Abstract
Summary
A 62-year-old female was admitted with severe left-sided chest pain, nausea and pre-syncope. She had widespread T wave inversion on ECG and elevated troponins and was suspected to have an acute coronary syndrome event. Invasive coronary angiogram revealed normal coronary anatomy with no flow-limiting lesions. Echocardiography and cardiac MRI revealed impaired left ventricular (LV) systolic impairment, a mobile LV apical thrombus and a moderate global pericardial effusion with no significant compromise. Full blood count analysis indicated the patient to have significant eosinophilia, and the patient was diagnosed with idiopathic eosinophilic myocarditis. She was commenced on Prednisolone and Apixaban, and eosinophil levels returned to normal after 10 days of steroids. Over the course of 3 months, the patient had a complete recovery of her LV function and resolution of the LV thrombus. This case highlights a rare, reversible case of idiopathic eosinophilic myocarditis which may present similar to acute coronary syndrome.
Learning points:
Eosinophilic myocarditis (EM) is a rare disease that can exhibit symptoms similar to acute coronary syndrome events.
The diagnosis of EM should be considered in patients with chest pain, normal coronary angiogram and pronounced eosinophilia levels.
Endomyocardial biopsy is the gold standard diagnostic tool; however, it has a low sensitivity detection rate and its use is not indicated in some patients.
Echocardiography is useful in the initial detection of cardiac involvement and complications. However, echocardiography lacks diagnostic specificity for all forms of myocarditis including EM.
Cardiac magnetic resonance is a useful method and may add in diagnosing all forms of myocarditis including EM.
Patients with EM should be identified promptly and treated with high doses of oral glucocorticoid to reduce the risk of permanent cardiac dysfunction.
Keywords: eosinophilic myocarditis, echocardiogram, magnetic resonance imaging, thrombus, idiopathic eosinophilic myocarditis
Background
Idiopathic eosinophilic myocarditis (IEM) is a rare and potentially life-threatening inflammatory cardiomyopathy characterized by abnormally high concentration levels of eosinophilic cells of an unidentified cause. The initial clinical presentation of IEM is variable and can mimic other acute pathologies and a timely diagnosis is of vital importance for best clinical outcome. This case highlights the challenges faced by clinicians in a patient presenting with a suspected acute coronary syndrome event who subsequently was diagnosed with IEM. The case is discussed in the context of the existing literature on IEM.
Case presentation
A 62-year-old Caucasian female was presented to the Accident and Emergency department after waking up with central chest pain radiating to her left arm, nausea and pre-syncope which persisted for 30 min. Her past medical history included hypothyroidism, vertigo, asthma and bronchiectasis. She was an ex-smoker with a family history of ischemic heart disease. Four months prior to this presentation, she was investigated for intermittent atypical chest pains, and a 12-lead ECG at this time showed sinus rhythm of heart rate 84 b.p.m. with no other abnormalities seen. A transthoracic echocardiogram showed a structurally normal heart with normal left ventricular (LV) size and systolic function and a visually estimated ejection fraction of 55–60%. Her blood tests were unremarkable. At this point, the patient was prescribed Ibuprofen analgesia as required and was discharged to the care of her general practitioner for follow-up if required. The patient’s regular medications included levothyroxine and fluticasone.
Investigation
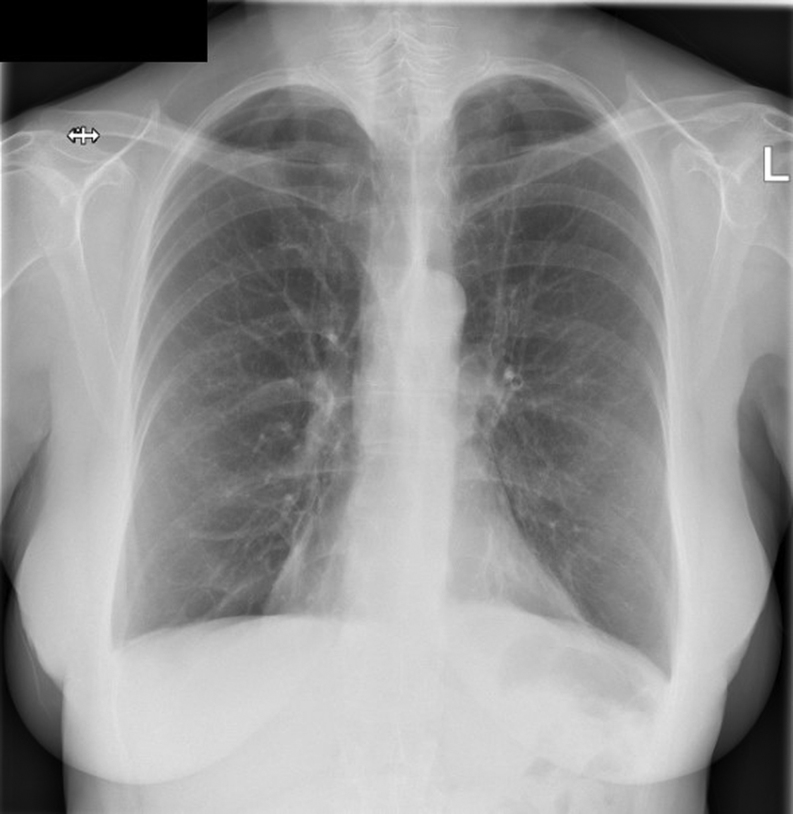
On presentation, the patient was clinically stable but apyrexial with a blood pressure of 127/75 mmHg, respiratory rate of 16 breaths per minute and oxygen saturation was 94% on air. A chest X-ray showed bi-basal pleural effusions with upper lobe vascular distension suggestive of pulmonary congestion (Fig. 1). Her 12-lead ECG showed sinus rhythm with a heart rate of 90 b.p.m., with new widespread T wave inversion in leads II, III, aVF and V2–V6. Cardiac troponin I was elevated at 817 and 891 ng/L (normal: 0–39 ng/L). In view of these findings, the patient was diagnosed with an acute coronary syndrome event. She was admitted to the coronary care unit where she was commenced on 300 mg Aspirin, 300 mg Clopidogrel and 2.5 mg Fondaparinux.
Figure 1.
Chest X-ray.
A repeat echocardiogram was undertaken 3 days after admission, which showed normal LV cavity dimensions with significant apical trabeculation and apical thickening, moderately impaired LV systolic function and a small pericardial effusion surrounding the right ventricular free wall with no features suggesting hemodynamic compromise (Fig. 2A and B).
Figure 2.
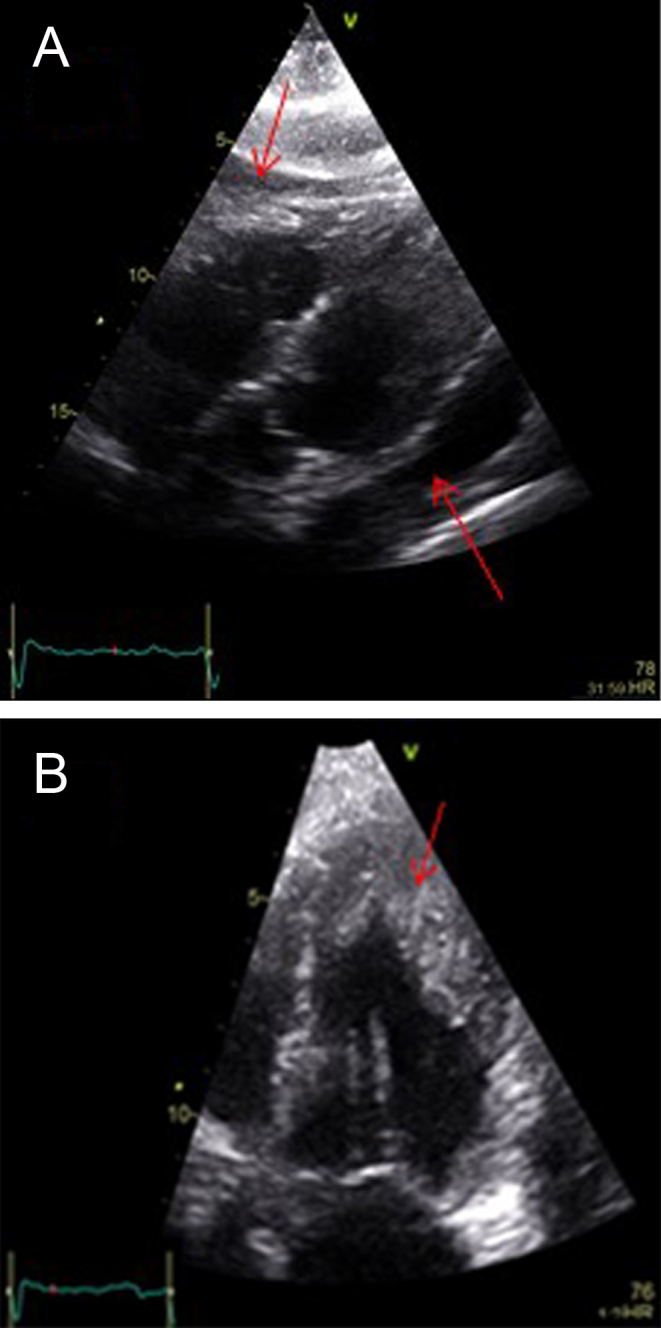
(A) Echo: Subcostal view showing pericardial effusion (arrowed). (B) Apical four chamber (focused view) showing apical trabeculation and apical thrombus (arrowed).
Coronary angiography was undertaken which demonstrated normal coronary anatomy with no flow-limiting lesions. On day 6 of admission the patient underwent a cardiac magnetic resonance (CMR) imaging study which showed a dilated left ventricle with prominent apical trabeculation, moderately impaired LV systolic function, estimated ejection fraction of 41% in the presence of mid-apical inferior and mid infero-lateral hypokinesia. There was late gadiolinium enhancement within the basal inferior segments in close proximetry to the RV insertion point but no evidence of infarction. A mobile LV apical thrombus measuring 27 mm by 15 mm by 14 mm and a global pericardial effusion with no significant hemodynamic compromise was also detected (Fig. 3A and B).
Figure 3.
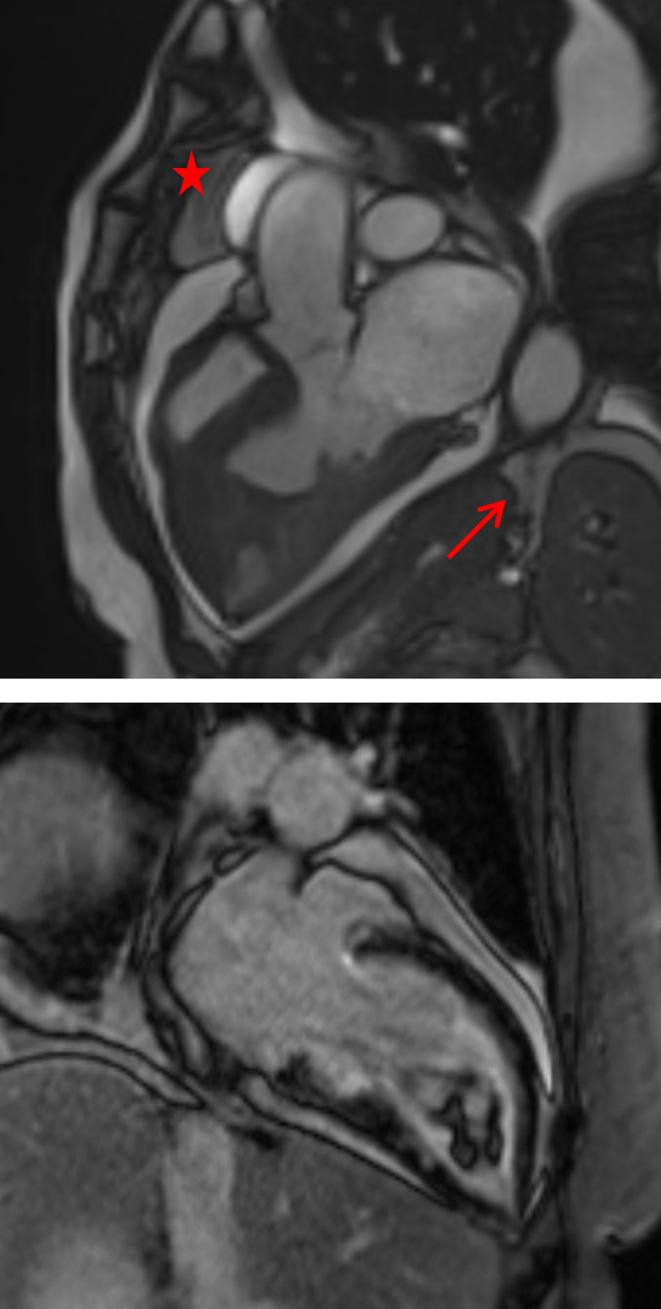
(A) Cardiac magnetic resonance imaging. Apical four chamber showing pericardial effusion (starred) and LV apical trabeculation (arrowed). (B) Cardiac magnetic resonance imaging. Apical four chamber showing LV apical thrombus (arrowed).
Laboratory test showed raised white cell count 17.2 (normal range: 4.0–11 × 109/L), C-reactive protein 46.7 (normal range: 0–5 mg/L) and erythrocyte sedimentation rate 37 (normal range: 0–20 mm/h). Eosinophil level was significantly elevated at 10.6/µL (normal range <1.5/µL) but was noted to be normal, 0.9/µL, in the previous test before this admission. The total serum immunoglobulin-E level was elevated at 216 (normal range: 0–100 kU/L). Other laboratory results were unremarkable, including anti-nuclear antibody screen (anti-Ro, anti-La, anti-Sm, anti-RNP, anti-Jo-1 and anti-Scl-70 antibodies). Anti-neutrophil cytoplasmic antibody was weakly positive but anti-Myeloperoxidase and anti-Proteinase 3 were within normal range (both less than 0.2 IU/mL). Therefore, vasculitis causes were excluded by the medical team. CT scan of the thorax, abdomen and pelvis was performed on day 9 which was unremarkable with no evidence of malignancy. A bone marrow biopsy also ruled out any myeloproliferative disorder. There was no stool sample obtained for microscopic examination to exclude parasitic infection. However, the patient had not traveled abroad and did not report to be in close contact with animals at risk of parasitic infection prior to the presentation. There were no other family members with similar symptoms, so the clinical team had low suspicion that a parasitic infection was the cause of her admission. Therefore, in view of significantly raised eosinophil count, hematology consultation was sought, and on multidisciplinary review a diagnosis of idiopathic IEM was made.
Treatment and outcome
The patient was started on once daily 50 mg Prednisolone, 30 mg Lansoprazole and 300 mg Allopurinol (300 mg oral OD). Apixaban (5 mg twice daily) was also initiated for the LV thrombus. The patient responded well and after only 4 days of Prednisolone, and the patient’s eosinophil cell levels returned to normal. The patient remained well and was discharged 10 days after admission. The patient was closely monitored in both cardiology and hematology outpatient clinics over a course of 12 months. From a hematology perspective, the eosinophil levels remained within normal limits and, thus, prednisolone was slowly tapered over a course of 10 months. The minimum dose which allowed for stable eosinophil count was 5 mg prednisolone on alternate days. From the cardiology perspective, a 3-month follow-up echocardiogram revealed normal LV size with a significant reduction in apical trabeculation. LV systolic function had improved with an estimated ejection fraction of 50%, and only basal-to-mid inferior hypokinesia was detected. There was no diastolic impairment and complete resolution of the pericardial effusion. A 12-month follow-up echocardiogram revealed a normal LV systolic function (estimated ejection fraction 60–65%) with no diastolic impairment, pericardial effusion or LV thrombus (Fig. 4A and B). Only minimal apical trabeculation remained. Over this time, the patient has clinically remained well with no further episodes of chest pains, pre-syncope or hospitalization.
Figure 4.
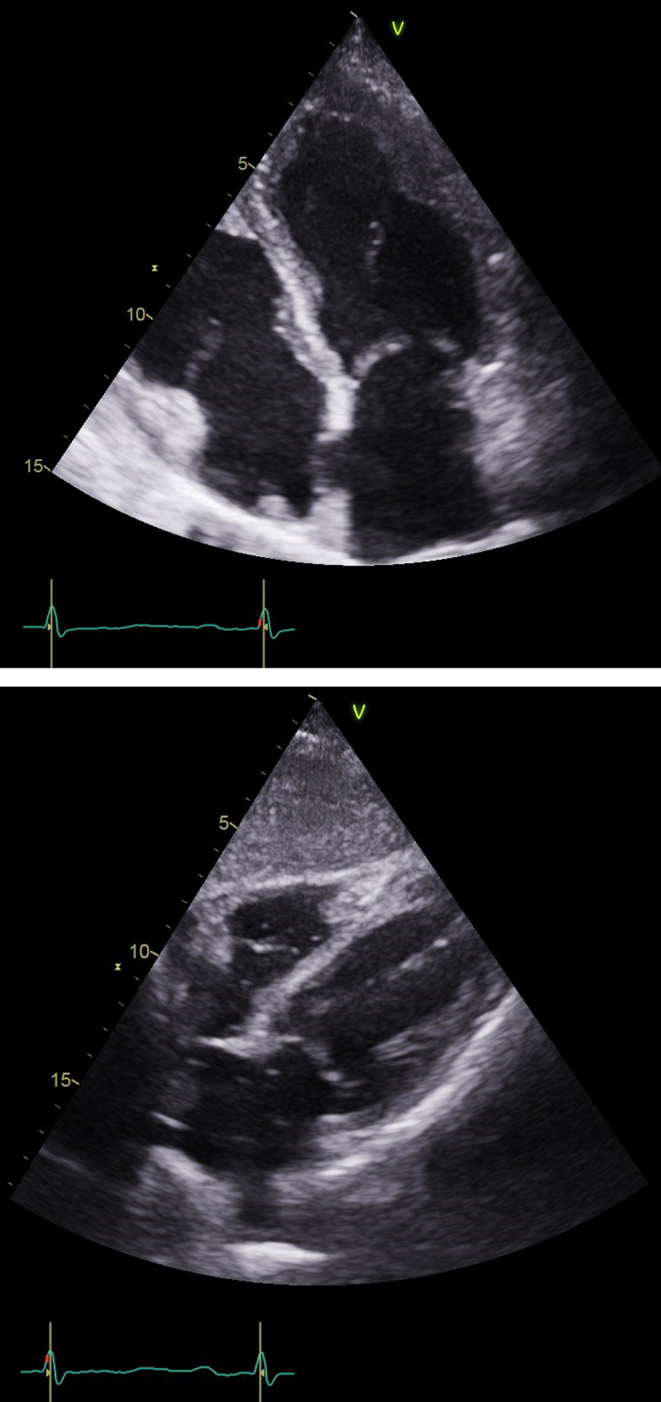
(A) Echo: Subcostal view at the most recent follow-up showing no further evidence of pericardial effusion. (B) Apical four-chamber view showing LV thrombus no longer present.
Discussion
This case highlights how the rare condition of IEM may present with features similar to an acute coronary syndrome event. This case is also important as it shows how prompt identification and treatment can result in good patient outcomes.
Eosinophils, a cellular component of the normal immune system, may initiate myocardial damage and dysfunction when the levels are elevated causing eosinophilic myocarditis (1). Eosinophilic myocarditis is a rare form of myocarditis with an estimated prevalence of 0.5% in unselected autopsy reports (1) and 0.1% in heart biopsied for suspected myocarditis (2). It is characterized by focal or diffuse inflammation with eosinophilic infiltration which is most commonly seen with peripheral blood eosinophilia (3). If untreated, EM carries a poor prognosis with a 5-year mortality rate of 50% (4). Although there are several known causes of EM including hypersensitivity, infection, malignancy and toxins (1), a large proportion of EM cases, as seen here, are labeled idiopathic. In a recent review of 179 cases of histologically proven EM, the prevalence of IEM was 35.7% (5).
The early diagnosis of IEM can be challenging. As seen in this case, test results prior to diagnosis maybe normal or non-specific, and as IEM can often mimic other disease states a diagnosis may be delayed. A review of eosinophilic myocarditis by Brambatti et al. indicated the most common presenting symptoms included dyspnea, chest pain and fever (59.4, 43.4 and 35.5%, respectively) (5).
It has been reported that there are three stages in eosinophilic myocarditis. The initial stage includes myocardial infiltration of eosinophils causing acute necrosis. Stage two, characterized with a hypercoagulation state leading to thrombus formation either within the coronary vasculature or the ventricles. Finally, stage three involves permanent cardiac dysfunction due to the formation of scar tissue (6). The patient presented here, most likely presented during stage two of EM due to the presence of an LV thrombus, echocardiography follow-up revealed improved LV function suggesting that the prompt treatment has, at present, not resulted in permanent cardiac dysfunction.
European cardiology guidelines recommend endomyocardial biopsy (EMB) as the gold standard investigation for definitive diagnosis of EM, especially in the presence of a rapid decline in cardiac function despite optimal medical therapy (6). However, EMB has a low sensitivity (50%) as eosinophilic infiltration is often focal and this can give rise to sampling errors and false-negative results (1). Furthermore, the procedure is not without risk, as endomyocardial biopsy carries risks including ventricular perforation and subsequent pericardial tamponade, heart block, tricuspid valve damage, supraventricular and ventricular arrhythmias (7). Additionally, EMB is not indicated in all cases in which EM is suspected (6). In this case, the biopsy was not performed due to the patient remaining well once corticosteroid therapy was initiated, the presence of LV thrombus was also a contributing factor.
Our case highlights the important role of imaging to supplement the detection of significant eosinophilia in a patient presenting with cardiac symptoms. As seen here, echocardiography allowed for the rapid assessment of cardiac structure and function. However, the diagnostic use of echocardiography in patients with myocarditis is limited due to the highly variable echocardiographic findings associated with myocarditis, in general, along with the notion that less severe forms of myocarditis, as initially seen with this patient, may also be normal thus further hindering diagnosis (8). As proposed by Debl et al., CMR is the only non-invasive imaging modality that can assess for endomyocardial involvement and aid in the initial diagnosis of EM prior to EMB (9). Although the use of CMR is unable to diagnose the presence of EM, CMR has the ability to assess for endocardial inflammation, perfusion and fibrosis which are advantageous in the prognostic outlook of patients (4).
Conclusion
Our case highlights how eosinophilic myocarditis is a rare cause of acute heart failure with LV thrombus. In our case, tests were performed to exclude acute coronary event and incidentally eosinophilia was found. Further cardiac imaging revealed evidence of moderate LV dysfunction, pericardial effusion and an LV thrombus. After multidisciplinary input, a diagnosis of eosinophilic myocarditis was made. The patient was treated with high-dose steroid and anticoagulation. There was a rapid return to normal levels of eosinophilia within 4 days of initiating treatment, and close echocardiography follow-up revealed a return to normal LV function and thrombus resolution over the course of 3 months.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this case report.
Funding
This work did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Patient consent
Written informed consent for publication of their clinical details clinical images was obtained from the patient.
Author contribution statement
Nam Tran is the main and first author of this case report. Authors who reviewed the articles are Dr Chun Shing Kwok, Dr Thanh Phan and cardiac physiologist Sadie Bennett who also helped to obtain imagings of this case report. We have permission from Dr Karim Ratib who was the physician responsible for the patient.
References
- 1.Al Ali AM, Straatman LP, Allard MF, Ignaszewski AP. Eosinophilic myocarditis: case series and review of literature. Canadian Journal of Cardiology 2006. 1233–1237. ( 10.1016/S0828-282X(06)70965-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maisch B., Pankuweit S. Current treatment options in (peri)myocarditis and inflammatory cardiomyopathy. Herz 2012. 644–656. ( 10.1007/s00059-012-3679-9) [DOI] [PubMed] [Google Scholar]
- 3.Ginsberg F, Parrillo JE. Eosinophilic myocarditis. Heart Failure Clinics 2005. 419–429. ( 10.1016/j.hfc.2005.06.013) [DOI] [PubMed] [Google Scholar]
- 4.Li H, Dai Z, Wang B, Huang W. A case report of eosinophilic myocarditis and a review of the relevant literature. BMC Cardiovascular Disorders 2015. 15 ( 10.1186/s12872-015-0003-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brambatti M, Matassini MV, Adler ED, Klingel K, Camici PG, Ammirati E. Eosinophilic myocarditis: characteristics, treatment, and outcomes. Journal of the American College of Cardiology 2017. 2363–2375. ( 10.1016/j.jacc.2017.09.023) [DOI] [PubMed] [Google Scholar]
- 6.Kuchynka P, Palecek T, Masek M, Cerny V, Lambert L, Vitkova I, Linhart A. Current diagnostic and therapeutic aspects of eosinophilic myocarditis. BioMed Research International 2016. 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Francis R, Lewis C. Myocardial biopsy: techniques and indications. Heart 2018. 950–958. ( 10.1136/heartjnl-2017-311382) [DOI] [PubMed] [Google Scholar]
- 8.Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper LT, White JA, Abdel-Aty H, Gutberlet M, Prasad S, et al Cardiovascular magnetic resonance in myocarditis: a JACC White Paper. Journal of the American College of Cardiology 2009. 1475–1487. ( 10.1016/j.jacc.2009.02.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Debl K, Djavidani B, Buchner S, Poschenrieder F, Heinicke N, Feuerbach S, Riegger G, Luchner A. Time course of eosinophilic myocarditis visualized by CMR. Journal of Cardiovascular Magnetic Resonance 2008. 21 ( 10.1186/1532-429X-10-21) [DOI] [PMC free article] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a