Abstract
目的
研究miR-671-3p对乳腺癌细胞增殖、侵袭生物学功能的影响及其可能的作用机制。
方法
以实时荧光定量PCR方法检测正常乳腺上皮细胞(MCF-10A)与乳腺癌细胞(MCF-7、MDA-MB-231、SK-BR3)中miR-671-3p表达情况;检测miR-671-3p在MCF-7细胞中的转染效率;通过CCK-8法检测miR-671-3p对MCF-7细胞增殖的影响;通过Transwell实验、划痕实验检测miR-671-3p对MCF-7细胞侵袭迁移能力的影响;Targetscan预测miR-671-3p的靶基因,并通过双荧光素酶法和Western blot法对靶基因DEPTOR进行验证,并检测DEPTOR的表达。
结果
miR-671-3p在乳腺癌细胞系MCF-7、MDA-MB-231、SK-BR3的表达量低于乳腺正常上皮细胞MCF-10A的表达。通过mimics、inhibitor分别促进、抑制miR-671-3p在MCF-7细胞中的表达后,结果显示miR-671-3p对乳腺癌细胞的增殖有抑制作用(P < 0.05),对肿瘤细胞的侵袭和迁移有抑制作用(P < 0.05)。双荧光素酶结果显示DEPTOR蛋白为miR-671-3p的直接作用靶点,过表达miR-671-3p可直接抑制DEPTOR蛋白的表达。
结论
miR-671-3p直接靶向作用于DEPTOR蛋白从而抑制乳腺癌细胞的增殖和侵袭。
Keywords: 乳腺癌, miR-671-3p, DEPTOR, 细胞增殖, 肿瘤浸润
Abstract
Objective
To investigate the effects of miR-671-3p on the proliferation and invasion of breast cancer cells and explore the possible mechanism.
Methods
We examined the expressions of miR-671-3p in human normal epithelial cells (MCF-10A) and breast cancer cell lines (MCF-7, MDA-MB-231, and SK-BR3) using RT-PCR. The effects of transfection with a miR-671-3p mimic or inhibitor on the proliferation, migration and invasion of MCF-7 cells were evaluated using CCK-8 assay and Transwell chamber assay. The target gene of miR-671-3p was predicated with Targetscan and validated by a dual luciferase reporter system and Western blotting.
Results
The expression of miR-671-3p was significantly lower in breast cancer cells than in normal breast epithelial cells. Compared with negative control group, MCF-7 cells with miR-671-3p overexpression exhibited significantly reduced proliferation and invasion, whereas inhibition of miR-671-3p obviously promoted the cell proliferation and invasion. Luciferase reporter assay demonstrated that DEPTOR was the target gene of miR-671-3p, and miR-671-3p overexpression caused significant down-regulation of the protein expression of DEPTOR.
Conclusion
MiR-671-3p suppresses the proliferation and invasion of breast cancer cell line MCF-7 by directly targeting DEPTOR protein.
Keywords: breast cancer, miR-671-3p, DEPTOR, cell proliferation, neoplasm invasiveness
乳腺癌是世界范围内最常见的女性恶性肿瘤,病死率是所有女性恶性肿瘤的第2位,我国发病率年平均增长速度约在3%~4%之间[1]。随着现代分子诊断技术的发展,乳腺癌的早期诊断及分子靶向药物治疗,虽已取得了实质性的进展,但其复发率及晚期患者的生存率仍不乐观[2]。因此,进一步阐明乳腺癌发生发展的分子机制,寻找新的治疗靶点,对提高乳腺癌的疗效具有极其重要的作用。
研究显示微小RNA(miRNA)与乳腺癌临床特征密切相关,可通过碱基互补配对原则与靶基因的3'非编码区域结合,从而发挥对靶基因的调节作用,参与调控乳腺癌的多种生理病理过程[3-5]。现约50%的miRNAs可作为致癌或抑癌基因而发挥促癌或抑癌作用[6]。最近研究表明,乳腺癌发生、发展的各阶段均有不同程度的miRNAs相对表达失调[7]。如:Bahena等[8]研究发现在乳腺癌细胞中过表达miR-10b可抑制PTEN的表达,促进细胞EMT和干细胞标志物表达,从而促进乳腺癌侵袭转移。Damiano等[9]发现发现miR-200b/c通过抑制ZEB2表达,促进间充质细胞向上皮细胞转化MET,促进乳腺癌细胞转移。Ding等[10]通过对乳腺癌和癌旁组织检测,发现miR-145过表达可降低乳腺癌细胞增殖并抑制TGF的表达。TOYAMA等[11]研究表明,miR-210高表达是日本乳腺癌患者预后不良的独立因素。miR-671作为调控网络中的重要分子涉及肿瘤的发生、发展[12],然而miR-671-3p在乳腺癌当中的作用及其机制还未明确。因此,本文旨在研究miR-671-3p对乳腺癌细胞MCF-7增殖与侵袭迁移作用的影响及其作用机制。为乳腺癌的诊断及靶向治疗提供新的靶点及实验依据,现报道如下。
1. 材料和方法
1.1. 材料
1.1.1. 细胞株
人乳腺癌细胞株MCF-7、MDA-MB-231、SK-BR-3和乳腺癌上皮细胞MCF-10A均来自中国科学院上海生命科学研究院细胞资源中心;荧光报告载体psiCHECK-2购于美国Promega公司。
1.1.2. 仪器和试剂
低温高速离心机(Thermo Fisher);二氧化碳培养箱(Thermo Fisher);实时荧光定量(RT-PCR)仪(Stratagene);Transwell小室(Corning);BCA蛋白浓度检测试剂盒(上海碧云天生物技术公司);RPMI 1640/ DMEM培养基(Life Techmologies);Trizol(Takara);DEPC(Bio Basic);胎牛血清(Gibco);Lipofectamine 3000(Invitrogen);miR-671-3p mimic/inhibitor(上海吉玛有限公司);双荧光素酶报告系统检测试剂盒(Promega);CCK-8试剂盒(同仁化学研究所)。
1.2. 方法
1.2.1. 细胞培养
乳腺癌细胞MCF-7和MDA-MB-231采用含10% FBS高糖DMEM培养基;SKBR-3采用含10% FBS的RPMI 1640培养基;MCF-10A采用DMEM/F12含5%马血清、10 μg/mL胰岛素、20 ng /mL EGF、100 ng/mL霍乱毒素、0.5 μg/mL氢化可的松培养基,37 ℃、5% CO2及相对湿度条件下培养。
1.2.2. 细胞转染
以(3~4)×105个细胞/孔的量取对数期生长细胞铺于6孔板中,待其生长至80%融合时,按lipofectmine 3000脂质体转染试剂盒说明书进行操作,转染20 nmol/LmiRNA到细胞中,分miR-671-3p mimic组、inhibitor组及其阴性对照组(NC)。转染后放于37 ℃,5% CO2环境中培养。转染6~8 h后更换含10%胎牛血清的DMEM培养基,细胞继续培养48 h后行后续实验。
1.2.3. RNA提取及RT-PCR
收集转染48 h后的各组细胞,用Trizol法提取总RNA,分光光度计Nanodrop上测定RNA浓度,并进行逆转录,逆转录具体操作步骤按照TaqMan MicroRNA逆转录试剂盒说明书进行。按照16 ℃ 30 min,42 ℃ 30 min,85 ℃ 5 min,反应条件进行逆转录,将得到的产物保存于4 ℃。根据Real-time PCR试剂盒说明书进行扩增,测定miR-671-3p的表达。PCR反应条件为95 ℃预变性2 min;95 ℃变性10 s,60 ℃退火30 s、72 ℃延伸30 s,共40个循环。每个实验重复3次,每次检测均以U6为内参照结果以2-△△CT法计算,用相对定量表示。
1.2.4. CCK-8法
通过CCK-8实验检测miR-671-3p对MCF-7细胞增殖的影响。将MCF-7细胞接种到96孔板中,将实验分为miR-671-3p mimic组、mimic NC组,miR-671-3p inhibitor组、inhibitor NC组,每组设5个复孔,培养24、48、72、96 h后分别向各孔中加入CCK-8溶液10 μL将培养板放入37 ℃孵箱中继续培养2 h。用酶标仪检测各组细胞吸光度(A450 nm)。
1.2.5. Transwell迁移实验
采用Transwell小室,预铺基质胶,取5×104/L的细胞接种于小室上层,加入无血清培养基,下室加入600 μL条件培养基(含有10% FBS),置于37 ℃、5% CO2培养箱培养。每个处理组设3个复孔。培养24 h后取出Transwell小室,弃小室内液体,棉签擦除里面细胞,结晶紫染色15 min,显微镜下计数细胞。
1.2.6. 划痕实验
取miR-671-3p mimic组、miR-671-3pinhibitor组和其对照组MCF-7细胞,采用0.25%胰蛋白酶消化,调整细胞浓度为1×105/mL,接种于6孔板中,置于37 ℃,5% CO2的培养箱中孵育培养,待细胞增长至汇合度80%以上时,用10 μL微量移液头消毒后在6孔细胞板上垂直划痕,采用PBS洗涤细胞3次,24 h后在倒置显微镜下观察细胞的迁移情况。
1.2.7. 双荧光素酶实验
将含有miR-671-3p靶点的DEPTOR 3'-UTR插入萤光素酶基因编码区下游的报告基因载体psiCHECK-2,构建psiCHECK-2-DEPTOR野生型重组报告基因载体(psiCHECK-2-DEPTOR-WT),后构建突变型报告基因载体(psiCHECK-2-DEPTORMut),将野生型和突变型报告基因载体与miR-671-3p mimic和阴性对照分别共转染MCF-7细胞,48 h后去除完全培养基,加入适量报告基因细胞裂解液,室温放置15 min;而后每孔加入100 μL萤火虫荧光素酶检测缓冲液(LARII),采用多功能酶标仪检测孔内相对光单位,后每孔加入100 μL海肾荧光素酶检测工作液(Stop & Glo Reagent)后再次测定。计算两种荧光素酶读数的比值,取3个平行孔的平均值,并计算标准差。
1.2.8. Western blot实验
收集培养72 h的miR-671-3pmimic组、miR-671-3p inhibitor组和其对照组MCF-7细胞,提取细胞总蛋白,采用BCA蛋白浓度测定试剂盒测定蛋白浓度。加入上样缓冲液,100 ℃煮沸10 min,取50 μg蛋白样品,经SDS-PAGE分离后,电转移至PVDF膜上,经5%脱脂奶粉室温置摇床封闭2 h;加入稀释好的抗体,4 ℃过夜,TBST洗膜3次,再于稀释后HRP标记的二抗工作液37 ℃孵育1 h。TBST再次清洗3次,ECL化学发光。
1.3. 统计学处理
采用SPSS 22.0统计软件,计量资料用均数±标准差表示,组间比较采用t检验,多组均数比较采用单因素方差分析,P < 0.05为差异有统计学意义。
2. 结果
2.1. miR-671-3p在乳腺癌细胞中的表达
Trizol法提取总RNA,Real-time PCR检测各种分型乳腺癌细胞系中miR-671-3p的表达。
乳腺癌细胞系MCF-7、MDA-MB-231、SK-BR-3中miR-671-3p表达水平低于正常乳腺上皮细胞系MCF- 10A,可见miR-671-3p在多个乳腺癌细胞系中水平均显著下调(t=9.587,t=7.107,t=5.084,P < 0.05,图 1)。
1.
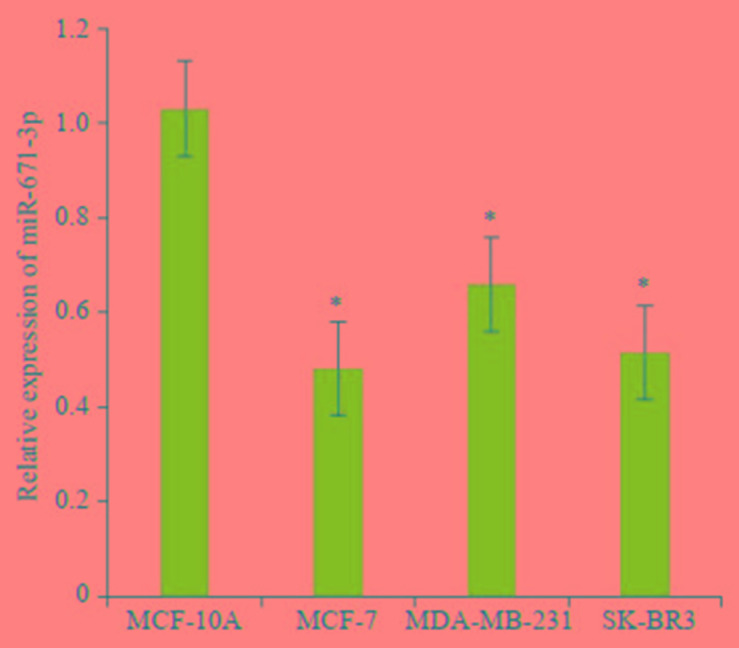
miR-671-3p在正常乳腺上皮细胞和不同乳腺癌细胞株中的表达水平
qPCR for comparing the expression of miR-671-3p between 4 cell lines. *P < 0.05 vs MCF-10Agroup.
2.2. miR-671-3p对乳腺癌细胞增殖活性的影响
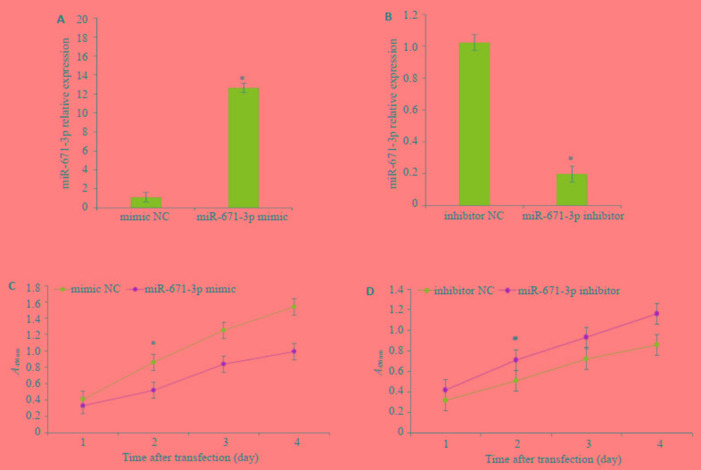
分别将miR-671-3p mimic及miR-671-3p inhibitor及对照组转染入MCF-7细胞中,采用RT-PCR检测不同组中miR-671-3p的表达水平,结果显示,miR-671-3p mimic组中miR-671-3p的表达水平显著升高(t=10.76,P < 0.01),miR-671-3p inhibitor组中miR-671-3p的表达水平显著降低(t=12.03,P < 0.01)。根据CCK-8法检测结果绘制MCF-7细胞的生长曲线,miR-671-3p mimic组细胞活力显著低于mimic NC组,48 h差异有统计学意义(t=9.831,P < 0.05),而miR-671-3p inhitibor组细胞活力显著高于inhibitor NC组,48 h差异有统计学意义(t=8.793,P < 0.05,图 2)。
2.
MCF-7细胞被转染后miR-671-3p相对表达水平及生长曲线图
A, B: qPCR for comparing the expression of MCF-7 cells transfected with NC-mimic/inhibitor and miR-671-3p mimics/inhibitor. *P < 0.05 vs mimic NC group; C, D: CCK-8 assay of MCF-7 cells transfected with NC-mimic/inhibitor and miR-671-3p mimics/inhibitor. *P < 0.05 vs inhibitor NC group.
2.3. miR-671-3p对乳腺癌细胞侵袭能力的影响
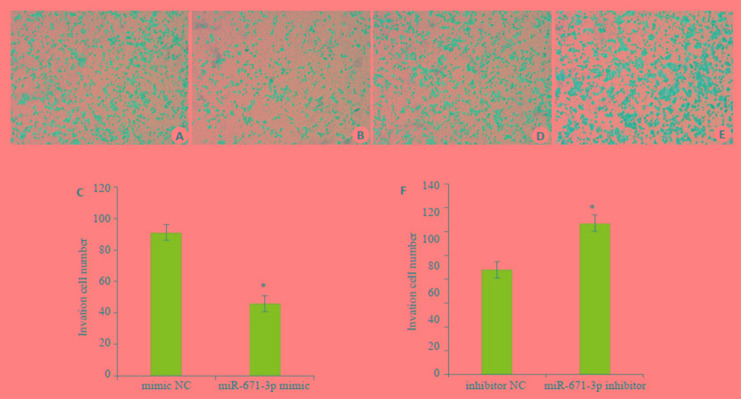
转染乳腺癌MCF-7细胞miR-671-3p mimic和miR-671-3p inhibitor,培养48 h后,miR-671-3p mimic组穿过小室的数目与mimic NC组比较,乳腺癌细胞的侵袭能力均显著降低,差异有统计学意义(t=8.405,P < 0.05);miR-671-3p inhibitor组较inhibitor NC组乳腺癌细胞的侵袭能力均显著升高,差异具有统计学意义(t= 5.866,P < 0.05,图 3)。
3.
Transwell侵袭实验观察miR-671-3p对MCF-7细胞侵袭能力的影响
Migration ability of MCF-7 cells after NC-mimic/inhibitor and miR-671-3p mimics/inhibitor transfection assessed by Transwell assay.A, B, D, E: Transwell assay; C, F: Quantitative analysis of cell migration. *P < 0.05 vs NC group.
2.4. miR-671-3p对乳腺癌细胞迁移能力影响
转染乳腺癌细胞MCF-7 miR-671-3p mimic和miR-671-3p inhibitor,培养24 h后,与NC对照组相比,miR-671-3p mimic组细胞的迁移能力明显降低,差异有统计学意义(t=5.464,P < 0.05);miR-671-3p inhibitor组细胞的迁移能力明显升高(t=4.151,P < 0.05,图 4)。
4.
miR-671-3p对MCF-7细胞迁移能力的影响
Migration ability of MCF-7 cells after NC-mimic/inhibitor and miR-671-3p mimics/inhibitor transfection assessed by wound healing assay.
2.5. 双荧光素酶实验验证miR-671-3p对蛋白的直接靶向作用
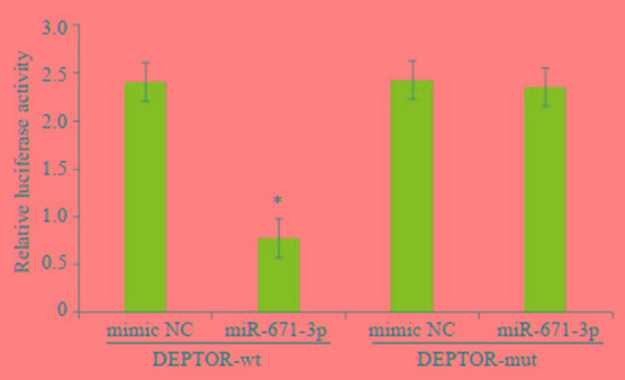
通过靶基因预测网站TargetScan(<a href="http://www.targetscan.org/" target="_blank">http://www.targetscan.org/</a>)和miRanda(<a href="http://www.microrna.org" target="_blank">http://www.microrna.org</a>)进行生物信息学预测,结果显示miR-671-3p与DEPTOR mRNA的3'UTR之间存在互补配对序列(<xref ref-type="fig" rid="Figure5">图 5</xref>)。WT组、Mut组和对照组质粒共转染MCF-7细胞48 h后进行双荧光素酶检测,结果表明共转染miR-671-3p mimic和psiCHECK-2的乳腺癌细胞中荧光素酶活性强度显著低于其他组(<italic>t</italic>=9.232,<italic>P</italic> < 0.01,<xref ref-type="fig" rid="Figure6">图 6</xref>)。
5.
生物信息学预测位于miR-671-3p与DEPTOR的3'UTR的匹配及突变位点
Matching of miR-671-3p with the target sites and mutations.
6.
miR-671-3p对DEPTOR萤光素酶活性的比较
Luciferase analysis showing the inhibitory effect of miR-671-3p on expression of the reporter gene by binding with DEPTOR 3'UTR. *P < 0.05 vs mimic NC group.
2.6. miR-671-3p对DEPTOR蛋白表达的影响
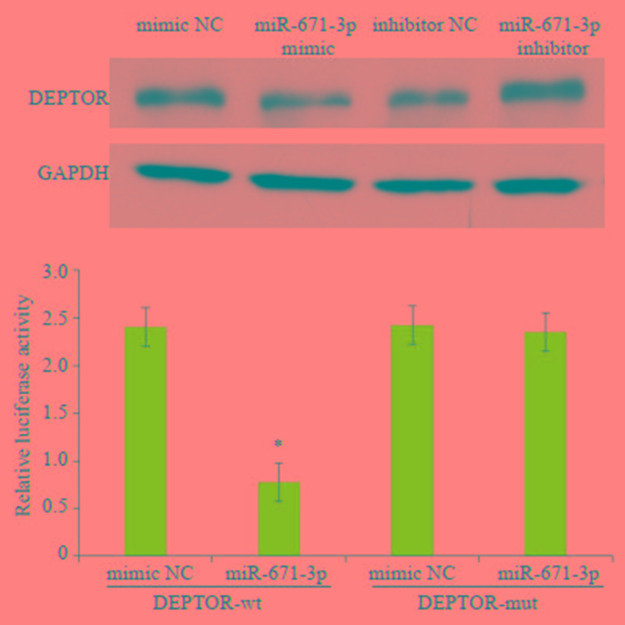
分别转染miR-671-3p mimic和miR-671-3p inhibitor至MCF-7细胞72 h后,通过Western blot检测DEPTOR蛋白的表达情况。结果显示,经转染后miR- 671-3p mimic组DEPTOR的相对表达水平下调,miR- 671-3p inhibitor组DEPTOR相对表达水平上调(图 7,P < 0.05)。
7.
miR-671-3p对乳腺癌MCF-7细胞DEPTOR蛋白表达的影响
Western blotting showing down- regulated DEPTOR proteins in MCF-7 cells overexpressing miR-671-3p. *P < 0.05 vs mimic NC group.
3. 讨论
近年来,乳腺癌发病率急剧上升,并呈年轻化趋势。由于其早期临床症状不明显,多数患者到确诊时已属中晚期,预后不理想。因此,乳腺癌的早期诊断对于提高乳腺癌的预后至关重要[13]。转移相关miRNA的发现,为我们研究乳腺癌转移提供了新的思路。Ma等[14]最早发现miR-10b可通过抑制转录因子HOXD10的翻译引发转移基因RHOC的表达,促进肿瘤细胞的侵袭和迁移。Wu等[15]发现诱导miR-340表达能够抑制肿瘤细胞的侵袭和转移。还有研究表明,机体的细胞因子和基因表达与患者治疗及预后密切相关[16]。因此,寻找影响乳腺癌患者病情进展和预后的相关因素并进行积极干预是改善预后的关键。
miRNA是一种长度约为22 nt的非编码RNA,能结合mRNA 3'UTR区,导致mRNA降解或翻译受阻,在多种肿瘤中扮演重要角色,它的调控作用参与到乳腺癌发生、侵袭和转移的各个过程。研究表明,恶性肿瘤的肿瘤组织、细胞系或血清样本中的miRNA表达谱较正常人有明显改变,这些miRNA可能来源于坏死或者凋亡细胞,或来自肿瘤细胞的主动释放,其中还包含mRNA,微泡或外泌体的形式进行细胞间的物质交换[17]。对于乳腺癌的筛查,缺乏血清学特异的标记,miRNA有望成为乳腺癌早期诊断的分子标记。miR-671-3p属于miR-671家族成员。有研究表明,miR-671-3p在胃癌组织和胃癌细胞中表达升高,且与胃癌临床分期及淋巴结转移相关[18]。唐佳佳等[19]研究发现,miR-671-3p可正向调控胃癌细胞的生长、迁移和侵袭。Yu等[12]研究发现,miR-671-3p通过靶向肿瘤抑制因子SOX6促进前列腺癌细胞增殖。雷奕等[20]研究表明miR-671-3p可能通过调控RHOU基因来促进神经胶质瘤发展。以上研究表明,miR-671-3p在胃癌、前列腺癌、神经胶质瘤等恶性肿瘤的发生、发展过程中具有重要作用。但目前关于miR-671-3p表达与乳腺癌细胞恶性生物学行为关系的报道较少。
DEPTOR来源于染色体8q24.12,分子量为48 kDa的蛋白[21],是雷帕霉素靶蛋白(mTOR)的特异性抑制蛋白。DEPTOR在体外以及细胞实验中均能够抑制mTOR的活性,是mTOR信号通路体内的调控开关[22],而mTOR是一种调节细胞生长和增殖的关键蛋白激酶[23]。DEPTOR在多种疾病,如肥胖、肝癌、甲状腺癌等中过表达[24-26]。也有文献报道DEPTOR与甲状腺肿瘤及肝恶性肿瘤的复发率也有相关联,且与患者的总体生存率呈负相关,是肿瘤不良预后的一个指标[27]。然而,DEPTOR在恶性肿瘤的表达水平因肿瘤的类型不同而有差异,所发挥的功能也不一样。不同于其他类型的肿瘤,最近研究发现DEPTOR高表达于多发性骨髓瘤细胞系,高表达DEPTOR可以活化磷脂酰肌醇三磷酸激酶PI3K/Akt通路,有研究从而调节细胞增殖、分化与凋亡[28]。由于DEPTOR在肿瘤细胞的生长、凋亡、自噬、EMT及耐药性等方面有着重要作用,它是否可以作为新的治疗恶性肿瘤的药物靶点,成为目前研究的热门[29]。在过表达的恶性肿瘤中,抑制剂的研究则是一种新的治疗策略。过表达的DEPTOR可以通过减弱mTOR1对于PI3K的负反馈抑制作用,从而激活PI3K/Akt通路,在肿瘤发生、侵袭和转移中发挥重要作用[30]。
本研究结果表明在过表达miR-671-3p的情况下,乳腺癌细胞的增殖、侵袭迁移能力受到明显抑制,而抑制乳腺癌细胞中miR-671-3p的表达,乳腺癌细胞的增殖、侵袭迁移能力则明显增加。进一步的机制研究中,证明了miR-671-3p的直接靶基因是DEPTOR蛋白,过表达miR-671-3p能显著抑制DEPTOR基因的表达,从而影响乳腺癌细胞的生物学行为,即乳腺癌细胞增殖、侵袭迁移能力受到抑制的原因是由于miR-671-3p通过直接靶向DEPTOR基因,从而影响其下游基因的表达,诱导乳腺癌细胞产生上述细胞学行为。在体外实验中,乳腺癌细胞侵袭迁移能力和增殖能力发生显著改变,提示乳腺癌的发生、发展与miR-671-3p表达水平密切相关,miR-671-3p表达水平的检测可以作为乳腺癌诊断的参考指标,对于临床上乳腺癌早期诊断和早期治疗提供了新的思路和方向。
Biography
夏巍,博士,主管技师,E-mail: xiawei123456@126.com
Funding Statement
广东省医学科学技术研究基金(A2017117);中国博士后科学基金(2016M602487);广东省自然科学基金(2018A030310434)
Contributor Information
夏 巍 (Wei XIA), Email: xiawei123456@126.com.
蔡 茁 (Zhuo CAI), Email: 2633749347@qq.com.
References
- 1.Li X, Xin P, Wang C, et al. Mechanisms of traditional Chinese medicine in the treatment of mammary gland hyperplasia. Am J Chin Med. 2017;45(3):443–58. doi: 10.1142/S0192415X17500276. [Li X, Xin P, Wang C, et al.Mechanisms of traditional Chinese medicine in the treatment of mammary gland hyperplasia[J].Am J Chin Med, 2017, 45(3):443-58.] [DOI] [PubMed] [Google Scholar]
- 2.Li L, Yuan L, Chen X, et al. Current treatments for breast cancerrelated lymphoedema:a systematic review. Asian Pac J Cancer Prev. 2016;17(11):4875–83. doi: 10.22034/APJCP.2016.17.11.4875. [Li L, Yuan L, Chen X, et al.Current treatments for breast cancerrelated lymphoedema:a systematic review[J].Asian Pac J Cancer Prev, 2016, 17(11):4875-83.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Z, Sun F, Hong Y, et al. MEG2 is regulated by miR-181a-5p and functions as a tumour suppressor gene to suppress the proliferation and migration of gastric cancer cells. Mol Cancer. 2017;16(1):133. doi: 10.1186/s12943-017-0695-7. [Liu Z, Sun F, Hong Y, et al.MEG2 is regulated by miR-181a-5p and functions as a tumour suppressor gene to suppress the proliferation and migration of gastric cancer cells[J].Mol Cancer, 2017, 16(1):133.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Z, Peng Z, Gu S, et al. Global analysis of miRNA-mRNA interaction network in breast cancer with brain metastasis. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=e5de86b4c07ffdbfdc631d922338a8d4. Anticancer Res. 2017;37(8):4455–68. doi: 10.21873/anticanres.11841. [Li Z, Peng Z, Gu S, et al.Global analysis of miRNA-mRNA interaction network in breast cancer with brain metastasis[J].Anticancer Res, 2017, 37(8):4455-68.] [DOI] [PubMed] [Google Scholar]
- 5.Zou Q, Tang Q, Pan Y, et al. MicroRNA-22 inhibits cell growth and metastasis in breast cancer via targeting of SIRT1. Exp Ther Med. 2017;14(2):1009–16. doi: 10.3892/etm.2017.4590. [Zou Q, Tang Q, Pan Y, et al.MicroRNA-22 inhibits cell growth and metastasis in breast cancer via targeting of SIRT1[J].Exp Ther Med, 2017, 14(2):1009-16.] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Bosco N, Pelliccia F, Rocchi A. Characterization of FRA7B, a human common fragile site mapped at the 7p chromosome terminal region. Cancer Genet Cytogenet. 2010;202(1):47–52. doi: 10.1016/j.cancergencyto.2010.06.008. [Bosco N, Pelliccia F, Rocchi A.Characterization of FRA7B, a human common fragile site mapped at the 7p chromosome terminal region[J].Cancer Genet Cytogenet, 2010, 202(1):47-52.] [DOI] [PubMed] [Google Scholar]
- 7.Nassar FJ, Nasr R, Talhouk R. MicroRNAs as biomarkers for early breast cancer diagnosis, prognosis and therapy prediction. Pharmacol Ther. 2017;172:34–49. doi: 10.1016/j.pharmthera.2016.11.012. [Nassar FJ, Nasr R, Talhouk R.MicroRNAs as biomarkers for early breast cancer diagnosis, prognosis and therapy prediction[J].Pharmacol Ther, 2017, 172:34-49.] [DOI] [PubMed] [Google Scholar]
- 8.Bahena-Ocampo I, Espinosa M, Ceballos-Cancino G, et al. miR-10b expression in breast cancer stem cells supports self-renewal through negative PTEN regulation and sustained AKT activation. EMBO Rep. 2016;17(7):1081. doi: 10.15252/embr.201642700. [Bahena-Ocampo I, Espinosa M, Ceballos-Cancino G, et al.miR-10b expression in breast cancer stem cells supports self-renewal through negative PTEN regulation and sustained AKT activation[J].EMBO Rep, 2016, 17(7):1081.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Damiano V, Brisotto G, Borgna S, et al. Epigenetic silencing of miR-200c in breast cancer is associated with aggressiveness and is modulated by ZEB1. Genes Chromosomes Cancer. 2017;56(2):147–58. doi: 10.1002/gcc.22422. [Damiano V, Brisotto G, Borgna S, et al.Epigenetic silencing of miR-200c in breast cancer is associated with aggressiveness and is modulated by ZEB1[J].Genes Chromosomes Cancer, 2017, 56(2):147-58.] [DOI] [PubMed] [Google Scholar]
- 10.Ding Y, Zhang C, Zhang J, et al. miR-145 inhibits proliferation and migration of breast cancer cells by directly or indirectly regulating TGF-β1 expression. Int J Oncol. 2017;50(5):1701–10. doi: 10.3892/ijo.2017.3945. [Ding Y, Zhang C, Zhang J, et al.miR-145 inhibits proliferation and migration of breast cancer cells by directly or indirectly regulating TGF-β1 expression[J].Int J Oncol, 2017, 50(5):1701-10.] [DOI] [PubMed] [Google Scholar]
- 11.Toyama T, Kondo N, Endo Y, et al. High expression of microRNA-210 is an Independent factor indicating a poor prognosis in Japanese triple-negative breast cancer patients. Jpn J Clin Oncol. 2012;42(4):256–63. doi: 10.1093/jjco/hys001. [Toyama T, Kondo N, Endo Y, et al.High expression of microRNA-210 is an Independent factor indicating a poor prognosis in Japanese triple-negative breast cancer patients[J].Jpn J Clin Oncol, 2012, 42(4):256-63.] [DOI] [PubMed] [Google Scholar]
- 12.Yu Y, Wang Z, Sun D, et al. miR-671 promotes prostate cancer cell proliferation by targeting tumor suppressor SOX6. Eur J Pharmacol. 2018;823:65–71. doi: 10.1016/j.ejphar.2018.01.016. [Yu Y, Wang Z, Sun D, et al.miR-671 promotes prostate cancer cell proliferation by targeting tumor suppressor SOX6[J].Eur J Pharmacol, 2018, 823:65-71.] [DOI] [PubMed] [Google Scholar]
- 13.Caughey AB, Doubeni CA, Epling JJ, et al. Medication use to reduce risk of breast cancer:US preventive services task force recommendation statement. JAMA. 2019;322(9):857–67. doi: 10.1001/jama.2019.11885. [Caughey AB, Doubeni CA, Epling JJ, et al.Medication use to reduce risk of breast cancer:US preventive services task force recommendation statement[J].JAMA, 2019, 322(9):857-67.] [DOI] [PubMed] [Google Scholar]
- 14.Ma L, Teruya-Feldstein J, Weinberg RA. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature. 2007;449(7163):682–8. doi: 10.1038/nature06174. [Ma L, Teruya-Feldstein J, Weinberg RA.Tumour invasion and metastasis initiated by microRNA-10b in breast cancer[J].Nature, 2007, 449(7163):682-8.] [DOI] [PubMed] [Google Scholar]
- 15.Wu ZS, Wu Q, Wang CQ, et al. miR-340 inhibition of breast cancer cell migration and invasion through targeting of oncoprotein c-Met. Cancer. 2011;117(13):2842–52. doi: 10.1002/cncr.25860. [Wu ZS, Wu Q, Wang CQ, et al.miR-340 inhibition of breast cancer cell migration and invasion through targeting of oncoprotein c-Met[J].Cancer, 2011, 117(13):2842-52.] [DOI] [PubMed] [Google Scholar]
- 16.Wieczorek E, Reszka EM. mRNA, microRNA and lncRNA as novel bladder tumor markers. Clin ChimActa. 2018;477:141–53. doi: 10.1016/j.cca.2017.12.009. [Wieczorek E, Reszka EM.mRNA, microRNA and lncRNA as novel bladder tumor markers[J].Clin ChimActa, 2018, 477:141-53.] [DOI] [PubMed] [Google Scholar]
- 17.Maziveyi M, Dong S, Baranwal S, et al. Exosomes from nischarinexpressing cells reduce breast cancer cell motility and tumor growth. Cancer Res. 2019;79(9):2152–66. doi: 10.1158/0008-5472.CAN-18-0842. [Maziveyi M, Dong S, Baranwal S, et al.Exosomes from nischarinexpressing cells reduce breast cancer cell motility and tumor growth[J].Cancer Res, 2019, 79(9):2152-66.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu GF, You CY, Chen YS, et al. MicroRNA-671-3p promotes proliferation and migration of glioma cells via targeting CKAP4. Onco Targets Ther. 2018;11:6217–26. doi: 10.2147/OTT.S177325. [Lu GF, You CY, Chen YS, et al.MicroRNA-671-3p promotes proliferation and migration of glioma cells via targeting CKAP4[J].Onco Targets Ther, 2018, 11:6217-26.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.唐 佳佳, 叶 林. MiRNA-671上调抑制胃癌细胞增殖与迁移. http://d.old.wanfangdata.com.cn/Periodical/dsjydxxb201716008. 第三军医大学学报. 2017;39(16):1643–7. [唐佳佳, 叶林.MiRNA-671上调抑制胃癌细胞增殖与迁移[J].第三军医大学学报, 2017, 39(16):1643-7.] [Google Scholar]
- 20.雷 奕, 邓 腾, 陈 海南, et al. MiR-671-3p对神经胶质瘤细胞增殖, 凋亡影响的实验研究. 临床肿瘤学杂志. 2019;24(9):791–4. doi: 10.3969/j.issn.1009-0460.2019.09.005. [雷奕, 邓腾, 陈海南, 等.MiR-671-3p对神经胶质瘤细胞增殖, 凋亡影响的实验研究[J].临床肿瘤学杂志, 2019, 24(9):791-4.] [DOI] [Google Scholar]
- 21.Panic N, Larghi A, Amore R, et al. Single nucleotide prolymorphisms within the 8Q24 region are not associated with the risk of intraductal papillary mucinous neoplasms of the pancreas. Gastrointestin Liver Dis. 2016;25(3):311–5. doi: 10.15403/jgld.2014.1121.253.q24. [Panic N, Larghi A, Amore R, et al.Single nucleotide prolymorphisms within the 8Q24 region are not associated with the risk of intraductal papillary mucinous neoplasms of the pancreas[J].Gastrointestin Liver Dis, 2016, 25(3):311-5.] [DOI] [PubMed] [Google Scholar]
- 22.Peterson TR, Laplante M, Thoreen CC, et al. DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell. 2009;137(5):873–86. doi: 10.1016/j.cell.2009.03.046. [Peterson TR, Laplante M, Thoreen CC, et al.DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival[J].Cell, 2009, 137(5):873-86.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guertin D, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12(1):9–22. doi: 10.1016/j.ccr.2007.05.008. [Guertin D, Sabatini DM.Defining the role of mTOR in cancer[J].Cancer Cell, 2007, 12(1):9-22.] [DOI] [PubMed] [Google Scholar]
- 24.Li H, Sun GY, Zhao Y, et al. DEPTOR has growth suppression activity against pancreatic cancer cells. Oncotarget. 2014;5(24):12811–9. doi: 10.18632/oncotarget.2659. [Li H, Sun GY, Zhao Y, et al.DEPTOR has growth suppression activity against pancreatic cancer cells[J].Oncotarget, 2014, 5(24):12811-9.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yonghui Y, Carolyne B, Patrick F, et al. DEPTOR is linked to a TOR C1-p21 survival proliferation pathway in multiple myeloma cells. https://www.ncbi.nlm.nih.gov/pubmed/24001224. Genes Cancer. 2014;5(12):407–19. doi: 10.18632/genesandcancer.44. [Yonghui Y, Carolyne B, Patrick F, et al.DEPTOR is linked to a TOR C1-p21 survival proliferation pathway in multiple myeloma cells[J].Genes Cancer, 2014, 5(12):407-19.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karly-Rai RB, Dimple C, George P, et al. Differential effects of rapalogues, dual kinase inhibitors on human ovarian carcinoma cellsin vitro. Int J Oncol. 2016;49(1):133–43. doi: 10.3892/ijo.2016.3531. [Karly-Rai RB, Dimple C, George P, et al.Differential effects of rapalogues, dual kinase inhibitors on human ovarian carcinoma cellsin vitro[J].Int J Oncol, 2016, 49(1):133-43.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou X, Guo J, Ji Y, et al. Reciprocal negative regulation between EGFR and DEPTOR plays an important role in progression of lung adenocarcinoma. Mol Cancer Res. 2016;14(5):448. doi: 10.1158/1541-7786.MCR-15-0480. [Zhou X, Guo J, Ji Y, et al.Reciprocal negative regulation between EGFR and DEPTOR plays an important role in progression of lung adenocarcinoma[J].Mol Cancer Res, 2016, 14(5):448.] [DOI] [PubMed] [Google Scholar]
- 28.Zhao Y, Xiong X, Deptor SY, et al. Is a physiological substrate of SCF (βTrCP) E3 ubiquitin ligase and regulates survival and autophagy. Mol Cell. 2011;44(2):304–16. doi: 10.1016/j.molcel.2011.08.029. [Zhao Y, Xiong X, Deptor SY, et al.Is a physiological substrate of SCF (βTrCP) E3 ubiquitin ligase and regulates survival and autophagy[J].Mol Cell, 2011, 44(2):304-16.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Z, Zhong J, Inuzuka H, et al. An evolving role for DEPTOR in tumor development and progression. Neoplasia. 2012;14(5):368–75. doi: 10.1593/neo.12542. [Wang Z, Zhong J, Inuzuka H, et al.An evolving role for DEPTOR in tumor development and progression[J].Neoplasia, 2012, 14(5):368-75.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Srinivas KP, Viji R, dan VM, et al. DEPTOR promotes survival of cervical squamous cell carcinoma cells and its silencing induces apoptosis through downregulating PI3K/AKT and by up-regulating p38 MAP kinase. Oncotarget. 2016;7(17):24154–71. doi: 10.18632/oncotarget.8131. [Srinivas KP, Viji R, dan VM, et al.DEPTOR promotes survival of cervical squamous cell carcinoma cells and its silencing induces apoptosis through downregulating PI3K/AKT and by up-regulating p38 MAP kinase[J].Oncotarget, 2016, 7(17):24154-71.] [DOI] [PMC free article] [PubMed] [Google Scholar]