Abstract
目的
探讨信号转导及转录活化因子2(STAT2)在卵巢癌中的表达及与患者预后的相关性,并研究STAT2对卵巢癌细胞转移能力的影响和作用机制。
方法
RT-qPCR分析62例新鲜冰冻卵巢癌组织和62例正常卵巢组织中STAT2的mRNA的表达,免疫组化法检测95例卵巢癌石蜡样本和33例正常卵巢石蜡样本中STAT2蛋白的表达,Kaplan-Meier法分析STAT2表达和患者的预后之间的相关性,分析Kaplan-Meier Plotter数据库中STAT2与患者预后之间的关系。Western blot检测STAT2在卵巢癌细胞株中的表达,在最高表达的A2780细胞株中干扰STAT2后,Transwell检测卵巢癌细胞迁移、侵袭能力的变化;以及下游分子EGFR表达水平的变化。
结果
卵巢癌组织中STAT2 mRNA的表达高于正常卵巢组织,STAT2 mRNA表达与较差的FIGO分期相关。免疫组化显示STAT2蛋白在卵巢癌组织中的高表达率为67.4%,高于正常卵巢组织的28.3%;卵巢癌患者中,STAT2蛋白的高表达与患者的腹水量、有无远处转移及FIGO分期相关(P < 0.05)。生存分析表明高表达STAT2蛋白的卵巢癌患者的整体生存期(P=0.021)及无进展生存期(P=0.018)较差。STAT2在卵巢癌细胞株中普遍呈高表达,A2780细胞株中表达最高,用于建立干扰细胞株;干扰STAT2后,A2780细胞迁移和侵袭能力减弱(P < 0.01),并且下游分子EGFR的mRNA和蛋白表达水平较对照组均降低。
结论
STAT2在卵巢癌中表达升高,与卵巢癌患者预后不良相关,STAT2可能通过促进EGFR的表达而促进卵巢癌的转移。
Keywords: 卵巢癌, STAT2, 肿瘤转移, 预后
Abstract
Objective
To investigate the expression of signal transduction and activator of transcription 2 (STAT2) in ovarian cancer and its correlation with the prognosis of ovarian cancer patients and explore the role of STAT2 inregulating metastasis of ovarian cancer cells.
Methods
RT-qPCR was performed to detect the expression of STAT2 mRNA in 62 fresh frozen ovarian cancer tissues and 62 normal ovarian tissues; immunohistochemistry was used to detect STAT2 protein expressions in 95 paraffin-embedded ovarian cancer samples and 33 normal ovarian tissues. Kaplan-Meier method was used to analyze the correlation between the expression of STAT2 and the prognosis of the patients. We also examined the relationship between STAT2 and the patients' prognosis by analyzing the data in Kaplan-Meier Plotter database. Western blotting was performed to detect the expression of STAT2 in different ovarian cancer cell lines. In A2780 cells with the highest STAT2 expression, we examined the effects of STAT2 interference on cell migration and invasiveness using Transwell migration assay and on the expressions of the downstream molecule epidermal growth factor receptor (EGFR).
Results
Ovarian cancer tissues expressed significantly higher levels of STAT2 mRNA than normal ovarian tissue. A high STAT2 mRNA expression was correlated with an advanced FIGO stage. Immunohistochemistry showed that 67.4% of the ovarian cancer samples, as compared with 28.3% of normal ovarian tissues, showed high STAT2 expressions. In ovarian cancer patients, a high expression of STAT2 protein was associated with ascites volume, distant metastasis and FIGO stage (P < 0.05). Survival analysis showed that ovarian cancer patients with a high expression of STAT2 protein had poor overall survival (P=0.021) and progression-free survival (P=0.018). STAT2 was overexpressed in all the ovarian cancer cell lines tested, and A2780 cell lines showed the highest expression. Interference of STAT2 significantly suppressed the migration and invasiveness (P < 0.01) and lowered the expression level of EGFR in A2780 cells.
Conclusion
STAT2 is overexpressed in ovarian cancer. A high expression of STAT2 is associated with a poor prognosis of ovarian cancer patients. STAT2 may promote the metastasis of ovarian cancer by enhancing the expression of EGFR.
Keywords: ovarian cancer, signal transduction and activator of transcription 2, tumor metastasis, prognosis
卵巢癌是妇科恶性肿瘤相关死亡的最常见原因,全世界每年有22万妇女患卵巢癌,手术和药物治疗后大多数晚期疾病患者会在18个月内复发,最终导致化疗耐药[1-3]。目前该疾病的主要筛查手段是B超和CA-125的检查,有可能使患者早期发现,但是没有提示能够明显改善患者的预后[1, 4],因此,阐明卵巢癌发生和发展的分子机制对于卵巢癌的早期诊断和治疗有重要意义。
信号转导及转录活化因子2(STAT2)是STATs蛋白家族中的一员,是构成JAK-STAT信号转导通路的重要组分,可介导多种细胞因子和生长因子的信号向细胞核内传导[5]。STAT2被IFN-Ⅰ和IFN-Ⅲ激活,与STAT1和IRF9形成三聚体复合物,也被称作IFN刺激基因因子3(ISGF3),转移进入细胞核,调控下游基因的转录[6-10]。STAT2在天然免疫、炎症反应、抗病毒、肿瘤血管生成、肿瘤细胞增殖和肿瘤细胞耐药等众多生物学行为中发挥重要作用[7, 11-16]。STAT2的表达增高与宫颈癌的进展相关[17],另外,STAT2是结直肠癌和皮肤癌发生的一个促进因子,可增加促炎介质如IL-6,趋化因子CCL2等基因表达和分泌,进而激活致癌的STAT3信号通路,促进肿瘤的发生[18]。但也有研究表明,STAT2缺失的宿主小鼠与野生型宿主小鼠相比倾向于形成更大的肿瘤和更易出现肺转移病灶,提示STAT2具有抗肿瘤作用[19]。但是STAT2在卵巢癌中如何发挥作用尚未有研究报道,本研究拟检测STAT2在卵巢癌组织样本中的表达,并分析STAT2的表达与相应的临床病理参数和预后的关系,探索STAT2对于卵巢癌的影响及潜在机制。
1. 资料和方法
1.1. 临床样本
1.1.1. 队列1
收集从2015年1月~2018年11月在福建医科大学附属泉州第一医院妇产科进行手术治疗的卵巢癌患者的新鲜冰冻卵巢癌组织62例,收集同期正常对照患者新鲜冰冻正常卵巢组织样本62例(因子宫肌瘤、子宫颈癌、子宫内膜癌等疾病切除卵巢者),以供检测目的基因mRNA表达水平。
1.1.2. 队列2
于病理科收集2007年3月~2013年3月于福建医科大学附属泉州第一医院妇产科进行手术治疗的石蜡标本,其中卵巢癌组织95例,正常卵巢组织33例(因子宫肌瘤、子宫颈癌、子宫内膜癌等疾病切除卵巢者)以供检通过免疫组化测目的基因的蛋白质表达水平及进行预后和临床病例特征分析等。病理学诊断资料由病理科检索获得,所有患者的临床相关资料由医院病历系统获得。卵巢癌患者的病理类型:浆液性腺癌65例,黏液性癌8例,子宫内膜样癌7例,透明细胞癌10例,其他类型5例;按国际妇产科联盟(FIGO)2009年的标准:FIGO Ⅰ~Ⅱ期28例,FIGO Ⅲ~Ⅳ期67例。所有患者术前均未接受放疗、化疗、生物治疗、激素治疗等相关抗肿瘤治疗,排除合并其他系统肿瘤患者。所有卵巢癌患者术后每半年随访1次,采用门诊随诊、电话等方式获得患者的生存状态和疾病进展情况,记录病逝患者死亡时间。总生存期(OS)定义为从手术至死亡的间期或至最后一次随访的间期。无疾病进展生存期(PFS)定义为从手术至卵巢癌疾病再次进展的间期。随访时间是从手术日期到2018年3月。
患者均签署知情同意书,组织标本的收集及应用通过福建医科大学附属泉州第一医院伦理委员会的审核。
1.1.3. 数据库资料
分析KM plotter数据库(网址:<a href="http://kmplot.com" target="_blank">http://kmplot.com</a>),进一步探讨STAT2与患者的预后关系,关于OS分析,纳入样本例数为655例,关于PFS分析,纳入样本例数为614例,对于卵巢癌组织类型,FIGO分期,组织分级以及是否涉及TP53突变等指标不做进一步区分,STAT2基因高低表达人群的区分标准为:自动选择最佳截断值。
1.2. 细胞株和主要试剂
卵巢癌细胞株SKOV3,HO-8910,A2780,Heye2,CP70购买于中国科学院典型培养物保藏委员会细胞库,兔抗人STAT2单克隆抗体(Abcam,ab134192)和兔抗人β-actin单克隆抗体(Abcam,ab179467),兔抗人EGFR多克隆抗体(Proteintech,18986-1-AP),组化试剂盒DAB显色剂(DAKO,K5007),TRIzol试剂(Sigma),mRNA逆转录试剂盒(TaKaRa),SYBR® Select Master Mix(Life Technologies)。
1.3. 总RNA的提取和实时荧光定量PCR检测STAT2mRNA水平的表达
使用TRIzol-酚-氯仿法从新鲜冰冻组织样本(队列1)中提取总RNA,使用mRNA逆转录试剂盒将mRNA反转录为cDNA,使用SYBR® Select Master Mix进行PCR反应,以GAPDH为内参,采用-ΔCt计算各样本STAT2 mRNA的相对表达水平,其中Ct为循环阈值。实验重复3次。引物序列如下:STAT2正向引物,5'-CCAGCTTTACTCGCACAGC-3',反向引物:5'-AG CCTTGGAATCATCACTCCC-3';GAPDH正向引物,5'-GCATTGCCCTCAACGACCAC-3',反向引物:5'-C CACCACCACCACCACCACCCTGTTGCTGTAG-3'。
1.4. 免疫组织化学染色及结果判读检测STAT2蛋白水平的表达
SP法检测不同卵巢组织中STAT2蛋白的表达:将石蜡切片在二甲苯中脱蜡,梯度乙醇再水合;然后用0.3%过氧化氢处理,阻断内源性过氧化物酶;然后进行抗原修复,用3%牛血清蛋白封闭后,将切片与兔抗人STAT2单克隆抗体(稀释比1:1000, Abcam, ab134192)在4 ℃下孵育过夜,然后用HRP标记的二抗在室温下孵育50 min。最后,用二氨基联苯胺四氯化合物进行显色反应,显微镜下控制显色时间,用苏木素进行细胞核反染色。免疫组化的结果由两名有经验的病理科医生分别进行判读,随机观察5个高倍镜视野,根据染色的阳性细胞百分比和染色强度评价蛋白表达情况(阳性细胞百分比: < 10%评分0,10%~25%评分为1,26%~50%评分2,51%~75%评分3,76%~100%评分4;染色强度评分:0分,无染色;1分,浅黄染色;2分,深黄染色;3分,棕褐色染色)。两项评分相乘即为该标本的免疫组化综合评分:0~4为低表达,6~12为高表达。
1.5. STAT2-shRNA质粒的获得与稳定干扰细胞株的构建
STAT2的shRNA和对照质粒均购自维真生物,shRNA的序列: shRNA-1: 5'-AAGCTGCACTTGGGA GTGATGAT-3'; shRNA-2: 5'-AAAGAGCAGAAGAT TCTGCAGGA-3'; shRNA-3: 5'-AAGGCAATGAGTC ACTGACTGTG-3'; shRNA-4: 5'-AACTGTAGGACT GAGGATCCATT-3'。
将卵巢癌细胞株A2780接种于6孔板,待细胞贴壁后,密度在60%进行转染;根据Lipo 3000转染试剂(Invitrogen)说明书进行操作, 转染后培养48 h更换含浓度为2 μg/mL嘌呤霉素的完全培养基筛选转染阳性的细胞,显微镜下观察绿色荧光细胞数目,占比达90%以上时,获得A2780-STAT2-shRNA1/2/3/4和对照组共5组细胞用于后续实验。
1.6. Western blot检测卵巢癌细胞株中STAT2及下游蛋白的表达
收集上述分组的处于对数生长期的5组细胞,加入细胞裂解液(RIPA加入蛋白酶抑制剂),提取总蛋白,BCA法测定各组蛋白样品浓度,加入上样缓冲液,将不同分组的蛋白样品调整为等浓度等体积,100 ℃加热变性,进行10%聚丙烯酰胺凝胶电泳(积层胶:60 V,30 min;分离胶120 V,80 min)分离蛋白,湿法转膜(100 V,120 min),脱脂牛奶封闭,一抗4 ℃孵育过夜,兔抗人STAT2单克隆抗体(稀释比1:1000)和兔抗人β-actin单克隆抗体(稀释比1:20000),另外兔抗人EGFR多克隆抗体(稀释比:1:2000),次日洗膜后加入二抗室温孵育1 h,ECL化学发光,Bio-Rad成像系统曝光显影获取图像。
1.7. Transwell法检测干扰STAT2对A2780细胞迁移和侵袭能力的影响
将对照组和STAT2干扰组细胞用无血清培养基培养饥饿处理12 h,将细胞消化,用无血清培养基将细胞重悬,浓度为2×105/mL;取200 μL细胞悬液加入Transwell上层小室(铺或未铺Matrigel胶),在下层皿中加入800 μL含10%胎牛血清的完全培养基,每组设置三个复孔;温箱中培养48 h,取出小室,弃去培养基,棉签擦除未穿膜细胞,4%多聚甲醛固定,结晶紫染色,PBS清洗晾干,倒置显微镜下拍照观察,随机取5个视野取平均值计算穿膜细胞数。
1.8. 统计学方法
采用SPSS 19.0软件进行统计分析,用GraphPad Prism 6软件进行图形绘制。正态分布的计量资料以均数±标准差表示,2独立样本间的比较采用t检验,非正态分布的计量资料以中位数和四分位数表示,采用曼惠特尼秩和检验。计数资料分析采用卡方检验或Fisher精确检验。Kaplan-Meier法计算生存率和绘制生存曲线,并行对数秩检验(Log-rank test)计算生存曲线之间的差异。采用Cox回归模型进行单因素和多因素生存分析来确定预后的危险因素,P < 0.05为差异有统计学意义。
2. 结果
2.1. 卵巢癌和正常对照卵巢组织中STAT2 mRNA的表达
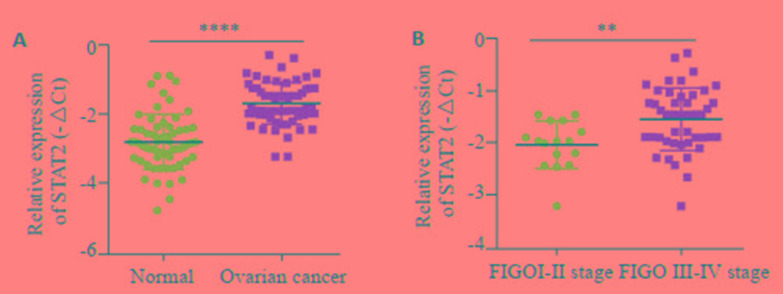
为了探索STAT2在卵巢癌中的表达,RT-qPCR法检测了队列1样本中STAT2的mRNA表达水平,结果提示,卵巢癌组织中STAT2 mRNA的表达(均数±标准差=-1.672±0.077,N=62)显著高于正常卵巢上皮组织(均数±标准差=-2.796±0.102,n=62)(t=8.793,P < 0.0001,图 1A),在卵巢癌患者中,STAT2的mRNA表达与临床FIGO分期有关,STAT2在FIGO Ⅰ-Ⅱ期(中位数=-1.968,n=16)卵巢癌组织中的mRNA的表达低于FIGO Ⅲ~Ⅳ期(中位数=-1.455,n=46),差异有统计学意义(P=0.0013,图 1B)。
1.
卵巢癌和正常对照卵巢组织中STAT2 mRNA的表达情况
Expression of STAT2 in different ovarian cancer andnormal ovarian tissues. A: STAT2 mRNA expression in cohort 1; B: STAT2 expression in ovarian cancer in different FIGO stages. **P < 0.01, ****P < 0.0001.
2.2. 卵巢癌和正常对照卵巢石蜡组织STAT2蛋白的表达
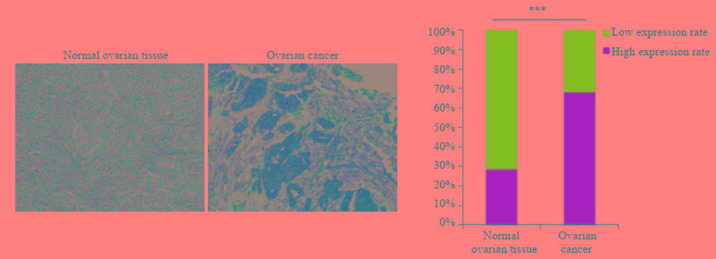
免疫组化染色显示STAT2蛋白在卵巢癌组织中的表达明显高于正常卵巢组织(图 2A),在卵巢癌样本中的高表达率为67.4%,正常对照卵巢组织中的高表达率为28.3%,差异有统计学意义(P < 0.001,图 2B)。
2.
免疫组化方法检测STAT2在正常卵巢组织和卵巢癌中的表达情况
Immunohistochemical detection of STAT2 expression in normal ovarian tissues and ovarian cancer. ***P < 0.001 (Original magnification: ×200).
2.3. 石蜡标本中STAT2蛋白的表达与卵巢癌患者临床病理特征的关系
在卵巢癌患者中,STAT2蛋白的高表达与患者的腹水量、有无远处转移及FIGO分期明显相关(P < 0.05),而与患者的年龄、Grade分级、血清CA-125、肿瘤病理类型及肿瘤直径无关(P>0.05,表 1)。
1.
STAT2表达与卵巢癌患者临床病理特征的关系
Relationship between STAT2 expression and clinicopathological features of patients with ovarian cancer
| Observation indicators | Number of samples | High expression of STAT2 | P | |
| No.(64) | (n/64) | |||
| Age (years) | 0 6553 | |||
| < 55 | 34 | 24 | 37.5% | |
| ≥55 | 61 | 40 | 62.5% | |
| CA-125 | 0.1883 | |||
| < 35 | 21 | 17 | 26. 6% | |
| ≥35 | 74 | 47 | 73. 4% | |
| Ascites (mL) | 0.0141 | |||
| < 100 | 40 | 21 | 32. 8% | |
| ≥100 | 55 | 43 | 67.2% | |
| Metastasis | 0.0038 | |||
| Absent | 29 | 13 | 20.3% | |
| Present | 66 | 51 | 79.7% | |
| FIGO stage | 0.0143 | |||
| Ⅰ-Ⅱ | 28 | 17 | 26. 6% | |
| Ⅱ-Ⅳ | 67 | 47 | 73. 4% | |
| Grade | 0 4691 | |||
| GⅠ-Ⅱ | 35 | 20 | 31.2% | |
| GⅢ | 60 | 44 | 68. 8% | |
| Tissue type | 0.3478 | |||
| Serous | 65 | 47 | 73.5% | |
| Mucous | 8 | 5 | 7.8% | |
| Endometrioid | 7 | 3 | 4.7% | |
| Clear cell | 10 | 5 | 7.8% | |
| Others | 5 | 4 | 6.2% | |
| Diameter | 0.2749 | |||
| < 10 cm | 42 | 31 | 48.4% | |
| ≥10 cm | 53 | 33 | 51.6% | |
2.4. STAT2表达水平与卵巢癌患者预后之间的关系
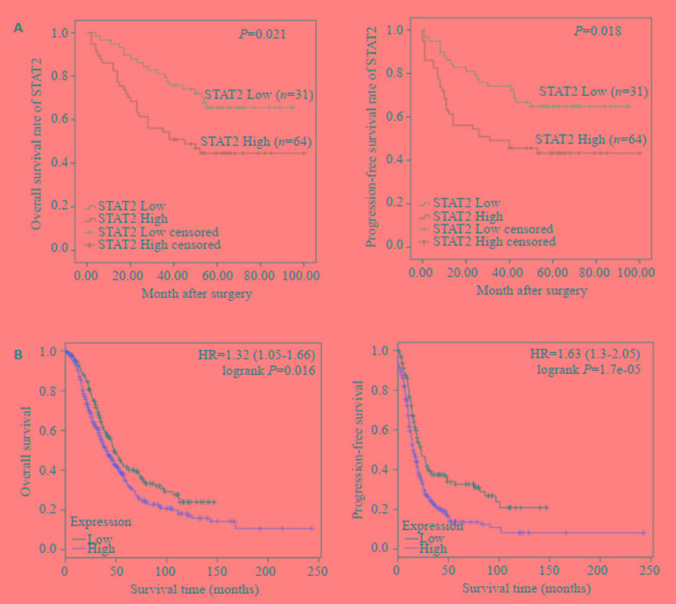
Kaplan-Meier方法分析STAT2蛋白表达和95例卵巢癌患者的整体生存期及无进展生存期之间的相关性,结果表明高表达STAT2蛋白的卵巢癌患者的整体生存期(<italic>P</italic>=0.021)及无进展生存期(<italic>P</italic>=0.018)均低于低表达的患者,差异有统计学意义(<xref ref-type="fig" rid="Figure3">图 3A</xref>)。另外,我们分析了<a href="http://kmplot.com" target="_blank">http://kmplot.com</a>数据库STAT2与卵巢癌患者预后之间的关系,结果提示,STAT2高表达的患者的整体生存期及无进展生存期均低于低表达者,相对危险度(Hazard ratio:HR)分别为:1.32(1.05~1.66),<italic>P</italic>=0.016和1.63(1.3~2.05),<italic>P</italic>=1.7e-05(<xref ref-type="fig" rid="Figure3">图 3B</xref>)。
3.
STAT2与卵巢癌患者整体生存期和无进展生存期之间的关系
Association of STAT2 with overall survival and progression-free survival of patients with ovarian cancer. A: STAT2 expression and prognosis of ovarian cancer patients in cohort 2; B: STAT2 expression and prognosis analysis of ovarian cancer patients in Kaplan-Meier Plotter database.
2.5. STAT2在卵巢癌细胞株中的表达与STAT2干扰效能的检测
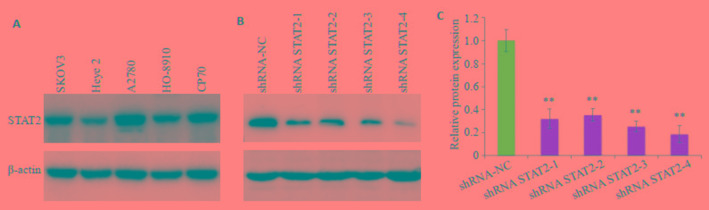
Western blot检测显示STAT2在卵巢癌细胞株SKOV3,HO-8910,A2780,Heye2,CP70中普遍呈高表达,其中在A2780细胞株中表达最高(图 4A)。选取A2780用建立干扰细胞株,获得A2780-shRNA STAT2- 1/2/3/4和A2780-NC共5组细胞,WB法检测转染干扰质粒后STAT2蛋白表达水平降低,其中shRNA STAT2-4降低最明显(图 4B,P < 0.01),用于后续实验。
4.
卵巢癌细胞株中STAT2的表达及干扰细胞株的构建
Expression of STAT2 in ovarian cancer cell lines and STAT2 interference in A2780 cells. A: STAT2 expression in ovarian cancer cell lines; B: Interference of STAT2 inA2780 cell line; C: Relative protein expression. **P < 0.01 vs shRNA-NC group.
2.6. 干扰STAT2对于卵巢癌细胞株A2780迁移、侵袭能力影响
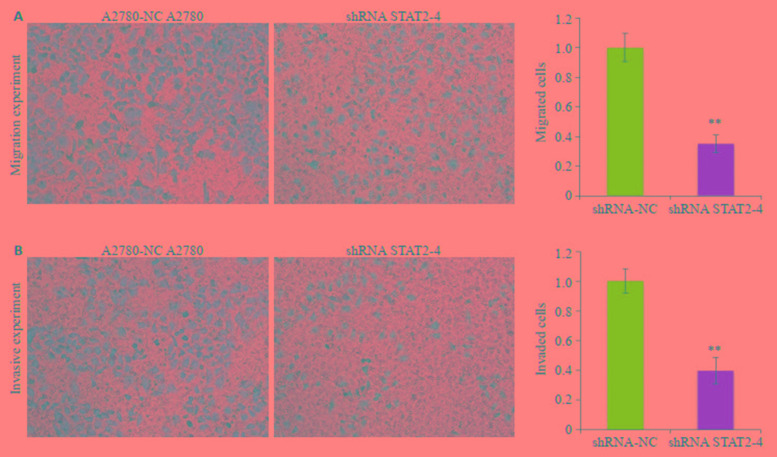
A2780细胞株中干扰STAT2后,Transwell法检测A2780细胞株迁移和侵袭能力的变化,与A2780-NC相比,A2780-shRNA STAT2-4组穿膜细胞数明显减少,细胞迁移能力(图 5A)和侵袭能力(图 5B)减弱,差异有统计学意义(P < 0.01,图 5)。
5.
干扰STAT2后卵巢癌细胞株A2780迁移和侵袭能力的变化
Transwell assay for detecting changes in migration and invasiveness of A2780 cells after interference of STAT2. A: Knockdown of STAT2 inhibits migration of A2780 cells; B: Knockdown of STAT2 inhibits invasion of A2780 cells (Original magnification: ×200). **P < 0.01 vs A2780-NC group.
2.7. STAT2对EGFR mRNA和蛋白表达的影响
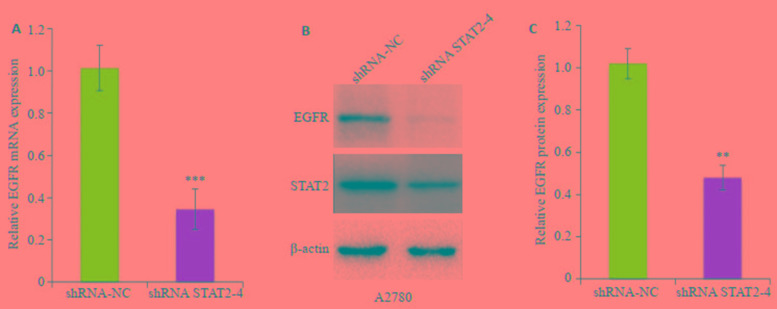
为了进一步分析STAT2促进卵巢癌A2780细胞转移的可能下游机制,我们检测发现干扰STAT2后,EGFR(epidermal growth factor receptor)的mRNA(图 6A)和蛋白(图 6B)表达水平较对照组均明显降低(P < 0.01)。
6.
干扰STAT2对于转移关键因子EGFR mRNA和蛋白表达的影响
Effects of STAT2 interference on expression of EGFR mRNA and protein. A: Expression of EGFR mRNA in A2780 cells after STAT2 interference; B: Expression of EGFR protein in A2780 cells after STAT2 interference. **P < 0.01, ***P < 0.001 vs shRNA-NC group.
3. 讨论
尽管近年来卵巢癌的手术和化疗取得了很大的进展,但卵巢癌的早期诊断仍比较困难,术后易发生肿瘤的局部浸润和远处转移[20-21],治疗失败和疾病复发仍相当常见,严重影响患者的生活质量和生存时间[2]。因此,深入开展卵巢癌转移相关的分子机制研究,寻找与卵巢癌的临床病例特征和预后相关的新的和有效的生物标志物对疾病的诊断和治疗具有重要意义。
STAT2通过JAK-STAT信号转导通路发挥功能,影响下游基因转录,STAT2在肿瘤增殖、血管生成、细胞凋亡中均发挥重要作用,但是既往研究报道,STAT2在肿瘤中可以发挥抑癌或促癌的作用<sup>[<xref ref-type="bibr" rid="b22">22</xref>-<xref ref-type="bibr" rid="b23">23</xref>]</sup>。在结肠癌中,抑制DNA甲基化转移酶可活化结肠癌细胞中的STAT2,引起细胞不受控制的增殖,导致结肠肿瘤的发生<sup>[<xref ref-type="bibr" rid="b24">24</xref>]</sup>。Pouyfung等<sup>[<xref ref-type="bibr" rid="b25">25</xref>]</sup>研究发现从灰葡萄球菌分离出的物质8αTGH可以通过抑制STAT3和STAT2磷酸化而导致G2/M细胞周期停滞,进而抑制口腔癌细胞的增殖。在胶质细胞瘤中,STAT2是miR-3908的直接靶向调控基因,miR-3908可以通过抑制STAT2的表达,降低STAT2下游通路AMPK/ SIRT1的活性,进而抑制胶质瘤细胞株U251和U87的致瘤性、迁移、生长和侵袭能力,并增加了这些细胞的凋亡<sup>[<xref ref-type="bibr" rid="b26">26</xref>]</sup>。Yue等<sup>[<xref ref-type="bibr" rid="b19">19</xref>]</sup>学者研究表明,在同源肿瘤移植模型中显示,与野生型小鼠相比,STAT2(-/-)小鼠形成了更大的肿瘤,提示STAT2可减少肿瘤生长。STAT2在不同的肿瘤中扮演不同的抑癌或者促癌作用,因此分析其在卵巢癌中的作用就显得尤为重要。本研究通过分析来自本院样本队列1的62例卵巢癌组织发现,STAT2 mRNA表达明显高于正常对照,并且在FIGO Ⅲ~Ⅳ期的患者中倾向于具有更高的表达;进一步通过对队列2石蜡样本免疫组化分析发现,STAT2在卵巢癌样本中蛋白表达水平升高,并且与卵巢癌患者的腹水量大于100 mL、有远处转移及FIGO Ⅲ~Ⅳ期明显相关。预后分析发现,在队列2样本与<a href="http://kmplot.com" target="_blank">http://kmplot.com</a>数据库中均提示高表达STAT2蛋白的卵巢癌患者的整体生存期及无进展生存期均低于低表达的患者,提示STAT2表达升高与患者预后不良相关。关于数据库的分析,有研究结果表明卵巢癌患者的OS与STAT2的表达不相关,研究采用STAT2表达均数的方法对患者进行分组<sup>[<xref ref-type="bibr" rid="b27">27</xref>]</sup>。而本研究采用最佳截断值方法对患者进行分组,另外,采用Li等<sup>[<xref ref-type="bibr" rid="b27">27</xref>]</sup>人的分组方法分析卵巢癌患者的PFS与STAT2的表达相关(Hazard ratio:HR=1.36(1.13~1.65),<italic>P</italic>=0.0012,因此仍提示STAT2与患者的预后不良相关。由于上述临床病理参数部分代表了肿瘤的恶化和发展,因此从临床角度来看,STAT2是卵巢癌进展的一个相关因素。
进一步的细胞实验表明,在卵巢癌细胞株中STAT2具有普遍的高表达,通过基因干扰技术下调STAT2的表达后,卵巢癌A2780细胞株的迁移和侵袭能力明显减弱。Yahata等研究者的报道也证实,阻断STAT2信号转导可抑制由VEGF及b-FGF诱导的血管内皮细胞增殖、迁移及微血管腔形成,从而发挥抗肿瘤血管生成作用,进而抑制肿瘤细胞的转移[28],机制分析我们发现干扰STAT2后,表皮生长因子受体家族成员之一EGFR的mRNA和蛋白表达水平较对照组均明显降低,而EGFR与肿瘤细胞的增殖、血管生成、肿瘤侵袭、转移及细胞凋亡的抑制等过程密切相关[29]。
综上所述,我们的研究证实STAT2在卵巢癌中表达上调并与卵巢癌患者生存不良有关,并且STAT2在卵巢癌中可能作为癌基因促进卵巢癌的转移和疾病的进展。本文的机制探讨发现STAT2可能影响EGFR的表达,但是关于STAT2是通过何种机制来调控EGFR的表达,还有待进一步的探讨,这也是本课题组后续研究的目标。我们的研究结果显示了STAT2在卵巢癌中的重要作用,为卵巢癌的诊断治疗和预后评估提供了新的分子靶点。
Biography
陈萱,在读硕士研究生,主治医师,E-mail: chenxuan_quanzhou@163.com
Funding Statement
泉州市科技项目(2018Z073)
Contributor Information
陈 萱 (Xuan CHEN), Email: chenxuan_quanzhou@163.com.
吕 育纯 (Yuchun LÜ), Email: 104955102@qq.com.
References
- 1.Jayson GC, Kohn EC, Kitchener HC, et al. Ovarian Cancer. Lancet. 2014;384(9951):1376–88. doi: 10.1016/S0140-6736(13)62146-7. [Jayson GC, Kohn EC, Kitchener HC, et al.Ovarian Cancer[J].Lancet, 2014, 384(9951):1376-88.] [DOI] [PubMed] [Google Scholar]
- 2.Menon U, Gentry-Maharaj A, Jacobs I. Ovarian Cancer screening and mortality. http://d.old.wanfangdata.com.cn/NSTLQK/NSTL_QKJJ0228921877/ JAMA. 2011;306(1544):1544–5. doi: 10.1001/jama.2011.1461. [Menon U, Gentry-Maharaj A, Jacobs I.Ovarian Cancer screening and mortality[J].JAMA, 2011, 306(1544):1544-5.] [DOI] [PubMed] [Google Scholar]
- 3.Holmes D. Ovarian Cancer:beyond resistance. Nature. 2015;527(7579):S217. doi: 10.1038/527S217a. [Holmes D.Ovarian Cancer:beyond resistance[J].Nature, 2015, 527(7579):S217.] [DOI] [PubMed] [Google Scholar]
- 4.Buys SS, Partridge E, Black A, et al. Effect of screening on ovarian cancer mortality:the prostate, lung, colorectal and ovarian (PLCO) cancer screening randomized controlled trial. JAMA. 2011;305(22):2295–303. doi: 10.1001/jama.2011.766. [Buys SS, Partridge E, Black A, et al.Effect of screening on ovarian cancer mortality:the prostate, lung, colorectal and ovarian (PLCO) cancer screening randomized controlled trial[J].JAMA, 2011, 305(22):2295-303.] [DOI] [PubMed] [Google Scholar]
- 5.Philip AM, Vijayan MM. Stress-immune-growth interactions:cortisol modulates suppressors of cytokine signaling and JAK/STAT pathway in rainbow trout liver. PLoS One. 2015;10(6):e0129299. doi: 10.1371/journal.pone.0129299. [Philip AM, Vijayan MM.Stress-immune-growth interactions:cortisol modulates suppressors of cytokine signaling and JAK/STAT pathway in rainbow trout liver[J].PLoS One, 2015, 10(6):e0129299.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Au-Yeung N, Mandhana R, Horvath CM. Transcriptional regulation by STAT1 and STAT2 in the interferon JAK-STAT pathway. JAKSTAT. 2013;2(3):e23931. doi: 10.4161/jkst.23931. [Au-Yeung N, Mandhana R, Horvath CM.Transcriptional regulation by STAT1 and STAT2 in the interferon JAK-STAT pathway[J].JAKSTAT, 2013, 2(3):e23931.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blaszczyk K, Nowicka H, Kostyrko K, et al. The unique role of STAT2 in constitutive and IFN-induced transcription and antiviral responses. Cytokine Growth Factor Rev. 2016;29(6):71–81. doi: 10.1016/j.cytogfr.2016.02.010. [Blaszczyk K, Nowicka H, Kostyrko K, et al.The unique role of STAT2 in constitutive and IFN-induced transcription and antiviral responses[J].Cytokine Growth Factor Rev, 2016, 29(6):71-81.] [DOI] [PubMed] [Google Scholar]
- 8.Nan J, Wang YX, Yang JB, et al. IRF9 and unphosphorylated STAT2 cooperate with NF-kappa B to drive IL6 expression. Proc Natl Acad Sci USA. 2018;115(15):3906–11. doi: 10.1073/pnas.1714102115. [Nan J, Wang YX, Yang JB, et al.IRF9 and unphosphorylated STAT2 cooperate with NF-kappa B to drive IL6 expression[J].Proc Natl Acad Sci USA, 2018, 115(15):3906-11.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rengachari S, Groiss S, Devos JM, et al. Structural basis of STAT2 recognition by IRF9 reveals molecular insights into ISGF3 function. Proc NatlAcad Sci USA. 2018;115(4):E601–9. doi: 10.1073/pnas.1718426115. [Rengachari S, Groiss S, Devos JM, et al.Structural basis of STAT2 recognition by IRF9 reveals molecular insights into ISGF3 function[J].Proc NatlAcad Sci USA, 2018, 115(4):E601-9.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Platanitis E, Demiroz D, Schneller A, et al. A molecular switch from STAT2-IRF9 to ISGF3 underlies interferon-induced gene transcription. Nat Commun. 2019;10(1):2921. doi: 10.1038/s41467-019-10970-y. [Platanitis E, Demiroz D, Schneller A, et al.A molecular switch from STAT2-IRF9 to ISGF3 underlies interferon-induced gene transcription[J].Nat Commun, 2019, 10(1):2921.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chi R, Chen X, Liu M, et al. Role of SNHG7-miR-653-5p-STAT2 feedback loop in regulating neuroblastoma progression. J Cell Physiol. 2019;234(8):13403–12. doi: 10.1002/jcp.28017. [Chi R, Chen X, Liu M, et al.Role of SNHG7-miR-653-5p-STAT2 feedback loop in regulating neuroblastoma progression[J].J Cell Physiol, 2019, 234(8):13403-12.] [DOI] [PubMed] [Google Scholar]
- 12.Gopal R, Lee B, Mchugh KJ, et al. STAT2 signaling regulates macrophage phenotype during influenza and bacterial superinfection. Front Immunol. 2018;25(9):2151. doi: 10.3389/fimmu.2018.02151. [Gopal R, Lee B, Mchugh KJ, et al.STAT2 signaling regulates macrophage phenotype during influenza and bacterial superinfection[J].Front Immunol, 2018, 25(9):2151.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manoochehrabadi S, Arsang-Jang S, Mazdeh M, et al. Analysis of STAT1, STAT2 and STAT3 mRNA expression levels in the blood of patients with multiple sclerosis. Hum Antibodies. 2019;27(2):91–8. doi: 10.3233/HAB-180352. [Manoochehrabadi S, Arsang-Jang S, Mazdeh M, et al.Analysis of STAT1, STAT2 and STAT3 mRNA expression levels in the blood of patients with multiple sclerosis[J].Hum Antibodies, 2019, 27(2):91-8.] [DOI] [PubMed] [Google Scholar]
- 14.Guo J, Chen D, Gao X, et al. Protein inhibitor of activated STAT2 restricts HCV replication by modulating viral proteins degradation. Viruses. 2017;9(10):pii:E285. doi: 10.3390/v9100285. [Guo J, Chen D, Gao X, et al.Protein inhibitor of activated STAT2 restricts HCV replication by modulating viral proteins degradation[J].Viruses, 2017, 9(10):pii:E285.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mariani MK, Dasmeh P, Fortin A, et al. The combination of IFN β and TNF induces an antiviral and immunoregulatory program via Non-Canonical pathways involving STAT2 and IRF9. Cells. 2019;8(8):pii:E919. doi: 10.3390/cells8080919. [Mariani MK, Dasmeh P, Fortin A, et al.The combination of IFN β and TNF induces an antiviral and immunoregulatory program via Non-Canonical pathways involving STAT2 and IRF9[J].Cells, 2019, 8(8):pii:E919.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu J, Lee MH, Chakhtoura M, et al. STAT2 is required for TLRInduced murine dendritic cell activation and cross-presentation. J Immunol. 2016;197(1):326–36. doi: 10.4049/jimmunol.1500152. [Xu J, Lee MH, Chakhtoura M, et al.STAT2 is required for TLRInduced murine dendritic cell activation and cross-presentation[J].J Immunol, 2016, 197(1):326-36.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang, Ze ng, Li LH, et al. Detection of STAT2 in early stage of cervical premalignancy and in cervical cancer. Asian Pac J Trop Med. 2012;5(9):738–42. doi: 10.1016/S1995-7645(12)60117-5. [Liang, Zeng, Li LH, et al.Detection of STAT2 in early stage of cervical premalignancy and in cervical cancer[J].Asian Pac J Trop Med, 2012, 5(9):738-42.] [DOI] [PubMed] [Google Scholar]
- 18.Gamero AM, Young MR, Mentor-Marcel R, et al. STAT2 contributes to promotion of colorectal and skin carcinogenesis. Cancer Prev Res (Phila) 2010;3(4):495–504. doi: 10.1158/1940-6207.CAPR-09-0105. [Gamero AM, Young MR, Mentor-Marcel R, et al.STAT2 contributes to promotion of colorectal and skin carcinogenesis[J].Cancer Prev Res (Phila), 2010, 3(4):495-504.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yue C, Xu J, Tan Estioko MD, et al. Host STAT2/type I interferon axis controls tumor growth. Int J Cancer. 2015;136(1):117–26. doi: 10.1002/ijc.29004. [Yue C, Xu J, Tan Estioko MD, et al.Host STAT2/type I interferon axis controls tumor growth[J].Int J Cancer, 2015, 136(1):117-26.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naora H, Montell DJ. Ovarian vancer metastasis:integrating insights from disparate model organisms. Nat Rev Cancer. 2005;5(5):355–66. doi: 10.1038/nrc1611. [Naora H, Montell DJ.Ovarian vancer metastasis:integrating insights from disparate model organisms[J].Nat Rev Cancer, 2005, 5(5):355-66.] [DOI] [PubMed] [Google Scholar]
- 21.Thibault B, Castells M, Delord JP, et al. Ovarian cancer microenvironment:implications for cancer dissemination and chemoresistance acquisition. Cancer Metastasis Rev. 2014;33(1):17–39. doi: 10.1007/s10555-013-9456-2. [Thibault B, Castells M, Delord JP, et al.Ovarian cancer microenvironment:implications for cancer dissemination and chemoresistance acquisition[J].Cancer Metastasis Rev, 2014, 33(1):17-39.] [DOI] [PubMed] [Google Scholar]
- 22.Stark GR, Darnell JE. The JAK-STAT pathway at twenty. Immunity. 2012;36(4):503–14. doi: 10.1016/j.immuni.2012.03.013. [Stark GR, Darnell JE.The JAK-STAT pathway at twenty[J].Immunity, 2012, 36(4):503-14.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanaka T, Arai M, Jiang X, et al. Downregulation of microRNA-431 by human interferon-β inhibits viability of medulloblastoma and glioblastoma cells via upregulation of SOCS6. Int J Oncol. 2014;44(5):1685–90. doi: 10.3892/ijo.2014.2317. [Tanaka T, Arai M, Jiang X, et al.Downregulation of microRNA-431 by human interferon-β inhibits viability of medulloblastoma and glioblastoma cells via upregulation of SOCS6[J].Int J Oncol, 2014, 44(5):1685-90.] [DOI] [PubMed] [Google Scholar]
- 24.Karpf AR, Peterson PW, Rawlins JT, et al. Inhibition of DNA methyltransferase stimulates the expression of signal transducer and activator of transcription 1, 2, and 3 genes in colon tumor cells. Proc NatlAcad Sci USA. 1999;96(24):14007–12. doi: 10.1073/pnas.96.24.14007. [Karpf AR, Peterson PW, Rawlins JT, et al.Inhibition of DNA methyltransferase stimulates the expression of signal transducer and activator of transcription 1, 2, and 3 genes in colon tumor cells[J].Proc NatlAcad Sci USA, 1999, 96(24):14007-12.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pouyfung P, Choonate S, Wongnoppavich A, et al. Anti-proliferative effect of 8α-tigloyloxyhirsutinolide-13-O-acetate (8αTGH) isolated from Vernonia cinerea on oral squamous cell carcinoma through inhibition of STAT3 and STAT2 phosphorylation. Phytomedicine. 2019;52(1):238–46. doi: 10.1016/j.phymed.2018.09.211. [Pouyfung P, Choonate S, Wongnoppavich A, et al.Anti-proliferative effect of 8α-tigloyloxyhirsutinolide-13-O-acetate (8αTGH) isolated from Vernonia cinerea on oral squamous cell carcinoma through inhibition of STAT3 and STAT2 phosphorylation[J].Phytomedicine, 2019, 52(1):238-46.] [DOI] [PubMed] [Google Scholar]
- 26.Liu X, Chen J, Zhang J. AdipoR1-mediated miR-3908 inhibits glioblastoma tumorigenicity through downregulation of STAT2 associated with the AMPK/SIRT1 pathway. Oncol Rep. 2017;37(6):3387–96. doi: 10.3892/or.2017.5589. [Liu X, Chen J, Zhang J.AdipoR1-mediated miR-3908 inhibits glioblastoma tumorigenicity through downregulation of STAT2 associated with the AMPK/SIRT1 pathway[J].Oncol Rep, 2017, 37(6):3387-96.] [DOI] [PubMed] [Google Scholar]
- 27.Li S, Sheng B, Zhao M, et al. The prognostic values of signal transducers activators of transcription family in ovarian cancer. Biosci Rep. 2017;37(4):pii:BSR20170650. doi: 10.1042/BSR20170650. [Li S, Sheng B, Zhao M, et al.The prognostic values of signal transducers activators of transcription family in ovarian cancer[J].Biosci Rep, 2017, 37(4):pii:BSR20170650.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yahata Y, Shirakata Y, Tokumaru S, et al. Nuclear translocation of phosphorylated STAT3 is essential for vascular endothelial growth factor-induced human dermal microvascular endothelial cell migration and tube formation. J Biol Chem. 2003;278(41):40026–31. doi: 10.1074/jbc.M301866200. [Yahata Y, Shirakata Y, Tokumaru S, et al.Nuclear translocation of phosphorylated STAT3 is essential for vascular endothelial growth factor-induced human dermal microvascular endothelial cell migration and tube formation[J].J Biol Chem, 2003, 278(41):40026-31.] [DOI] [PubMed] [Google Scholar]
- 29.Rosa DD, Clamp AR, Collinson F, et al. Antiangiogenic therapy for ovarian cancer. Curr Opin Oncol. 2007;19(5):497–505. doi: 10.1097/CCO.0b013e32827035f0. [Rosa DD, Clamp AR, Collinson F, et al.Antiangiogenic therapy for ovarian cancer[J].Curr Opin Oncol, 2007, 19(5):497-505.] [DOI] [PubMed] [Google Scholar]