHARIDE
Structural analysis of bacterial PSs is the foundation for several fields of microbial-focus research, such as serotype designation, genetics, virulence, immunochemistry and diagnostics. Knowledge of PS fine structure is also key in the development of anti-bacterial PS-protein conjugate vaccines, in that only after the structural features are known can one devise effective and consistent chemical conjugation strategies. So far, our studies have revealed that C. bolteae expresses a specific capsular PS composed of disaccharide repeating units of 4-linked rhamnose [→4)-Rha-(1→] and 3-linked mannose [→3)-Man-(1→] as shown in (Fig. 1) [26, 27]. The non-reducing end of the PS is terminated by a Man residue in a Man-(1→4)-Rha linkage: α-D-Manp-(1→[-4)-β-D-Rhap-(1→3)-α-D-Manp-(1→]n. To assess C. bolteae PS immunogenicity, New Zealand rabbits were immunized with purified capsule PS. Serum samples from the animals were collected and used in immunological studies that revealed that antibodies were raised against the C. bolteae PS, with a strong interaction between antibody and PS down to a 1:1000 dilution [26, 27].
Fig. (1).
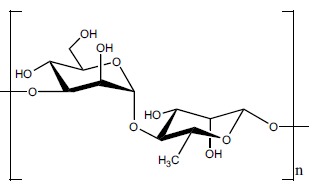
The structure of a proposed C. bolteae disaccharide repeating block: [→3)-α-D-Manp-(1→4)-β-D-Rhap-(1→].
3. CONJUGATION OF CLOSTRIDIUM BOLTEAE POLYSACCHARIDE TO PROTEIN
In conjugate vaccine development, periodate-based oxidation (to generate aldehydes) of bacterial PSs has become a go-to approach for conjugation of carbohydrates to proteins [28]. However, this methodology poses difficulties when vicinal diols are widely distributed throughout the PS backbone, in that, even when stoichiometric amounts of periodate are used, it is difficult to achieve consistency in batch-to-batch oxidation levels. In the case of C. bolteae capsule PS, the presence of 4-substituted Rha unit presented such a challenge and thus another process to activate the PS for conjugation was needed. In our laboratory, we have devised a scheme for direct conjugation of PSs to protein by selectively oxidizing primary hydroxyls (e.g. C-6 in hexoses or C-7 in heptoses) using 2, 2, 6, 6-tetramethylpiperidin-1-oxyl (TEMPO)-mediated oxidation [29]. This PS activation method allows for full or stoichiometric oxidation of PS primary hydroxyls, to aldehydes or carboxylic acid moieties, without disrupting the structural integrity of the PS backbone [29].
C. bolteae capsule PS was thus activated via TEMPO-mediation oxidation (Fig. 2) [30]. Stoichiometric and selective oxidation of the PS could be affected at the sole primary hydroxyl group in the disaccharide repeating block, the C-6 of Man [30]. The TEMPO-mediation oxidation was shown to solely derivatize the primary hydroxyl present at C-6 of the Man residues, with the 1D-1H NMR confirming that the original disaccharide structure was left intact after oxidation [30]. Carbodiimide chemistry was then used to conjugate the activated PS to a carrier protein (CRM197) in a 2:1 ratio by weight, respectively [30]. The integrity of the conjugated PS was confirmed following conjugation by 1D-1H NMR, in which resonances characteristic of CRM197 protein were also present [30].
Fig. (2).
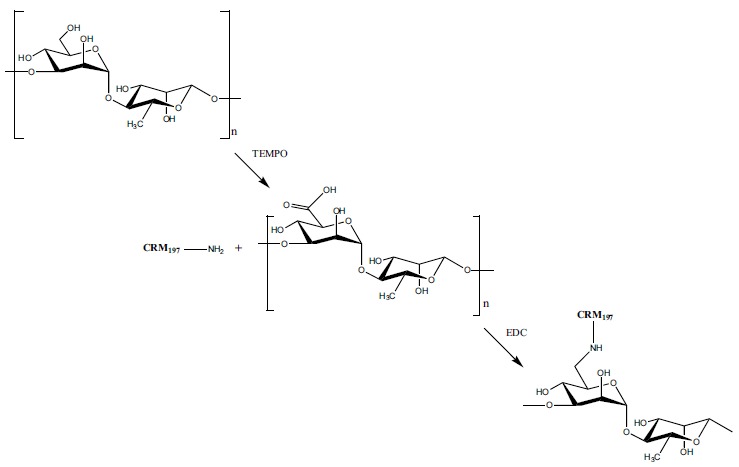
Conjugation of the C. bolteae PS to CRM197 protein. First, TEMPO-mediated oxidation activates the primary hydroxyl on the Man residue and then carbodiimide-like chemistry is used to conjugate the activated PS to the carrier protein CRM197.
4. SYNTHESIS OF THE NON-REDUCING END LINKAGE OF CLOSTRIDIUM BOLTEAE POLYSACCHARIDE
The chemical syntheses of bacterial capsule PS repeating oligosaccharides, or subunits thereof, afford well defined homogenous glycan structures that are useful in evaluating the immunochemistry of PS epitopes. To this end, the first exploratory synthesis of a C. bolteae PS substructure is also described here. Due to the fact that antibodies are readily generated against the non-reducing end of PSs, the first synthesis was that of a disaccharide containing the non-reducing end D-Man-(1→4)-D-Rha linkage of C. bolteae PS [30]. This PS substructure was incorporated in a α-D-Manp-(1→4)-α-D-Rhap-(1→O(CH2)5NH2 format, 1 (Scheme 1), with a aminopentyl linker at the reducing end for potential conjugation experiments. In our synthesis of Campylobacter jejuni capsule PS substructures [31] we have observed that the anomeric configuration of the unit carrying the aminopentyl linker at C-1 does not play a role in the immunodetection of the other glycosidic linkages in the oligosaccharide (OS). Thus, in this first attempt, the Rha unit in disaccharide 1 is present in the α configuration. Ongoing synthetic strategies to produce lengthier C. bolteae PS capsule fragments (di- and tetra-disaccharide repeats) including Rha in the β configuration are ongoing.
Scheme 1.
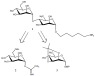
Generation of C. bolteae disaccharide 1 equipped with an aminopentyl linker at the reducing end [30].
The trichloroacetimidate activated mannosyl donor 2 was prepared from D-Man [32], and the 4-hydroxyl rhamnosyl acceptor 3 was prepared from D-Rha [33]. Glycosylation between donor 2 and acceptor 3 was carried out using activator TMSOTf at -10 °C, resulting in a 69% yield of disaccharide 4 with a newly formed α linkage [34]. The β-Man anomer was not observed. Disaccharide 4 was treated with ammonium cerium (IV) nitrate (CAN) at 0 °C to remove the 4-methoxyphenol protecting group [35]. After 30 minutes, disaccharide 5, with a free hydroxyl at the anomeric center of D-Rha, was obtained with a 50% yield (Scheme 2).
Scheme 2.
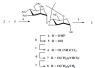
Reagents and conditions [30]: (a) TMSOTf, CH2Cl2, -10°C, 69% (b) CAN, CH3CN:H2O, 0°C, 50%, (c) K2CO3, CH2Cl2, CCl3CN, RT, 29%, (d) TMSOTf, 5-aminio-N-benzyloxycarbonyl pentanol, CH2Cl2, RT, 46%, (e) NH3 (l), Na (s), THF, -78°C, 21%, (f) AcOH:H2O, 80°C, 81%.
Disaccharide 5 was activated with trichloroacetonitrile, and after 48 hours of stirring the reaction mixture at room temperature, trichloro-acetimidate disaccharide donor 6, was obtained with a yield of 29% (α product). Disaccharide 6 was subsequently activated with TMSOTf, at room temperature, and an aminopentyl linker was introduced to disaccharide donor 6. After one hour at room temperature, disaccharide 7 with the α-oriented aminopentyl linker was afforded with a 46% yield (Scheme 2). Disaccharide 7 was subjected to global deprotection to yield 21% of product 8 (Scheme 2). The 2,3-O-isoproprylidene was not removed with basic deprotection and 8 was treated with acetic acid to yield the final product 1 with an 81% yield (Scheme 2) [30]. A variant of disaccharide 1 containing L-Rha (instead of D-Rha) was also synthesized following the same reaction steps and with comparable yields (Pequegnat and Monteiro, unpublished results).
5. REMARKS AND FUTURE DIRECTIONS
Although still at early stages, investigations into the relationship between the human gut microbiome and health have revealed interesting findings, especially those that are related to a likely association between gut-flora compositions and neurological disorders, in a so-called ‘gut-brain’ axis [36]. One of the most intriguing findings has been the detection of a set of gut bacteria in autistic patients, which also suffer from GI disorders [5]. These gut pathogens, especially Clostridia pathogens, excrete metabolites, such as short-chain fatty-acids, that cross the brain barrier, and are believed to act as neurotoxins instigating autism-like symptoms, especially during early stages of brain development [5]. C. bolteae is one of the pathogens that regularly show up in stool material of autistic subjects [3].
During the past decade, we have researched the surface PSs displayed by a clinical important Clostridia pathogen, C. difficile [37-40]. C. difficile PSs (capsules PS-I and PS-II, and lipoteichoic acid PS-III) were found to be immunogenic and circulating antibodies specific for C. difficile PSs have also been detected in humans and horses [38, 41]. These native capsular PSs, and synthetic substructures have also been incorporated into anti-C. difficile conjugate vaccines, with pre-clinical tests showing a marked reduction in disease burden and colonization levels [25, 40, 42]. Our experience with C. difficile encouraged us to investigate the cell-surface carbohydrates of C. bolteae. We discovered that C. bolteae produces a specific capsule PS immunogen that so far seems to be shared by C. bolteae strains: α-D-Manp-(1→[-4)-β-D-Rhap-(1→3)-α-D-Manp-(1→]n. This C. bolteae capsule PS differs from those expressed by other Clostridia species [37, 43]. The conserved nature of the C. bolteae capsule PS follows the trend observed in C. difficile, in that diverse ribotypes also expose a regular cell-wall capsule PS [37].
The uncommon D-Rha observed here is also a key component of Pseudomonas aeruginosa lipopolysaccharide (A-band), in which it is biosynthesized from GDP-D-Man [44, 45]. Since D-Man is also a member of C. bolteae PS, perhaps a similar genetic mechanism may be responsible for furnishing D-Rha from GDP-D-Man in C. bolteae. Conversely, L-Rha may be generated from UDP-D-Glc [46]. Preliminary genome analysis of a C. bolteae strain 14578 has identified, albeit with low percentage homology, enzymes that may be capable of yielding L-Rha [47]. However, within the limits of detection, we have only been able to characterize D-Rha in C. bolteae through the detection of the corresponding diastereomer (as the butyl-glycoside) by gas chromatography-mass spectrometry. To further probe the presence of D-Rha and/or L-Rha in C. bolteae capsule PSs, we have also incorporated L-Rha in a α-D-Manp-(1→4)-α-L-Rhap-(1→O(CH2)5NH2 format, and the syntheses of longer C. bolteae PS substructures for molecular modelling and high-field NMR studies are ongoing by our collaborator, France-Isabelle Auzanneau [48].
CONCLUSION
The C. bolteae capsule PS immunogen described here represents a key tool that may be used in research strategies aimed at evaluating the significance of this microbe in ASD-related health. At the outset, this C. bolteae PS, and related synthetic fragments, may be used in experiments to assess the presence of natural anti-C. bolteae PS circulating antibodies or in stool material. The capsule PS can also be used to raise antibody preparations that could be used in the development of a C. bolteae serotyping system, and in diagnostic kits to detect and assess the incidence of this pathogen in a clinical setting. Ultimately, if C. bolteae is determined to be a pathogen in part responsible for the onset or aggravation of certain ASD disorders, one could envisage the usage of this capsule PS as a vaccine to control colonization levels and disease burden.
ACKNOWLEDGEMENTS
Declared none.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
This work was supported by Natural Sciences and Engineering Research Council (NSERC) Canada (Grant No. 2016-04472).
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Bradley E., Caldwell P., Underwood L. In: Handbook of Psychopathology in Intellectual Disability. Tsakanikos E., McCarthy J., editors. New York: Springer; 2014. pp. 237–264. [http://dx.doi.org/10.1007/978-1-4614-8250-5_16] [Google Scholar]
- 2.Finegold S.M. Desulfovibrio species are potentially important in regressive autism. Med. Hypotheses. 2011;77(2):270–274. doi: 10.1016/j.mehy.2011.04.032. [http://dx.doi.org/10.1016/j.mehy.2011.04.032]. [PMID: 21592674]. [DOI] [PubMed] [Google Scholar]
- 3.Parracho H.M., Bingham M.O., Gibson G.R., McCartney A.L. Differences between the gut microflora of children with autistic spectrum disorders and that of healthy children. J. Med. Microbiol. 2005;54(Pt 10):987–991. doi: 10.1099/jmm.0.46101-0. [http://dx.doi.org/10.1099/jmm.0.46101-0]. [PMID: 16157555]. [DOI] [PubMed] [Google Scholar]
- 4.Guarner F., Malagelada J-R. Gut flora in health and disease. Lancet. 2003;361(9356):512–519. doi: 10.1016/S0140-6736(03)12489-0. [http://dx.doi.org/10.1016/S0140-6736(03)12489-0]. [PMID: 12583961]. [DOI] [PubMed] [Google Scholar]
- 5.Toh M.C., Allen-Vercoe E. The human gut microbiota with reference to autism spectrum disorder: considering the whole as more than a sum of its parts. Microb. Ecol. Health Dis. 2015;26:26309. doi: 10.3402/mehd.v26.26309. [PMID: 25634609]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finegold S.M., Downes J., Summanen P.H. Microbiology of regressive autism. Anaerobe. 2012;18(2):260–262. doi: 10.1016/j.anaerobe.2011.12.018. [http://dx.doi.org/10.1016/j.anaerobe.2011.12.018]. [PMID: 22202440]. [DOI] [PubMed] [Google Scholar]
- 7.Bercik P., Denou E., Collins J., Jackson W., Lu J., Jury J., Deng Y., Blennerhassett P., Macri J., McCoy K.D., Verdu E.F., Collins S.M. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology. 2011;141(2):599–609. doi: 10.1053/j.gastro.2011.04.052. 609.e1-609.e3. [DOI] [PubMed] [Google Scholar]
- 8.Arranga T., Viadro C.I., Underwood L., Herbert M. Bugs, Bowels, and Behavior: The Groundbreaking Story of the Gut-Brain Connection. Skyhorse Publishing Company, Incorporated; 2013. [Google Scholar]
- 9.Valicenti-McDermott M., McVicar K., Rapin I., Wershil B.K., Cohen H., Shinnar S. Frequency of gastrointestinal symptoms in children with autistic spectrum disorders and association with family history of autoimmune disease. J. Dev. Behav. Pediatr. 2006;27(2) Suppl.:S128–S136. doi: 10.1097/00004703-200604002-00011. [http://dx.doi.org/10.1097/00004703-200604002-00011]. [PMID: 16685179]. [DOI] [PubMed] [Google Scholar]
- 10.Finegold S.M., Molitoris D., Song Y., Liu C., Vaisanen M-L., Bolte E., McTeague M., Sandler R., Wexler H., Marlowe E.M., Collins M.D., Lawson P.A., Summanen P., Baysallar M., Tomzynski T.J., Read E., Johnson E., Rolfe R., Nasir P., Shah H., Haake D.A., Manning P., Kaul A. Gastrointestinal microflora studies in late-onset autism. Clin. Infect. Dis. 2002;35(Suppl. 1):S6–S16. doi: 10.1086/341914. [http://dx.doi.org/10.1086/341914]. [PMID: 12173102]. [DOI] [PubMed] [Google Scholar]
- 11.Song Y., Liu C., Molitoris D.R., Tomzynski T.J., Lawson P.A., Collins M.D., Finegold S.M. Clostridium bolteae sp. nov., isolated from human sources. Syst. Appl. Microbiol. 2003;26(1):84–89. doi: 10.1078/072320203322337353. [http://dx.doi.org/10.1078/072320203322337353]. [PMID: 12747414]. [DOI] [PubMed] [Google Scholar]
- 12.MacFabe D.F., Cain D.P., Rodriguez-Capote K., Franklin A.E., Hoffman J.E., Boon F., Taylor A.R., Kavaliers M., Ossenkopp K-P. Neurobiological effects of intraventricular propionic acid in rats: possible role of short chain fatty acids on the pathogenesis and characteristics of autism spectrum disorders. Behav. Brain Res. 2007;176(1):149–169. doi: 10.1016/j.bbr.2006.07.025. [http://dx.doi.org/10.1016/j.bbr.2006.07.025]. [PMID: 16950524]. [DOI] [PubMed] [Google Scholar]
- 13.MacFabe D.F., Rodríguez-Capote K., Hoffman J.E., Franklin A.E., Mohammad-Asef Y., Taylor A.R., Boon F., Cain D.P., Kavaliers M., Possmayer F. A novel rodent model of autism: intraventricular infusions of propionic acid increase locomotor activity and induce neuroinflammation and oxidative stress in discrete regions of adult rat brain. Am. J. Biochem. Biotechnol. 2008;4(2):146–166. [http://dx.doi.org/10.3844/ajbbsp.2008.146.166]. [Google Scholar]
- 14.Macfabe D.F. Short-chain fatty acid fermentation products of the gut microbiome: implications in autism spectrum disorders. Microb. Ecol. Health Dis. 2012;23 doi: 10.3402/mehd.v23i0.19260. [Epub ahead of print]. [http://dx.doi.org/10.3402/mehd.v3423i3400.19260]. [PMID: 23990817]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shultz S.R., MacFabe D.F., Ossenkopp K-P., Scratch S., Whelan J., Taylor R., Cain D.P. Intracerebroventricular injection of propionic acid, an enteric bacterial metabolic end-product, impairs social behavior in the rat: implications for an animal model of autism. Neuropharmacology. 2008;54(6):901–911. doi: 10.1016/j.neuropharm.2008.01.013. [http://dx.doi.org/10.1016/j.neuropharm.2008.01.013]. [PMID: 18395759]. [DOI] [PubMed] [Google Scholar]
- 16.Bolte E.R. Autism and Clostridium tetani. Med. Hypotheses. 1998;51(2):133–144. doi: 10.1016/s0306-9877(98)90107-4. [http://dx.doi.org/10.1016/S0306-9877(98)90107-4]. [PMID: 9881820]. [DOI] [PubMed] [Google Scholar]
- 17.Song Y., Liu C., Finegold S.M. Real-time PCR quantitation of clostridia in feces of autistic children. Appl. Environ. Microbiol. 2004;70(11):6459–6465. doi: 10.1128/AEM.70.11.6459-6465.2004. [http://dx.doi.org/10.1128/AEM.70.11.6459-6465.2004]. [PMID: 15528506]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Finegold S.M., Dowd S.E., Gontcharova V., Liu C., Henley K.E., Wolcott R.D., Youn E., Summanen P.H., Granpeesheh D., Dixon D., Liu M., Molitoris D.R., Green J.A., III Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe. 2010;16(4):444–453. doi: 10.1016/j.anaerobe.2010.06.008. [http://dx.doi.org/10.1016/j.anaerobe.2010.06.008]. [PMID: 20603222]. [DOI] [PubMed] [Google Scholar]
- 19.Wang L., Christophersen C.T., Sorich M.J., Gerber J.P., Angley M.T., Conlon M.A. Increased abundance of Sutterella spp. and Ruminococcus torques in feces of children with autism spectrum disorder. Mol. Autism. 2013;4(1):42–42. doi: 10.1186/2040-2392-4-42. [http://dx.doi.org/10.1186/2040-2392-4-42]. [PMID: 24188502]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Finegold S.M., Song Y., Liu C., Hecht D.W., Summanen P., Könönen E., Allen S.D. Clostridium clostridioforme: a mixture of three clinically important species. Eur. J. Clin. Microbiol. Infect. Dis. 2005;24(5):319–324. doi: 10.1007/s10096-005-1334-6. [http://dx.doi.org/10.1007/s10096-005-1334-6]. [PMID: 15891914]. [DOI] [PubMed] [Google Scholar]
- 21.Sandler R.H., Finegold S.M., Bolte E.R., Buchanan C.P., Maxwell A.P., Väisänen M-L., Nelson M.N., Wexler H.M. Short-term benefit from oral vancomycin treatment of regressive-onset autism. J. Child Neurol. 2000;15(7):429–435. doi: 10.1177/088307380001500701. [http://dx.doi.org/10.1177/088307380001500701]. [PMID: 10921511]. [DOI] [PubMed] [Google Scholar]
- 22.Venugopal A.A., Johnson S. Current state of Clostridium difficile treatment options. Clin. Infect. Dis. 2012;55(Suppl. 2):S71–S76. doi: 10.1093/cid/cis355. [http://dx.doi.org/10.1093/cid/cis355]. [PMID: 22752868]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Srinivasan A., Dick J.D., Perl T.M. Vancomycin resistance in staphylococci. Clin. Microbiol. Rev. 2002;15(3):430–438. doi: 10.1128/CMR.15.3.430-438.2002. [http://dx.doi.org/10.1128/CMR.15.3.430-438.2002]. [PMID: 12097250]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cetinkaya Y., Falk P., Mayhall C.G. Vancomycin-resistant enterococci. Clin. Microbiol. Rev. 2000;13(4):686–707. doi: 10.1128/cmr.13.4.686-707.2000. [http://dx.doi.org/10.1128/CMR.13.4.686]. [PMID: 11023964]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monteiro M.A. The design of a Clostridium difficile carbohydrate-based vaccine. Methods Mol. Biol. 2016;1403:397–408. doi: 10.1007/978-1-4939-3387-7_21. [http://dx.doi.org/10.1007/978-1-4939-3387-7_21]. [PMID: 27076143]. [DOI] [PubMed] [Google Scholar]
- 26.Pequegnat B., Sagermann M., Valliani M., Toh M., Chow H., Allen-Vercoe E., Monteiro M.A. A vaccine and diagnostic target for Clostridium bolteae, an autism-associated bacterium. Vaccine. 2013;31(26):2787–2790. doi: 10.1016/j.vaccine.2013.04.018. [http://dx.doi.org/10.1016/j.vaccine.2013.04.018]. [PMID: 23602537]. [DOI] [PubMed] [Google Scholar]
- 27.Pequegnat B. A Diagnostic Target Against Clostridium bolteae, Towards a Multivalent Vaccine for Autism-Related Gastric Bacteria. University of Guelph; 2013. [Google Scholar]
- 28.Bertolo L., Ewing C.P., Maue A., Poly F., Guerry P., Monteiro M.A. The design of a capsule polysaccharide conjugate vaccine against Campylobacter jejuni serotype HS15. Carbohydr. Res. 2013;366:45–49. doi: 10.1016/j.carres.2012.11.017. [http://dx.doi.org/10.1016/j.carres.2012.11.017]. [PMID: 23261782]. [DOI] [PubMed] [Google Scholar]
- 29.Ma Z., Bertolo L., Arar S., Monteiro M.A. TEMPO-mediated glycoconjugation: a scheme for the controlled synthesis of polysaccharide conjugates. Carbohydr. Res. 2011;346(2):343–347. doi: 10.1016/j.carres.2010.11.021. [http://dx.doi.org/10.1016/j.carres.2010.11.021]. [PMID: 21167478]. [DOI] [PubMed] [Google Scholar]
- 30.Pequegnat B. Polysaccharide Vaccines for Enteric Pathogens: The Next Generation Multivalent Diarrhea Vaccine. University of Guelph; 2016. [Google Scholar]
- 31.Jiao Y. Syntheses of Carbohydrate Antigens Expressed by Gastric-intestinal Bacteria and Conjugates Thereof. University of Guelph; 2016. [Google Scholar]
- 32.Kerékgyártó J., Kamerling J.P., Bouwstra J.B., Vliegenthart J.F., Lipták A. Synthesis of four structural elements of xylose-containing carbohydrate chains from N-glycoproteins. Carbohydr. Res. 1989;186(1):51–62. doi: 10.1016/0008-6215(89)84004-2. [http://dx.doi.org/10.1016/0008-6215(89)84004-2]. [PMID: 2720704]. [DOI] [PubMed] [Google Scholar]
- 33.Fauré R., Shiao T.C., Damerval S., Roy R. Practical synthesis of valuable d-rhamnoside building blocks for oligosaccharide synthesis. Tetrahedron Lett. 2007;48(13):2385–2388. [http://dx.doi.org/10.1016/j.tetlet.2007.01.122]. [Google Scholar]
- 34.Ma Z., Zhang J., Kong F. Concise syntheses of β-GlcNAcp-(1→ 6)-α-Manp-(1→ 6)-Manp and its dimer, and β-GlcNAcp-(1→ 2)-α-Manp-(1→ 6)-. Manp. Tetrahedron: Asymmetry. 2003;14(17):2595–2603. [http://dx.doi.org/10.1016/S0957-4166(03)00570-6]. [Google Scholar]
- 35.Ning J., Zhang W., Yi Y., Yang G., Wu Z., Yi J., Kong F. Synthesis of β-(1-->6)-branched β-(1-->3) glucohexaose and its analogues containing an α-(1-->3) linked bond with antitumor activity. Bioorg. Med. Chem. 2003;11(10):2193–2203. doi: 10.1016/s0968-0896(03)00118-4. [http://dx.doi.org/10.1016/S0968-0896(03)00118-4]. [PMID: 12713829]. [DOI] [PubMed] [Google Scholar]
- 36.Johnson K.V., Foster K.R. Why does the microbiome affect behaviour? Nat. Rev. Microbiol. 2018;16(10):647–655. doi: 10.1038/s41579-018-0014-3. [http://dx.doi.org/10.1038/s41579-018-0014-3]. [PMID: 29691482]. [DOI] [PubMed] [Google Scholar]
- 37.Ganeshapillai J., Vinogradov E., Rousseau J., Weese J.S., Monteiro M.A. Clostridium difficile cell-surface polysaccharides composed of pentaglycosyl and hexaglycosyl phosphate repeating units. Carbohydr. Res. 2008;343(4):703–710. doi: 10.1016/j.carres.2008.01.002. [http://dx.doi.org/10.1016/j.carres.2008.01.002]. [PMID: 18237724]. [DOI] [PubMed] [Google Scholar]
- 38.Bertolo L., Boncheff A.G., Ma Z., Chen Y-H., Wakeford T., Friendship R.M., Rosseau J., Weese J.S., Chu M., Mallozzi M., Vedantam G., Monteiro M.A. Clostridium difficile carbohydrates: glucan in spores, PSII common antigen in cells, immunogenicity of PSII in swine and synthesis of a dual C. difficile-ETEC conjugate vaccine. Carbohydr. Res. 2012;354:79–86. doi: 10.1016/j.carres.2012.03.032. [http://dx.doi.org/10.1016/j.carres.2012.03.032]. [PMID: 22533919]. [DOI] [PubMed] [Google Scholar]
- 39.Jiao Y., Ma Z., Hodgins D., Pequegnat B., Bertolo L., Arroyo L., Monteiro M.A. Clostridium difficile PSI polysaccharide: synthesis of pentasaccharide repeating block, conjugation to exotoxin B subunit, and detection of natural anti-PSI IgG antibodies in horse serum. Carbohydr. Res. 2013;378(0):15–25. doi: 10.1016/j.carres.2013.03.018. [http://dx.doi.org/10.1016/j.carres.2013.03.018]. [PMID: 23597587]. [DOI] [PubMed] [Google Scholar]
- 40.Monteiro M.A., Ma Z., Bertolo L., Jiao Y., Arroyo L., Hodgins D., Mallozzi M., Vedantam G., Sagermann M., Sundsmo J., Chow H. Carbohydrate-based Clostridium difficile vaccines. Expert Rev. Vaccines. 2013;12(4):421–431. doi: 10.1586/erv.13.9. [http://dx.doi.org/10.1586/erv.13.9]. [PMID: 23560922]. [DOI] [PubMed] [Google Scholar]
- 41.Oberli M.A., Hecht M.L., Bindschädler P., Adibekian A., Adam T., Seeberger P.H. A possible oligosaccharide-conjugate vaccine candidate for Clostridium difficile is antigenic and immunogenic. Chem. Biol. 2011;18(5):580–588. doi: 10.1016/j.chembiol.2011.03.009. [http://dx.doi.org/10.1016/j.chembiol.2011.03.009]. [PMID: 21609839]. [DOI] [PubMed] [Google Scholar]
- 42.Broecker F., Martin C.E., Wegner E., Mattner J., Baek J.Y., Pereira C.L., Anish C., Seeberger P.H. Synthetic lipoteichoic acid glycans are potential vaccine candidates to protect from Clostridium difficile infections. Cell Chem. Biol. 2016;23(8):1014–1022. doi: 10.1016/j.chembiol.2016.07.009. [http://dx.doi.org/10.1016/j.chembiol.2016.07.009]. [PMID: 27524293]. [DOI] [PubMed] [Google Scholar]
- 43.Kalelkar S., Glushka J., van Halbeek H., Morris L.C., Cherniak R. Structure of the capsular polysaccharide of Clostridium perfringens Hobbs 5 as determined by NMR spectroscopy. Carbohydr. Res. 1997;299(3):119–128. doi: 10.1016/s0008-6215(97)00010-4. [http://dx.doi.org/10.1016/S0008-6215(97)00010-4]. [PMID: 9163894]. [DOI] [PubMed] [Google Scholar]
- 44.Rocchetta H.L., Burrows L.L., Lam J.S. Genetics of O-antigen biosynthesis in Pseudomonas aeruginosa. Microbiol. Mol. Biol. Rev. 1999;63(3):523–553. doi: 10.1128/mmbr.63.3.523-553.1999. [PMID: 10477307]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramm M., Wolfender J-L., Queiroz E.F., Hostettmann K., Hamburger M. Rapid analysis of nucleotide-activated sugars by high-performance liquid chromatography coupled with diode-array detection, electrospray ionization mass spectrometry and nuclear magnetic resonance. J. Chromatogr. A. 2004;1034(1-2):139–148. doi: 10.1016/j.chroma.2004.02.023. [http://dx.doi.org/10.1016/j.chroma.2004.02.023]. [PMID: 15116923]. [DOI] [PubMed] [Google Scholar]
- 46.Watt G., Leoff C., Harper A.D., Bar-Peled M. A bifunctional 3,5-epimerase/4-keto reductase for nucleotide-rhamnose synthesis in Arabidopsis. Plant Physiol. 2004;134(4):1337–1346. doi: 10.1104/pp.103.037192. [http://dx.doi.org/10.1104/pp.103.037192]. [PMID: 15020741]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Institute B. [Clostridium] bolteae WAL-14578. Human Microbiome Project. 2015 [Google Scholar]
- 48.Davidson J. Synthesis of Clostridium bolteae Capsular Polysaccharide Fragments: A Repeating Disaccharide Unit. University of Guelph; 2016. [Google Scholar]


