Abstract
Objective:
Chronic thromboembolic pulmonary hypertension (CTEPH) is one of the leading causes of pulmonary hypertension (PH). We aimed to investigate the outcome of CTEPH patients who were followed-up by a PH outpatient clinic.
Methods:
We screened the medical records of 29 PH patients who were followed-up by a PH outpatient clinic between 2009 and 2018. The patients’ demographics and their clinical, echocardiographic, and hemodynamic characteristics were recorded.
Results:
Our study group consisted of 16 females (55.2%) and 13 males (44.8%). The mean age was 59.5±13.7 years and the median follow-up duration was 44 months (1-113 months). The mean initial 6-minute walking distance (6MWD) was 321.4±119.9 m. The initial median N-terminal pro brain natriuretic peptide (NT-proBNP) level was 2468 pg/mL (46.1-20.564 pg/mL). All patients were on oral anticoagulant therapy. Pulmonary endarterectomy (PEA) was performed in 17 of 29 patients (58.6%). Twelve patients (41.4%) were not operated upon due to distal disease, comorbidities, or their own preference. The operated patients were younger than the non-operated patients (55 years & 65 years, p=0.04). At the follow-up, the 6MWD in the operated patients increased (+76 m) and decreased in non-operated patients (-46 m). The survival rate at 10-year follow-up was 58.6% for the whole group. Twelve patients died during the follow-up period. While 7 of 12 not-operated patients died (58.3%), just 5 of 17 operated patients (4 perioperatively and 1 at follow-up) died (29%). Advanced-stage final functional capacity (FC) [New York Heart Association (NYHA) III-IV], inoperability, lower final 6MWD, higher final NT-proBNP, and reduced tricuspid annular plane systolic excursion (TAPSE) were associated with an increased mortality rate. Univariate Cox regression analysis showed that patients with NYHA I-II final FC showed a 166-fold decreased mortality rate.
Conclusion:
The long-term prognosis of operated patients is better than the outcome of not-operated patients. The strongest predictor associated with mortality was a worse final FC (NYHA III-IV).
Keywords: chronic thromboembolic pulmonary hypertension, endarterectomy, follow-up, mortality
Introduction
Chronic thromboembolic pulmonary hypertension (CTEPH) is one of the leading causes of pulmonary hypertension (PH). CTEPH is classified within group 4 PH (1) and can be defined as pre-capillary PH with at least one segmental perfusion defect (as seen in scintigraphy) and typical findings [as seen on conventional or computed tomography (CT) or pulmonary angiography] after a minimum of 3 months of anticoagulation therapy (2). Organized thromboembolic material and altered vascular remodeling are the pathological characteristics of the disease. These pathological changes are initiated or potentiated by a combination of defective angiogenesis, impaired fibrinolysis, and endothelial dysfunction (3,4). These changes lead to PH and right ventricular failure (4).
The annual incidence of CTEPH was four million and more than six million adults per year in the German and French PH registries, respectively (5). The prevalence ranges from 0.4% to 9.1% (6). An international CTEPH registry (Europe and Canada) indicated that 75% of patients with CTEPH had a history of acute pulmonary embolism (2).
Surgical removal of obstructive material by pulmonary endarterectomy (PEA) definitively cures the disease (7). The prognosis can be improved by medical therapy (8), but remains poor without intervention and depends mostly on the hemodynamic severity of PH (9). Pulmonary endarterectomy (PEA) is recommended as the first-choice therapy, whereas specific pulmonary arterial hypertension (PAH) therapy and balloon pulmonary angioplasty (BPA) are restricted to inoperable patients or patients with persistent/recurrent PH after PEA (4,10).
A single-center series reported a 3-year survival rate of 76% and 91% in patients undergoing surgery (11-15). Inoperable patients who were treated with intravenous prostacyclin analogs or oral PAH targeted drugs had a 3-year survival rate varying from 41% to 80% (11, 16, 17). In older series, patients receiving only oral anticoagulants had a 3-year survival rate as low as 30% (9).
We aimed to investigate the baseline characteristics and outcome of CTEPH patients (including operated and non-operated patients) who were followed up at the PH outpatient clinic of İstanbul University-Cerrahpaşa Institute of Cardiology.
Methods
We screened the medical records of PH patients who were followed-up by the PH outpatient clinic of our hospital between 2009 and 2018. Twenty-nine patients with a diagnosis of CTEPH were included in the study. The diagnosis of PH was confirmed by right heart catheterization (RHC). To qualify for inclusion, patients had to be ≥18 years with confirmed PH, demonstrating a mean pulmonary artery pressure (PAPm) of ≥ 25 mm Hg at rest, and a pulmonary capillary wedge pressure (PCWP) of ≤15 mm Hg. CTEPH had to be confirmed as the cause of PH by abnormalities in a ventilation/perfusion scan (at least 1 mismatched segmental perfusion defect) and in a CT scan or a pulmonary angiography. Before the diagnosis, patients were required to have undergone at least 3 months of anticoagulation therapy. Data were obtained retrospectively from medical records of the CTEPH patients who were followed-up regularly after the diagnosis. Medical history, clinical signs and symptoms, laboratory results, and radiological, echocardiographic, and RHC findings were recorded.
PEA was performed using principles similar to those used by the University of California, San Diego group. All patients underwent surgical intervention with deep hypothermia and complete circulation arrest (18).
Statistical analysis
Continuous variables were reported as mean±standard deviation or as median and inter-quartile range. Categorical variables were reported as percentages and compared using the χ2 test. Continuous variables were compared using the t-test or the Mann-Whitney U-test. A p-value of <0.05 was considered statistically significant. All tests were two-sided. Analyses were performed with SPSS for Windows software version 22.0 (SPSS Inc, Chicago, IL, USA). The time to death (all causes) over a 10-year period was estimated by the Kaplan-Meier method and was analyzed using the log-rank test to compare the operated and not-operated patients. Univariate Cox regression analysis was performed to predict the factors associated with death.
Results
The study group consisted of 16 females (55.2%) and 13 males (44.8%). The mean age was 59.5±13.7 years and the median follow-up duration was 44 months (1–113 months). Most of the patients (22/29, 75.8%) were classified under the New York Heart Association (NYHA) functional class III or IV. Most of the patients were on sinus rhythm (n=23, 79.3%) and 6 patients (20.7%) were in atrial fibrillation (AF). The mean systolic PAP was 66.1±26.7 mm Hg and the mean tricuspid annular plane systolic excursion (TAPSE) was 16±4 mm on transthoracic echocardiography (TTE). Three patients had pericardial effusion as seen on TTE. The mean initial 6-minute walking distance (6MWD) was 321.4±119.9 m. On RHC, the systolic, diastolic, and mean PAP were 79.4±22.9 mm Hg, 35.1±13.2 mm Hg, and 50.9±16.1 mm Hg, respectively. The mean pulmonary vascular resistance (PVR) was 8.6±5.9 WU, the mean PCWP was 14.3±5.6 mm Hg, mean right atrial pressure (RAP) was 15.1±5.7 mm Hg, and mean cardiac output (CO) was 4.8±1.5 L/per minute. On laboratory examination, the initial median N-terminal pro brain natriuretic peptide (NT-proBNP) level was 2468 pg/mL (46.1-20.564 pg/mL) and the final median NT-proBNP level was 2382 pg/mL (67.0-23.368 pg/mL) (Table 1).
Table 1.
Demographics of the study group
| Variables | n=29 |
|---|---|
| Sex (n, %) | Female (16, 55.2%) |
| Male (13, 44.8%) | |
| Age (years) | 59.5±13.7 |
| Median follow-up (months) | 44 (1-113) |
| NYHA III-IV (%) | 22 (75.8%) |
| Rhythm (n, %) | Sinus (23, 79.3%) |
| AF (6, 20.7%) | |
| TTE | |
| • mPAP (mm Hg) | 66.1±26.7 |
| • TAPSE (mm) | 16±4 |
| Initial 6MWD (m) | 321.4±119.9 |
| Final 6MWD (m) | 356.1±132.8 |
| RHC | |
| • dPAP (mm Hg) | 35.1±13.2 |
| • mPAP (mm Hg) | 50.9±16.1 |
| • sPAP (mm Hg) | 79.4±22.9 |
| • PVR (WU) | 8.6±5.9 |
| • PCWP (mm Hg) | 14.3±5.6 |
| • RAP (mm Hg) | 15.1±5.7 |
| • CO (L/per minute) | 4.8±1.5 |
AF - atrial fibrillation, CO - cardiac output, dPAP - diastolic pulmonary artery pressure, mPAP - mean pulmonary artery pressure, NYHA - New York Heart Association, PCWP - pulmonary capillary wedge pressure, PVR - pulmonary vascular resistance, RAP - right atrial pressure, RHC - right heart catheterization, 6MWD - six-minute walking distance, sPAP - systolic pulmonary artery pressure, TAPSE - tricuspid annular peak systolic excursion, TTE - transthoracic echocardiography
Pulmonary endarterectomy (PEA) was performed in 17 of 29 patients (58.6%). Twelve patients (41.4%) were not operated upon due to distal disease, comorbidities, and/or their own preference. None of our patients underwent BPA. Operated patients were younger than non-operated patients (55 years vs. 65 years, p=0.04). About 52.9% of operated patients and 50% of non-operated patients were female. At the follow-up, the 6MWD in the operated patients increased (+76 m) and decreased in non-operated patients (-46 m) (Table 2). Further, the functional capacity (FC) was improved in operated patients. There were 12 patients (41.4%) with NYHA class III or IV at the follow-up. All patients were under oral anticoagulant therapy and 12 not-operated patients were on PAH-specific treatment. While 7 of these not-operated patients were on monotherapy (3 endotelin receptor antagonists-ERAs, 2 phosphodiesterase type-5 inhibitors-PDE-5is, and 2 inhaled prostanoids), the other 5 patients were taking combination therapy (2 patients with ERAs+PDE-5is combination, 1 patient with ERAs+inhaled prostanoid, 1 patient with ERAs+PDE-5is+inhaled prostanoid, and 1 patient with ERAs+sGC stimulator+inhaled prostanoid) (Fig. 1).
Table 2.
Clinical characteristics of operated and non-operated patients
| Variable | Operated | Not-operated (17, 58.6%) | P-value (12, 41.4%) |
|---|---|---|---|
| Median age (years) | 55 | 65 | 0.04 |
| Females, n (%) | 9 (52.9) | 6 (50.0) | 0.876 |
| i6MWD (m) | 309.8±127.0 | 339.6±112.8 | 0.564 |
| f6MWD (m) | 385.1±147.2 | 293.3±67.5 | 0.08 |
| Delta 6MWD (m) | +76 | -46 | 0.01 |
| iNYHA III-IV (%) | 87.5 | 72.7 | 0.219 |
| fNYHA III-IV (%) | 29.4 | 58.4 | 0.44 |
| TAPSE (mm) | 16±5 | 18±2 | 0.302 |
| RHC | |||
| • dPAP (mm Hg) | 35.6±13.3 | 34.3±13.9 | |
| • mPAP (mm Hg) | 52.9±16.3 | 47.3±15.8 | |
| • sPAP (mm Hg) | 81.7±23.5 | 75.9±22.8 | |
| • PVR (WU) | 10.1±6.2” | 5.7±4.2 | |
| • PCWP (mm Hg) | 14.3±5.0 | 14.4±6.8 | |
| • RAP (mm Hg) | 14.9±5.0 | 15.7±7.3 | |
| • CO (L/per minute) | 4.4±1.5 | 4.6±1.5 | |
CO - cardiac output, dPAP - diastolic pulmonary artery pressure, f6MWD - final six-minute walking distance, fNYHA - final New York Heart Association, i6MWD - initial six-minute walking distance, iNYHA - initial New York Heart Association, mPAP - mean pulmonary artery pressure, PVR - pulmonary vascular resistance, RAP - right atrial pressure, RHC - right heart catheterization, sPAP - systolic pulmonary artery pressure, TAPSE - tricuspid annular plane systolic excursion
Figure 1.
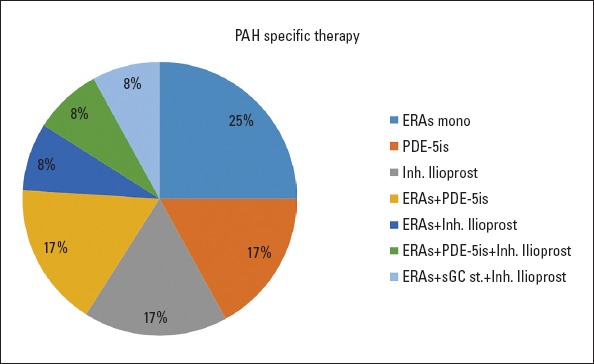
Pulmonary arterial hypertension-specific treatment of not-operated patients
ERA - endothelin receptor antagonist, Inh. - inhaled, mono. - monotherapy, PAH - pulmonary arterial hypertension, PDE-5is - phosphodiesterase type 5 inhibitors, sGC ST. - soluble guanylate cyclase stimulator
The Kaplan-Meier estimates of the 10-year survival rates was 58.6% for the whole group (Fig. 2). Twelve patients died during the 10-year follow-up duration. While 7 of the 12 non-operated patients died (58.3%), only 5 of 17 operated patients (4 perioperatively and 1 at follow-up) died (29.4%). Right heart failure was the main and most common reason of death in non-operated patients. While the 10-year survival was 70.6% in operated patients, it was 41.7% in non-operated patients. After exclusing the patients who died perioperatively, the survival rate improved with pulmonary endarterectomy and it reached statistical significance (p<0.05). Advanced-stage final FC (NYHA III-IV), lower final 6MWD, higher final NT-proBNP, reduced TAPSE, and inoperability were associated with a higher mortality rate (Table 3). Univariate Cox regression analysis showed that patients with improved final FC (NYHA I-II) had a 166-fold decreased mortality rate.
Figure 2.
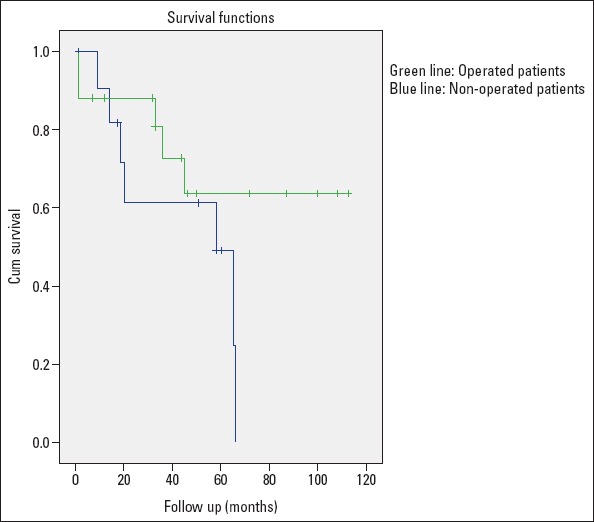
Kaplan-Meier estimates of 10-year survival in both operated and non-operated patients
Table 3.
Factors associated with mortality
| Univariate Cox regression | P-value | ||
|---|---|---|---|
| HR | 95% CI | ||
| TAPSE (mm) | 0.898 | 0.776-1.038 | 0.145 |
| f6MWD (m) (Δ: Last-First) | 0.994 | 0.987-1.00 | 0.051 |
| fNT-proBNP (pg/mL) (Δ: Last-First) | 1.00 | 1.00-1.0001 | 0.353 |
| Endarterectomy (%) | 2.38 | 0.740-7.682 | 0.141 |
| fNYHA III-IV (First) Class (1-2) vs. (3-4) | 0.387 | 0.317-19.4 | 0.327 |
| fNYHA III-IV (Last) Class (1-2) vs. (3-4) | 17.36 | 2.23-135.09 | <0.001 |
fNYHA - final New York Heart Association, fNT-proBNP - final N-terminal pro brain natriuretic peptide, f6MWD - final six-minute walking distance, TAPSE - tricuspid annular plane systolic excursion
Discussion
In this single-center 10-year CTEPH experiment, we aimed to retrospectively investigate the baseline demographics, clinical characteristics, and outcome of CTEPH patients who were followed-up by the PH outpatient department of our university hospital from 2009 to 2018. Both operated and non-operated CTEPH patients were included in the study. The main finding of this analysis was that patients who underwent PEA showed better long-term survival rates than non-operated patients, despite having a similar initial hemodynamic severity and FC.
Pulmonary endarterectomy (PEA) is the first-line and curative treatment of choice for CTEPH. It consists of removing all thrombotic endoluminal material, neo-intima, and few elastic lamellae from the inner layers of the tunica media of pulmonary arteries using a surgical approach. However, between 10% and 50% of referred patients may not be eligible for surgery (19). In the international prospective CTEPH registry, 36.6% of evaluated patients are not eligible for PEA (2). This statistic was a little bit higher in our study population (41.4%). The most important prohibitive factor was the presence of distal disease (seen in 9 of 12 not-operated patients, 75%).
CTEPH is occurs with equal frequency in men and women, most often in their sixth decade of life (2). The mean age of occurrence was similar (59.5 years) in our study population, although there was an insignificant female predominance in the whole group (55% vs. 45%). Operated patients were younger than non-operated patients but presented with similar disease severity, as assessed by hemodynamic variables and NYHA functional class. This is consistent with other CTEPH registries from Europe and United Kingdom (UK) (2, 11, 20, 21,).
The current multi-center CTEPH registry showed a 3-year survival rate of 89% in operated patients (22). In two large single-center cohorts, the 3-year survival rates were reported as 81% (23) and 76% (11). In the UK National CTEPH cohort, the overall survival was 86%, 84%, 79%, and 72% at 1 year, 3 years, 5 years, and 10 years for the entire cohort (21). The overall survival rates at 1 year and 3 years after surgery were 91% and 90%, respectively. In our study, the 10-year survival was 58.6% for entire cohort. Operated patients had a better 10-year survival (70.6%) than not-operated patients (41.7%). Non-operated patients consisted of 41.4% of our study population. They were older than operated patients and most of them (75%) had a distal disease, which was the main prohibitive factor for inoperability. Although their hemodynamic severity was similar to operated patients, non-operated patients had a worse 10-year survival rate. All non-operated patients were using anticoagulant and PAH-specific therapy. Non-operated patients were on various PAH-specific drugs, namely endothelin receptor antagonists, phosphodiesterase 5 inhibitors, and prostacylin analogs. Only one of the non-operated patients was on riociguat in combination with other drugs, which is the only approved drug for medical treatment of CTEPH. The reason for this was that riociguat was approved in our country in 2015 and most of the patients had been prescribed with other drugs before that time period. Most of the deaths in operated patients (4/5, 80%) occurred in the perioperative period. Only 1 patient died during follow-up due to respiratory failure that was associated with chronic obstructive pulmonary disease. Patients who survived surgery did not require PAH-specific therapy.
The independent factors associated with long-term survival were: advanced-stage final FC (NYHA III-IV), inoperability, lower final 6MWD, higher final NT-proBNP, and reduced TAPSE. Univariate Cox regression analysis showed that patients with improved final FC (NYHA I-II) showed a 166-fold decreased mortality rate.
Study limitations
The main limitation of our study was its retrospective nature. We were not able to record thrombotic risk factors associated with CTEPH. Although we tried to record all hemodynamic data, they were not 100% complete. We also were not able to use multivariate Cox regression analysis because only one variable has been found to be associated with mortality to date. Instead, univariate analysis was performed to predict factors associated with mortality.
Conclusion
Our study presents the long-term CTEPH experience in a university hospital. Operated patients showed better survival rates than non-operated patients (70.6% vs. 41.7% over 10 years), despite the fact that all not-operated patients were using PAH-specific therapy. The predictors of mortality were: a worse final NYHA FC, inoperability, lower 6MWD at follow-up, higher NT-proBNP at follow-up, and reduced TAPSE, all of which are also poor prognostic factors for PAH. After performing univariate Cox regression analysis, the relation of high mortality was still constant for advanced-stage final NYHA FC (III-IV).
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept – M.S.K.; Design – Ü.Y.S.; Supervision – M.S.K.; Funding – Ü.Y.S.; Materials – B.Y.; Data collection and/or processing – Ü.Y.S.; Analysis and/or interpretation – Ü.Y.S.; Literature search – Ü.Y.S.; Writing – Ü.Y.S.; Critical review – Ü.Y.S.
References
- 1.Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53:pii:1801913. doi: 10.1183/13993003.01913-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pepke-Zaba J, Delcroix M, Lang I, Mayer E, Jansa P, Ambroz D, et al. Chronic thromboembolic pulmonary hypertension (CTEPH):results from an international prospective registry. Circulation. 2011;124:1973–81. doi: 10.1161/CIRCULATIONAHA.110.015008. [DOI] [PubMed] [Google Scholar]
- 3.Dorfmüller P, Günther S, Ghigna MR, Thomas de Montpreville, Boulate D, Paul JF, et al. Microvascular disease in chronic thromboembolic pulmonary hypertension:a role for pulmonary veins and systemic vasculature. Eur Respir J. 2014;44:1275–88. doi: 10.1183/09031936.00169113. [DOI] [PubMed] [Google Scholar]
- 4.Kim NH, Delcroix M, Jenkins DP, Channick R, Dartevelle P, Jansa P, et al. Chronic thromboembolic pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):D92–9. doi: 10.1016/j.jacc.2013.10.024. [DOI] [PubMed] [Google Scholar]
- 5.Hoeper MM, Humbert M, Souza R, Idrees M, Kawut SM, Sliwa-Hahnle K, et al. A global view of pulmonary hypertension. Lancet Respir Med. 2016;4:306–22. doi: 10.1016/S2213-2600(15)00543-3. [DOI] [PubMed] [Google Scholar]
- 6.Lang IM, Pesavento R, Bonderman D, Yuan JX. Risk factors and basic mechanisms of chronic thromboembolic pulmonary hypertension:a current understanding. Eur Respir J. 2013;41:462–8. doi: 10.1183/09031936.00049312. [DOI] [PubMed] [Google Scholar]
- 7.Jamieson SW, Kapelanski DP, Sakakibara N, Manecke GR, Thistlethwaite PA, Kerr KM, et al. Pulmonary endarterectomy:experience and lessons learned in 1,500 cases. Ann Thorac Surg. 2003;76:1457–62. doi: 10.1016/s0003-4975(03)00828-2. [DOI] [PubMed] [Google Scholar]
- 8.Ghofrani HA, D'Armini AM, Grimminger F, Hoeper MM, Jansa P, Kim NH. CHEST-1 Study Group Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med. 2013;369:319–29. doi: 10.1056/NEJMoa1209657. [DOI] [PubMed] [Google Scholar]
- 9.Lewczuk J, Piszko P, Jagas J, Porada A, Wojciak S, Sobkowicz B, et al. Prognostic factors in medically treated patients with chronic pulmonary embolism. Chest. 2001;119:818–23. doi: 10.1378/chest.119.3.818. [DOI] [PubMed] [Google Scholar]
- 10.Kim NH, Delcroix M, Jais X, Madani MM, Matsubara H, Mayer E, et al. Chronic thromboembolic pulmonary hypertension. Eur Respir J. 2019;53:pii:1801915. doi: 10.1183/13993003.01915-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Condliffe R, Kiely DG, Gibbs JS, Corris PA, Peacock AJ, Jenkins DP, et al. Improved outcomes in medically and surgically treated chronic thromboembolic pulmonary hypertension. Am J Respir Crit Care Med. 2008;177:1122–7. doi: 10.1164/rccm.200712-1841OC. [DOI] [PubMed] [Google Scholar]
- 12.Skoro-Sajer N, Marta G, Gerges C, Hlavin G, Nierlich P, Taghavi S, et al. Surgical specimens, haemodynamics and long-term outcomes after pulmonary endarterectomy. Thorax. 2014;69:116–22. doi: 10.1136/thoraxjnl-2013-203746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corsico AG, D'Armini AM, Cerveri I, Klersy C, Ansaldo E, Niniano R, et al. Long-term outcome after pulmonary endarterectomy. Am J Respir Crit Care Med. 2008;178:419–24. doi: 10.1164/rccm.200801-101OC. [DOI] [PubMed] [Google Scholar]
- 14.Saouti N, Morshuis WJ, Heijmen RH, Snijder RJ. Long-term outcome after pulmonary endarterectomy for chronic thromboembolic pulmonary hypertension:a single institution experience. Eur J Cardiothorac Surg. 2009;35:947–52. doi: 10.1016/j.ejcts.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 15.Wieteska M, Biederman A, Kurzyna M, Dyk W, Burakowski J, Wawrzyńska L, et al. Outcome of medically versus surgically treated patients with chronic thromboembolic pulmonary hypertension. Clin Appl Thromb Hemost. 2016;22:92–9. doi: 10.1177/1076029614536604. [DOI] [PubMed] [Google Scholar]
- 16.Cabrol S, Souza R, Jais X, Fadel E, Ali RH, Humbert M, et al. Intravenous epoprostenol in inoperable chronic thromboembolic pulmonary hypertension. J Heart Lung Transplant. 2007;26:357–62. doi: 10.1016/j.healun.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 17.Skoro-Sajer N, Bonderman D, Wiesbauer F, Harja E, Jakowitsch J, Klepetko W, et al. Treprostinil for severe inoperable chronic thromboembolic pulmonary hypertension. J Thromb Haemost. 2007;5:483–9. doi: 10.1111/j.1538-7836.2007.02394.x. [DOI] [PubMed] [Google Scholar]
- 18.Madani MM, Auger WR, Pretorius V, Sakakibara N, Kerr KM, Kim NH, et al. Pulmonary endarterectomy:recent changes in a single institution's experience of more than 2,700 patients. Ann Thorac Surg. 2012;94:97–103. doi: 10.1016/j.athoracsur.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 19.Peacock A, Simonneau G, Rubin L. Controversies, uncertainties and future research on the treatment of chronic thromboembolic pulmonary hypertension. Proc Am Thorac Soc. 2006;3:608–14. doi: 10.1513/pats.200605-114LR. [DOI] [PubMed] [Google Scholar]
- 20.Bonderman D, Skoro-Sajer N, Jakowitsch J, Adlbrecht C, Dunkler D, Taghavi S, et al. Predictors of outcome in chronic thromboembolic pulmonary hypertension. Circulation. 2007;115:2153–8. doi: 10.1161/CIRCULATIONAHA.106.661041. [DOI] [PubMed] [Google Scholar]
- 21.Cannon JE, Su L, Kiely DG, Page K, Toshner M, Swietlik E, et al. Dynamic Risk Stratification of Patient Long-Term Outcome After Pulmonary Endarterectomy Results from the United Kingdom National Cohort. Circulation. 2016;133:1761–71. doi: 10.1161/CIRCULATIONAHA.115.019470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Delcroix M, Lang I, Pepke-Zaba J, Jansa P, D'Armini AM, Snijder R, et al. Long-Term Outcome of Patients with Chronic Thromboembolic Pulmonary Hypertension Results from an International Prospective Registry. Circulation. 2016;133:859–71. doi: 10.1161/CIRCULATIONAHA.115.016522. [DOI] [PubMed] [Google Scholar]
- 23.Archibald CJ, Auger WR, Fedullo PF, Channick RN, Kerr KM, Jamieson SW, et al. Long-term outcome after pulmonary thromboendarterectomy. Am J Respir Crit Care Med. 1999;160:523–8. doi: 10.1164/ajrccm.160.2.9808109. [DOI] [PubMed] [Google Scholar]


