Abstract
Background
Chronic obstructive pulmonary disease (COPD) is associated with risk of venous thromboembolism (VTE). It remains unknown whether individual respiratory symptoms and lowered oxygen saturation (SpO2), individually and in combination with COPD, affect the risk of VTE.
Objectives
To investigate whether measures of respiratory impairments including respiratory symptoms and SpO2, individually and combined with COPD, were associated with an increased risk of VTE.
Methods
Spirometry, SpO2, and self‐reported respiratory symptoms were collected in 8686 participants from the fifth (2001‐2002) and sixth (2007‐2008) surveys of the Tromsø Study. Incident VTE events were registered from the date of inclusion to December 31, 2016. Cox regression models with exposures and confounders as time‐varying covariates (for repeated measurements) were used to estimate hazard ratios (HRs) with 95% confidence intervals (CIs) for VTE.
Results
During a median follow‐up of 9.1 years, 330 participants developed incident VTE. Subjects with SpO2 ≤ 96% (lowest 20th percentile) had a 1.5‐fold higher risk of VTE (adjusted HR, 1.48; 95% CI, 1.13‐1.93) compared with those with SpO2 ≥ 98%. Severe respiratory symptoms (dyspnea, cough, and phlegm) were associated with a 1.4‐ to 2.0‐fold higher risk of VTE compared with no such symptoms. COPD, combined with respiratory symptoms or lowered SpO2, had an additive effect on the VTE risk.
Conclusions
Lowered SpO2 and severe respiratory symptoms were associated with increased VTE risk. COPD combined with respiratory impairments had an additive effect on VTE risk, and may suggest particular attention on VTE preventive strategies in COPD patients with respiratory impairments.
Keywords: chronic obstructive pulmonary disease, oxygen saturation, respiratory symptoms, risk, venous thromboembolism
Essentials.
Whether respiratory symptoms and oxygen saturation (SpO2) affect the risk of venous thromboembolism (VTE) is not known.
We explored the association in a cohort of 8686 participants followed for a median of 9.1 years.
Lowered SpO2 and severe respiratory symptoms were associated with increased VTE risk.
Chronic obstructive pulmonary disease combined with respiratory impairments had an additive effect on VTE risk.
1. INTRODUCTION
The global prevalence of chronic obstructive pulmonary disease (COPD) has increased substantially during the past decades1 and represents a major challenge to health care systems. COPD is associated with frequent hospitalizations, grave comorbidities, and a high mortality rate.2, 3 Around 5%–10% of patients hospitalized for acute COPD exacerbation die during the hospital stay, and 20% die during the first year after hospital discharge.4, 5
Results from registry‐based studies have reported that COPD is associated with a 2‐ to 5‐fold increased risk of venous thromboembolism (VTE),6, 7, 8 and the prevalence of acute pulmonary embolism (PE) is high (15%–30%) in patients with COPD hospitalized with suspected acute exacerbation.7, 9, 10 Recent population‐based cohorts, with respiratory function assessed by spirometry and validated evaluation of confounding factors and VTE events, confirmed that COPD was associated with a moderately increased risk of VTE11 and showed that the VTE risk increased with the severity of COPD.11, 12 Furthermore, respiratory symptoms (eg, cough, phlegm, and dyspnea) were associated with VTE risk, even in subjects with normal spirometry measurements,11 suggesting that respiratory symptoms may represent risk markers for VTE.
Although the mechanism by which COPD causes VTE remains unknown, COPD‐related complications such as lower respiratory tract infections, repeated hospitalizations, and immobilization are potential mediators. Moreover, impaired lung function and hypoxemia may result in pulmonary hypertension and systemic inflammation, which contribute to increase the risk.13, 14 Patients with COPD with hypoxemia have been shown to have a larger mean platelet volume15 and increased platelet aggregation16 compared with those with normal oxygen saturation. In addition, COPD patients had higher coagulation activation than age‐ and sex‐matched controls17, 18 and exposure to short‐term hypoxia further augmented coagulation activation.19 Severe hypoxia was also shown to increase the incidence and size of thrombi in the inferior vena cava stenosis model in mice.20
To the best of our knowledge, no study has investigated the association between oxygen saturation and future risk of VTE. The aims of the present study were to investigate whether measures of respiratory impairments, such as respiratory symptoms and oxygen saturation (SpO2), individually and combined with COPD, were associated with increased risk of VTE.
2. METHODS
2.1. Study population
Study participants were derived from the fifth (2001‐2002) and sixth (2007‐2008) surveys of the Tromsø Study. To these surveys, fractions of the population aged ≥30 years living in the municipality of Tromsø, Norway, were invited to participate in an extensive screening where measurements of oxygen saturation and spirometry were included. A detailed description of study participation in the Tromsø study has been published elsewhere.21 Overall, 9577 unique individuals aged 32 to 89 years participated in ≥1 of the surveys. All subjects gave their written consent to participate, and the study was approved by the regional committee of medical and health research ethics. We excluded subjects who had officially moved out of Tromsø before the date of study enrollment (n = 7), subjects with VTE before baseline (n = 111), and subjects with missing values for SpO2 (n = 773). Consequently, 8686 subjects were included in the study and were followed from the date of inclusion until the end of follow‐up (December 31, 2016). Of these, 2752 participated in both surveys, while 2328 participated only in Tromsø 5 and 3606 participated only in Tromsø 6.
2.2. Measurement of peripheral capillary SpO2
SpO2values were measured at baseline with a digital handheld pulse oximeter (Onyx II, model 9550, Nonin Medical, Inc, Plymouth, MN, USA). The participants rested at least 15 minutes before the measurement, and the best of 3 measurements were recorded.
2.3. Respiratory symptoms
At baseline, the participants completed a questionnaire with questions about respiratory symptoms, including presence of dyspnea in various situations, daily cough for periods of the year, chronic cough (ie, cough with continuous duration of more than 3 months during the past 2 years), and productive cough (ie, phlegm) for periods of the year. Dyspnea was categorized into “none,” “dypsnea when walking calmly or flat or when washing and dressing,” and “dyspnea at rest.” Cough was categorized into “none,” “daily cough for periods of the year,” and “chronic cough for periods of the year” (ie, periods of daily cough lasting continuously for more than 3 months in the past 2 years). Phlegm was categorized as “none” and “productive cough for periods of the year.”
2.4. Chronic obstructive pulmonary disease
Spirometry was assessed at enrollment in the Tromsø study, as previously described in detail.12 The American Thoracic Society’s criteria for spirometry testing were followed.22 Current drug therapy was not interrupted before the test, and reversibility testing was not performed. The equations proposed by Langhammer et al23 were used to calculate predicted values of forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC), and FEV1/FVC ratio. Those with FEV1/FVC <0.7 or predicted FEV1 < 80% were excluded from the analyses if peak expiratory flow (PEF) was <3× forced expiratory flow when 75% of the air had been expired (PEF <3× FEF75).24 The subjects were allocated into groups based on lung function according to the Global Initiative of Chronic Obstructive Lung Disease guidelines.25
2.5. Other measurements
Height and weight were measured at enrollment in the Tromsø study, with subjects wearing light clothes and no shoes, and body mass index (BMI) was calculated (kg/m2). Information on smoking status (current, former, never) and history of cardiovascular disease (CVD; ie, myocardial infarction, stroke, or angina pectoris), was collected from a self‐administered questionnaire.
2.6. Venous thromboembolism
All incident VTE events during follow‐up were identified by searching the hospital discharge diagnosis registry, the autopsy registry, and the radiology procedure registry at the University Hospital of North Norway as previously described.29 VTE events were adjudicated by thorough review of medical records, and the adjudication criteria included clinical signs and symptoms of deep vein thrombosis or PE combined with objective confirmation tests (imaging or autopsy), which resulted in a VTE diagnosis that required treatment.29 Information on date of death was obtained from the National Population Registry of Norway.
2.7. Statistical analysis
Statistical analyses were performed with STATA version 13.0 (Stata Corporation, College Station, TX, USA). For each participant, person‐years for follow‐up were accrued from the date of enrollment (in Tromsø 5 or 6) to the date a VTE was diagnosed, the date the participant died or officially moved from the municipality of Tromsø, or to the end of the study period (December 31, 2016). We used a time‐varying analysis, which allowed for update of exposure and covariates in participants who were remeasured in Tromsø 6 (n = 2752). Thus, 8686 individuals contributed, with 11 438 observational periods. Subjects who died (n = 1706) or moved from Tromsø (n = 501) during follow‐up were censored at the date of migration or death.
SpO2 was categorized into 3 categories based on the following cutoffs: ≤96%; 97%, and ≥98% based on the ≤20th percentile, 20th to 60th percentile, and 60th to 100th percentile. Incidence rates (IRs) of VTE with 95% confidence intervals (CIs) were calculated and expressed as the number of events per 1000 person‐years. Cox proportional hazards regression models were used to estimate hazard ratios (HRs) of VTE with 95% CIs according to the different exposures (ie, oxygen saturation and respiratory symptoms). Age was used as the time scale in the Cox model, as the risk of VTE is strongly related to age. Model 1 was adjusted for sex, and Model 2 was adjusted for sex, BMI, history of CVD, and history of cancer at baseline. We additionally performed analyses stratified by COPD status (airflow without obstruction and COPD stage I‐IV). The proportional hazards assumption was evaluated using Schoenfeld residuals, and no violation was found.
3. RESULTS
During a median follow‐up of 9.1 years, 330 participants developed an incident VTE (overall IR, 3.5; 95% CI, 2.3‐3.2). Baseline characteristics according to categories of SpO2 are shown in Table 1. The mean age, the mean BMI levels, and the proportion of men increased over decreasing levels of SpO2. As expected, the proportion of subjects with COPD and respiratory symptoms increased over decreasing levels of SpO2 (Table 1).
Table 1.
Baseline characteristics according to categories of oxygen saturation
| Oxygen saturation level (%) | |||
|---|---|---|---|
| ≥98 | 97 | ≤96 | |
| Observation periods | 6169 (53.9) | 2951 (28.8) | 2318 (20.3) |
| Age (y) | 63.1 (9.7) | 65.7 (8.9) | 67.1 (8.3) |
| Sex (male) | 2386 (38.6) | 1435 (48.6) | 1136 (49.0) |
| Daily smoking | 1288 (21.1) | 612 (21.0) | 558 (24.3) |
| Body mass index (kg/m2) | 26.0 (3.8) | 27.7 (4.0) | 28.5 (4.7) |
| History of CVD | 768 (12.5) | 472 (16.0) | 465 (20.1) |
| History of cancer | 480 (7.8) | 264 (9.0) | 212 (9.2) |
| COPD stages | |||
| Airflow without obstruction | 4744 (80.9) | 2127 (75.8) | 1483 (68.0) |
| Stage I‐II | 1066 (18.2) | 607 (21.7) | 555 (25.4) |
| Stage III‐IV | 52 (0.9) | 69 (2.5) | 144 (6.6) |
| Dyspnea when walking calmly | 131 (2.1) | 95 (3.2) | 130 (5.6) |
| Dyspnea while washing and dressing | 150 (2.4) | 120 (4.1) | 183 (7.9) |
| Dyspnea at rest | 96 (1.6) | 53 (1.8) | 56 (2.4) |
| Cough daily in periods of the year | 965 (15.6) | 554 (18.8) | 564 (24.3) |
| Chronic cough | 580 (9.4) | 361 (12.2) | 405 (17.5) |
| Productive cough (in periods of the year) | 649 (10.5) | 379 (12.8) | 435 (18.8) |
Values are n (%) or mean ± SD.
Abbreviations: COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease.
The risk of VTE according to categories of SpO2 and respiratory symptoms are shown in Table 2. The IR of VTE was 2.7 per 1000 person‐years in those with SpO2 ≥98% and 5.3 per 1000 person‐years in those with SpO2 ≤96%, with a corresponding age and sex‐adjusted HR of 1.58 (95% CI, 1.22‐2.05) for SpO2 ≤96% vs. SpO2 ≥98% (reference category). Further adjustment for BMI, CVD, and history of cancer only slightly attenuated the HR. The risk of VTE increased according to the degree of dyspnea. Those who reported dyspnea when performing light activities (walking calmly, washing, or dressing) had a 1.5‐fold increased risk of VTE, while those with dyspnea at rest had a 2‐fold higher risk of VTE (HR, 2.18; 95% CI, 1.22‐3.88) compared with those without dyspnea. Chronic cough and phlegm were both associated with a 1.4‐fold higher risk of VTE (Table 2 and Figure 1). Subgroup analyses revealed that the associations between respiratory symptoms and VTE were mainly driven by an increased risk of PE (Figure 1).
Table 2.
Risk of VTE according to oxygen saturation and respiratory symptoms
| Person‐years | Events | IR (95% CI) |
Model 1 HR (95% CI) |
Model 2 HR (95% CI) |
|
|---|---|---|---|---|---|
| Oxygen saturation (SpO2) | |||||
| SpO2 ≥ 98% | 50 450 | 137 | 2.72 (2.30‐3.21) | Reference | Reference |
| SpO2 97% | 23 778 | 93 | 3.91 (3.19‐4.79) | 1.26 (0.97‐1.65) | 1.19 (0.91‐1.56) |
| SpO2 ≤ 96% | 18 835 | 100 | 5.31 (4.36‐6.46) | 1.58 (1.22‐2.05) | 1.48 (1.13‐1.93) |
| Dyspnea | |||||
| None | 86 989 | 290 | 3.33 (2.97‐3.74) | Reference | Reference |
| Dypsnea when walking calmly, washing or dressing | 4439 | 28 | 6.31 (4.36‐9.13) | 1.49 (1.00‐2.20) | 1.37 (0.92‐2.04) |
| Dyspnea at rest | 1636 | 12 | 7.33 (4.17‐12.9) | 2.18 (1.22‐3.88) | 2.04 (1.14‐3.66) |
| Cough | |||||
| None | 75 678 | 251 | 3.32 (2.93‐3.75) | Reference | Reference |
| Cough daily (in periods of the year) | 6800 | 27 | 3.97 (2.72‐5.79) | 1.21 (0.81‐1.80) | 1.21 (0.81‐1.80) |
| Chronic cougha | 10 585 | 52 | 4.91 (3.74‐6.44) | 1.39 (1.03‐1.87) | 1.39 (1.03‐1.88) |
| Phlegm | |||||
| None | 81 638 | 271 | 3.32 (2.95‐3.74) | Reference | Reference |
| Productive cough (in periods of the year) | 11 426 | 59 | 5.16 (4.00‐6.66) | 1.40 (1.05‐1.85) | 1.39 (1.04‐1.85) |
Model 1: adjusted for age (time scale) and sex. Model 2: adjusted for age (time scale), sex, body mass index, history of cardiovascular disease, and history of cancer.
Abbreviations: CI, confidence interval; HR, hazard ratio; IR, incidence rate per 1000 person‐years; VTE, venous thromboembolism.
Cough daily >3 months past 2 years.
Figure 1.
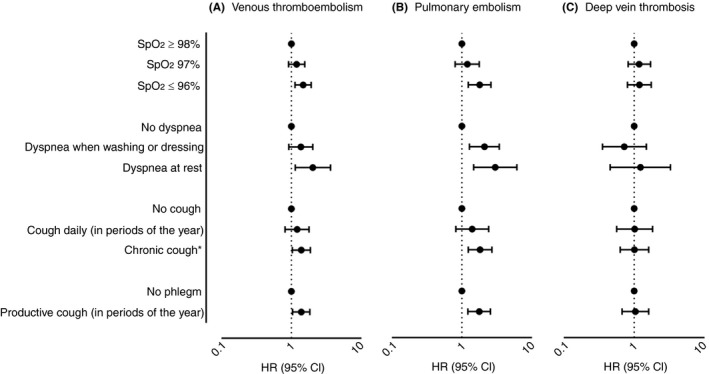
Risk of venous thromboembolism (A), pulmonary embolism (B), and deep vein thrombosis (C), according to oxygen saturation and respiratory symptoms. Hazard ratios (HRs) with 95% confidence intervals (CIs) adjusted for age (time scale), sex, BMI, history of CVD, and history of cancer. BMI, body mass index; CVD, cardiovascular disease
Information on COPD status, assessed by spirometry, was available in 8129 subjects (contributing with 10 849 observation periods) in whom 315 VTEs occurred during follow‐up. Overall, 2493 participants had COPD, and COPD was associated with an 18% increased risk of VTE (HR, 1.18; 95% CI, 0.92‐1.50) compared to those with airflow without obstruction in a model adjusted for age and sex. However, the risk of VTE was substantially higher in those with severe COPD. The HR of VTE was 1.09 (95% CI, 0.85‐1.42) in those with stage I to II COPD, and 1.92 (95% CI, 1.15‐3.21) in those with stage III to IV COPD, compared to those with airflow without obstruction. Table 3 shows the risk of VTE according to categories of SpO2 and respiratory symptoms in subjects with and without COPD. SpO2 ≤96% was associated with increased risk of VTE both in subjects with airflow without obstruction and in subjects with COPD. The degree of dyspnea was associated with increased risk of VTE in subjects with airflow without obstruction, and particularly among those with COPD. Subjects with COPD and dyspnea at rest had a >3‐fold higher risk of VTE (multivariable HR, 3.30; 95% CI, 1.35‐8.04) compared with those with airflow without obstruction and no dyspnea. Chronic cough with phlegm was associated with VTE only in subjects with concomitant COPD (Table 3).
Table 3.
Risk of VTE according to oxygen saturation and categories of COPD
| Person‐years | Events | IR (95% CI) |
Model 1 HR (95% CI) |
Model 2 HR (95% CI) |
||
|---|---|---|---|---|---|---|
| Oxygen saturation (SpO2) | ||||||
| Airflow without obstruction | SpO2 ≥ 98% | 38 847 | 100 | 2.57 (2.12‐3.13) | Reference | Reference |
| SpO2 97% | 17 322 | 60 | 3.46 (2.69‐4.46) | 1.20 (0.87‐1.65) | 1.12 (0.81‐1.55) | |
| SpO2 ≤ 96% | 12 270 | 60 | 4.88 (3.79‐6.30) | 1.54 (1.12‐2.13) | 1.40 (1.01‐1.96) | |
| COPD | SpO2 ≥ 98% | 9039 | 31 | 3.43 (2.41‐4.89) | 1.03 (0.70‐1.55) | 1.06 (0.70‐1.60) |
| SpO2 97% | 5249 | 30 | 5.71 (4.00‐8.17) | 1.52 (1.00‐2.30) | 1.51 (0.99‐2.29) | |
| SpO2 ≤ 96% | 5446 | 36 | 6.61 (4.77‐9.16) | 1.77 (1.20‐2.61) | 1.72 (1.17‐2.54) | |
| Dyspnea | ||||||
| Airflow without obstruction | None | 64 777 | 200 | 3.09 (2.69‐3.55) | Reference | Reference |
| Dypsnea when walking calmly, washing or dressing | 2585 | 14 | 5.42 (3.21‐9.15) | 1.39 (0.81‐2.40) | 1.25 (0.72‐2.18) | |
| Dyspnea at rest | 1077 | 6 | 5.57 (2.50‐12.4) | 1.75 (0.78‐3.95) | 1.59 (0.70‐3.59) | |
| COPD | None | 17 687 | 79 | 4.47 (3.58‐5.57) | 1.12 (0.86‐1.45) | 1.18 (0.90‐1.54) |
| Dypsnea when walking calmly, washing or dressing | 1631 | 13 | 7.97 (4.63‐13.7) | 1.76 (1.00‐3.10) | 1.66 (0.94‐2.93) | |
| Dyspnea at rest | 417 | 5 | 12.0 (5.00‐28.8) | 3.12 (1.28‐7.58) | 3.30 (1.35‐8.04) | |
| Cough | ||||||
| Airflow without obstruction | None | 57 540 | 179 | 3.11 (2.69‐3.60) | Reference | Reference |
| Cough daily (in periods of the year) | 4411 | 18 | 4.08 (2.57‐6.48) | 1.33 (0.82‐2.16) | 1.30 (0.80‐2.11) | |
| Cough daily > 3 months last two years | 6489 | 23 | 3.54 (2.35‐5.33) | 1.08 (0.70‐1.67) | 1.06 (0.69‐1.64) | |
| COPD | None | 14 043 | 63 | 4.49 (3.50‐5.74) | 1.09 (0.82‐1.46) | 1.14 (0.85‐1.54) |
| Cough daily (in periods of the year) | 2092 | 6 | 2.87 (1.29‐6.38) | 0.76 (0.34‐1.72) | 0.81 (0.36‐1.82) | |
| Cough daily > 3 months last two years | 3599 | 28 | 7.78 (5.37‐11.3) | 1.95 (1.30‐2.91) | 2.08 (1.39‐1.53) | |
| Phlegm | ||||||
| Airflow without obstruction | None | 61 833 | 195 | 3.15 (2.74‐3.63) | Reference | Reference |
| Productive cough (in periods of the year) | 6606 | 25 | 3.78 (2.55‐5.60) | 1.11 (0.73‐1.68) | 1.08 (0.71‐1.64) | |
| COPD | None | 15 431 | 66 | 4.28 (3.36‐5.44) | 1.04 (0.79‐1.38) | 1.10 (0.82‐1.46) |
| Productive cough (in periods of the year) | 4303 | 31 | 7.20 (5.06‐10.2) | 1.73 (1.17‐2.53) | 1.82 (1.24‐2.69) | |
Model 1: adjusted for age (time scale) and sex. Model 2: adjusted for age (time scale), sex, body mass index, history of cardiovascular disease, and history of cancer. Information on COPD was available in 8129 subjects in whom 315 VTEs occurred during follow‐up.
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; HR, hazard ratio; IR, incidence rate per 1000 person‐years; VTE, venous thromboembolism.
4. DISCUSSION
In the present study, we investigated how individual respiratory symptoms (ie, dyspnea, cough, and phlegm) and oxygen saturation, alone and in combination with COPD, affected the VTE risk. We found that subjects with SpO2 ≤96% (lowest 20th percentile) had a 1.5‐fold higher risk of VTE than subjects with SpO2 ≥98% (highest 40th percentile). In addition, severe manifestations of individual respiratory symptoms were associated with a 1.4 to 2.0‐fold higher risk of VTE compared with those without such symptoms. COPD combined with severe respiratory symptoms or lowered SpO2 had an additive effect on the VTE risk, suggesting that COPD patients with severe respiratory symptoms or lowered SpO2 should attract particular attention with regard to prevention strategies for VTE.
In the present study, we observed that moderately lowered oxygen saturation was associated with a higher risk of VTE among subjects recruited from the general population. There are several conditions that could lead to lowered SpO2, such as obesity,26, 27 heart failure,28 COPD,14, 27 and cancers affecting the cardiorespiratory system.29 These conditions are all associated with VTE risk,11, 12, 30, 31, 32 and may act as confounders for the relationship between lowered SpO2 and VTE risk. However, the risk estimates for VTE by categories of SpO2 were only marginally affected in the multivariable model taking obesity, history of CVD, and cancer into account. Moreover, SpO2 was associated with increased risk of VTE in analyses restricted to subjects with airflow without obstruction. This suggest that a skewed distribution of obesity, CVD, COPD and cancer between subjects with high and low SpO2 does not explain the apparent association between low SpO2 and VTE risk. Even though the underlying mechanism(s) are unknown, several lines of evidence support a direct relationship between hypoxia or hypoxemia and VTE risk. First, hypoxia induces secretion of Weibel‐Palade bodies in endothelial cells,33 with subsequent expression at the cell surface and release of von Willebrand factor and P‐selectin,34, 35 both of which are associated with VTE risk.36, 37 Second, hypoxia is reported to affect key components in the pathogenesis of thrombus formation. Hypoxia increases platelet reactivity38, 39 and, though controversial, hypoxia has been shown to increase coagulation activation in some40 but not all studies.41 Third, systemic hypoxia has been shown to accelerate thromboembolic events in mice through induction of the nucleotide‐binding domain, leucine‐rich–containing family, pyrin domain–containing 3 inflammasome complex.42 Fourth, severe hypoxia is also reported to increase the incidence and size of thrombi in the inferior vena cava stenosis model in mice.20
In coherence with previous cohort studies,11, 12 COPD was found to be associated with a moderately higher risk of VTE that increased with the severity of COPD. In the present study, we additionally found that the combination of COPD and lowered SpO2 had an additive effect on the risk of VTE. Patients with COPD with hypoxemia are known to have larger mean platelet volume (MPV)15 and increased platelet reactivity16 compared to those with normal oxygen saturation. High MPV has been identified as a biomarker of increased VTE risk,43 and the protective effect of antiplatelet drugs strongly suggests that platelet reactivity plays an important role in the pathogenesis of VTE.44 Furthermore, patients with COPD have higher coagulation activation than age‐ and sex‐matched controls,17, 18 and exposure to short‐term hypoxia further augments coagulation activation.19 In light of these latter findings, it is pertinent to speculate that the additive effect of COPD and lowered SpO2 on VTE risk is mediated by the same pathophysiological mechanism.
Previously, Kubota et al11 reported that the presence of any respiratory symptom (dyspnea, cough, and phlegm) was associated with 1.4‐fold higher VTE risk in subjects with normal spirometry compared to those without respiratory symptoms. In the present study, we extended the knowledge by showing that all respiratory symptoms (dyspnea, cough, and phlegm) were individually associated with VTE risk, and that the VTE risk increased with the severity of the dyspnea and cough. Even though the mechanism for the association between respiratory symptoms and VTE risk is unknown, it is obvious that these respiratory symptoms merely are markers rather than mediators of the VTE risk. It could be speculated that the VTE risk by respiratory symptoms is attributed to associated comorbidities, such as immobility, cancer, heart failure, and others. The risk estimates for VTE by respiratory symptoms, however, remained unchanged after adjustments for BMI, cancer, and CVD. However, we cannot rule out the possibility of residual confounding, in particular by heart failure, as we only adjusted for CVD in general.
Our findings revealed an additive impact of COPD and other measures of respiratory impairments on the risk of VTE. Both severe respiratory symptoms (cough, dyspnea, and phlegm) and lowered SpO2 had a detrimental effect on the VTE risk in patients with COPD. This may imply a need for close monitoring and consideration of VTE‐preventing strategies in patients with COPD with severe respiratory symptoms or decreased SpO2.
Recruitment of participants from a general population, long‐term follow‐up with repeated measurements of exposure and confounders, temporal sequence between exposure and outcome, and thorough adjudication of outcomes are major strengths of our study. Objective methods were used to assess COPD and SpO2, as spirometry was used for measurement of airflow patterns, and SpO2 was measured by a digital handheld pulse oximeter. The personnel who performed outcome adjudication were blinded to the baseline variable, and potential exposure misclassification would likely be nondifferential (ie, not related to the outcome). The study also had some limitations. Unfortunately, the statistical power was too low to perform subgroup analyses. For some estimates, the CIs were wide, and our findings should therefore be interpreted with caution. Moreover, participants with asthma could potentially have been misclassified as having COPD, as spirometry was performed without reversibility testing. Presence of respiratory symptoms was registered on a self‐administered questionnaire and this could lead to nondifferential misclassification, which could bias our estimates toward null.
In conclusion, lowered SpO2 and individual respiratory symptoms (dyspnea, cough, and phlegm) were associated with an increased risk of VTE. COPD combined with severe respiratory symptoms or lowered SpO2 had an additive effect on the VTE risk. Our findings may suggest that particular attention with regard to VTE preventive strategies should be considered for patients with COPD with severe respiratory symptoms or lowered SpO2.
RELATIONSHIP DISCLOSURE
The authors report nothing to disclose.
AUTHOR CONTRIBUTIONS
Conception and design: JBH. Data collection: TB, LE, SKB, HM, and JBH. Statistical analyses: TB and SKB. Draft of manuscript: TB, JBH. Interpretation of results: TB, LE, WMM, HM, SKB, and JBH. Critical revision of manuscript: LE, WMM, HM, SKB, and JBH.
Børvik T, Evensen LH, Morelli VM, Melbye H, Brækkan SK, Hansen J‐B. Impact of respiratory symptoms and oxygen saturation on the risk of incident venous thromboembolism—the Tromsø study. Res Pract Thromb Haemost. 2020;4:255–262. 10.1002/rth2.12299
Handling Editor: Suzanne Cannegieter
Funding information
KG Jebsen TREC is supported by an independent grant from Stiftelsen Kristian Gerhard Jebsen.
Contributor Information
Line H. Evensen, htps://twitter.com/EvensenL.
Sigrid K. Brækkan, Email: Sigrid.brakkan@uit.no, htps://twitter.com/TREC_UiT.
REFERENCES
- 1. GBD 2015 Chronic Respiratory Disease Collaborators . Global, regional, and national deaths, prevalence, disability‐adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990‐2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir Med. 2017;5(9):691–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cavailles A, Brinchault‐Rabin G, Dixmier A, Goupil F, Gut‐Gobert C, Marchand‐Adam S, et al. Comorbidities of COPD. Eur Respir Rev. 2013;22(130):454–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sogaard M, Madsen M, Lokke A, Hilberg O, Sorensen HT, Thomsen RW. Incidence and outcomes of patients hospitalized with COPD exacerbation with and without pneumonia. Int J Chron Obstruct Pulmon Dis. 2016;11:455–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Groenewegen KH, Schols AM, Wouters EF. Mortality and mortality‐related factors after hospitalization for acute exacerbation of COPD. Chest. 2003;124(2):459–67. [DOI] [PubMed] [Google Scholar]
- 5. Gunen H, Hacievliyagil SS, Kosar F, Mutlu LC, Gulbas G, Pehlivan E, et al. Factors affecting survival of hospitalised patients with COPD. Eur Respir J. 2005;26(2):234–41. [DOI] [PubMed] [Google Scholar]
- 6. Sidney S, Sorel M, Quesenberry CP Jr, DeLuise C, Lanes S, Eisner MD. COPD and incident cardiovascular disease hospitalizations and mortality: Kaiser Permanente medical care program. Chest. 2005;128(4):2068–75. [DOI] [PubMed] [Google Scholar]
- 7. Schneider C, Bothner U, Jick SS, Meier CR. Chronic obstructive pulmonary disease and the risk of cardiovascular diseases. Eur J Epidemiol. 2010;25(4):253–60. [DOI] [PubMed] [Google Scholar]
- 8. Chen WJ, Lin CC, Lin CY, Chang YJ, Sung FC, Kao CH, et al. Pulmonary embolism in chronic obstructive pulmonary disease: a population‐based cohort study. COPD. 2014;11(4):438–43. [DOI] [PubMed] [Google Scholar]
- 9. Gunen H, Gulbas G, In E, Yetkin O, Hacievliyagil SS. Venous thromboemboli and exacerbations of COPD. Eur Respir J. 2010;35(6):1243–8. [DOI] [PubMed] [Google Scholar]
- 10. Winter JH, Buckler PW, Bautista AP, Smith FW, Sharp PF, Bennett B, et al. Frequency of venous thrombosis in patients with an exacerbation of chronic obstructive lung disease. Thorax. 1983;38(8):605–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kubota Y, London SJ, Cushman M, Chamberlain AM, Rosamond WD, Heckbert SR, et al. Lung function, respiratory symptoms and venous thromboembolism risk: the atherosclerosis risk in communities study. J Thromb Haemost. 2016;14(12):2394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Borvik T, Braekkan SK, Enga K, Schirmer H, Brodin EE, Melbye H, et al. COPD and risk of venous thromboembolism and mortality in a general population. Eur Respir J. 2016;47(2):473–81. [DOI] [PubMed] [Google Scholar]
- 13. Ambrosetti M, Ageno W, Spanevello A, Salerno M, Pedretti RF. Prevalence and prevention of venous thromboembolism in patients with acute exacerbations of COPD. Thromb Res. 2003;112(4):203–7. [DOI] [PubMed] [Google Scholar]
- 14. Kent BD, Mitchell PD, McNicholas WT. Hypoxemia in patients with COPD: cause, effects, and disease progression. Int J Chron Obstruct Pulmon Dis. 2011;6:199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wedzicha JA, Rudd RM, Apps MC, Cotter FE, Newland AC, Empey DW. Erythrapheresis in patients with polycythaemia secondary to hypoxic lung disease. Br Med J (Clin Res Ed). 1983;286(6364):511–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wedzicha JA, Syndercombe‐Court D, Tan KC. Increased platelet aggregate formation in patients with chronic airflow obstruction and hypoxaemia. Thorax. 1991;46(7):504–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Alessandri C, Basili S, Violi F, Ferroni P, Gazzaniga PP, Cordova C. Hypercoagulability state in patients with chronic obstructive pulmonary disease. Chronic obstructive bronchitis and haemostasis Group. Thromb Haemost. 1994;72(3):343–6. [PubMed] [Google Scholar]
- 18. Davi G, Basili S, Vieri M, Cipollone F, Santarone S, Alessandri C, et al. Enhanced thromboxane biosynthesis in patients with chronic obstructive pulmonary disease. The Chronic Obstructive Bronchitis and Haemostasis Study Group. Am J Respir Crit Care Med. 1997;156(6):1794–9. [DOI] [PubMed] [Google Scholar]
- 19. Sabit R, Thomas P, Shale DJ, Collins P, Linnane SJ. The effects of hypoxia on markers of coagulation and systemic inflammation in patients with COPD. Chest. 2010;138(1):47–51. [DOI] [PubMed] [Google Scholar]
- 20. Brill A, Suidan GL, Wagner DD. Hypoxia, such as encountered at high altitude, promotes deep vein thrombosis in mice. J Thromb Haemost. 2013;11(9):1773–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jacobsen BK, Eggen AE, Mathiesen EB, Wilsgaard T, Njolstad I. Cohort profile: the Tromso study. Int J Epidemiol. 2012;41(4):961–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Standardization of Spirometry, 1994 Update. American Thoracic Society. Am J Respir Crit Care Med. 1995;152(3):1107–36. [DOI] [PubMed] [Google Scholar]
- 23. Langhammer A, Johnsen R, Gulsvik A, Holmen TL, Bjermer L. Forced spirometry reference values for Norwegian adults: the Bronchial Obstruction in Nord‐Trondelag Study. Eur Respir J. 2001;18(5):770–9. [DOI] [PubMed] [Google Scholar]
- 24. Melbye H, Joensen L, Risor MB, Halvorsen PA. Symptoms of respiratory tract infection and associated care‐seeking in subjects with and without obstructive lung disease; the Tromso Study: Tromso 6. BMC Pulm Med. 2012;12:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fromer L, Cooper CB. A review of the GOLD guidelines for the diagnosis and treatment of patients with COPD. Int J Clin Pract. 2008;62(8):1219–36. [DOI] [PubMed] [Google Scholar]
- 26. Parameswaran K, Todd DC, Soth M. Altered respiratory physiology in obesity. Can Respir J. 2006;13(4):203–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vold ML, Aasebo U, Melbye H. Low FEV1, smoking history, and obesity are factors associated with oxygen saturation decrease in an adult population cohort. Int J Chron Obstruct Pulmon Dis. 2014;9:1225–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cleland JG, McGowan J. Heart failure due to ischaemic heart disease: epidemiology, pathophysiology and progression. J Cardiovasc Pharmacol. 1999;33(suppl 3):S17–29. [DOI] [PubMed] [Google Scholar]
- 29. Nichols L, Saunders R, Knollmann FD. Causes of death of patients with lung cancer. Arch Pathol Lab Med. 2012;136(12):1552–7. [DOI] [PubMed] [Google Scholar]
- 30. Borch KH, Braekkan SK, Mathiesen EB, Njolstad I, Wilsgaard T, Stormer J, et al. Anthropometric measures of obesity and risk of venous thromboembolism: the Tromso study. Arterioscler Thromb Vasc Biol. 2010;30(1):121–7. [DOI] [PubMed] [Google Scholar]
- 31. Howell MD, Geraci JM, Knowlton AA. Congestive heart failure and outpatient risk of venous thromboembolism: a retrospective, case‐control study. J Clin Epidemiol. 2001;54(8):810–6. [DOI] [PubMed] [Google Scholar]
- 32. Blix K, Gran OV, Severinsen MT, Cannegieter SC, Jensvoll H, Overvad K, et al. Impact of time since diagnosis and mortality rate on cancer‐associated venous thromboembolism: the Scandinavian Thrombosis and Cancer (STAC) cohort. J Thromb Haemost. 2018;16(7):1327–35. [DOI] [PubMed] [Google Scholar]
- 33. Pinsky DJ, Naka Y, Liao H, Oz MC, Wagner DD, Mayadas TN, et al. Hypoxia‐induced exocytosis of endothelial cell Weibel‐Palade bodies. A mechanism for rapid neutrophil recruitment after cardiac preservation. J Clin Invest. 1996;97(2):493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wagner DD, Olmsted JB, Marder VJ. Immunolocalization of von Willebrand protein in Weibel‐Palade bodies of human endothelial cells. J Cell Biol. 1982;95(1):355–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bonfanti R, Furie BC, Furie B, Wagner DD. PADGEM (GMP140) is a component of Weibel‐Palade bodies of human endothelial cells. Blood. 1989;73(5):1109–12. [PubMed] [Google Scholar]
- 36. Tsai AW, Cushman M, Rosamond WD, Heckbert SR, Tracy RP, Aleksic N, et al. Coagulation factors, inflammation markers, and venous thromboembolism: the longitudinal investigation of thromboembolism etiology (LITE). Am J Med. 2002;113(8):636–42. [DOI] [PubMed] [Google Scholar]
- 37. Pabinger I, Ay C. Biomarkers and venous thromboembolism. Arterioscler Thromb Vasc Biol. 2009;29(3):332–6. [DOI] [PubMed] [Google Scholar]
- 38. Rahangdale S, Yeh SY, Novack V, Stevenson K, Barnard MR, Furman MI, et al. The influence of intermittent hypoxemia on platelet activation in obese patients with obstructive sleep apnea. J Clin Sleep Med. 2011;7(2):172–8. [PMC free article] [PubMed] [Google Scholar]
- 39. Tyagi T, Ahmad S, Gupta N, Sahu A, Ahmad Y, Nair V, et al. Altered expression of platelet proteins and calpain activity mediate hypoxia‐induced prothrombotic phenotype. Blood. 2014;123(8):1250–60. [DOI] [PubMed] [Google Scholar]
- 40. Bendz B, Rostrup M, Sevre K, Andersen TO, Sandset PM. Association between acute hypobaric hypoxia and activation of coagulation in human beings. Lancet. 2000;356(9242):1657–8. [DOI] [PubMed] [Google Scholar]
- 41. Toff WD, Jones CI, Ford I, Pearse RJ, Watson HG, Watt SJ, et al. Effect of hypobaric hypoxia, simulating conditions during long‐haul air travel, on coagulation, fibrinolysis, platelet function, and endothelial activation. JAMA. 2006;295(19):2251–61. [DOI] [PubMed] [Google Scholar]
- 42. Gupta N, Sahu A, Prabhakar A, Chatterjee T, Tyagi T, Kumari B, et al. Activation of NLRP3 inflammasome complex potentiates venous thrombosis in response to hypoxia. Proc Natl Acad Sci U S A. 2017;114(18):4763–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Braekkan SK, Mathiesen EB, Njolstad I, Wilsgaard T, Stormer J, Hansen JB. Mean platelet volume is a risk factor for venous thromboembolism: the Tromso Study, Tromso, Norway. J Thromb Haemost. 2010;8(1):157–62. [DOI] [PubMed] [Google Scholar]
- 44. Simes J, Becattini C, Agnelli G, Eikelboom JW, Kirby AC, Mister R, et al. Aspirin for the prevention of recurrent venous thromboembolism: the INSPIRE collaboration. Circulation. 2014;130(13):1062–71. [DOI] [PubMed] [Google Scholar]


