Abstract
Background:
Development of polymeric micelles for the management of allergic conjunctivitis to overcome the limitations of topical installation, such as poor patient compliance, poor stromal permeability, and significant adverse effects, increase precorneal residence time and efficacy, and also control the release of drug at the target site.
Objective:
The investigation was aimed at developing a polymeric micellar system of Azelastine HCl for Ocular Delivery.
Methods:
Drug loaded micelles of tri-block copolymers Pf 127 were prepared by Thin Film hydration method. The polymeric micelles formulations (F1 to F9) were assessed for entrapment efficiency, micelle size, in vitro permeation, ex vivo transcorneal permeation, in vivo Ocular Irritation, and Histology.
Results:
Optimized micelles formulation (F3), with the lowest micelle size of 92 nm, least polydispersity value of 0.135, highest entrapment efficiency of 95.30 ± 0.17%, and a cumulative drug permeation of 84.12 ± 1.26% in 8h, was selected to develop pH-sensitive micelles loaded carbopol in situ gel. The optimized in situ gel (G4) proved to be superior in its ex vivo transcorneal permeation when compared with Market Preparation and pure drug suspension, exhibiting 43.35 ± 1.48% Permeation with zero-order kinetics (r2 = 0.9944) across goat cornea. Transmission Electron microscopy revealed spherical polymeric micelles trapped in the gel matrix. A series of experiments showed hydration capability, non-irritancy, and histologically safe gel formulation that had appropriate handling characteristics.
Conclusion:
A controlled release pH-sensitive ocular formulation capable of carrying the drug to the anterior section of the eye via topical delivery was successfully developed for the treatment of allergic conjunctivitis.
Keywords: Azelastine HCl, Entrapment efficiency, x vivo Transcorneal permeation, Histology, in vitro Permeation, in vivo Ocular irritation, Transmission electron microscopy, Tri-block copolymers
1. Introduction
Ophthalmic drug delivery systems (ODDSs) as eye drops and visual additions are linked to the corneal surface to treat sicknesses of both the anterior and posterior portions of the eye [1]. If visual infections are left untreated, life-changing visual disability and visual impairment may develop [2- 4]. After organization, just 1%-5% of the medication achieves the ideal site of activity, making eye-drop-based ODDSs extremely inefficient [5]. Poor visual bioavailability and the short duration of action limit the utilization of eye drops to illnesses found in the front fragments of the eye [6]. Volume loss occurs by eye blinking, nasolacrimal drainage, and systemic absorption by the conjunctiva [7-9]. The simulation results showed that contact-lens-based ODDSs are more effective than eye drops [10]. Clinical studies have shown that soft contact lenses can result in significantly higher drug penetration than subconjunctival injections, thereby increasing ocular drug bioavailability and minimizing side effects [11].
Conventional treatments of posterior ailments such as posterior uveitis, diabetic macular edema, and age-related macular degeneration include invasive and repetitive intravitreal injections and/or surgical implants [12]. It was recently demonstrated that sustained drug release from drug-loaded contact lenses enables significant drug levels to be achieved within tissues in the posterior segment of the eye. Because of their ability to enhance drug bioavailability and provide longer drug residence times, drug-eluting contact lenses may be used as an alternative to the methods commonly used to treat both anterior and posterior ailments of the eye [13]. Allergic conjunctivitis is caused by an allergen-induced inflammatory response in which allergens interact with IgE bound to sensitized mast cells resulting in the clinical ocular allergic expression. The pathogenesis of allergic conjunctivitis is predominantly an IgE-mediated hypersensitivity reaction. Activation of mast cells induces enhanced tear levels of histamine, tryptase, prostaglandins, and leukotrienes [14]. The drug loading capacity of polymeric microspheres is governed by the concentration of the polymer and the drug-polymer ratio.
Azelastine hydrochloride (AZT HCl) is a relatively selective histamine H1 antagonist and inhibitor of histamine release and other mediators involved in allergic response [15]. In vitro studies using human cell lines demonstrated that AZT HCl can inhibit mediators like leukotrienes and platelet-activating factor (PAF) which are involved in allergic reactions. Decreased chemotaxis and activation of eosinophil have also been demonstrated. In the present study, AZT HCl was selected as a model drug. It is available as an eye drop in the market as Azelast, SunPharma, India [16]. The anti-allergic agent azelastine exhibits antagonistic activity against leukotrienes and platelet-activating factor, as well as inhibiting both the actions and the release of histamine. Azelastine is orally effective in various allergic disorders [17]. It is also available as a nasal spray, which provides rapid and long-lasting relief of nasal and ocular symptoms without sedation in children with seasonal or perennial allergic conjunctivitis [18]. In a new formulation as eye drops, azelastine HCl was found to be highly potent in double-blind studies in adults with allergic conjunctivitis [19, 20].
2. MATERIAL AND METHOD
2.1. Material
Azelastine HCl was obtained as a gift sample from Sun Pharma Pvt. Ltd. Ponta-sahib India. Pluronic F127 was obtained from BASF Corporation, NJ, USA. Methanol, Acetone, Acetonitrile, and phosphotungstic acid were obtained from Qualigens FineChem.Pvt.Ltd., Mumbai, India. Sodium chloride and sodium hydroxide pellets were obtained from Central Drug house Ltd. New Delhi, India. Carbopol 940 was purchased from Central Drug house Ltd. New Delhi, India. Sudan III Dye AR and dialysis membrane 150 were purchased from HiMedia Laboratories, Mumbai, India.
2.2. Method
2.2.1. Critical Micelle Concentration
Critical micelle concentration of pluronic F-127 was determined by dye micellization method using Sudan III dye. Stock solution of Sudan III (1% w/v) in water was prepared. 1 ml of Sudan III solution was added to 2 ml of the polymeric surfactant solutions of different concentrations in the range of 1-6 mg/ml. The samples were allowed to reach equilibrium by shaking for 24 h at 37°C [21]. The absorbance of polymeric surfactant solution was measured at 520 nm using UV/VIS spectrophotometer (Electronic India, 2375, Himachal Pradesh, India). The X-intercept of the linear fit for the plot of solubilized Sudan III versus polymer concentration was used to determine the critical micelle concentration.
2.2.2. Preparation of Azelastine HCl Loaded Polymeric Micelles
Azelastine HCl polymeric micelles were formulated by Thin Film hydration method. The amount of Azelastine HCl drug and copolymer Pf 127 dissolved into the solvent, i.e. methanol acetone, and acetonitrile (10 ml), was varied according to formulation design (Table 1). The mixture was transferred to a 250 ml round flask after stirring at room temperature for 1-2 hr. Then the solvent was evaporated at 50°C with the help of a rotary evaporator (Hicon®, New Delhi, India). Then thin film formation formed around the flask and further film was hydrated with 50ml distilled water before being heated at 60°C and the solution was stirred strongly at 37°C and rotated at 100 rpm until polymeric micelles containing Azelastine HCl were formed. Then the unentrapped drug was removed by dialysis. Dialysis was performed with dialysis bag (used dialysis membrane) of (2.6 nm), M. wt. cut off (12-16 kDa) (HImedia Ltd., Mumbai, India) until complete removal of the free form of the drug took place and then the solution was filtered with Cellulose nitrate filter paper (0.45 µm, Sertorrius A.G. 37070, Goettingen, Germany). All microparticulate impurities were removed and the filtrate contained a pure colloidal suspension of Azelastine HCl loaded polymeric micelles [22].
Table 1. Characterization of Azelastine HCl loaded polymeric micelles (F1-F9).
| Formulation Code | Amount of Drug in Formulation | Water Miscible Solvent | Micelle Size (nm) | Poly Dispersity Index |
%Entrapment Efficiency ± (SD) | % CDP at 8th hr ± (SD) | Best Fit Model | r2 Value |
|---|---|---|---|---|---|---|---|---|
| F1 | 2 | Methanol | 99.7 | 0.175 | 81.50 ± 1.06 | 70.52 ± 1.17 | Higuchi | 0.992 |
| F2 | 4 | Methanol | 97.6 | 0.181 | 84.24 ± 0.36 | 64.94 ± 1.17 | Higuchi | 0.993 |
| F3 | 6 | Methanol | 92.0 | 0.135 | 95.30 ± 0.17 | 84.12 ± 1.26 | Higuchi | 0.997 |
| F4 | 2 | Acetone | 103.9 | 0.231 | 73.52 ± 2.43 | 81.30 ± 1.16 | Higuchi | 0.994 |
| F5 | 4 | Acetone | 106.8 | 0.127 | 88.62 ± 1.45 | 78.12 ± 0.77 | Higuchi | 0.991 |
| F6 | 6 | Acetone | 104.7 | 0.237 | 93.04 ± 0.52 | 72.62 ± 2.39 | Higuchi | 0.981 |
| F7 | 2 | Acetonitrile | 121.3 | 0.135 | 69.36 ± 1.56 | 61.17 ± 0.90 | Higuchi | 0.988 |
| F8 | 4 | Acetonitrile | 123.9 | 0.166 | 73.48 ± 1.56 | 60.8 ± 2.51 | Higuchi | 0.987 |
| F9 | 6 | Acetonitrile | 129.4 | 0.223 | 79.01 ± 1.66 | 55.38 ± 0.94 | Higuchi | 0.986 |
2.2.3. Entrapment Efficiency
Entrapment efficiency (EE) was determined by 1ml polymeric micellar formulation dissolved in 5 ml ethanol to confirm complete micelle destruction and drug release. The volume makeup up to 10 ml with pH 7.4 phosphate buffer. The Azelastine HCl content was calculated spectrophotometrically at 286 nm and % EE was calculated using the following equation:
% EE = Amount of drug present in micelles /Total drug incorporated x 100
2.2.4. Differential Scanning Calorimetry
Thermogram of the sample was recorded by Differential scanning calorimetry [DSC Q20 V24.4 Build 116 (Universal V4.5A TA instrument)]. The sample was weighed directly in Aluminum (Al) pan and scanned at 50-300°C temperature under the dry nitrogen environment at the heating rate of 10°C/mint.
2.2.5. Particle Size
The size of pluronic micelles and polydispersity index were measured by Zetasizer (Malvern Instruments Ltd, Worcestershire, UK). The scattering angle was fixed at 173º and temperature was maintained at 25ºC. For analysis of particle size, each sample was diluted with deionized water and filtered through Millipore 0.45µm pore size membrane.
2.2.6. In vitro Permeation
In vitro permeation was carried out using fabricated Franz diffusion cell. Franz diffusion cell consists of donor and receptor compartment using dialysis membrane (Hi Media Ltd. India). 1 ml of Azelastine HCl loaded micellar formulation was placed in the donor compartment and the receptor compartment was filled with phosphate buffer pH 7.4 continuously stirred with a magnetic stirrer. Donor and receptor chambers were separated by Dialysis membrane of pore size 0.22 µm (HIMEDIA Ltd., Mumbai). The temperature was kept constant at 37 ± 1.0°C as mimic body conditions. 1 ml of sample was withdrawn through the sampling port of the diffusion cell at 0, 1, 2, 3, 4, 6, 8, 10, and 12 hr time intervals and evaluated spectrophotometrically at 286 nm. An equal volume of fresh phosphate buffer pH 7.4 was replaced into the receptor compartment after each sampling. All results from in vitro permeation were reported as mean with standard deviation (n = 3) [23].
2.2.7. Selection of the Optimized Formulation
The optimal formulation was selected on behalf of the high entrapment efficiency and percentage of cumulative drug release with lowest Polydispersity index, optimal particle size, and Transmission Electron Microscopy.
2.2.8. Transmission Electron Microscopy
One drop of the micellar suspension was spread on a 400-mesh carbon-coated copper cxzgrid and the excess droplets were removed with filter paper. A drop of 4% w/v phosphotungstic acid solution was dropped into the grid. The negatively stained sample was air-dried at room temperature and examined at 4000 X magnification. TEM images were obtained at an acceleration voltage of 120 kV.
2.2.9. Preparation of Micellar Gel
Aqueous formulations reduce drug concentration up to 10-fold as a result of blinking and tear drainage thus resulting in a decrease in ocular therapeutic efficacy. Thus the gel formulation was aimed in order to enhance the viscosity of best formulation by adding a polymer such as carbopol 940 that will undergo sol-gel transition at ocular pH, thus enhancing the contact time of the drug with the cornea, improving therapeutic efficiency. Gel base solutions were prepared by dispersing different weighed amounts of carbopol 940 in deionized water with continuous stirring until it gets completely dissolved and finally made up the volume using deionized water to obtain gel base strength of (0.1-0.5% w/v). The best selected polymeric micelles formulation (F3) equivalent to 0.6 mg of Azelastine HCl containing polymeric micelles was subjected to cooling centrifugation (REMI Instrument Ltd, Vasai, India) at 16000 rpm for 15 min and the pellets obtained were incorporated into the prepared 10 ml in situ gel base of different strength (0.1%-0.5% w/v) to get final in situ gel strength of 0.6 mg/ml [24]. Thus different formulations of Azelastine HCl loaded ocular polymeric micellar in situ gel were obtained using direct dispersion method and were coded as (G1- G5) as mentioned in Table 2.
Table 2. Formulation design of Azelastine HCl loaded in situ gel formulation of polymeric micelles as carrier system (G1- G5).
| Formulation Code | Carbopol 940 (%w/v) |
G5 Equivalent to Azelastine HCl (mg) | In situ Gel Strength (%w/v) |
|---|---|---|---|
| G1 | 0.1 | 0.6 | 1 |
| G2 | 0.2 | 0.6 | 1 |
| G3 | 0.3 | 0.6 | 1 |
| G4 | 0.4 | 0.6 | 1 |
| G5 | 0.5 | 0.6 | 1 |
2.2.10. Clarity, pH and Drug Content
The clarity of the developed gel formulations was determined before and after gelation by visual examination of the formulations under light alternatively against a white and black background. The pH of developed gel formulations was determined using Digital pH meter model 111 E (Hicon®, New Delhi, India). For drug content, weighed amount (100 mg) of Azelastine HCl loaded ocular polymeric micellar in situ gel formulations were diluted using 5 ml of methanol. The resultant dispersion was vortexed using Vortex shaker (Hicon®, New Delhi, India) and shook for 10 min. The volume was made up to 10 ml with methanol and analyzed via UV spectrophotometry at 286 nm. All readings were made in triplicate and represented as (± S.D).
2.2.11. In vitro Gelation
Prepared gel formulations were evaluated for in vitro gelation to identify the composition that is best suited for use as in situ gel. The in vitro gelling efficiency was determined by mixing the in situ gel with STF (pH 7.4) in the ratio 25:7 (25 µl of PM-ISG’s and normal tear volume 7 µL) to mimic the in vivo ocular conditions equilibrated at 37 ± 0.5°C. The gelation was assessed visually and simultaneously recorded the time required for gelation, as well as the time taken for dissolution of the formed gel [25]. Flow behavior of the gelling system was determined by various signs obtained by visual inspection.
2.2.12. Selection of Optimized Formulation
Optimized formulation was selected on the basis of formulation that showed clarity, maximum gelling capacity along with maximum drug content.
2.2.13. Ex vivo Transcorneal Permeation
The ex vivo transcorneal permeation study of optimized Azelastine HCl loaded polymeric micelles, micelle loaded in situ gel, Azelastine eye drop (ITRAL; Jawa pharmaceuticals), and of pure drug suspension was performed using Franz diffusion cell consisting of donor and receptor compartment using goat cornea. Cornea used in ex vivo permeation studies were obtained from goat eyes which were collected as the whole eyeball from local butcher shop immediately after the animal was slaughtered and transferred to the laboratory in Ringer’s salt solution within one hour of slaughtering. The cornea was carefully excised along with 2-4 mm of surrounding scleral tissues and then washed with Ringer’s salt solution until washings were free from protein and adhering tissues and then kept in freshly prepared simulated tear fluid (STF, pH 7.4). The upper compartment which served as donor compartment was filled with 2 ml of formulations equivalent to 0.6 mg/ml of Azelastine Hcl and lower compartment i.e., receptor medium filled with 10 ml of freshly prepared STF and stirred continuously using a small magnetic bar. Both upper and lower compartments were separated by goat cornea (area 0.785 cm2) in such a way that its epithelial surface faced the donor compartment and continuously remained intact with the release medium. The whole system was maintained at 37 ± 0.5°C to mimic in vivo ocular condition [26]. At a predetermined time interval, 1 ml of aliquots were withdrawn and analyzed by UV spectrophotometry at 286 nm. An equal volume of fresh STF was replaced into the receptor compartment after each sampling. The cumulative amount of drug permeated through goat cornea was plotted as a function of time [27].
2.2.14. In vivo Ocular Irritation Studies
Institutional Animal Ethics Committee guidelines were severely followed to carry out experiments (MMCP-IAEC-20). Draize technique was used to perform in vivo ocular irritation studies. Under examination, Albino rabbits (2-3 kg) were placed in an animal house. Proper diet and water were given for 24 hr. 50 μl solution was introduced in a single administration in the left eye of every rabbit while protecting the untouched eye as the control. The sterile G4 was introduced two times daily for a period of 21 days (1, 2, 3, 4, 7, 10, 15, 18, and 21 days). The rabbits were examined periodically for redness, swelling, and watering of the eye.
2.2.15. Histology
This study was performed to evaluate the effect of formulations on corneal structure and integrity. Whole eyeballs of goat were collected from the slaughterhouse and immediately transported to the laboratory in normal buffered saline in cold condition within 1h of slaughtering. Corneas from excised eyes were immediately rinsed with isotonic NaCl (0.9% w/v) solution for 1 min. and incubated for 30 min in the optimized G4 formulation at 34°C, phosphate buffer saline (PBS) pH 7.4 as negative control, and 75% isopropyl alcohol in PBS as positive control were taken as reference. The corneas were washed with PBS pH 7.4 and immediately fixed with 10% v/v formalin solution for 24 h. The corneas were dehydrated with ethyl alcohol gradient (70-90-100%) and xylene, put in melted paraffin, and solidified in block forms. Then, cross-sections (<1 mm) were made, mounted on a glass slide and observed under a microscope for any histological changes after staining with hematoxylin and eosin for any histological damage if any [28, 29].
2.2.16. Stability Study
Stability test of micelles consisted of visual control and analytical measurement of drug content. For this purpose, Azelastine HCl loaded micelles were placed in a climatic chamber at 25 ± 2°C, 60% relative humidity for three months. Micelle suspension was placed in an amber-colored bottle (5 ml) with a lid. Further, samples were taken to determine the drug content at the beginning and at the end of three months. Drug content was measured spectrophotometrically at λmax 286 nm.
3. RESULT AND DISCUSSION
3.1. Critical Micelle Concentration
The CMC values of Pf 127 were found to be 4 mg/ml as shown in Fig. (1). Different CMC values calculated for pluronic in various literature reports depend on the method of determination. The literature reports we referred to indicated CMC value of Pf 10 mg/ml [26]. The increase in dye absorbance gives the evidence that its solubilization via incorporation into the micellar phase resulted in the appearance of micelles in the system. Below CMC dye did not dissolve, a heterogeneous mixture consisting of water phase having low absorbance was obtained. An increase in the absorbance was seen as the concentration of polymeric surfactant was expanded until a sharp expression because of micellization procedure was recognized. Pf 127 propylene oxide (PO) has a chain length of 65.17. It was found that an increase in the length of the hydrophobic PO block elevates the net hydrophobicity of Pluronic molecule which favored the segregation of the PO chains into the micelle core and resulted in a decrease in CMC value. Conversely, ethylene oxide (EO) chain length of Pf 127 was 200.45. An increase in the length of the EO blocks increases the probability of contact of the PO units with the EO units within the core of the micelles. Therefore, the CMC value increases with an increase in the hydrophilic EO block length. Thus, low CMC value of Pf 127 indicates its better stability against possible dilution by body fluids. Further, on increasing the length of the hydrophobic segment the stability, as well as overall hydrophobicity of the amphiphilic block copolymer, also increased [30]. The reason for the selection of amphiphilic surfactants rather than common low molecular weight surfactants lies in its low CMC value which provides better stability.
Fig. (1).

CMC determination of Pf 127 using Sudan III dye.
3.2. Preparation of Azelastine HCl Loaded Polymeric Micelles
Azelastine HCl loaded polymeric micelles were prepared by using the thin film hydration method. Polymeric micelles have a high drug loading capacity. The solvent used in the preparation of the micelles can be essentially eliminated [31]. Different parameters were examined for the selection of the best formulation. Parameters like particle size, entrapment efficiency and in vitro permeation studies of all the formulation.
3.3. Micelle Size and Polydispersity Index of the Polymeric Micelles
Table 1 shows the typical size distribution of Azelastine HCl loaded polymeric micelles of all formulations (F1-F9). The particle size of Azelastine HCl loaded polymeric micelles varied in the range from 92 -129.4 nm with PDI value ranging from 0.135-0.223. The results showed that the amount of polymer and Azelastine HCl were critical parameters for governing particle size. Since particle size and uniform size distribution are the most pivotal parameters for topical delivery. Formulation F3 showed an optimum particle size for ocular delivery i.e. 92 nm with low PDI value of 0.135. Mean particle size of Pf 127 developed micelles (F1-F3) ranged from 92- 99.7 nm using methanol and 103.7- 106.2 nm (F4- F6) using Acetone and 121.3-129.4nm using Acetonitrile. This was observed as Pf 127 ethylene oxide (EO) chain was 200.45, and ethylene oxide (EO) chain governs the micelles aggregation number and micellar size. In general, for a fixed PO block, the block copolymers with a higher EO block content are less aggregated and have a smaller core size with same copolymer Pf 127. But different organic solvent, Acetonitrile and Acetone as an organic solvent were found to produce large-sized polymeric micellar particles rather than methanol. This effect was observed due to the difference in miscibility between water and organic solvent because methanol possesses high water miscibility rather than Acetonitrile and Acetone [31]. And within the same copolymer, the effect of varying drug: copolymer weight ratio was observed on micelles size. Also the effect of varying drug: copolymer weight ratio was observed on micelles size i.e. first it increased in size with an increase in drug: copolymer weight ratio then on further increasing ratio, it decreased in size. The further increase in drug: copolymer weight ratio leads to precipitation of drug and decrease in its loading capacity into micellar core thus, decrease in polymeric micelles size [32]. Thus formulation F3 with the optimum size range of polymeric micelles particles for ocular delivery with low PDI value indicating a narrow and mono-disperse pattern.
3.4. Entrapment Efficiency
The drug entrapment efficiency was found to be in the range of (69.36 ± 1.56-95.30 ± 0.17) as shown in Table 1. As Azelastine HCl weight ratio against triblock copolymer (Pf 127) with methanol was increased from 2/500 mg to 4/500 mg in (F1-F3), drug entrapment efficiency increased from (81.50-95.30%), with increase in acetone from 2/500 mg to 4/500 mg in (F4-F6), drug entrapment efficiency increased from (73.52-93.04%), and with increase in acetonitrile from 2/500 mg to 4/500 mg in (F7-F9), drug entrapment efficiency increased from (69.36-79.01%). Lowering of drug entrapment efficiency was observed on further increasing drug- copolymer weight ratio due to precipitation of drug thus overcoming its loading capability into micellar core [23]. Using Pf 127 and methanol as organic solvent (F1-F3), higher entrapment efficiency was observed than other formulations. Drug and copolymer weight ratio is slightly affected by the type of organic solvent used to prepare polymeric micelles. This result was observed for better interaction of the drug with a hydrophobic core formed by copolymer with methanol and thus better drug loading rather acetonitrile and acetone.
3.5. Differential Scanning Calorimetry
To study the binding strength of Azelastine HCl in the micelles, differential scanning calorimetry (DSC) was performed. The thermogram of Azelastine HCl displayed a sharp endothermic peak at 226.95°C as shown in the Fig. (2). The DSC thermogram of Pf 127 showed an endothermic peak at 54.31°C. This broad peak is attributable to dehydration of the EO chains with increased temperature. In the case of F3, the DSC thermogram of Azelastine HCl micelles showed an endothermic peak at 54.91°C and at 232.88°C [33]. As explained in literature, high loading of a poorly soluble drug into micelles normally leads to the crys-
Fig. (2).

DSC thermogram of Pluronic F-127, Azelastine HCl loaded polymeric micelles (F3) and Azelastine HCl.
tallization of the loaded drug, whereas drug molecules are present in the molecularly dispersed state at low loading.
3.6. In vitro Permeation
The in vitro drug permeation profiles (Fig. 3) were used to determine % cumulative drug permeated (CDP) at 8th h. The %CDP varied widely between 55.38 ± 0.94-84.12 ± 1.26%. F3 displayed maximum CDP of 84.12 ± 1.26% probably due to the small size of micelles and optimum entrapment efficiency. The determinants of this variability may be attributed to micelle size and PDI that was in turn affected by drug: copolymer weight ratio and type of organic solvent used. Thus F4-F9 prepared with Acetone and Acetonitrile as organic solvent produced large-sized micelles than methanol (F1-F3). This led to incomplete drug permeation which varied from 55.38 ± 0.94-84.12 ± 1.26%.
Fig. (3).
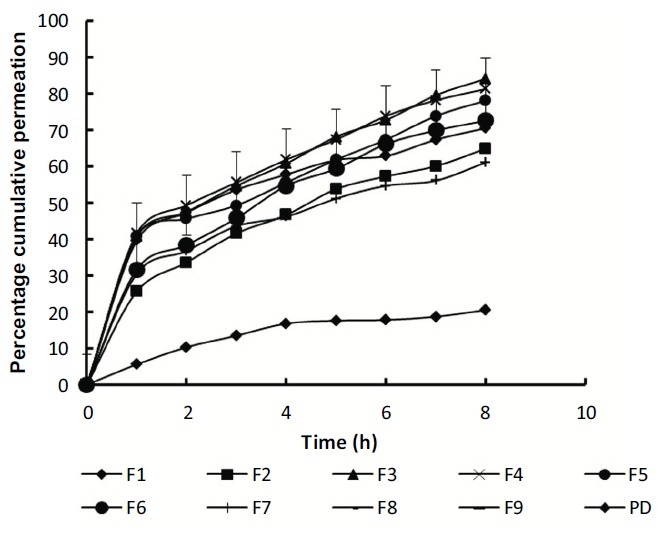
Comparative in vitro drug permeation profiles of Azelastine HCl from developed polymeric micelles (F1-F9) and pure drug suspension in STF pH 7.4 using Franz diffusion cell.
The in vitro permeation profiles were fitted to various kinetic models, however, most of the formulation subjected followed Higuchi model as shown in Table 1 indicating that released drug in the shell is rapidly replaced by the drug available in the core of the reservoir meaning thereby, the drug concentration will not change as a function of time. Although percent cumulative drug permeation of F3 was more than 82% and fitted best to Higuchi model. F3 was selected as the optimized one with maximum entrapment efficiency (95.30 ± 0.17%), i.e. more than 90%, describing homogeneous polymeric micelles suggest that drug-polymer interaction leads to more regular polymeric micelles with micelle size 92 nm. Thus, F3 was selected for in situ gel formulation containing optimum size range of polymeric micelles particles for ocular delivery indicating mono-disperse unimodal pattern with the highest encapsulating capability [34].
3.7. Selection of Optimized Formulation
Formulation F3 showed the highest percent cumulative drug permeation (90.28 ± 0.31%) entrapment efficiency (95.30 ± 0.17%) with optimum particle size (92 nm). Thus, F3 formulation was considered as the best among all twelve micelle formulations and this optimized formulation was incorporated into gel after characterization.
3.8. Transmission Electron Microscopy
Transmission electron microscopic image of F3 indicated spherical shaped particles with narrow size distribution as shown in Fig. (4). All the particles were less than 130 nm, a size particularly suitable for ocular delivery.
Fig. (4).

TEM image of optimized Azelastine HCl polymeric micelles (F3).
3.9. Preparation of Azelastine HCl Loaded Micellar In situ Gel
The optimized Azelastine HCl loaded polymeric micelle formulation F3 was further incorporated into pH controlled carbopol 940 in situ gelling system by direct dispersion method with the aim of achieving more controlled release of polymeric micelles from the gelling system.
3.10. Characterization of Azelastine HCl Loaded Micellar In situ Gel
3.10.1. Clarity, pH and Drug Content
As we are developing a pH triggered ocular in situ gel, clarity, pH, and gelling capacity become the important prerequisite parameters. Thus, the formed gel should be clear and not cause blurring of vision. As for a successful in situ gelling system, the pH of the solution should be such which allows easy installation of a liquid solution that will undergo sol to gel transformation on exposure to ocular physiological conditions. Thus, Carbopol 940 with satisfactory attributes of viscosity and gelling capacity was selected as pH-sensitive in-situ gelling agent [35]. The results of clarity, pH, and gelling capacity of G1 to G5 mentioned in G4 containing 0.4% w/v concentration of carbopol 940 exhibited rapid gel formation and remained so for an extended period of time. Solutions having concentration below 0.5% exhibited free-flowing liquid at non-physiological conditions and G5 was more viscous. On visual observation, no contamination was found and all formulations (F1-F5) were transparent. The pH of all formulations before gelation was more towards the acidic side (3.20 ± 0.60 to 3.80 ± 0.30) and after gelation it
shifted to 6.90 ± 0.11 to 7.1 ± 0.54 (Table 3). This confirms the ability of sol to gel transition on ocular instillation. Moreover, formulations within a pH range of 6.8 to 7.4 are considered safe and acceptable for ocular delivery. The drug content varied in the narrow range of 77.89 ± 0.98 to 83.22 ± 2.39%. These features were acceptable but the gelling characteristics are of utmost importance for in situ gelling systems.
Table 3. Characterization of Azelastine HCl loaded polymeric micellar in situ gel (G1-G5).
| Formulation Code |
Carbopol 940 (%w/v) |
Gelling Time (Seconds) |
pH of Sol | pH of Gel | %Drug Content |
|---|---|---|---|---|---|
| G1 | 0.1% | -_______ | 3.20 ± 0.60 | 6.90 ± 0.11 | 77.89 ± 0.98 |
| G2 | 0.2% | 79.6 ± 4.42 | 3.38 ± 0.12 | 6.98 ± 0.28 | 78.16 ± 5.88 |
| G3 | 0.3% | 66.6 ± 3.70 | 3.55 ± 0.47 | 7.0 ± 0.45 | 80.91 ± 2.47 |
| G4 | 0.4% | 48.6 ± 4.94 | 3.62 ± 0.41 | 7.2 ± 0.25 | 83.22 ± 2.39 |
| G5 | 0.5% | 55.3 ± 4.77 | 3.80 ± 0.30 | 7.1 ± 0.54 | 79.22 ± 1.71 |
3.11. In vitro Gelation
The gelling ability increased on increasing carbopol concentration and G4 containing 0.4% w/v concentration of carbopol exhibited rapid gelation with the least gelling time of 48.6 ± 4.94 seconds (Table 3). The higher the polymer concentration, the stronger will be the expanded polymeric network. Gelation mainly occurs due to increase in ionization as a result of increased pH which in turn results in electrostatic repulsion between adjacent carboxyl groups and the subsequent expander polymeric network of carbopol 940, thus showing better gelation capacity in comparison to other in situ gel formulations. On further increasing the concentration of carbopol, stiffer gel was found to be formed due to hydrophobic nature, backbone of carbopol 940 which may form hydrophobic interchain aggregation and this cross-linking phenomenon may result in the formation of more viscous gel with higher viscosity, the resulting solution becomes highly acidic and cannot be neutralized even by the buffering action of tear fluid [33].
3.12. Selection of Optimized Formulation
Hence, formulation G4 with maximum gelling capacity and highest drug content was considered as best amongst the Azelastine HCl loaded micellar in situ gel formulations. It was further characterized for ex vivo transcorneal permeation, in vitro antifungal activity, irritation potential, and histological study.
3.13. Ex vivo Transcorneal Permeation Study
The ex vivo transcorneal permeation plot of G4 was compared against the plots of F3, market preparation, and Azelastine HCl pure drug suspension. G4 was able to sustain the release and achieved permeation of 50.71 ± 0.87% in 8h (Fig. 5). This clearly indicates that the gel matrix did not impede the release of Azelastine HCl loaded polymeric micelles from G4 [26]. The results are quite in contrast to the permeation profiles obtained with market preparation and Azelastine HCl suspension and were able to achieve only 14.64 ± 0.61% and 9.8 ± 0.45% cumulative drug permeated (CDP) (Table 4). Compared to marketed eye drops and Azelastine HCl pure drug suspension, M5 exhibited 5.87 folds and F5 exhibited 4.89 folds increase in ex vivo permeation across goat corneal membrane that was significantly higher than both marketed eye drops and Azelastine HCl pure drug suspension claiming the efficiency of polymeric micelles. On fitting the data to various kinetic models, both F3 and G4 followed zero-order kinetics.
Fig. (5).

Comparative ex vivo drug permeation profiles of Azelastine HCl loaded polymeric micelles (F3), Micelle loaded in situ gel (G4), Marketed preparation (MP) and Azelastine HCl pure drug suspension in STF pH 7.4 using Franz diffusion cell.
Table 4. Ex vivo permeability parameters of micellar formulation (F3), micelle loaded in situ gel (G4) and marketed preparation of Azelastine HCl across goat cornea.
| Formulation Code | % CDP (8th h) | % Enhancement Ratio (ER) |
r2 Value | Permeation Kinetics |
|---|---|---|---|---|
| F3 | 50.71 ± 0.87 | 5.87 | 0.9963 | Zero order |
| G4 | 43.35 ± 1.48 | 4.89 | 0.9944 | Zero order |
| MP | 14.64 ± 0.61 | 1.38 | 0.9632 | Zero order |
| Azelastine HCl suspension |
9.8 ± 0.45 | - | 0.9879 | Zero order |
3.14. In vivo Ocular Irritation
Eye irritation potential of the introducing agent is practically divided into four grades: non-irritating, slightly irritating, moderately irritating, and severely irritating. Non-irritating ranges between 0-3; slightly irritating between 4-8; moderately irritating between 9-12; and severely irritating between 13-16 [20]. The eye irritation range is determined by separating the total range of all rabbits by the number of rabbits tested. The observed eye irritation range in the control is 0.38 and the range of the G4 is 0.72, which shows good ocular tolerance [36]. Besides irritation, irregular clinical signs or no ocular harm relating to the cornea, iris, or conjunctivae were noticeable. Moreover, no watering, no redness, and swelling of the eye were observed for both control and G4. Generally, the outcomes of this in vivo Ocular Irritation study discovered that G4 is safe and harmless for ocular application.
3.15. Histology
The effect of optimized in situ gel formulation G4 on the structural integrity of corneal epithelial tissue was evaluated using histological sections of the cornea. Fig. (6a-c) represents cross sections of freshly excised goat corneas after 8h treatment with PBS pH 7.4 (negative control), 75% isopropyl alcohol (positive control), and optimized in situ gel formulation G4, respectively.
Fig. (6).

Histological cross section of excised goat cornea, (a) PBS pH 7.4 (negative control) (b) 75% isopropyl alcohol (positive control), (c) G4.
After incubating corneal cross-section in 75% isopropyl alcohol, an irritant 6(b), widening of narrow intercellular spaces with deformation, distortion of superficial epithelial cells, and finally detachment from the tissue assembly were observed. Incubation of cornea with G4 6(c) did not show any destructive effect on corneal epithelium as well as on stromal structure while maintaining intact corneal structure and integrity [26, 36]. The above effect shown by optimized formulations was found to have a similar effect as shown after incubating corneal tissue in PBS pH 7.4 6(a). The above results indicated the ocular safety of polymeric micelles in situ gel formulation on corneal structure and integrity.
3.16. Stability Study
Stability test of optimized micellar formulation (F3) was performed. The formulation exhibited no significant difference in drug content at zero (90.77 ± 0.926) and at 3rd month (89.39 ± 3.60) indicated no chemical degradation, sedimentation, and phase separation took place during this time. It is concluded that micelles formed by amphiphilic block copolymer (Pf 127) maintained their integrity until it reached the aqueous humour due to low critical micelle concentration (CMC) value.
CONCLUSION
Polymeric micelle loaded in situ gel of Azelastine HCl (G4) can be considered as an effective and superior alternative topical dosage form that overcomes the drawbacks associated with marketed preparation of Azelastine HCl and also minimizes systemic side effects linked to oral administration of Azelastine HCl without compromising safety and patient compliance. The developed formulation shows a longer duration of action (up to 8 hr) without any irritation and corneal toxicity. The system proved to have better permeability than the marketed preparation and capable of carrying the drug to the anterior segment of the eye via topical delivery. Furthermore, presence of carbopol 940 as in situ gelling polymer results in an increase in residence time of drug over the corneal surface due to the transformation of polymeric micelles (F3) into a gel and is not associated with limitations of conventional eye drops, i.e., not easily eroded or cleared by tear. Thus, Pf 127 micelles have the potential to be carriers of the hydrophobic drug to the anterior segment of the eye and capable of treating allergic conjunctivitis.
ACKNOWLEDGEMENTS
The authors thank the M M College of Pharmacy Maharishi Markandeshwar (Deemed to be University), Mullana, Ambala-133207, Haryana for providing the facilities and encouragement.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study is approved by the committee Institutional Animal Ethics Committee of CPCSEA, India (MMCP-IAEC-20).
HUMAN AND ANIMAL RIGHTS
No humans were used in the study. The reported experiments on animals were in accordance with the guidelines of IAEC/ CPCSEA (India).
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The data that support the findings of this study are available from the corresponding author, [Dr. Manish Kumar], upon request.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Kuno N., Fujii S. Recent advances in ocular drug delivery systems. Polymers (Basel) 2011;3(1):193–221. [Google Scholar]
- 2.Danion A., Arsenault I., Vermette P. Antibacterial activity of contact lenses bearing surface-immobilized layers of intact liposomes loaded with levofloxacin. J. Pharm. Sci. 2007;96(9):2350–2363. doi: 10.1002/jps.20871. [DOI] [PubMed] [Google Scholar]
- 3.Lavik E., Kuehn M.H., Kwon Y.H. Novel drug delivery systems for glaucoma. Eye (Lond.) 2011;25(5):578–586. doi: 10.1038/eye.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Short B.G. Safety evaluation of ocular drug delivery formulations: techniques and practical considerations. Toxicol. Pathol. 2008;36(1):49–62. doi: 10.1177/0192623307310955. [DOI] [PubMed] [Google Scholar]
- 5.Gulsen D., Chauhan A. Ophthalmic drug delivery through contact lenses. Invest. Ophthalmol. Vis. Sci. 2004;45(7):2342–2347. doi: 10.1167/iovs.03-0959. [DOI] [PubMed] [Google Scholar]
- 6.Gulsen D., Chauhan A. Dispersion of microemulsion drops in HEMA hydrogel: a potential ophthalmic drug delivery vehicle. Int. J. Pharm. 2005;292(1-2):95–117. doi: 10.1016/j.ijpharm.2004.11.033. [DOI] [PubMed] [Google Scholar]
- 7.Kapoor Y., Chauhan A. Ophthalmic delivery of Cyclosporine A from Brij-97 microemulsion and surfactant-laden p-HEMA hydrogels. Int. J. Pharm. 2008;361(1-2):222–229. doi: 10.1016/j.ijpharm.2008.05.028. [DOI] [PubMed] [Google Scholar]
- 8.Kim J., Chauhan A. Dexamethasone transport and ocular delivery from poly(hydroxyethyl methacrylate) gels. Int. J. Pharm. 2008;353(1-2):205–222. doi: 10.1016/j.ijpharm.2007.11.049. [DOI] [PubMed] [Google Scholar]
- 9.Li C.C., Chauhan A. Modeling ophthalmic drug delivery by soaked contact lenses. Ind. Eng. Chem. Res. 2006;45(10):3718–3734. [Google Scholar]
- 10.Del Amo E.M., Urtti A. Current and future ophthalmic drug delivery systems. A shift to the posterior segment. Drug Discov. Today. 2008;13(3-4):135–143. doi: 10.1016/j.drudis.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Jain M.R. Drug delivery through soft contact lenses. Br. J. Ophthalmol. 1988;72(2):150–154. doi: 10.1136/bjo.72.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schultz C., Breaux J., Schentag J., Morck D. Drug delivery to the posterior segment of the eye through hydrogel contact lenses. Clin. Exp. Optom. 2011;94(2):212–218. doi: 10.1111/j.1444-0938.2010.00553.x. [DOI] [PubMed] [Google Scholar]
- 13.Dart J.K., Buckley R.J., Monnickendan M., Prasad J. Perennial allergic conjunctivitis: definition, clinical characteristics and prevalence. A comparison with seasonal allergic conjunctivitis. Trans. Ophthalmol. Soc. U. K. 1986;105(Pt 5):513–520. [PubMed] [Google Scholar]
- 14.Leonardi A., Secchi A.G. Vernal keratoconjunctivitis. Int. Ophthalmol. Clin. 2003;43(1):41–58. doi: 10.1097/00004397-200343010-00007. [DOI] [PubMed] [Google Scholar]
- 15.Bielory B., Bielory L. Atopic dermatitis and keratoconjunctivitis. Immunol. Allergy Clin. North Am. 2010;30(3):323–336. doi: 10.1016/j.iac.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Shinde U.A., Shete J.N., Nair H.A., Singh K.H. Eudragit RL100 based microspheres for ocular administration of azelastine hydrochloride. J. Microencapsul. 2012;29(6):511–519. doi: 10.3109/02652048.2012.665088. [DOI] [PubMed] [Google Scholar]
- 17.McTavish D., Sorkin E.M. Azelastine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential. Drugs. 1989;38(5):778–800. doi: 10.2165/00003495-198938050-00005. [DOI] [PubMed] [Google Scholar]
- 18.Lassig W., Wober W., Höflich C., Bähre M., Roloff A. Topical therapy of allergic rhinitis in childhood: Allergodil nasal spray--non-sedating in children. Curr. Med. Res. Opin. 1996;13(7):391–395. doi: 10.1185/03007999609111558. [DOI] [PubMed] [Google Scholar]
- 19.Rosario N., Bielory L. Epidemiology of allergic conjunctivitis. Curr. Opin. Allergy Clin. Immunol. 2011;11(5):471–476. doi: 10.1097/ACI.0b013e32834a9676. [DOI] [PubMed] [Google Scholar]
- 20.Lenhard G, Mivsek-Music E, M PerrinFayolle, A. Secchi. Double-blind, randomised, placebo controlled study of two concentrations of azelastine eye drops in seasonal allergic conjunctivitis or rhinoconjunctivitis Curr Med Res Opin. 1997;14(1):21–8. doi: 10.1185/03007999709113339. [DOI] [PubMed] [Google Scholar]
- 21.Jiang W., Wang Y.D., Gan Q. Preparation and characterization of copolymer micelles formed by poly (ethylene glycol)-polylactide block copolymers as novel drug carriers. CJPE. 2006;6:289–295. [Google Scholar]
- 22.Wu Y., Yang W., Wang C., Hu J., Fu S. Chitosan nanoparticles as a novel delivery system for ammonium glycyrrhizinate. Int. J. Pharm. 2005;295(1-2):235–245. doi: 10.1016/j.ijpharm.2005.01.042. [DOI] [PubMed] [Google Scholar]
- 23.Known S.K., Kim S.Y., Ha K.W., et al. Pharmaceutical evaluation of Genistein-loaded pluronic micelles for oral delivery. Pharm. Res. 2007;30(9):1138–1143. doi: 10.1007/BF02980249. [DOI] [PubMed] [Google Scholar]
- 24.Patel R., Patel H. Formulation and evaluation of carbopol gel containing liposomes of ketoconazole. Int J Drug Deliv Tech. 2009;1:42–45. [Google Scholar]
- 25.Suryawanshi S.S., Kunjwani H.K., Kawade J.V., Alkunte M.A., Yadav D.J. Novel polymeric in-situ gels for ophthalmic drug delivery system. Int J Res Pharm Sci. 2012;2:67–83. [Google Scholar]
- 26.Jaiswal M., Kumar M., Pathak K. Zero order delivery of itraconazole via polymeric micelles incorporated in situ ocular gel for the management of fungal keratitis. Colloids Surf. B Biointerfaces. 2015;130:23–30. doi: 10.1016/j.colsurfb.2015.03.059. [DOI] [PubMed] [Google Scholar]
- 27.Sun Y., Du L., Liu Y., et al. Transdermal delivery of the in situ hydrogels of curcumin and its inclusion complexes of hydroxypropyl-β-cyclodextrin for melanoma treatment. Int. J. Pharm. 2014;469(1):31–39. doi: 10.1016/j.ijpharm.2014.04.039. [DOI] [PubMed] [Google Scholar]
- 28.Ammar H.O., Salama H.A., Ghorab M., Mahmoud A.A. Development of dorzolamide hydrochloride in situ gel nanoemulsion for ocular delivery. Drug Dev. Ind. Pharm. 2010;36(11):1330–1339. doi: 10.3109/03639041003801885. [DOI] [PubMed] [Google Scholar]
- 29.Li J., Wu L., Wu W., et al. A potential carrier based on liquid crystal nanoparticles for ophthalmic delivery of pilocarpine nitrate. Int. J. Pharm. 2013;455(1-2):75–84. doi: 10.1016/j.ijpharm.2013.07.057. [DOI] [PubMed] [Google Scholar]
- 30.Kumar P., Mohan C., Kanamsrinivasan Uma Shankar M., Gulati M. Physiochemical characterization and release rate studies of soliddispersions of ketoconazole with pluronic F127 and PVP K-30. Iran. J. Pharm. Res. 2011;10(4):685–694. [PMC free article] [PubMed] [Google Scholar]
- 31.Shawn C.O., Dianna P.Y.C., Shoichet M.S. Polymeric micelle stability. Nano Today. 2011;7:53–65. [Google Scholar]
- 32.Kabanov A.V., Batrakova E.V., Alakhov V.Y. Pluronic block copolymers as novel polymer therapeutics for drug and gene delivery. J. Control. Release. 2002;82(2-3):189–212. doi: 10.1016/s0168-3659(02)00009-3. [DOI] [PubMed] [Google Scholar]
- 33.Vaidya F.U., Sharma R., Shaikh S., Ray D., Aswal V.K. Pluronic micelles encapsulated curcumin manifests apoptotic cell death and inhibit pro-inflammatory cytokines in human breast adenocarcinoma cells. Cancer Rep. 2018;2(1):1–17. doi: 10.1002/cnr2.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao X., Poon Z., Engler A.C., Bonner D.K., Hammond P.T., Paula T. Enhanced stability of polymeric micelles based on postfunctionalized poly(ethylene glycol)-b-poly(γ-propargyl L-glutamate): the substituent effect. Biomacromolecules. 2012;13(5):1315–1322. doi: 10.1021/bm201873u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sahoo S.K., Dilnawaz F., Krishnakumar S. Nanotechnology in ocular drug delivery. Drug Discov. Today. 2008;13(3-4):144–151. doi: 10.1016/j.drudis.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 36.Khalil R.M., Abdelbary G.A., Basha M., Awad G.E.A., El-Hashemy H.A. Enhancement of lomefloxacin HCL ocular efficacy via niosomal encapsulation: in vitro characterization and in vivo evaluation. J. Liposome Res. 2017;27(4):312–323. doi: 10.1080/08982104.2016.1191022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, [Dr. Manish Kumar], upon request.


