Abstract
Introduction
Macroscopic hematuria is considered a significant risk factor for urologic disease, and it is highly prevalent in people with hemophilia.
Aim
To determine whether prophylactic factor replacement therapy is associated with reduced occurrence of macroscopic hematuria in people with hemophilia in a post hoc analysis using data from a cross‐sectional study conducted by the Age‐Related Developments and Comobordities in Hemophilia (ADVANCE) Working Group that included males with hemophilia ≥40 years of age.
Methods
Data from 16 contributing centers, in 13 European countries and Israel, were analyzed using logistic regression. Of 532 recruited individuals, this analysis included 370 patients with moderate or severe hemophilia who received on‐demand or prophylactic therapy.
Results
For patients with a history of macroscopic hematuria, we analyzed the association between prophylaxis and reoccurrence of macroscopic hematuria within the past 5 years (n = 235 patients). Frequent (≥3 times/wk) prophylaxis was negatively associated with a recent episode of macroscopic hematuria (odds ratio [OR], 0.38; 95% confidence interval [CI], 0.18‐0.76). We also analyzed whether prophylaxis corresponded to a lower lifetime number of macroscopic hematuria episodes (n = 285 patients). Frequent prophylaxis for >15 years was associated with a lower number of episodes compared to on‐demand treatment (OR, 0.29; 95% CI, 0.16‐0.54), whereas nonsteroidal anti‐inflammatory drugs (NSAIDs) and severe hemophilia were associated with a higher number. There was no association of prophylaxis <3 times/wk with hematuria.
Conclusion
Frequent prophylaxis was negatively associated with the number of episodes of macroscopic hematuria in people with hemophilia. Prevalence of macroscopic hematuria was higher among individuals with severe hemophilia and those regularly using NSAIDs.
Keywords: aging, blood coagulation factors, hematuria, hemophilia A, hemophilia B
Essentials.
Macroscopic hematuria is highly prevalent in people with hemophilia.
We analyzed data from 370 individuals with hemophilia from 13 countries.
Frequent prophylaxis for the past 5 years is associated with a reduced reoccurrence of macroscopic hematuria.
Frequent long‐standing prophylaxis is associated with fewer episodes of macroscopic hematuria than on demand.
1. INTRODUCTION
Hemophilia is an inherited deficiency of factor VIII or IX that is associated with recurrent and spontaneous bleeding. People with hemophilia use replacement therapy with clotting factor concentrates to treat bleeding episodes. The most common regimes are on demand, which is episodic replacement therapy in response to an acute bleed, and prophylaxis, which is regular replacement therapy to prevent bleeding.1
Hematuria is the presence of blood or blood cells in the urine that is either visible (macroscopic) or nonvisible (microscopic). Several underlying conditions may cause hematuria, the most common of which are inflammation or infection of the prostate or bladder, stones, and in older patients, urologic malignancy.2, 3 In the general male population, reported prevalence of microscopic hematuria ranges from 2.5%4 to 20%5 in high‐risk patients undergoing urinary dipstick screening for bladder cancer. As even a single episode of macroscopic hematuria is considered a significant risk factor for urologic disease,6 macroscopic hematuria requires the involvement of several specialists to investigate for an underlying disorder.
To our knowledge, there are few studies of hematuria in people with hemophilia. Two important studies from the 1970s7, 8 showed that macroscopic hematuria is more prevalent in the hemophilia population than in the general population. Prentice et al7 examined the underlying renal function and structure in 35 people with hemophilia (age range, 12‐60 years) showing that 66% of the patients had a history of either severe or moderate hematuria. In a survey of the renal status in 26 people with hemophilia (age range, 17‐82 years), Beck and Evans8 reported a history of macroscopic hematuria in 69% of the patients. Later studies confirmed the high prevalence of hematuria among people with hemophilia.9, 10, 11, 12, 13 Although usually considered a benign condition,14 studies have found macroscopic hematuria associated with a reduction in renal function15 and an increased risk of hypertension11, comorbid conditions that may complicate treatment in aging people with hemophilia.
Previous research has mainly considered hematuria and its association with the development or presence of renal disease and hypertension, and has not assessed the associations between different factor replacement therapies and macroscopic hematuria. As several studies have identified macroscopic hematuria as a risk factor for renal damage, an assessment of treatment that may prevent hematuria is important. Using data from the Age‐Related Developments and Comorbidities in Hemophilia (ADVANCE) Working Group's H3 Study,12 we examined whether frequent prophylactic factor replacement therapy corresponded with a reduced occurrence of macroscopic hematuria in this large cohort of people with hemophilia.
2. MATERIALS AND METHODS
2.1. Data collection
The data set, consisting of 532 men aged 40 years and older, was collected between June 2011 and September 2013 from researchers in 16 participating centers, in 13 European countries and Israel, where Germany had 3 centers and Italy had 2. The study had an observational, noninterventional, non–product‐specific, and cross‐sectional design. All data were gathered from consecutive patients attending their routine clinical visit using a case report form and from laboratory data collected no earlier than 1 year prior to the clinical visit. The case report form included items about patient characteristics, demographics, past and current treatment, and medical history including a lifetime history of comorbidities. A history of macroscopic hematuria was recorded as either present or absent, as reported by the patient. The lifetime number of episodes, and whether the last episode of bleeding occurred <5 years ago, were determined from a combination of patient recollections and review of existing medical records. Use of nonsteroidal anti‐inflammatory drugs (NSAIDs), including selective cyclooxygenase‐2 inhibitors, for >3 months per year was recorded as a history of regular NSAID use. Treatment was classified as either on demand or prophylactic therapy. Duration of treatment was categorized into 5‐year intervals from ≤5 years to >20 years of treatment, where the latter category included those who started prophylactic therapy in early childhood. Patients with any severity of hemophilia A or B, consenting to provide information to researchers compiling epidemiologic data on the link between hemophilia, hypertension, and hematuria, were included in the study. Respective national ethical committees or the institutional review boards approved the study.12, 16
2.2. Study design and statistical analyses
In this post hoc analysis, we assessed the association between macroscopic hematuria and treatment, comparing prophylactic treatment to on demand. In contrast to people with moderate or severe hemophilia, almost all individuals with mild hemophilia received on‐demand treatment. Therefore, this group could not contribute to explaining the association of interest, and hence only individuals with moderate and severe hemophilia were included in the analysis.
In this paper, we endeavored to provide evidence to answer the following 2 questions:
Is frequent prophylactic treatment associated with lower odds of macroscopic hematuria reoccurring in people with hemophilia with a history of macroscopic hematuria?
Is frequent long‐standing prophylactic treatment associated with a lower number of episodes of macroscopic hematuria?
We conducted 2 separate analyses to answer these questions. In Analysis 1, we analyzed reoccurrence of macroscopic hematuria in the past 5 years. We limited the sample to patients receiving either on‐demand or >5 years of prophylactic treatment to know with certainty whether they were on prophylactic therapy for the entire period during which the relevant diagnosis could be set (see Figure 1 for sample selection). To observe an association of prophylaxis with the number of episodes of macroscopic hematuria, it is reasonable to require that the treatment be of a minimum duration. From a clinical perspective, we have considered >15 years of prophylaxis to be long‐standing treatment and ≤5 years of treatment to be too short to observe a significant association with number of episodes of macroscopic hematuria. Therefore, on demand and ≤5 years of treatment together constitute the reference level against which long‐standing treatment was compared in Analysis 2 (see Figure 2 for sample selection).
Figure 1.
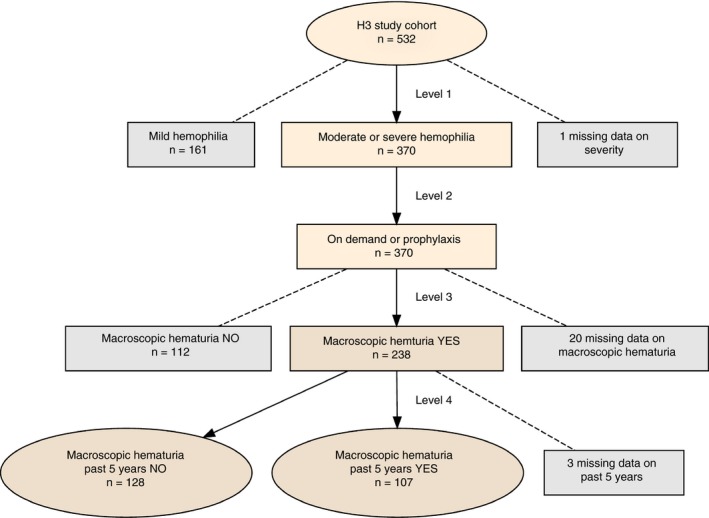
Patient selection for Analysis 1. The figure shows the filtering process that leads to the leaf nodes that constitute the levels of the response variable in Analysis 1
Figure 2.
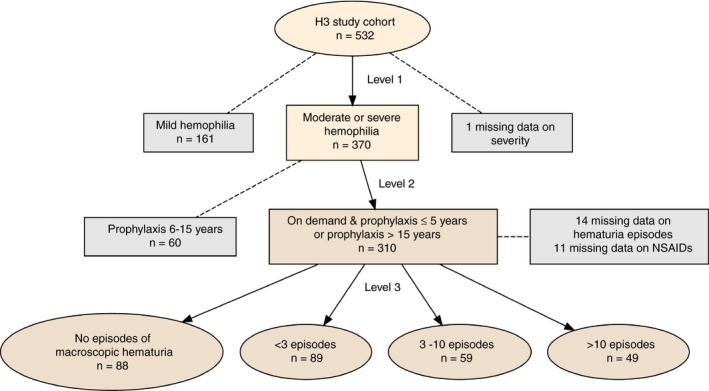
Patient selection for Analysis 2. The figure shows the filtering process that leads to the leaf nodes that constitute the levels of the response variable in Analysis 2
The frequency of prophylactic treatment ranged from 1 to 7 times per week, and prophylaxis was on average administered less frequently for hemophilia B than for hemophilia A (2.4 vs. 3 times/wk). Welch's unequal variances t‐test showed that the difference in average frequency between hemophilia A and B was significant. We corrected for this by defining frequent prophylaxis as ≥3 times per week for hemophilia A and ≥2 times per week for hemophilia B. No patients in this cohort used enhanced half‐life factor replacement therapy.
We estimated a multivariable logistic regression model to analyze the dichotomous outcome variable in Analysis 1. For the ordinal categorical response with 4 levels in Analysis 2, we estimated a proportional odds model.
We used R version 3.6.017 for all analyses, and we considered a 2‐tailed P value <0.05 to be statistically significant.
3. RESULTS
3.1. Study population
The patients in the study population were 98% white, with median age 51 years (range, 40‐98). Of the 370 patients with either moderate or severe hemophilia receiving either on‐demand or prophylactic treatment (Level 2 in Figure 1), 320 (86.5%) had hemophilia A and 313 (84.6%) had severe disease. Table 1 shows descriptive statistics grouped by severity of hemophilia. The majority of patients with moderate factor deficiency received on‐demand therapy, while prophylactic therapy was the most common treatment regime for patients with severe hemophilia. For patients with moderate and severe hemophilia, more than 50% have experienced an episode of macroscopic hematuria, with a higher average number of hematuria episodes in the severe group. Following an episode of macroscopic hematuria, 69% of the patients received factor replacement therapy, 32% were hospitalized, 8.5% required red blood cell transfusion, and 2.5% needed surgery. Twenty‐three individuals (6.2%) had inhibitors. Some of them failed treatment with immune tolerance induction, but the majority (82.6%) were never treated.
Table 1.
Descriptive statistics by severity of hemophilia
| Moderate | Severe | Total | ||||
|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | |
| Type | ||||||
| Hemophilia A | 43 | (75.4) | 277 | (88.5) | 320 | (86.5) |
| Hemophilia B | 14 | (24.6) | 36 | (11.5) | 50 | (13.5) |
| Total | 57 | (100) | 313 | (100) | 370 | (100) |
| Measures | ||||||
| Age, median (IQR) | 54 | (46‐65) | 51 | (45‐58) | 51 | (45‐59) |
| EGFR, median (IQR) | 95 | (83‐102) | 101 | (90‐109) | 100 | (88‐109) |
| Treatment | ||||||
| On demand | 49 | (86) | 138 | (44.1) | 187 | (50.5) |
| Prophylaxis | 8 | (14) | 175 | (55.9) | 183 | (49.5) |
| Comorbidities | ||||||
| Hypertension | 21 | (36.8) | 141 | (45) | 162 | (43.8) |
| History of renal disease | 1 | (1.8) | 15 | (4.8) | 16 | (4.3) |
| NSAIDs | 10 | (17.5) | 86 | (27.5) | 96 | (25.9) |
| Macroscopic hematuria | 31 | (54.4) | 207 | (66.1) | 238 | (64.3) |
| <3 bleeds | 18 | (31.6) | 85 | (27.2) | 103 | (27.8) |
| 3‐10 bleeds | 10 | (17.5) | 60 | (19.2) | 70 | (18.9) |
| >10 bleeds | 3 | (5.3) | 62 | (19.8) | 65 | (17.6) |
| Past 5 years | 16 | (28.1) | 91 | (29.1) | 107 | (28.9) |
The columns of this table show descriptive statistics for patients with moderate hemophilia, severe hemophilia, and the sum total. The numbers in parentheses report the number in the neighboring left column as a fraction of the total number of patients in the corresponding severity category.
Abbreviations: EGFR, estimated glomerular filtration rate; IQR, interquartile range; NSAIDs, nonsteroidal anti‐inflammatory drugs.
Table 2 shows characteristics of the patients selected for analysis in Analysis 1 and Analysis 2. To highlight differences, we compared the reference levels in Analysis 1 and Analysis 2 with frequent prophylaxis of an appropriate duration. Under the header Analysis 1, where all patients have a history of macroscopic hematuria (Level 3 in Figure 1), we compared on‐demand with >5 years of frequent prophylaxis, thus filtering out patients with other treatments. The majority of the patients (51.6%) treated on demand experienced hematuria during the past 5 years compared to 25.5% on prophylactic therapy. Under the header Analysis 2, we compared on‐demand and ≤5 years of prophylaxis (reference group in Analysis 2) to >15 years of frequent prophylactic therapy, again excluding patients with other treatments (Level 2 in Figure 2). Macroscopic hematuria occurred in 72.7% of the patients in the reference group in Analysis 2, 20.6 percentage points more than the group that received long‐standing frequent prophylaxis. The percentage of patients with >3 bleeds was approximately twice as high in the reference group as in the frequent long‐standing prophylaxis group.
Table 2.
Descriptive statistics by on‐demand and frequent prophylaxis
| Patients with a history of macroscopic hematuria (Analysis 1) |
All patients (Analysis 2) |
|||||||
|---|---|---|---|---|---|---|---|---|
| On demand |
>5 y frequent prophylaxis |
On demand & ≤5 y prophylaxis |
>15 y frequent prophylaxis | |||||
| n | (%) | n | (%) | n | (%) | n | (%) | |
| Type | ||||||||
| Hemophilia A | 103 | (84.4) | 34 | (72.3) | 190 | (86.4) | 38 | (79.2) |
| Hemophilia B | 19 | (15.6) | 13 | (27.7) | 30 | (13.6) | 10 | (20.8) |
| Total | 122 | (100) | 47 | (100) | 220 | (100) | 48 | (100) |
| Severity | ||||||||
| Moderate | 25 | (20.5) | 0 | (0) | 49 | (22.3) | 1 | (2.1) |
| Severe | 97 | (79.5) | 47 | (100) | 171 | (77.7) | 47 | (97.9) |
| Measures | ||||||||
| Age, median (IQR) | 52 | (46‐62) | 54 | (45‐58) | 51 | (45‐61) | 50 | (45‐58) |
| EGFR, median (IQR) | 100 | (90‐110) | 103 | (89‐108) | 98 | (86‐107) | 103 | (89‐111) |
| Comorbidities | ||||||||
| Hypertension | 52 | (42.6) | 17 | (36.2) | 92 | (41.8) | 19 | (39.6) |
| History of renal disease | 4 | (3.3) | 2 | (4.3) | 9 | (4.1) | 2 | (4.2) |
| NSAIDs | 34 | (27.9) | 14 | (29.8) | 54 | (24.5) | 16 | (33.3) |
| Macroscopic hematuria | 122 | (100) | 47 | (100) | 160 | (72.7) | 25 | (52.1) |
| <3 bleeds | 54 | (44.3) | 23 | (48.9) | 68 | (30.9) | 15 | (31.2) |
| 3‐10 bleeds | 37 | (30.3) | 12 | (25.5) | 50 | (22.7) | 6 | (12.5) |
| >10 bleeds | 31 | (25.4) | 12 | (25.5) | 42 | (19.1) | 4 | (8.3) |
| Past 5 years | 63 | (51.6) | 12 | (25.5) | 78 | (35.5) | 7 | (14.6) |
In Analysis 1, we compared on demand to >5 y of frequent prophylaxis. In Analysis 2, we compared the reference level, defined as on demand and ≤5 y of prophylaxis, to >15 y of frequent prophylaxis. Patients on prophylaxis that was either infrequent or outside of the specified durations were excluded. The numbers in parentheses report the number in the neighboring left column as a fraction of the total number of patients in the corresponding treatment category.
Abbreviations: EGFR, estimated glomerular filtration rate; IQR, interquartile range; NSAIDs, nonsteroidal anti‐inflammatory drugs.
In Germany, the country that recruited most patients, 62% experienced macroscopic hematuria, and among the 60% receiving prophylaxis, 41% had frequent prophylaxis. Austria, Slovenia, and Israel had the highest bleeding rates. Although 73% of patients in Austria used prophylaxis, it appears that frequent treatment is a recent phenomenon. Israel was the country with the highest use of on‐demand (79%). Except for 1 patient in Greece, Norway and Sweden had the highest fractions of long‐standing frequent prophylaxis, followed by Germany.
3.2. Regression models
Table 3 shows results pertaining to the occurrence of macroscopic hematuria obtained from multivariable logistic regressions. In Analysis 1, the response variable was whether a patient with a history of macroscopic hematuria experienced another episode in the most recent 5 years (Level 4 in Figure 1). Frequent prophylaxis was negatively associated with macroscopic hematuria (odds ratio [OR], 0.38; 95%confidence interval, 0.18‐0.76; P = .008), suggesting that frequent prophylaxis reduced the reoccurrence of hematuria. In Analysis 2, the outcome variable was the number of episodes of macroscopic hematuria, an ordered categorical variable with levels in ascending order (Level 3 in Figure 2): no episodes, <3 episodes, 3 to 10 episodes, and >10 episodes. The OR significantly <1 in this model suggests that >15 years of frequent prophylaxis contributed to limiting the number of episodes of macroscopic hematuria (OR, 0.29; 95% CI, 0.16‐0.54; P < .001). In contrast, use of NSAIDs and severe hemophilia were both associated with additional episodes of hematuria.
Table 3.
Association between macroscopic hematuria and prophylactic treatment, adjusting for risk factors
| Analysis 1 (n = 235) | Analysis 2 (n = 285) | |||
|---|---|---|---|---|
| Odds ratio (95% CI) | P value | OR (95% CI) | P value | |
| Age | 0.98 (0.95‐1.00) | .08 | 1.03 (1.01‐1.05) | .007 |
| Frequent prophylaxis | 0.38 (0.18‐0.76) | .008 | 0.29 (0.16‐0.54) | <.001 |
| Infrequent prophylaxis | 1.43 (0.60‐3.50) | .43 | 0.87 (0.34‐2.26) | .78 |
| NSAIDs | … | … | 2.37 (1.46‐3.85) | <.001 |
| Severe hemophilia | … | … | 2.68 (1.49‐4.80) | <.001 |
This table shows the results from the 2 separate logistic regression models. In Analysis 1, the dichotomous response variable is whether the patient experienced hematuria within the past 5 years. In Analysis 2, the ordinal response variable is the number of macroscopic hematuria episodes. The variables in the first column are the covariates for each model.
Abbreviations: CI, confidence interval; NSAIDs, nonsteroidal anti‐inflammatory drugs.
On average, macroscopic hematuria was more prevalent among individuals with inhibitors. To assess the difference, we reestimated the logistic regression coefficients in Table 3 after excluding individuals with inhibitors. The association between macroscopic hematuria and frequent prophylaxis weakened only slightly by not including individuals with inhibitors (OR, 0.44; P = .03 in Analysis 1; and OR, 0.31; P < .001 in Analysis 2). Qualitatively, the results were identical, and infrequent prophylaxis remained nonsignificant.
Chi‐squared tests confirmed the inferences from the multivariate regressions. For both models, Pearson's chi‐squared test rejected the null hypothesis of independence between macroscopic hematuria and frequent long‐standing prophylaxis.
3.3. Sensitivity of results to different cutoff values for years of prophylactic treatment
In Analysis 2 in Table 3, we defined the reference level as individuals who received on‐demand or ≤5 years of prophylaxis. We compared this reference level to patients who received >15 years of prophylaxis, the cutoff value we considered long‐standing treatment based on clinical experience.
In Table 4, we examined the robustness of the result for frequent prophylaxis in Table 3 by estimating separate ordinal regression models using the same variables as in Analysis 2 in Table 3, but with different cutoff values for years of prophylactic treatment. Table 4 shows odds ratios only for the covariate frequent prophylaxis, with rows corresponding to different patient groups included in the reference level and columns corresponding to different cutoff values for duration of prophylaxis treatment. As the table shows, the results are robust to our specific choice of cutoff in Table 3. Only when we included all patients on prophylaxis, including those who started <5 years ago, did the effect become statistically insignificant. The strongest effect (lowest OR), highlighted in bold, corresponded to the cutoff choices in Table 3.
Table 4.
Association between frequent prophylaxis and number of macroscopic hematuria episodes for varying duration of treatment
| Reference level | Years of prophylaxis treatment | ||||
|---|---|---|---|---|---|
| >20 y | >15 y | >10 y | >5 y | >0 y | |
|
OR (95% CI), (n) |
OR (95% CI), (n) |
OR (95% CI), (n) |
OR (95% CI), (n) |
OR (95% CI), (n) |
|
| On demand |
0.34 (0.17‐0.68), (218) |
0.32 (0.17‐0.60), (235) |
0.34 (0.19‐0.61), (256) |
0.48 (0.29‐0.80), (288) |
0.68 (0.43, 1.06), (338) |
|
On demand and ≤5 y prophylaxis |
0.32 (0.16‐0.62), (268) |
0.29 (0.16‐0.54), (285) |
0.31 (0.19‐0.55), (306) |
0.44 (0.27‐0.72), (338) |
– |
|
On demand and ≤10 y prophylaxis |
0.33 (0.17‐0.64), (300) |
0.31 (0.17‐0.56), (317) |
0.33 (0.19‐0.56), (338) |
… | – |
This table shows odds ratios for frequent prophylaxis from separate ordinal regression models with varying cutoff values for long‐standing prophylaxis and varying definitions for the reference level. The first column shows the reference levels, while the headers indicate the cutoff values for years of prophylactic treatment. The number of observations for each model is in parentheses.
Abbreviations: CI, confidence interval; OR, odds ratio.
3.4. Frequency vs. number of infusions
In the regression models above, we found that the efficacy of prophylaxis with respect to reducing the risk of macroscopic hematuria depended on the frequency of treatment. It is natural to investigate whether it was the frequency or the number of infusions that constituted the effective treatment. We estimated a linear regression with the reported annual number of infusions as the dependent variable and the reported frequency per week as the independent variable. We found an almost perfect linear relation with a coefficient approximately equal to 52 with P value <2e −16. Hence, the 2 measures provide virtually the same information. We have focused on the frequency, as we place greater confidence in patients and physicians correctly reporting frequency than an estimated number of annual infusions.
4. DISCUSSION
While different strategies for factor replacement exist, the primary aim in hemophilia treatment is to reduce and treat bleeds. This study found that dosing frequency and duration of prophylactic therapy was associated with fewer episodes of macroscopic hematuria in aging people with hemophilia. Several pediatric studies have recognized the benefits of primary prophylaxis, which is now the preferred treatment for young individuals with hemophilia to prevent joint destruction.18, 19 Fewer studies exist for the increasing adult hemophilia population. Collins et al20 and Valentino et al21 reported on the safety and efficacy of prophylaxis compared to on‐demand and showed reduced bleeding rates in the prophylactic treatment groups. In addition, both the Prophylaxis Versus On‐Demand Therapy Through Economic Report (POTTER)22 and the Trial to Evaluate the Effect of Secondary Prophylaxis With rFVIII Therapy in Severe Hemophilia A Adult and/or Adolescent Subjects Compared to That of Episodic Treatment (SPINART)23 studies demonstrated a significant reduction in joint bleeds and less joint damage when using prophylaxis compared with on‐demand treatment. However, these studies focused on joint disease and included mainly adolescents and young adults. In contrast, our study included only older people with hemophilia and compared occurrence of macroscopic hematuria for patients receiving different factor replacement therapies.
Our hypothesis was that frequent prophylaxis could prevent episodes of macroscopic hematuria by ensuring higher peak factor levels and increased plasma factor levels for a longer time between treatments.24 We assumed that an effect from prophylaxis was only statistically observable if administered for a minimum duration. As there were only adults in our data, individuals with few years on their current prophylaxis regimen must necessarily have received on‐demand treatment most of their life. For example, if a 70‐year‐old patient has received on‐demand for 69 years and frequent prophylaxis for only 1 year, the observed statistical effect on macroscopic hematuria would presumably be indistinguishable from zero in a sample of our size. In contrast, if a patient received frequent prophylaxis for the past 20 years, this duration should be sufficient to observe an association.
In the first model, in which all patients have a history of macroscopic hematuria, frequent prophylaxis was a significant covariate, and >5 years of treatment with frequent prophylaxis was negatively associated with the condition reoccurring. In the second model, we studied the effect on the number of hematuria episodes of >15 years of prophylaxis compared to on‐demand therapy or ≤5 years of prophylaxis. Consistent with our hypothesis, we found frequent long‐standing prophylaxis negatively correlated with the number of episodes of macroscopic hematuria, suggesting a beneficial effect from maintaining an adequate factor level over time.
The significant risk factors were not identical across the regression models. In the first model, in contrast to the second, all patients had a history of macroscopic hematuria, and hence any risk factor responsible for the initial presence of the condition could have been equally distributed among those with an episode in the most recent 5 years and those without. This could explain why NSAIDs and severe hemophilia appeared as risk factors only in the second model.
The long‐term impact of hematuria on renal function is uncertain in people with hemophilia. Suggested causes of renal damage induced by macroscopic hematuria are toxicity of hemoglobin, heme, or iron released from red blood cells via oxidative stress. In addition to being cytotoxic, heme can promote renal damage by inducing inflammation and fibrosis.25, 26 Multiple episodes of macroscopic hematuria are associated with a decline in renal function and progression of chronic renal disease.27, 28, 29 Reducing macroscopic hematuria may thus improve renal function and limit future renal damage. Although management of macroscopic hematuria depends on etiology, the recommended initial treatment is increased fluid intake, either orally or by intravenous hydration. For persistent macroscopic hematuria, use of factor concentrate is the appropriate treatment.30
4.1. Limitations
This study has some limitations. We acknowledge that causality cannot be inferred from these cross‐sectional data. However, the timeline of certain events is deducible, and we believe associations obtained while considering this can provide important probable evidence. Properly measuring and comparing the outcomes of the 2 treatments, and ensuring no systematic differences among the treatment groups, would require a randomized controlled trial. Since we have no information regarding the reasons for the physician's choice of either on‐demand or prophylactic therapy, we cannot rule out bias in the choice of treatment. We can only identify associations between type of treatment and macroscopic hematuria. A possible limitation is that, apart from blood, certain foods and drugs may cause red urine discoloration. We have assumed that patients and physicians have ruled out other probable causes before recording macroscopic hematuria in the case report form. To minimize recall bias, a ratio scale rather than exact numbers was used for the lifetime number of macroscopic hematuria episodes. Patient reporting was supplemented with information from existing medical records. Although prostate cancer was reported in only 6 patients, 2 of whom were in remission or without relapse, it is a limitation that the case report form did not capture prostate pathology, such as benign hyperplasia, as this condition may be associated with either micro‐ or macroscopic hematuria. Furthermore, we had no data on patient pharmacokinetic analyses and expected trough levels. On average, prophylaxis treatment was 26.4 units/kg for the total cohort, with 25.3 units/kg for hemophilia A and 33.5 for hemophilia B. However, according to Collins et al31, and consistent with our hypothesis, frequency of dosing has a much higher effect on factor trough levels and time per week with increased plasma factor levels than the infused dose. Another limitation was that most data were collected retrospectively. These limitations notwithstanding, the H3 study12 represents a comprehensive multicountry hemophilia sample that adds to the generalizability of the strong association between frequent prophylaxis and reduction in occurrence of hematuria.
5. CONCLUSION
In this study, frequent prophylaxis was negatively associated with occurrence of macroscopic hematuria compared with on‐demand. Infrequent prophylactic treatment had no significant effect. This is in agreement with the current consensus32 that lifelong prophylactic therapy should be the standard treatment for people with hemophilia (Table S1).
RELATIONSHIP DISCLOSURES
The ADVANCE Working Group is supported by grant funding from Bayer Healthcare. The sponsor played no role in data collection but was present in meetings and discussions related to the H3 study. CQ reports honoraria and nonfinancial support from Bayer HealthCare, outside the submitted work. RCT reports honoraria and nonfinancial support from Bayer HealthCare, CSL Behring, and Novo Nordisk, and honoraria from Pfizer, Sanofi, Shire, and Sobi, outside the submitted work. PdM reports honoraria from Bayer HealthCare, Baxalta, CSL Behring, Novo Nordisk, Shire, and Sobi, outside the submitted work. PAH reports honoraria or research support from Bayer HealthCare, CSL Behring, Novo Nordisk, Octapharma, Pfizer, Shire, and Sobi, outside the submitted work.
AUTHOR CONTRIBUTIONS
CQ designed the research study, performed the research, analyzed the data and wrote the paper. RCT, PdM, and PAH designed the research study and participated in writing the manuscript.
Supporting information
ACKNOWLEDGMENTS
The authors thank the centers that contributed data: Austria (Vienna Adult Hemophilia Center), Germany (Vivantes Klinikum im Friedrichshain, Berlin; University Clinic Bonn; Kurpfalz Hospital and Haemophilia Centre, Heidelberg), Greece (Hippokration Hospital, Athens), Israel (Sheba Medical Center, Tel Hashomer; Chaim Sheba Medical Center, Tel Aviv), Italy (IRCCS Maggiore Hospital, Mangiagalli and Regina Elena Foundation, Milan; University Hospital of Parma), The Netherlands (University Medical Center Utrecht), Norway (Oslo University Hospital), Poland (Institute of Haematology and Blood Transfusion, Warsaw) Slovenia (University Medical Centre, Ljubljana), Spain (Virgen del Rocıo Hospital, Seville), Sweden (Lund University, Malmo), Switzerland (University Hospital of Geneva), United Kingdom (Royal Infirmary, Glasgow).
APPENDIX 1.
Members involved in ADVANCE at the time of the H3 study
Ingrid Pabinger, Austria
Cedric Hermans, Belgium
Roseline d’Oiron, France
Robert Klamroth, Germany
Johannes Oldenburg, Natascha Marquardt, Germany
Peter Staritz, Germany
Olga Katsarou, Greece
Uri Martinowitz, Aharon Lubetsky, Gili Kenet, Israel
Annarita Tagliaferri, Italy
Maria Elisa Mancuso, Italy
Roger Schutgens, The Netherlands
Pal Andre Holme, Norway
Jerzy Windyga, Poland
Irena Zupan, Slovenia
Victor Jimenez Yuste, Spain
Ramiro Nunez, Spain
Philippe de Moerloose, Switzerland
Erik Berntorp, Jan Astermark, Sweden
Campbell Tait, UK
Gerry Dolan, UK
Qvigstad C, Tait RC, de Moerloose P, Holme PA; On behalf of the ADVANCE Working Group . Hematuria in aging men with hemophilia: Association with factor prophylaxis. Res Pract Thromb Haemost. 2020;4:309–317. 10.1002/rth2.12298
Handling Editor: Pantep Angchaisuksiri
REFERENCES
- 1. Srivastava A, Brewer A, Mauser‐Bunschoten E, Key N, Kitchen S, Llinas A, et al. Guidelines for the management of hemophilia. Haemophilia. 2013;19(1):e1–e47. [DOI] [PubMed] [Google Scholar]
- 2. Grossfeld GD, Carroll PR. Evaluation of asymptomatic microscopic hematuria. Urol Clin North Am. 1998;25:661–76. [DOI] [PubMed] [Google Scholar]
- 3. Schmidt‐Hansen M, Berendse S, Hamilton W. The association between symptoms and bladder or renal tract cancer in primary care: a systematic review. Br J Gen Pract. 2015;65:e769–e775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ritchie CD, Bevan EA, Collier SJ. Importance of occult haematuria found at screening. Br Med J (Clin Res Ed). 1986;292:681–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Britton JP, Dowell AC, Whelan P, Harris CM. A community study of bladder cancer screening by the detection of occult urinary bleeding. J Urol. 1992;148:788–90. [DOI] [PubMed] [Google Scholar]
- 6. Nielsen M, Qaseem A. Hematuria as a marker of occult urinary tract cancer: advice for high‐value care from the American College of Physicians. Ann Intern Med. 2016;164:488–97. [DOI] [PubMed] [Google Scholar]
- 7. Prentice C, Lindsay R, Barr R, Forbes C, Kennedy A, McNicol G, et al. Renal complications in haemophilia and Christmas disease. QJM. 1971;40:47–61. [PubMed] [Google Scholar]
- 8. Beck P, Evans K. Renal abnormalities in patients with haemophilia and Christmas disease. Clin Radiol. 1972;23:349–54. [DOI] [PubMed] [Google Scholar]
- 9. Kulkarni R, Soucie JM, Evatt B. Renal disease among males with haemophilia. Haemophilia. 2003;9:703–10. [DOI] [PubMed] [Google Scholar]
- 10. Benedik‐Dolničar M, Benedik M. Haematuria in patients with haemophilia and its influence on renal function and proteinuria. Haemophilia. 2007;13:489–92. [DOI] [PubMed] [Google Scholar]
- 11. van de Putte DEF, Fischer K, Makris M, Tait RC, Collins PW, Meijer K, et al. Increased prevalence of hypertension in haemophilia patients. Thromb Haemost. 2012;108:750–5. [DOI] [PubMed] [Google Scholar]
- 12. Holme PA, Combescure C, Tait RC, Berntorp E, Rauchensteiner S, de Moerloose P, et al. Hypertension, haematuria and renal functioning in haemophilia – a cross‐sectional study in Europe. Haemophilia. 2016;22:248–55. [DOI] [PubMed] [Google Scholar]
- 13. Sun H, Yang M, Sait A, Drygalski A, Jackson S. Haematuria is not a risk factor of hypertension or renal impairment in patients with haemophilia. Haemophilia. 2016;22:549–55. [DOI] [PubMed] [Google Scholar]
- 14. Small S, Rose P, McMillan N, Belch J, Rolfe E, Forbes C, et al. Haemophilia and the kidney: assessment after 11‐year follow‐up. Br Med J (Clin Res Ed). 1982;285:1609–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Forbes CD, Prentice CR. Renal disorders in haemophilia A and B. Scand J Haematol. 1977;19:43–50. [DOI] [PubMed] [Google Scholar]
- 16. Qvigstad C, Tait RC, Rauchensteiner S, Berntorp E, de Moerloose P, Schutgens RE, et al. The elevated prevalence of risk factors for chronic liver disease among ageing people with hemophilia and implications for treatment. Medicine. 2018;97:e12551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Core R, Team R. A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing, 2019. [Google Scholar]
- 18. Manco‐Johnson MJ, Abshire TC, Shapiro AD, Riske B, Hacker MR, Kilcoyne R, et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med. 2007;357:535–44. [DOI] [PubMed] [Google Scholar]
- 19. Gringeri A, Lundin B, Von Mackensen S, Mantovani L, Mannucci P. A randomized clinical trial of prophylaxis in children with hemophilia A (the ESPRIT study). J Thromb Haemost. 2011;9:700–10. [DOI] [PubMed] [Google Scholar]
- 20. Collins P, Faradji A, Morfini M, Enriquez M, Schwartz L. Efficacy and safety of secondary prophylactic vs. on‐demand sucrose‐formulated recombinant factor VIII treatment in adults with severe hemophilia A: results from a 13‐month crossover study. J Thromb Haemost. 2010;8:83–9. [DOI] [PubMed] [Google Scholar]
- 21. Valentino L, Mamonov V, Hellmann A, Quon D, Chybicka A, Schroth P, et al. A randomized comparison of two prophylaxis regimens and a paired comparison of on‐demand and prophylaxis treatments in hemophilia A management. J Thromb Haemost. 2012;10:359–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tagliaferri A, Feola G, Molinari AC, Santoro C, Rivolta GF, Cultrera DB, et al. Benefits of prophylaxis versus on‐demand treatment in adolescents and adults with severe haemophilia A: the POTTER study. Thromb Haemost. 2015;114:35–45. [DOI] [PubMed] [Google Scholar]
- 23. Manco‐Johnson MJ, Lundin B, Funk S, Peterfy C, Raunig D, Werk M, et al. Effect of late prophylaxis in hemophilia on joint status: a randomized trial. J Thromb Haemost. 2017;15:2115–24. [DOI] [PubMed] [Google Scholar]
- 24. Valentino LA, Pipe SW, Collins PW, Blanchette VS, Berntorp E, Fischer K, et al. Association of peak factor VIII levels and area under the curve with bleeding in patients with haemophilia A on every third day pharmacokinetic‐guided prophylaxis. Haemophilia. 2016;22:514–20. [DOI] [PubMed] [Google Scholar]
- 25. Tracz MJ, Alam J, Nath KA. Physiology and pathophysiology of heme: implications for kidney disease. J Am Soc Nephrol. 2007;18:414–20. [DOI] [PubMed] [Google Scholar]
- 26. Moreno JA, Martin‐Cleary C, Gutierrez E, Toldos O, Blanco‐Colio LM, Praga M, et al. AKI associated with macroscopic glomerular hematuria: clinical and pathophysiologic consequences. Clin J Am Soc Nephrol. 2012;7:175–84. [DOI] [PubMed] [Google Scholar]
- 27. Gutierrez E, Gonzalez E, Hernandez E, Morales E, Martinez MA, Usera G, et al. Factors that determine an incomplete recovery of renal function in macrohematuria‐induced acute renal failure of IgA nephropathy. Clin J Am Soc Nephrol. 2007;2:51–7. [DOI] [PubMed] [Google Scholar]
- 28. Yuste C, Rubio‐Navarro A, Barraca D, Aragoncillo I, Vega A, Abad S, et al. Haematuria increases progression of advanced proteinuric kidney disease. PLoS ONE. 2015;10:e0128575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Orlandi PF, Fujii N, Roy J, Chen H‐Y, Hamm LL, Sondheimer JH, et al. Hematuria as a risk factor for progression of chronic kidney disease and death: findings from the chronic renal insufficiency cohort (CRIC) Study. BMC Nephrol. 2018;19:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Quon D, Konkle B. How we treat: Haematuria in adults with haemophilia. Haemophilia. 2010;16:683–5. [DOI] [PubMed] [Google Scholar]
- 31. Collins PW, Björkman S, Fischer K, Blanchette V, Oh M, Schroth P, et al. Factor VIII requirement to maintain a target plasma level in the prophylactic treatment of severe hemophilia A: influences of variance in pharmacokinetics and treatment regimens. J Thromb Haemost. 2010;8:269–75. [DOI] [PubMed] [Google Scholar]
- 32. Makris M. Prophylaxis in haemophilia should be life‐long. Blood Transfus. 2012;10:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


