Abstract
A State of the Art lecture, “VTE Risk Assessment in Pregnancy,” was presented at the ISTH congress in Melbourne, Australia, in 2019. Venous thromboembolism (VTE) remains a leading cause of death in pregnancy and in the postpartum period. Moreover, VTE can result in lifelong disability. The elevated baseline pregnancy‐associated VTE risk is further increased by additional maternal, pregnancy, and delivery characteristics, highlighting the importance of VTE risk assessment in early pregnancy, at delivery, and if risk factors change. This review will provide an overview of the impact and epidemiology of VTE in pregnancy (including reported risk factors for pregnancy‐associated VTE), will address VTE risk‐reduction strategies (including ongoing studies), and will provide a summary of critical knowledge gaps. Finally, throughout this review, relevant new data presented during the 2019 ISTH annual congress in Melbourne will be summarized.
Keywords: postpartum, pregnancy, prevention, risk factors, venous thromboembolism
Essentials.
The following knowledge gaps need to be addressed by current and future research to better prevent pregnancy‐associated VTE events:
Pregnancy‐associated venous thromboembolism (VTE) is a leading cause of maternal mortality and morbidity.
Knowledge and awareness of VTE risk factors is vital to ensure that appropriate risk‐reduction measures are implemented.
VTE risk assessment should be performed in early pregnancy, at delivery, and if risk factors change.
Several important knowledge gaps remain and should be prioritized for research to protect the health and lives of pregnant and postpartum women.
1. INTRODUCTION
Venous thromboembolism (VTE) remains a leading cause of death in pregnancy and in the postpartum period.1. During 2014‐2016, VTE was reported to be the top cause of direct maternal death in the United Kingdom and Ireland, occurring in 1.39 (95% confidence interval [CI], 0.95‐1.96) per 100 000 pregnancies.2 A maternal death due to pulmonary embolism is a devastating event with wide‐reaching consequences for the woman’s family, friends, and society. Importantly, VTE can also result in lifelong disability.3 VTE risk rises during pregnancy and peaks in the postpartum period. In a recent systematic review and meta‐analysis restricted to recent studies in which VTE cases were validated, the pooled incidence rate of VTE during the antepartum period was 118 (95% CI, 101‐137) per 100 000 person‐years and 424 (95% CI, 238‐755) per 100 000 person‐years during the postpartum period.4 Mechanisms underlying this pregnancy‐related increase in VTE risk include venous stasis, pelvic venous compression by the gravid uterus, pulsatile compression of the left iliac vein by the right iliac artery and changes in pro‐ and anticoagulant and fibrinolytic pathways.5, 6, 7, 8 For example, plasma endogenous thrombin potential and plasminogen activator inhibitor‐1 levels are significantly higher in pregnant women compared with nonpregnant controls.7, 8
In pregnancy, platelets undergo morphological changes and their activation is increased.9 Upon activation, platelets release a multitude of signaling (soluble and vesicular) factors collectively termed the platelet releasate (PR),10 which plays important roles in hemostasis, wound healing, and inflammatory response.11 Maternal PR enhances trophoblast invasion and drives placental bed angiogenesis, critical processes during normal placentation.12 Recently, PR contents of 18 healthy pregnant and 13 nonpregnant women were characterized using comparative label‐free quantitative proteomic profiling and shown to be altered in pregnant compared with nonpregnant women.13 Sixty‐nine PR proteins were differentially released, and 11 PR proteins were capable of discriminating pregnant and nonpregnant women with an area under the curve of 0.876, sensitivity of 88.9%, and specificity of 84.6%. It remains to be determined whether this change in PR contents contributes to pregnancy‐associated VTE (PA‐VTE) risk.
1.1. ISTH 2019 (Melbourne) report
De Laurenzo and colleagues14 evaluated thromboelastography parameters in 70 healthy pregnant or postpartum women, reporting a lower “K” parameter and higher “alpha angle” and “maximum amplitude (MA)” parameters in the first trimester compared with the postpartum period, overall suggesting a prothrombotic state using this assay in the postpartum period. Another multicenter cross‐ sectional cohort study by Obeng‐Tuudah and colleagues including 175 women showed that platelet function (measured by light transmission aggregometry in response to 6 agonists) demonstrated a non–statistically significant increase with advancing pregnancy, reaching highest levels in the third trimester and reverting to control levels postpartum.15
2. ADDITIONAL PA‐VTE RISK FACTORS
This elevated baseline PA‐VTE risk is further increased by additional maternal, pregnancy, and delivery characteristics (Table 1),5, 16, 17, 18, 19, 20, 21, 22, 23 highlighting the importance of VTE risk assessment in early pregnancy, at delivery, and throughout pregnancy if risk factors change (eg, during extended antenatal immobilization, as will be discussed later).24
Table 1.
aOR and IRR reported for selected maternal, pregnancy, and delivery characteristics in the listed studies
| Maternal characteristics | |
|---|---|
| Risk factor | Selected aOR/IRR (95% CI) |
| Age > 35 y | 1.3 (1.0‐1.7)16 |
| Parity ≥ 3 | 2.4 (1.8‐3.1)16 |
| BMI ≥ 25kg/m2 | 1.8 (1.3‐2.4)a , 17 |
| BMI ≥ 25kg/m2 + antepartum immobilization | 62.3 (11.5‐337.6)a , 17 |
| Smokerb | 2.1 (1.3‐3.4)a , 17 |
| ART (singleton) | 4.3 (2.0‐9.4)a , 17 |
| Varicose veinsd | 2.21 (1.55‐4.76)a , 23 |
| Inherited thrombophilia | Variable (0.7‐34.4)26 |
| Prior VTE | 24.8 (17.1‐36.0)18 |
| Antiphospholipid syndrome | 15.8 (10.9‐22.8)18 |
| Sickle cell disease | 6.7 (4.4‐10.1)18 |
| Preexisting diabetesd | 3.54 (1.13‐11.0)23 |
| Pregnancy Characteristics | |
|---|---|
| Risk factor | Selected aOR (95% CI) |
| IUGR | 3.8 (1.4‐10.2)c , 17 |
| Preeclampsia | 3.1 (1.8‐5.3)c , 17 |
| Twins | 2.6 (1.1‐6.2)a , 17 |
| Delivery characteristics | |
|---|---|
| Risk factor | Sample aOR/IRR (95% CI) |
| Preterm deliveryd | 2.28 (1.66‐3.14)c , 23 |
| Emergency CS | 2.7 (1.8‐4.1)c , 17 |
| Stillbirthd | 4.07 (1.73‐9.56)c , 23 |
| Infection (CS) | 6.2 (2.4‐16.2)c , 17 |
| PPH ≥ 1000 mL | 4.1 (2.3‐7.3)c , 17 |
| Infection (VD) | 20.2 (6.4‐63.5)c , 17 |
The reported strength of these characteristics is highly variable between studies: the intention of this table is to raise awareness of the need to consider individualized risk assessment at these times in the pregnancy journey rather than to comprehensively review all reported risk factors.
Abbreviations: aOR, adjusted odds ratio; ART, assisted reproductive technology; BMI, body mass index; CI, confidence interval; CS, cesarean section; FHx, family history of VTE; IRR, incidence rate ratio; IUGR, intrauterine growth restriction; PPH, postpartum haemorrhage; VD, vaginal delivery.
As a predictor of antenatal VTE events.
10‐30 cigarettes per day.
As a predictor of postnatal VTE events.
Denotes IRR rather than aOR.
2.1. Thrombophilia
PA‐VTE risk is higher in women with inherited and acquired thrombophilia compared with those without these conditions, particularly if associated with a family history of VTE.25, 26, 27, 28 The reported increase in PA‐VTE risk varies widely depending on the type of thrombophilia and between studies. The American Society of Hematology (ASH) guideline panel has suggested that the appropriate threshold for initiation of thromboprophylaxis during pregnancy is approximately 2%.25 Absolute risks of VTE during pregnancy appear not to reach this threshold in the case of some thrombophilias but do with others. For example, in a recent case‐control study, the absolute reported risk of PA‐VTE in women heterozygous for the factor V Leiden (FVL) polymorphism was 0.5% (95% CI, 0.23%‐0.72%) in women aged <35 years 28 and in a pooled analysis of published studies including thrombophilic women with a family VTE history reported in the 2018 ASH guideline, an absolute risk of 0.5% (95% CI, 0.06%‐1.21%) was estimated.25 In contrast, the combination of compound heterozygosity for FVL and the prothrombin gene promoter region polymorphism (G20210A) is reported to be associated with an absolute PA‐VTE risk of 5.5% (95% CI, 0%‐21.92%).28
2.1.1. ISTH 2019 (Melbourne) report
VTE risk in women with antithrombin deficiency was highlighted by Abbattista and colleagues in a single‐center retrospective Italian study including women with quantitative (type I) antithrombin deficiency who had at least 1 pregnancy. Eighty women without current VTE had 189 pregnancies; 43 of whom were managed with low‐molecular‐weight heparin (LMWH) thromboprophylaxis and 146 without. PA‐VTE occurred in 7.0% and 11.6%, respectively (relative risk [RR], 0.6; 95% CI, 0.2‐1.9).
2.2. Prior VTE
Women with a personal VTE history have a higher risk of recurrent VTE during pregnancy.18, 29 The absolute reported recurrence risk varies widely30, 31, 32, 33 but appears highest for women with an unprovoked or a hormone‐provoked VTE, in which the reported absolute risk in the absence of thromboprophylaxis exceeded ~2% to 6% in some studies.29, 30, 31, 32, 34 Two retrospective studies reported a higher VTE recurrence rate (although not reaching statistical significance) during pregnancy in women with a history of a VTE event provoked by oral hormonal contraceptive use or pregnancy than in women with an unprovoked or nonhormonal transient risk factor–provoked event.31, 32 Moreover, a large retrospective cohort study reported a higher risk of recurrence during pregnancy in women with pregnancy‐associated VTE than women with unprovoked VTE (4.5% vs. 2.7%; RR, 1.7; 95% CI, 1.0‐2.8).35 In contrast, VTE recurrence risk during pregnancy was estimated to be 1.0% (95% CI, 1.9%‐5.7%) in women whose prior event was provoked by a major transient nonhormonal VTE risk factor.6
2.3. Interaction of PA‐VTE risk factors
The interaction of VTE risk factors remains an important knowledge gap. However, an interesting insight was provided by a large hospital‐based case‐control study in which VTE risk factors were validated by review of medical records. This study included 559 women with objectively verified VTE during pregnancy or the postpartum period and 1229 controls.17 Some risk factors exhibited additive interaction (as observed with the combination of assisted reproductive technology with multiple pregnancy, and emergency cesarean section with infection), while others appeared to act as multipliers, as with the combination of antepartum immobilization and elevated body mass index (the adjusted OR of antepartum immobilization in women with a body mass index of <25 and ≥25 kg/m2 were 7.7 [95% CI, 3.2‐19.0] and 62.3 [95% CI, 11.5‐337.6], respectively).
In particular, understanding how these VTE risk factors translate into absolute PA‐VTE is essential. A risk prediction model for postpartum VTE was recently developed using UK Clinical Practice Research Datalink data linked to Hospital Episode Statistics and including 433 353 deliveries. This model was externally validated using Swedish data sets and including 662 387 deliveries. Emergency cesarean section, stillbirth, varicose veins, preeclampsia/eclampsia, infection, and medical comorbidities were the strongest VTE predictors in the final multivariable model. The risk prediction model was able to discriminate postpartum women with and without VTE with a C statistic of 0.70 (95% CI, 0.67‐0.73) in the UK cohort and 0.73 (95% CI, 0.71‐0.75) in the Swedish cohort, with excellent calibration of observed vs. predicted VTE risks.22
2.4. How frequently do PA‐VTE risk factors occur?
VTE risk factors vary in their association with PA‐VTE risk but appear to be common. In a recently published cross‐sectional study of prospectively collected data from 21 019 sequential postpartum VTE risk assessments completed over a 3‐year period in the Rotunda Hospital, Dublin, Ireland, the most common VTE risk factors related to maternal characteristics and delivery characteristics included overweight and obesity (36%), age ≥ 35 (35%) and cesarean delivery (32%). Over three‐quarters of women had at least 1 VTE risk factor (78%), and over 40% had multiple (2 or more) VTE risk factors. In 19% of women, all VTE risk developed during delivery or in the postpartum period (and were not present prior to the peripartum period; Figure 1),36 highlighting the critical importance of performing VTE risk assessment after delivery.
Figure 1.

Frequency of VTE risk factors identified in postpartum women. Green: No VTE risk factors identified; Orange: at least one VTE risk factor identified; Red: VTE risk factors were not identifiable prior to labor and delivery or the postpartum period. FHx, family history; VTE, venous thromboembolism
3. REDUCING THE RISK OF VTE IN PREGNANCY
Addressing the question “does pharmacological thromboprophylaxis reduce the risk of PA‐VTE” has been challenging, despite some progress in specific areas, which will be discussed. Indeed, the authors of a 2014 Cochrane review concluded that “there is insufficient evidence on which to base recommendations for thromboprophylaxis during pregnancy (and that) large scale, high‐quality randomised trials of currently used interventions are warranted.”37 However, the experience of the PROSPER investigators has demonstrated that conducting randomized controlled trials (RCTs) for women with (in this case, postpartum) VTE risk factors can prove extremely challenging38, 39: Rodger et al38 conducted a multinational, double‐blind pilot RCT comparing LMWH for 21 days vs. placebo injections in postpartum women at high VTE risk to determine the feasibility of conducting a full‐scale multicenter randomized double‐blind study. Of 378 eligible women, only 25 (6.6%) were randomized, with a recruitment rate of 0.7 per center per month, leading the authors to conclude that a double‐blind RCT design for this intervention was not feasible. A second pilot by the same group explored the feasibility of a randomized, open‐label trial comparing 10 days of LMWH vs. no treatment for postpartum thromboprophylaxis in women at risk of VTE.39 Of 343 eligible women, only 37 were randomized over 4.9 months, with an overall recruitment rate of 0.9 per center per month. The authors noted that “poor recruitment is a common and major threat to the completion of RCTs, especially notable in the peri‐partum population.”
Consequently, to date, guideline recommendations are mainly based on expert opinion rather than high‐quality evidence.24, 25, 40, 41, 42 This can be extremely difficult for care providers, particularly given the competing risks and challenges of pharmacological thromboprophylaxis, which are relatively common 43 and include bleeding, bruising, skin reactions, pain, and, in many jurisdictions, high out‐of‐pocket costs.
3.1. What is the effect of LMWH on bleeding risk during pregnancy?
Despite the fact that the stakes are so high, there is a dearth of high‐quality data surrounding the impact of LMWH on bleeding risk during pregnancy. A 2005 systematic review that aimed to assess the safety and efficacy of LMWH during pregnancy reported a 2.0% (95% CI, 1.50%‐2.61%) overall risk of “significant maternal bleeding” for women receiving LMWH for thromboprophylaxis, adverse pregnancy outcome, or unspecified indications.43 These events included antenatal bleeding (0.42%; 95% CI, 0.21%‐0.75%), “primary obstetric causes” for bleeding (0.92%; 95% CI, 0.59%‐1.37%), and wound hematoma (0.65%; 95% CI, 0.38%‐1.04%). There is frequently no consistent definition of bleeding in studies evaluating LMWH in pregnancy. In a systematic review of RCTs in pregnant women evaluating the effect of LMWH, 16 studies met eligibility criteria, which included 2690 women. Critically, bleeding events were prospectively recorded using a standardized definition in only one‐third (912 women).44 The risk of major bleeding with LMWH appears low in both the heparin and no‐heparin arms of RCTs.6 Moreover, while minor bleeding is common in women in the heparin and no‐heparin arms of RCTs comparing standard care or placebo to LMWH, it remains uncertain whether the risk is significantly higher with LMWH. Indeed, highly variable rates of minor bleeding have been reported in the heparin and no‐heparin arms of RCTs.44 Arising from this unmet clinical need, a new classification of anticoagulant‐related bleeding has recently been proposed by the ISTH Scientific and Standardization Subcommittee on Control of Anticoagulation.44
3.2. PA‐VTE risk reduction in women with prior VTE
Women with a personal VTE history have a higher risk of recurrent VTE during pregnancy.18, 29 The absolute reported recurrence risk varies widely 30, 31, 32, 33 but appears highest for women with an unprovoked or a hormone‐provoked VTE, in which the reported absolute risk in the absence of thromboprophylaxis has exceeded ~2% to 6% in some studies.29, 30, 31, 32, 34 It appears that this risk may be reduced with LMWH: Pooled proportions of recurrent major VTE reported in studies evaluating LMWH during the antepartum and postpartum periods in women with prior VTE (including provoked [nonestrogen], unprovoked, or estrogen‐associated VTE as a single group) reported antepartum recurrence risks of 0.9% (95% CI, 0.5%‐1.8%) with LMWH and 4.2% (95% CI, 0.3%‐6.0%) without LMWH and postpartum recurrence risks of 1.7% (95% CI, 1.2%‐2.7%) with LMWH and 6.5% (95% CI, 4.3%‐9.7%) without LMWH.6 Current American College of Chest Physicians (ACCP) and ASH guidelines recommend that all pregnant women with a history of VTE receive postpartum pharmacologic thromboprophylaxis with LMWH, while women with prior VTE that is either unprovoked or estrogen/pregnancy‐associated receive both antepartum and postpartum thromboprophylaxis (Figure 2).25, 40 These recommendations reflect lower reported recurrence risks for women with provoked (nonhormonal) VTE than for women whose VTE was estrogen related or unprovoked: In a pooled analysis of 4 cohort studies, major antenatal VTE recurrence rates without prophylaxis were 1.1% (95% CI, 0.2%‐5.8%) 6.4% (95% CI, 3.9%‐10.4%) and 3.6% (95% CI, 1.4%‐8.9%) for these 3 groups, respectively.6
Figure 2.
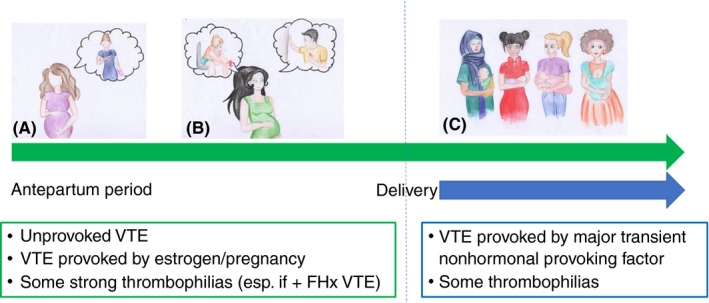
Prevention of VTE in women with prior VTE or thrombophilia. Guideline recommendations around VTE and thrombophilia can be broadly summarized as follows: Antepartum + postpartum thromboprophylaxis is recommended for women with prior unprovoked VTE or VTE provoked by estrogen or pregnancy (A) and for some strong thrombophilias, particularly if associated with a family history of VTE (B) due to a higher predicted recurrence risk than for women with a VTE provoked by major transient nonhormonal provoking factors and some weaker thrombophilias, for whom only postnatal thromboprophylaxis is recommended (C). FHx, family history; VTE, venous thromboembolism
Previous guidelines have suggested various approaches to VTE prevention in these women, with strategies including a low prophylactic or an intermediate (half‐therapeutic) dose.25, 40 The optimal LMWH dose is unknown. The ongoing Highlow study (NCT 01828697; clinicaltrials.gov) is addressing this question in an investigator‐initiated, multicenter, multinational RCT comparing a fixed low dose of LMWH with an intermediate weight‐adjusted dose in the prevention of pregnancy‐related VTE recurrence in women ≥18 years with a history of VTE and an indication for ante‐ and postpartum thromboprophylaxis who are recruited ≤14 weeks’ gestation (Figure 3).29
Figure 3.
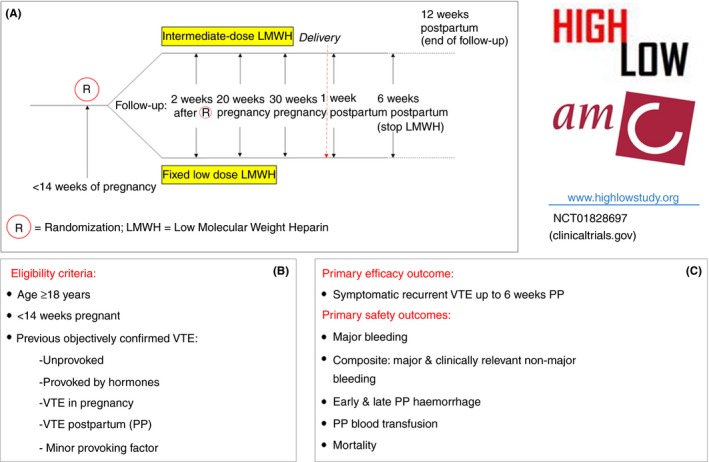
Overview of the Highlow study (http://www.highlowstudy.org; NCT01828697, clinicaltrials.gov). (A) Study flowchart from randomization to follow up; (B and C) Eligibility criteria, primary efficacy outcome, and primary safety outcomes
3.2.1. ISTH 2019 (Melbourne) report
Interim data from the Highlow RCT on obstetric outcome and anesthetic use in 541 women using antepartum LMWH were presented by Dr Bistervels on behalf of the Highlow investigators.45 In this study, LMWH is discontinued at first signs of labor or 12 hours (for patients randomized to low‐dose LMWH) or 24 hours (for patients randomized to intermediate‐dose LMWH) prior to planned delivery. In this analysis, the median time interval between the last LMWH injection and delivery was 34 hours. Spontaneous onset of delivery occurred in 43.5% and 50% of women receiving low‐ and intermediate‐dose LMWH (OR, 0.8; 95% CI, 0.6‐1.1). Neuraxial anesthesia was administered to 53.7% of women with no difference between low and intermediate doses (53.3% vs. 54.2%; OR, 1.0; 95% CI, 0.7‐1.4).
3.3. PA‐VTE risk reduction in women with thrombophilia
No randomized studies have explicitly evaluated the efficacy of pharmacological thromboprophylaxis in reducing PA‐VTE risk in women with inherited thrombophilia. The TIPPS (Antepartum Dalteparin Versus No Antepartum Dalteparin for the Prevention of Pregnancy Complications in Pregnant Women With Thrombophilia) multinational RCT enrolled pregnant women with thrombophilia who were at increased risk of VTE or with previous placenta‐mediated pregnancy complications and randomized participants to antepartum prophylactic LMWH dalteparin or to no antepartum dalteparin.46 There was no significant difference in the primary composite outcome (severe or early‐onset preeclampsia, small‐for‐gestational‐age infant [birth weight < 10th percentile], pregnancy loss, or VTE). However, the study was not powered to specifically evaluate VTE risk reduction, and a relatively small number of participants were enrolled due to prior VTE (secondary VTE or calf deep vein thrombosis (DVT); 21 of 146 in the dalteparin arm and 15 of 143 in the no‐dalteparin arm). Consequently, guideline recommendations for PA‐VTE risk reduction in women with thrombophilia vary.24, 25, 40, 41, 47 However, a consistent theme is the suggestion for implementation of ante‐ and postpartum thromboprophylaxis in pregnant women with “high‐risk” thrombophilias, especially when accompanied by a strong family history (Figure 2), in which the predicted absolute VTE risk is above the ~2% threshold accepted in recent international guidelines but not for “low‐risk” thrombophilias (eg, those associated with a <1% absolute predicted VTE risk).25
3.4. PA‐VTE risk reduction in women with multiple, common VTE risk factors
Although arguably the best‐quality data exist to address PA‐VTE risk reduction in women with a personal VTE history or inherited thrombophilia, these 2 categories of risk factor are rare (<1% in a recent large prospective study.36) The optimal strategy for PA‐VTE prevention in women with more common VTE risk factors remains a critical knowledge gap, as evidenced (again) by widely varying international guideline recommendations24, 25, 40, 47, 48 and intense debate.49, 50 In particular, the balance of thrombosis and bleeding risk is uncertain, and it is noteworthy that the 2018 ASH guideline panel highlighted important research needs, including a requirement for more data on the absolute VTE risk in women with combinations of known risk factors.25 Reflecting the lack of data, we recently performed an analysis of prospectively collected data from 21 019 consecutive comprehensive postpartum VTE risk assessments, applying the recommendations of representative international guidelines and calculating the proportion of women who would have received a recommendation for postpartum thromboprophylaxis under each guideline.36 This proportion ranged from 7% under American College of Obstetrics and Gynecologists guidelines51 to 37% under UK Royal College of Obstetricians and Gynaecologists guidelines24 (Table 2).
Table 2.
Estimated proportion of women recommended postpartum thromboprophylaxis according to international guidelines
| Guideline | Year | Jurisdiction | Estimated proportion of women recommended postpartum thromboprophylaxis (N = 20 775) | |||||
|---|---|---|---|---|---|---|---|---|
| Total (N = 21 019) | Cesarean delivery (n = 6717) | Vaginal delivery (n = 14 302) | ||||||
| n | % (95% CI) | n | % (95% CI) | n | % (95% CI) | |||
| Australia and New Zealand 48 | 2012 | Australia and New Zealand | 4895 | 23 (23‐24) | 4559 | 68 (67‐69) | 336 | 2.3 (2.1‐2.6) |
| American College of Chest Physicians (ACCP) 40 | 2012 | United States | 1521 | 7 (6.9‐7.6) | 1435 | 21 (20‐22) | 86 | 0.6 (0.5‐0.7) |
| American College of Obstetricians and Gynecologists (ACOG) 51 | 2018 | United States | 1678 | 8 (7.6‐8.4) | 1594 | 24 (23‐25) | 84 | 0.6 (0.5‐0.7) |
| National Partnership for Maternal Safety (NPMS) 58 | 2016 | United States | 4381 | 21 (20‐21) | 4268 | 63 (62‐65) | 113 | 0.8 (0.7‐1.0) |
| Royal College of Obstetricians and Gynaecologists (RCOG) 24 | 2015 | United Kingdom | 7858 | 37 (37‐38) | 5673 | 85 (84‐85) | 2185 | 15 (15‐16) |
| Swedish Society of Obstetrics and Gynecology (SFOG) 42 | 2011 | Sweden | 2302 | 11 (11‐11) | 2074 | 31 (30‐32) | 228 | 1.6 (1.4‐1.8) |
| Society of Obstetricians and Gynecologists of Canada (SOGC) 59 | 2014 | Canada | 3091 | 15 (14‐15) | 2306 | 34 (33‐36) | 785 | 5.5 (5.1‐5.9) |
Reproduced, with permission, from O'Shaughnessy et al.36
Several international groups are prioritizing this research question (see, in particular, ISTH Melbourne report, below). In a recent retrospective cohort study by Cox and colleagues including 172 pregnancies in 123 women with a variety of VTE risk factors who qualified for enoxaparin thromboprophylaxis, the rate of VTE despite thromboprophylaxis was low at 1.2% (95% CI, 0.32‐4.14).33 A multicenter study performed by the STRATHEGE investigators of the French INNOVTE Network compared PA‐VTE and placental vascular complication rates before and after implementation of a risk scoring system to determine thromboprophylaxis strategies in 2085 women. Vascular events occurred in 190 (19.2%) women before and 140 (13%) after implementation of risk score–driven prophylaxis (RR, 0.68; 95% CI, 0.55‐0.83). Moreover, the incidence of PA‐DVT was reduced (RR, 0.30; 95% CI, 0.14‐0.67). Postpartum hemorrhage was recorded in 3.2% of women before and 4.5% after implementation (RR, 1.38; 95% CI, 0.89‐2.13; P = 0.15).
3.4.1. ISTH 2019 (Melbourne) report
Dr O’Shaughnessy of the Rotunda Hospital, Dublin, Ireland, presented pilot data evaluating the impact of systematic electronic VTE risk assessment 52 in all postpartum women combined with a strong positive campaign to increase VTE awareness among multidisciplinary colleagues on VTE prevention strategies in a large maternity hospital.53 Introduction of these measures resulted in an increase in use of risk‐appropriate pharmacological thromboprophylaxis (RA‐TPX) from 68% to 89% (P < 0.001). This was primarily driven by increased use of RA‐TPX after vaginal delivery (10%‐72%) following the introduction of this tool (P < .001). Moreover, in this preliminary sample, hospital‐level VTE events recorded at the institution during the delivery admission or in the postpartum period reduced from 22 (0.08%) in the 3‐year period before introduction of “Thrombocalc” to 4 (0.02%) in the 3‐year period after introduction of Thrombocalc (RR, 0.19; 95% CI, 0.07‐0.56; P < 0.001), with the caveat that these VTE events were not adjudicated.
In an Australian single‐center retrospective study including 153 women, Banahene and colleagues54 evaluated the efficacy and safety of standard‐dose enoxaparin (40 mg) in prevention of pregnancy‐associated VTE in obese women (defined in this study as body mass index ≥30 kg/m2 or weight >90 kg) compared to normal‐weight women, approximately half of whom had a previous VTE history. A total of 77 pregnancies were included in the antepartum analysis, and 145 pregnancies in the postpartum analysis. [Corrections added on February 25, 2020, after first online publication: The values for above sentence updated.] No new antenatal VTE events occurred; however, 2 postpartum events were recorded, 1 in the obese group (1/53; 1.9%) and 1 in the nonobese group (1/93; 1.1%). Both patients had a history of prior VTE. Bleeding rates were similar in both groups.
Mezzarobba and colleagues55 highlighted the potential overuse of LMWH during pregnancy in a 5‐year retrospective analysis of women presenting to hematology and obstetrics outpatient clinics in Buenos Aires, Argentina. Only 37% of prescriptions were appropriate, according to ACCP 2012 recommendations.
The International Network of Venous Thromboembolism Clinical Research Networks (INVENT‐VTE; https://www.invent-vte.com/) hosted an innovative “Dragon's Den” competition aimed at providing an international collaboration grant for an ambitious clinical trial addressing an important knowledge gap. This competition was won by Dr Leslie Skeith (University of Calgary and Canadian Venous Thromboembolism Clinical Trials and Outcomes Research) for the Pilot PARTUM Trial: Postpartum Aspirin to Reduce Thromboembolism Undue Morbidity. PARTUM is a pilot study assessing the feasibility of an RCT evaluating aspirin in postpartum women at risk of developing VTE.
4. VTE AWARENESS DURING PREGNANCY AND THE IMPORTANCE OF RISK ASSESSMENT
The international World Thrombosis Day campaign (https://www.worldthrombosisday.org/; @thrombosisday) aims to increase awareness of thrombosis and prevent death and disability due to this condition. This is highly relevant for pregnant women, their families, and their care providers. In a recent review of international guidelines on PA‐VTE, 8 of 9 guidelines assessed recommended that all women should also undergo VTE risk factor assessment.56 As discussed earlier, a substantial proportion of risk factors may arise for the first time in the peripartum or postpartum period, highlighting the importance of VTE awareness, not just in early pregnancy but also around the time of delivery. The reality of performing VTE risk assessment can be incredibly challenging, particularly in the often chaotically busy environment of the delivery suite or postnatal ward. However, we have recently demonstrated that, when this complex, multistep process is streamlined and tailored to this extremely busy environment with intense multidisciplinary stakeholder involvement, systematic risk assessment of a very high proportion of women is feasible.52
4.1. ISTH 2019 (Melbourne) report
“Emphasis must be put on education and (VTE) awareness of pregnant women.” This was the important message delivered by Ms Christine Ashimwe, World Thrombosis Day ambassador and founder, Rwanda Clot Awareness Network, who conducted a questionnaire‐based cross‐sectional study in 3 referral hospitals in Kigali city, which showed that knowledge of VTE was very low among pregnant women in Kigali, with only 9.33% and 15.7%, respectively, having “good knowledge” of VTE and treatment options respectively.57
5. KNOWLEDGE GAPS
Optimal LMWH dose for prevention of VTE recurrence in women with prior VTE
Validation of clinical prediction tools evaluating absolute risk of VTE during antepartum and postpartum periods
Optimal risk threshold for initiating pharmacological thromboprophylaxis in the antepartum and postpartum periods, particularly in women with lower‐risk thrombophilic traits and multiple (common) VTE risk factors.
Optimal duration of pharmacological thromboprophylaxis in the postpartum period
Absolute bleeding risks and type of bleeding with pharmacologic thromboprophylaxis (eg, incisional versus uterine bleeding).
6. CONCLUSIONS
Pregnancy‐associated VTE can be a devastating event, with significant associated morbidity and mortality. Knowledge and awareness of VTE risk factors is vital to ensure that appropriate risk‐reduction measures are implemented. Well‐designed studies are ongoing and are likely to address important research questions in specific areas. However, important knowledge gaps remain, and these research areas should be prioritized to protect the health and lives of pregnant and postpartum women.
RELATIONSHIP DISCLOSURE
Dr Ewins reports nonfinancial support from LEO Pharma and Amgen, outside the submitted work. Dr Ni Ainle reports grants from Actelion, Leo Pharma, and Bayer, outside the submitted work.
AUTHOR CONTRIBUTION
Both KE and FNA wrote the manuscript.
ACKNOWLEDGEMENT
The authors thank Ms Renata Donciu, who produced the original artwork illustrating Figure 2.
Ewins K, Ní Ainle F. VTE risk assessment in pregnancy. Res Pract Thromb Haemost. 2020;4:183–192. 10.1002/rth2.12290
Handling Editor: Susan Kahn
Contributor Information
Karl Ewins, https://twitter.com/kewins.
Fionnuala Ní Ainle, Email: fniainle@mater.ie, https://twitter.com/fniainle.
REFERENCES
- 1. Berg CJ, Callaghan WM, Syverson C, Henderson Z. Pregnancy‐related mortality in the United States, 1998 to 2005. Obstet Gynecol. 2010;116(6):1302–9. [DOI] [PubMed] [Google Scholar]
- 2. Knight M, Bunch K, Tuffnell D, Jayakody H, Shakespeare J, Kotnis R, et al. Saving lives, improving mothers’ care: lessons learned to inform maternity care from the UK and Ireland Confidential Enquiries into Maternal Deaths and Morbidity 2014–16. Oxford: National Perinatal Epidemiology Unit, University of Oxford; 2018. [Google Scholar]
- 3. Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing GJ, Harjola VP, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2019: 41–44. [DOI] [PubMed] [Google Scholar]
- 4. Abdul Sultan A, Tata LJ, Grainge MJ, West J. The incidence of first venous thromboembolism in and around pregnancy using linked primary and secondary care data: a population based cohort study from England and comparative meta‐analysis. PLoS ONE. 2013;8(7):e70310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kevane B, Donnelly J, D'Alton M, Cooley S, Preston RJ, Ni AF. Risk factors for pregnancy‐associated venous thromboembolism: a review. J Perinat Med. 2014;42(4):417–25. [DOI] [PubMed] [Google Scholar]
- 6. Rodger M. Pregnancy and venous thromboembolism: “TIPPS” for risk stratification. Hematology Am Soc Hematol Educ Program. 2014;2014(1):387–92. [DOI] [PubMed] [Google Scholar]
- 7. Egan K, O’Connor H, Kevane B, Malone F, Lennon A, Zadjali A, et al. Elevated plasma TFPI activity causes attenuated TF‐dependent thrombin generation in early onset preeclampsia. Thromb Haemost. 2017;117(8):1549–57. [DOI] [PubMed] [Google Scholar]
- 8. Haire G, Egan K, Parmar K, McKinnon T, Monteith C, O’Connor H, et al. Alterations in fibrin formation and fibrinolysis in early onset‐preeclampsia: association with disease severity. Eur J Obstet Gynecol Reprod Biol. 2019;241:19–23. [DOI] [PubMed] [Google Scholar]
- 9. Morrison R, Crawford J, MacPherson M, Heptinstall S. Platelet behaviour in normal pregnancy, pregnancy complicated by essential hypertension and pregnancy‐induced hypertension. Thromb Haemost. 1985;54(3):607–11. [PubMed] [Google Scholar]
- 10. Heijnen HF, Schiel AE, Fijnheer R, Geuze HJ, Sixma JJ. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha‐granules. Blood. 1999;94(11):3791–9. [PubMed] [Google Scholar]
- 11. Nurden AT. Platelets, inflammation and tissue regeneration. Thromb Haemost. 2011;105(Suppl 1):S13–33. [DOI] [PubMed] [Google Scholar]
- 12. Sato Y, Fujiwara H, Konishi I. Role of platelets in placentation. Med Mol Morphol. 2010;43(3):129–33. [DOI] [PubMed] [Google Scholar]
- 13. Szklanna PB, Parsons ME, Wynne K, O'Connor H, Egan K, Allen S, et al. The platelet releasate is altered in human pregnancy. Proteomics Clin Appl. 2019;13(3):e1800162. [DOI] [PubMed] [Google Scholar]
- 14. De Laurenzo A, Favuzzi G, Chinni E, Sciannamè N, Vergura P, Cappucci F, et al. Thromboelastography parameters in normal pregnancies. Res Pract Thromb Haemost. 2019;3(Suppl. 1):1–4. [Google Scholar]
- 15. Obeng‐Tuudah D, Hussein B, Lanning L, Riddell A, Gomez K, Abdul‐Kadir R. The changes in platelet function during the three trimesters of uncomplicated pregnancy and puerperium compared to nonpregnant controls. Res Pract Thromb Haemost. 2019;3(Suppl. 1):1–4. [Google Scholar]
- 16. Lindqvist P, Dahlbäck B, Marŝál K. Thrombotic risk during pregnancy: a population study. Obstet Gynecol. 1999;94(4):595–9. [DOI] [PubMed] [Google Scholar]
- 17. Jacobsen AF, Skjeldestad FE, Sandset PM. Ante‐ and postnatal risk factors of venous thrombosis: a hospital‐based case‐control study. J Thromb Haemost. 2008;6(6):905–12. [DOI] [PubMed] [Google Scholar]
- 18. James AH, Jamison MG, Brancazio LR, Myers ER. Venous thromboembolism during pregnancy and the postpartum period: incidence, risk factors, and mortality. Am J Obstet Gynecol. 2006;194(5):1311–5. [DOI] [PubMed] [Google Scholar]
- 19. Liu S, Rouleau J, Joseph KS, Sauve R, Liston RM, Young D, et al. Epidemiology of pregnancy‐associated venous thromboembolism: a population‐based study in Canada. J Obstet Gynaecol Canada. 2009;31(7):611–20. [DOI] [PubMed] [Google Scholar]
- 20. Danilenko‐Dixon DR, Heit JA, Silverstein MD, Yawn BP, Petterson TM, Lohse CM, et al. Risk factors for deep vein thrombosis and pulmonary embolism during pregnancy or post partum: a population‐based, case‐control study. Am J Obstet Gynecol. 2001;184(2):104–10. [DOI] [PubMed] [Google Scholar]
- 21. Simpson E, Lawrenson R, Nightingale A, Farmer R. Venous thromboembolism in pregnancy and the puerperium: incidence and additional risk factors from a London perinatal database. BJOG. 2001;108(1):56–60. [DOI] [PubMed] [Google Scholar]
- 22. Sultan AA, West J, Grainge MJ, Riley RD, Tata LJ, Stephansson O, et al. Development and validation of risk prediction model for venous thromboembolism in postpartum women: multinational cohort study. BMJ. 2016;355:i6253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sultan AA, Tata LJ, West J, Fiaschi L, Fleming KM, Nelson‐Piercy C, et al. Risk factors for first venous thromboembolism around pregnancy: a population‐based cohort study from the United Kingdom. Blood. 2013;121(19):3953–61. [DOI] [PubMed] [Google Scholar]
- 24. Reducing the Risk of Venous Thromboembolism during Pregnancy and the Puerperium. Royal College of Obstetricians and Gynaecologists Green‐top Guideline No 37a; 2015. [Google Scholar]
- 25. Bates SM, Rajasekhar A, Middeldorp S, McLintock C, Rodger MA, James AH, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: venous thromboembolism in the context of pregnancy. Blood Adv. 2018;2(22):3317–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Robertson L, Wu O, Langhorne P, Twaddle S, Clark P, Lowe GDO, et al. Thrombophilia in pregnancy: a systematic review. Br J Haematol. 2006;132(2):171–96. [DOI] [PubMed] [Google Scholar]
- 27. Martinelli I, Legnani C, Bucciarelli P, Grandone E, De Stefano V, Mannucci PM. Risk of pregnancy‐related venous thrombosis in carriers of severe inherited thrombophilia. Thromb Haemost. 2001;86(3):800–3. [PubMed] [Google Scholar]
- 28. Gerhardt A, Scharf RE, Greer IA, Zotz RB. Hereditary risk factors for thrombophilia and probability of venous thromboembolism during pregnancy and the puerperium. Blood. 2016;128(19):2343–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bleker SM, Buchmüller A, Chauleur C, Áinle FN, Donnelly J, Verhamme P, et al. Low‐molecular‐weight heparin to prevent recurrent venous thromboembolism in pregnancy: rationale and design of the highlow study, a randomised trial of two doses. Thromb Res. 2016;144:62–8. [DOI] [PubMed] [Google Scholar]
- 30. Brill‐Edwards P, Ginsberg JS, Gent M, Hirsh J, Burrows R, Kearon C, et al. Safety of withholding heparin in pregnant women with a history of venous thromboembolism. Recurrence of Clot in This Pregnancy Study Group. N Engl J Med. 2000;343(20):1439–44. [DOI] [PubMed] [Google Scholar]
- 31. Pabinger I, Grafenhofer H, Kaider A, Kyrle PA, Quehenberger P, Mannhalter C, et al. Risk of pregnancy‐associated recurrent venous thromboembolism in women with a history of venous thrombosis. J Thromb Haemost. 2005;3(5):949–54. [DOI] [PubMed] [Google Scholar]
- 32. Stefano VD, Martinelli I, Rossi E, Battaglioli T, Za T, Mannuccio Mannucci P, et al. The risk of recurrent venous thromboembolism in pregnancy and puerperium without antithrombotic prophylaxis. Br J Haematol. 2006;135(3):386–91. [DOI] [PubMed] [Google Scholar]
- 33. Cox S, Eslick R, McLintock C. Effectiveness and safety of thromboprophylaxis with enoxaparin for prevention of pregnancy‐associated venous thromboembolism. J Thromb Haemost. 2019;17(7):1160–70. [DOI] [PubMed] [Google Scholar]
- 34. Roeters van Lennep JE, Meijer E, Klumper FJ, Middeldorp JM, Bloemenkamp KW, Middeldorp S. Prophylaxis with low‐dose low‐molecular‐weight heparin during pregnancy and postpartum: is it effective? J Thromb Haemost. 2011;9(3):473–80. [DOI] [PubMed] [Google Scholar]
- 35. White RH, Chan WS, Zhou H, Ginsberg JS. Recurrent venous thromboembolism after pregnancy‐associated versus unprovoked thromboembolism. Thromb Haemost. 2008;100(2):246–52. [PubMed] [Google Scholar]
- 36. O'Shaughnessy F, Donnelly JC, Bennett K, Damkier P, Ainle FN, Cleary BJ. Prevalence of postpartum venous thromboembolism risk factors in an Irish urban obstetric population. J Thromb Haemost. 2019;17(11):1875–85. [DOI] [PubMed] [Google Scholar]
- 37. Bain E, Wilson A, Tooher R, Gates S, Davis LJ, Middleton P. Prophylaxis for venous thromboembolic disease in pregnancy and the early postnatal period. Cochrane Database Syst Rev. 2014;2:CD001689. [DOI] [PubMed] [Google Scholar]
- 38. Rodger MA, Phillips P, Kahn SR, James AH, Konkle BA, Investigators P. Low‐molecular‐weight heparin to prevent postpartum venous thromboembolism. A pilot randomised placebo‐controlled trial. Thromb Haemost. 2015;113(1):212–6. [DOI] [PubMed] [Google Scholar]
- 39. Rodger MA, Phillips P, Kahn SR, Bates S, McDonald S, Khurana R, et al. Low molecular weight heparin to prevent postpartum venous thromboembolism: a pilot study to assess the feasibility of a randomized, open‐label trial. Thromb Res. 2016;142:17–20. [DOI] [PubMed] [Google Scholar]
- 40. Bates SM, Greer IA, Middeldorp S, Veenstra DL, Prabulos AM, Vandvik PO, et al. VTE, thrombophilia, antithrombotic therapy, and pregnancy. Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e691S–736S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chan W‐S, Rey E, Kent NE, Corbett T, David M, Douglas MJ, et al. Venous thromboembolism and antithrombotic therapy in pregnancy. J Obstet Gynaecol Canada. 2014;36(6):527–53. [DOI] [PubMed] [Google Scholar]
- 42. Lindqvist PG, Hellgren M. Obstetric thromboprophylaxis: the Swedish guidelines. Adv Hematol. 2011;2011:157483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Greer IA, Nelson‐Piercy C. Low‐molecular‐weight heparins for thromboprophylaxis and treatment of venous thromboembolism in pregnancy: a systematic review of safety and efficacy. Blood. 2005;106(2):401–7. [DOI] [PubMed] [Google Scholar]
- 44. Tardy BL, Richardson JJ, Nithipipat V, Kempe K, Guo J, Cho KL, et al. Protein adsorption and coordination‐based end‐tethering of functional polymers on metal‐phenolic network films. Biomacromol. 2019;20(3):1421–8. [DOI] [PubMed] [Google Scholar]
- 45. Bistervels I, Buchmüller A, Ní Áinle F, Blecker S, Chauleur C, Donnelly J, et al. Management of anticoagulant therapy around delivery: results from the ongoing Highlow study. Res Pract Thromb Haemost. 2019;3(Suppl. 1):1–4. [Google Scholar]
- 46. Rodger MA, Hague WM, Kingdom J, Kahn SR, Karovitch A, Sermer M, et al. Antepartum dalteparin versus no antepartum dalteparin for the prevention of pregnancy complications in pregnant women with thrombophilia (TIPPS): a multinational open‐label randomised trial. Lancet. 2014;384(9955):1673–83. [DOI] [PubMed] [Google Scholar]
- 47. James A. Committee on Practice B‐O. Practice bulletin no. 123: thromboembolism in pregnancy. Obstet Gynecol. 2011;118(3):718–29. [DOI] [PubMed] [Google Scholar]
- 48. McLintock C, Brighton T, Chunilal S, Dekker G, McDonnell N, McRae S, et al. Recommendations for the prevention of pregnancy‐associated venous thromboembolism. Aust N Z J Obstet Gynaecol. 2012;52(1):3–13. [DOI] [PubMed] [Google Scholar]
- 49. Kotaska A. Postpartum venous thromboembolism prophylaxis may cause more harm than benefit: a critical analysis of international guidelines through an evidence‐based lens. BJOG. 2018;125(9):1109–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Arya R. Postpartum venous thromboembolism prophylaxis: harm versus benefit. BJOG. 2018;125(9):1117. [DOI] [PubMed] [Google Scholar]
- 51. James A, Birsner M, Kaimal A, in collaboration with American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins‐Obstetrics. ACOG Practice Bulletin No. 196: thromboembolism in pregnancy. Obstet Gynecol. 2018;132(1):e1–17. [DOI] [PubMed] [Google Scholar]
- 52. O'Shaughnessy F, Donnelly JC, Cooley SM, Deering M, Raman A, Gannon G, et al. Thrombocalc: implementation and uptake of personalized postpartum venous thromboembolism risk assessment in a high‐throughput obstetric environment. Acta Obstet Gynecol Scand. 2017;96:1382–90. [DOI] [PubMed] [Google Scholar]
- 53. O’Shaughnessy F, Ní Áinle F, Donnelly J, Bennett K, Cleary B. Preventing postpartum venous thromboembolism; impact of systematic VTE risk assessment. Res Pract Thromb Haemost. 2019;3(Suppl. 1):1–4. [Google Scholar]
- 54. Banahene P, Tey A, Cheah R, Yuen A, Choong J, Chunilal S, et al. Performance of standard dose enoxaparin in preventing pregnancy‐associated venous thromboembolism. Res Pract Thromb Haemost. 2019;3(Suppl. 1):1–4. [Google Scholar]
- 55. Mezzarobba D, Penchasky D, Viñuales S, Privitera V, Schutz N, Otero V, et al. Enoxaparin prescription during pregnancy. are we using it according to international guidelines? Res Pract. Thromb Haemost. 2019;3(Suppl. 1):1–4. [Google Scholar]
- 56. Okoroh EM, Azonobi IC, Grosse SD, Grant AM, Atrash HK, James AH. Prevention of venous thromboembolism in pregnancy: a review of guidelines, 2000–2011. J Womens Health (Larchmt). 2012;21(6):611–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ashimwe C. Assessment of the knowledge about venous thromboembolism (VTE) on pregnant women attending Kigali Referral Hospitals. Res Pract Thromb Haemost. 2019;3(Suppl. 1):1–4. [Google Scholar]
- 58. DʼAlton ME, Friedman AM, Smiley RM, Montgomery DM, Paidas MJ, DʼOria R, et al. National partnership for maternal safety: consensus bundle on venous thromboembolism. Obstet Gynecol. 2016;128(4):688–98. [DOI] [PubMed] [Google Scholar]
- 59. Chan W‐S, Rey E, Kent NE, Chan W‐S, Kent NE, Rey E, et al. Venous thromboembolism and antithrombotic therapy in pregnancy. JOGC. 2014;36(6):527–53. [DOI] [PubMed] [Google Scholar]


