Abstract
Abstract: Within the last two decades, the field of nanomedicine has not developed as successfully as has widely been hoped for. The main reason for this is the immense complexity of the biological systems, including the physico-chemical properties of the biological fluids as well as the biochemistry and the physiology of living systems. The nanoparticles’ physico-chemical properties are also highly important. These differ profoundly from those of freshly synthesized particles when applied in biological/living systems as recent research in this field reveals. The physico-chemical properties of nanoparticles are predefined by their structural and functional design (core and coating material) and are highly affected by their interaction with the environment (temperature, pH, salt, proteins, cells). Since the coating material is the first part of the particle to come in contact with the environment, it does not only provide biocompatibility, but also defines the behavior (e.g. colloidal stability) and the fate (degradation, excretion, accumulation) of nanoparticles in the living systems. Hence, the coating matters, particularly for a nanoparticle system for biomedical applications, which has to fulfill its task in the complex environment of biological fluids, cells and organisms. In this review, we evaluate the performance of different coating materials for nanoparticles concerning their ability to provide colloidal stability in biological media and living systems.
Keywords: Nanoparticles, coating materials, colloidal stability, biological media, biopolymers, polymeric coatings, protein corona
1. INTRODUCTION
1.1. Perspectives and Requirements of Functionalized Nano-particles
It was Paul Ehrlich in the beginning of the 20th century, who envisioned the creation of “magic bullets”, active agents that would seek out and destroy their target (disease-causing cells), avoiding healthy cells and having no harmful effects on the organism [1]. The evolution of this idea has led us to the field of nanomedicine, which is aiming for targeted drug delivery systems based on functional nanoparticles (NPs) and thus attempting to realize Paul Ehrlich´s concept [2]. NPs are in the size range of bio-molecules (1-100 nm) that are encountered at the cellular level. They are orders of magnitude smaller than biological cells, which are mostly between 5-15 μm in size. Accordingly, nanodevices (nanocarriers/nanosensors) consisting of NPs are supposed to travel into cells to deliver drugs or to probe bio-molecules such as deoxyribonucleic acid (DNA), lipids, proteins and receptors inside or outside of the cell [3-9].
This field has gained pace in the recent years, because of new developments and the joint ventures in the fields of nanotechnology, colloidal and polymer science and biomedicine, including pharmacology and immunology [10, 11]. Additionally, the advances in optical imaging [12, 13] and sensing techniques [14, 15] have accelerated this progress [16]. These developments have opened up new perspectives to create such magic bullets with the promise to heal incurable diseases and detect illnesses at an early stage, thus leading to the field of theranostics (therapy and diagnostics) [17, 18].
In the design of such nanodevices, their architecture can be divided into two main parts: the core and the coating. The core provides the size, shape, porosity and eventually the addressability/traceability via physical methods. [19]. The coating defines the physicochemical properties of the NPs, determining the interaction of NPs with the environment and thus the overall behavior during their application. The essential physicochemical properties are suitable surface wettability (i.e. hydrophilicity) and colloidal stability (at different pH values, in the presence of salt and proteins) [20-22]. Furthermore, the coating can generate, enhance and tailor smart properties like responsiveness [23] (pH, [24-27] temperature, [28-30] light [31, 32]) and opportunities for further functionalization, e.g. markers, [33, 34] targeting agents [35, 36] and drugs [37, 38]. There are plenty of concepts for smart nanodevices to be applied in the field of nanomedicine and many of them can be or have been realized as a proof of concept (Table 1) [39, 40]. However, a variety of fundamental problems still prevent the effective therapeutic application of NPs and cause clinical trial failures.
Some of the main problems are related to the complexity of the application field, i.e. the alteration of the physicochemical properties in biological fluids (NP aggregation, protein corona formation etc.), non-specific clearance by the reticuloendothelial system (RES) (lack of stealth behavior =, i.e. non-immunogenicity) and NP-biointerface interactions (internalization pathways, biological barriers, etc.). The prediction of the NPs’ behavior in biological systems has failed until now; mainly because of the variability of NPs inside of a biological system. This is the result of the protein corona formation on the NPs, which can be highly undefined and variable (Fig. 1). This process has a huge influence on the interactions of the NPs with the biological systems [41, 42]. Research on this will surely provide new insights in the coming years.
Fig. (1).
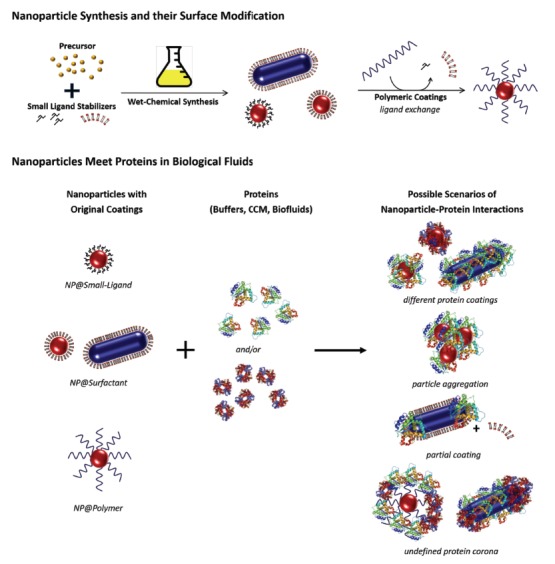
Schematic illustration of a typical approach for the synthesis of NPs and their surface modification prior to their biomedical applications. For the biomedical applications, the NP dispersions with their original coatings (small ligands, surfactants, polymers) are suspended either into biological media (e.g. buffers, cell culture media) or directly into the biofluids of the organism (e.g. blood), where the NPs eventually meet proteins. Depending on the surface chemistry and the coating material, the NP-protein interactions can differ and thus lead to different final properties of the NPs.
Other fundamental problems arise from the choice of the NPs’ building materials, such as a) biocompatibility (material toxicity, dose-response behavior) and b) colloidal stability of the NPs throughout the application pathway (from in vitro to in vivo). For any serious biomedical application, the uttermost prerequisite is the biocompatibility of both, the core and the coating material. Predicting long term toxicities and side effects of such nanosystems is difficult at this early stage of research. However, by using a biocompatible core and coating materials, a direct toxicity can be excluded from these materials. Of course, coating NPs, e.g. with proteins, can also induce changes in the coating material itself by adsorption processes, e.g. in the conformation, enabling a recognition by the immune system. In general, the acute toxicity can be attributed to the coating material as it gets in contact with any biological interface first [43-46].
Ultimately, it is the coating material which not only has to provide an immediate biocompatibility, but also colloidal stability during the entire course of the application. Thanks to the advances in organic and polymer chemistry, a plethora of tailor-made coating materials and coating techniques for NPs are available. However, the most fundamental requirement for these coatings is to provide high colloidal stability throughout the whole pathway, i.e. from the NPs’ production to their application. In the biomedical applications, the colloidal stability of the NPs has to be particularly ensured during all application steps and relevant times and conditions. This implies that the NPs have to remain colloidally stable not only in salt and protein containing media, such as buffer solutions or cell culture media, but also during their incubation with biological cells (in vitro tests) or their injection in the blood stream of the animal models (in vivo tests). In this context, colloidal stability of the NPs is required for suitable handling and long-term storage times as well as for long cell incubation and blood circulation times. For the in vivo tests, the colloidal stability of the NPs and consequently their nanoscale size are also paramount for their elimination/excretion out of the organism. Hence, to reach and to pass clinical tests with NPs, the right choice of the coating matters, because this ultimately enables NPs to fulfill their task in the complex environment of biological fluids, cells and organisms.
Organic coatings for NPs can be classified according to various categories, e.g. the size of a single unit of the coating material, which is defined by the molecular weight (MW). This can vary from monomeric type (small molecules, MW < 1000 g/mol) to polymeric type (MW > 1000 g/mol) coatings. The widely used
monomeric coatings are usually comprised of either multivalently charged small molecules (e.g. succinic acid, citric acid), or of amphiphilic surfactant molecules consisting of a polar head group (e.g., thiolates, dithiolates, amines, ammoniums, carboxylates, cya-nides, isothiocyanates, phosphines) and a hydrocarbon chain. The former stabilize NPs electrostatically whereas the latter can self-assemble into monolayers (SAMs) [47-49] or bilayers [50] to allow the stable dispersion of particles in both organic and aqueous media, respectively. Besides the stabilization of the NPs in the synthesis media, such amphiphilic coatings (cetrimonium bromide (CTAB), [51, 52] benzyl dimethyl ammonium chloride (BDAC), [53] oleate [54] etc.) also provide a precise control of particle growth with regard to their size (4-200nm) and morphology (spheres, rods, triangles, etc.). They are therefore inevitable for the NPs’ synthesis. However, various problems arise from these small ligands, rendering these coatings unsuitable for medical application. For example, these molecules bind weakly to the NPs’ surface and can therefore easily be displaced or replaced by other molecules, which can induce the NPs’ aggregation. In particular, an uncontrolled and unspecific adsorption of large biomolecules such as proteins cannot be prevented by these molecules, which leads to ambiguous results [55]. Furthermore, in biological media with physiological salt concentration, the stabilizing surface charges are screened by the increasing ionic strength. This leads to their destabilization and aggregation. Additionally, the amphiphilic surfactant coatings are cytotoxic, [56, 57] due to their strong interactions with cell membranes and biomolecules such as proteins. In conclusion, these coatings are highly unsuitable in biomedical applications and have to be exchanged for suitable coatings, preferentially polymeric coatings that provide the required properties, in particular enhanced colloidal stability in complex biological media [58].
Indeed, the polymer coatings lead to a higher colloidal stability compared to that of small molecules [59, 60]. It comprises steric and electrostatic stabilization, depending on whether the polymers are uncharged or charged, respectively [61]. The steric repulsion between two polymer-coated particles occurs when the NPs come closer and the outermost layers of their polymer coatings begin to interpenetrate, which results in increased osmotic pressure between the NPs and reduced conformational entropy of the polymer chains (i.e. increased Gibbs energy of the system), making the NPs repel each other. [62-64]. In the case of charged polymers, i.e. polyelectrolytes, the stabilization of the NPs arises from both steric and electrostatic interactions. This is referred to as electrosteric stabilization [62, 65].
The quality of the (electro-)steric stabilization also strongly depends on the type of polymer grafting and the architecture of the polymer coating on the NPs’ surface. Polymers can either be adsorbed via physisorption (van der Waals and electrostatic interactions) or they can be covalently bonded/grafted onto the NPs’ surface, with one or multiple surface binding/anchoring groups. By tethering polymer chains to the particle with one end and by leaving the polymer dangling in the solution, strong steric stabilization can be achieved. At very low grafting densities, polymers attach to the NPs in a relatively flat orientation (pancake regime) [66]. With increasing grafting density, the polymer chains start crowding on the surface, which leads to conformational changes in the polymer layer. Thus, the polymers change from the pancake regime over the mushroom regime to brush regime with fully stretched polymer chains poking out from the surface [67-69]. In general, high grafting densities lead to strong steric stabilization, and thus to higher colloidal stability of the NPs. Furthermore, polymer brushes also reveal excellent protein-repellent (non-fouling) properties [70-72]. Another way of providing a high steric stabilization to colloids is to coat them with a relatively thick layer of a crosslinked polymer gel. The most prominent example for such gel networks are hydrogels, which consist of hydrophilic, crosslinked polymer networks swollen with water. The high degree of hydration of the hydrogel coatings (>95%) does not only lead to high colloidal stability of NPs, but also to low protein adsorption (stealth behavior) on the NPs’ surface [70, 73]. They are thus very attractive for biomedical applications [74, 75]. Furthermore, the porous structure of the gel coating can be used to trap drug molecules for drug delivery applications [76].
Taking advantage of controlled polymerization techniques, in particular of atom-transfer radical polymerization (ATRP), [77] ring-opening metathesis polymerization (ROMP) [78, 79], nitroxide-mediated living radical polymerization (NMP) [80] and reversible addition-fragmentation chain transfer (RAFT) polymerization [81, 82], polymer coatings with tunable architecture can be achieved by simply adding the suitable functionalized monomers to the polymerization mixture. This can lead to cross-linked polymer gel coatings or multiplex functionalization, for instance conjugation with various fluorophores and biological markers. There are excellent reviews and books/book chapters on coating colloids with polymer brushes and polymer gels, which the reader can consult [69, 74-76].
While the synthetic polymers can be precisely designed and their properties can be adjusted according to the demand, biopolymers have highly defined structures and functions, but with huge variety and complexity. Although highly complex, the main advantage of a biopolymer is the intrinsic biocompatibility and bio-degradability. Similar to synthetic polymers, biopolymers can feature various physicochemical properties such as polar/nonpolar, charged/uncharged, positive/negative charges, and even stimuli-responsive behavior. As a surplus, biopolymers can feature more sophisticated properties and functions, catalytic activity, recognition, specific binding and structure formation. Here, synthetic polymers cannot compete with them (yet) [83]. In terms of providing colloidal stability to NPs in physiological media, biopolymers are per se highly ideal candidates, since these polymers are intrinsically stable in biological fluids and can exhibit protein-repellent [84-86] behavior naturally.
The great advantage of synthetic coatings is their tailor-made design of their molecular structure, which leads to a precise tuning of the physico-chemical properties, e.g. responsiveness. Still, their application in biomedicines is not yet as advanced as might have been expected. One of the main reasons for the missing breakthrough is the high complexity and variability of the biological systems. Another one is the lack of real-time monitoring techniques inside the biological entities and organisms.
To understand the role of proteins for the behavior of the NPs in biological fluids and living systems, is one of the major current challenges. It is one of the major current challenges to understand the role of proteins for the behavior of NPs in biological fluids and living systems. In biological systems, the NPs have to face more extreme environmental conditions, i.e. high ionic strength, alternating pH and the presence of proteins and other biomolecules, compared to conditions under which they are synthesized or stored. For example, in the presence of salts or under extreme pH conditions, NPs that are stabilized by charge may lose their colloidal stability as a result of charge-screening effects [87]. The presence of proteins leads to their unspecific adsorption onto the NPs, forming an undefined protein corona, which results in complex and highly variable effects on the NPs’ stability. The adsorption of proteins can cause an aggregation of NPs on the one hand, but can lead to their stabilization on the other hand, depending on the protein, its concentration and the environmental conditions [59, 88]. It has also been shown that proteins can induce incomplete agglomeration of NPs, leading to stable NPs’ clusters or agglomerates of finite sizes [89]. Furthermore, it is known that the extracellular protein corona around NPs evolves with time, [90] and that proteins associated with internalized particles are degraded intracellularly, influencing the NPs’ properties and interactions inside biological systems [91]. For this reason, coating materials that provide high colloidal stability to the NPs, repel proteins and confer stealth behavior to NPs are highly sought. [70-72, 92-95].
Hence, the leading criterion for this review’s structure is the synthetic origin of the coating material. In the first part, synthetic polymer coatings are reviewed. The second part deals with different types of biopolymers, which are usually applied as coating materials for colloids. The focus here lies on biocompatible organic coating materials that are widely used in biomedical applications. The most prominent examples and candidates are discussed and their performance is evaluated with regard to the colloidal stability in biological fluids, cells and organisms.
2. SYNTHETIC COATINGS
2.1. PEG
Polyethylene glycol (PEG), also known as polyethylene oxide (PEO), is by far the most widely used polymer for coating colloidal particles in order to apply them in the field of bio-nanomedicine. PEG is polymerized via anionic ring opening polymerization, which allows precise control of the molecular weight [123]. Due to its hydrophilic polyether backbone, it holds a strong and stable hydration layer through hydrogen bonding to water molecules. Depending on its architecture, [124, 125] this hydration layer in combination with PEG's flexible chains can resist protein adsorption to the underlying surfaces [126]. Therefore, it has been seen as a biologically inert material with no immunogenicity and antigenicity in early studies [127, 128]. The first publication to use PEG in order to alter the protein immunogenicity tested PEGylated bovine serum albumin (BSA) in rabbits. Neither anti-BSA nor anti-PEG antibodies could be found [127]. PEG has also been categorized as a classical “non-fouling” material that resists non-specific protein adsorption [129]. For this reason it is used to coat various surfaces ranging from biomedical devices to colloidal particles as drug delivery systems. Furthermore, PEG is a polymer approved by the FDA (U.S Food and Drug Administration). Thus, PEG-coated NPs are believed to pass clinical tests easily. As a consequence, a variety of PEG-coated NPs is commercially available and therapeutically used. (Table 1).
Table 1. Nanoparticles in applications.
| Product | Core | Coating | Size | Application | Year approved/ Status | Refs. | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| PEG based coatings | ||||||||||
| Adagen® | Adenosine deaminase | PEG | n.a. | Severe combined immunodeficiency | 1990 | [96, 97] | ||||
| Neulasta®/Filgrastim | G-CSF | PEG | n.a. | Febrile neutropenia, cancer chemotherapy associated with neutropenia | 2002 | [98] | ||||
| Oncaspar® | l-asparaginase | PEG | n.a. | Leukemia acute lymphoblastic leukemia | 1994 | [99] | ||||
| Cimzia® | Certolizumab anti-TNFα Fab′ | PEG | n.a. | Crohn's disease rheumatoid arthritis | 2008 | [100] | ||||
| Peg-intron® | Interferon alpha 2b | PEG | n.a. | Hepatitis C | 2001 | [101] | ||||
| Pegasys® | Interferon alpha 2a | PEG | n.a. | Hepatitis C & B, HIV | 2002 | [102] | ||||
| Mircera® | Epoetin® | PEG | n.a. | Anemia associated with chronic kidney disease | 2007 | [103] | ||||
| Krystexxa® | Pegloticase | PEG | n.a. | Refractory chronic gout | 2010 | [104] | ||||
| Omontys® | Peginesatide | PEG | n.a. | Anemia associated with chronic kidney disease | 2012 | [105] | ||||
| Uricase-PEG 20 | Uricase | PEG | n.a. | Hyperuricemia associated with tumor lysis syndrome | 2009 | [106] | ||||
| Macugen® | Anti-VEGF aptamer | PEG | n.a. | Age-related macular degeneration | 2004 | [107] | ||||
| Somavert®/ Pegvisomant | Growth hormonereceptor antagonist | PEG | n.a. | Acromegaly | 2003 | [108] | ||||
| BIND-014 | PLGA-Docetaxel | PEG+targeting ligand | 100 nm | Solid tumors | Phase II | [109] | ||||
| CALAA-01 | Cyclodextrin containing polymer | PEG+targeting ligand | 50-70 nm | Solid tumors | Phase I | [110-112] | ||||
| Erbitux®/EDVsPAC | Bacterially derived mini-cell + Paclitaxel bound to polyglutamate polymer | PEG+bispecific monoclonal antibody | 400 nm | Solid tumors | Phase II | [113] | ||||
| Estrasorb® | Estradiol in soybean oil | PEG | 20-200 nm | Estrogen receptor | 2003 | [114] | ||||
| CYT-6091: Cyt-immune | Gold | Human tumor necrosis factor alpha + PEG-SH | 75 nm | Advanced solid tumors | Phase I | [115] | ||||
| Protein or peptide based coatings | ||||||||||
| Abraxane® | Paclitaxel | Albumin (immobilized) | 70-130 nm | Breast cancer, non-small cell lung cancer, pancreatic cancer | 2005 | [116, 117] | ||||
| SEL-068 | PLGA | Nicotine antigen T-helper cell peptide TLR agonist | 150–250 nm | Smoking cessation vaccine | Phase I | [118] | ||||
| Sugar based coatings | ||||||||||
| Resovist® | Iron oxide | Carboxydextrane | 60 nm | Imaging (MRI) | 2001 | [119] | ||||
| Product | Core | Coating | Size | Application | Year approved/ Status | Refs. | ||||
| Venofer® | Iron oxide | Sucrose | 7 -23 nm | Anemia | 2000 | [120] | ||||
| Cosmofer® | Iron oxide | Dextran | 10-15 nm | Anemia | 2008 | [121] | ||||
| Ferrlecit® | Iron oxide | Sodium gluconate | 3-12 nm | Anemia | 1999 | [120] | ||||
| Feridex® | Iron oxide | Dextran, mannitol, citrate | 120-180 nm | Imaging (MRI) | 1996 | [119] | ||||
| Other | ||||||||||
| Rexin-G® | Cytocidal dominant negative cyclin-G1 DNA construct | Pathotropic NP | 100 nm | Sarcoma, osteosarcoma pancreatic cancer | Phase IIa | [118] | ||||
| Gastromark®/Lumirem® | Iron oxide | [N-(2-aminoethyl)-3-aminopropyl] siloxane | 300 nm | Imaging | 1996 | [122] | ||||
Protein-based drugs are frequently applied medically [130]. Although they are biopharmaceuticals, they usually face great challenges, e.g. instability, inadequate circulation half-life and immunogenicity. The
most successful strategy to overcome these shortcomings is the conjugation of the protein drugs to PEG (PEGylated proteins) [130, 131]. Hence, most of the commercial protein drugs are available as PEG-conjugates (Table 1).
Encouraged by the great success of PEGylation, most of the NP systems that are relevant for biomedical applications are usually coated with PEG. When applied to semi-conductor NPs (quantum dots (QDs)), the PEG coating serves to suppress their cytotoxicity and to enhance the solubility in water, PBS (phosphate buffered saline) buffer and BSA solution [132-134]. PEGylated QDs are of particularly high interest in tumor imaging due to their fluorescence properties [133]. However, even if PEGylation can provide an immediate biocom-patibility to the semiconductor NPs, the long term toxicity of such NPs always has to be taken into account, prior to their application.
Widely applied NP systems for the biomedical applications are the superparamagnetic iron oxide NPs, (SPIONs), which can serve as contrast agents for magnetic resonance imaging (MRI). Xie et al. synthesized 9 nm Fe3O4 NPs in organic solvents and coated them subsequently with PEG. The PEG-coated Fe3O4 NPs were stable in aqueous media in physiological environment, e.g. in PBS + FBS (fetal bovine serum) [135]. Tromsdorf et al. synthesized smaller iron oxide NPs (4 nm core size) and coated them with PEG. They discovered that the PEG coating could not completely avoid the adsorption of serum proteins. The magnetic properties were comparable to the clinically used Resovist® NPs, which bear a carboxydextrane coating. Compared to these, PEGylated NPs show lower cytotoxicity and lower levels of unspecific uptake into cells of the RES [136].
PEGylation is also widely performed on silica NPs, in order to generate a nano-platform – usually in combination with other functional materials like dyes or magnetic NPs for tumor imaging, labeling, and therapeutic applications [137]. He et al. prepared mesoporous silica NPs which were PEGylated by covalently grafting PEG–silanes with different molecular weights (4, 6, 10, 20kDa) and chain densities (0.05 wt%–3.75 wt%). Both show not only an influence on the has (human serum albumin) adsorption but also on phagocytosis and hemolysis: The lowest HSA adsorption, phagocytosis and hemolysis was found for PEG coatings with 10kDA and with 0.75 wt% chain density. The lowest amount of HSA adsorption was 2.5% of a 0.2 mg/mL solution. On the one hand the adsorbed amount is very low, on the other hand this proves once more that PEG is not really anti-fouling [138]. It is not only the PEG length and grafting density that can have an impact on the hemolytic activity of the PEG-NPs. Lin et al. show that nonporous and porous silica PEG-NPs exhibit dose- and size-dependent hemolytic activity on red blood cells [139].
Metal NPs, such as Au NPs, are also promising candidates for biomedical applications, particularly for targeted drug delivery, and as carriers for contrast agents or radiotherapy enhancers [141]. Wang et al. synthesized PEGylated Au NPs and tested them in biological media. They show that Au@PEG NPs are stable in BSA, HBSS (Hank's Balanced Salt Solution) but not in DMEM (Dulbecco's Modified Eagle Medium) + 10% serum and 3 M NaCl.(Fig. 2) [140]. Combining these results with the findings of He et al. [138] shows that the adsorption of albumins does not lower the colloidal stability. In contrast to this, the interaction of NPs with serum leads to their destabilization, which might be due to the adsorption of serum proteins on the NPs’ surface. This serves as a good example for the role of proteins in nano-bio interactions, as they can enhance [88, 89, 142-146] or decrease the colloidal stability by adsorption onto the NPs [138, 140]. When using PEG as coating, albumins do not change the colloidal stability drastically, which might be due to a low adsorption onto the NPs. Serum consists of a large variety of proteins. Some of them interact strongly with PEG (adsorption), resulting in a destabilization of the NPs. A detailed study on protein adsorption onto PEG-NPs by Schöttler et al. will be discussed at the end of this chapter [147].
Fig. (2).

Ultraviolet–visible spectroscopy (UV/VIS) spectra of redispersed PEG–Au colloid in different dispersion media: (a) Dulbecco's Modified Eagle Medium (DMEM) containing 10% serum; (b) BSA; (c) HBSS and (d) NaCl (3 M). Reprinted with permission from ref. [140].
Anisotropic gold NPs, such as gold nanorods (Au NRs) are promising NP systems for in vivo imaging when using near-infrared light scattering [148]. They can also be used in photothermal therapy, for which near infrared laser light is applied [149]. The synthesis of Au rods requires toxic auxiliaries like CTAB [51, 52] to direct the crystal growth and can therefore not be used in biomedical applications directly. A PEGylation can in this case reduce the toxicity [150-152] The anisotropic gold NPs are, moreover, as stable in media as spherical ones [152, 153].
So far, it has been shown that PEGylation successfully leads to NPs which are sufficiently stable in biological media. However, recent studies have disclosed that PEG also bears various drawbacks, e.g. immunogenicity and long-term stability – as discussed in the following:
Animal studies have shown that anti-PEG antibodies can be found in the body after immunization with PEGylated proteins. Whether this was clinically relevant, remained unclear at the time of the study [154, 155]. This generation of anti-PEG antibodies might have been one reason for inflammatory effects of gold NPs observed in mouse liver [156]. In contrast to this, Pissuwan et al. studied inflammatory effects of Au NRs by measuring the secretion of cytokines. Beside good stability in cell culture media, the PEGylated Au NRs showed low toxicity and no significant secretion of the monitored cytokines IL-6 and TNF- α [150].
It is likely that the biological effects are caused by protein adsorption onto PEG. Therefore, a detailed study of this seems necessary. Parak et al. investigated the effect of PEGylation on the adsorption of proteins onto polymer-coated NPs and their uptake by cultured cells [126]. They demonstrated that the PEG coatings did not completely prevent protein adsorption, but the PEGylated NPs still displayed a pronounced reduction of cellular uptake with respect to bare NPs. Very recently, Schöttler et al. investigated the protein adsorption of serum proteins on polystyrene NPs coated with PEG and poly(phosphoester) (Fig. 3). Compared to non-modified nanocarriers, the particles showed a reduction and modification of protein adsorption after incubation with human plasma. Schöttler et al. identified clusterin as the major protein in the corona of both polymer-modified NPs. Furthermore, pre-loading of the PEGylated nanocarriers with clusterin reduced macrophage uptake (thus: “don’t eat me proteins”). These results indicate that the stealth effect is not only a polymeric effect, but also involves a secondary effect that depends on the characteristics of the protein corona. However, the chemical structure of the “stealth polymer” can trigger the amount and type of the adsorbed plasma protein and thus the biological fate of the NPs [147].
Fig. (3).
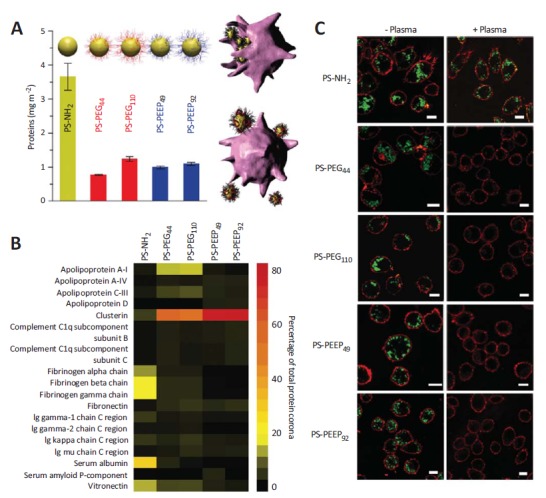
A) Quantification of human plasma proteins adsorbed at the NPs’ surface. The NPs consist of a polystyrene (PS) core and different shells (PEG or poly(ethyl ethylene phosphate)(PEEP)). B), Heat map of the most abundant proteins in the protein corona of PS-NH2, PS-PEG44, PS-PEG110, PS-PEEP49 and PS-PEEP92 determined by proteomic mass spectrometry. The indices represent the polymerization degree. C), Laser scanning microscopy images of RAW264.7 cells incubated with PS-NH2, PS-PEEP49, PS-PEEP92, PS-PEG44 and PS-PEG110 for 1 h in 100% human plasma(+Plasma) or DMEM without plasma(-Plasma). Figure reprinted with the permission of Nature Publishing Group [147].
Unwanted side effects [157] as severe hyper sensitivity [158] or anaphylactic reactions [159] and non long-term biocompatibility of PEG coatings are still problematic. Furthermore, PEG rapidly auto oxidizes ex vivo (particularly in the presence of oxygen and transition metal ions) [160-162] and the termal hydroxyl group of PEG can also be oxidized enzymatically to aldehydes and acids in vivo, allowing proteins and cells to attach [163,164].
Nevertheless, PEG possesses all necessary properties for biomedical applications, i.e. water solubility, immediate biocompatibility and high colloidal stability [165, 166]. Unfortunately, PEGylation withdraws the option for further functionalization and is not responsive to external stimuli. Furthermore, end-functionalized PEG polymers (e.g. terminal thiol, amine, silane functionalities) which can be grafted onto NPs and other surfaces are highly expensive. For these reasons, polymer research is now increasingly focusing on PEG-analogues, which exhibit sophisticated and smart properties, i.e. stimuli-responsiveness, tunable molecular and physico-chemical properties and facile synthesis with different architectures.
2.2. PEG Analogues
One prominent class of PEG-analogue polymers are poly(oligo ethylene glycol (meth)acrylate)s (POEG(M)A)s, which consist of a poly(meth)acrylate backbone with oligo ethylene glycol side chains. They exhibit thermoresponsive behavior, with a lower critical solution temperature (LCST). Such polymers that undergo an LCST transition are soluble in water at low temperatures and phase separate when increasing the temperature above the LCST [167]. The LCST and thus the thermoresponsive behavior of POEGMA can be tailored exactly around the body temperature, i.e. 37-38°C by the variation of the number of ethylene glycol repeat units and/or by the systematic variation of the oligo ethylene glycol chain-ends [168, 169]. Furthermore, POEGMA polymers do not show a significant hysteresis between the heating and cooling cycles as is the case for poly(N –isopropylacrylamide) (PNIPAM). In contrast to PEG, the synthesis of POEGMA polymers is quite facile, since any simple radical polymerization technique can be applied. By using controlled radical polymerization techniques, such as ATRP or RAFT polymerization, POEGMA polymers of defined molecular weights and terminal-functionalities can be synthesized in solution [68, 170, 171] (free chains) or even directly from the NPs’ surface (end-grafted via surface-initiated polymerization) [145]. The straightforward synthesis, the high tunability of the chemical and physical properties, e.g. the thermoresponsiveness, and the commercial availability of the OEGMA are all reasons for the popularity of POEGMA, which can nowadays strongly compete with PEG and PNIPAM [172, 173]. Nonetheless, POEGMA is not yet used as frequently as PEG.
Tests on POEGMA-coated NPs show that they become highly surface active in the presence of salt and/or at elevated temperatures and can even cross the oil-water interface bi-directionally by changing the temperature [170, 171]. Furthermore, POEGMA coated NPs withstand high ionic strength and different pH values [28]. As the different salts can also have an influence, it is convenient to test the stability in biological growth media. Tedja et al. for example coated TiO2 NPs with POEGMA and characterized them by dynamic light scattering (DLS) in distilled water, DMEM, keratinocyte serum-free medium (KSFM) and cell culture media RPMI1640 with 10% FBS and 1% L-glutamine. The NPs remained stable in all media [174]. In contrast to this, Basuki et al. showed that POEGA-SPIONS are not stable in FBS [175]. Shen et al. used RAFT aqueous dispersion polymerization to prepare core-shell nanogels mainly composed of PEGMA or PEG. Both, PEG- and PEGMA nanogels, are stable in 1.5 M NaCl solution for more than three months and in BSA solution for one week. In FBS, PEGMA-nanogels are stable for four days while PEG-nanogels are stable only for two days [176]. Boyer et al. coated gold NPs with different POEGMA/ (2-(2-methoxyethoxy)ethyl methacrylate) (MEO2MA) di-block copolymers displaying a broad range of LCST values (from 15 to 90°C). These NPs do not only show tunable thermosensitive behavior but also protein resistant surfaces
against BSA [177]. In 2009, Chanana et al. showed that MEO2MA-co-OEGMA copolymer coated iron oxide NPs with varied ratios of MEO2MA to OEGMA stay stable in buffer and physiological solution. Furthermore, the NPs can agglomerate reversibly in response to the environmental temperature change, even inside biological cells such as red blood cells (Fig. 4) [68]. A POEGMA- poly(acrylic acid) (PAA) coating was used for magnetite NPs with different diameter (10-25 nm). These NPs exhibited a long-term colloidal stability in either pure deionized water or physiological buffer. The smallest NPs were investigated in rats by MRI. They did not accumulate in the liver, which hints at a long circulation in the bloodstream [178]. Abulateefeh et al. used NPs coated with poly(lactic-co-glycolic acid) (PLGA)-block-(poly(ethylene glycol) methyl ether methacrylate (PEGMEMA)-co-poly(propylene glycol)methacrylate(PPGMA)) and showed a reduced cytotoxicity of encapsulated paclitaxel and its temperature controlled delivery (Fig. 4). This might be of importance for therapy of inflammation or tumors because hyperthermic tissues are characteristic for these diseases [179].
Fig. (4).
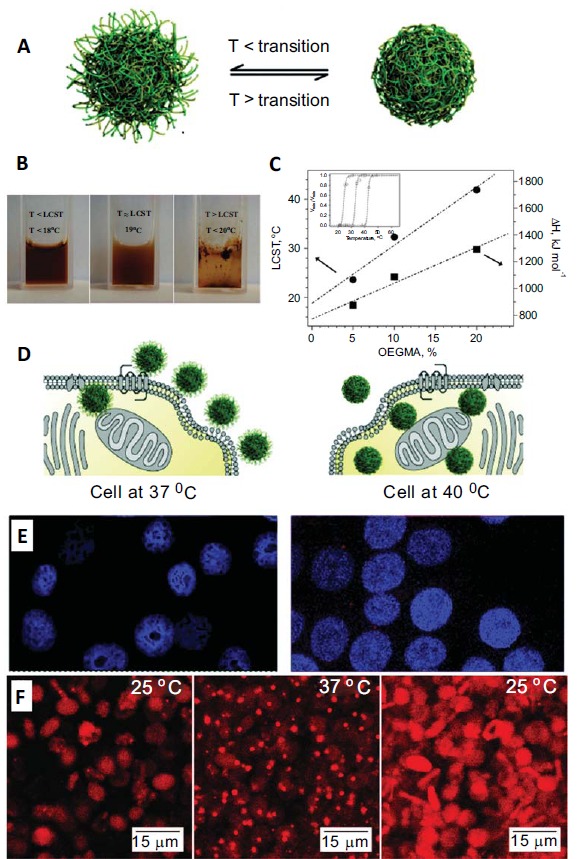
Thermoresponsive NPs in biomedical studies. A) Schematic illustration of the phase transition of a thermoresponsive NP system. The size and surface properties of the NPs’ change below and above the transition temperature. B) Colloidal stability of Fe3O4@P(MEO2MA100-OEGMA10) NPs (POEGMA) at T below, equal to and above the LCST of the polymer coating. C) The transition temperature of the P(MEOMA-OEGMA)-coatings can be tuned by the molar ratio of the two monomers. The cellular uptake of such thermoresponsive NPs depends on the LCST of the polymer and the environmental temperature. As illustrated in D) Scheme of PLGA-b-(PEGMEMA-co-PPGMA) NP at temperatures below and above the LCST. Above the LCST the particles are more hydrophobic and therefore the uptake of cells is higher E)This was proven by confocal micrographs of MCF-7 cells treated with R6G-loaded NPs at 37 °C and 40 °C, showing enhanced signal at 40°C. F) The NPs retain their thermoresponsive behavior inside the cells and agglomerate and disagglomerate reversibly inside the cells, as shown by rhodamine labelled Fe3O4@P(MEO2MA90-OEGMA10) NPs. Reprinted with permission form ref. [68, 179, 180].
Since the PEG analogues contain similar chemical entities as PEG, all advantages apply (e.g. sufficient colloidal stability in biological media, low protein adsorption) and the same is likely for all the disadvantages (e.g. non-immunogenicity). A further potential limitation for biomedical applications of the PEG analogues could be the non-degradability of the carbon-carbon backbone of such polymers [181, 182]. Hence, polymer science and nanoscience as well as nanomedical research all concentrate on finding new and more sophisticated coating materials.
2.3. PNIPAM
Another widely used uncharged polymer in the fields of biomedicine and biotechnology is poly(N –isopropylacrylamide) - PNIPAM. PNIPAM is not only popular because its LCST lies between body and room temperature (LCST ~32°), making it very interesting for biomedical applications, but also because of its robust phase behavior. This means that the LCST of PNIPAM does not shift with variations in chain length and pH [167]. Simple polymerization of NIPAM can be achieved by free radical polymerization. In recent years, controlled radical polymerization has been developed leading to defined polymers. The most common techniques are RAFT [183], ATRP [184] and anionic polymerization [169].
Various types of NPs have been coated with PNIPAM in the form of micro/nanogels [185-194] or core-shell particles. [193, 195-197]. In general, PNIPAM hydrogel coated NPs exhibit a good colloidal stability in various salt containing buffers. It has for example been shown that the PNIPAM coated NPs were stable in pH 4 in bicarbonate buffer [186] and in 0.01 M PBS (pH 7.4), independent of the architecture of the polymer coating, i.e. hydrogel or brush type coated particles [190]. Zyuzin et al. used spherical Au NPs and coated them with different polyelectrolytes and PNIPAM. In comparison to poly(allylamine hydrochloride) (PAH) and polystyrene sulfonate (PSS), PNIPAM coated NPs stayed stable in protein-free PBS buffer, in PBS buffer with BSA and in Eagle's minimum essential medium (EMEM). They monitored the hydrodynamic radius over a cooling and heating cycle for 2h, without any aggregation for the PNIPAM coated particles, whereas aggregation was observed for PAH and PSS coated NPs in PBS. [198]. In the category of core-shell NPs, Au NRs coated with PNIPAM have been applied in in vivo studies. For example, Zhang et al. used Au@SiO2 NRs with a thermoresponsive PNIPAM shell for controlled drug release inside the tissue, upon heating the NPs with a near-infrared (NIR) laser. The NPs showed minimal cytotoxicity and high biocompatibility in cell experiments and a prolonged blood circulation time [199]. In terms of protein corona formation on PNIPAM-coated NPs, Shamim et al. showed that the adsorption of BSA onto PNIPAM coated iron oxide particles depends on temperature, pH, and ionic strength. More protein was adsorbed at higher temperatures and less below the LCST. This can be explained by the hydrophobicity of the NPs above the LCST of PNIPAM. The adsorption of BSA was highest at the isoelectric point (pI) of the protein, because the hydrophobic contribution of the proteins is at a maximum here. Raising the ionic strength leads to a lower amount of adsorbed properties, which is due to the salting in effect of BSA [200].
To conclude, PNIPAM seems to be a smart choice for NP coating, as it ensures the particles’ stability in biological media [198] and adds a thermoresponsive behavior to the NPs, similar to POEGMA systems. One potential limitation for biomedical applications could be the non-degradability of the carbon-carbon backbone [181, 182].
2.4. PVP
Polyvinylpyrrolidone (PVP) is well known in medical applications e.g. as Povidone-iodine for antiseptics. Usually, it is radically polymerized from vinylpyrrolindone [201, 202]. A controlled radical polymerization of PVP has been reported by several groups using ATRP, [203]. RAFT, [204-206] and organometal-mediated living radical polymerization [207, 208]. As toxic auxiliaries are applied, a reliable purification must be implemented. PVP is soluble in water and in some organic solvents and therefore enables phase transfer from organic to aqueous solutions.
PVP is frequently drawn upon for directing the nanoparticle growth of complex structures such as metal-organic framework (MOF) NPs assemblies [209] or Pd bars and rods [210]. LSMO (La0.7Sr0.3MnO3) NPs with sizes ranging from 20–25 nm were synthesized and successfully coated with PVP as a stabilizing
agent. DLS measurements showed that the hydrodynamic diameter was stable in PBS [211] PVP coated particles exhibit a higher colloidal stability than the as-prepared citrate capped silver NPs [212]. Unfortunately, PVP coating does not fully prevent protein adsorption, which has up to now been shown on 2D surfaces only [213, 214]. Covaliu et al. show a comparable biocompatibility of the PVP coating compared to PEG on cobalt ferrite(CoFe2O4) particles [215].
To conclude, PVP seems to behave very similarly to PEG with regard to the colloidal stability. As it is not as sufficiently investigated as PEG, it is not surprising that no negative behavior is known up to now. However, one problem might be the non-degradability, which could generate long-term biocompatibility problems. Therefore, the application of degradable systems seems to be more sensible.
2.5. PVA
Polyvinyl alcohol (PVA) is a water soluble polymer, which is made from polyvinyl acetate through hydrolysis and can easily be degraded by biological organisms. It has been applied in the industrial, commercial, medical, and food sector and to produce various end products, such as resins, lacquers, surgical threads, and food packaging materials [216]. The variety of applications is due to the low toxicity of the polymer, [217, 218] and it is approved by the FDA to be GRAS (generally recognized as safe) [217]. The chemical and physical properties of PVA mainly depend on the percentage of hydrolysis (usually 80%-99%). This determines the PVA grade and its molecular weight, which is usually between 20,000 and 400,000 g/mol, depending on the length of the initial vinyl acetate polymer and on whether it occurs under alkaline or acidic conditions [217]. PVA is a biodegradable polymer as hydroxyl groups are present on the carbon atoms [216]. PVA allows control over the shape and size of iron oxide NPs in the synthesis [219]. All the advantages of biodegradability [220] are of no use if the NPs are not colloidally stable in biological buffer. The few publications in this field show an immediate agglomeration of the particles in serum, PBS and DMEM [221]. This suggests that PVA is most likely not an alternative for PEG.
2.6. Polyelectrolyte
Compared to the polymers mentioned above, polyelectrolytes are charged. This allows a functionalization by loading of charged dyes or drugs into a polyelectrolyte coating. The most popular examples of positively charged synthetic polyelectrolytes, i.e. polycations, are polyallylamine, polyvinylpyridin (P4VP), poly(2-(dimethylamino)ethyl-methacrylate) (PDMAEMA) [222], polydiallyldimethylammonium chloride (PolyDADMAC), polyethylenimine (PEI). The most commonly used synthetic polyanions are poly(meth)acrylic acid (P(M)AA) and PSS [223].
The biophysical interactions of polyelectrolyte NPs strongly depend on on their physiochemical properties, especially size and charge. Other properties such as shape [224-226] and surface topography (roughness) [227] are still under investigation. Polyelectrolyte coatings are very popular, because of their ability to build multilayered structures. The charge of the particles depends on the polyelectrolyte of the outer shell. Several studies show that positively charged NPs should be avoided as they attract negatively charged biological matter such as luminal surfaces, [228] kidney membranes, [110] or epithelial/endothelial cell membrane surfaces [229, 230]. Therefore, it is convenient to concentrate on outer polyanionic coatings as the inner cationic layers are not important for colloidal stability and cell uptake.
Polyanionic coatings or layer by layer coatings with an outer polyanionic layer of NPs have been realized for a plethora of particles. Leonov et al. show a significant reduction of toxicity of PSS coated Au NRs that goes along with colloidal stability in PBS buffer. Unfortunately, they show that there is free PSS ligand in solution in their DLS measurements [231]. PAA coatings render NPs stable in nearly all pH regimes. Jans et al. showed PAA coated Au NPs exhibit colloidal stability in the range of pH 2-14 [232] which, unfortunately, aggregated at a concentration of NaCl of 30mM. They stated that this is due the PAA shrinking caused by dehydration. However, a charge screening seems much more likely. In contrast to the instability induced by 30mM of NaCl found by Jans et al., the groups of Chanteau and Safi show that the colloidal stability for PAA seems to be sufficient in cell culture medium [233, 234].
Charged surfaces always bear the problem that they attract matter from the opposite charge in biological systems e.g. proteins. Therefore, nearly all polyelectrolyte coatings are combined with PEG when used in vivo to suppress protein adsorption. Polyelectrolytes are used in vivo as their charge alters with pH, thus they are pH responsive.
In order to shield the polyanionic layer of PSS or carboxylated QDs, an outer layer of iminobiotin-labelled PEG was applied by Poon et al. This cell-penetrating surface was revealed at hypoxic tumor pH conditions so that a significant higher uptake in the tumor regions could be observed in comparison to standard PEG particles. The stability of NPs was not investigated in cell media buffer, but was found to be freely circulating in mice for 24 h. [235-238]. This goes along with the findings of Yang et al. who have developed an efficient delivery of Layer-by-Layer modified NPs into human breast cancer cells. The NPs consisted of polysulfonamide/poly(ethylenimine)/pDNA layers, which were shielded with PEG. [239].
As mentioned above, polyelectrolyte coated NPs can be functionalized with targeting ligands. This includes small molecules, [240] peptides [240] and aptamers, [241, 242] that do not seem to influence the colloidal stability drastically, whereas a functionalization with antibodies [243] or glycopolymers [244] will generate a completely new interface so that this behavior can be traced back to the functionalization substances.
Besides classical core-shell structure, a combination of polyanions and polycations can also be used to generate interpolyelectrolyte complexes (IPEC) [245]. Stoichiometric polyelectrolyte complexes possess both, cationic and anionic groups in close proximity. These are known as bulk zwitterions or zwittersolids [246, 247].
2.7. Zwitterionic Polymers
Zwitterionic polymers raise two expectations: First of all, they might be an alternative to PEG as their overall charge is zero. At first, of course, it seems to be paradox to generate no charge by using charges. This can, however, easily be achieved when ensuring a stoichiometric composition of charges by choosing a zwitterionic monomer. Secondly, zwitterionic polymers can serve to tailor the surface charge by copolymerization of classic polyelectrolytes, which then induce a responsivity for pH. This can also be seen as an attempt to mimic proteins. Typically used zwitterionic polymers are: poly(carboxybetaine) (PCB), poly(sulfo-betaine) (PSB), phosphorylcholine-based copolymers and derivatives of classical polyelectrolytes such as PAA or poly(maleic anhydride-alt-1-alkene) [247, 248].
Zwitterionic polymers have been used to coat several cores through a ligand exchange process: Yang et al. prepared Au NPs with zwitterionic polymers. Unfortunately, their particles aggregated during their coating procedure. Once coated, these aggregates stayed stable in human serum [249]. There are lots of publications stating that the single protein adsorption is reduced for zwitterionic polymers, compared to other ligands e.g. PEG. [250]. Right now it is being discussed whether these zwitterionic polymers can serve as anti-fouling polymers. This application can be seen as a pre-stage and might eventually lead to an application in nanomedicine [251, 252]. Although the material is quite new, in vivo tests of zwitterionic polymer-based systems are already available. They show sufficient physicochemical properties, i.e. colloidal stability, anti-protein adsorption ability and prolonged circulation half-lives and therefore improved pharmacokinetics and increased drug accumulation in tumors that are comparable to PEG-based systems [253-258].
It seems as if the use of synthetic polymers was mainly driven by a trial and error process. Thus, it seems more sensible to concentrate in inherent biocompatible materials, i.e. biopolymers.
3. NATURAL POLYMERIC COATINGS
Due to their inherent biocompatibility, biomolecules have been of particular interest as coating material for colloids; especially biomacromolecules or biopolymers. These molecules exhibit very well-defined molecular structures and provide a huge variety of chemical functionalities, physico-chemical properties and biological activities. Motivated by these features, researchers of different scientific communities took advantage of the materials’ complexity and manipulated them to direct the formation of intricate nanostructures. Thereby, the physicochemical interactions in these biological macromolecules that give rise to the structural and functional diversity in nature are adopted for creating smart nanodevices, employing nature´s concepts. The main classes of biopolymers are the polynucleotides (DNA and ribonucleic acid (RNA)), polysaccharides/glycanes (dextran, cellulose, chitosan) and polypeptides (proteins, enzymes). In the following, these three biopolymer classes are briefly described and their feasibility for using them as coating material for NPs is summarized.
3.1. Polynucleotides
The polynucleotides DNA and RNA are used for several purposes in NP coating. They are not as frequently applied to render them biocompatible and colloidally stable in biological fluids as they are used in the transfection into living cells or DNA origami. Herein, DNA is used for template-assisted self-assembly of colloidal particles into 2D and 3D nanostructures, making use of their specific recognition properties, known as the Watson-Crick base-pairing interactions [259]. The highly specific interactions of its four building blocks (adenine, thymine, guanine, and cytosine) are translated into the characteristic double helical structure and folding. Hereby, the nucleic base adenine pairs exclusively with thymine, and guanine pairs with cytosine via intermolecular hydrogen bonds, allowing for highly specific recognition and thermosensitive reversible bonding [260, 261].
There are several reports, where DNA has been employed to coat colloidal particles, such as Au NPs or microspheres made of PS, poly(methyl methacrylate) (PMMA) or silica. Although indications exist that these DNA coated colloids are stable in the presence of salts (0.1 M NaCl, 50 mM phosphate buffer), [262, 263]. DNA is not the first choice for making NPs colloidally stable for biomedical applications. One of the main reasons for this is the cost-expensive and arduous extraction.
3.2. Neutral Polysaccharides: Dextrans
From the variety of polysaccharides (Table 2), dextran is the most common coating material for NPs, e.g. as MRI contrasting agent like Resovist (Table 1) [119]. Therefore, it serves here as a representative for neutral polysaccharides. Dextran has the chemical formula of H(C6H10O5)nOH. It is a type of α-glucan produced from sucrose by the bacterial extracellular enzyme dextransucrase. Usually, it is synthesized by bacteria like Leuconostoc, Gluconobacter, Streptococcus and Lactobacillus [265-273]. Dextran is well soluble in water and several other organic solvents, such as methyl sulfoxide, formamide, ethylene glycol and glycerol. It bears no charge, so that it can be compared with PEG in this aspect [274]. For about hundred years it has now been used in medicine as plasma expander [275-277] and as preservation agent [278]. It adsorbs onto the blood platelets [279, 280] and deteriorates the blood coagulation [281]. The great success of dextran can be explained by good biocompatibility, resulting in low toxicity and the ability of good stabilization of NP [282].
Table 2. Properties of several polysaccharides commonly used for coating or encapsulation of NPs. Reprinted with permission from Ref. [264].
| Polysaccharide | Natural Source | Charge | Functional Groups |
|---|---|---|---|
| Alginate | Distributed widely in the cell walls of brown algae | Negative | OH,COOH |
| Chitosan | From the exoskeletons of shrimp and other crustaceans treated with sodium hydroxide | Positive | OH, NH2 |
| Dextran | First discovered by Louis Pasteur as a microbial product in wine | Neutral | OH |
| Hyaluronic acid | Distributed widely throughout connective, epithelial, and neural tissues | Negative | OH, COOH |
| Heparin | Extracted from animal tissues | Negative | OH, OSO3H |
| Mannan | Plant polysaccharide | Neutral | OH |
| Pullulan | Produced from starch by the fungus Aureobasidium pullulans | Neutral | OH |
| Starch | Produced by most green plants to store energy | Neutral | OH |
Pure dextran particles do not seem to be stable in PBS buffer. [283]. By adding an additional chitosan coating, Qi et al. were able to generate stable NPs (130–230 nm diameter) with BSA/chitosan/doxoru-bicin core under physiological conditions [284].
This is why dextran derivates are usually drawn upon. For example, carboxymethyl substituted dextran can help to stabilize liposomes in physiological buffer, which results in a slower clearance from blood circulation than the unmodified form [285]. Beside the colloidal stability, the carboxymethyl groups allow for covalent functionalization by an epidermal growth factor receptor onto the NPs’ surface [286].
Unfortunately, lots of in vivo tests have been prepared without monitoring the colloidal stability and protein adsorption. It is thus rather difficult to name the impact of the dextran coatings in this case [287-292].
The findings of Delgado et al. show that dextran–protamine–solid lipid NPs are stable in the bloodstream of mice [293]. It remains, however, unclear if dextran alone generates a sufficient stabilization or if this is reached by the adsorption or substitution of proteins [294].
3.3. Positively Charged Polysaccharides: Chitosan
Chitosan is a biocompatible hydrophilic polysaccharide that is found in sea crustaceans and is investigated widely because of its biodegradability, [295] nonimmunogenicity, [296] and nontoxic properties [297, 298]. Besides, chitosan is reported to have other biological properties, such as antitumor, [299] antimicrobial, [300] and antioxidant [301] activities. Chitosan is a copolymer of a 2-amino-2-deoxy-D-glucose and 2-acetamido-2-deoxy-D-glucose units with 𝛽 (1-4) linkages and can be obtained by the deacetylation of chitin. It possesses plenty of available amino and hydroxyl groups, which enable it to coat NPs [302]. Its pI depends on the molecular weight and the degree of acetylation (higher degree of acetylation = lower pI) and lies around 6-7 [303, 304]. Consequently, the charge of chitosan can be close to zero in most biological fluids, which may be a reason for aggregation.
Nevertheless, previous studies have demonstrated that a chitosan coating can improve the colloidal stability of NPs, even at the pI of chitosan. This stabilization can neither be explained by the DLVO theory (named after Boris Derjaguin and Lev Landau, Evert Verwey and Theodoor Overbeek) nor by steric repulsions. It seems be due to short-range repulsive interactions, more specifically hydration forces [303, 305], which have to be taken into account because of chitosans’ hydrophilicity [306].
The physicochemical properties of chitosan strongly depend on its degree of acetylation (DA). For low DA values (< 20%) chitosan can be understood as a hydrophilic polyelectrolyte. The intramolecular electrostatic repulsions favor the formation of an open, stiff structure. With increasing DA, chitosan becomes more hydrophobic so that hydrophilic and hydrophobic interactions are counterbalanced. This leads to a decrease of the intramolecular repulsions and the conformation of the polymer becomes more flexible. If the DA exceeds 50%, hydrophobic interactions dominate, generating attractive intramolecular interactions which then promote the formation of loop-like assemblies [307]. This behavior is also affected by the MW of the polymer [308]. The DA does not only affect the structure of chitosan in solution but also its conformation when it is adsorbed onto a hydrophobic core [309].
It was shown that chitosan coated NPs exhibit remarkable stability in Minimum Essential Medium (MEM) and Endothelial Cell Growth Medium (ECGM), and that those with a DA >27% are also stable in Roswell Park Memorial Institute medium (RPMI)-1640 [303]. As a result of the high colloidal stability evoked by the chitosan coating, the bioavailability of NPs increases [310-312].
Looking at in vivo experiments it seems as if chitosan-coated metal oxide NPs could easily cross cell membranes, e.g. by chitosan mediated opening of epithelial cells’ tight junctions [313, 314]. Moreover, the possibility of further functionalization has been used quite often to optimize in vivo experiments. For gene therapy applications, nucleic acid was loaded in the chitosan shell, which was then protected against degradation [315, 316]. These nucleic acid-loaded chitosan NPs can then permeate cell membranes in order to deliver gene therapeutics. It seems as if this was due to the higher biocompatibility and non-immunogenicity compared to conventional approaches (e.g. by liposomes) [317]. Using this concept, different peptides and proteins were encapsulated in chitosan NPs, such as: BSA as a model protein for overcoming enzymatic and absorption barriers [318] or insulin by ionotropic gelation for its delivery. [319-323]. Moreover, chitosan was variously modified: [324] with DNA to generate vectors; [325-327] with exendin-4 (LAM1-Ex4) to prolong the hypoglycemic effect in mice; [328] with N-trimethyl-chitosan for brain-targeting of an anti-neuroexcitation peptide; [329] with linoleic acid for gene delivery by SPIONs [330] and by ceramic calcination to adjust hydrophilicity [331]. Chitosan has also been used to conjugate polymers to the surface of the particles [324, 332, 333]. In most cases PEG is used for conjugation to reduce plasma protein adhesion and phagocytosis by RES [334].
Apart from cationic chitosan, negatively charged polysaccharides may also serve to coat NPs for biomedical applications, e.g. alginate or hyaluronic acid.
3.4. Negatively Charged Polysaccharides: Alginate and Hyaluronic Acid
Alginate, an anionic polysaccharide, is obtained from cell walls of brown algae. It contains 𝛽 (1-4) linked D-mannuronic acid and 𝛼 (1-4) linked L-guluronic acid residues. One problem with alginates is that the industrial processes used for the seaweed extraction could introduce additional endotoxic contaminants like polyphenols, proteins, or lipopolysaccharide, which can induce severe inflammatory reactions [335]. However, alginate purification has led to a reduced - if still existent – inflammatory reaction [336]. In the presence of divalent cations, alginates form ionotropic gels, which have widely been studied [324, 337, 338]. Alginates offer the possibility of functionalization by covalent [339, 340] or physical attachments, i.e. ionic interactions [341, 342]. Many different molecules have been attached such as growth factors [343] and cell adhesive ligands [344, 345].
Alginate was used as “pure” coating agent [346] and after its functionalization with thiol groups [344]. Au NPs coated with thiolated alginate were tested in PBS and show the same stability and cell viability as PEG NPs. Unfortunately, the colloidal stability in the cells was not measured [344]. With an alginate coating it was possible to enhance the solubility of chitosan NPs. Bagre et al. show that alginate coated chitosan core shell NPs can be used for the delivery of enoxaparin, a low molecular weight heparin. The particles seem to be stable in phosphate buffer (10 mg/mL) and exhibit an uptake by intestinal epithelium [346]. In contrast to this, Bar-Shir et al. synthesized iron oxide NPs and coated them with alginate, which surprisingly showed that the NPs are not stable at 0.5 M Ca2+. By this, however, Bar-Shir et al. could show a non-invasive detection of extracellular Ca2+ by conventional MRI. It seems to be doubtful, whether this could lead to a clinical study due to unsolvable safety issues [347].
Hyaluronic acid (HA) is comparable to alginate. It is a polysaccharide composed of alternating (1-4)- β-D-glucuronic acid and (1-3)-β-Nacetyl-D-glucosamine [348]. HA is abundant in the body, especially in the skin, the lungs, in the synovial fluid and in blood [349]. The biological role of HA varies with the molecular weight [350]. High molecular weight HA in the extracellular matrix has an antiangiogenic effect [351] whereas the HA fragments can be a stimulator of angiogenesis [352] and of endothelial differentiation [353].
The biological functions and receptors, render HA interesting for nanomedical constructs – especially for anti-cancer drugs, because the HA receptors CD44 and RHAMM are overexpressed in tumor cells [354, 355]. Therefore, HA has already been used successfully for the delivery of anticancer drugs such as paclitaxel [356, 357] by increasing the colloidal stability in PBS buffer [358]. The same applies for doxorubicin [354, 359, 360]. and camptothecin [361]. Here, HA enhances the stability of the NPs in acetate buffer so that no significant changes were observed over the course of five days [361]. Park et al. showed that the presence of HA prevented particle aggregation in blood and death related to pulmonary embolism [324].
Due to high amounts of carboxylic groups on the surface of hyaluronic acid, it is also possible to functionalize it. For example, HA was conjugated with polyethyleneimine and used to deliver siRNA in vitro [362] and in vivo [363, 364]. HA can also improve gene transfection by increasing extracellular stability of NPs: HA does not only protect the NPs from protein adsorption but it also helps them to deliver the gene once they are internalized by cells [365].
Alginate and hyaluronic acid are suitable coatings for NPs as they provide sufficient colloidal stability in biological media and have already successfully been tested in vivo.
3.5. Polypeptides (Polyaminoacids, Peptides, Proteins)
Polypeptides and proteins are another class of biopolymers, which are highly diverse in their molecular composition and architecture, their structural organization and biochemical and physical functions. Proteins are macromolecules consisting of 21 different monomers, i.e. amino acids. They have hydrophilic and hydrophobic residues and are connected to a polypeptide chain. Furthermore, the 21 monomers provide a plethora of chemical functionalities, such as carboxylic, amino, thiol, methionine, hydroxyl, phenyl and various heterocyclic groups. In order to minimize the conformational energy of individual amino acid residues in the chain, to maximize hydrogen bonding of polar groups, and to bury hydrophobic residues away from the aqueous environment, these chains fold and organize into highly ordered 3D architectures. In addition to this, the folded proteins can further self-assemble to protein superstructures and protein complexes or incorporate other molecules such as porphyrins and metal ions giving rise to unique functional and catalytic entities, such as enzymes [366].
Proteins and polypeptide structures have been widely employed to render NPs biocompatible, to allow for targeting and catalytic activity (enzyme immobilization). In the field of nanomedicine and colloid science, proteins have been considered as rather problematic, owed to their unspecific adsorption on almost any kind of surface, leading either to the NPs’ stabilization or to their destabilization (aggregation). One widely used approach is to hinder proteins from adsorbing onto NPs’ surfaces by conferring stealth properties to the NPs. Here, NPs are coated with a polymer such as PEG. Although, PEG (see section PEG above) has been considered to be a quite promising candidate as a stealth coating for NPs over the past years, many studies have shown that a certain amount of serum proteins is always detected on these coatings. Thus, an overall stealth behavior is not given [147]. These factors have given rise to a search for alternatives to PEG, and other biocompatible polymers, such as zwitterionic polybetaines (see section zwitterionic polymers), polysaccharides (see section polysaccharides) including chitosan, hyaluronic acid and dextran [157].
Since the protein adsorption and protein corona formation onto the NPs seems to be unavoidable, regardless of the coating material and the fine-tuned surface properties, [147, 367] the search for alternative approaches becomes all the more essential. One approach is to use the proteins themselves as coating material. Proteins are in the range of small NPs, but under physiological conditions they do not aggregate. They bear both negative and positive charges and other non-ionic hydrophilic groups, including the polyamide/polypeptide backbone. Indeed, by coating proteins directly onto NPs, systems with a defined and highly robust protein corona can be obtained. Such protein-coated NPs exhibit high colloidal stability at physiological conditions of the protein, which is in the range of pH 7.4 with a ionic strength of 150 mM [88, 89, 142-146, 368, 369].
In general, there are three main strategies to coat NPs with proteins, namely 1) covalently, 2) via His-tag-binding and 3) non-covalently via physisorption. For attaching proteins covalently to NPs, they require chemical functionalities such as carboxylates, amines, alcohols, and thiols that enable protein attachment via organic coupling reactions [370, 371]. However, due to the colloidal instability issues related to NPs and the protein denaturation problems under reaction conditions that are beyond the ideal conditions for proteins, only few coupling reactions can be employed. The most frequently applied reaction is the amide coupling reaction, where a primary amine group (-NH2) reacts with an activated carboxylic acid group (-COOH) in aqueous media. For the activation of the carboxylic acid group, carbodiimide compounds provide the most popular and versatile method. The most commonly used carbodiimides are the water-soluble 1-ethyl-3-(-3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) for aqueous crosslinking and the water-insoluble N',N'-dicyclohexylcarbodiimide (DCC) for non-aqueous organic synthesis methods. N-hydroxy-succinimide (NHS) or its water-soluble analogue (sulfo-NHS) is often included in carbodiimide coupling protocols to improve efficiency or create dry-stable (amine-reactive) intermediates. The amine coupling reaction is highly versatile with regard to proteins and particle types, since proteins bear both amine and carboxylic groups and NPs can easily be decorated with amine or carboxylic functionalities. Besides amine-coupling reactions, sulfhydryl-reactive chemical groups such as maleimides, haloacetyls, and pyridyl disulfides can also be used for attaching proteins covalently to NPs. The proteins are then covalently attached to NPs via thioether or disulfide bonds, respectively [371].
The second strategy for decorating particles with proteins or peptides makes use of metal affinity binding entities such as oligohistidine tags (His-tags), which have a high binding affinity toward metal surfaces [371]. Such His-tags are engineered biotechnologically or genetically into the protein sequence. Peptide synthesizers (solid phase) or the above-mentioned coupling reactions (liquid phase) can also be used to add His-tags to peptide sequences. Eventually, those proteins attach onto the NP surface with the His-tag. The NPs that can be employed are usually metal and metal oxide NPs, due to the high affinity of His-tags toward metals. However, the method is expensive and yields a very low amount of labeled proteins, owed to the multiple and arduous protein engineering steps [372].
The third method of decorating NPs with proteins simply comprises the physisorption of proteins onto NPs by using electrostatic and van der Waals forces (Fig. 5). Since proteins readily adsorb onto all kinds of NPs, a protein coating can easily be achieved. Depending on the surface chemistry of the NPs, the proteins bind either weakly or robustly. In the case of metal NPs, the protein layer strongly adsorbs onto the metal surface, yielding a robust protein coating [88, 142-145]. Chanana et al. have reported on coating isotropic and anisotropic metal NPs, in particular spherical Au NPs and Au NRs with various proteins in a ligand exchange process. The original stabilizing agents such as the negatively charged citrate or the positively charged surfactants (CTAB, CTAC) are entirely replaced by the protein coating, which has been shown via surface enhanced Raman spectroscopy (SERS) [146].
Fig. (5).

Synthesis and physicochemical properties of protein-coated Au NPs and Au NRs. A) Schematic illustration of the synthesis of protein-coated NPs in a ligand exchange process. B) Transmission electron microscopy (TEM) image of 15 nm spherical Au NPs coated with the 2 nm protein coating (insulin). C) The localized surface plasmon resonance (LSPR) band indicates no aggregation during the coating process, revealing a successful coating. D) By taking the environmental parameters into account, various types of proteins and enzymes can be coated onto Au NPs. The photograph shows dispersions of Au NPs coated with proteins of different pI (increasing from left to right). TEM images (E) and LSPR band of Au NRs of different aspect ratios. Similar to the Au NPs, protein-coated Au NRs are also highly stable in various buffers and cell culture media (G) and in serum containing media (H). They can be freeze dried and are stable at high particle concentrations (H). Reprinted with permission from ref. [88, 142, 146].
For achieving a successful protein coating, i.e. a thin layer thickness (≈ 2 nm) and to avoid particle ag- gregation during the coating process, the environmental parameters such as pH, temperature and ionic strength have to be precisely controlled. This is because the sur- face chemistry of the NPs (surface charge) and the physicochemical parameters of the protein, in particu- lar its pI (pH value, where the protein bears no charge), are strongly interrelated. Dewald et al. have reported on the interrelationship between the NP identity, pro- tein identity and the environmental parameters to suc- cessfully coat Au NPs with different proteins and en- zymes. Depending on the pI of the proteins, the pro- tein-coated Au NPs exhibit different stability profiles and surface charges with regard to the environmental
pH. NPs coated with proteins of pI ≈ 4.8 exhibit high colloidal stability under physiological conditions, i.e. pH 7.4 and a ionic strength of 150 mM. At pH≈pI, the protein-coated NPs agglomerate reversibly and disagglomerate by moving the pH of the dispersion away from the pI. The resulting protein-coated NPs are highly stable at extreme pH values, at high salt concentrations and in cell culture media containing serum proteins. Furthermore, the Au@protein NP dispersions can be concentrated to extremely high particle concentrations or even be freeze-dried to stable powders, facilitating their storage, handling and application. [143, 145, 146].
In the view of biomedical applications, protein-coated NPs are colloidally stable, responsive (pH) and highly biocompatible nanosystems that allow for novel applications in the fields of drug delivery and sensing. The protein coatings are fully degradable, which was shown by an enzymatic drug delivery assay by Chanana et al. ex vivo (Fig. 6A and B) and in vitro (Fig. 6C and D). Here, various fluorescent dyes were attached to the protein-coating of the Au NPs as model for drug compounds. The fluorescence of the dyes was quenched due to being in close vicinity to the plasmonic Au NP’s surface. In the presence of proteolytic enzymes in a vial or in the lysosomal compartment of a cell (in vitro), the quenched dyes were gradually released upon the enzymatic degradation of the protein coating, thus emitting a fluorescence signal. Strikingly, ex vivo tests showed that the protein-coated NPs disagglomerated and became colloidally stable at physiological pH, even after proteolytic digestion at acidic pH. These studies show that such protein-coated NPs are colloidally stable in biological fluids and compartments of living cells or organisms as long as the environmental pH correlates with the stability profile of the NPs.
Fig. (6).
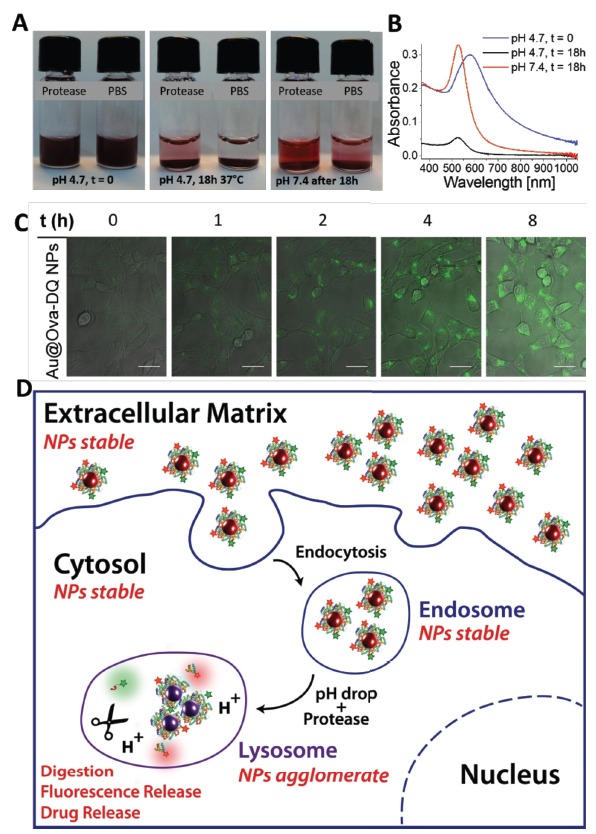
A) Stability of Au@protein NPs (Au@Ovalbumin- 4,4-difluoro-5,7-dimethyl-4-bora-3a,4a-diaza-s-indacene-3-pro-pionic acid (Au@Ova-Bodipy, Au@Ova-DQ) under lysosomal conditions, i.e. under acidic conditions (pH 4.7) in the presence of proteases. The NPs aggregate because of their pH-sensitive character. After incubation for 18 h at 37°C (central photograph), the pH of the dispersions was raised to the physiological pH of 7.4 (right photograph). B) UV/VIS spectra of the protease-containing mixtures in (A). C) Time-dependent fluorescence release of Au@Ova-DQ in 3T3 fibroblasts upon enzymatic digestion of the fluorescently labelled protein coating. Confocal laser scanning microscopy (CLSM) images of 3T3 fibroblasts incubated with the Au@Ova-DQ NPs. The images were recorded at the same position at different times, as indicated. The images are an overlay of the fluorescence (green) and transmission channel. Scale bars: 20 m. D) Schematic illustration of the behavior of protein-coated NPs inside cells and cellular compartments and the prediction of their colloidal stability in the various biological environments. Reprinted with permission from Ref. [143]. (The color version of the figure is available in the electronic copy of the article).
CONCLUSION
For any type of biomedical application of NPs the biocompatibility and the colloidal stability of the NPs have to be ensured throughout all relevant steps, which involve their design, production, storage and application, including NP administration and excretion. Whereas long term biocompatibility still needs to be assessed and quantified for most of the clinically applied NPs, the immediate biocompatibility, which is sufficient for most of the in vitro tests, can be ensured by selecting biocompatible constitutes, i.e. core and coating material. Among these two, it is the coating material, which not only provides an apparent biocompatibility, but also colloidal stability during the entire course of the application, if designed properly. Hence, the choice of the coating material with defined physicochemical and biochemical properties is extremely important to render NPs applicable in nanomedicine.
The physicochemical and the biochemical properties of the coating material are defined by the molecular structure and their architecture on the NP surface. Whereas the monomeric coatings, such as small charged ligands (e.g. citrate) or surfactant molecules (CTAB, oleate) do not stabilize the NPs sufficiently, particularly at high dilutions and in salt and/or protein containing media, polymeric coatings perform better, due to their steric stabilization mechanism. From the plethora of various hydrophilic polymers, non-ionic ones, such as PEG or their analogues, e.g. POEGMA, with the capability of building a strong hydration layer render NPs stable in buffer solutions and cell culture media with physiological salt content and even free proteins. The strong hydration layer of these non-ionic polymer coatings (PEG, POEGMA) is believed to be the reason for the good colloidal stability of the NPs at high ionic strengths, as well as for the stealth behavior against unspecific protein adsorption (i.e. protein corona formation) and against non-fouling properties. Another class of polymers that features this capability of forming a strong hydration layer are the zwitterionic polymers, which do not exhibit any overall charge, but possess both negatively and positively charged groups in stoichiometric ratios. Indeed, these zwitterionic coatings confer high colloidal stability to NPs in salt and protein containing media and are also believed to feature stealth properties. Nevertheless, the non-biological chemical structure and consequently, the low biodegradability of such synthetic polymers can have negative long-term effects on living beings (cells, organisms).
Therefore, nowadays, instead of searching for smart synthetic polymers, research tends to rather study natural biopolymers and their derivatives, which can be easily degraded in living systems, thus avoiding long-term incompatibilities. Dextran, a polysaccharide, represents the biological equivalent of the non-ionic hydrophilic polymers, exhibiting good NP stabilizing properties in water. However, it usually fails in salt containing buffers such as PBS. Although many in vivo tests have been performed with dextran-coated NPs, the colloidal stability issues were not necessarily taken in account, making the results of many studies questionable [283, 287, 288, 290]. For achieving a better colloidal stability in salt containing buffers and biological media, but also for maintaining it over long periods, charged polysaccharides such as chitosan, hyaluronic acid, alginate or charged derivatives of dextran have been successfully employed [264, 323, 347, 373-375].
Proteins are other biopolymers that gain significance in the colloid chemistry and nanomedicine. Their composition of 21 amino acids provides a plethora of chemical functionalities which can be used to coat NPs of different surface chemistries. Furthermore, hydrophobic and hydrophilic (ionic and non-ionic) residues in the same polymer chain lead to an intrinsic stability in physiological media at an ionic strength of at least 150 mM and in complex protein cocktails such as
blood, cytoplasma etc. Thus, protein-coated NPs exhibit high colloidal stability in biological environments, in the presence of salt and of other proteins. The stability profile of the protein-coated NPs goes along with that of the protein. As the proteins are highly sensitive to the environmental pH, the protein-coated NPs also exhibit pH-dependent colloidal stability. NPs coated with proteins are highly stable under physiological conditions in various biological media. Thus, proteins represent a rather newly discovered class of coating material for NPs, which will surely attract more attention in the coming years, particularly in the field of personalized medicine.
However, the properties and behavior of NPs in a cell free biological fluid may change completely when they are introduced into living cells. Moreover, most of the results of in vitro studies are not transferable to in vivo systems. The main reason for this is the intrinsic complexity of biological systems through all levels of hierarchy – from biomolecular level over subcellular, cellular and tissue level up to the level of a full organism. For example, when NPs meet proteins, they tend to immediately adsorb onto the NP’s surface. Even if protein-repellent coatings, the so-called stealth coatings, such as PEG, are used, the unspecific protein adsorption and the formation of a protein corona are unavoidable. While some proteins, e.g. albumins, are repelled to the greatest extent, other proteins can still adsorb. Recent studies have shown that proteins such as clusterin are actually responsible for the final stealth behavior of the NPs [147]. Thus, it is obvious that predictions of the NPs’ behavior in biological systems have to be corrected. Further research is required in order to better understand the fundamental problems of the NPs’ physicochemical properties in biological systems. Only then one can advance significantly in the field of nanomedicine.
ACKNOWLEDGEMENTS
The authors thank Corinna Link for proof reading. J.S. thanks the German Federal Environmental Foundation (DBU) and the BMBF (“Biotechnologie 2020+ Strukturvorhaben: Leibniz Research Cluster”, 031A360C) for funding.
LIST OF ABBREVIATIONS
- ATRP
Atom-transfer radical polymerization
- BDAC
Benzyl dimethyl ammonium chloride
- BODIPY
4,4-Difluoro-5,7-dimethyl-4-bora-3a,4a-diaza-s-indacene-3-pro-pionic acid
- BSA
Bovine serum albumin
- CLSM
Confocal laser scanning microscope
- CTAB
Cetrimonium bromide
- DA
Degree of acetylation
- DCC
N',N'-dicyclohexylcarbodiimide
- DLS
Dynamic light scattering
- DLVO
Named after Boris Derjaguin and Lev Landau, Evert Verwey and Theodoor Overbeek
- DNA
Deoxyribonucleic acid
- DMEM
Dulbecco's Modified Eagle Medium
- DQ
BODIPY
- ECGM
Endothelial cell growth medium
- EDC
1-Ethyl-3-(-3-dimethylaminopropyl) carbodiimide hydrochloride
- EMEM
Eagle's minimum essential medium
- FBS
Fetal bovine serum
- FDA
U.S Food and Drug administration
- GRAS
Generally recognized as safe
- HA
Hyaluronic acid
- HBSS
Hank's Balanced Salt Solution
- HSA
Human serum albumin
- IPEC
Interpolyelectrolyte complexes
- KSFM
Keratinocyte serum-free medium
- LCST
Lower critical solution temperature
- LSPR
Localized surface plasmon resonance
- MEM
Minimum Essential Media
- MEO2MA
Poly(2-(2-methoxyethoxy)ethyl methacrylate)
- MOF
Metal-organic framework
- MRI
Magnetic resonance imaging
- MW
Molecular weight
- NHS
N-hydroxysuccinimide
- NIR
Near-infrared
- NMP
Nitroxide-mediated living radical polymerization
- NRs
Nanorods
- NPs
Nanoparticles
- Ova
Ovalbumin
- P4VP
Poly(4-vinylpyridin)
- P(M)AA
Poly(meth)acrylic acid
- PAH
Poly(allylamine hydrochloride)
- PBS
phosphate buffered saline
- PCB
poly(carboxybetaine
- PDMAEMA
poly(2-(dimethylamino)ethyl-methacrylate)
- PEG
polyethylene glycol
- PEGMEMA
Poly(ethylene glycol) methyl ether methacrylate
- PEI
Polyethylenimine
- PEO
Polyethylene oxide
- pI
Isoelectric point
- PLGA
Poly(lactic-co-glycolic acid)
- PMMA
Poly(methyl methacrylate)
- PNIPAM
Poly(N–isopropylacrylamide)
- POEG(M)A
Poly(oligo ethylene glycol (meth)acrylate)
- PolyDADMAC
Poly(diallyldimethylammonium chloride)
- PPGMA
Poly(propylene glycol)methacrylate)
- PSB
Poly(sulfobetaine)
- PSS
Polystyrenesulfonate
- PVA
Polyvinyl alcohol
- PVP
Polyvinylpyrrolidone
- QDs
Quantum dots
- RAFT
Reversible addition-fragmentation chain transfer polymerization
- RES
Reticuloendothelial system
- RNA
Ribonucleic acid
- ROMP
Ring-opening metathesis polymerization
- RPMI
Roswell Park Memorial Institute medium
- SAMs
Self-assemble into monolayers
- SERS
Surface enhanced Raman spectroscopy
- SPIONs
Superparamagnetic ironoxide NPs
- TEM
Transmission electron microscopy
- UV/VIS
Ultraviolet–visible spectroscopy
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Winau F., Westphal O., Winau R. Paul Ehrlich--in search of the magic bullet. Microbes Infect. 2004;6(8):786–789. doi: 10.1016/j.micinf.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Abbott N.J., Romero I.A. Transporting therapeutics across the blood-brain barrier. Mol. Med. Today. 1996;2(3):106–113. doi: 10.1016/1357-4310(96)88720-x. [DOI] [PubMed] [Google Scholar]
- 3.Brannon-Peppas L., Blanchette J.O. Nanoparticle and targeted systems for cancer therapy. Adv. Drug Deliv. Rev. 2004;56(11):1649–1659. doi: 10.1016/j.addr.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 4.Sun C., Lee J.S.H., Zhang M. Magnetic nanoparticles in MR imaging and drug delivery. Adv. Drug Deliv. Rev. 2008;60(11):1252–1265. doi: 10.1016/j.addr.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fabio S., Catherine D., Paolo C., Patrick C. Metallic Col-loid Nanotechnology, Applications in Diagnosis and Thera-peutics. Curr. Pharm. Des. 2005;11(16):2091–2105. [Google Scholar]
- 6.West J.L., Halas N.J. Engineered nanomaterials for biophotonics applications: Improving sensing, imaging, and therapeutics. Annu. Rev. Biomed. Eng. 2003;5(1):285–292. doi: 10.1146/annurev.bioeng.5.011303.120723. [DOI] [PubMed] [Google Scholar]
- 7.Xu Z.P., Zeng Q.H., Lu G.Q., Yu A.B. Inorganic nano-particles as carriers for efficient cellular delivery. Chem. Eng. Sci. 2006;61(3):1027–1040. [Google Scholar]
- 8.Morphy R., Kay C., Rankovic Z. From magic bullets to designed multiple ligands. Drug Discov. Today. 2004;9(15):641–651. doi: 10.1016/S1359-6446(04)03163-0. [DOI] [PubMed] [Google Scholar]
- 9.Alivisatos P. The use of nanocrystals in biological detection. Nat. Biotechnol. 2004;22(1):47–52. doi: 10.1038/nbt927. [DOI] [PubMed] [Google Scholar]
- 10.Suri G.S., Kaur A., Sen T. A recent trend of drug-nanoparticles in suspension for the application in drug delivery. Nanomedicine (Lond.) 2016 doi: 10.2217/nnm-2016-0238. [DOI] [PubMed] [Google Scholar]
- 11.Tuomela A., Saarinen J., Strachan C.J., Hirvonen J., Pel-tonen L. Production, applications and in vivo fate of drug nanocrystals. J. Drug Deliv. Sci. Technol. 2016;34:21–31. [Google Scholar]
- 12.Miller M.A., Weissleder R. Imaging the pharmacology of nanomaterials by intravital microscopy: Toward understand-ing their biological behavior. Adv. Drug Deliv. Rev. 2017;113:61–86. doi: 10.1016/j.addr.2016.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Savla R., Minko T. Nanoparticle design considerations for molecular imaging of apoptosis: Diagnostic, prognostic, and therapeutic value. Adv. Drug Deliv. Rev. 2017;113:122–140. doi: 10.1016/j.addr.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 14.Gontero D., Lessard-Viger M., Brouard D., Bracamonte A.G., Boudreau D., Veglia A.V. Smart multifunctional na-noparticles design as sensors and drug delivery systems based on supramolecular chemistry. Microchem. J. 2017;130:316–328. [Google Scholar]
- 15.Ng S.M., Koneswaran M., Narayanaswamy R. A review on fluorescent inorganic nanoparticles for optical sensing ap-plications. RSC Advances. 2016;6(26):21624–21661. [Google Scholar]
- 16.Bawa R., Audette G.F., Rubinstein I. Handbook of Clinical Nanomedicine: Nanoparticles, Imaging, Therapy, and Clini-cal Applications. CRC Press; 2016. [Google Scholar]
- 17.Huang H., Lovell J.F. Advanced functional nanomaterials for theranostics. Adv. Funct. Mater. 2016 doi: 10.1002/adfm.201603524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dykman L.A., Khlebtsov N.G. Multifunctional gold-based nanocomposites for theranostics. Biomaterials. 2016;108:13–34. doi: 10.1016/j.biomaterials.2016.08.040. [DOI] [PubMed] [Google Scholar]
- 19.Arnida; Janát-Amsbury, M.M.; Ray, A.; Peterson, C.M.; Ghandehari, H. Geometry and surface characteristics of gold nanoparticles influence their biodistribution and uptake by macrophages. Eur. J. Pharm. Biopharm. 2011;77(3):417–423. doi: 10.1016/j.ejpb.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Navya P.N., Daima H.K. Rational engineering of physicochemical properties of nanomaterials for biomedical applications with nanotoxicological perspectives. Nano Converg. 2016;3(1):1. doi: 10.1186/s40580-016-0064-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shin S.W., Song I.H., Um S.H. Role of Physicochemical Properties in Nanoparticle Toxicity. Nanomaterials (Basel) 2015;5(3):1351–1365. doi: 10.3390/nano5031351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dominguez-Medina S., Kisley L., Tauzin L.J., Hoggard A., Shuang B., Indrasekara A.S., Chen S., Wang L.Y., Derry P.J., Liopo A., Zubarev E.R., Landes C.F., Link S. Adsorption and unfolding of a single protein triggers nanoparticle aggregation. ACS Nano. 2016;10(2):2103–2112. doi: 10.1021/acsnano.5b06439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alvarez-Lorenzo C., Concheiro A. Smart drug delivery systems: From fundamentals to the clinic. Chem. Commun. (Camb.) 2014;50(58):7743–7765. doi: 10.1039/c4cc01429d. [DOI] [PubMed] [Google Scholar]
- 24.Bachelet M. Design of pH-responsive gold nanoparticles in oncology. Mater. Sci. Technol. 2016;32(8):794–804. [Google Scholar]
- 25.Kanamala M., Wilson W.R., Yang M., Palmer B.D., Wu Z. Mechanisms and biomaterials in pH-responsive tumour targeted drug delivery: A review. Biomaterials. 2016;85:152–167. doi: 10.1016/j.biomaterials.2016.01.061. [DOI] [PubMed] [Google Scholar]
- 26.Pang X., Jiang Y., Xiao Q., Leung A.W., Hua H., Xu C. pH-responsive polymer-drug conjugates: Design and progress. J. Control. Release. 2016;222:116–129. doi: 10.1016/j.jconrel.2015.12.024. [DOI] [PubMed] [Google Scholar]
- 27.Karimi M., Eslami M., Sahandi-Zangabad P., Mirab F., Farajisafiloo N., Shafaei Z., Ghosh D., Bozorgomid M., Dashkhaneh F., Hamblin M.R. pH-Sensitive stimulus-responsive nanocarriers for targeted delivery of therapeutic agents. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016;8(5):696–716. doi: 10.1002/wnan.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gambinossi F., Mylon S.E., Ferri J.K. Aggregation kinetics and colloidal stability of functionalized nanoparticles. Adv. Colloid Interface Sci. 2015;222:332–349. doi: 10.1016/j.cis.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 29.Al-Ahmady Z., Kostarelos K. Chemical Components for the Design of Temperature-Responsive Vesicles as Cancer Therapeutics. Chem. Rev. 2016;116(6):3883–3918. doi: 10.1021/acs.chemrev.5b00578. [DOI] [PubMed] [Google Scholar]
- 30.Karimi M., Sahandi Zangabad P., Ghasemi A., Amiri M., Bahrami M., Malekzad H., Ghahramanzadeh Asl H., Mahdieh Z., Bozorgomid M., Ghasemi A., Rahmani Taji Boyuk M.R., Hamblin M.R. Temperature-responsive smart nanocarriers for delivery of therapeutic agents: Applications and recent advances. ACS Appl. Mater. Interfaces. 2016;8(33):21107–21133. doi: 10.1021/acsami.6b00371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y., Huang L., Li Z., Ma G., Zhou Y., Han G. Illuminating cell signaling with near-infrared light-responsive nanomaterials. ACS Nano. 2016;10(4):3881–3885. doi: 10.1021/acsnano.6b02284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang H., Zhou S. Magnetic and fluorescent carbon-based nanohybrids for multi-modal imaging and magnetic field/NIR light responsive drug carriers. Biomater. Sci. 2016;4(7):1062–1073. doi: 10.1039/c6bm00262e. [DOI] [PubMed] [Google Scholar]
- 33.Sperling R.A., Parak W.J. Surface modification, function-alization and bioconjugation of colloidal inorganic nanoparti-cles. 2010 doi: 10.1098/rsta.2009.0273. [DOI] [PubMed] [Google Scholar]
- 34.Beija M., Charreyre M-T., Martinho J.M.G. Dye-labelled polymer chains at specific sites: Synthesis by liv-ing/controlled polymerization. Prog. Polym. Sci. 2011;36(4):568–602. [Google Scholar]
- 35.Li J., Wang F., Sun D., Wang R. A review of the ligands and related targeting strategies for active targeting of paclitaxel to tumours. J. Drug Target. 2016;24(7):590–602. doi: 10.3109/1061186X.2016.1154561. [DOI] [PubMed] [Google Scholar]
- 36.Hudlikar M.S., Li X., Gagarinov I.A., Kolishetti N., Wolfert M.A., Boons G.J. Controlled multi-functionalization facilitates targeted delivery of nanoparticles to cancer Cells. Chemistry. 2016;22(4):1415–1423. doi: 10.1002/chem.201503999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.José L.A. In Nanotechnology and Drug Delivery, Volume One. CRC Press; 2014. pp. 1–27. [Google Scholar]
- 38.Jiang Y., Stenzel M. Drug delivery vehicles based on albumin-polymer conjugates. Macromol. Biosci. 2016;16(6):791–802. doi: 10.1002/mabi.201500453. [DOI] [PubMed] [Google Scholar]
- 39.Hare J.I., Lammers T., Ashford M.B., Puri S., Storm G., Barry S.T. Challenges and strategies in anti-cancer nano-medicine development: An industry perspective. Adv. Drug Deliv. Rev. 2016 doi: 10.1016/j.addr.2016.04.025. [DOI] [PubMed] [Google Scholar]
- 40.Sheridan C. Proof of concept for next-generation nanoparticle drugs in humans. Nat. Biotechnol. 2012;30(6):471–473. doi: 10.1038/nbt0612-471. [DOI] [PubMed] [Google Scholar]
- 41.Parveen R., Shamsi T.N., Fatima S. Nanoparticles-protein interaction: Role in protein aggregation and clinical implications. Int. J. Biol. Macromol. 2017;96(Pt A):386–395. doi: 10.1016/j.ijbiomac.2016.10.024. [DOI] [PubMed] [Google Scholar]
- 42.Henriksen-Lacey M., Carregal-Romero S., Liz-Marzán L.M. Current challenges toward in vitro cellular validation of inorganic nanoparticles. Bioconjug. Chem. 2017;28(1):212–221. doi: 10.1021/acs.bioconjchem.6b00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mahmoudi M., Saeedi-Eslami S.N., Shokrgozar M.A., Azadmanesh K., Hassanlou M., Kalhor H.R., Burtea C., Rothen-Rutishauser B., Laurent S., Sheibani S., Vali H. Cell “vision”: complementary factor of protein corona in nanotoxicology. Nanoscale. 2012;4(17):5461–5468. doi: 10.1039/c2nr31185b. [DOI] [PubMed] [Google Scholar]
- 44.Laurent S., Burtea C., Thirifays C., Häfeli U.O., Mahmoudi M. Crucial ignored parameters on nanotoxicology: The importance of toxicity assay modifications and “cell vision”. PLoS One. 2012;7(1):e29997. doi: 10.1371/journal.pone.0029997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walczyk D., Bombelli F.B., Monopoli M.P., Lynch I., Dawson K.A. What the cell “sees” in bionanoscience. J. Am. Chem. Soc. 2010;132(16):5761–5768. doi: 10.1021/ja910675v. [DOI] [PubMed] [Google Scholar]
- 46.Laurent S., Burtea C., Thirifays C., Rezaee F., Mahmoudi M. Significance of cell “observer” and protein source in nanobiosciences. J. Colloid Interface Sci. 2013;392:431–445. doi: 10.1016/j.jcis.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 47.Badia A., Singh S., Demers L., Cuccia L., Brown G.R., Lennox R.B. Self-assembled monolayers on gold nano-particles. Chemistry. 1996;2(3):359–363. [Google Scholar]
- 48.Srisombat L., Jamison A.C., Lee T.R. Stability: A key issue for self-assembled monolayers on gold as thin-film coatings and nanoparticle protectants. Colloids Surf. A Physicochem. Eng. Asp. 2011;390(1–3):1–19. [Google Scholar]
- 49.Daniel M-C., Astruc D. Gold nanoparticles: Assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem. Rev. 2004;104(1):293–346. doi: 10.1021/cr030698+. [DOI] [PubMed] [Google Scholar]
- 50.Gao J., Bender C.M., Murphy C.J. Dependence of the gold nanorod aspect ratio on the nature of the directing surfactant in aqueous solution. Langmuir. 2003;19(21):9065–9070. [Google Scholar]
- 51.Kozek K.A., Kozek K.M., Wu W-C., Mishra S.R., Tracy J.B. Large-scale synthesis of gold nanorods through continuous secondary growth. Chem. Mater. 2013;25(22):4537–4544. doi: 10.1021/cm402277y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scarabelli L., Sánchez-Iglesias A., Pérez-Juste J., Liz-Marzán L.M.A. “Tips and Tricks” PRACTICAL GUIDE TO THE SYNTHESIS OF GOLD NANorods. J. Phys. Chem. Lett. 2015;6(21):4270–4279. doi: 10.1021/acs.jpclett.5b02123. [DOI] [PubMed] [Google Scholar]
- 53.Ye X., Zheng C., Chen J., Gao Y., Murray C.B. Using binary surfactant mixtures to simultaneously improve the dimensional tunability and monodispersity in the seeded growth of gold nanorods. Nano Lett. 2013;13(2):765–771. doi: 10.1021/nl304478h. [DOI] [PubMed] [Google Scholar]
- 54.Ye X., Gao Y., Chen J., Reifsnyder D.C., Zheng C., Murray C.B. Seeded growth of monodisperse gold nanorods using bromide-free surfactant mixtures. Nano Lett. 2013;13(5):2163–2171. doi: 10.1021/nl400653s. [DOI] [PubMed] [Google Scholar]
- 55.Mnif I., Ghribi D. High molecular weight bioemulsifiers, main properties and potential environmental and biomedical applications. World J. Microbiol. Biotechnol. 2015;31(5):691–706. doi: 10.1007/s11274-015-1830-5. [DOI] [PubMed] [Google Scholar]
- 56.Cortesi R., Esposito E., Menegatti E., Gambari R., Nas-truzzi C. Effect of cationic liposome composition on in vitro cytotoxicity and protective effect on carried DNA. Int. J. Pharm. 1996;139(1):69–78. [Google Scholar]
- 57.Mirska D., Schirmer K., Funari S.S., Langner A., Dobner B., Brezesinski G. Biophysical and biochemical properties of a binary lipid mixture for DNA transfection. Colloids Surf. B Biointerfaces. 2005;40(1):51–59. doi: 10.1016/j.colsurfb.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 58.Lehner R., Wang X., Marsch S., Hunziker P. Intelligent nanomaterials for medicine: carrier platforms and targeting strategies in the context of clinical application. Nanomedicine (Lond.) 2013;9(6):742–757. doi: 10.1016/j.nano.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 59.Moore T.L., Rodriguez-Lorenzo L., Hirsch V., Balog S., Urban D., Jud C., Rothen-Rutishauser B., Lattuada M., Petri-Fink A. Nanoparticle colloidal stability in cell culture media and impact on cellular interactions. Chem. Soc. Rev. 2015;44(17):6287–6305. doi: 10.1039/c4cs00487f. [DOI] [PubMed] [Google Scholar]
- 60.Mogoşanu G.D., Grumezescu A.M., Bejenaru C., Bejenaru L.E. Polymeric protective agents for nanoparticles in drug delivery and targeting. Int. J. Pharm. 2016;510(2):419–429. doi: 10.1016/j.ijpharm.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 61.Ai H., Jones S.A., Lvov Y.M. Biomedical applications of electrostatic layer-by-layer nano-assembly of polymers, enzymes, and nanoparticles. Cell Biochem. Biophys. 2003;39(1):23–43. doi: 10.1385/CBB:39:1:23. [DOI] [PubMed] [Google Scholar]
- 62.Norde W. Colloids and interfaces in life sciences and bionanotechnology. CRC Press; 2011. [Google Scholar]
- 63.Milner S.T. Polymer brushes. Science. 1991;251(4996):905–914. doi: 10.1126/science.251.4996.905. [DOI] [PubMed] [Google Scholar]
- 64.Advincula R.C., Brittain W.J., Caster K.C., Rühe J. Poly-mer brushes. Wiley Online Library; 2004. [Google Scholar]
- 65.Rühe J., Ballauff M., Biesalski M., Dziezok P., Gröhn F., Johannsmann D., Houbenov N., Hugenberg N., Konradi R., Minko S., Motornov M., Netz R.R., Schmidt M., Seidel C., Stamm M., Stephan T., Usov D., Zhang H. In: Polyelectrolytes with Defined Molecular Architecture I. Heidelberg S.B., Schmidt M., editors. Berlin: Hei-delberg; 2004. pp. 79–150. [Google Scholar]
- 66.Vilanova N. Voets, I.K. Soft Matter at Aqueous Interfaces. Springer; 2016. pp. 3–27. [Google Scholar]
- 67.Advincula R.C. Surface initiated polymerization from nanoparticle surfaces. J. Dispers. Sci. Technol. 2003;24(3-4):343–361. [Google Scholar]
- 68.Chanana M., Jahn S., Georgieva R., Lutz J-F., Bäumler H., Wang D. Chem. Mater. 2009;21(9):1906–1914. [Google Scholar]
- 69.Kim M., Schmitt S., Choi J., Krutty J., Gopalan P. From Self-Assembled Monolayers to Coatings: Advances in the Synthesis and Nanobio Applications of Polymer Brushes. Polymers (Basel) 2015;7(7):1346–1378. [Google Scholar]
- 70.Chen H., Zhao C., Zhang M., Chen Q., Ma J., Zheng J. Molecular understanding and structural-based design of polyacrylamides and polyacrylates as antifouling materials. Langmuir. 2016;32(14):3315–3330. doi: 10.1021/acs.langmuir.6b00386. [DOI] [PubMed] [Google Scholar]
- 71.Zhao C., Zhao J., Li X., Wu J., Chen S., Chen Q., Wang Q., Gong X., Li L., Zheng J. Probing structure-antifouling activity relationships of polyacrylamides and polyacrylates. Biomaterials. 2013;34(20):4714–4724. doi: 10.1016/j.biomaterials.2013.03.028. [DOI] [PubMed] [Google Scholar]
- 72.Yang J., Zhang M., Chen H., Chang Y., Chen Z., Zheng J. Probing the structural dependence of carbon space lengths of poly(N-hydroxyalkyl acrylamide)-based brushes on antifouling performance. Biomacromolecules. 2014;15(8):2982–2991. doi: 10.1021/bm500598a. [DOI] [PubMed] [Google Scholar]
- 73.Chen S., Li L., Zhao C., Zheng J. Surface hydration: Principles and applications toward low-fouling/nonfouling biomaterials. Polymer (Guildf.) 2010;51(23):5283–5293. [Google Scholar]
- 74.Lai W.F., He Z.D. Design and fabrication of hydrogel-based nanoparticulate systems for in vivo drug delivery. J. Control. Release. 2016;243:269–282. doi: 10.1016/j.jconrel.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 75.Karg M. Functional materials design through hydrogel encapsulation of inorganic nanoparticles: Recent developments and challenges. Macromol. Chem. Phys. 2016;217(2):242–255. [Google Scholar]
- 76.da Silva M.A., Dreiss C.A. Soft nanocomposites: Nanoparticles to tune gel properties. Polym. Int. 2016;65(3):268–279. [Google Scholar]
- 77.Matyjaszewski K., Xia J. Atom transfer radical polymerization. Chem. Rev. 2001;101(9):2921–2990. doi: 10.1021/cr940534g. [DOI] [PubMed] [Google Scholar]
- 78.Choi T-L., Grubbs R.H. Controlled living ring-opening-metathesis polymerization by a fast-initiating ruthenium catalyst. Angew. Chem. Int. Ed. Engl. 2003;42(15):1743–1746. doi: 10.1002/anie.200250632. [DOI] [PubMed] [Google Scholar]
- 79.Bielawski C.W., Grubbs R.H. Living ring-opening metathesis polymerization. Prog. Polym. Sci. 2007;32(1):1–29. [Google Scholar]
- 80.Hawker C.J., Bosman A.W., Harth E. New polymer synthesis by nitroxide mediated living radical polymerizations. Chem. Rev. 2001;101(12):3661–3688. doi: 10.1021/cr990119u. [DOI] [PubMed] [Google Scholar]
- 81.Chiefari J., Chong Y.K., Ercole F., Krstina J., Jeffery J., Le T.P.T., Mayadunne R.T.A., Meijs G.F., Moad C.L., Moad G., Rizzardo E., Thang S.H. Living free-radical polymerization by reversible addition-fragmentation chain transfer: The RAFT Process. Macromolecules. 1998;31(16):5559–5562. [Google Scholar]
- 82.Braunecker W.A., Matyjaszewski K. Controlled/living radical polymerization: Features, developments, and perspec-tives. Prog. Polym. Sci. 2007;32(1):93–146. [Google Scholar]
- 83.Bhatia S. In Natural Polymer Drug Delivery Systems: Na-noparticles, Plants, and Algae. Cham: Springer International Pub-lishing; 2016. pp. 95–118. [Google Scholar]
- 84.Chelmowski R., Köster S.D., Kerstan A., Prekelt A., Grunwald C., Winkler T., Metzler-Nolte N., Terfort A., Wöll C. Peptide-based SAMs that resist the adsorption of proteins. J. Am. Chem. Soc. 2008;130(45):14952–14953. doi: 10.1021/ja8065754. [DOI] [PubMed] [Google Scholar]
- 85.Chen S., Cao Z., Jiang S. Ultra-low fouling peptide surfaces derived from natural amino acids. Biomaterials. 2009;30(29):5892–5896. doi: 10.1016/j.biomaterials.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 86.Leng C., Buss H.G., Segalman R.A., Chen Z. Surface Structure and Hydration of Sequence-Specific Amphiphilic Polypeptoids for Antifouling/Fouling Release Applications. Langmuir. 2015;31(34):9306–9311. doi: 10.1021/acs.langmuir.5b01440. [DOI] [PubMed] [Google Scholar]
- 87.Pfeiffer C., Rehbock C., Hühn D., Carrillo-Carrion C., de Aberasturi D.J., Merk V., Barcikowski S., Parak W.J. Interaction of colloidal nanoparticles with their local environment: the (ionic) nanoenvironment around nanoparticles is different from bulk and determines the physico-chemical properties of the nanoparticles. J. R. Soc. Interface. 2014;11(96):20130931. doi: 10.1098/rsif.2013.0931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dewald I., Isakin O., Schubert J., Kraus T., Chanana M. Protein Identity and Environmental Parameters Determine the Final Physicochemical Properties of Protein-Coated Metal Nanoparticles. J. Phys. Chem. C. 2015;119(45):25482–25492. [Google Scholar]
- 89.Moerz S.T., Kraegeloh A., Chanana M., Kraus T. Formation mechanism for stable hybrid clusters of proteins and nanoparticles. ACS Nano. 2015;9(7):6696–6705. doi: 10.1021/acsnano.5b01043. [DOI] [PubMed] [Google Scholar]
- 90.Casals E., Pfaller T., Duschl A., Oostingh G.J., Puntes V. Time evolution of the nanoparticle protein corona. ACS Nano. 2010;4(7):3623–3632. doi: 10.1021/nn901372t. [DOI] [PubMed] [Google Scholar]
- 91.Rivera-Gil P., De Koker S., De Geest B.G., Parak W.J. Intracellular processing of proteins mediated by biodegradable polyelectrolyte capsules. Nano Lett. 2009;9(12):4398–4402. doi: 10.1021/nl902697j. [DOI] [PubMed] [Google Scholar]
- 92.Jiang S., Cao Z. Ultralow-fouling, functionalizable, and hydrolyzable zwitterionic materials and their derivatives for biological applications. Adv. Mater. 2010;22(9):920–932. doi: 10.1002/adma.200901407. [DOI] [PubMed] [Google Scholar]
- 93.Zhao C., Li L., Zheng J. Achieving highly effective nonfouling performance for surface-grafted poly(HPMA) via atom-transfer radical polymerization. Langmuir. 2010;26(22):17375–17382. doi: 10.1021/la103382j. [DOI] [PubMed] [Google Scholar]
- 94.Zhao C., Zheng J. Synthesis and characterization of poly(N-hydroxyethylacrylamide) for long-term antifouling ability. Biomacromolecules. 2011;12(11):4071–4079. doi: 10.1021/bm2011455. [DOI] [PubMed] [Google Scholar]
- 95.Chen H., Zhang M., Yang J., Zhao C., Hu R., Chen Q., Chang Y., Zheng J. Synthesis and characterization of antifouling poly(N-acryloylaminoethoxyethanol) with ultralow protein adsorption and cell attachment. Langmuir. 2014;30(34):10398–10409. doi: 10.1021/la502136q. [DOI] [PubMed] [Google Scholar]
- 96.Bax B.E., Bain M.D., Fairbanks L.D., Webster A.D.B., Chalmers R.A. In vitro and in vivo studies with human carrier erythrocytes loaded with polyethylene glycol-conjugated and native adenosine deaminase. Br. J. Haematol. 2000;109(3):549–554. doi: 10.1046/j.1365-2141.2000.02059.x. [DOI] [PubMed] [Google Scholar]
- 97.Hershfield M.S., Buckley R.H., Greenberg M.L., Melton A.L., Schiff R., Hatem C., Kurtzberg J., Markert M.L., Kobayashi R.H., Kobayashi A.L., Abuchowski A. Treatment of adenosine deaminase deficiency with polyethylene glycol-modified adenosine deaminase. N. Engl. J. Med. 1987;316(10):589–596. doi: 10.1056/NEJM198703053161005. [DOI] [PubMed] [Google Scholar]
- 98.Lambertini M., Ferreira A.R., Del Mastro L., Danesi R., Pronzato P. Pegfilgrastim for the prevention of chemotherapy-induced febrile neutropenia in patients with solid tumors. Expert Opin. Biol. Ther. 2015;15(12):1799–1817. doi: 10.1517/14712598.2015.1101063. [DOI] [PubMed] [Google Scholar]
- 99.Rytting M. Peg-asparaginase for acute lymphoblastic leukemia. Expert Opin. Biol. Ther. 2010;10(5):833–839. doi: 10.1517/14712591003769808. [DOI] [PubMed] [Google Scholar]
- 100.Dhillon S. Certolizumab pegol: a review of its use in patients with axial spondyloarthritis or psoriatic arthritis. Drugs. 2014;74(9):999–1016. doi: 10.1007/s40265-014-0239-z. [DOI] [PubMed] [Google Scholar]
- 101.Ahn J., Flamm S. Peginterferon-α(2b) and ribavirin. Expert Rev. Anti Infect. Ther. 2004;2(1):17–25. doi: 10.1586/14787210.2.1.17. [DOI] [PubMed] [Google Scholar]
- 102.Asmuth D.M., Utay N.S., Pollard R.B. Peginterferon α-2a for the treatment of HIV infection. Expert Opin. Investig. Drugs. 2016;25(2):249–257. doi: 10.1517/13543784.2016.1132699. [DOI] [PubMed] [Google Scholar]
- 103.Perugini M., Varelias A., Sadlon T., D’Andrea R.J. Hematopoietic growth factor mimetics: from concept to clinic. Cytokine Growth Factor Rev. 2009;20(1):87–94. doi: 10.1016/j.cytogfr.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 104.Shannon J.A., Cole S.W. Pegloticase: A novel agent for treatment-refractory gout. Ann. Pharmacother. 2012;46(3):368–376. doi: 10.1345/aph.1Q593. [DOI] [PubMed] [Google Scholar]
- 105.Valliant A., Hofmann R.M. Managing dialysis patients who develop anemia caused by chronic kidney disease: focus on peginesatide. Int. J. Nanomedicine. 2013;8:3297–3307. doi: 10.2147/IJN.S44944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sherman M.R., Saifer M.G., Perez-Ruiz F. PEG-uricase in the management of treatment-resistant gout and hyperuricemia. Adv. Drug Deliv. Rev. 2008;60(1):59–68. doi: 10.1016/j.addr.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 107.Ferrara N., Adamis A.P. Ten years of anti-vascular endothelial growth factor therapy. Nat. Rev. Drug Discov. 2016;15(6):385–403. doi: 10.1038/nrd.2015.17. [DOI] [PubMed] [Google Scholar]
- 108.Franck S.E., Muhammad A., van der Lely A.J., Neggers S.J. Combined treatment of somatostatin analogues with pegvisomant in acromegaly. Endocrine. 2016;52(2):206–213. doi: 10.1007/s12020-015-0810-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Von Hoff D.D., Mita M.M., Ramanathan R.K., Weiss G.J., Mita A.C., LoRusso P.M., Burris H.A., III, Hart L.L., Low S.C., Parsons D.M., Zale S.E., Summa J.M., Youssoufian H., Sachdev J.C. Phase I study of PSMA-targeted docetaxel-containing nanoparticle bind-014 in patients with advanced solid tumors. Clin. Cancer Res. 2016;22(13):3157–3163. doi: 10.1158/1078-0432.CCR-15-2548. [DOI] [PubMed] [Google Scholar]
- 110.Zuckerman J.E., Choi C.H., Han H., Davis M.E. Polycation-siRNA nanoparticles can disassemble at the kidney glomerular basement membrane. Proc. Natl. Acad. Sci. USA. 2012;109(8):3137–3142. doi: 10.1073/pnas.1200718109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zuckerman J.E., Gritli I., Tolcher A., Heidel J.D., Lim D., Morgan R., Chmielowski B., Ribas A., Davis M.E., Yen Y. Correlating animal and human phase Ia/Ib clinical data with CALAA-01, a targeted, polymer-based nanoparticle containing siRNA. Proc. Natl. Acad. Sci. USA. 2014;111(31):11449–11454. doi: 10.1073/pnas.1411393111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Davis M.E. The first targeted delivery of siRNA in humans via a self-assembling, cyclodextrin polymer-based nanoparticle: from concept to clinic. Mol. Pharm. 2009;6(3):659–668. doi: 10.1021/mp900015y. [DOI] [PubMed] [Google Scholar]
- 113.Lahlou A., Blanchet B., Carvalho M., Paul M., Astier A. Mechanically-induced aggregation of the monoclonal antibody cetuximab. Ann. Pharm. Fr. 2009;67(5):340–352. doi: 10.1016/j.pharma.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 114.Patravale V., Dandekar P., Jain R. Nanoparticulate systems as drug carriers: The need. 2012:1–28. [Google Scholar]
- 115.Al Zaki A., Joh D., Cheng Z., De Barros A.L.B., Kao G., Dorsey J., Tsourkas A. Gold-loaded polymeric micelles for computed tomography-guided radiation therapy treatment and radiosensitization. ACS Nano. 2014;8(1):104–112. doi: 10.1021/nn405701q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bhattacharyya J., Bellucci J.J., Weitzhandler I., McDaniel J.R., Spasojevic I., Li X., Lin C.C., Chi J.T., Chilkoti A. A paclitaxel-loaded recombinant polypeptide nanoparticle outperforms Abraxane in multiple murine cancer models. Nat. Commun. 2015;6:7939. doi: 10.1038/ncomms8939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Desai N. Challenges in development of nanoparticle-based therapeutics. AAPS J. 2012;14(2):282–295. doi: 10.1208/s12248-012-9339-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.van der Meel R., Vehmeijer L.J., Kok R.J., Storm G., van Gaal E.V. Ligand-targeted particulate nanomedicines undergoing clinical evaluation: current status. Adv. Drug Deliv. Rev. 2013;65(10):1284–1298. doi: 10.1016/j.addr.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 119.Wang Y.X. Superparamagnetic iron oxide based MRI contrast agents: Current status of clinical application. Quant. Imaging Med. Surg. 2011;1(1):35–40. doi: 10.3978/j.issn.2223-4292.2011.08.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kudasheva D.S., Lai J., Ulman A., Cowman M.K. Structure of carbohydrate-bound polynuclear iron oxyhydroxide nanoparticles in parenteral formulations. J. Inorg. Biochem. 2004;98(11):1757–1769. doi: 10.1016/j.jinorgbio.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 121.Jahn M.R., Andreasen H.B., Fütterer S., Nawroth T., Schünemann V., Kolb U., Hofmeister W., Muñoz M., Bock K., Meldal M., Langguth P. A comparative study of the physicochemical properties of iron isomaltoside 1000 (Monofer), a new intravenous iron preparation and its clinical implications. Eur. J. Pharm. Biopharm. 2011;78(3):480–491. doi: 10.1016/j.ejpb.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 122.Reddy L.H., Arias J.L., Nicolas J., Couvreur P. Magnetic nanoparticles: design and characterization, toxicity and biocompatibility, pharmaceutical and biomedical applications. Chem. Rev. 2012;112(11):5818–5878. doi: 10.1021/cr300068p. [DOI] [PubMed] [Google Scholar]
- 123.Philippe Dubois O.C. Jean-Marie Raquez In Handbook of Ring-Opening Polymerization. Wiley-VCH Verlag GmbH & Co. KGaA; 2009. [Google Scholar]
- 124.Unsworth L.D., Sheardown H., Brash J.L. Protein resistance of surfaces prepared by sorption of end-thiolated poly(ethylene glycol) to gold: effect of surface chain density. Langmuir. 2005;21(3):1036–1041. doi: 10.1021/la047672d. [DOI] [PubMed] [Google Scholar]
- 125.Gillich T., Acikgöz C., Isa L., Schlüter A.D., Spencer N.D., Textor M. PEG-stabilized core-shell nanoparticles: Impact of linear versus dendritic polymer shell architecture on colloidal properties and the reversibility of temperature-induced aggregation. ACS Nano. 2013;7(1):316–329. doi: 10.1021/nn304045q. [DOI] [PubMed] [Google Scholar]
- 126.Pelaz B., del Pino P., Maffre P., Hartmann R., Gallego M., Rivera-Fernández S., de la Fuente J.M., Nienhaus G.U., Parak W.J. Surface functionalization of nanoparticles with polyethylene glycol: Effects on protein adsorption and cellular uptake. ACS Nano. 2015;9(7):6996–7008. doi: 10.1021/acsnano.5b01326. [DOI] [PubMed] [Google Scholar]
- 127.Abuchowski A., van Es T., Palczuk N.C., Davis F.F. Alteration of immunological properties of bovine serum albumin by covalent attachment of polyethylene glycol. J. Biol. Chem. 1977;252(11):3578–3581. [PubMed] [Google Scholar]
- 128.Abuchowski A., McCoy J.R., Palczuk N.C., van Es T., Davis F.F. Effect of covalent attachment of polyethylene glycol on immunogenicity and circulating life of bovine liver catalase. J. Biol. Chem. 1977;252(11):3582–3586. [PubMed] [Google Scholar]
- 129.Lowe S., O’Brien-Simpson N.M., Connal L.A. Antibio-fouling polymer interfaces: Poly(ethylene glycol) and other promising candidates. Polym. Chem. 2015;6(2):198–212. [Google Scholar]
- 130.Zelikin A.N., Ehrhardt C., Healy A.M. Materials and methods for delivery of biological drugs. Nat. Chem. 2016;8(11):997–1007. doi: 10.1038/nchem.2629. [DOI] [PubMed] [Google Scholar]
- 131.Chirino A.J., Ary M.L., Marshall S.A. Minimizing the immunogenicity of protein therapeutics. Drug Discov. Today. 2004;9(2):82–90. doi: 10.1016/S1359-6446(03)02953-2. [DOI] [PubMed] [Google Scholar]
- 132.Zhang P., Han H. Compact PEGylated polymer-caged quantum dots with improved stability. Colloids Surf. A Physicochem. Eng. Asp. 2012;402:72–79. [Google Scholar]
- 133.Ulusoy M., Jonczyk R., Walter J.G., Springer S., Lavrentieva A., Stahl F., Green M., Scheper T. Aqueous synthesis of PEGylated quantum dots with increased colloidal stability and reduced cytotoxicity. Bioconjug. Chem. 2016;27(2):414–426. doi: 10.1021/acs.bioconjchem.5b00491. [DOI] [PubMed] [Google Scholar]
- 134.Yu W.W., Chang E., Falkner J.C., Zhang J., Al-Somali A.M., Sayes C.M., Johns J., Drezek R., Colvin V.L. Forming biocompatible and nonaggregated nanocrystals in water using amphiphilic polymers. J. Am. Chem. Soc. 2007;129(10):2871–2879. doi: 10.1021/ja067184n. [DOI] [PubMed] [Google Scholar]
- 135.Xie J., Xu C., Kohler N., Hou Y., Sun S. Controlled PEGylation of monodisperse FE3O4 nanoparticles for reduced non-specific uptake by macrophage cells. Adv. Mater. 2007;19(20):3163–3166. [Google Scholar]
- 136.Tromsdorf U.I., Bruns O.T., Salmen S.C., Beisiegel U., Weller H. A highly effective, nontoxic T1 MR contrast agent based on ultrasmall PEGylated iron oxide nanoparticles. Nano Lett. 2009;9(12):4434–4440. doi: 10.1021/nl902715v. [DOI] [PubMed] [Google Scholar]
- 137.Lee J.E., Lee N., Kim H., Kim J., Choi S.H., Kim J.H., Kim T., Song I.C., Park S.P., Moon W.K., Hyeon T. Uniform mesoporous dye-doped silica nanoparticles decorated with multiple magnetite nanocrystals for simultaneous enhanced magnetic resonance imaging, fluorescence imaging, and drug delivery. J. Am. Chem. Soc. 2010;132(2):552–557. doi: 10.1021/ja905793q. [DOI] [PubMed] [Google Scholar]
- 138.He Q., Zhang J., Shi J., Zhu Z., Zhang L., Bu W., Guo L., Chen Y. The effect of PEGylation of mesoporous silica nanoparticles on nonspecific binding of serum proteins and cellular responses. Biomaterials. 2010;31(6):1085–1092. doi: 10.1016/j.biomaterials.2009.10.046. [DOI] [PubMed] [Google Scholar]
- 139.Lin Y-S., Haynes C.L. Impacts of mesoporous silica nanoparticle size, pore ordering, and pore integrity on hemolytic activity. J. Am. Chem. Soc. 2010;132(13):4834–4842. doi: 10.1021/ja910846q. [DOI] [PubMed] [Google Scholar]
- 140.Chang-Hai W., Chi-Jen L., Cheng-Liang W., Tzu-En H., Judy M.O., Lee K.H., Hwu Y., Chung-Shi Y., Ru-Shi L., Hong-Ming L., Jung-Ho J., Margaritondo G. Optimizing the size and surface properties of polyethylene glycol (PEG)-gold nanoparticles by intense x-ray irradiation. J. Phys. D Appl. Phys. 2008;41(19):195301 [Google Scholar]
- 141.Dreaden E.C., Austin L.A., Mackey M.A., El-Sayed M.A. Size matters: gold nanoparticles in targeted cancer drug delivery. Ther. Deliv. 2012;3(4):457–478. doi: 10.4155/tde.12.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chanana M., Correa-Duarte M.A., Liz-Marzán L.M. Insulin-coated gold nanoparticles: A plasmonic device for studying metal-protein interactions. Small. 2011;7(18):2650–2660. doi: 10.1002/smll.201100735. [DOI] [PubMed] [Google Scholar]
- 143.Chanana M., Rivera Gil P., Correa-Duarte M.A., Liz-Marzán L.M., Parak W.J. Physicochemical properties of protein-coated gold nanoparticles in biological fluids and cells before and after proteolytic digestion. Angew. Chem. Int. Ed. Engl. 2013;52(15):4179–4183. doi: 10.1002/anie.201208019. [DOI] [PubMed] [Google Scholar]
- 144.Höller R.P., Dulle M., Thomä S., Mayer M., Steiner A.M., Förster S., Fery A., Kuttner C., Chanana M. Protein-assisted assembly of modular 3d plasmonic raspberry-like core/satellite nanoclusters: correlation of structure and optical properties. ACS Nano. 2016;10(6):5740–5750. doi: 10.1021/acsnano.5b07533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Strozyk M.S., Chanana M., Pastoriza-Santos I., Pérez-Juste J., Liz-Marzán L.M. Protein/polymer-based dual-responsive gold nanoparticles with ph-dependent thermal sensitivity. Adv. Funct. Mater. 2012;22(7):1436–1444. [Google Scholar]
- 146.Tebbe M., Kuttner C., Männel M., Fery A., Chanana M. Colloidally stable and surfactant-free protein-coated gold nanorods in biological media. ACS Appl. Mater. Interfaces. 2015;7(10):5984–5991. doi: 10.1021/acsami.5b00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Schöttler S., Becker G., Winzen S., Steinbach T., Mohr K., Landfester K., Mailänder V., Wurm F.R. Protein adsorption is required for stealth effect of poly(ethylene glycol)- and poly(phosphoester)-coated nanocarriers. Nat. Nanotechnol. 2016;11(4):372–377. doi: 10.1038/nnano.2015.330. [DOI] [PubMed] [Google Scholar]
- 148.Versiani A.F., Andrade L.M., Martins E.M.N., Scalzo S., Geraldo J.M., Chaves C.R., Ferreira D.C., Ladeira M., Guatimosim S., Ladeira L.O., da Fonseca F.G. Gold na-noparticles and their applications in biomedicine. Future Virol. 2016;11(4):293–309. [Google Scholar]
- 149.Abadeer N.S., Murphy C.J. Recent progress in cancer thermal therapy using gold nanoparticles. J. Phys. Chem. C. 2016;120(9):4691–4716. [Google Scholar]
- 150.Pissuwan D., Kumagai Y., Smith N.I. Effect of surface-modified gold nanorods on the inflammatory cytokine response in macrophage cells. Particle & Particle Systems Characterization. 2013;30(5):427–433. [Google Scholar]
- 151.Niidome T., Yamagata M., Okamoto Y., Akiyama Y., Takahashi H., Kawano T., Katayama Y., Niidome Y. PEG-modified gold nanorods with a stealth character for in vivo applications. J. Control. Release. 2006;114(3):343–347. doi: 10.1016/j.jconrel.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 152.Kinnear C., Dietsch H., Clift M.J., Endes C., Rothen-Rutishauser B., Petri-Fink A. Gold nanorods: controlling their surface chemistry and complete detoxification by a two-step place exchange. Angew. Chem. Int. Ed. Engl. 2013;52(7):1934–1938. doi: 10.1002/anie.201208568. [DOI] [PubMed] [Google Scholar]
- 153.Wang W., Ji X., Burns H., Mattoussi H. A multi-coordinating polymer ligand optimized for the functionalization of metallic nanocrystals and nanorods. Faraday Discuss. 2016;191:481–494. doi: 10.1039/c6fd00056h. [DOI] [PubMed] [Google Scholar]
- 154.Richter A.W., Åkerblom E. Antibodies against polyethylene glycol produced in animals by immunization with monomethoxy polyethylene glycol modified proteins. Int. Arch. Allergy Appl. Immunol. 1983;70(2):124–131. doi: 10.1159/000233309. [DOI] [PubMed] [Google Scholar]
- 155.Zhang P., Sun F., Liu S., Jiang S. Anti-PEG antibodies in the clinic: Current issues and beyond PEGylation. J. Control.Release. 2016;244(Pt B):184–193. doi: 10.1016/j.jconrel.2016.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cho W.S., Cho M., Jeong J., Choi M., Cho H.Y., Han B.S., Kim S.H., Kim H.O., Lim Y.T., Chung B.H., Jeong J. Acute toxicity and pharmacokinetics of 13 nm-sized PEG-coated gold nanoparticles. Toxicol. Appl. Pharmacol. 2009;236(1):16–24. doi: 10.1016/j.taap.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 157.Pelegri-O’Day E.M., Lin E.W., Maynard H.D. Therapeutic protein-polymer conjugates: advancing beyond PEGylation. J. Am. Chem. Soc. 2014;136(41):14323–14332. doi: 10.1021/ja504390x. [DOI] [PubMed] [Google Scholar]
- 158.Shah S., Prematta T., Adkinson N.F., Ishmael F.T. Hypersensitivity to polyethylene glycols. J. Clin. Pharmacol. 2013;53(3):352–355. doi: 10.1177/0091270012447122. [DOI] [PubMed] [Google Scholar]
- 159.Hamad I., Hunter A.C., Szebeni J., Moghimi S.M. Poly(ethylene glycol)s generate complement activation products in human serum through increased alternative pathway turnover and a MASP-2-dependent process. Mol. Immunol. 2008;46(2):225–232. doi: 10.1016/j.molimm.2008.08.276. [DOI] [PubMed] [Google Scholar]
- 160.Crouzet C., Decker C., Marchal J. Caractérisation de réac-tions primaires de dégradation oxydante au cours de l’au-toxydation des poly(oxyéthylène)s à 25°C: Étude en solution aqueuse avec amorçage par radiolyse du solvant, 8. Étude ci-nétique en fonction du ph compris entre 1 et 13. Makromol. Chem. 1976;177(1):145–157. [Google Scholar]
- 161.Hamburger R., Azaz E., Donbrow M. Autoxidation of polyoxyethylenic non-ionic surfactants and of polyethylene glycols. Pharm. Acta Helv. 1975;50(1-2):10–17. [PubMed] [Google Scholar]
- 162.Gerhardt W., Martens C. Zur Oxydation von Polyeth-ylenoxiden und Polyethylenoxidethern; Die Bildung von Ac-etaldehyd bei der Oxydation von Diethylenglycol mit Sauer-stoff. Zeitschrift für Chemie. 1985;25(4):143–143. [Google Scholar]
- 163.Talarico T., Swank A., Privalle C. Autoxidation of pyridoxalated hemoglobin polyoxyethylene conjugate. Biochem. Biophys. Res. Commun. 1998;250(2):354–358. doi: 10.1006/bbrc.1998.9312. [DOI] [PubMed] [Google Scholar]
- 164.Herold D.A., Keil K., Bruns D.E. Oxidation of polyethylene glycols by alcohol dehydrogenase. Biochem. Pharmacol. 1989;38(1):73–76. doi: 10.1016/0006-2952(89)90151-2. [DOI] [PubMed] [Google Scholar]
- 165.Jokerst J.V., Lobovkina T., Zare R.N., Gambhir S.S. Nanoparticle PEGylation for imaging and therapy. Nanomedicine (Lond.) 2011;6(4):715–728. doi: 10.2217/nnm.11.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hak S., Helgesen E., Hektoen H.H., Huuse E.M., Jarzyna P.A., Mulder W.J.M., Haraldseth O. Davies, Cde.L. The effect of nanoparticle polyethylene glycol surface density on ligand-directed tumor targeting studied in vivo by dual modality imaging. ACS Nano. 2012;6(6):5648–5658. doi: 10.1021/nn301630n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hoogenboom R. Temperature-responsive polymers: Properties, synthesis and applications. 2014:15–44. [Google Scholar]
- 168.Han S., Hagiwara M., Ishizone T. Synthesis of thermally sensitive water-soluble polymethacrylates by living anion-ic polymerizations of oligo(ethylene glycol) methyl ether methacrylates. Macromolecules. 2003;36(22):8312–8319. [Google Scholar]
- 169.Ishizone T., Seki A., Hagiwara M., Han S., Yokoyama H., Oyane A., Deffieux A., Carlotti S. Anionic polymeri-zations of oligo(ethylene glycol) alkyl ether methacrylates: Effect of side chain length and ω-Alkyl group of side chain on cloud point in water. Macromolecules. 2008;41(8):2963–2967. [Google Scholar]
- 170.Edwards E.W., Chanana M., Wang D., Möhwald H. Stimuli-responsive reversible transport of nanoparticles across water/oil interfaces. Angew. Chem. Int. Ed. Engl. 2008;47(2):320–323. doi: 10.1002/anie.200702597. [DOI] [PubMed] [Google Scholar]
- 171.Stocco A., Chanana M., Su G., Cernoch P., Binks B.P., Wang D. Bidirectional nanoparticle crossing of oil-water interfaces induced by different stimuli: insight into phase transfer. Angew. Chem. Int. Ed. Engl. 2012;51(38):9647–9651. doi: 10.1002/anie.201203493. [DOI] [PubMed] [Google Scholar]
- 172.Vancoillie G., Frank D., Hoogenboom R. Thermorespon-sive poly(oligo ethylene glycol acrylates). Prog. Polym. Sci. 2014;39(6):1074–1095. [Google Scholar]
- 173.Weber C., Hoogenboom R., Schubert U.S. Temperature responsive bio-compatible polymers based on poly(ethylene oxide) and poly(2-oxazoline)s. Prog. Polym. Sci. 2012;37(5):686–714. [Google Scholar]
- 174.Tedja R., Soeriyadi A.H., Whittaker M.R., Lim M., Mar-quis C., Boyer C., Davis T.P., Amal R. Effect of TiO2 nanoparticle surface functionalization on protein adsorption, cellular uptake and cytotoxicity: The attachment of PEG comb polymers using catalytic chain transfer and thiol–ene chemis-try. Polym. Chem. 2012;3(10):2743. [Google Scholar]
- 175.Basuki J.S., Esser L., Zetterlund P.B., Whittaker M.R., Boyer C., Davis T.P. Grafting of P(OEGA) onto magnetic nanoparticles using cu(0) mediated polymerization: comparing grafting “from” and “to” approaches in the search for the optimal material design of nanoparticle MRI contrast agents. Macromolecules. 2013;46(15):6038–6047. [Google Scholar]
- 176.Shen W., Chang Y., Liu G., Wang H., Cao A., An Z. Biocompatible, antifouling, and thermosensitive core−shell nanogels synthesized by RAFT aqueous dispersion polymerization. Macromolecules. 2011;44(8):2524–2530. [Google Scholar]
- 177.Boyer C., Whittaker M.R., Luzon M., Davis T.P. Design and synthesis of dual thermoresponsive and antifouling hybrid polymer/gold nanoparticles. Macromolecules. 2009;42(18):6917–6926. [Google Scholar]
- 178.Lutz J-F., Stiller S., Hoth A., Kaufner L., Pison U., Cartier R. One-pot synthesis of pegylated ultrasmall iron-oxide nanoparticles and their in vivo evaluation as magnetic resonance imaging contrast agents. Biomacromolecules. 2006;7(11):3132–3138. doi: 10.1021/bm0607527. [DOI] [PubMed] [Google Scholar]
- 179.Abulateefeh S.R., Spain S.G., Thurecht K.J., Aylott J.W., Chan W.C., Garnett M.C., Alexander C. Enhanced uptake of nanoparticle drug carriers via a thermoresponsive shell en-hances cytotoxicity in a cancer cell line. Biomater. Sci. 2013;1(4):434. doi: 10.1039/c2bm00184e. [DOI] [PubMed] [Google Scholar]
- 180.Gambinossi F., Chanana M., Mylon S.E., Ferri J.K. Pro-gramming nanoparticle aggregation kinetics with poly(MeO2MA-co-OEGMA) copolymers. Soft Matter. 2013;9(46):11046. [Google Scholar]
- 181.Delplace V., Nicolas J. Degradable vinyl polymers for biomedical applications. Nat. Chem. 2015;7(10):771–784. doi: 10.1038/nchem.2343. [DOI] [PubMed] [Google Scholar]
- 182.Lutz J-F. Polymerization of oligo(ethylene glycol) (meth)acrylates: Toward new generations of smart biocom-patible materials. J. Polym. Sci. A Polym. Chem. 2008;46(11):3459–3470. [Google Scholar]
- 183.Lowe A.B., McCormick C.L. Reversible addition–fragmentation chain transfer (RAFT) radical polymerization and the synthesis of water-soluble (co)polymers under ho-mogeneous conditions in organic and aqueous media. Prog. Polym. Sci. 2007;32(3):283–351. [Google Scholar]
- 184.Xia Y., Burke N.A.D., Stöver H.D.H. (N-isopropylacrylamide) Prepared by Atom Transfer Radical Polymerization. Macromolecules. 2006;39(6):2275–2283. [Google Scholar]
- 185.Fernández-López C., Polavarapu L., Solís D.M., Taboada J.M., Obelleiro F., Contreras-Cáceres R., Pastoriza-Santos I., Pérez-Juste J. Gold Nanorod-pNIPAM Hybrids with Reversible Plasmon Coupling: Synthesis, Modeling, and SERS Properties. ACS Appl. Mater. Interfaces. 2015;7(23):12530–12538. doi: 10.1021/am5087209. [DOI] [PubMed] [Google Scholar]
- 186.Gorelikov I., Field L.M., Kumacheva E. Hybrid microgels photoresponsive in the near-infrared spectral range. J. Am. Chem. Soc. 2004;126(49):15938–15939. doi: 10.1021/ja0448869. [DOI] [PubMed] [Google Scholar]
- 187.Lu Y., Yuan J., Polzer F., Drechsler M., Preussner J. In situ growth of catalytic active Au-Pt bimetallic nanorods in thermoresponsive core-shell microgels. ACS Nano. 2010;4(12):7078–7086. doi: 10.1021/nn102622d. [DOI] [PubMed] [Google Scholar]
- 188.Karg M., Lu Y., Carbó-Argibay E., Pastoriza-Santos I., Pérez-Juste J., Liz-Marzán L.M., Hellweg T. Multiresponsive hybrid colloids based on gold nanorods and poly(NIPAM-co-allylacetic acid) microgels: temperature- and pH-tunable plasmon resonance. Langmuir. 2009;25(5):3163–3167. doi: 10.1021/la803458j. [DOI] [PubMed] [Google Scholar]
- 189.Karg M., Pastoriza-Santos I., Pérez-Juste J., Hellweg T., Liz-Marzán L.M. Nanorod-coated PNIPAM microgels: thermoresponsive optical properties. Small. 2007;3(7):1222–1229. doi: 10.1002/smll.200700078. [DOI] [PubMed] [Google Scholar]
- 190.Das M., Sanson N., Fava D., Kumacheva E. Microgels loaded with gold nanorods: photothermally triggered volume transitions under physiological conditions. Langmuir. 2007;23(1):196–201. doi: 10.1021/la061596s. [DOI] [PubMed] [Google Scholar]
- 191.Fernández-López C., Pérez-Balado C., Pérez-Juste J. Pas-toriza-Santos, I.; de Lera, Á.R.; Liz-Marzán, L.M., A general LbL strategy for the growth of pNIPAM microgels on Au nanoparticles with arbitrary shapes. Soft Matter. 2012;8(15):4165–4170. [Google Scholar]
- 192.Rodríguez-Fernández J., Fedoruk M., Hrelescu C., Lutich A.A., Feldmann J. Triggering the volume phase transition of core-shell Au nanorod-microgel nanocomposites with light. Nanotechnology. 2011;22(24):245708. doi: 10.1088/0957-4484/22/24/245708. [DOI] [PubMed] [Google Scholar]
- 193.Dong X., Zou X., Liu X., Lu P., Yang J., Lin D., Zhang L., Zha L. Temperature-tunable plasmonic property and SERS activity of the monodisperse thermo-responsive com-posite microgels with core-shell structure based on gold nanorod as core. Colloids Surf. A Physicochem. Eng. Asp. 2014;452:46–50. [Google Scholar]
- 194.Kawano T., Niidome Y., Mori T., Katayama Y., Niidome T. PNIPAM gel-coated gold nanorods for targeted delivery responding to a near-infrared laser. Bioconjug. Chem. 2009;20(2):209–212. doi: 10.1021/bc800480k. [DOI] [PubMed] [Google Scholar]
- 195.Marcelo G., Fernández-García M. Direct preparation of PNIPAM coating gold nanoparticles by catechol redox and surface adhesion chemistry. RSC Advances. 2014;4(23):11740. [Google Scholar]
- 196.Contreras-Cáceres R., Sánchez-Iglesias A., Karg M. Pas-toriza-Santos, I.; Pérez-Juste, J.; Pacifico, J.; Hellweg, T.; Fernández-Barbero, A.; Liz-Marzán, L.M. Encapsulation and Growth of Gold Nanoparticles in Thermoresponsive Mi-crogels. Adv. Mater. 2008;20(9):1666–1670. [Google Scholar]
- 197.Contreras-Cáceres R., Pacifico J., Pastoriza-Santos I. Pé-rez-Juste, J.; Fernández-Barbero, A.; Liz-Marzán, L.M. Au@pNIPAM thermosensitive nanostructures: control over shell cross-linking, overall dimensions, and core growth. Adv. Funct. Mater. 2009;19(19):3070–3076. [Google Scholar]
- 198.Zyuzin M.V., Honold T., Carregal-Romero S., Kantner K., Karg M., Parak W.J. Influence of temperature on the colloidal stability of polymer-coated gold nanoparticles in cell culture media. Small. 2016;12(13):1723–1731. doi: 10.1002/smll.201503232. [DOI] [PubMed] [Google Scholar]
- 199.Zhang Z., Wang J., Nie X., Wen T., Ji Y., Wu X., Zhao Y., Chen C. Near infrared laser-induced targeted cancer therapy using thermoresponsive polymer encapsulated gold nanorods. J. Am. Chem. Soc. 2014;136(20):7317–7326. doi: 10.1021/ja412735p. [DOI] [PubMed] [Google Scholar]
- 200.Shamim N., Hong L., Hidajat K., Uddin M.S. Thermosensitive-polymer-coated magnetic nanoparticles: adsorption and desorption of bovine serum albumin. J. Colloid Interface Sci. 2006;304(1):1–8. doi: 10.1016/j.jcis.2006.08.047. [DOI] [PubMed] [Google Scholar]
- 201.Shamim N., Hong L., Hidajat K., Uddin M.S. Thermosensitive-polymer-coated magnetic nanoparticles: adsorption and desorption of bovine serum albumin. J. Colloid Interface Sci. 2006;304(1):1–8. doi: 10.1016/j.jcis.2006.08.047. [DOI] [PubMed] [Google Scholar]
- 202.Teodorescu M., Bercea M. Poly(vinylpyrrolidone) - A Versatile Polymer for Biomedical and Beyond Medical Ap-plications. Polym. Plast. Technol. Eng. 2015;54(9):923–943. [Google Scholar]
- 203.Lu X., Gong S., Meng L., Li C., Yang S., Zhang L. Controllable synthesis of poly(N-vinylpyrrolidone) and its block copolymers by atom transfer radical polymerization. Polymer (Guildf.) 2007;48(10):2835–2842. [Google Scholar]
- 204.Devasia R., Bindu R.L., Borsali R., Mougin N., Gnanou Y. Controlled Radical Polymerization ofN-Vinylpyrrolidone by Reversible Addition-Fragmentation Chain Transfer Pro-cess. Macromol. Symp. 2005;229(1):8–17. [Google Scholar]
- 205.Pound-Lana G. Klumperman, B.Controlled/Living Radi-cal Polymerization: Progress in RAFT, DT, NMP & OMRP. Vol. 1024. American Chemical Society; 2009. pp. 167–179. [Google Scholar]
- 206.Aroua S., Tiu E.G.V., Ayer M., Ishikawa T., Yamakoshi Y. RAFT synthesis of poly(vinylpyrrolidone) amine and preparation of a water-soluble C60-PVP conjugate. Polym. Chem. 2015;6(14):2616–2619. [Google Scholar]
- 207.Ray B., Kotani M., Yamago S. Highly Controlled Synthe-sis of Poly(N-vinylpyrrolidone) and Its Block Copolymers by Organostibine-Mediated Living Radical Polymerization. Macromolecules. 2006;39(16):5259–5265. [Google Scholar]
- 208.Yamago S., Ray B., Iida K., Yoshida J., Tada T., Yoshizawa K., Kwak Y., Goto A., Fukuda T. Highly versatile organostibine mediators for living radical polymerization. J. Am. Chem. Soc. 2004;126(43):13908–13909. doi: 10.1021/ja044787v. [DOI] [PubMed] [Google Scholar]
- 209.Lu G., Li S., Guo Z., Farha O.K., Hauser B.G., Qi X., Wang Y., Wang X., Han S., Liu X., DuChene J.S., Zhang H., Zhang Q., Chen X., Ma J., Loo S.C., Wei W.D., Yang Y., Hupp J.T., Huo F. Imparting functionality to a metal-organic framework material by controlled nanoparticle encapsulation. Nat. Chem. 2012;4(4):310–316. doi: 10.1038/nchem.1272. [DOI] [PubMed] [Google Scholar]
- 210.Xiong Y., Cai H., Wiley B.J., Wang J., Kim M.J., Xia Y. Synthesis and mechanistic study of palladium nanobars and nanorods. J. Am. Chem. Soc. 2007;129(12):3665–3675. doi: 10.1021/ja0688023. [DOI] [PubMed] [Google Scholar]
- 211.Jadhav S.V., Nikam D.S., Khot V.M., Thorat N.D., Phadatare M.R., Ningthoujam R.S., Salunkhe A.B., Pawar S.H. Studies on colloidal stability of PVP-coated LSMO nanoparticles for magnetic fluid hyperthermia. New J. Chem. 2013;37(10):3121. [Google Scholar]
- 212.Huynh K.A., Chen K.L. Aggregation kinetics of citrate and polyvinylpyrrolidone coated silver nanoparticles in monovalent and divalent electrolyte solutions. Environ. Sci. Technol. 2011;45(13):5564–5571. doi: 10.1021/es200157h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Wu Z., Chen H., Liu X., Zhang Y., Li D., Huang H. Protein adsorption on poly(N-vinylpyrrolidone)-modified silicon surfaces prepared by surface-initiated atom transfer radical polymerization. Langmuir. 2009;25(5):2900–2906. doi: 10.1021/la8037523. [DOI] [PubMed] [Google Scholar]
- 214.Yuan H., Qian B., Zhang W., Lan M. Protein adsorption resistance of PVP-modified polyurethane film prepared by surface-initiated atom transfer radical polymerization. Appl. Surf. Sci. 2016;363:483–489. [Google Scholar]
- 215.Covaliu C.I., Jitaru I., Paraschiv G., Vasile E., Biriş S-Ş., Diamandescu L., Ionita V., Iovu H. Core–shell hybrid na-nomaterials based on CoFe2O4 particles coated with PVP or PEG biopolymers for applications in biomedicine. Powder Technol. 2013;237:415–426. [Google Scholar]
- 216.Gaaz T.S., Sulong A.B., Akhtar M.N., Kadhum A.A., Mohamad A.B., Al-Amiery A.A. Properties and applications of polyvinyl alcohol, halloysite nanotubes and their nanocomposites. Molecules. 2015;20(12):22833–22847. doi: 10.3390/molecules201219884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.DeMerlis C.C., Schoneker D.R. Review of the oral toxicity of polyvinyl alcohol (PVA). Food Chem. Toxicol. 2003;41(3):319–326. doi: 10.1016/s0278-6915(02)00258-2. [DOI] [PubMed] [Google Scholar]
- 218.Asghar W., El Assal R., Shafiee H., Anchan R.M., Demirci U. Preserving human cells for regenerative, reproductive, and transfusion medicine. Biotechnol. J. 2014;9(7):895–903. doi: 10.1002/biot.201300074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Guo Z., Zhang D., Wei S., Wang Z., Karki A.B., Li Y., Bernazzani P., Young D.P., Gomes J.A., Cocke D.L., Ho T.C. Effects of iron oxide nanoparticles on polyvinyl alco-hol: interfacial layer and bulk nanocomposites thin film. J. Nanopart. Res. 2009;12(7):2415–2426. [Google Scholar]
- 220.Westedt U., Kalinowski M., Wittmar M., Merdan T., Unger F., Fuchs J., Schäller S., Bakowsky U., Kissel T. Poly(vinyl alcohol)-graft-poly(lactide-co-glycolide) nanoparticles for local delivery of paclitaxel for restenosis treatment. J. Control. Release. 2007;119(1):41–51. doi: 10.1016/j.jconrel.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 221.Liong M., Shao H., Haun J.B., Lee H., Weissleder R. Carboxymethylated polyvinyl alcohol stabilizes doped ferrofluids for biological applications. Adv. Mater. 2010;22(45):5168–5172. doi: 10.1002/adma.201002219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Synatschke Christopher V., Plamper Felix A., Müller Axel H.E. In. Nachr. Chem. 2013;61:1008. [Google Scholar]
- 223.Fales A.M., Norton S.J., Crawford B.M., DeLacy B.G., Vo-Dinh T. Fano resonance in a gold nanosphere with a J-aggregate coating. Phys. Chem. Chem. Phys. 2015;17(38):24931–24936. doi: 10.1039/c5cp03277f. [DOI] [PubMed] [Google Scholar]
- 224.Chithrani B.D., Ghazani A.A., Chan W.C.W. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006;6(4):662–668. doi: 10.1021/nl052396o. [DOI] [PubMed] [Google Scholar]
- 225.Shimoni O., Yan Y., Wang Y., Caruso F. Shape-dependent cellular processing of polyelectrolyte capsules. ACS Nano. 2013;7(1):522–530. doi: 10.1021/nn3046117. [DOI] [PubMed] [Google Scholar]
- 226.Zan X., Garapaty A., Champion J.A. Engineering polyelectrolyte capsules with independently controlled size and shape. Langmuir. 2015;31(27):7601–7608. doi: 10.1021/acs.langmuir.5b01578. [DOI] [PubMed] [Google Scholar]
- 227.Wurster E.C., Liebl R., Michaelis S., Robelek R., Wastl D.S., Giessibl F.J., Goepferich A., Breunig M. Oligolayer-coated nanoparticles: impact of surface topography at the nanobio interface. ACS Appl. Mater. Interfaces. 2015;7(15):7891–7900. doi: 10.1021/am508435j. [DOI] [PubMed] [Google Scholar]
- 228.Srinivasan S., Sawyer P.N. Role of surface charge of the blood vessel wall, blood cells, and prosthetic materials in intravascular thrombosis. J. Colloid Interface Sci. 1970;32(3):456–463. doi: 10.1016/0021-9797(70)90131-1. [DOI] [PubMed] [Google Scholar]
- 229.Alkilany A.M., Nagaria P.K., Hexel C.R., Shaw T.J., Murphy C.J., Wyatt M.D. Cellular uptake and cytotoxicity of gold nanorods: molecular origin of cytotoxicity and surface effects. Small. 2009;5(6):701–708. doi: 10.1002/smll.200801546. [DOI] [PubMed] [Google Scholar]
- 230.Chanana M., Gliozzi A., Diaspro A., Chodnevskaja I., Huewel S., Moskalenko V., Ulrichs K., Galla H-J., Krol S. Interaction of polyelectrolytes and their composites with living cells. Nano Lett. 2005;5(12):2605–2612. doi: 10.1021/nl0521219. [DOI] [PubMed] [Google Scholar]
- 231.Leonov A.P., Zheng J., Clogston J.D., Stern S.T., Patri A.K., Wei A. Detoxification of gold nanorods by treatment with polystyrenesulfonate. ACS Nano. 2008;2(12):2481–2488. doi: 10.1021/nn800466c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Jans H., Jans K., Lagae L., Borghs G., Maes G., Huo Q. Poly(acrylic acid)-stabilized colloidal gold nanoparticles: Synthesis and properties. Nanotechnology. 2010;21(45):455702. doi: 10.1088/0957-4484/21/45/455702. [DOI] [PubMed] [Google Scholar]
- 233.Chanteau B., Fresnais J., Berret J.F. Electrosteric enhanced stability of functional sub-10 nm cerium and iron oxide particles in cell culture medium. Langmuir. 2009;25(16):9064–9070. doi: 10.1021/la900833v. [DOI] [PubMed] [Google Scholar]
- 234.Safi M., Courtois J., Seigneuret M., Conjeaud H., Berret J.F. The effects of aggregation and protein corona on the cellular internalization of iron oxide nanoparticles. Biomaterials. 2011;32(35):9353–9363. doi: 10.1016/j.biomaterials.2011.08.048. [DOI] [PubMed] [Google Scholar]
- 235.Shah N.J. Hammond Layer-by-Layer Films for Biomedical Applications. Wiley-VCH Verlag GmbH & Co. KGaA; 2015. pp. 131–170. [Google Scholar]
- 236.Poon Z., Chang D., Zhao X., Hammond P.T. Layer-by-layer nanoparticles with a pH-sheddable layer for in vivo targeting of tumor hypoxia. ACS Nano. 2011;5(6):4284–4292. doi: 10.1021/nn200876f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Dreaden E.C., Morton S.W., Shopsowitz K.E., Choi J-H., Deng Z.J., Cho N-J., Hammond P.T. Bimodal tumor-targeting from microenvironment responsive hyaluronan layer-by-layer (LbL) nanoparticles. ACS Nano. 2014;8(8):8374–8382. doi: 10.1021/nn502861t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Dreaden E.C., Kong Y.W., Morton S.W., Correa S., Choi K.Y., Shopsowitz K.E., Renggli K., Drapkin R., Yaffe M.B., Hammond P.T. Tumor-targeted synergistic blockade of MAPK and PI3K from a layer-by-layer nanoparticle. Clin. Cancer Res. 2015;21(19):4410–4419. doi: 10.1158/1078-0432.CCR-15-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Yang X-Z., Du J-Z., Dou S., Mao C-Q., Long H-Y., Wang J. Sheddable ternary nanoparticles for tumor acidity-targeted siRNA delivery. ACS Nano. 2012;6(1):771–781. doi: 10.1021/nn204240b. [DOI] [PubMed] [Google Scholar]
- 240.Kim J.S., Rieter W.J., Taylor K.M.L., An H., Lin W., Lin W. Self-assembled hybrid nanoparticles for cancer-specific multimodal imaging. J. Am. Chem. Soc. 2007;129(29):8962–8963. doi: 10.1021/ja073062z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Xie L., Tong W., Yu D., Xu J., Li J., Gao C. Bovine serum albumin nanoparticles modified with multilayers and aptamers for pH-responsive and targeted anti-cancer drug delivery. J. Mater. Chem. 2012;22(13):6053. [Google Scholar]
- 242.Liao W-C., Lu C-H., Hartmann R., Wang F., Sohn Y.S., Parak W.J., Willner I. Adenosine Triphosphate-Triggered Release of Macromolecular and Nanoparticle Loads from Aptamer/DNA-Cross-Linked Microcapsules. ACS Nano. 2015;9(9):9078–9086. doi: 10.1021/acsnano.5b03223. [DOI] [PubMed] [Google Scholar]
- 243.Masereel B., Dinguizli M., Bouzin C., Moniotte N., Feron O., Gallez B., Vander Borght T., Michiels C., Lu-cas S. Antibody immobilization on gold nanoparticles coated layer-by-layer with polyelectrolytes. J. Nanopart. Res. 2010;13(4):1573–1580. [Google Scholar]
- 244.Boyer C., Bousquet A., Rondolo J., Whittaker M.R., Stenzel M.H., Davis T.P. Glycopolymer decoration of gold nanoparticles using a lbl approach. Macromolecules. 2010;43(8):3775–3784. [Google Scholar]
- 245.Müller M. In: Polyelectrolyte Complexes in the Dispersed and Solid State II: Application Aspects; Müller, M. Heidelberg S.B., editor. Berlin, Heidelberg: 2014. pp. 197–260. [Google Scholar]
- 246.Schlenoff J.B., Rmaile A.H., Bucur C.B. Hydration contributions to association in polyelectrolyte multilayers and complexes: Visualizing hydrophobicity. J. Am. Chem. Soc. 2008;130(41):13589–13597. doi: 10.1021/ja802054k. [DOI] [PubMed] [Google Scholar]
- 247.Schlenoff J.B. Zwitteration: coating surfaces with zwitterionic functionality to reduce nonspecific adsorption. Langmuir. 2014;30(32):9625–9636. doi: 10.1021/la500057j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Pombo García K., Zarschler K., Barbaro L., Barreto J.A., O’Malley W., Spiccia L., Stephan H., Graham B. Zwitterionic-coated “stealth” nanoparticles for biomedical applications: recent advances in countering biomolecular corona formation and uptake by the mononuclear phagocyte system. Small. 2014;10(13):2516–2529. doi: 10.1002/smll.201303540. [DOI] [PubMed] [Google Scholar]
- 249.Yang W., Zhang L., Wang S., White A.D., Jiang S. Functionalizable and ultra stable nanoparticles coated with zwitterionic poly(carboxybetaine) in undiluted blood serum. Biomaterials. 2009;30(29):5617–5621. doi: 10.1016/j.biomaterials.2009.06.036. [DOI] [PubMed] [Google Scholar]
- 250.Robinson K.J., Coffey J.W., Muller D.A., Young P.R., Kendall M.A., Thurecht K.J., Grondahl L., Corrie S.R. Comparison between polyethylene glycol and zwitterionic polymers as antifouling coatings on wearable devices for selective antigen capture from biological tissue. 2015;10(4):04A305. doi: 10.1116/1.4932055. [DOI] [PubMed] [Google Scholar]
- 251.Higaki Y., Kobayashi M., Murakami D., Takahara A. Antifouling behavior of polymer brush immobilized surfaces. Polym. J. 2016;48(4):325–331. [Google Scholar]
- 252.Hartleb W., Saar J.S., Zou P., Lienkamp K. Just antimicrobial is not enough: Toward bifunctional polymer surfaces with dual antimicrobial and protein-repellent functionality. Macromol. Chem. Phys. 2016;217(2):225–231. [Google Scholar]
- 253.Zhang L., Cao Z., Bai T., Carr L., Ella-Menye J.R., Irvin C., Ratner B.D., Jiang S. Zwitterionic hydrogels implanted in mice resist the foreign-body reaction. Nat. Biotechnol. 2013;31(6):553–556. doi: 10.1038/nbt.2580. [DOI] [PubMed] [Google Scholar]
- 254.Zhang L., Liu Y., Liu G., Xu D., Liang S., Zhu X., Lu Y., Wang H. Prolonging the plasma circulation of proteins by nano-encapsulation with phosphorylcholine-based poly-mer. Nano Res. 2016;9(8):2424–2432. [Google Scholar]
- 255.Sun R., Du X.J., Sun C.Y., Shen S., Liu Y., Yang X.Z., Bao Y., Zhu Y.H., Wang J. A block copolymer of zwitterionic polyphosphoester and polylactic acid for drug delivery. Biomater. Sci. 2015;3(7):1105–1113. doi: 10.1039/c4bm00430b. [DOI] [PubMed] [Google Scholar]
- 256.Zhang P., Sun F., Tsao C., Liu S., Jain P., Sinclair A., Hung H.C., Bai T., Wu K., Jiang S. Zwitterionic gel encapsulation promotes protein stability, enhances pharmacokinetics, and reduces immunogenicity. Proc. Natl. Acad. Sci. USA. 2015;112(39):12046–12051. doi: 10.1073/pnas.1512465112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Wu L., Ni C., Zhang L., Shi G., Bai X., Zhou Y., He F. Surface charge convertible and biodegradable synthetic zwitterionic nanoparticles for enhancing cellular drug uptake. Macromol. Biosci. 2016;16(3):308–313. doi: 10.1002/mabi.201500299. [DOI] [PubMed] [Google Scholar]
- 258.Yuan Y.Y., Mao C.Q., Du X.J., Du J.Z., Wang F., Wang J. Surface charge switchable nanoparticles based on zwitterionic polymer for enhanced drug delivery to tumor. Adv. Mater. 2012;24(40):5476–5480. doi: 10.1002/adma.201202296. [DOI] [PubMed] [Google Scholar]
- 259.Peng Z., Liu H. Bottom-up Nanofabrication Using DNA Nanostructures. Chem. Mater. 2016;28(4):1012–1021. [Google Scholar]
- 260.Watson J.D., Crick F.H.C. Molecular structure of nucleic acids; a structure for deoxyribose nucleic acid. Nature. 1953;171(4356):737–738. doi: 10.1038/171737a0. [DOI] [PubMed] [Google Scholar]
- 261.Seeman N.C. Nucleic acid junctions and lattices. J. Theor. Biol. 1982;99(2):237–247. doi: 10.1016/0022-5193(82)90002-9. [DOI] [PubMed] [Google Scholar]
- 262.Wang Y., Wang Y., Zheng X., Ducrot É., Yodh J.S., Weck M., Pine D.J. Crystallization of DNA-coated colloids. Nat. Commun. 2015;6:7253. doi: 10.1038/ncomms8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Kanaras A.G., Wang Z., Bates A.D., Cosstick R., Brust M. Towards multistep nanostructure synthesis: Programmed enzymatic self-assembly of DNA/gold systems. Angew. Chem. Int. Ed. Engl. 2003;42(2):191–194. doi: 10.1002/anie.200390075. [DOI] [PubMed] [Google Scholar]
- 264.Uthaman S., Lee S.J., Cherukula K., Cho C.S., Park I.K. Polysaccharide-coated magnetic nanoparticles for imaging and gene therapy. BioMed Res. Int. 2015;2015:959175. doi: 10.1155/2015/959175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Robyt J.F., Walseth T.F. Production, purification, and properties of dextransucrase from Leuconostoc mesenteroides NRRL B-512F. Carbohydr. Res. 1979;68(1):95–111. doi: 10.1016/s0008-6215(00)84059-8. [DOI] [PubMed] [Google Scholar]
- 266.Lindberg B., Svensson S. Structural studies on dextran from Leuconostoc mesenteroides NRRL B-512. Acta Chem. Scand. 1968;22(6) [Google Scholar]
- 267.Jeanes A., Haynes W.C., Wilham C.A., Rankin J.C., Mel-vin E.H., Austin M.J., Cluskey J.E., Fisher B.E., Tsuchi-ya H.M., Rist C.E. Characterization and classification of dextrans from ninety-six strains of bacteria1b. J. Am. Chem. Soc. 1954;76(20):5041–5052. [Google Scholar]
- 268.Sidebotham R.L. Dextrans. Adv. Carbohydr. Chem. Biochem. 1974;30:371–444. doi: 10.1016/s0065-2318(08)60268-1. [DOI] [PubMed] [Google Scholar]
- 269.Takagi K., Ioroi R., Uchimura T., Kozaki M., Komagata K. Purification and some properties of dextransucrase from Streptococcus bovis 148. J. Ferment. Bioeng. 1994;77(5):551–553. [Google Scholar]
- 270.Chludzinski A.M., Germaine G.R., Schachtele C.F. Purification and properties of dextransucrase from Streptococcus mutans. J. Bacteriol. 1974;118(1):1–7. doi: 10.1128/jb.118.1.1-7.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Hehre E.J. Natural synthesis of low molecular weight (clinical type) dextran by a Streptococcus strain. J. Biol. Chem. 1956;222(2):739–750. [PubMed] [Google Scholar]
- 272.Ruckel E.R., Schuerch C. Chemical synthesis of a dextran model, poly-α-(1 → 6)-anhydro-D-glucopyranose. Biopolymers. 1967;5(6):515–523. [Google Scholar]
- 273.Barker S.A., Bourne E.J., Bruce G.T., Stacey M. Immunopolysaccharides. Part XI. Structure of an Acetobacter capsulatum dextran Journal of the Chemical Society (Resumed) 1958;(0):4414–4416. [Google Scholar]
- 274.de Belder A.N. Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH Verlag GmbH & Co. KGaA; 2000. [Google Scholar]
- 275.Leung A.D., Wong K.H., Tien J. Plasma expanders stabilize human microvessels in microfluidic scaffolds. J. Biomed. Mater. Res. A. 2012;100(7):1815–1822. doi: 10.1002/jbm.a.34137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Bowman H.W. Clinical evaluation of dextran as a plasma volume expander. J. Am. Med. Assoc. 1953;153(1):24–26. doi: 10.1001/jama.1953.02940180026009. [DOI] [PubMed] [Google Scholar]
- 277.Couch N.P. The clinical status of low molecular weight dextran: a critical review. Clin. Pharmacol. Ther. 1965;6(5):656–665. doi: 10.1002/cpt196565656. [DOI] [PubMed] [Google Scholar]
- 278.Ashwood-Smith M.J., Warby C., Connor K.W., Becker G. Low-temperature preservation of mammalian cells in tissue culture with polyvinylpyrrolidone (PVP), dextrans, and hydroxyethyl starch (HES). Cryobiology. 1972;9(5):441–449. doi: 10.1016/0011-2240(72)90161-7. [DOI] [PubMed] [Google Scholar]
- 279.Rothman S., Adelson E., Schwebel A., Langdell R.D. Adsorption of carbon-14 dextran to human blood platelets and red blood cells, in vitro. Vox Sang. 1957;2(2):104–109. doi: 10.1111/j.1423-0410.1957.tb03441.x. [DOI] [PubMed] [Google Scholar]
- 280.Bäumler H., Donath E., Krabi A., Knippel W., Budde A., Kiesewetter H. Electrophoresis of human red blood cells and platelets. Evidence for depletion of dextran. Biorheology. 1996;33(4-5):333–351. doi: 10.1016/0006-355x(96)00026-1. [DOI] [PubMed] [Google Scholar]
- 281.Levi M., Jonge, editors. Clinical relevance of the effects of plasma expanders on coagulation. Semin. Thromb. Hemost. 2007;33(8):810–815. doi: 10.1055/s-2007-1000370. [DOI] [PubMed] [Google Scholar]
- 282.Ravikumar C., Kumar S., Bandyopadhyaya R. Aggrega-tion of dextran coated magnetic nanoparticles in aqueous me-dium: Experiments and Monte Carlo simulation. Colloids Surf. A Physicochem. Eng. Asp. 2012;403:1–6. [Google Scholar]
- 283.Yu F., Yang V.C. Size-tunable synthesis of stable superparamagnetic iron oxide nanoparticles for potential biomedical applications. J. Biomed. Mater. Res. A. 2010;92(4):1468–1475. doi: 10.1002/jbm.a.32489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Qi J., Yao P., He F., Yu C., Huang C. Nanoparticles with dextran/chitosan shell and BSA/chitosan core--doxorubicin loading and delivery. Int. J. Pharm. 2010;393(1-2):176–184. doi: 10.1016/j.ijpharm.2010.03.063. [DOI] [PubMed] [Google Scholar]
- 285.Ning S., Huang Q., Sun X., Li C., Zhang Y., Li J., Liu Y-N. Carboxymethyl dextran-coated liposomes: Toward a robust drug delivery platform. Soft Matter. 2011;7(19):9394. [Google Scholar]
- 286.Creixell M., Bohórquez A.C., Torres-Lugo M., Rinaldi C. EGFR-targeted magnetic nanoparticle heaters kill cancer cells without a perceptible temperature rise. ACS Nano. 2011;5(9):7124–7129. doi: 10.1021/nn201822b. [DOI] [PubMed] [Google Scholar]
- 287.Passirani C., Barratt G., Devissaguet J-P., Labarre D. Long-circulating nanoparticles bearing heparin or dextran covalently bound to poly(methyl methacrylate). Pharm. Res. 1998;15(7):1046–1050. doi: 10.1023/a:1011930127562. [DOI] [PubMed] [Google Scholar]
- 288.Alhareth K., Vauthier C., Bourasset F., Gueutin C., Ponchel G., Moussa F. Conformation of surface-decorating dextran chains affects the pharmacokinetics and biodistribution of doxorubicin-loaded nanoparticles. Eur. J. Pharm. Biopharm. 2012;81(2):453–457. doi: 10.1016/j.ejpb.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 289.Malzahn K., Jamieson W.D., Dröge M., Mailänder V., Jenkins A.T.A., Weiss C.K., Landfester K. Advanced dex-tran based nanogels for fighting Staphylococcus aureus in-fections by sustained zinc release. J. Mater. Chem. B Mater. Biol. Med. 2014;2(15):2175–2183. doi: 10.1039/c3tb21335h. [DOI] [PubMed] [Google Scholar]
- 290.Wang Q., Perez J.M., Webster T.J. Inhibited growth of Pseudomonas aeruginosa by dextran- and polyacrylic acid-coated ceria nanoparticles. Int. J. Nanomedicine. 2013;8:3395–3399. doi: 10.2147/IJN.S50292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Mandal S.M., Porto W.F., De D., Phule A., Korpole S., Ghosh A.K., Roy S.K., Franco O.L. Screening of serine protease inhibitors with antimicrobial activity using iron oxide nanoparticles functionalized with dextran conjugated trypsin and in silico analyses of bacterial serine protease inhibition. Analyst (Lond.) 2014;139(2):464–472. doi: 10.1039/c3an01132a. [DOI] [PubMed] [Google Scholar]
- 292.Kiruthika V., Maya S., Suresh M.K., Kumar V.A., Jayakumar R., Biswas R. Comparative efficacy of chloramphenicol loaded chondroitin sulfate and dextran sulfate nanoparticles to treat intracellular Salmonella infections. Colloids Surf. B Biointerfaces. 2015;127:33–40. doi: 10.1016/j.colsurfb.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 293.Delgado D., Gascón A.R., Del Pozo-Rodríguez A., Echevarría E., Ruiz de Garibay A.P., Rodríguez J.M., Solinís M.A. Dextran-protamine-solid lipid nanoparticles as a non-viral vector for gene therapy: In vitro characterization and in vivo transfection after intravenous administration to mice. Int. J. Pharm. 2012;425(1-2):35–43. doi: 10.1016/j.ijpharm.2011.12.052. [DOI] [PubMed] [Google Scholar]
- 294.Rahman M., Laurent N., Tawil S., Yahia L.H., Mahmoudi M. Protein-Nanoparticle Interactions: The Bio-Nano Interface. Berlin: HeidelbergSpringer Berlin Heidelberg; 2013. pp. 21–44. [Google Scholar]
- 295.Cristallini C., Barbani N., Bianchi F., Silvestri D., Guerra G.D. Biodegradable bioartificial materials made by chitosan and poly (vinyl alcohol). Part II: enzymatic degradability and drug-releasing ability. Biomed. Eng. (N.Y.) 2008;20(5):321–328. [Google Scholar]
- 296.Li X., Min M., Du N., Gu Y., Hode T., Naylor M., Chen D., Nordquist R.E., Chen W.R. Chitin, chitosan, and glycated chitosan regulate immune responses: The novel adjuvants for cancer vaccine. Clin. Dev. Immunol. 2013;2013:387023. doi: 10.1155/2013/387023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Kean T., Thanou M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug Deliv. Rev. 2010;62(1):3–11. doi: 10.1016/j.addr.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 298.Rodrigues S., Dionísio M., López C.R., Grenha A. Biocompatibility of chitosan carriers with application in drug delivery. J. Funct. Biomater. 2012;3(3):615–641. doi: 10.3390/jfb3030615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Azuma K., Osaki T., Minami S., Okamoto Y. Anticancer and anti-inflammatory properties of chitin and chitosan oligosaccharides. J. Funct. Biomater. 2015;6(1):33–49. doi: 10.3390/jfb6010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Martins A.F., Facchi S.P., Follmann H.D., Pereira A.G., Rubira A.F., Muniz E.C. Antimicrobial activity of chitosan derivatives containing N-quaternized moieties in its backbone: a review. Int. J. Mol. Sci. 2014;15(11):20800–20832. doi: 10.3390/ijms151120800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Ngo D.H., Kim S.K. Antioxidant effects of chitin, chitosan, and their derivatives. Adv. Food Nutr. Res. 2014;73:15–31. doi: 10.1016/B978-0-12-800268-1.00002-0. [DOI] [PubMed] [Google Scholar]
- 302.Shen C-R., Wu S-T., Tsai Z-T., Wang J-J., Yen T-C., Tsai J-S., Shih M-F., Liu C-L. Characterization of quaternized chitosan-stabilized iron oxide nanoparticles as a novel potential magnetic resonance imaging contrast agent for cell tracking. Polym. Int. 2011;60(6):945–950. [Google Scholar]
- 303.Goycoolea F.M., Valle-Gallego A., Stefani R., Menchic-chi B., David L., Rochas C., Santander-Ortega M.J., Alonso M.J. Chitosan-based nanocapsules: Physical charac-terization, stability in biological media and capsaicin encapsu-lation. Colloid Polym. Sci. 2012;290(14):1423–1434. [Google Scholar]
- 304.López-León T., Carvalho E.L., Seijo B., Ortega-Vinuesa J.L., Bastos-González D. Physicochemical characterization of chitosan nanoparticles: Electrokinetic and stability behavior. J. Colloid Interface Sci. 2005;283(2):344–351. doi: 10.1016/j.jcis.2004.08.186. [DOI] [PubMed] [Google Scholar]
- 305.Santander-Ortega M.J., Lozano-López M.V., Bastos-González D., Peula-García J.M., Ortega-Vinuesa J.L. Novel core-shell lipid-chitosan and lipid-poloxamer nanocap-sules: Stability by hydration forces. Colloid Polym. Sci. 2009;288(2):159–172. [Google Scholar]
- 306.López-León T., Santander-Ortega M.J., Ortega-Vinuesa J.L., Bastos-González D. Hofmeister effects in colloidal systems: Influence of the surface nature. J. Phys. Chem. C. 2008;112(41):16060–16069. [Google Scholar]
- 307.Schatz C., Viton C., Delair T., Pichot C., Domard A. Typical physicochemical behaviors of chitosan in aqueous solution. Biomacromolecules. 2003;4(3):641–648. doi: 10.1021/bm025724c. [DOI] [PubMed] [Google Scholar]
- 308.Lamarque G., Lucas J-M., Viton C., Domard A. Physicochemical behavior of homogeneous series of acetylated chitosans in aqueous solution: Role of various structural parameters. Biomacromolecules. 2005;6(1):131–142. doi: 10.1021/bm0496357. [DOI] [PubMed] [Google Scholar]
- 309.Quemeneur F., Rammal A., Rinaudo M., Pépin-Donat B. Large and giant vesicles “decorated” with chitosan: effects of pH, salt or glucose stress, and surface adhesion. Biomacromolecules. 2007;8(8):2512–2519. doi: 10.1021/bm061227a. [DOI] [PubMed] [Google Scholar]
- 310.Bernkop-Schnürch A., Dünnhaupt S. Chitosan-based drug delivery systems. Eur. J. Pharm. Biopharm. 2012;81(3):463–469. doi: 10.1016/j.ejpb.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 311.Dai T., Tanaka M., Huang Y.Y., Hamblin M.R. Chitosan preparations for wounds and burns: Antimicrobial and wound-healing effects. Expert Rev. Anti Infect. Ther. 2011;9(7):857–879. doi: 10.1586/eri.11.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Jayakumar R., Menon D., Manzoor K., Nair S.V., Tamu-ra H. Biomedical applications of chitin and chitosan based nanomaterials-A short review. Carbohydr. Polym. 2010;82(2):227–232. [Google Scholar]
- 313.Kuroiwa T., Noguchi Y., Nakajima M., Sato S., Muka-taka S., Ichikawa S. Production of chitosan oligosaccha-rides using chitosanase immobilized on amylose-coated mag-netic nanoparticles. Process Biochem. 2008;43(1):62–69. [Google Scholar]
- 314.Zhu L., Ma J., Jia N., Zhao Y., Shen H. Chitosan-coated magnetic nanoparticles as carriers of 5-fluorouracil: preparation, characterization and cytotoxicity studies. Colloids Surf. B Biointerfaces. 2009;68(1):1–6. doi: 10.1016/j.colsurfb.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 315.Liu G. W.; De Yao, K. Chitosan and its derivatives--a promising non-viral vector for gene transfection. J. Control. Release. 2002;83(1):1–11. doi: 10.1016/s0168-3659(02)00144-x. [DOI] [PubMed] [Google Scholar]
- 316.van der Lubben I.M., Verhoef J.C., Borchard G., Junginger H.E. Chitosan and its derivatives in mucosal drug and vaccine delivery. Eur. J. Pharm. Sci. 2001;14(3):201–207. doi: 10.1016/s0928-0987(01)00172-5. [DOI] [PubMed] [Google Scholar]
- 317.Mao S., Sun W., Kissel T. Chitosan-based formulations for delivery of DNA and siRNA. Adv. Drug Deliv. Rev. 2010;62(1):12–27. doi: 10.1016/j.addr.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 318.Coppi G., Iannuccelli V., Leo E., Bernabei M.T., Cameroni R. Chitosan-alginate microparticles as a protein carrier. Drug Dev. Ind. Pharm. 2001;27(5):393–400. doi: 10.1081/ddc-100104314. [DOI] [PubMed] [Google Scholar]
- 319.Ma Z., Lim T.M., Lim L.Y. Pharmacological activity of peroral chitosan-insulin nanoparticles in diabetic rats. Int. J. Pharm. 2005;293(1-2):271–280. doi: 10.1016/j.ijpharm.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 320.Mukhopadhyay P., Mishra R., Rana D., Kundu P.P. Strategies for effective oral insulin delivery with modified chitosan nanoparticles: A review. Prog. Polym. Sci. 2012;37(11):1457–1475. [Google Scholar]
- 321.Pan Y., Li Y.J., Zhao H.Y., Zheng J.M., Xu H., Wei G., Hao J.S., Cui F.D. Bioadhesive polysaccharide in protein delivery system: chitosan nanoparticles improve the intestinal absorption of insulin in vivo. Int. J. Pharm. 2002;249(1-2):139–147. doi: 10.1016/s0378-5173(02)00486-6. [DOI] [PubMed] [Google Scholar]
- 322.Wong T.W. Chitosan and its use in design of insulin delivery system. Recent Pat. Drug Deliv. Formul. 2009;3(1):8–25. doi: 10.2174/187221109787158346. [DOI] [PubMed] [Google Scholar]
- 323.Diop M., Auberval N., Viciglio A., Langlois A., Bietiger W., Mura C., Peronet C., Bekel A., Julien David D., Zhao M., Pinget M., Jeandidier N., Vauthier C., Marchioni E., Frere Y., Sigrist S. Design, characterisation, and bioefficiency of insulin-chitosan nanoparticles after stabilisation by freeze-drying or cross-linking. Int. J. Pharm. 2015;491(1-2):402–408. doi: 10.1016/j.ijpharm.2015.05.065. [DOI] [PubMed] [Google Scholar]
- 324.Park J.H., Saravanakumar G., Kim K., Kwon I.C. Targeted delivery of low molecular drugs using chitosan and its derivatives. Adv. Drug Deliv. Rev. 2010;62(1):28–41. doi: 10.1016/j.addr.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 325.Zhou X., Zhang X., Yu X., Zha X., Fu Q., Liu B., Wang X., Chen Y., Chen Y., Shan Y., Jin Y., Wu Y., Liu J., Kong W., Shen J. The effect of conjugation to gold nanoparticles on the ability of low molecular weight chitosan to transfer DNA vaccine. Biomaterials. 2008;29(1):111–117. doi: 10.1016/j.biomaterials.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 326.Bhattarai S.R., Kim S.Y., Jang K.Y., Lee K.C., Yi H.K., Lee D.Y., Kim H.Y., Hwang P.H. Laboratory formulated magnetic nanoparticles for enhancement of viral gene expression in suspension cell line. J. Virol. Methods. 2008;147(2):213–218. doi: 10.1016/j.jviromet.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 327.Kumar A., Jena P.K., Behera S., Lockey R.F., Mohapatra S., Mohapatra S. Multifunctional magnetic nanoparticles for targeted delivery. Nanomedicine (Lond.) 2010;6(1):64–69. doi: 10.1016/j.nano.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Son S., Lim S.M., Chae S.Y., Kim K., Park E.J., Lee K.C., Na D.H. Mono-lithocholated exendin-4-loaded glycol chitosan nanoparticles with prolonged antidiabetic effects. Int. J. Pharm. 2015;495(1):81–86. doi: 10.1016/j.ijpharm.2015.08.084. [DOI] [PubMed] [Google Scholar]
- 329.Wang S., Jiang T., Ma M., Hu Y., Zhang J. Preparation and evaluation of anti-neuroexcitation peptide (ANEP) loaded N-trimethyl chitosan chloride nanoparticles for brain-targeting. Int. J. Pharm. 2010;386(1-2):249–255. doi: 10.1016/j.ijpharm.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 330.Cheong S.J., Lee C.M., Kim S.L., Jeong H.J., Kim E.M., Park E.H., Kim D.W., Lim S.T., Sohn M.H. Superparamagnetic iron oxide nanoparticles-loaded chitosan-linoleic acid nanoparticles as an effective hepatocyte-targeted gene delivery system. Int. J. Pharm. 2009;372(1-2):169–176. doi: 10.1016/j.ijpharm.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 331.Bhattarai S.R., Bahadur K.C.R., Aryal S., Khil M.S., Kim H.Y. N-Acylated chitosan stabilized iron oxide nanoparticles as a novel nano-matrix and ceramic modification. Carbohydr. Polym. 2007;69(3):467–477. [Google Scholar]
- 332.Arami H., Stephen Z., Veiseh O., Zhang M. Chitosan for Biomaterials. 2011;243:163–184. [Google Scholar]
- 333.Bodnar M., Hartmann J.F., Borbely J. Synthesis and study of cross-linked chitosan-N-poly(ethylene glycol) nanoparticles. Biomacromolecules. 2006;7(11):3030–3036. doi: 10.1021/bm0605053. [DOI] [PubMed] [Google Scholar]
- 334.Qu G., Yao Z., Zhang C., Wu X., Ping Q. PEG conjugated N-octyl-O-sulfate chitosan micelles for delivery of paclitaxel: In vitro characterization and in vivo evaluation. Eur. J. Pharm. Sci. 2009;37(2):98–105. doi: 10.1016/j.ejps.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 335.van Schilfgaarde R., de Vos P. Factors influencing the properties and performance of microcapsules for immunoprotection of pancreatic islets. J. Mol. Med. (Berl.) 1999;77(1):199–205. doi: 10.1007/s001090050336. [DOI] [PubMed] [Google Scholar]
- 336.Dusseault J., Tam S.K., Ménard M., Polizu S., Jourdan G., Yahia L., Hallé J.P. Evaluation of alginate purification methods: effect on polyphenol, endotoxin, and protein contamination. J. Biomed. Mater. Res. A. 2006;76(2):243–251. doi: 10.1002/jbm.a.30541. [DOI] [PubMed] [Google Scholar]
- 337.Sreeram K. Studies on the nature of interaction of iron(III) with alginates. Biochim. Biophys. Acta. 2004;1670(2):121–125. doi: 10.1016/j.bbagen.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 338.Menakbi C., Quignard F., Mineva T. Complexation of trivalent metal cations to mannuronate type alginate models from a density functional study. J. Phys. Chem. B. 2016;120(15):3615–3623. doi: 10.1021/acs.jpcb.6b00472. [DOI] [PubMed] [Google Scholar]
- 339.Morgan S.M., Al-Shamkhani A., Callant D., Schacht E., Woodley J.F., Duncan R. Alginates as drug carriers: Covalent attachment of alginates to therapeutic agents containing primary amine groups. Int. J. Pharm. 1995;122(1):121–128. [Google Scholar]
- 340.Eiselt P., Lee K.Y., Mooney D.J. Rigidity of Two-Component Hydrogels Prepared from Alginate and Poly(ethylene glycol)−Diamines. Macromolecules. 1999;32(17):5561–5566. [Google Scholar]
- 341.Yang J., Chen S., Fang Y. Viscosity study of interactions between sodium alginate and CTAB in dilute solutions at different pH values. Carbohydr. Polym. 2009;75(2):333–337. [Google Scholar]
- 342.Stockwell A.F., Davis S.S., Walker S.E. In vitro evaluation of alginate gel systems as sustained release drug delivery systems. J. Control. Release. 1986;3(1):167–175. [Google Scholar]
- 343.Gu F., Amsden B., Neufeld R. Sustained delivery of vascular endothelial growth factor with alginate beads. J. Control. Release. 2004;96(3):463–472. doi: 10.1016/j.jconrel.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 344.Kodiyan A., Silva E.A., Kim J., Aizenberg M., Mooney D.J. Surface modification with alginate-derived polymers for stable, protein-repellent, long-circulating gold nanoparticles. ACS Nano. 2012;6(6):4796–4805. doi: 10.1021/nn205073n. [DOI] [PubMed] [Google Scholar]
- 345.Rowley J.A., Madlambayan G., Mooney D.J. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials. 1999;20(1):45–53. doi: 10.1016/s0142-9612(98)00107-0. [DOI] [PubMed] [Google Scholar]
- 346.Bagre A.P., Jain K., Jain N.K. Alginate coated chitosan core shell nanoparticles for oral delivery of enoxaparin: In vitro and in vivo assessment. Int. J. Pharm. 2013;456(1):31–40. doi: 10.1016/j.ijpharm.2013.08.037. [DOI] [PubMed] [Google Scholar]
- 347.Bar-Shir A., Avram L., Yariv-Shoushan S., Anaby D., Cohen S., Segev-Amzaleg N., Frenkel D., Sadan O., Offen D., Cohen Y. Alginate-coated magnetic nanoparticles for noninvasive MRI of extracellular calcium. NMR Biomed. 2014;27(7):774–783. doi: 10.1002/nbm.3117. [DOI] [PubMed] [Google Scholar]
- 348.Chong B.F., Blank L.M., Mclaughlin R., Nielsen L.K. Microbial hyaluronic acid production. Appl. Microbiol. Biotechnol. 2005;66(4):341–351. doi: 10.1007/s00253-004-1774-4. [DOI] [PubMed] [Google Scholar]
- 349.Oh E.J., Park K., Kim K.S., Kim J., Yang J.A., Kong J.H., Lee M.Y., Hoffman A.S., Hahn S.K. Target specific and long-acting delivery of protein, peptide, and nucleotide therapeutics using hyaluronic acid derivatives. J. Control. Release. 2010;141(1):2–12. doi: 10.1016/j.jconrel.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 350.Stern R., Asari A.A., Sugahara K.N. Hyaluronan fragments: an information-rich system. Eur. J. Cell Biol. 2006;85(8):699–715. doi: 10.1016/j.ejcb.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 351.Feinberg R.N., Beebe D.C. Hyaluronate in vasculogenesis. Science. 1983;220(4602):1177–1179. doi: 10.1126/science.6857242. [DOI] [PubMed] [Google Scholar]
- 352.West D.C., Hampson I.N., Arnold F., Kumar S. Angiogenesis induced by degradation products of hyaluronic acid. Science. 1985;228(4705):1324–1326. doi: 10.1126/science.2408340. [DOI] [PubMed] [Google Scholar]
- 353.Takahashi Y., Li L., Kamiryo M., Asteriou T., Moustakas A., Yamashita H., Heldin P. Hyaluronan fragments induce endothelial cell differentiation in a CD44- and CXCL1/GRO1-dependent manner. J. Biol. Chem. 2005;280(25):24195–24204. doi: 10.1074/jbc.M411913200. [DOI] [PubMed] [Google Scholar]
- 354.Luo Y., Bernshaw N.J., Lu Z-R., Kopecek J., Prestwich G.D. Targeted delivery of doxorubicin by HPMA copolymer-hyaluronan bioconjugates. Pharm. Res. 2002;19(4):396–402. doi: 10.1023/a:1015170907274. [DOI] [PubMed] [Google Scholar]
- 355.Doh K-O., Yeo Y. Application of polysaccharides for surface modification of nanomedicines. Ther. Deliv. 2012;3(12):1447–1456. doi: 10.4155/tde.12.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Rivkin I., Cohen K., Koffler J., Melikhov D., Peer D., Margalit R. Paclitaxel-clusters coated with hyaluronan as selective tumor-targeted nanovectors. Biomaterials. 2010;31(27):7106–7114. doi: 10.1016/j.biomaterials.2010.05.067. [DOI] [PubMed] [Google Scholar]
- 357.Luo Y., Prestwich G.D. Synthesis and selective cytotoxicity of a hyaluronic acid-antitumor bioconjugate. Bioconjug. Chem. 1999;10(5):755–763. doi: 10.1021/bc9900338. [DOI] [PubMed] [Google Scholar]
- 358.Bajaj G., Kim M.R., Mohammed S.I., Yeo Y. Hyaluronic acid-based hydrogel for regional delivery of paclitaxel to intraperitoneal tumors. J. Control. Release. 2012;158(3):386–392. doi: 10.1016/j.jconrel.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Wu J.L., Liu C.G., Wang X.L., Huang Z.H. Preparation and characterization of nanoparticles based on histidine-hyaluronic acid conjugates as doxorubicin carriers. J. Mater. Sci. Mater. Med. 2012;23(8):1921–1929. doi: 10.1007/s10856-012-4665-8. [DOI] [PubMed] [Google Scholar]
- 360.Eliaz R.E., Szoka F.C. Cancer Res. 2001;61(6):2592–2601. [PubMed] [Google Scholar]
- 361.Choi K.Y., Yoon H.Y., Kim J-H., Bae S.M., Park R-W., Kang Y.M., Kim I-S., Kwon I.C., Choi K., Jeong S.Y., Kim K., Park J.H. Smart nanocarrier based on PEGylated hyaluronic acid for cancer therapy. ACS Nano. 2011;5(11):8591–8599. doi: 10.1021/nn202070n. [DOI] [PubMed] [Google Scholar]
- 362.Han S.E., Kang H., Shim G.Y., Kim S.J., Choi H.G., Kim J., Hahn S.K., Oh Y.K. Cationic derivatives of biocompatible hyaluronic acids for delivery of siRNA and antisense oligonucleotides. J. Drug Target. 2009;17(2):123–132. doi: 10.1080/10611860802472461. [DOI] [PubMed] [Google Scholar]
- 363.Park K., Hong S.W., Hur W., Lee M.Y., Yang J.A., Kim S.W., Yoon S.K., Hahn S.K. Target specific systemic delivery of TGF-β siRNA/(PEI-SS)-g-HA complex for the treatment of liver cirrhosis. Biomaterials. 2011;32(21):4951–4958. doi: 10.1016/j.biomaterials.2011.03.044. [DOI] [PubMed] [Google Scholar]
- 364.Jiang G., Park K., Kim J., Kim K.S., Hahn S.K. Target specific intracellular delivery of siRNA/PEI-HA complex by receptor mediated endocytosis. Mol. Pharm. 2009;6(3):727–737. doi: 10.1021/mp800176t. [DOI] [PubMed] [Google Scholar]
- 365.Xu P., Quick G.K., Yeo Y. Gene delivery through the use of a hyaluronate-associated intracellularly degradable crosslinked polyethyleneimine. Biomaterials. 2009;30(29):5834–5843. doi: 10.1016/j.biomaterials.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Stryer L., Berg J. Tymoczko, J. Biochemistry. 5th ed. W H Freeman; 2002. [Google Scholar]
- 367.Pino P.d., Pelaz B., Zhang Q., Maffre P., Nienhaus G.U., Parak W.J. Protein corona formation around nanoparticles - from the past to the future. Mater. Horiz. 2014;1(3):301–313. [Google Scholar]
- 368.Ashraf S., Abbasi A.Z., Pfeiffer C., Hussain S.Z., Khalid Z.M., Gil P.R., Parak W.J., Hussain I. Protein-mediated synthesis, pH-induced reversible agglomeration, toxicity and cellular interaction of silver nanoparticles. Colloids Surf. B Biointerfaces. 2013;102:511–518. doi: 10.1016/j.colsurfb.2012.09.032. [DOI] [PubMed] [Google Scholar]
- 369.Mannel M.J., Kreuzer L.P., Goldhahn C., Schubert J., Hartl M.J., Chanana M. Catalytically active protein coatings: Towards enzymatic cascade reactions at inter-colloidal level. ACS Catal. 2017;7(3):1664–1672. [Google Scholar]
- 370.Sheldon R.A. Enzyme immobilization: The quest for op-timum performance. Adv. Synth. Catal. 2007;349(8-9):1289–1307. [Google Scholar]
- 371.Liu W., Wang L., Jiang R. Specific enzyme immobiliza-tion approaches and their application with nanomaterials. Top. Catal. 2012;55(16-18):1146–1156. [Google Scholar]
- 372.Hochuli E., Bannwarth W., Dobeli H., Gentz R., Stuber D. Genetic approach to facilitate purification of recombi-nant proteins with a novel metal chelate adsorbent. Nat Bi-otech. 1988;6(11):1321–1325. [Google Scholar]
- 373.Sun J., Tan H. Alginate-based biomaterials for regenerative medicine applications. Materials (Basel) 2013;6(4):1285–1309. doi: 10.3390/ma6041285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Su C-H., Cheng F-Y. In vitro and in vivo applications of alginate/iron oxide nanocomposites for theranostic molecular imaging in a brain tumor model. RSC Advances. 2015;5(109):90061–90064. [Google Scholar]
- 375.Cheung R.C., Ng T.B., Wong J.H., Chan W.Y. Chitosan: An update on potential biomedical and pharmaceutical applications. Mar. Drugs. 2015;13(8):5156–5186. doi: 10.3390/md13085156. [DOI] [PMC free article] [PubMed] [Google Scholar]


