Abstract
Objectives:
This study aimed to assess the dentin adaptability of a certain type of fluoride varnish, as a novel root canal sealer, in comparison with AH-Plus sealer.
Materials and Methods:
Twenty-four extracted single-rooted, single-canal human permanent teeth with straight and fully formed roots and no internal calcification, resorption or cracks, were selected and decoronated such that the remaining root length was 14 mm in all teeth. Root canals were prepared using the Mtwo rotary file system according to the manufacturer’s instructions and filled with gutta-percha and either AH-Plus (n=12) or fluoride varnish (n=12) via the lateral compaction technique. Each root was then sectioned at 4 and 8 mm distances from the apex for evaluation under a scanning electron microscope (SEM). The gap size between the sealer and dentin was measured. Statistical analysis was performed using the Kolmogorov-Smirnov test and t-test with the significance level set at 0.05.
Results:
The mean gap size was 14.407±1.402 μm and 8.342±0.694 μm in the roots obturated with AH-Plus and fluoride varnish sealers, respectively. The t-test revealed a statistically significant difference (P<0.001) in this regard between the two groups.
Conclusion:
Fluoride varnish, as a root canal sealer, has a superior adaptation to dentinal canal walls compared to the AH-Plus sealer.
Keywords: AH Plus, Root Canal Filling Materials, Root Canal Sealants, Fluoride Varnish, Scanning Electron Microscopy
INTRODUCTION
The major goal of root canal obturation is to seal the root canal system to prevent reinfection of the root canal because of leakage of microorganisms and their by-products [1]. The sealing ability of a root canal filling material is, therefore, an important factor to ensure long-term stability of treatment outcome. The root filling material should be adapted to the canal walls, and the whole canal should be filled with a homogeneous mass of gutta-percha and sealer. Without a sealer, the filling material cannot adapt to the canal walls and may leave voids and gaps [2,3].
Some of the most important features of an ideal sealer include optimal adhesion, sealability, tissue compatibility, insolubility in tissue fluids, and antibacterial properties. Ideally, a root canal sealer should be able to create a strong bond between the filling material and dentinal canal walls to prevent microleakage [4].
Although several types of sealers are used in the clinical setting, they all have their limitations. Due to the different compositions of sealers, the level of adhesion of sealers to dentin or gutta-percha is expected to vary among different sealer types. AH-Plus (Dentsply DeTrey, Konstanz, Germany) is one of the most popular sealers due to its ideal properties, including high dentin adhesion and optimal sealability [5,6].
Fluoride varnishes have several applications including prevention of dental caries [7,8], application as an intracanal medicament [9], treatment of dentin hypersensitivity [10], and filling the root canals of deciduous teeth in combination with calcium hydroxide and zinc oxide [11]. Fluoride decreases the permeability of dentin by blocking the dentinal tubules through the production of calcium fluoride (CaF2) and its deposition over the tubules. In addition, the natural resins in the composition of fluoride varnishes could create an additional protective barrier on the dentin surface [12].
Recently, two studies used fluoride varnish as a root canal sealer and reported satisfactory results concerning its sealing properties compared to AH 26 and AH-Plus [13,14]. Duofluoride XII (FGM, Joinville, SC, Brazil) is a dual fluoride varnish with proven efficacy for prevention of dental caries [15].
To the best of the authors’ knowledge, no previous study has examined the dentin adaptability of fluoride varnish when used as a root canal sealer. This study aimed to measure the gap size between dentinal canal walls and fluoride varnish used as a sealer compared to the application of AH-Plus sealer using scanning electron microscopy (SEM).
MATERIALS AND METHODS
This in-vitro study has been approved by the Human Research Ethics Committee of Mazandaran University of Medical Sciences, Mazandaran, Iran (IR.MAZUMS.REC.94.2064). Twenty-four extracted single-rooted, single-canal human permanent teeth with straight, fully formed roots and no internal calcification, resorption or cracks were selected based on clinical and radiographic examinations.
Preparation of teeth:
Initially, the root surface of the extracted teeth was cleaned with a periodontal curette to eliminate calculi and soft tissue debris. Then, the samples were immersed in 5.25% sodium hypochlorite solution (NaOCl; Golrang, Tehran, Iran) for one hour to remove organic debris from root surfaces. Subsequently, all samples were radiographed from the proximal aspect to ensure the existence of a single canal. The crown of the selected teeth was cut to standardize the root length of the teeth using a diamond disc (Jota AG, Rüthi, Switzerland) such that the remaining root length was 14 mm. Next, the working length was determined by introducing a #15 K-file (Dentsply Maillefer, Switzerland) into the canal until the tip of the file was visible at the apical foramen. The actual working length was considered to be 1 mm shorter than the measured length.
A rotary file system (Mtwo, VDW Co., Munich, Germany) was used to prepare the canals according to the manufacturer’s instructions. First, Gates Glidden drills (#2, #3, and #4) were used to prepare the coronal third of the canals. Subsequently, the middle and apical parts were prepared with Mtwo rotary files (#20, #25, #35, and #40, respectively), a rotary handpiece (NSK, Nakanishi Inc., Tokyo, Japan), and an electric motor (NSK Endo-Mate DT, Nakanishi Inc., Tokyo, Japan) operating at 350 revolutions per minute (rpm) and 1.5-Ncm torque using the single-length technique. After using each file, the canal was rinsed with 1 ml of 5.25% NaOCl.
To remove the smear layer, 17% ethylenediaminetetraacetic acid (EDTA; CinaBartar Co., Tehran, Iran) and 5.25% NaOCl were used according to the Crumpton’s method [16]. Accordingly, 1 ml of 17% EDTA solution was left in the canal for one minute, and then, 3 ml of 5.25% NaOCl solution was used for irrigation. A final rinse with 3 ml of distilled water was also performed. This was followed by drying the canals with paper points (Meta Biomed Co. Ltd., Chungcheongbuk-do, Korea). The samples were then randomly divided into two groups of 12 roots each:
Group 1- Gutta-percha (Meta Biomed Co. Ltd., Chungcheongbuk-do, Korea) was used in combination with AH-Plus sealer (Dentsply DeTrey, Konstanz, Germany) to fill the canals after mixing the sealer according to the manufacturer’s instructions.
Group 2- Gutta-percha was used along with a fluoride varnish (Doufluoride XII, FGM, Joinville, SC, Brazil) to fill the canals. The fluoride varnish was prepared according to the manufacturer’s instructions.
The lateral compaction technique was used to fill the canals in all samples. First, the canal walls were coated with the sealer using a finger spreader (Dentsply Maillefer, Ballaigues, Switzerland). A #40 master guttapercha cone with 0.02 taper (Meta Biomed Co. Ltd., Chungcheongbuk-do, Korea) was dipped in the sealer and introduced into the canal to the appropriate working length. Then, the lateral cones were placed into the canals until the spreader could not reach the coronal third of the root canal.
The remaining gutta-percha was cut using a heat carrier (Dentsply Maillefer, Ballaigues, Switzerland). Coronal sealing for all samples was performed using a 2- to 3-mm-thick temporary restorative material (Coltosol; Ariadent, Tehran, Iran). Then, a radiograph was taken to confirm the quality of the obturation. Proper obturation was defined as the presence of a dense filling without voids. Next, all teeth were incubated at 37°C with 100% humidity for 72 hours. Afterwards, the roots were transversely sectioned at 4 and 8 mm distances from the apex with a 200-μm-thick diamond disc (CNC; Nemo Fanavaran Pars, Mashhad, Iran) mounted on a slow-speed handpiece under water coolant. By doing so, a section with a 4-mm height from the middle third of each root was dissected for examination under an SEM.
Microscopic examination:
The coronal surfaces of the specimens were ground. The specimens were then sonicated for 7 minutes in distilled water to remove the smear layer from the exposed dentinal surfaces [16]. The coronal side of the specimens was coated with a thin gold-palladium film, and they were then mounted on the aluminum stub of the sputter coater (SC7620, Emitech, UK) operating at 20 milliamperes (mA) for 3 minutes for sputter-coating. Finally, the specimens were examined under an SEM (LEO1450VP, Zeiss, Oberkochen, Germany) with a 205-nm resolution at 20 kilovoltages (kV). A single operator performed all the above-mentioned procedures for standardization.
The specimens were first examined at low magnification (×150) to have an inclusive view of the entire root area. Subsequently, the root canal cross-sections were hypothetically divided into four equal quadrants. The greatest and the smallest gap sizes between the sealer and root canal dentinal wall were measured in each quadrant at ×1000 and ×2000 magnifications and recorded. Ultimately, the mean minimum gap size and the mean maximum gap size were calculated for each sample. The gap size was measured in micrometers (μm) using the calibrated measurement instrument of the microscope. The samples were observed by two trained endodontists who were blinded to the group allocation of specimens (AH-Plus or fluoride varnish). The ideal percentage of inter-observer agreement was considered to be over 95%. In case of a lower percentage, a consensus was obtained through inter-observer discussion.
The Kolmogorov-Smirnov test was used to evaluate the normal distribution of data, and the independent t-test was applied to compare the two groups. The significance level was set at P<0.05.
RESULTS
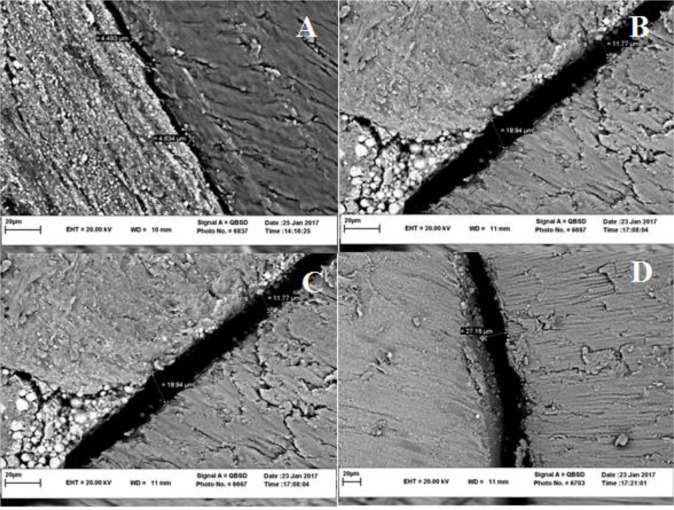
The normal distribution of data was assessed using the Kolmogorov-Smirnov and Shapiro-Wilk tests. The data in both groups had a normal distribution (P>0.05). Mean (standard deviation) gap sizes were calculated as 14.407 (1.402) μm with a range of 12.19–16.369 μm for AH-Plus and 8.342 (0.699) μm ranging from 7.562–9.933 μm for fluoride varnish (N=12 in both groups). The AH-Plus group showed significantly greater gap sizes between the sealer and dentinal walls as compared to the fluoride varnish group (P<0.001). The microscopic images are demonstrated in Figure 1.
Fig. 1:
Scanning electron microscopic micrograph showing the dentin-sealer interface. (A) and (B) indicate the largest and the smallest gaps between the fluoride varnish and dentinal wall, respectively. The largest and the smallest gaps between AH-Plus and dentinal wall are marked as (C) and (D), respectively.
DISCUSSION
The present study examined the dentin adaptability of fluoride varnish (Duofluoride XII) as a root canal sealer. The results showed that this type of sealer has superior dentin adaptability in comparison with AH-Plus sealer. AH-plus sealer is known as the gold standard sealer due to its ideal properties, including good adhesion to dentin and sealability [17].
Some methods, including gap size measurement [18], bond strength measurement [19,20], and calculation of tubular penetration rate [21,22] are used to evaluate the sealer penetration ability and its adaptation to root canal dentinal walls. The gap size measurement approach was the method of choice in the current study.
Accordingly, a slice of the root was dissected by sectioning the root at 4 and 8 mm distances from the apex to facilitate the mounting of the samples for SEM assessment. The coronal surface was evaluated as the area undergoing the greatest condensation forces during root canal filling using the lateral compaction technique [23].
Undetected gaps in some areas of root canal walls are a potential adverse occurrence, which might confound the findings of the studies investigating the gap size at certain cross-sections of the root. To overcome this problem in the current study, each cross-section was divided into four quadrants to enhance the accuracy of gap detection [18].
In the present study, an SEM was used to evaluate the dentin adaptability of AH-Plus versus the fluoride varnish. This microscope allows the examiner to observe the fine relevant details regarding the integrity and surface appearance of the sealer. In addition, the adaptation of the sealer to the dentin can be evaluated under higher magnifications, allowing for observation and measurement of the gap(s) formed between the sealer and the canal wall [24].
Gap sizes are often reported at ×2000 magnification. In a microscopic study, Steier et al [25] observed that an increase in magnification from ×150 to ×1000 had no significant effect on the detection of a greater number of gaps. However, in the current study, ×150 magnification was first used to obtain an overview followed by ×2000 magnification for enhanced accuracy.
The primary characteristics emphasized by Grossman et al [26] concerning an ideal sealer include optimal adhesion quality and sealing properties. Fluoride varnish has shown satisfying adhesion and durability on the dentin surface [12]. Its application as a root canal sealer indicated favorable results in studies conducted by Parirokh et al [13] and Rao et al [14]. In the current study, the mean gap size was 14.407±1.0402 μm in the AH-Plus group and 8.342±0.694 μm in the fluoride varnish group. At the time of conducting this study, no similar study seemed to be available to compare our results regarding gap size following the application of fluoride varnish as a root canal sealer. The results of some previous SEM studies showed that AH-Plus has proper dentinal adaptation [18,21, 25]. Steier et al [25] evaluated the sealer-dentin interface of AH-Plus and RealSeal. According to their findings, only 21% to 30% of the cases had a visible gap [25]. Similarly, Balguerie et al [21] found no gaps between AH-Plus sealer and dentin in most of the transverse sections of the teeth. In a similar study, Mohammadian et al [18] evaluated the dentin-sealer interfaces of BC sealer, AH-Plus, and Dorifill using an SEM at ×300 magnification. They found that AH-Plus had a mean gap size of 5.63±2.56 μm in the middle section of the teeth [18]. Accordingly, resin-based sealers seem to have an appropriate adaptation to the canal for two reasons, namely mechanical retention [27,28] and no polymerization shrinkage [29].
Parirokh et al [13] and Rao et al [14] used a 5% fluoride varnish as a root canal sealer and compared the results versus using AH-26 and AH-Plus sealers in terms of bacterial leakage. They observed that fluoride varnish, used as a root canal sealer, had satisfying sealing quality [13,14]. The favorable adaptation quality of sealers may enhance their sealing properties because of the increased contact surface area between the sealer and dentin [30]. Parirokh et al [13] examined eight roots to evaluate the adaptation quality of sealers using an SEM. They applied fluoride varnish on the root canal walls of split roots and found that their experimental varnish had successfully covered the dentinal tubules [13]. In the present study, despite using a different method for preparation of the samples, similar results were obtained. Sen and Büyükyilmaz [31] used a 4% titanium fluoride solution to cover root canal walls in the presence and absence of a smear layer. The microscopic evaluation using an SEM revealed that this solution had successfully sealed the dentinal tubules. They hypothesized that this finding might be due to the presence of titanium ion with complex-binding capacity concurrently with fluoride and apatite in dentin [31]. However, similar results were obtained using Doufluoride XII (6% CaF2+6% NaF) in the present study. According to the manufacturer, calcium fluoride is added to increase the precipitation of CaF2 on the tooth surface, which may be a reason for the good adaptation quality.
CONCLUSION
The present study indicated that the examined fluoride varnish has better dentin adaptability than AH-Plus sealer. Further in-vitro and invivo studies are required to examine other properties of this varnish.
ACKNOWLEDGMENTS
This paper was based on a thesis supported by a grant from the Research Council of Mazandaran University of Medical Sciences, Sari, Iran.
Footnotes
CONFLICT OF INTEREST STATEMENT
None declared
REFERENCES
- 1.Sjogren U, Figdor D, Persson S, Sundqvist G. Influence of infection at the time of root filling on the outcome of endodontic treatment of teeth with apical periodontitis. Int Endod J. 1997. September;30(5):297–306. [DOI] [PubMed] [Google Scholar]
- 2.Li GH, Niu LN, Zhang W, Olsen M, De-Deus G, Eid AA, et al. Ability of new obturation materials to improve the seal of the root canal system: a review. Acta Biomater. 2014. March;10(3):1050–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.ØRSTAVIK D. Materials used for root canal obturation: technical, biological and clinical testing. Endod Topics. 2005. November;12(1):25–38. [Google Scholar]
- 4.Grossman LI, Oliet S, Del Rio CE. Endodontic Practice. Lea & Febiger, Philadelphia, PA, USA, 1988:179–227. [Google Scholar]
- 5.Tyagi S, Mishra P, Tyagi P. Evolution of root canal sealers: An insight story. Eur J Gen Dent. 2013. August;2(3):199–218. [Google Scholar]
- 6.Sevimay S, Dalat D. Evaluation of penetration and adaptation of three different sealers: a SEM study. J Oral Rehabil. 2003. September;30(9):951–5. [DOI] [PubMed] [Google Scholar]
- 7.Agouropoulos A, Twetman S, Pandis N, Kavvadia K, Papagiannoulis L. Caries-preventive effectiveness of fluoride varnish as adjunct to oral health promotion and supervised tooth brushing in preschool children: a double-blind randomized controlled trial. J Dent. 2014. October;42(10):1277–83. [DOI] [PubMed] [Google Scholar]
- 8.Fontana M, Gonzalez-Cabezas C, Haider A, Stookey GK. Inhibition of secondary caries lesion progression using fluoride varnish. Caries Res. 2002. Mar-Apr;36(2):129–35. [DOI] [PubMed] [Google Scholar]
- 9.Omidi S, Iomee M, Eshaghi F, Ahanjan M, Cherati JY. Antibacterial effect of stannous fluoride (SnF) 0.63% as an intracanal medicament against Enterococcus faecalis: An in vitro study. Ann Dent Spec. 2018. Jan-Mar:6(1):35–39. [Google Scholar]
- 10.Lochaiwatana Y, Poolthong S, Hirata I, Okazaki M, Swasdison S, Vongsavan N.The synthesis and characterization of a novel potassium chloride-fluoridated hydroxyapatite varnish for treating dentin hypersensitivity. Dent Mater J. 2015;34(1):31–40. [DOI] [PubMed] [Google Scholar]
- 11.Chawla HS, Setia S, Gupta N, Gauba K, Goyal A. Evaluation of a mixture of zinc oxide, calcium hydroxide, and sodium fluoride as a new root canal filling material for primary teeth. J Indian Soc Pedod Prev Dent. 2008. June;26(2):53–8. [DOI] [PubMed] [Google Scholar]
- 12.Ritter AV, de L Dias W, Miguez P, Caplan DJ, Swift EJ., Jr. Treating cervical dentin hypersensitivity with fluoride varnish: a randomized clinical study. J Am Dent Assoc. 2006. July;137(7):1013–20; quiz 1029. [DOI] [PubMed] [Google Scholar]
- 13.Parirokh M, Talebizad M, Forghani FR, Haghdoost AA, Asgary S, Eghbal MJ, et al. Fluoride varnish as root canal sealer: a scanning electron microscopy and bacterial penetration study. Iran Endod J. 2015. Winter;10(1):64–8. [PMC free article] [PubMed] [Google Scholar]
- 14.Rao DG, Trivedi MV, Havale R, Shrutha SP. New fluoride MI Varnish as root canal sealer: An in vitro analysis of bacterial leakage. J Indian Soc Pedod Prev Dent. 2016. Oct-Dec;34(4):359–63. [DOI] [PubMed] [Google Scholar]
- 15.Olympio KP, Cardoso VE, Bijella MF, Pessan JP, Delbem AC, Buzalaf MA. Urinary fluoride output in children following the use of a dual-fluoride varnish formulation. J Appl Oral Sci. 2009. May-Jun;17(3):179–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moradi S, Ghoddusi J, Forghani M. Evaluation of dentinal tubule penetration after the use of dentin bonding agent as a root canal sealer. J Endod. 2009. November;35(11):1563–6. [DOI] [PubMed] [Google Scholar]
- 17.Silva EJ, Perez R, Valentim RM, Belladonna FG, De-Deus GA, Lima IC, et al. Dissolution, dislocation and dimensional changes of endodontic sealers after a solubility challenge: a micro-CT approach. Int Endod J. 2017. April;50(4):407–14. [DOI] [PubMed] [Google Scholar]
- 18.Mohammadian F, Farahanimastary F, Dibaji F, Kharazifard MJ. Scanning Electron Microscopic Evaluation of the Sealer-Dentine Interface of Three Sealers. Iran Endod J. 2017. Winter;12(1):38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forough Reyhani M, Ghasemi N, Rahimi S, Salem Milani A, Mokhtari H, Shakouie S, et al. Push-Out Bond Strength of Dorifill, Epiphany and MTA-Fillapex Sealers to Root Canal Dentin with and without Smear Layer. Iran Endod J. 2014. Fall;9(4):246–50. [PMC free article] [PubMed] [Google Scholar]
- 20.Carvalho CN, Martinelli JR, Bauer J, Haapasalo M, Shen Y, Bradaschia-Correa V, et al. Micropush-out dentine bond strength of a new gutta-percha and niobium phosphate glass composite. Int Endod J. 2015. May;48(5):451–9. [DOI] [PubMed] [Google Scholar]
- 21.Balguerie E, van der Sluis L, Vallaeys K, Gurgel-Georgelin M, Diemer F. Sealer penetration and adaptation in the dentinal tubules: a scanning electron microscopic study. J Endod. 2011. November;37(11):1576–9. [DOI] [PubMed] [Google Scholar]
- 22.Shokouhinejad N, Sabeti M, Gorjestani H, Saghiri MA, Lotfi M, Hoseini A. Penetration of Epiphany, Epiphany self-etch, and AH Plus into dentinal tubules: a scanning electron microscopy study. J Endod. 2011. September;37(9):1316–9. [DOI] [PubMed] [Google Scholar]
- 23.Vassiliadis LP, Sklavounos SA, Stavrianos CK. Depth of penetration and appearance of Grossman sealer in the dentinal tubules: an in vivo study. J Endod. 1994. August;20(8):373–6. [DOI] [PubMed] [Google Scholar]
- 24.Mamootil K, Messer HH. Penetration of dentinal tubules by endodontic sealer cements in extracted teeth and in vivo. Int Endod J. 2007. November;40(11):873–81. [DOI] [PubMed] [Google Scholar]
- 25.Steier L, de Figueiredo JAP, Belli S. Comparison of the interface dentinendodontic sealer using two SEM magnifications. Rev Odonto Ciênc. 2010. December;25(3):296–9. [Google Scholar]
- 26.Grossman LI, Shepard LI, Pearson LA. Roentgenologic and clinical evaluation of endodontically treated teeth. Oral Surg Oral Med Oral Pathol. 1964. March;17:368–74. [DOI] [PubMed] [Google Scholar]
- 27.Barbizam JV, Trope M, Tanomaru-Filho M, Teixeira EC, Teixeira FB. Bond strength of different endodontic sealers to dentin: push-out test. J Appl Oral Sci. 2011. Nov-Dec;19(6):644–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sagsen B, Ustun Y, Demirbuga S, Pala K. Push-out bond strength of two new calcium silicate-based endodontic sealers to root canal dentine. Int Endod J. 2011. December;44(12):1088–91. [DOI] [PubMed] [Google Scholar]
- 29.Marciano MA, Guimaraes BM, Ordinola-Zapata R, Bramante CM, Cavenago BC, Garcia RB, et al. Physical properties and interfacial adaptation of three epoxy resin-based sealers. J Endod. 2011. October;37(10):1417–21. [DOI] [PubMed] [Google Scholar]
- 30.Wu MK, de Gee AJ, Wesselink PR. Effect of tubule orientation in the cavity wall on the seal of dental filling materials: an in vitro study. Int Endod J. 1998. September;31(5):326–32. [PubMed] [Google Scholar]
- 31.Sen BH, Büyükyilmaz T. The effect of 4% titanium tetrafluoride solution on root canal walls--a preliminary investigation. J Endod. 1998. April;24(4):239–43. [DOI] [PubMed] [Google Scholar]