Abstract
Objective
We aimed to investigate the potential risk factors for residual lesions after uterine artery chemotherapy and embolization (UACE) in combination with dilatation and curettage (D&C) in patients with cesarean scar pregnancy (CSP).
Settings
Retrospective case-control study.
Method
Univariate analysis and logistic analysis were applied to analyze these data to assess the risk factor of residue after UACE in combination with D&C.
Results
Gestational age, human chorionic gonadotropin (HCG) level, and the gestation sac (GS) evagination to the bladder were the risk factors for the postoperative lesion. The most relevant was GS evagination to the bladder, followed by the preoperative HCG level and the gestational age. We defined the cut-off value of gestational age as 28 days with a sensitivity and specificity of 100 and 0, respectively.
Conclusion
In cases with GS evagination to bladder and HCG of >28,113.65 mIU/ml as well as a gestational age of >28 days, the possibility of residual lesions was high.
Keywords: Caesarean scar pregnancy, risk factors, dilatation and curettage, residue, uterine artery embolization
1. INTRODUCTION
Cesarean scar pregnancy (CSP), a rare type of ectopic pregnancy, refers to the implantation of zygote(s) in the myometrium at the site of a previous cesarean section scar [1, 2]. Nowadays, the incidence of CSP is on a rising trend with a range of 1:1,800 to 1:2,216 in women with at least one previous cesarean section [3-6]. With the progress of pregnancy, many patients may present villi, myometrium adhesions and implantation, which then cause high risks of uterine rupture, invasive placenta, uncontrolled hemorrhage, loss of future fertility, and life-threatening complications [2, 7-9].
Many treatment options are available for the treatment of CSP, but there is no consensus on the treatment until now. Based on a systemic review, Birch et al. recommended treatment options for CSP in clinical practice based on efficacy and safety [10]. One of the treatment options is suction curettage following uterine artery chemotherapy and embolization (UACE). As a minimally invasive nonsurgical treatment [11, 12], UACE can effectively treat CSP by controlling haemorrhage, and at the same time, it provides a possibility to preserve the uterus and the patient’s future fertility [13, 14]. Despite the fact that the uterus is reserved for CSP patients, it is necessary to evaluate the risk factors of residual lesions, in order to improve patient compliance and the prognosis. In this study, we aimed to identify the potential risk factors for residual lesions after UACE in combination with dilatation and curettage (D&C) in the treatment of CSP. Besides, a retrospective case-control study was designed to clarify the risk factors.
2. MATERIALS AND METHODS
2.1. Patients
We retrospectively analyzed the perioperative characteristics of patients diagnosed with CSP after bilateral UACE combined with D&C from April 2014 to March 2017 in our hospital. The diagnosis was based on sonographic and Doppler flow findings according to the previously described criteria [15]. Patients were divided into two groups according to the residual lesions. The identification of residual lesions was based on clinical manifestations such as vaginal hemorrhage after curettage and persistent elevation of serum human chorionic gonadotrophin (HCG). After curettage, pathological analysis revealed that the residual tissues were degenerated villi.
For ultrasonography, part of the uterine cavity line was vague or not persistent. Moderate and high-density echoes or uneven echoes were identified in the uterine cavity, and the shape was not regular. The boundary was not clear, and the internal echoes were not evenly distributed. Near the nidation site, abundant blood flow was observed in local muscular layers, together with the arterial flow spectrum with low resistance.
The exclusion criteria were as follows: (i) those with a twin pregnancy or compound pregnancy; (ii) those with an inevitable abortion or with an incomplete abortion with the previous caesarean delivery; (iii) patients with myoma or adenomyoma; (iv) patients with significant maternal cardiac, renal, hepatic and blood system diseases [16]; (v) patients with the gestational trophoblastic disease (vi) those with a gestational age of more than 12 weeks.
2.2. Patient and Public Involvement
Written informed consent was obtained from the patients. The study was approved by the Ethical Committee of Tianjin Hospital.
2.3. Surgical Approaches
UACE was carried out by two sophisticated gynecologists with at least 5 years of experiences. Upon puncture using improved Seldinger technique of the right femoral artery, a Cobra catheter (5.0 F) was placed into the bilateral uterine artery under the guidance of a 0.889-mm guidewire. After intubation and angiography, methotrexate (MTX, 50 mg/m2 body surface area) was infused bilaterally prior to the embolism procedures using gelatin sponge particles with a diameter of 1–3.0 mm (Alicon, Hangzhou, China). With the slow blood flow in bilateral uterine artery, we embolized the arteries with fresh gelatin sponge particles. Extubation was conducted after embolization was confirmed by angiography. Embolization was terminated in the presence of complete stasis of uterine artery flow. After completing the embolization on the left side, the right uterine artery catheterization was performed using a similar technique (Fig. 1 and 2). The puncture point was locally bandaged by an artery hemostatic instrument with pressure for 8 hrs. Close monitoring was carried out of the blood pressure, heart rate, and consciousness during the operation. Diclofenac sodium plugging into the anus, or intramuscular injection of pethidine was utilized to relieve severe abdominal pain after the operation. About 24–48 hrs after UACE, D&C was performed on all the patients under the guidance of ultrasonography and hysteroscopy.
Fig. (1).
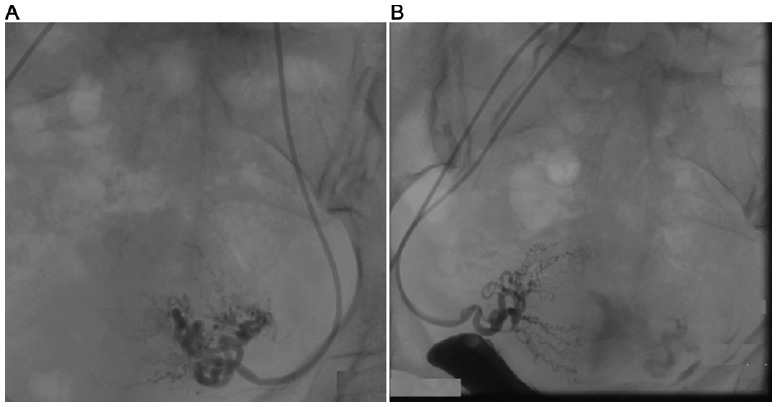
Before embolization of the bilateral uterine artery with gelatin sponge, blood perfusion was left in situ. A. left side; B. right side.
Fig. (2).
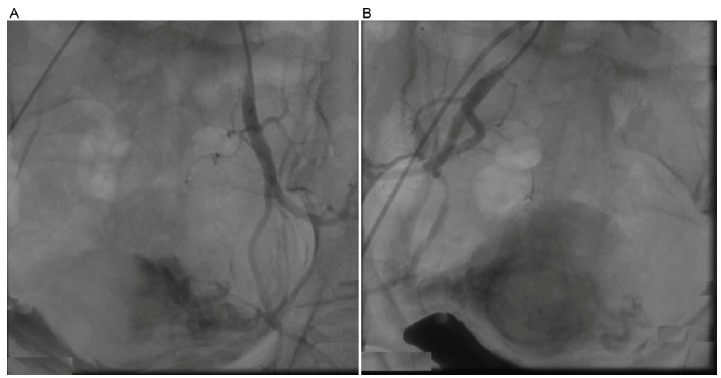
After embolization of the bilateral uterine artery with gelatin sponge,no blood perfusion was left in situ. A. left side; B. right side.
2.4. Data Collection
The following information was collected from each patient: age, gestational age, abdominal pain, colporrhagia, cesarean section times, interval between the last caesarean section and this pregnancy, haematocrit (Hct), hemoglobin (Hgb), platelet (Plt), fibrin (Fib), HCG level before D&C, blood flow, muscular invasion, GS evagination to the bladder, muscular continuity, embryo sac size, myometrial thickness, time duration between UACE and D&C, and hemorrhage during operation.
Serum β-HCG and transabdominal ultrasound were monitored before the intervention, on day 1 after UACE and D&C, every 3 days during hospitalization, and finally every week until the recovery of normal value (ECLIA method, 0-5.3IU/L).
2.5. Statistical Analysis
Statistical analyses were performed using SPSS software. Numeration data were presented as mean ± standard deviation. Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated using logistic regression analysis. Quantitative variables were analyzed using Student’s t-test. Qualitative variables were evaluated using Chi-square test. The positive predictive value and negative predictive value of risk factors were calculated using the ROC curve. The ROC curve was analyzed with MedCalc software. P<0.05 was considered to be statistically significant.
3. RESULTS
There were no statistical differences in age, gestational age, abdominal pain, colporrhagia, cesarean section times, interval between the last caesarean section and this pregnancy, Hct, Hgb, Plt, FIb, rich blood flow, muscular invasion, boundary with the bladder, embryo sac size, myometrial thickness, as well as time range between UACE and D&C (Table 1). HCG level before dilatation and curettage, muscular continuity, evagination, and hemorrhage during the operation were statistically significant. The preoperative HCG of the residual group was higher than the non-residue group. The intraoperative hemorrhage increased in those with poor muscle continuity. We combined the above three indicators into clinically meaningful indicators for logistic regression (Table 1).
Table 1. Single factor analysis of lesion residues.
| Variable | No residual(n=35) | Residual(n=30) | p |
|---|---|---|---|
| Age(year) | 35.51±5.84 | 35.30±6.26 | 0.89 |
| Gestational age (day) | 49.29±9.05 | 53.03±10.67 | 0.13 |
| Abdominal pain(%) | 6(17.14) | 4(13.33) | 0.67 |
| Colporrhagia(%) | 26(74.29) | 21(70.00) | 0.70 |
| Cesarean section(times) | 1.40±0.60 | 1.53±0.63 | 0.39 |
| Interval between last caesarean section and this pregnancy(year) | 6.43±4.35 | 6.48±4.85 | 0.96 |
| Hct(L/L) | 0.36±0.04 | 0.36±0.04 | 0.78 |
| Hgb(g/L) | 122.66±14.86 | 122.70±13.54 | 0.99 |
| Plt(10^9/L) | 247.20±50.36 | 244.50±60.87 | 0.85 |
| FIb(g/L) | 3.14±0.67 | 3.08±0.45 | 0.68 |
| HCG(mIU/mL) | 20,548.55±23,618.07 | 52,748.97±34,735.45 | 0.00 |
| Rich blood flow(%) | 25(71.43) | 23(76.67) | 0.63 |
| Muscular invasion(%) | 10(28.57) | 8(26.67) | 0.86 |
| Boundary with the bladder (%) | 3(8.57) | 5(16.67) | 0.32 |
| Muscular continuity(%) | 7(20.00) | 13(43.33) | 0.04 |
| Embryo sac size(cm) | 3.62±1.89 | 4.20±1.80 | 0.21 |
| Myometrial thickness(cm) | 0.26±0.17 | 0.20±0.08 | 0.12 |
| Evagination | 9(25.71) | 23(76.67) | 0.00 |
| Range intervention time (hour) | 108.40±43.17 | 108.93±51.14 | 0.96 |
| Hemorrhage during operation(%) | 3(8.57) | 17(56.67) | 0.00 |
According to logistic regression analysis, gestational age, HCG level, and the GS evagination to the bladder were the risk factors for the postoperative lesion. The most relevant is whether the GS evagination to the bladder is followed by the preoperative HCG level, and finally the gestational age (Table 2).
Table 2. Logistic regression analysis.
| Variable | B | S.E. | Wald | df | Sig. | Exp(B) | 95% CI for EXP(B) | |
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| Gestational age (day) | .101 | .051 | 3.918 | 1 | .048 | 1.11 | 1.001 | 1.223 |
| HCG (mIU/mL) | .000 | .000 | 6.272 | 1 | .012 | 1.00 | 1.000 | 1.000 |
| Rich blood flow | .213 | .936 | .052 | 1 | .820 | 1.24 | .198 | 7.748 |
| Muscular invasion | -.229 | 1.199 | .037 | 1 | .848 | .80 | .076 | 8.332 |
| Boundary with the bladder | -2.76 | 1.071 | .066 | 1 | .797 | .76 | .093 | 6.193 |
| Muscular continuity | -.879 | .881 | .994 | 1 | .319 | .42 | .074 | 2.337 |
| Embryo sac size(cm) | -.128 | .246 | .273 | 1 | .601 | .88 | .544 | 1.423 |
| Evagination | 2.594 | .929 | 7.792 | 1 | .005 | 13.38 | 2.166 | 82.723 |
| Range intervention time (hour) | -.004 | .010 | .145 | 1 | .703 | .99 | .977 | 1.016 |
| Hemorrhage during operation | -2.076 | 1.178 | 3.107 | 1 | .078 | .13 | .012 | 1.262 |
AUC of the preoperative HCG and gestational age were 0.78 and 0.59, respectively (Fig. 3 and Table 3). In cases of HCG of >28,113.06 mIU/ml, the sensitivity and specificity were 80.00 and 74.29, respectively. In cases of gestational age of >56 days, the sensitivity and specificity were 36.67 and 80.00, respectively. After taking the risks of CSP misdiagnosis into consideration, we defined the cut-off value of gestational age as 28 days according to the positive and negative predictive values (Table 4 and Table 5).
Fig. (3).
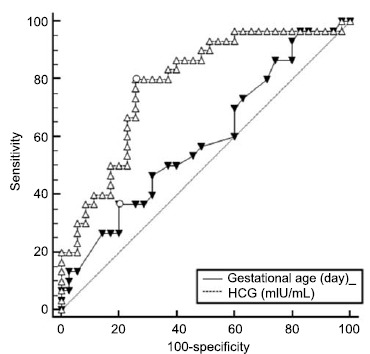
Comparison of ROC curves of gestational age and HCG level.
Table 3. The AUC of gestational age and HCG.
| Variable | AUC | SE | 95%CI |
|---|---|---|---|
| Gestational age(day) | 0.59 | 0.07 | 0.46-0.71 |
| HCG(mIU/mL) | 0.78 | 0.06 | 0.66-0.88 |
Table 4. The cut-off of gestational age and HCG.
| Variable | Criterion | Sensitivity | Specificity |
|---|---|---|---|
| Gestational age(day) | >28 | 100 | 0 |
| HCG(mIU/mL) | >28113.65 | 80.00 | 74.29 |
Table 5. Positive predictive and negative predictive values of residual lesions.
| Variable | +value | +pv | 95%CI | -value | -pv | 95%CI |
|---|---|---|---|---|---|---|
| Gestational Age (day) |
76 | 100 | 15.8-100.0 | 28 | 100 | 2.5-100.0 |
| HCG (mIU/mL) |
79025.47 | 100 | 54.1-100.0 | 166.95 | 100 | 2.5-100.0 |
4. DISCUSSION
The exponential rise in CSP incidence during the past 20 years may be due to the result of the increasing worldwide cesarean section rate. In China, more families decide to have their second children
regardless of receiving a cesarean section or not. Nowadays, the detection ratio of CSP is more accurate with the advances of MRI and ultrasonography techniques.
Nowadays, the incidence of CSP is rare, and most of the studies are case reports or involving a small sample size, without a consensus on the preferred mode of treatment [17]. The ideal treatment strategy should meet the criteria including safety, effectiveness, and a quick recovery of uterine function [18]. UACE has been utilized for decades to treat postpartum hemorrhage and pelvic trauma with the goal of avoiding hysterectomy and preserving fertility [19]. UACE involves less trauma, instant hemostasis and high success rates. Application of UACE in the bilateral uterine artery could trigger platelet condensation and fibrin deposit as well as thrombosis [20-24]. Therefore, several measures were taken for the hemostasis of precapillary arteriole and its branches. Ischemic necrosis was noticed in the tissues near the scar. In clinical practice, MTX perfusion and particulate embolization using gelfoam were used. As a folic acid reductase inhibitor, MTX killed the embryonic tissues, which then inhibited the DNA synthesis of embryo, which finally resulted in pregnancy failure. Intra-arterial chemotherapy contributed to the local drug concen-tration, which then enhanced the embryo termination effects. However, for those with implantation of zygote(s) in the myometrium at the site of a previous cesarean section scar, dilatation and curettage contributed to uterine perforation, organ injuries, and even uterine rupture. In this study, among the 65 cases, 48% showed residual lesions. Gestational age, HCG level, and the GS evagination to the bladder were observed to be the risk factors for the postoperative lesion. The most relevant was GS evagination to the bladder, followed by the preoperative HCG level, and the gestational age [25]. Related literature reported that there were 48% of the patients with residual scar lesions of 10-30 mm. Gestational age, embryo sac size, myometrial thickness, and blood flow were associated with lesions residue after dilatation and curettage in which the embryo sac size was the key to predict any residues [26].
In order to prevent uterine perforation, the gynecologists could not give a complete D&C. The external convexity of the lesion triggered potential difficulties during the operation. HCG is a kind of glycoprotein hormone secreted by trophoblast cells in the placenta and is positively proportional to the number of trophoblast cells. In early pregnancy, villi trophoblast cells divide and proliferate into the uterine muscle layer, and intervillous space becomes full of maternal blood [27]. High serum HCG value means that trophoblast cell growth is strong and aggressive, and the GS is more closely related to the muscle layer and increases the risk of residues after D&C. It is more correlated with the grassroots. Muscularis continuity and intraoperative bleeding have significance in a preliminary calculation, but these will be meaningless after many indicators are selected into the binary logistic regression. This may be related to the sample size. In this study, residual lesions were not associated with intraoperative hemorrhage, which may be related to different blood coagulation function, uterine contractions, as well as technical differences.
Persistent monitoring should be given to the patients with satisfactory HCG, and for those, with poor HCG, their willingness to the pregnancy should be considered in advance, together with the sac position, size, scale and HCG concentration. If no pregnancy plan is proposed by the patients, regular follow up is given after informing the potential risks and benefits. Besides, diphereline and/or mifepristone should be given accordingly. For patients with the formation of the diverticulum, intima repairment can be suggested by an artificial cycle regimen. Regarding those with pregnancy plan, curettage or transvaginal repair of cesarean scar repairment should be considered after taking the blood supply near the mass, the distance between the sac and the external cervix orifice into consideration. In the presence of obvious GS evagination to the bladder, laparoscopic lumpectomy and repairment were considered. In cases of massive mass blocking of the laparoscopic procedures, transabdominal lumpectomy is considered.
Indeed, there are still limitations in our study. For example, we could not take all the factors into consideration. When reviewing the specific ultrasound data in our hospital information system, there were not adequate information on the vascularization index, which could be evaluated by three-dimensional color Doppler imaging. There might be difficulties in the imaging of the non-typical residuals on ultrasono-graphy. For example, some residuals were not easy to distinguish from normal endomembrane if they adhered to the endometrium. Misdiagnosis may occur if the residuals were close to the echoes of the endometrium. Meanwhile, this study is a retrospective study rather than a randomized controlled one. Besides, the sample size is comparatively small. In future studies, we will focus on the randomized controlled studies with a large sample size to investigate the treatment efficiency and complications of different methods.
CONCLUSION
Gestational age, HCG level, and the GS evagination to the bladder were observed to be the risk factors for the postoperative lesion. The most relevant is GS evagination to the bladder, followed by the preoperative HCG level, and finally the gestational age. For those with no GS evagination to the bladder, a lower possibility of the residual lesion was considered in the presence of HCG of <28,113.65 mIU/ml, and the gestational age of 28 days. For those with long-term residual lesions, comprehensive treatment should be given after taking the lesion position, size, peripheral blood supply, HCG level as well as patient’ conditions into consideration.
ACKNOWLEDGEMENTS
Declared none.
LIST OF ABBREVIATIONS
- UACE
Uterine Artery Chemotherapy and Embolization
- D&C
Dilatation and Curettage
- CSP
Caesarean Scar Pregnancy
- HCG
Human Chorionic Gonadotropin
- F
“French” Gauge
- MTX
Methotrexate
- Hct
Haematocrit
- Hgb
Hemoglobin
- Plt
Platelet
- Fib
Fibrin
- GS
Gestation Sac
- Ors
Odds Ratios
- CIs
Confidence Intervals
- B
Partial Regression Coefficient
- S.E
Standard Error
- df
Degree of Freedom
- sig
p Value
- Exp(B)
Odds Ratios
- AUC
Area Under Curve
- +pv
Positive Predictive Values
- -PV
Negative Predictive Values
- ROC
Receiver Operating Characteristic Curve
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the Ethical Committee of Tianjin Hospital (Tianjin, China). The approval No. is 2019-075].
HUMAN AND ANIMAL RIGHTS
No animals were used in this study. All reported experiments on humans were followed in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
CONSENT FOR PUBLICATION
Informed consent was obtained from all individual participants included in the study.
AVAILABILITY OF DATA AND MATERIALS
The raw data were available upon appropriate requests.
FUNDING
The fund information is Name: The Scientific and Technology Fund of Tianjin Hospital Grant No.: TJYY1707.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
AUTHORS' CONTRIBUTIONS
CF wrote the manuscript; HTX revised the manuscript; SD, GSJ, CS did the data analysis; YHW, HJL did the data collection.
REFERENCES
- 1.Timor-Tritsch I.E., Monteagudo A. Unforeseen consequences of the increasing rate of cesarean deliveries: early placenta accreta and cesarean scar pregnancy. A review. Am. J. Obstet. Gynecol. 2012;207:14–29. doi: 10.1016/j.ajog.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 2.Ko J.K., Li R.H., Cheung V.Y. Caesarean scar pregnancy: a 10-year experience. Aust. N. Z. J. Obstet. Gynaecol. 2015;55:64–69. doi: 10.1111/ajo.12273. [DOI] [PubMed] [Google Scholar]
- 3.Sekiguchi A., Okuda N., Kawabata I., Nakai A., Takeshita T. Ultrasound detection of lacunae-like image of a cesarean scar pregnancy in the first trimester. J. Nippon Med. Sch. 2013;80:70–73. doi: 10.1272/jnms.80.70. [DOI] [PubMed] [Google Scholar]
- 4.Zhuang Y., Huang L. Uterine artery embolization compared with methotrexate for the management of pregnancy implanted within a cesarean scar. Am. J. Obstet. Gynecol. 2009;201:152.e151–152.e153. doi: 10.1016/j.ajog.2009.04.038. [DOI] [PubMed] [Google Scholar]
- 5.Seow K.M., Wang P.H., Huang L.W., Hwang J.L. Transvaginal sono-guided aspiration of gestational sac concurrent with a local methotrexate injection for the treatment of unruptured cesarean scar pregnancy. Arch. Gynecol. Obstet. 2013;288:361–366. doi: 10.1007/s00404-013-2765-4. [DOI] [PubMed] [Google Scholar]
- 6.An X., Ming X., Li K., Wang J. The analysis of efficacy and failure factors of uterine artery methotrexate infusion and embolization in treatment of cesarean scar pregnancy. ScientificWorldJournal. 2013;2013:213603. doi: 10.1155/2013/213603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chetty M., Elson J. Treating non-tubal ectopic pregnancy. Best Pract. Res. Clin. Obstet. Gynaecol. 2009;23:529–538. doi: 10.1016/j.bpobgyn.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 8.Yin X., Su S., Dong B., Ban Y., Li C., Sun B. Angiographic uterine artery chemoembolization followed by vacuum aspiration: an efficient and safe treatment for managing complicated cesarean scar pregnancy. Arch. Gynecol. Obstet. 2012;285:1313–1318. doi: 10.1007/s00404-011-2132-2. [DOI] [PubMed] [Google Scholar]
- 9.Seow K.M., Huang L.W., Lin Y.H., Lin M.Y., Tsai Y.L., Hwang J.L. Cesarean scar pregnancy: issues in management. Ultrasound Obstet. Gynecol. 2004;23:247–253. doi: 10.1002/uog.974. [DOI] [PubMed] [Google Scholar]
- 10.Birch Petersen K., Hoffmann E., Rifbjerg Larsen C., Svarre Nielsen H. Cesarean scar pregnancy: a systematic review of treatment studies. Fertil. Steril. 2016;105:958–967. doi: 10.1016/j.fertnstert.2015.12.130. [DOI] [PubMed] [Google Scholar]
- 11.Shen L., Tan A., Zhu H., Guo C., Liu D., Huang W. Bilateral uterine artery chemoembolization with methotrexate for cesarean scar pregnancy. Am. J. Obstet. Gynecol. 2012;207:386.e381–386.e386. doi: 10.1016/j.ajog.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 12.Le A., Shan L., Xiao T., Zhuo R., Xiong H., Wang Z. Transvaginal surgical treatment of cesarean scar ectopic pregnancy. Arch. Gynecol. Obstet. 2013;287:791–796. doi: 10.1007/s00404-012-2617-7. [DOI] [PubMed] [Google Scholar]
- 13.Hong Y., Guo Q., Pu Y., Lu D., Hu M. Outcome of high-intensity focused ultrasound and uterine artery embolization in the treatment and management of cesarean scar pregnancy: A retrospective study. Medicine (Baltimore) 2017;96:e7687. doi: 10.1097/MD.0000000000007687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li C., Li C., Feng D., Jia C., Liu B., Zhan X. Transcatheter arterial chemoembolization versus systemic methotrexate for the management of cesarean scar pregnancy. Int. J. Gynaecol. Obstet. 2011;113:178–182. doi: 10.1016/j.ijgo.2010.11.027. [DOI] [PubMed] [Google Scholar]
- 15.Fylstra D.L. Ectopic pregnancy within a cesarean scar:areview. Obstet. Gynecol. Surv. 2002;57(8):537–543. doi: 10.1097/00006254-200208000-00024. [DOI] [PubMed] [Google Scholar]
- 16.Du Y.J., Zhang X.H., Wang L.Q. Risk Factors for Haemorrhage during Suction Curettage after Uterine Artery Embolization for Treating Caesarean Scar Pregnancy: A Case-Control Study. Gynecol. Obstet. Invest. 2015;80:259–264. doi: 10.1159/000381263. [DOI] [PubMed] [Google Scholar]
- 17.Lan W., Hu D., Li Z., Wang L., Yang W., Hu S. Bilateral uterine artery chemoembolization combined with dilation and curettage for treatment of cesarean scar pregnancy: A method for preserving the uterus. J. Obstet. Gynaecol. Res. 2013;39:1153–1158. doi: 10.1111/jog.12051. [DOI] [PubMed] [Google Scholar]
- 18.Litwicka K., Greco E. Caesarean scar pregnancy: a review of management options. Curr. Opin. Obstet. Gynecol. 2013;25:456–461. doi: 10.1097/GCO.0000000000000023. [DOI] [PubMed] [Google Scholar]
- 19.Wang H., Garmel S. Successful term pregnancy after bilateral uterine artery embolization for postpartum hemorrhage. Obstet. Gynecol. 2003;102:603–604. doi: 10.1016/s0029-7844(03)00230-8. [DOI] [PubMed] [Google Scholar]
- 20.Marcus S., Cheng E., Goff B. Extrauterine pregnancy resulting from early uterine rupture. Obstet. Gynecol. 1999;94:804–805. doi: 10.1016/s0029-7844(99)00492-5. [DOI] [PubMed] [Google Scholar]
- 21.Cao S., Zhu L., Jin L., Gao J., Chen C. Uterine artery embolization in cesarean scar pregnancy: safe and effective intervention. Chin. Med. J. (Engl.) 2014;127:2322–2326. [PubMed] [Google Scholar]
- 22.Qian Z.D., Huang L.L., Zhu X.M. Curettage or operative hysteroscopy in the treatment of cesarean scar pregnancy. Arch. Gynecol. Obstet. 2015;292:1055–1061. doi: 10.1007/s00404-015-3730-1. [DOI] [PubMed] [Google Scholar]
- 23.Gao L., Huang Z., Gao J., Mai H., Zhang Y., Wang X. Uterine artery embolization followed by dilation and curettage within 24 hours compared with systemic methotrexate for cesarean scar pregnancy. Int. J. Gynaecol. Obstet. 2014;127:147–151. doi: 10.1016/j.ijgo.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 24.Wozniak S., Pyra K., Kludka-Sternik M., et al. Uterine artery embolization using gelatin sponge particles performed due to massive vaginal bleeding caused by ectopic pregnancy within a cesarean scar: a case study. Ginekol. Pol. 2013;84:966–969. doi: 10.17772/gp/1668. [DOI] [PubMed] [Google Scholar]
- 25.Ananthakrishnan G., Murray L., Ritchie M., et al. Randomized comparison of uterine artery embolization (UAE) with surgical treatment in patients with symptomatic uterine fibroids (REST trial): subanalysis of 5-year MRI findings. Cardiovasc. Intervent. Radiol. 2013;36:676–681. doi: 10.1007/s00270-012-0485-y. [DOI] [PubMed] [Google Scholar]
- 26.He Y., Wu X., Zhu Q., et al. Combined laparoscopy and hysteroscopy vs. uterine curettage in the uterine artery embolization-based management of cesarean scar pregnancy: a retrospective cohort study. BMC Womens Health. 2014;14:116. doi: 10.1186/1472-6874-14-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang Z., Su J., Yang H. [Feasibility of treatment of cesarean scar pregnancy with dilatation and curettage under ultrasound guidance]. Zhonghua Yi Xue Za Zhi. 2015;95:3045–3049. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data were available upon appropriate requests.


