Abstract
Background
Global assays measure the interactions of coagulants, anticoagulants, and platelets on thrombin generation and may reflect the comprehensive coagulation potential in patients with hemophilia better than conventional assays.
Objectives
The objectives of the current study were to investigate the value of global assays for measuring and monitoring the coagulation potential of patients with hemophilia A (HA).
Patients/Methods
Rotational thromboelastometry, thrombin generation assay (TGA), and activated partial thromboplastin time (APTT) clot waveform analysis were investigated in a cohort of patients with severe, moderate, and mild HA and compared with conventional assays.
Results
The maximum velocity (MaxVel) parameter of modified thromboelastometry analysis, initiated by tissue factor and in the presence of corn trypsin inhibitor (CTI), had 92% sensitivity and 95% specificity for hemophilia diagnosis. The MaxVel also strongly correlated with factor VIII (FVIII) levels of patients with HA (r = .805, P < .0001). CTI improved the sensitivity of TGA, providing more accurate results. In particular, peak height parameter of platelet‐rich plasma samples with CTI had a sensitivity and specificity of 100% and 94%, respectively, in all patients with HA. APTT clot waveform analysis minimum value of first derivative (Min1) and minimum value of second derivative (Min2) parameters (representing speed and acceleration of clot formation, respectively) were sensitive and correlated more strongly with FVIII levels than APTT clotting times did (Min1: r = 0.786, P < 0.0001; Min2: r = 0.759, P < 0.0001; APTT: r = −0.513, P = 0.001).
Conclusions
The sensitivity and specificity of the global assays was method dependent. Correlation between clinical end points and thrombin generation might also be valuable in the era of non–factor replacement therapy.
Keywords: blood coagulation tests, factor VIII, hemophilia A, thrombin, thromboelastometry
Essentials.
Three global assays were used to measure the coagulability of hemophilia A (HA) blood.
Specific conditions in ROTEM analysis were sensitive for the heterogeneity of coagulation profiles.
Peak height in thrombin generation assay was most sensitive for severity of HA.
Clot waveform analysis correlated more strongly with factor VIII levels than did the APTT.
1. BACKGROUND
Hemophilia A (HA) is one of the most common severe hereditary coagulation disorders and is caused by factor VIII (FVIII) abnormalities. The hallmark of severe HA is spontaneous bleeding into joints and muscles that can be painful and destructive if inadequately treated. Most patients with severe HA require regular replacement therapy, but a small proportion of patients exhibit a mild clinical presentation and need only occasional treatment.1, 2 The FVIII and Factor IX Subcommittee of the International Society of Thrombosis and Hemostasis has recommended that plasma levels be used to classify hemophilia severity into 3 groups: mild, FVIII >5 to 40 IU/dL; moderate, FVIII 1 to 5 IU/dL; and severe, FVIII <1 IU/dL.3, 4
Management of severe HA includes infusion of FVIII on a regular basis to convert the bleeding pattern from a severe to a moderate phenotype, maintaining FVIII trough levels above 1 IU/dL,5 and reducing spontaneous bleeds.6 Accurate measurement of coagulation potential is thus critical to diagnose and manage HA. Clinical laboratories most commonly measure FVIII activity (FVIII:C) by one‐stage activated partial thromboplastin time (APTT) clotting assays (OSA).7 The quality of the deficient plasma, activating reagent, type of standard, and other analytical variables define the lower assay limit.8 As a result, the lower limit of quantitation for conventional assays is approximately 1.0 IU/dL,9 although trough levels are titrated to maintain FVIII ≥1 IU/dL. Furthermore, OSAs mainly capture the initial thrombin generation required for clot formation. Nevertheless, 95% of thrombin generation occurs after initial clot formation.10 Thus, OSAs do not simulate in vivo hemostasis11 and may fail to characterize the true severity of the HA phenotype. Of patients diagnosed with severe HA, about 10% present with a milder phenotype.1
In contrast, global assays such as rotational thromboelastometry (ROTEM), the thrombin generation assay (TGA), and clot waveform analysis reflect a greater range of interactions among procoagulants, anticoagulants, and platelets. Results obtained by thromboelastography are dependent on the activity of the plasma coagulation system, platelet function, and fibrinolysis. TGA reflects the interaction of procoagulants and select anticoagulants and can be performed on both platelet‐poor plasma (PPP) and platelet‐rich plasma (PRP).12 Clot waveforms are derived from constant light‐transmittance measurement taken during routine APTT assays. These sigmoid patterns reflect parameters from the clotting process such as minimum value of first derivative (Min1; coagulation acceleration) and minimum value of second derivative (Min2; coagulation velocity).13, 14, 15
Many recent advances in hemophilia treatment have sparked new interest in global assays. Half‐life extending modifications on recombinant coagulation factors have led to discrepancies in FVIII:C measurements with conventional assays.16 Novel nonreplacement therapies such as FVIII‐mimetic bispecific antibodies17 and tissue factor (TF) pathway inhibitor antibodies18 may be difficult to measure using conventional assays. Researchers have explored the use of global assays to measure coagulation potential with these products.19, 20 Furthermore, evidence suggests that global assays are a useful tool to detect bleeding tendency in people with HA,2, 21, 22, 23, 24, 25 might facilitate individualized treatment of patients with HA,26, 27 and help monitor bypassing agents in patients with inhibitors.28, 29
To investigate and understand differences in global and conventional laboratory assays in the diagnosis and management of HA, we measured the coagulation potential of patients with severe, moderate, and mild HA using thromboelastometry, TGA, and clot waveform analysis and compared the parameters to conventional APTT tests and FVIII levels.
2. PATIENTS/METHODS
2.1. Patients and controls
Patients who participated in this research had previously identified HA and were registered at the Katharine Dormandy Haemophilia and Thrombosis Centre, Royal Free Hospital, London, United Kingdom. All patient material was collected with informed consent. This study was approved by the local ethical committee (the Royal Free Hospital Ethics Committee; reference number: 04/Q050/120) and was conducted in accordance with the International Conference on Harmonization and World Health Organization Good Clinical Practice standards.
Patients from the United Kingdom; ≥16 years old; previously diagnosed with severe, moderate, or mild HA; without inhibitors were enrolled between November 2005 and January 2007 after informed consent was obtained. Samples were collected from 2007 to 2011. Patients with established liver disease and high lipids were excluded. Patients were asked to withhold hemostatic treatment 72 hours before planned sample collection. The local ethics committee recommended this washout period, as there was a potential risk for bleeding with longer washout periods. Some patients with severe HA had FVIII:C >1 IU/dL after the washout period. This group is referred to as severe HA FVIII >1 IU/dL. To calculate reference ranges, healthy male volunteers from Royal Free Hospital staff gave informed consent and provided blood samples.
2.2. Blood sample collection and plasma preparation
Blood samples were collected in S‐Monovette tubes (Sarstedt, Leicester, UK) containing 0.106 mol/L trisodium citrate (1:9, V:V) alone or in combination with corn trypsin inhibitor (CTI) (Haematologic Technologies Inc., Essex Junction, VT, USA) (final concentration 20 μg/mL whole blood). Details on PRP and PPP preparation are reported in the supplemental methods. For tests on whole blood or PRP, samples were analyzed immediately after collection. All other analyses were performed in batches within 6 to 8 weeks of collection. Confirmatory tests were performed within 6 months of collection.
2.3. FVIII activity measurements
Factor VIII activity was measured using APTT lyophilized reagent (Instrumentation Laboratory Ltd, Warrington, UK) and Coatest C/4 kit (Chromogenix, Quadratech, Epsom, Surrey, UK) according to the manufacturer’s instructions. All samples were analyzed on an ACL 300R (Instrumentation Laboratory Ltd). For details, please see the supplemental methods.
2.4. Thromboelastometry analysis
Thromboelastometry using ROTEM (Pentapharm GmbH, Munich, Germany) analysis was performed immediately after collection of citrated whole blood (CWB) samples with or without CTI and activated with recombinant TF (Innovin, Dade Behring, Marburg, Germany), using the nonactivated thromboelastometry protocol. As samples were taken prior to the availability of commercial CTI tubes, CTI was added to tubes before sample collection. All samples taken for ROTEM analysis were rested for 30 minutes (as instructed by the manufacturer) before the test was performed as per protocol. ROTEM in this study was performed as described by Sørensen et al.30 Based on their methodology, the final TF (Innovin, Dade Behring) dilution was 1:17 000, corresponding to a theoretical level of approximately 13 pg/mL (∼0.35 pmol/L). CWB was recalcified by 20 µL 0.2 mol/L CaCl2.
The above method was validated in‐house to check reproducibility and precision. TF concentration was determined by Actichrome TF activity assay (American Diagnostica Inc, Greenwich, CT, USA). TF was added to the thromboelastometry cup, immediately followed by a 300‐µL test sample (with/without CTI) and 20‐µL 200 mmol/L CaCl2. Samples and solutions were mixed and immediately returned to the thromboelastometry cup according to the manufacturer’s instructions. Clotting time (CT), clot formation time (CFT; the time for the clot to reach a width of 20 mm) and alpha angle (α°; to measure the kinetics of clot formation) were measured and analyzed by the software provided with the ROTEM analyzer. Thromboelastometry curves were analyzed using a software program provided with the ROTEM analyzer, which was first described by Sørensen et al.30 The raw data are imported into the ROTEM software CalcuRo (Pentapharm GmbH, Munich, Germany) for analysis of maximum velocity (MaxVel), time to maximum velocity (tMaxVel), and area under the curve (AUCVel) 31.
2.5. Thrombin generation assay
Thrombin generation assay was performed by an in‐house method based on Hemker et al.32 TGA was activated with 1 pmol/L of TF (final concentration) for PPP or 0.5 pmol/L of TF (final concentration) for PRP. PRP was analyzed within 2 hours of collection after a 30‐minute rest period. TF was diluted in working buffer—20 mmol/L of 4‐(2‐hydroxyethyl)‐1‐piperazineethanesulfonic acid (HEPES), 140 mmol/L of NaCl, 5 mg/mL of bovine serum albumin, pH 7.35 (Severn Biotech, Kidderminster, UK). Synthetic phospholipids: phosphatidylcholine, phosphatidylethanolamine, and phosphatidylserine (Avanti Polar Lipids, Alabaster, AL, USA) were prepared using an extrusion method.33 Final phospholipid concentration was 4 mmol/L (20% phosphatidylserine, 20% phosphatidylethanolamine, 60% phosphatidylcholine; a ratio optimized for Xase assembly in the absence of platelets34). TF and phospholipids were mixed in a 96‐well plate (Greiner Bio‐One, Stonehouse, UK), with PPP samples. Only TF was mixed with PRP samples. Mixtures then were incubated at 37°C for 10 minutes; 2.5 mmol/L of fluorogenic substrate (Z‐Gly‐Gly‐Arg‐AMC; Bachem, Bubendorf, Switzerland) in 0.1 mol/L of CaCl2, 20 mmol/L of HEPES, and 60 mg/mL of bovine serum albumin, pH 7.35 (Severn Biotech) was used as a starting reagent and was dispensed into each well. Reactions were measured in a plate reader at the excitation wavelength of 390 nm and the reading wavelength of 460 nm at 30‐second intervals for 1 hour (Spectramax Gemini XS, Molecular Devices, Wokingham, UK). The amount of thrombin generated was calculated according to the method of Hemker and Beguin.35 Peak height (PH), area under the curve (AUC), and time to PH were calculated.36 All parameters were normalized against pooled control plasma prepared using the same method as patient PPP samples (controls were run with each test).
2.6. Clot waveform analysis
APTT tests were performed on the MDA coagulometer (Organon Teknika, Cambridge, UK) using MDA Platelin LS and Platelin LS CaCl2 reagents (Organon Teknika), according to the manufacturer’s protocol. Aliquots of patient and control group plasma were stored at −80°C immediately after processing and were thawed before testing. Min1 (coagulation velocity, percent change in transmittance [T]/s) and Min2 (coagulation acceleration, percent change in T/s2) were observed and analyzed as previously described.37
2.7. Statistical analysis
Statistical analysis was performed using GraphPad Prism version 5.00 (GraphPad Software, La Jolla, CA, USA). Mann‐Whitney U tests were performed to compare the different patient groups to each other and to the controls (mild vs. moderate, moderate vs. severe, severe vs. severe >1 IU/dL). Wilcoxon rank tests were performed to compare paired parameters across the test categories. Kruskal‐Wallis nonparametric analyses of variance tests were performed to analyze the variance among three test categories. Correlation coefficients were determined using the Spearman rank method. The sensitivity, specificity, and likelihood ratio were calculated for the conventional and derived parameters by receiver operating characteristic (ROC) analysis. Sensitivity and specificity analyses were not performed for diagnosis but to investigate the proportion of individuals who had abnormal clot formation (sensitivity) and proportion of normal individuals who had normal clot formation (specificity). ROC analyses were performed on all patients with HA in each test category and compared to 22 healthy individuals. For all statistical analyses, P < 0.05 was considered significant.38
3. RESULTS
A total of 68 patients with previously diagnosed severe HA (n = 41), moderate HA (n = 11), or mild HA (n = 16), were included in the final analysis. The median (range) of FVIII levels measured by OSA were 11 (5‐48 IU/dL) for patients with mild HA and 3 (1‐5 IU/dL) for patients with moderate HA. Eleven patients with severe HA had FVIII levels >1 IU/dL due to ongoing prophylaxis and inadequate washout. These patients formed the >1 IU/dL severe HA group and had a median (range) of FVIII levels of 4 (1.3‐11 IU/dL). Chromogenic FVIII activity assays showed good agreement with OSA results for all patient groups (data not shown).
3.1. Thromboelastometry analysis
Thromboelastometry tests were performed in all HA patient groups under 3 different sample and analytic combinations: CWB, CWB+TF, and CWB+CTI+TF for CT, CFT, α°, MaxVel, tMaxVel, and AUCVel.
In all patient and healthy volunteer samples, CWB+TF condition had shorter CT and CFT than CWB or CWB+CTI+TF (Tables 1 and 2). HA patient groups and the control group were significantly different for CT, CFT, α°, MaxVel, and tMaxVel in all 3 test conditions (Tables 1 and 2).
Table 1.
Thromboelastometry conventional parameters. Median (5th–95th percentile) values are shown for each parameter
| Thromboelastometry parameters | Test category | Normal, n = 22 | Severe, HA, n = 30 | Severe, HA FVIII > 1, n = 11 | Moderate HA, n = 11 | Mild, HA, n = 16 |
|---|---|---|---|---|---|---|
| Clotting time (seconds) | CWB | 597 (354‐937) | 1783** (1132‐5524) | 1174 (852‐1620) | 1262 (772‐1580) | 1019 (641‐1328) |
| CWB+TF | 311 (186‐437) | 754 (372‐1779) | 795 (371‐929) | 486 (348‐757) | 483 (288‐1398) | |
| CWB+CT+TF | 412 (201‐612) | 1018 (464‐2832) | 983 (389‐2759) | 768.5 (374‐1652) | 727 (409‐1554) | |
| Clot formation time (seconds) | CWB | 247 (130‐459) | 537* (288‐1434) | 423 (326‐678) | 348 (216‐522) | 359 (177‐557) |
| CWB+TF | 117 (95‐290) | 291 (180‐587) | 304 (203‐395) | 211 (159‐326) | 211 (102‐323) | |
| CWB+CTI+TF | 178 (112‐280) | 544* (256‐1773) | 536 (235‐1372) | 413 (211‐524) | 366 (183‐738) | |
| α° | CWB | 49.5 (30.4‐61.9) | 28.5* (14.4‐44.6) | 32.5 (24‐42) | 36.5 (28‐44) | 37 (26‐57) |
| CWB+TF | 64 (51.0‐71.0) | 45 (26.5‐57) | 42 (34‐54) | 53 (40‐58) | 53 (42‐69) | |
| CWB+CTI+TF | 57.5 (47.0‐67.7) | 27.5 (10.9‐50.6) | 27 (20‐50) | 35 (28‐52) | 39 (26‐58) |
The median and the range (5th‐95th percentiles) thromboelastometry conventional parameters were calculated in the control group and 6 patient groups in 3 test categories.
Abbreviations: α°, alpha angle; CFT, clot formation time; CT, clotting time; CTI, corn trypsin inhibitor; CWB, citrated whole blood; HA, hemophilia A; TF, tissue factor.
P < 0.05 vs. moderate.
P <0.01 vs. moderate.
Table 2.
Thromboelastometry‐derived parameters. Median (5th–95th percentile) values are shown for each parameter
| Thromboelastometry parameters | Test category | Normal, n = 22 | Severe HA, n = 30 | Severe HA FVIII > 1, n = 11, median (5th‐95th percentile) | Moderate HA, n = 11, median (5th‐95th percentile) | Mild HA, n = 16 |
|---|---|---|---|---|---|---|
| Maximum velocity (mm/min) | CWB | 7.4 (5.1‐12.5) | 3 (1.2‐5.2) | 4 (3.0‐6.0) | 3.3 (2.0‐4.0) | 4 (3.0‐8.0) |
| CWB+TF | 10 (7.0‐15.2) | 5 (2.6‐8.3) | 6 (4.0‐7.3) | 5 (3.4‐8.3) | 7 (5.0‐12.0) | |
| CWB+CTI+TF | 9.0 (6.5‐13.7) | 3.0 (1.9‐6.3) | 4 (1.0‐5.8) | 3.5# (2.90‐4.9) | 5 (3.0‐8.0) | |
| Time to maximum velocity (minutes) | CWB | 9 (5.5‐13.5) | 38.7* (21.9‐92.0) | 23.1 (9.5‐31.6) | 25.9## (18.3‐33.0) | 19.7 (13.3‐26.8) |
| CWB+TF | 7.7 (3.5‐12.3) | 16.9 (9.8‐35.4) | 13.5 (8.4‐20) | 12.4## (10.8‐18.9) | 10.5 (6.6‐13.9) | |
| CWB+CTI+TF | 8.4 (4.7‐14.9) | 22.6 (9.3‐52.2) | 17.5 (8.9‐50) | 19.2 (12.5‐32.2) | 15.8 (10.1‐26.9) | |
| Area under the first derivative curve (mm) | CWB | 55 (43.6‐60.0) | 47.6 (27.8‐61.0) | 43.6 (30.8‐53.4) | 44.2 (39.7‐58.7) | 48.1 (35.4‐60.0) |
| CWB+TF | 58.8 (44.1‐82.0) | 51.6 (39.2‐69.5) | 48.8 (39.9‐56.9) | 49.1 (43.0‐63.4) | 53.6 (46.8‐62.6) | |
| CWB+CTI+TF | 55.5 (48.4‐62.3) | 48 (40.5‐68.3) | 49.4 (45.5‐57.4) | 51.6 (44.6‐65.0) | 52.5 (42.8‐62.0) |
The median and the range (5th‐95th percentiles) thromboelastometry‐derived parameters were calculated in control group and HA patients in 3 categories.
Abbreviations: CTI, corn trypsin inhibitor; CWB, citrated whole blood; FVIII, factor VIII; HA, hemophilia A; MaxVel, maximum velocity; TF, tissue factor.
P < 0.05 vs. moderate.
P < 0.05 vs. mild.
P < 0.01 vs. mild.
For AUCVel, however, the HA patient groups and the control group were significantly different in all conditions except for mild CWB HA samples and moderate and mild CWB+CTI+TF HA samples (Tables 1 and 2).
For CWB samples, CT, CFT, α°, and tMaxVel median differences (95% confidence interval) between severe vs. moderate HA were 563 seconds (330‐1375), 189 seconds (40‐449), 11° (2‐18), and 12.9 minutes (5.5‐28.4), all significant (P = 0.002, 0.02, 0.04, and 0.002, respectively) (Table 2). The median tMaxVel difference for moderate HA vs. mild HA was 6.1 minutes (2.9‐10.5; P = 0.010). In CWB+TF samples, median tMaxVel was 2.0 minutes (0.3‐5.8) longer in moderate HA compared to mild HA (P = 0.007) (Tables 1 and 2).
For CWB+CTI+TF samples, severe HA median CFT 138 seconds (25‐399) was longer than moderate HA patient group (P = 0.02). Median MaxVel was markedly reduced in severe HA, but increased proportionally in patients with moderate and mild HA (Figure 1A‐C). TF initiation dramatically increased variability, particularly in the severe HA patient group (Figure 1B,C). Patients with mild HA were a median of 4 mm/min (2‐5) higher than moderate HA patients (P = 0.019) (Figure 1C), and MaxVel strongly correlated with individual FVIII levels (r = 0.805, P < 0.0001). Furthermore, linear regression analysis showed a significant coefficient of determination (r 2 = 0.648, P < 0.0001) (Figure 1D).
Figure 1.
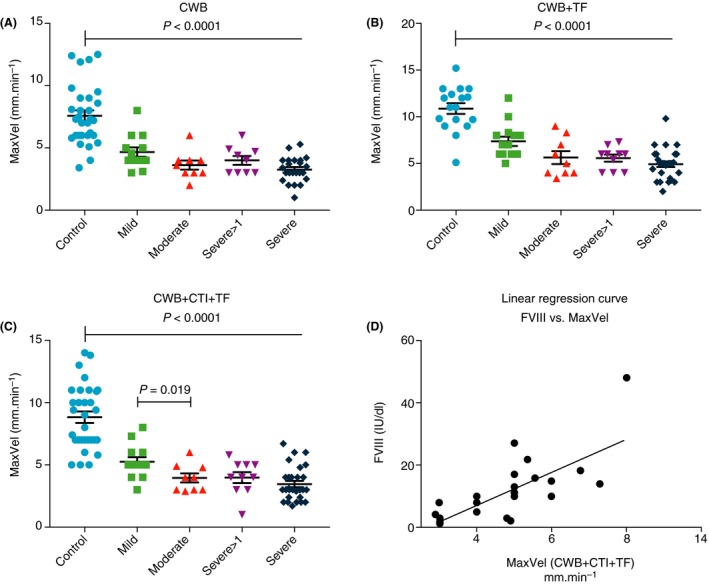
MaxVel parameter of thromboelastometry analysis in haemophilia A patient blood samples treated with the following conditions: (A) CWB, (B) CWB+TF and (C) CWB+CTI+TF. (D) Linear regression curve for FVIII concentration vs thromboelastometry MaxVel in sample condition CWB+CTI+TF showed a significant coefficient of determination (r 2 = .648, P < 0.0001). CTI, corn trypsin inhibitor; CWB, citrated whole blood; FVIII, factor VIII; MaxVel, maximum velocity; TF, tissue factor
Across all severities of HA, the sensitivity for MaxVel was the highest in CWB+CTI+TF (92%; 85% in CWB+TF and 90% in CWB). The specificity was 95% for all test conditions. The positive likelihood ratio, therefore, was highest in CWB+CTI+TF (20.31), followed CWB (18.08) and CWB+TF (16.19).
To avoid skewed results, the ROC analysis was repeated for the mild HA patient group (n = 16) only compared with the control group (n = 22). This analysis found that tMaxVel in sample condition CWB was the most sensitive parameter (100%) of thromboelastometry (95% specificity, 20.0 likelihood ratio). This is compared to sensitivity, specificity, and likelihood ratio of 71%, 89%, and 6.79 in CWB+TF, and 54%, 95%, and 11.85 in CWB+TF+CTI. The sensitivity and specificity of CWB+TF+CTI MaxVel in mild HA was 85% and 95%, respectively.
Wilcoxon rank tests were performed to investigate if MaxVel was different between test categories. The results showed a significant difference between CWB+TF and CWB+CTI+TF (P = 0.0004) where median MaxVel was higher in CWB+TF. This indicates that the addition of TF, without exclusion of contact activation by CTI, may cause overestimation of the results. There was no significant difference between MaxVel in CWB and CWB+CTI+TF. MaxVel in CWB and CWB+TF was significantly different (P = 0.0005).
3.2. TGA analysis
HA patient groups had significantly lower PH and AUC, and longer PH than control samples (Table 3, Table S1).
Table 3.
Thrombin generation assay (in‐house) parameters. Median (5th–95th percentile) values are shown for each parameter
| TGA |
Test Category |
Normal n = 22 |
Severe HA n = 30 |
Severe HA >1 IU/dL n = 11 |
Moderate HA n = 8 |
Mild HA n = 15 |
|---|---|---|---|---|---|---|
| Peak height % | PPP+CTI | 95 (66–152) | 16**## (6.4–31.5) | 24 (15–49) | 28.5 (16–50) | 32.3 (17.8–60.5) |
| PPP | 97 (64–159) | 19.6** (6.7–39.5) | 27 (10.7–46.8) | 44.4 (18.2–47.9) | 42.5 (16.6–84.1) | |
| PRP+CTI | 97 (67–133) | 28.8 (9.3–57.6) | 28.8 (20–35.8) | 30.8 (15.2–70) | 41.6 (29.4–65.2) | |
| PRP | 104 (65–134) | 31.7 (9.5–56.9) | 31.7 (22.1–34.3) | 36.3 (24.9–65.4) | 53.5 (33.1–69.7) | |
|
Area under the thrombin generation curve % |
PPP+CTI | 90.5 (78–133) | 34.4 (12.1–56.7) | 46 (29–78) | 40 (32–70) | 53.3 (27.1–90.8) |
| PPP | 100 (72–124) | 38.8 (14.6–61.5) | 51.5 (23.2–74) | 66.5 (33.6–81.7) | 59.1 (27.9–119.5) | |
| PRP+CTI | 103 (71–115) | 37.2 (10.0–71.4) | 29.7 (22.9–42.5) | 39.7 (21.3–64.4) | 49.4 (28.6–83.8) | |
| PRP | 93 (86–114) | 39.7 (9.0–77.3) | 39.3 (21.3–46.2) | 54.7 (35.0–89.2) | 76.9 (52.0–93.7) | |
|
Time to peak thrombin generation minutes |
PPP+CTI | 12 (9–15) | 20.5 (14.4–52.6) | 18.5 (11–51) | 18.3 (11.5–28) | 17.5 (13–22) |
| PPP | 12 (9–15.5) | 20 (13.5–54.5) | 18.3 (11–39) | 15.3 (11.5–17) | 14.5 (9.5–20) | |
| PRP+CTI | 29 (20.5–49) | 50.8 (37–61) | 58.5 (53–60) | 47.5 (23.5–58) | 38.5 (32.5–56) | |
| PRP | 22 (15–41) | 50 (34.5–60) | 58.5 (54–60) | 41 (25–47.5) | 32.5 (24.5–41) |
The median and the range (5th–95th percentiles) for in‐house TGA were calculated in control group and Hemophilia patients in 4 categories. Significant difference (P value) is shown between mild HA and normal groups only. **P < .01 vs moderate; ## P < .01 vs severe >1 IU/dL.
Abbreviations: CTI, corn trypsin inhibitor; HA, hemophilia A; PPP, platelet poor plasma; PRP, platelet rich plasma; TGA, thrombin generation assay.
In PPP, PHs of severe HA patients were 7% (3%‐20%) lower with CTI and 28% (3.3%‐34.2%) lower with no CTI than moderate HA patients (P < 0.002 for both). In PPP+CTI samples, median PH was 8% (4%‐13%) lower for the severe HA group than severe >1 IU/dL patient group (P = 0.0015) (Figure 2B). However, median of PPP for the mild and moderate groups were similar (Figure 2A‐D). Interestingly, in the PRP and PRP+CTI groups, severe and moderate PHs were similar.
Figure 2.
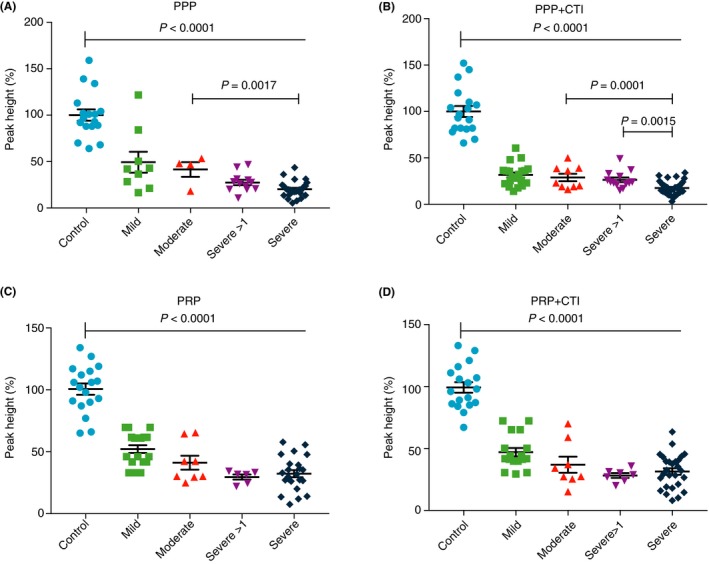
TGA peak height of blood samples from patients with hemophilia A under the following conditions: (A) PPP, (B) PPP+CTI, (C) PRP, and (D) PRP+CTI. CTI, corn trypsin inhibitor; PPP; platelet‐poor plasma, PRP; platelet‐rich plasma, TGA, thrombin generation assay
Although the differences between the groups with and without CTI were not significant, ROC analysis demonstrated an improved sensitivity for diagnosing hemophilia under conditions tested with CTI.
ROC analysis for the mild HA patient group (n = 16) compared with the control group (n = 22) showed that PH provided the best sensitivity with CTI (94% in PPP+CTI vs. 80% in PPP only). Within PH, CTI improved sensitivity further, with PRP+CTI achieving 100% sensitivity (likelihood ratio, 18.0), compared with 83% sensitivity (likelihood ratio, 15.0) in PRP only. Specificity for TP and AUC parameters was 94% in all test conditions.
Using the Wilcoxon paired test, PH values for HA patient PPP samples were significantly lower than PRP samples without CTI (P = 0.0004) and with CTI (P = 0.02). No differences were found between the PHs of PPP and PPP+CTI samples (P = 0.14), and PRP and PRP+CTI (P = .73) samples (Figure 2A‐D).
3.3. Clot waveform analysis
Min1 and Min2 were significantly lower in all HA patient groups compared to control samples (Table 4). Furthermore, Min1 and Min2 of severe HA patients (1.5% T/s [0.56‐3.14]), 0.11% T/s2) were lower than moderate HA patients (2.52% T/s [1.39‐4.28], 0.25% T/s2; P = 0.003 and 0.001). Median Min1 and Min2 of moderate HA patients were slightly lower than that of mild patients (3.54% T/s [2.15‐5.57], 0.37% T/s2 [0.19‐0.59], P > 0.05) (Table 4).
Table 4.
Clot waveform analysis of HA and control group. Median (5th–95th percentile) values are shown for each parameter
| Clot wave form | Normal, n = 22 | Severe, HA, n = 30 | Severe, HA, >1 IU/dL, n = 11 | Moderate, HA, n = 8 | Mild, HA, n = 15 |
|---|---|---|---|---|---|
| Min1, %T/s | 6.62 (5.42‐8.67) | 1.50* (0.56‐3.14) | 2.79 (1.86‐3.82) | 2.52** (1.39‐4.28) | 3.54 (2.15‐5.57) |
| Min2, %T/s2 | 0.58 (0.51‐0.76) | 0.11* (0.03‐0.29) | 0.28 (0.19‐0.36) | 0.25** (0.12‐0.45) | 0.37 (0.19‐0.59) |
| APTT, s | 29.0 (24‐36) | 70.2 (55.5‐111.6) | 56.5 (44.1‐76.5) | 53.1 (45‐66.1) | 42.2 (35.8‐75.9) |
| FVIII, IU/dL | 100 (50‐150) | <1 | 4 (1.4‐11) | 3 (1‐5) | 11 (5‐48) |
The median and the range (5th‐95th percentiles) Clot waveform parameters, APTT, and FVIII/factor IX levels were calculated in patient and control groups.
Abbreviations: APTT, activated partial thromboplastin time; FVIII, factor VIII; HA, hemophilia A; Min1, minimum value of first derivative (coagulation velocity, percent change in transmitance (T)/s); Min2, minimum value of second derivative (coagulation acceleration, percent change in transmitance (T)/s2).
Significant vs. moderate (P < 0.05).
Significant vs. mild.
As with thromboelastometry and TGA analyses, ROC was performed for the mild HA patients group compared to the healthy patient group, resulting in a 93% sensitivity, 96% specificity, and 21.5 likelihood ratio for Min1 and 80% sensitivity, 96% specificity, and 18.4 likelihood ratio for Min2.
Min1 correlated stronger with FVIII levels than with APTT (Min1, r = .786, P < 0.0001 vs. APTT, r = −0.513, P = 0.001). This was also true for Min2 (r = 0.759, P < 0.0001). The correlation between FVIII levels and APTT was only moderately strong, indicating that Min1 or Min2 may more accurately reflect FVIII levels than APTT. Similar results were seen with linear regressions (Figure S1).
4. DISCUSSION
In this evaluation of clinical HA samples measured by global assays, we have demonstrated that thromboelastometry differentiates between moderate and mild HA, while TGA and clot waveform analysis were better able to distinguish between severe and moderate HA.
The accuracy and precision of thromboelastometry measurements can be affected by preanalytical variables, including specimen collection techniques, transport, and storage. In all patient and healthy volunteer samples, CWB+TF condition had the lowest CT and CFT values, suggesting early coagulation and simultaneous activation via both the extrinsic and intrinsic pathway. Thromboelastometry analysis with CWB and CWB+CTI+TF was the most useful for identifying the heterogeneity of patients’ global coagulation profiles.
The tMaxVel of CWB samples distinguished individuals with HA from the healthy population with 100% sensitivity and 94% specificity. However, the CWB test condition does not add discriminatory or diagnostic value to conventional assays. CWB is dominated by contact activation and coagulation through the intrinsic pathway, mimicking the OSAs. Addition of CTI and TF ensures activation through the extrinsic pathway followed by the intrinsic pathway, simulating in vivo coagulation. In CWB+CTI+TF samples, MaxVel differentiated between the severe, moderate, and mild hemophilia populations and strongly correlated with individual FVIII levels. Furthermore, CWB+CTI+TF MaxVel had an 85% sensitivity and 95% specificity for the diagnosis of mild HA. In CWB+TF samples, however, MaxVel was less sensitive (57%) but specific (95%), indicating that CTI is essential to improve the sensitivity when TF is used to activate coagulation.
The MaxVel was markedly depressed in severe HA, but increased proportionally in patients with moderate and mild hemophilia. The absence of statistical significance between severe and moderate HA may be related to lack of complete washout, or represent underlying variability contributing to fewer bleeding episodes in some severe patients and marked bleeding tendency in some moderate patients. TF initiation improved the tracing, but dramatically increased variability, particularly in the severe HA group. This variability might be due to clot formation by other components of blood (such as red blood cells, platelets, and white blood cells). It is also possible that thromboelastometry identified changes in FVIII levels at <1.0 IU/dL in this study. Indeed, the correlation between the MaxVel in CWB+CTI+TF and the level of FVIII was strong and significant, and the linear regression analysis showed a significant coefficient of determination.
Clinical application of the TGA has increased in recent years, but its utility and reliability in various clinical scenarios remains unclear 22, 23, 24, 39, 40, 41, 42. TGA appears to be a reliable test for excluding individuals with lower than normal coagulation FVIII levels. In this study, the use of CTI to eliminate the interference of contact‐activated coagulation improved the accuracy and precision of thrombin generation measures. In particular, PH with PRP+CTI samples achieved 100% sensitivity and a likelihood ratio of 18.0 (PRP: 83% sensitivity, likelihood ratio, 15.0).
Although the results generally confirmed differences between severe, moderate, and mild groups, there were a number of crossovers in both thromboelastometry and TGA results. This may suggest that not all patients with severe, moderate, or mild HA have the same coagulation potential. Modified thromboelastometry analysis with CTI and TF mimics in vivo hemostasis and thus may predict clinical phenotype more accurately. This is in line with Chitlur et al,43 who concluded that thromboelastometry parameters showed evidence of faster and better clot formation by a higher maximum thrombin/fibrin generation in patients with mild bleeding manifestations compared to those with severe bleeding tendency. Furthermore, Santagostino et al2 demonstrated that TGA was able to detect increased thrombin generation in patients with severe HA who had a mild phenotype. Thus, additional information provided by global assays capturing the effects of other coagulation factors and platelets might help improve management of patients with hemophilia.2, 44, 45, 46
In the present study, the clot waveform parameters, Min1 and Min2, appeared to be reliable predictors of FVIII levels <1.0 IU/dL. The wide ranges of Min1 (5th‐95th percentile = 8%‐47%) and Min2 (5th‐95th percentile = 5%‐48%) suggests that heterogeneity exists in patients with severe HA, and categorizing all patients with severe HA under the same <1.0 IU/dL FVIII activity level may not reflect the manifestation of their clinical phenotype.
Furthermore, Min1 and Min2 correlated better with FVIII levels than APTT did,14 suggesting that APTT might be variably affected by other plasma factors15 and raises the possibility that Min1 and Min2 could be used interchangeably with APTT as an end point for factor assays. Other studies have also indicated that clot waveform analysis may have greater discriminatory power in assessing low‐clotting‐factor‐activity samples, providing additional information in patients with severe HA.37, 47, 48 The increased sensitivity of Min1 and Min2 may also be valuable for other uses, such as monitoring patients treated with novel coagulation factor products or nonreplacement products49 or in emergencies when technical support is limited.
The heterogeneity of thrombin generation, in particular in severe HA, was demonstrated by all 3 global assays. This indicates that the rate and amount of thrombin generation are not exclusively dependent on the amount of FVIII or level in the samples. The use of CTI to eliminate the interference of the contact system was also shown to play an important role in improving the accuracy and precision of thrombin generation. This confirms earlier reports by other groups that the use of CTI at low TF concentration (1.0 ρM) for initiation of thrombin generation improves assay variations.24, 50, 51
Clot waveform analysis was carried out in this study and showed that heterogeneity exists among individuals with severe HA and was more pronounced in Min1 and Min2 parameters when compared to the APTT. Min1 and Min2 appear to be sensitive, simple, fast, and cost effective in diagnosing hypocoagulability and may be useful in the monitoring of patients with hemophilia.
A major limitation of our study was related to the washout period. As the ethics committee restricted the washout period to 72 hours, a correlation between the global assays and clinical phenotype could not be undertaken. Furthermore, no analysis was performed on interoccasion variability to ascertain the robustness of these assays for use in routine clinical practice. Currently, the company (Organon Teknika) that made the instrument and APTT reagents used to perform clot waveform measurements in this study has ceased production. However, we have previously compared methods used in this study to clot waveform measurements made using an instrument and APTT reagent widely in use today (ACL TOP series analyzer and the APTT reagent SynthASil, Instrumentation Laboratory Ltd, Warrington, UK). Min1 and Min2 strongly correlated with the Max1 and Max2 parameters of the ACT TOP series analyzer (r = 0.88, P < 0.0001 for both parameters).52
Differences in the coagulation profile of samples measured with global assays and conventional OSAs suggests that various factors contribute to severity of clinical HA phenotype. A better understanding of patients’ global coagulation profile may help individualize prophylaxis treatment53 and improve clinical outcomes.54. However, the small number of subjects in this study, and other limitations, means that further research with robust clinical end points is required to define the roles of these assays in clinical care.
5. CONCLUSION
The sensitivity and specificity of the global assays was method dependent. The use of CTI and low doses of TF enhanced the sensitivity of both TGA and thromboelastometry. TGA of PRP with TF in the presence of CTI was able to discriminate between severe and moderate HA. Similarly, with thromboelastometry, MaxVel in CWB+CTI+TF samples differentiated between the severe, moderate, and mild HA populations and strongly correlated with individual FVIII levels. Furthermore, clot waveform analysis was more sensitive and correlated better with FVIII level than APTT and could potentially be incorporated into the methodology for factor assay measurement. Correlation between clinical end points and thrombin generation might also be valuable in the era of non–replacement product therapy.
RELATIONSHIP DISCLOSURE
SA was an employee of KDHT Royal Free Hospital, where this research was carried out. He is now an employee of Novo Nordisk Ltd. PC has served on advisory boards for Bayer, Baxalta/Shire, Biogen Idec, CSL Behring, Chugai, Freeline, Novo Nordisk, Pfizer, Roche, Sanofi, and Sobi; and has received research funding unrelated to this manuscript from Bayer, CSL Behring, Novo Nordisk, Pfizer and Sobi. All other authors report no conflicts of interest.
AUTHOR CONTRIBUTIONS
All authors contributed to the conception, design, and interpretation of the research presented here and participated in the preparation and revision of the manuscript. All authors read and approved the final version.
Supporting information
ACKNOWLEDGMENTS
Coagulation assays were performed in the Haemostasis Laboratory, Addenbrooke's Hospital, Cambridge, UK with kind permission of Dr Roger Luddington. This work was submitted for a doctorate in biomedical science thesis. Editorial and writing support for this manuscript was provided by Physicians World Europe (Mannheim, Germany) funded by a grant from Novo Nordisk A/S (Bagsvaerd, Denmark). We thank Professor Edward Tuddenham, Dr Vincent Jenkins, Dr Dale Owen, Dr Alex Gatt, Dr Thynn Yee, and Dr Keith Gomez, Ms Chris Harrington, Ms Debra Pollard, Ms Barbara Suebel and the late Ms Patricia Lilley for their support. We would like to thank the patients and center staff.
Aghighi S, Riddell A, Lee CA, Brown SA, Tuddenham E, Chowdary P. Global coagulation assays in hemophilia A: A comparison to conventional assays. Res Pract Thromb Haemost. 2020;4:298–308. 10.1002/rth2.12295
Handling Editor: Pantep Angchaisuksiri
Funding information
Funding for the study was provided by Katharine Dormandy Trust and Royal Free charity (TF35).
Contributor Information
Saman Aghighi, Email: sxag@novonordisk.com.
Pratima Chowdary, https://twitter.com/chowdarypm.
REFERENCES
- 1. Aledort LM, Haschmeyer RH, Pettersson H. A longitudinal study of orthopaedic outcomes for severe factor‐VIII‐deficient haemophiliacs. The Orthopaedic Outcome Study Group. J Intern Med. 1994;236:391–9. [DOI] [PubMed] [Google Scholar]
- 2. Santagostino E, Mancuso ME, Tripodi A, Chantarangkul V, Clerici M, Garagiola I, et al. Severe hemophilia with mild bleeding phenotype: molecular characterization and global coagulation profile. J Thromb Haemost. 2010;8:737–43. [DOI] [PubMed] [Google Scholar]
- 3. White G, Rosendaal F, Aledort L, Lusher J, Rothschild C, Ingerslev J. Definitions in hemophilia. Recommendation of the scientific subcommittee on factor VIII and factor IX of the scientific and standardization committee of the International Society on Thrombosis and Haemostasis. Thromb Haemost. 2001;85:560. [PubMed] [Google Scholar]
- 4. Makris M, Oldenburg J, Mauser‐Bunschoten EP, Peerlinck K, Castaman G, Fijnvandraat K. The definition, diagnosis and management of mild hemophilia A: communication from the SSC of the ISTH. J Thromb Haemost. 2018;16:2530–3. [DOI] [PubMed] [Google Scholar]
- 5. National Hemophilia Foundation, Medical and Scientific Advisory Council . MASAC recommendations concerning prophylaxis. New York, NY: National Hemophilia Foundation, Medical and Scientific Advisory Council; 2016. [Google Scholar]
- 6. Nilsson IM, Berntorp E, Lofqvist T, Pettersson H. Twenty‐five years' experience of prophylactic treatment in severe haemophilia A and B. J Intern Med. 1992;232:25–32. [DOI] [PubMed] [Google Scholar]
- 7. Kitchen S, Gray E, Mertens K. Monitoring of modified factor VIII and IX products. Haemophilia. 2014;20(Suppl 4):36–42. [DOI] [PubMed] [Google Scholar]
- 8. Mackie I, Cooper P, Lawrie A, Kitchen S, Gray E, Laffan M, et al. Guidelines on the laboratory aspects of assays used in haemostasis and thrombosis. Int J Lab Hematol. 2013;35:1–13. [DOI] [PubMed] [Google Scholar]
- 9. Yatuv R, Dayan I, Baru M. A modified chromogenic assay for the measurement of very low levels of factor VIII activity (FVIII:C). Haemophilia. 2006;12:253–7. [DOI] [PubMed] [Google Scholar]
- 10. Mann KG, Brummel K, Butenas S. What is all that thrombin for? J Thromb Haemost. 2003;1:1504–14. [DOI] [PubMed] [Google Scholar]
- 11. Mann KG, Butenas S, Brummel K. The dynamics of thrombin formation. Arterioscler Thromb Vasc Biol. 2003;23:17–25. [DOI] [PubMed] [Google Scholar]
- 12. Luddington RJ. Thrombelastography/thromboelastometry. Clin Lab Haematol. 2005;27:81–90. [DOI] [PubMed] [Google Scholar]
- 13. Bates SM, Weitz JI. Coagulation assays. Circulation. 2005;112:e53–60. [DOI] [PubMed] [Google Scholar]
- 14. Shima M, Thachil J, Nair SC, Srivastava A. Towards standardization of clot waveform analysis and recommendations for its clinical applications. J Thromb Haemost. 2013;11:1417–20. [DOI] [PubMed] [Google Scholar]
- 15. Shima M, Matsumoto T, Ogiwara K. New assays for monitoring haemophilia treatment. Haemophilia. 2008;14(Suppl 3):83–92. [DOI] [PubMed] [Google Scholar]
- 16. Gu JM, Ramsey P, Evans V, Tang L, Apeler H, Leong L, et al. Evaluation of the activated partial thromboplastin time assay for clinical monitoring of PEGylated recombinant factor VIII (BAY 94–9027) for haemophilia A. Haemophilia. 2014;20:593–600. [DOI] [PubMed] [Google Scholar]
- 17. Shima M, Hanabusa H, Taki M, Matsushita T, Sato T, Fukutake K, et al. Factor VIII‐mimetic function of humanized bispecific antibody in hemophilia A. N Engl J Med. 2016;374:2044–53. [DOI] [PubMed] [Google Scholar]
- 18. Chowdary P, Lethagen S, Friedrich U, Brand B, Hay C, Abdul Karim F, et al. Safety and pharmacokinetics of anti‐TFPI antibody (concizumab) in healthy volunteers and patients with hemophilia: a randomized first human dose trial. J Thromb Haemost. 2015;13:743–54. [DOI] [PubMed] [Google Scholar]
- 19. Waters EK, Hilden I, Sorensen BB, Ezban M, Holm PK. Thrombin generation assay using factor XIa to measure factors VIII and IX and their glycoPEGylated derivatives is robust and sensitive. J Thromb Haemost. 2015;13:2041–52. [DOI] [PubMed] [Google Scholar]
- 20. Waters EK, Sigh J, Friedrich U, Hilden I, Sorensen BB. Concizumab, an anti‐tissue factor pathway inhibitor antibody, induces increased thrombin generation in plasma from haemophilia patients and healthy subjects measured by the thrombin generation assay. Haemophilia. 2017;23:769–76. [DOI] [PubMed] [Google Scholar]
- 21. Dargaud Y, Beguin S, Lienhart A, Al Dieri R, Trzeciak C, Bordet JC, et al. Evaluation of thrombin generating capacity in plasma from patients with haemophilia A and B. Thromb Haemost. 2005;93:475–80. [DOI] [PubMed] [Google Scholar]
- 22. Dargaud Y, Bordet JC, Francillon S, Negrier C. Haemophilia patients exhibit prolonged coagulation time but normal lag time of thrombin generation test: Are these results really discordant? Thromb Haemost. 2007;97:675–6. [PubMed] [Google Scholar]
- 23. Lewis SJ, Stephens E, Florou G, Macartney NJ, Hathaway LS, Knipping J, et al. Measurement of global haemostasis in severe haemophilia A following factor VIII infusion. Br J Haematol. 2007;138:775–82. [DOI] [PubMed] [Google Scholar]
- 24. Luddington R, Baglin T. Clinical measurement of thrombin generation by calibrated automated thrombography requires contact factor inhibition. J Thromb Haemost. 2004;2:1954–9. [DOI] [PubMed] [Google Scholar]
- 25. van Dijk K, van der Bom JG, Lenting PJ, de Groot PG, Mauser‐Bunschoten EP, Roosendaal G, et al. Factor VIII half‐life and clinical phenotype of severe hemophilia A. Haematologica. 2005;90:494–8. [PubMed] [Google Scholar]
- 26. Aledort LM. Why thrombin generation? From bench to bedside. Pathophysiol Haemost Thromb. 2003;33:2–3. [DOI] [PubMed] [Google Scholar]
- 27. Al Hawaj MA, Martin EJ, Venitz J, Barrett JC, Kuhn JG, Nolte ME, et al. Monitoring rFVIII prophylaxis dosing using global haemostasis assays. Haemophilia. 2013;19:409–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Haku J, Nogami K, Matsumoto T, Ogiwara K, Shima M. Optimal monitoring of bypass therapy in hemophilia A patients with inhibitors by the use of clot waveform analysis. J Thromb Haemost. 2014;12:355–62. [DOI] [PubMed] [Google Scholar]
- 29. Furukawa S, Nogami K, Ogiwara K, Yada K, Minami H, Shima M. Systematic monitoring of hemostatic management in hemophilia A patients with inhibitor in the perioperative period using rotational thromboelastometry. J Thromb Haemost. 2015;13:1279–84. [DOI] [PubMed] [Google Scholar]
- 30. Sorensen B, Johansen P, Christiansen K, Woelke M, Ingerslev J. Whole blood coagulation thrombelastographic profiles employing minimal tissue factor activation. J Thromb Haemost. 2003;1:551–8. [DOI] [PubMed] [Google Scholar]
- 31. Landskroner KA, Olson NC, Jesmok GJ. Thromboelastography measurements of whole blood from factor VIII‐deficient mice supplemented with rFVIII. Haemophilia. 2005;11:346–52. [DOI] [PubMed] [Google Scholar]
- 32. Hemker HC, Giesen P, Al Dieri R, Regnault V, de Smedt E, Wagenvoord R, et al. Calibrated automated thrombin generation measurement in clotting plasma. Pathophysiol Haemost Thromb. 2003;33:4–15. [DOI] [PubMed] [Google Scholar]
- 33. Hope MJ, Bally MB, Webb G, Cullis PR. Production of large unilamellar vesicles by a rapid extrusion procedure: characterization of size distribution, trapped volume and ability to maintain a membrane potential. Biochim Biophys Acta. 1985;812:55–65. [DOI] [PubMed] [Google Scholar]
- 34. Gilbert GE, Novakovic VA, Shi J, Rasmussen J, Pipe SW. Platelet binding sites for factor VIII in relation to fibrin and phosphatidylserine. Blood. 2015;126:1237–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hemker HC, Beguin S. Thrombin generation in plasma: its assessment via the endogenous thrombin potential. Thromb Haemost. 1995;74:134–8. [PubMed] [Google Scholar]
- 36. Chantarangkul V, Clerici M, Bressi C, Giesen PLA, Tripodi A. Thrombin generation assessed as endogenous thrombin potential in patients with hyper‐ or hypocoagulability. Haematologica. 2003;88:547–54. [PubMed] [Google Scholar]
- 37. Shima M, Matsumoto T, Fukuda K, Kubota Y, Tanaka I, Nishiya K, et al. The utility of activated partial thromboplastin time (aPTT) clot waveform analysis in the investigation of hemophilia A patients with very low levels of factor VIII activity (FVIII:C). Thromb Haemost. 2002;87:436–41. [PubMed] [Google Scholar]
- 38. Zweig MH, Campbell G. Receiver‐operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem. 1993;39:561–77. [PubMed] [Google Scholar]
- 39. Dargaud Y, Luddington R, Gray E, Lecompte T, Siegemund T, Baglin T, et al. Standardisation of thrombin generation test ‐ which reference plasma for TGT?: An international multicentre study. Thromb Res. 2010;125:353. [DOI] [PubMed] [Google Scholar]
- 40. Hemker HC, Al Dieri R, De Smedt E, Beguin S. Thrombin generation, a function test of the haemostatic‐thrombotic system. Thromb Haemost. 2006;96:553–61. [PubMed] [Google Scholar]
- 41. van Veen JJ, Gatt A, Bowyer AE, Cooper PC, Kitchen S, Makris M. Calibrated automated thrombin generation and modified thromboelastometry in haemophilia A. Thromb Res. 2009;123:895–901. [DOI] [PubMed] [Google Scholar]
- 42. Bolton‐Maggs PH, Pasi KJ. Haemophilias A and B. Lancet. 2003;361:1801–9. [DOI] [PubMed] [Google Scholar]
- 43. Chitlur M, Warrier I, Rajpurkar M, Hollon W, Llanto L, Wiseman C, et al. Thromboelastography in children with coagulation factor deficiencies. Br J Haematol. 2008;142:250–6. [DOI] [PubMed] [Google Scholar]
- 44. Driessler F, Miguelino MG, Pierce GF, Peters RT, Sommer JM. Evaluation of recombinant factor VIII Fc (Eloctate) activity by thromboelastometry in a multicenter phase 3 clinical trial and correlation with bleeding phenotype. Blood Coagul Fibrinolysis. 2017;28:540–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chitlur M, Young G. Global assays in hemophilia. Semin Hematol. 2016;53:40–5. [DOI] [PubMed] [Google Scholar]
- 46. Young G, Sorensen B, Dargaud Y, Negrier C, Brummel‐Ziedins K, Key NS. Thrombin generation and whole blood viscoelastic assays in the management of hemophilia: current state of art and future perspectives. Blood. 2013;121:1944–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Siegemund T, Scholz U, Schobess R, Siegemund A. Clot waveform analysis in patients with haemophilia A. Hamostaseologie. 2014;34(Suppl 1):S48–52. [DOI] [PubMed] [Google Scholar]
- 48. Matsumoto T, Nogami K, Tabuchi Y, Yada K, Ogiwara K, Kurono H, et al. Clot waveform analysis using CS‐2000i distinguishes between very low and absent levels of factor VIII activity in patients with severe haemophilia A. Haemophilia. 2017;23:e427–e435. [DOI] [PubMed] [Google Scholar]
- 49. Nogami K, Matsumoto T, Tabuchi Y, Soeda T, Arai N, Kitazawa T, et al. Modified clot waveform analysis to measure plasma coagulation potential in the presence of the anti‐factor IXa/factor X bispecific antibody emicizumab. J Thromb Haemost. 2018;16:1078–88. [DOI] [PubMed] [Google Scholar]
- 50. van Veen JJ, Gatt A, Makris M. Thrombin generation testing in routine clinical practice: are we there yet? Br J Haematol. 2008;142:889–903. [DOI] [PubMed] [Google Scholar]
- 51. Dargaud Y, Lienhart A, Meunier S, Hequet O, Chavanne H, Chamouard V, et al. Major surgery in a severe haemophilia A patient with high titre inhibitor: use of the thrombin generation test in the therapeutic decision. Haemophilia. 2005;11:552–8. [DOI] [PubMed] [Google Scholar]
- 52. Riddell AF, Aghighi S, Gandhi T, Vijayakumar E, Brooks S, Chowdary P. Evaluation of APTT clot waveform analysis by IL ACL TOP® in patients with Haemophilia and comparison to clot waveform analysis with MDA® 180. J Thromb Haemost. 2011;9:946. [Google Scholar]
- 53. Collins PW, Blanchette VS, Fischer K, Bjorkman S, Oh M, Fritsch S, et al. Break‐through bleeding in relation to predicted factor VIII levels in patients receiving prophylactic treatment for severe hemophilia A. J Thromb Haemost. 2009;7:413–20. [DOI] [PubMed] [Google Scholar]
- 54. Valentino LA. Considerations in individualizing prophylaxis in patients with haemophilia A. Haemophilia. 2014;20:607–15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


