Abstract
Background
In this study, we aimed to detect the changes in the level of interleukin (IL)-4 and IL-13 cytokines and their downstream genes including interleukin-13 receptor subunit alpha-2 (IL13Ra2), interleukin-4 receptor subunit alpha-1 (IL4Ra1), dual oxidase 1 (DUOX1) and dual oxidase 2 (DUOX2). The protective effects of Selenium-L-methionine on radiation-induced histopathological damages and changes in the level of these cytokines and genes were detected.
Methods
Four groups of 20 rats (5 rats in each) namely, control; Selenium-L-methionine, radiation and radiation plus Selenium-L-methionine were used in this study. 4 mg/kg of Selenium-L-methionine was administered 1 day before irradiation and five consecutive days after irradiation. Irradiation was done using a dose of 15 Gy 60Co gamma rays at 109 cGy/min. All rats were sacrificed 10 weeks after irradiation for detecting changes in IL-4 and IL-13 cytokines, the expressions of IL13Ra2, IL4Ra1, Duox1 and Duox2 and histopathological changes.
Results
The level of IL-4 but not IL-13 increased after irradiation. This was associated with increased expression of IL4Ra1, Duox1 and Duox2, in addition to changes in morphological properties. Selenium-L-methionine could attenuate all injury markers following lung irradiation.
Conclusion
Selenium-L-methionine can protect lung tissues against toxic effects of ionizing radiation. It is possible that the modulation of immune responses and redox interactions are involved in the radioprotective effect of this agent.
Keywords: Selenium-L-methionine, lung, radiation, IL-4. IL-13, radiotherapy, pneumonitis, interleukin
1. INTRODUCTION
Protection of lung tissues against toxic effects of radiotherapy or chemotherapy is one of the most studied investigations in experimental studies. The lung is one of the most
sensitive organs to ionizing radiation. Although, damages to the lungs during the course of radiotherapy may not cause severe problems, the appearance of the signs of lung toxicity months to years after exposure poses a threat to patients’ life [1]. In addition to clinical implications in cancer radiotherapy, lung injury is one of the most dangerous consequences of exposure to external or internal radiation during a nuclear or radiation disaster [2]. The major side effects of radiotherapy in the lungs are pneumonitis and fibrosis. Pneumonitis occurs due to acute upregulation of inflammatory mediators and cytokines while fibrosis appears following long-term T2-helper cytokines such as interleukin (IL)-4, IL-13, transforming growth factor beta (TGF-β) as well as some growth factors like platelet-derived growth factor (PDGF) [3, 4].
Experimental studies have shown that exposure to radiation can lead to oxidative DNA damage in both nucleus and mitochondria. If cells cannot repair DNA damages, it may lead to cell death mainly through apoptosis, mitotic catastrophe and necrosis. Apoptosis and necrosis stimulate the immune system, leading to an increased level of cytokines and chemokines, as well as immune mediators and transcription factors [5]. In response to apoptosis, macrophages release tolerogenic cytokines such as IL-4, IL-10, IL-13, and TGF-β while necrosis which is more obvious following higher doses of radiation induces inflammatory responses. Danger alarms like oxidized DNA and other products of necrosis induce upregulation of toll-like receptors (TLRs), transcription factors, inflammatory mediators and cytokines [6]. Both inflammatory and anti-inflammatory cytokines are able to induce reduction/oxidation (redox) interactions and amplify oxidative injury by ionizing radiation [7, 8]. Chronic oxidative stress by redox mediators stimulates edema and collagen deposition, dramatic upregulation of inflammatory and anti-inflammatory cytokines as well as prostaglandins in a positive feedback loop [9]. These changes disrupt normal lung action months to years after exposure to a high dose of ionizing radiation [10].
With regard to the mechanisms of lung injury following exposure to radiation, several studies have been conducted to alleviate radiation toxicity in this organ. Targeting of immune system mediators by inhibition of cyclooxygenase-2 (COX-2), inducible nitric oxide synthase (iNOS), renin-angiotensin system, as well as cytokines such as IL-4, IL-13 and TGF-β has shown promising results [11, 12]. Moreover, suppression of chronic oxidative stress by targeting pro-oxidant enzymes such as NADPH oxidase and mitochondria can ameliorate late effects of radiation to the lung [13]. NADPH oxidase comprises five subfamilies which include NADPH oxidase 1-5 (NOX1-5) and dual oxidase 1 and 2 (Duox1&2) [14]. Duox1 and Duox2 are usually identified in thyroid cells. These enzymes through the production of H2O2 stimulate the production of thyroid hormones [15]. It is known that both IL-4 and IL-13 play key roles in late effects of radiation-induced lung injury [16]. On the other hand, some studies have shown that increased levels of IL-4 and IL-13 play key roles in stimulating continuous production of free radicals following exposure to radiation. A study by Hassani et al. showed that IL-4 and IL-13, through upregulation of interleukin-13 receptor subunit alpha-2 (IL13Ra2) and interleukin-4 receptor subunit alpha-1 (IL4Ra1) lead to the stimulation of Duox2 and Duox1 as well as chronic production of H2O2 and damage thyroid cells [17]. IL13Ra2 is able to upregulate Duox1, while IL4Ra1 can induce upregulation of both Duox1 and Duox2 [18]. This study proposed that attenuation of these genes can help mitigate radiation injury and chronic oxidative damage. Modulation of NADPH oxidase enzymes such as Duox1 and Duox2 has been proposed for suppressing redox interactions, inflammatory responses and fibrosis [8, 19, 20].
Selenium-L-methionine can be incorporated into proteins containing methionine, without any detrimental effects on the functions of proteins. Free selenium can also be used for the development of selenocysteine, an amino acid that plays a key role in stimulating antioxidant enzymes such as superoxide dismutase (SOD), thioredoxin reductase (TR), glutathione peroxidases (GPx), and others [21]. In addition to neutralization of free radicals by these antioxidant enzymes, methionine itself is able to scavenge ROS via its sulfur, and also act as a metal chelator [22]. Selenium-L-methionine is a potent antioxidant that has shown interesting radioprotective and mitigatory effects. In recent years, it has shown the ability to mitigate radiation-induced hematopoietic and gastrointestinal injury as well as nephrotoxicity in animal models [23, 24]. In the present study, our aim was to assess changes in the regulation of IL-4, IL-13, IL4Ra1, IL13Ra2, Duox2 and Duox1 following local chest irradiation of rats, in addition to evaluating the modulatory effects of Selenium-L-methionine on these genes and its radioprotective effect on inflammatory and fibrosis markers.
2. MATERIALS AND METHODS
2.1. Irradiation and Treatment with Selenium-L-methionine
Selenium-L-methionine, purchased from Sigma Aldrich company (USA) was dissolved in distilled water at a concentration of 0.8 mg/ml. Intraperitoneal injection of Selenium-L-methionine at a dose of 4 mg/kg (1 ml for each rat) was administered to the rats 1 day before and five consecutive days after irradiation. This dose of Selenium-L-methionine was chosen based on a previous study [25]. On the day of irradiation, 30 minutes before commencement, Selenium-L-methionine was injected to each rat followed by anesthesia using a combination of ketamine and xylazine. The rats were positioned supine on the table of a 60Co gamma rays source. Irradiation of 15 Gy was performed using a 60Co source (1.25Mev) at a dose rate of 109 cGy/min and a source to skin distance of 60 cm. This radiation dose was selected similar to previous studies [26, 27].
2.2. Experimental Design
Twenty male Wistar rats weighing 200±20 g were provided from Razi Institute, Tehran, Iran. All rats were kept under standard humidity and temperature (55% and 220C, respectively) at Pharmacy Animal Laboratory, Tehran University of Medical Sciences, Tehran, Iran. All rats were divided into 4 groups (5 rats in each) namely: control; Selenium-L-methionine treatment; radiation; and radiation plus Selenium-L-methionine. 10 weeks after irradiation, all rats were sacrificed using spinal dislocation followed by removal of lung tissues. The lower parts of their left lungs were fixed in 10% normal buffer formalin while their right lungs were frozen for ELISA assay and real-time PCR.
2.3. Histopathological Evaluation
The fixed lung samples were embedded in paraffin blocks. Afterward, tissue samples were cut into 5-micron sizes and placed on the slides. For evaluating the usual histological changes caused by radiation such as infiltration of inflammatory cells, edema, as well as damages to alveolar and vascular, the slides were stained with hematoxylin and eosin (H&E). For the evaluation of collagen deposition as a marker of fibrosis, other slides were provided and stained with Masson’s trichrome. Slides were evaluated by a pathologist with the aid of a light microscope. The severity of histological changes was scored from 0 to 3, while fibrosis was reported as absent, mild and severe.
2.4. Real-time PCR
Total RNA was extracted from lung cells after homogenization in TRIzol reagent (RiboExTM, GeneAll, South Korea). The cDNA was generated using cDNA Synthesis Kits (GeneAll, South Korea) via a thermocycler. The expression of each gene was detected using Applied Biosystems real-time PCR (USA). The sequence of each primer was designed using Gene Runner and NCBI Blast. GAPDH was chosen as an internal control primer. The sequences of confirmed primers are shown in Table 1.
Table 1. The sequences of primers for Real-time PCR.
| Gene | Forward Sequence | Reverse Sequence |
|---|---|---|
| IL-4R1 | GAGTGAGTGGAGTCCCAGCATC | GCTGAAGTAACAGGTCAGGC |
| IL- 13Ra2 | TCGTGTTAGCGGATGGGGAT | GCCTGGAAGCCTGGATCTCTA |
| Duox1 | AAGAAAGGAAGCATCAACACCC | ACCAGGGCAGTCAGGAAGAT |
| Duox2 | AGTCTCATTCCTCACCCGGA | GTAACACACACGATGTGGCG |
| GAPDH | AGTGCCAGCCTCGTCTCATA | ATGAAGGGGTCGTTGATGGC |
2.5. ELISA
The levels of both IL-4 and IL-13 were detected by ELISA kit provided from Zellbio Company (Gold Biotechnology, Inc, Germany). The complete protocol was carried out based on the manufacturer’s instruction. Colorimetric kit results were converted into pictogram per milliliter (pg/ml) and reported.
2.6. Statistical Analysis
All statistical analyses were done using SPSS software (version 16). Pathological results were analyzed using the Mann-Whitney test. The significance of changes in the expression of genes reported by real-time PCR was analyzed using T-Test. Finally, ELISA results were analyzed by one-way ANOVA and Tukey's HSD post HOC with P values <0.05 considered statistically significant.
3. RESULTS
3.1. Histopathological Evaluation
Pathological studies with H&E stained slides showed a severe increase in infiltration of macrophages and lymphocytes as well as damages to vascular and alveolar in the radiation group compared to the control group. Moreover, trichrome slides showed a mild accumulation of collagen following irradiation, compared to control. Treatment with Selenium-L-methionine led to significant alleviation of all mentioned parameters Figs. (1)-(3).
Fig. (1).
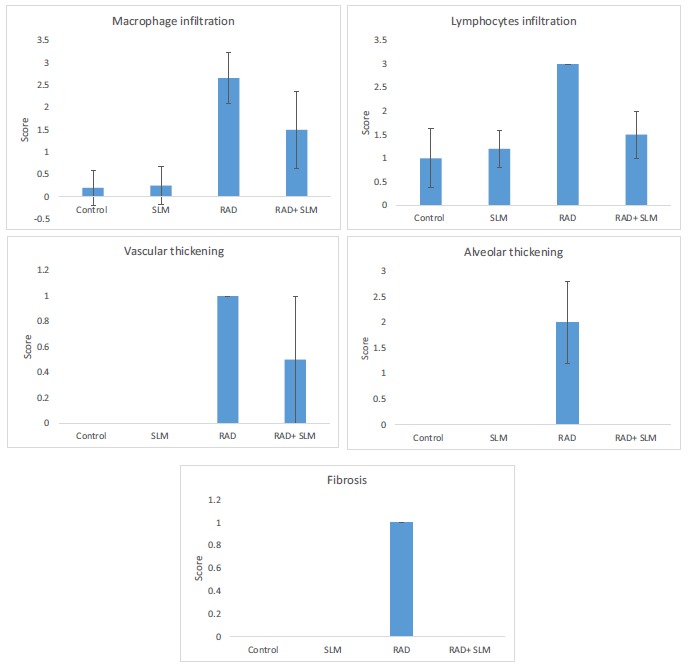
The histopathological results of lung irradiation with 15 Gy gamma rays and radioprotective effect of Selenium-L-methionine. Results were scored from 0 to 3 (absent; mild; moderate; severe) (a: significant change compared to control group; b: significant change compared to radiation group (p<0.05). (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Fig. (3).
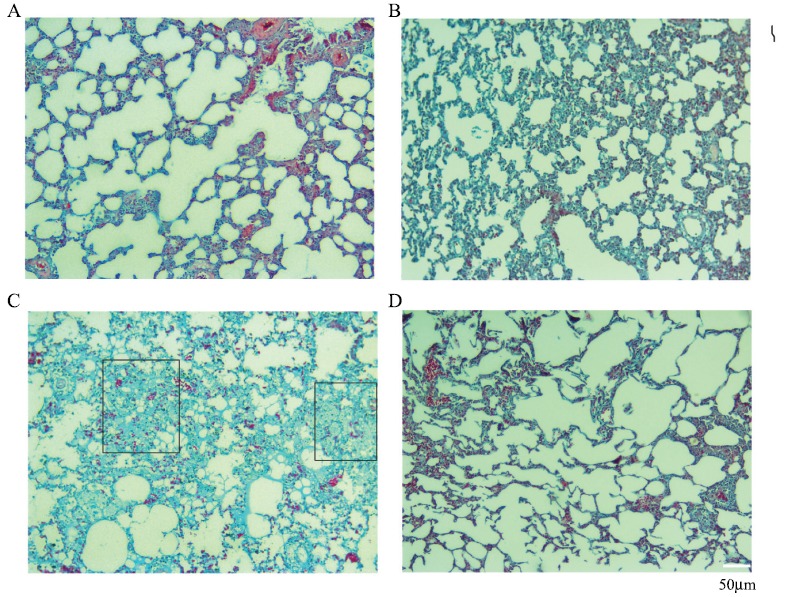
Radioprotective effect of Selenium-L-methionine on rat’s lung tissues fibrosis following local irradiation by gamma rays (Trichrome staining ×100 magnification). A: Control; B: Selenium-L-methionine treatment; C: Irradiation; D: Irradiation plus Selenium-L-methionine. Irradiation caused mild fibrosis, while treatment with Selenium-L-methionine reversed it. Fibrosis in radiation plus Selenium-L-methionine was similar to control and Selenium-L-methionine treatment groups. Rectangular shapes show collagen accumulation. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
3.2. Real-time PCR
QPCR results showed that when rats were irradiated with 15 Gy gamma rays, the level of IL4Ra1 increased significantly (4.9±1.3) compared to the control group (p<0.05). Treatment with Selenium-L-methionine could significantly reduce the expression of IL4Ra1 (1.78±0.51) (p<0.05). However, when rats were treated with only Selenium-L-methionine, the level of IL4Ra1 did not show any significant change (1.12±0.54). Lung tissue irradiation caused an 8.37±1.37-fold increase in the expression of Duox1 compared to control group (p<0.05). Treatment with only Selenium-L-methionine did not change the expression of Duox1 (1.40±24) while in administering Selenium-L-methionine with radiation, the expression of Duox1 reduced significantly (4.2±0.76) compared to radiation alone (p<0.05). Similar to IL4Ra1 and Duox1, the expression of Duox2 was upregulated following irradiation and attenuated when rats were treated with Selenium-L-methionine before and after irradiation. Results showed a 5.87±2-fold increase in the expression of Duox2 following irradiation while Selenium-L-methionine reduced it to 2.26±0.64-fold (p<0.05). Treatment with only Selenium-L-methionine did not cause any change in the expression of Duox2 (1.02±0.36) Fig. (4).
Fig. (4).
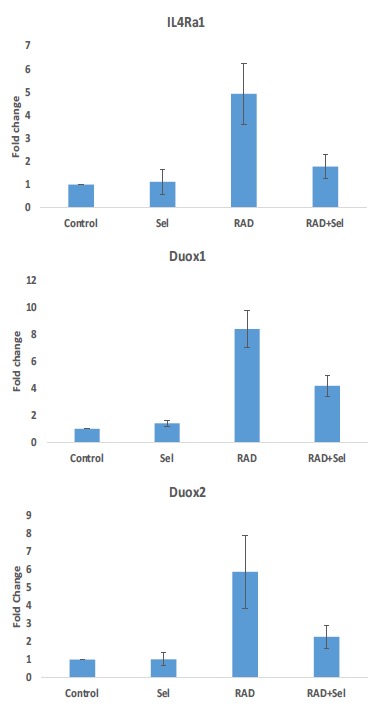
The changes in the expressions of IL4Ra1, Duox1 and Duox2 following rat’s lung irradiation with or without treatment with Selenium-L-methionine. (a: significant in comparison to control group; b: significant in comparison to radiation group). (A higher resolution / colour version of this figure is available in the electronic copy of the article).
3.3. ELISA
Results of enzyme immunoassay of cytokines showed that irradiation with gamma rays led to a significant increase in the level of IL-4 (633±48) compared to the basal level of this cytokine in the control group (422±71) (p<0.05). When rats were treated with Selenium-L-methionine before and after exposure to gamma rays, the level of this cytokine decreased significantly (468±44) (p<0.05). Treatment with only Selenium-L-methionine did not cause any significant increase or decrease in IL-4 compared to control group (477±49). ELISA assay results for IL-13 showed no significant change in the level of this cytokine following irradiation (215±3) compared to control (223±8). Also, treatment with only Selenium-L-methionine (237±5) before and after irradiation (243±14) had no significant change in the level of IL-13 Fig. (5).
Fig. (5).
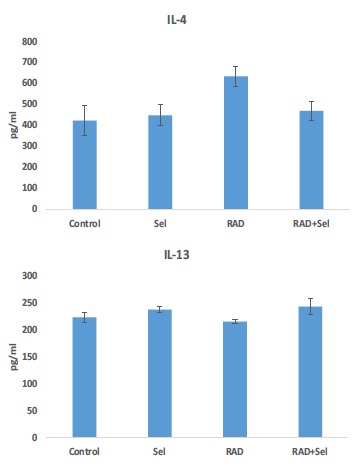
The changes in the levels of IL-4 and IL-13 following rat’s lung irradiation with or without treatment with Selenium-L-methionine. (a: significant in comparison to control group; b: significant in comparison to radiation group). (A higher resolution / colour version of this figure is available in the electronic copy of the article).
4. DISCUSSION
As previously mentioned, lung toxicity is a major side effect for chest cancer patients who underwent radiotherapy or chemo/radiation therapy. In addition, lung injury may appear in patients who had been exposed to whole body irradiation for bone marrow transplantation. Development of some mitigators for preventing death due to radiation-induced lung injury in an accidental nuclear disaster has been on the increase in recent years. Knowledge about the
mechanisms of radiation-induced pneumonitis or fibrosis can improve the management of these side effects. For example, dramatic upregulation of renin-angiotensin system in lung tissue following exposure to radiation can be modulated by captopril, leading to attenuation of lung toxicity and increased survival in animal models. Some studies have proposed antioxidants such as genistein, hesperidin, melatonin, superoxide dismutase-catalase mimetic (EUK-207) etc. [13, 28, 29]. Selenium-L-methionine is a potent antioxidant that has shown interesting properties for protection and mitigation of radiation injury [30]. It has shown the ability to protect against micronuclei formation in bone marrow and also mitigate radiation injury in hematopoietic and gastrointestinal system as well as alleviating nephropathy [23, 31-33]. These studies may indicate the important roles of inflammatory reaction and redox system in late effects of ionizing radiation in lung tissues.
Studies have proposed the involvement of a large number of cytokines and pro-oxidants in radiation-induced lung pneumonitis and fibrosis. It has been confirmed that chronic upregulation of some subfamilies of NADPH oxidase genes such as NOX1, NOX4 and NOX5 is involved in the continuous production of free radicals, genomic instability as well as lung injury [34, 35]. Duox1 and Duox2 are other subfamilies of NADPH oxidase that are involved in oxidative injury induced by some agents such as ionizing radiation [36].
Studies have shown that exposure to ionizing radiation leads to massive oxidative injury, DNA break and cell death. The release of cell contents such as oxidized DNA, apoptotic bodies and necrotic products like high mobility group box 1 (HMGB1) lead to the activation of immune responses by macrophages and lymphocytes. In response to these danger alarms, macrophages, dendritic cells and lymphocytes trigger the release of several pro-inflammatory and pro-fibrotic cytokines such as IL-1, IL-2, IL-4, IL-6, IL-8, IL-10, IL-13, IL-18, IL-33, TNF-α, TGF-β and IFN-γ. Evidences have shown that chronic upregulation of some cytokines such as IL-1, IL-4, IL-10, IL-13, and TGF-β cause continuous production of prostaglandins, free radicals, etc., which mediate inflammation and fibrosis induction from months to years after exposure to radiation [37]. For example, TGF-β upregulates the expression of NOX1, NOX2, NOX4, NOX5, COX-2 and iNOS, leading to chronic oxidative injury, cell senescence, genomic instability and fibrosis [38]. IL-4 and IL-13 stimulate the upregulation of Duox1 and Duox2 via stimulation of some pathways such as JAK1-STAT6 cascade, STAT1, p38MAPK, etc. [39, 40]. Moreover, IFN-γ may induce upregulation of Duox2 through STAT-1 [41].
In the present study, we aimed to detect changes in the levels of IL-4 and IL-13; two potent pro-fibrotic cytokines, following local lung irradiation. Also, we evaluated changes in the expression of their downstream genes such as IL4Ra1, IL13Ra2, Duox1 and Duox2. The modulatory effect of Selenium-L-methionine on these parameters associated with histopathological changes was observed. Results of this study showed that irradiation of lung tissues led to an increase in the level of IL-4 but not IL-13. Also, the expression of IL13Ra2 was not detected. Results showed that the expression of IL4Ra1, Duox1 and Duox2 increased following lung irradiation. As earlier mentioned, IL4Ra1 can upregulate both Duox1 and Duox2. It is possible that IL-4, through upregulation of IL4Ra1, plays a key role in the increased expressions of Duox1 and Duox2, while IL-13 and IL-13Ra2 do not have this effect. However, some other pathways such as IFN-γ may be involved in the upregulation of these pro-oxidant genes. Moreover, treatment with Selenium-L-methionine reduced the expression of all of them. These results were associated with infiltration of macrophages, lymphocytes, fibrosis as well as vascular and alveolar thickening following irradiation and amelioration of them when rats were treated with Selenium-L-methionine. Selenium-L-methionine was able to reverse vascular and alveolar thickening as well as fibrosis completely.
Selenium-L-methionine, as well as selenium alone, has shown abilities to potently mitigate radiation injury via modulating chronic production of free radicals. The inhibition of redox reactions may play a key role in the radioprotective effects of Selenium-L-methionine. Upregulation of antioxidant enzymes by selenium as well as direct chelating of ROS by methionine can alleviate radiation-induced DNA damage and cell death. Previous studies confirmed potent antioxidant effects, as well as stimulatory effects on antioxidant enzymes [42]. These are associated with attenuation, leading to the release of pro-inflammatory and pro-fibrotic cytokines, which reduce redox activity and chronic free radicals’ production by pro-oxidant enzymes. Further studies are needed to illustrate the possible antioxidant role of Selenium-L-methionine on lung tissues following exposure to ionizing radiation. The suppression of pro-oxidant enzymes such as Duox1 and Duox2 is involved in the radioprotective property of Selenium-L-methionine.
CONCLUSION
This study showed upregulation of IL-4–Duox2 pathways after irradiation of rats’ lungs. Also, results indicated an increased level of Duox1 without upregulation of IL-13. Selenium-L-methionine could reduce the increased level of IL-4 and attenuate the upregulation of IL4Ra1, Duox1 and Duox2. Moreover, the administration of Selenium-L-methionine attenuated the infiltration of macrophages and lymphocytes. It reversed vascular and alveolar thickening as well as fibrosis. These results may be an indication that Selenium-L-methionine can be proposed for protection against radiation-induced lung toxicity. The suppression of chronic oxidative stress, mediated by some pro-oxidant enzymes such as Duox1 and Duox2 is involved in radioprotective property of Selenium-L-methionine.
Fig. (2).
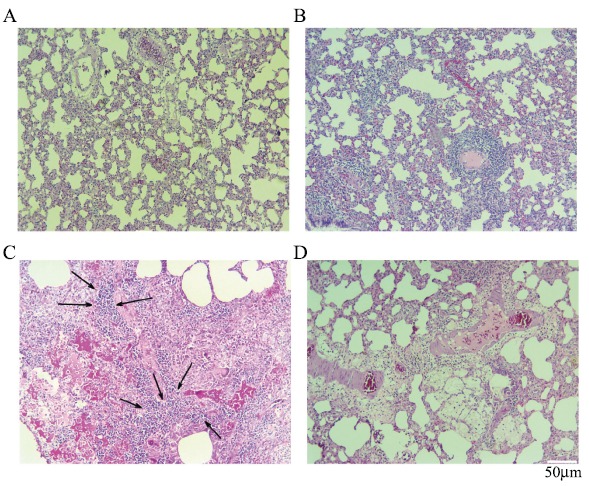
Histological images showing the radioprotective effect of Selenium-L-methionine on rat’s lung tissues following local irradiation by gamma rays (H&E staining ×100 magnification). A: Control; B: Selenium-L-methionine treatment; C: Irradiation; D: Irradiation plus Selenium-L-methionine. Irradiation of lung tissues led to significant augment in the numbers of macrophages and lymphocytes. This was more obvious for lymphocytes. Also, mild vascular thickening and moderate alveolar thickening were observed. In irradiation plus treatment with Selenium-L-methionine, only a mild to moderate infiltration of macrophages and lymphocytes were observed. Arrows indicate infiltration of lymphocytes and macrophages. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
ACKNOWLEDGEMENTS
Declared none.
ETHICAL STATEMENT AND APPROVAL
This study was in accordance with the ethical laws provided by Tehran University of Medical Sciences, Tehran, Iran. The ethical approval number is IR.TUMS.VCR.REC.1396.4419.
HUMAN AND ANIMAL RIGHTS
No humans were used in this research. All animal research procedures followed were in accordance with the standards set forth in the eighth edition of Guide for the Care and Use of Laboratory Animals published by the National Academy of Sciences, The National Academies Press, and Washington, D.C.).
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
The study was funded by Tehran University of Medical Sciences and Health Service; grant number: 36668.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Rezaeyan A., Fardid R., Haddadi G.H., et al. Evaluating radioprotective effect of hes-peridin on acute radiation damage in the lung tissue of rats. J. Biomed. Phys. Eng. 2016;6(3):165–174. [PMID: 27853724]. [PMC free article] [PubMed] [Google Scholar]
- 2.Mahmood J., Jelveh S., Calveley V., Zaidi A., Doctrow S.R., Hill R.P. Mitigation of lung injury after accidental exposure to radiation. Radiat. Res. 2011;176(6):770–780. doi: 10.1667/rr2562.1. [http://dx.doi.org/10.1667/RR2562.1]. [PMID: 22013884]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farhood B., Mortezaee K., Goradel N.H., et al. Curcumin as an anti-inflammatory agent: Implications to radiotherapy and chemotherapy. J. Cell. Physiol. 2019;234(5):5728–5740. doi: 10.1002/jcp.27442. [http://dx.doi.org/10.1002/jcp.27442]. [PMID: 30317564]. [DOI] [PubMed] [Google Scholar]
- 4.Keywan Mortezaee N.H.G. Peyman Amini, Dheyauldeen Shabeeb, Ahmed Eleojo Musa, Masoud Najafi, NADPH oxi-dase as a target for modulation of radiation response; implica-tions to carcinogenesis and radiotherapy. Curr. Mol. Pharmacol. 2018;12(1) doi: 10.2174/1874467211666181010154709. [http://dx.doi.org/10.2174/1874467211666181010154709]. [DOI] [PubMed] [Google Scholar]
- 5.Yahyapour R., Salajegheh A., Safari A., et al. Radiation-induced Non-targeted Effect and Carcinogenesis; Implications in Clinical Radiotherapy. J. Biomed. Phys. Eng. 2018;8(4):435–446. [http://dx.doi.org/10.31661/jbpe.v0i0.713]. [PMID: 30568933]. [PMC free article] [PubMed] [Google Scholar]
- 6.Di Maggio F.M., Minafra L., Forte G.I., et al. Portrait of inflammatory response to ionizing radiation treatment. J. Inflamm. (Lond.) 2015;12:14. doi: 10.1186/s12950-015-0058-3. [http://dx.doi.org/10.1186/s12950-015-0058-3]. [PMID: 25705130]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yahyapour R., Motevaseli E., Rezaeyan A., et al. Reduction-oxidation (redox) system in radiation-induced normal tissue injury: molecular mechanisms and implications in radiation therapeutics. Clin. Transl. Oncol. 2018;20(8):975–988. doi: 10.1007/s12094-017-1828-6. [http://dx.doi.org/10.1007/s12094-017-1828-6]. [PMID: 29318449]. [DOI] [PubMed] [Google Scholar]
- 8.Farhood B., Goradel N.H., Mortezaee K., et al. Intercellular communications-redox interactions in radiation toxicity; potential targets for radiation mitigation. J. Cell Commun. Signal. 2019;13(1):3–16. doi: 10.1007/s12079-018-0473-3. [http://dx.doi.org/10.1007/s12079-018-0473-3]. [PMID: 29911259]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frey B., Hehlgans S., Rödel F., Gaipl U.S. Modulation of inflammation by low and high doses of ionizing radiation: Implications for benign and malign diseases. Cancer Lett. 2015;368(2):230–237. doi: 10.1016/j.canlet.2015.04.010. [http://dx.doi.org/10.1016/j.canlet.2015.04.010]. [PMID: 25888451]. [DOI] [PubMed] [Google Scholar]
- 10.Abratt R.P., Morgan G.W. Lung toxicity following chest irradiation in patients with lung cancer. Lung Cancer. 2002;35(2):103–109. doi: 10.1016/s0169-5002(01)00334-8. [http://dx.doi.org/10.1016/S0169-5002(01)00334-8]. [PMID: 11804681]. [DOI] [PubMed] [Google Scholar]
- 11.Jakubzick C., Kunkel S.L., Puri R.K., Hogaboam C.M. Therapeutic targeting of IL-4- and IL-13-responsive cells in pulmonary fibrosis. Immunol. Res. 2004;30(3):339–349. doi: 10.1385/IR:30:3:339. [http://dx.doi.org/10.1385/IR:30:3:339]. [PMID: 15531774]. [DOI] [PubMed] [Google Scholar]
- 12.Mahmood J., Jelveh S., Zaidi A., Doctrow S.R., Medhora M., Hill R.P. Targeting the Renin-angiotensin system combined with an antioxidant is highly effective in mitigating radiation-induced lung damage. Int. J. Radiat. Oncol. Biol. Phys. 2014;89(4):722–728. doi: 10.1016/j.ijrobp.2014.03.048. [http://dx.doi.org/10.1016/j.ijrobp.2014.03.048]. [PMID: 24867538]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahmood J., Jelveh S., Zaidi A., Doctrow S.R., Hill R.P. Mitigation of radiation-induced lung injury with EUK-207 and genistein: effects in adolescent rats. Radiat. Res. 2013;179(2):125–134. doi: 10.1667/RR2954.1. [http://dx.doi.org/10.1667/RR2954.1]. [PMID: 23237541]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lambeth J.D. NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 2004;4(3):181–189. doi: 10.1038/nri1312. [http://dx.doi.org/10.1038/nri1312]. [PMID: 15039755]. [DOI] [PubMed] [Google Scholar]
- 15.Yoshihara A., Hara T., Kawashima A., et al. Regulation of dual oxidase expression and H2O2 production by thyroglobulin. Thyroid. 2012;22(10):1054–1062. doi: 10.1089/thy.2012.0003. [http://dx.doi.org/10.1089/thy.2012.0003]. [PMID: 22874065]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Groves A.M., Johnston C.J., Misra R.S., Williams J.P., Finkelstein J.N. Effects of IL-4 on pulmonary fibrosis and the accumulation and phenotype of macrophage subpopulations following thoracic irradiation. Int. J. Radiat. Biol. 2016;92(12):754–765. doi: 10.1080/09553002.2016.1222094. [http://dx.doi.org/10.1080/09553002.2016.1222094]. [PMID: 27539247]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ameziane-El-Hassani R., Talbot M., de Souza Dos Santos M.C., et al. NADPH oxidase DUOX1 promotes long-term persistence of oxidative stress after an exposure to irradiation. Proc. Natl. Acad. Sci. USA. 2015;112(16):5051–5056. doi: 10.1073/pnas.1420707112. [http://dx.doi.org/10.1073/pnas.1420707112]. [PMID: 25848056]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eskalli Z., Achouri Y., Hahn S., et al. Overexpression of interleukin-4 in the thyroid of transgenic mice upregulates the expression of Duox1 and the anion transporter pendrin. Thyroid. 2016;26(10):1499–1512. doi: 10.1089/thy.2016.0106. [http://dx.doi.org/10.1089/thy.2016.0106]. [PMID: 27599561]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Azmoonfar R., Amini P., Saffar H., et al. Metformin protects against radiation-induced pneumonitis and fibrosis and attenuates upregulation of dual oxidase genes expression. Adv. Pharm. Bull. 2018;8(4):697–704. doi: 10.15171/apb.2018.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yahyapour R, Amini P, Saffar H, et al. Metformin Protects Against Radiation-Induced Heart Injury and Attenuates the Up-regulation of Du-al Oxidase Genes Following Rat’s Chest Irradiation. nt J Mol Cell Med. 2018;7(3):0. doi: 10.22088/IJMCM.BUMS.7.3.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stỳblo M., Walton F.S., Harmon A.W., Sheridan P.A., Beck M.A. Activation of superoxide dismutase in selenium-deficient mice infected with influenza virus. J. Trace Elem. Med. Biol. 2007;21(1):52–62. doi: 10.1016/j.jtemb.2006.11.001. [http://dx.doi.org/10.1016/j.jtemb.2006.11.001]. [PMID: 17317526]. [DOI] [PubMed] [Google Scholar]
- 22.Bourdon E., Loreau N., Lagrost L., Blache D. Differential effects of cysteine and methionine residues in the antioxidant activity of human serum albumin. Free Radic. Res. 2005;39(1):15–20. doi: 10.1080/10715760400024935. [http://dx.doi.org/10.1080/10715760400024935]. [PMID: 15875807]. [DOI] [PubMed] [Google Scholar]
- 23.Sieber F., Muir S.A., Cohen E.P., et al. Dietary selenium for the mitigation of radiation injury: effects of selenium dose escalation and timing of supplementation. Radiat. Res. 2011;176(3):366–374. doi: 10.1667/rr2456.1. [http://dx.doi.org/10.1667/RR2456.1]. [PMID: 21867430]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sieber F., Muir S.A., Cohen E.P., et al. High-dose selenium for the mitigation of radiation injury: a pilot study in a rat model. Radiat. Res. 2009;171(3):368–373. doi: 10.1667/0033-7587-171.3.368. [http://dx.doi.org/10.1667/0033-7587-171.3.368]. [PMID: 19267564]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weiss J.F., Srinivasan V., Kumar K.S., Landauer M.R. Radioprotection by metals: selenium. Adv. Space Res. 1992;12(2-3):223–231. doi: 10.1016/0273-1177(92)90112-b. [http://dx.doi.org/10.1016/0273-1177(92)90112-B]. [PMID: 11537012]. [DOI] [PubMed] [Google Scholar]
- 26.Szabo S., Ghosh S.N., Fish B.L., et al. Cellular inflammatory infiltrate in pneumonitis induced by a single moderate dose of thoracic x radiation in rats. Radiat. Res. 2010;173(4):545–556. doi: 10.1667/RR1753.1. [http://dx.doi.org/10.1667/RR1753.1]. [PMID: 20334527]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Medhora M., Haworth S., Liu Y., et al. Biomarkers for Radiation Pneumonitis Using Noninvasive Molecular Imaging. J. Nucl. Med. 2016;57(8):1296–1301. doi: 10.2967/jnumed.115.160291. [http://dx.doi.org/10.2967/jnumed.115.160291]. [PMID: 27033892]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahmood J., Jelveh S., Calveley V., Zaidi A., Doctrow S.R., Hill R.P. Mitigation of lung injury after accidental exposure to radiation. Radiat. Res. 2011;176(6):770–780. doi: 10.1667/rr2562.1. [http://dx.doi.org/10.1667/RR2562.1]. [PMID: 22013884]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farhood B., Goradel N.H., Mortezaee K., Khanlarkhani N., Najafi M., Sahebkar A. Melatonin and cancer: From the promotion of genomic stability to use in cancer treatment. J. Cell. Physiol. 2019;234(5):5613–5627. doi: 10.1002/jcp.27391. [http://dx.doi.org/10.1002/jcp.27391]. [PMID: 30238978]. [DOI] [PubMed] [Google Scholar]
- 30.Yahyapour R., Shabeeb D., Cheki M., et al. Radiation protection and mitigation by natural antioxidants and flavo-noids; implications to radiotherapy and radiation disasters. Curr. Mol. Pharmacol. 2018;11(4):285–304. doi: 10.2174/1874467211666180619125653. [http://dx.doi.org/10.2174/1874467211666180619125653]. [PMID: 29921213]. [DOI] [PubMed] [Google Scholar]
- 31.Sieber F., Muir S.A., Cohen E.P., et al. High-dose selenium for the mitigation of radiation injury: a pilot study in a rat model. Radiat. Res. 2009;171(3):368–373. doi: 10.1667/0033-7587-171.3.368. [http://dx.doi.org/10.1667/0033-7587-171.3.368]. [PMID: 19267564]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown S.L., Kolozsvary A., Liu J., Jenrow K.A., Ryu S., Kim J.H. Antioxidant diet supplementation starting 24 hours after exposure reduces radiation lethality. Radiat. Res. 2010;173(4):462–468. doi: 10.1667/RR1716.1. [http://dx.doi.org/10.1667/RR1716.1]. [PMID: 20334518]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bagheri H., Rezapour S., Najafi M., et al. Protection against radia-tion-induced micronuclei in rat bone marrow erythrocytes by Curcumin and selenium L-methionine. Iran. J. Med. Sci. 2018 [PMC free article] [PubMed] [Google Scholar]
- 34.Choi S.H., Kim M., Lee H.J., Kim E.H., Kim C.H., Lee Y.J. Effects of NOX1 on fibroblastic changes of endothelial cells in radiation-induced pulmonary fibrosis. Mol. Med. Rep. 2016;13(5):4135–4142. doi: 10.3892/mmr.2016.5090. [http://dx.doi.org/10.3892/mmr.2016.5090]. [PMID: 27053172]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pazhanisamy S.K., Li H., Wang Y., Batinic-Haberle I., Zhou D. NADPH oxidase inhibition attenuates total body irradiation-induced haematopoietic genomic instability. Mutagenesis. 2011;26(3):431–435. doi: 10.1093/mutage/ger001. [http://dx.doi.org/10.1093/mutage/ger001]. [PMID: 21415439]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu Y, Doroshow JH. AACR Cancer Progress Report. 2014 [Google Scholar]
- 37.Yahyapour R., Amini P., Rezapour S., et al. Radiation-induced inflammation and autoimmune diseases. Mil. Med. Res. 2018;5(1):9. doi: 10.1186/s40779-018-0156-7. [http://dx.doi.org/10.1186/s40779-018-0156-7]. [PMID: 29554942]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yahyapour R., Amini P., Rezapoor S., et al. Targeting of Inflammation for Radiation Protection and Mitigation. Curr. Mol. Pharmacol. 2018;11(3):203–210. doi: 10.2174/1874467210666171108165641. [http://dx.doi.org/10.2174/1874467210666171108165641]. [PMID: 29119941]. [DOI] [PubMed] [Google Scholar]
- 39.Raad H., Eskalli Z., Corvilain B., Miot F., De Deken X. Thyroid hydrogen peroxide production is enhanced by the Th2 cytokines, IL-4 and IL-13, through increased expression of the dual oxidase 2 and its maturation factor DUOXA2. Free Radic. Biol. Med. 2013;56:216–225. doi: 10.1016/j.freeradbiomed.2012.09.003. [http://dx.doi.org/10.1016/j.freeradbiomed.2012.09.003]. [PMID: 23010498]. [DOI] [PubMed] [Google Scholar]
- 40.Hodny Z., Reinis M., Hubackova S., Vasicova P., Bartek J. Interferon gamma/NADPH oxidase defense system in immunity and cancer. OncoImmunology. 2015;5(2):e1080416. doi: 10.1080/2162402X.2015.1080416. [http://dx.doi.org/10.1080/2162402X.2015.1080416]. [PMID: 27057461]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu Y, Antony S, Juhasz A, et al. Upregulation and sustained activation of STAT1 are essential for interferon-gamma (IFN-gamma)- induced dual oxidase-2 (DUOX2) and dual oxidase A2 (DUOXA2) expression in human pancreatic cancer cell lines. J Biol Chem. 2011 doi: 10.1074/jbc.M110.191031. jbc. M110. 191031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schnabel R., Lubos E., Messow C.M., et al. Selenium supplementation improves antioxidant capacity in vitro and in vivo in patients with coronary artery disease: The SElenium Therapy in Coronary Artery disease Patients (SETCAP) Study. Am. Heart J. 2008;156(6):1201–1211. doi: 10.1016/j.ahj.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


