Abstract
Background
Preeclampsia is a major pregnancy complication that results in significant maternal and infant mortality, most of which occurs in low and middle-income countries. The accurate and timely diagnosis of preeclampsia is critical in management of affected pregnancies to reduce maternal and fetal/neonatal morbidity and mortality, yet difficulties remain in establishing the rigorous diagnosis of preeclampsia based on clinical parameters alone. Biomarkers that detect biochemical disease have been proposed as complements or alternatives to clinical criteria to improve diagnostic accuracy. This cohort study assessed the performance of several biomarkers, including glycosylated fibronectin (GlyFn), to rule-in or rule-out preeclampsia within 4 weeks in a cohort of women at increased risk for preeclampsia.
Methods
151 women with risk factors for or clinical signs and symptoms of preeclampsia were selected from a prospective cohort. Maternal serum samples were collected between 20 and 37 weeks of gestation. Clinical suspicion of preeclampsia was defined as presence of new-onset proteinuria, or clinical symptoms of preeclampsia. Subjects with a clinical diagnosis of preeclampsia at the time of enrollment were excluded. GlyFn, pregnancy-associated plasma protein-A2 (PAPPA2), placental growth factor (PlGF), and soluble fms-like tyrosine kinase-1 (sFlt-1) were measured by immunoassay. GlyFn was also determined using a rapid point-of care (POC) test format. Receiver-operating characteristic (ROC) curves derived from logistic regression analysis were used to determine the classification performance for each analyte.
Results
32 of 151 (21%) women developed a clinical diagnosis of preeclampsia within 4 weeks. All biomarkers exhibited good classification performance [GlyFn (area under the curve (AUROC) = 0.94, 91% sensitivity, 86% specificity); PAPPA2 AUC = 0.92, 87% sensitivity, 77% specificity; PlGF AUC = 0.90, 81% sensitivity, 83% specificity; sFlt-1 AUC = 0.92, 84% sensitivity, 91% specificity. The GlyFn immunoassay and the rapid POC test showed a correlation of r = 0.966.
Conclusions
In this prospective cohort, serum biomarkers of biochemical disease were effective in short-term prediction of preeclampsia, and the performance of GlyFn in particular as a POC test may meet the needs of rapid and accurate triage and intervention.
Background
Preeclampsia (PE) is associated with 10–15% of all maternal deaths during pregnancy and childbirth, making it the second-leading cause of maternal mortality, resulting in an estimated 76,000 maternal deaths annually [1–3]. PE also accounts for 25% of stillbirths and 25% of neonatal deaths [4]. Over 99% of this maternal and fetal/neonatal mortality attributed to PE occurs in low-and middle-income countries, in particular Africa and the Indian subcontinent [5]. Previous studies suggest that mortality rates could be considerably reduced if clinicians were more aware of the likelihood that PE could develop [6, 7]. PE was redefined by the American College of Obstetricians and Gynaecologists (ACOG) in 2013 [8]. Specifically, the “traditional” diagnostic criteria of new-onset hypertension > 140/90 mmHg and proteinuria > 300 mg/24 h after 20 weeks of gestation were revised, and proteinuria is no longer required as long as other maternal organ dysfunction (i.e., renal insufficiency, liver involvement, neurological and hematological complications) is present. The International Society for the Study of Hypertension in Pregnancy (ISSHP), the Australasian Society for the Study of Hypertension in Pregnancy, and the Society of Obstetricians and Gynaecologists of Canada added utero-placental dysfunction or intrauterine growth restriction (IUGR) to the diagnostic criteria for PE [9–11].
Identification of the clinical features consistently associated with PE is further complicated by the existence of cases of PE with the same underlying placental pathology, but that exhibit no signs of hypertension [12]. Eclampsia and the syndrome of Hemolysis, Elevated Liver enzymes, and Low Platelets (HELLP) can also occur in the absence of hypertension or proteinuria [13]. These “non-traditional” constellations of symptoms contribute to the difficulty in obtaining an accurate diagnosis of PE solely based on clinical criteria. This is particularly problematic in women with pre-existing proteinuria and pre-existing or gestational hypertension, in whom accurate diagnosis of PE is critical. More objective measures to help clinicians make a final and accurate diagnosis would greatly improve clinical care and in many cases could be lifesaving.
An important alternative to diagnoses based on observable clinical presentation is the determination of the levels of predictive biomarkers that can be measured in body fluids such as blood, urine, or saliva. A number of circulating factors have been shown to be associated with PE, including soluble endoglin, placental growth factor (PlGF), soluble fms-like tyrosine kinase-1 (sFlt-1), vascular endothelial growth factor (VEGF), pregnancy-associated plasma protein A-2 (PAPPA2), glycosylated fibronectin (GlyFn), vasopressin, and copeptin [14–18].
In this study, we evaluated the ability of several of the biomarkers GlyFn, PAPPA2, PlGF, and sFlt-1, to predict the development of PE within 40 days of maternal sampling. The hypothesis is that GlyFn and PAPPA2 have comparable test performance as the known biomarkers PlGF and sFlt-1. We also describe a point-of-care (POC) test for GlyFn (Lumella™) and determine its test performance in comparison to the standard GlyFn immunoassay.
Methods
Study design and patients
We present a prospective, observational study which was conducted at the University Hospitals in Basel and Geneva, Switzerland [19]. The Competent Ethics Committee of Northwestern Switzerland and Geneva (IRB approval numbers EKNZ PB_2016_02490 and GE 14–216) approved the study protocol, and written informed consent was obtained from all participants. Women who were > 18 years of age with a singleton pregnancy were included if they had at least one PE risk factor: nulliparous overweight or obese women with body mass index (BMI) > 26.1 kg/m2, nulliparous women > 40 years of age, pre-existing diabetes, essential hypertension or renal disease, pregnancy-induced hypertension, gestational diabetes (defined by at least one pathological value of fasting glucose (> 5.1 mmol/l) or at one (> 10.0 mmol/l) or two hours (> 8.5 mmol/l) after a 75-g glucose load, utero-placental dysfunction (defined by abnormal uterine perfusion with mean pulsatility index >95th percentile in the second trimester and/or bilateral uterine artery notching), previous PE, eclampsia, or HELLP, thrombophilia with high risk for PE (homozygous factor V Leiden or methylenetetrahydrofolate reductase (MTHFR) C677T defects, or the combination of heterozygous factor II G20210A and heterozygous factor V Leiden defects diagnosed in a DNA analysis prior pregnancy), antiphospholipid antibodies, or family history of PE, eclampsia, or HELLP in first-degree relatives. Additionally, women who had symptoms suspicious of PE (two combined findings of clinical symptoms like headache and/or scotoma and/or epigastric pain and/or excessive edema and/or new onset proteinuria (> 1+ in dipstick)) were asked to participate. Exclusion criteria included diagnosis of PE at sample collection, chromosomal aberrations, fetal malformations, abortion, or stillbirth at < 22 weeks of gestation. All eligible women were followed regularly with recording of demographic characteristics, medical history, clinical examinations, and blood draws for biomarker analysis (GlyFn, PAPPA2, PlGF, and sFlt-1). High-risk women with suggestive clinical findings and symptomatic women were treated expectantly, depending on their clinical condition, until delivery. The results of the biomarker analysis were not available until the end of study and did not, therefore, influence management decisions.
Diagnostic criteria for hypertensive diseases in pregnancy
Pre-existing hypertension was defined as systolic blood pressure > 140 mmHg and/or diastolic blood pressure > 90 mmHg diagnosed before conception or < 20 weeks of gestation. Gestational hypertension was determined as new onset of hypertension developing > 20 weeks of gestation without proteinuria. The following criteria for PE were used to establish the diagnosis: New-onset systolic blood pressure > 140 mmHg and/or diastolic blood pressure > 90 mmHg measured on two occasions at least 6 h apart but within one week and new-onset proteinuria with > 30 mg/24-h urine protein collection or > 2+ in dipstick or spot urine (> 3 mg/dL or protein/creatinine ratio > 3 mg protein/mmol creatinine) > 20 weeks of gestation. Eclampsia was defined as new onset of tonic-clonic seizures associated with PE, which could not be assigned to any other cause. HELLP syndrome was considered when haemolysis (lactic acid dehydrogenase > 600 IU/L, and/or lowered haptoglobin), elevated liver enzymes (aspartate amino transferase exceeding 70 IU/L) and low platelets (platelet counts < 100,000/μL) occurred.
Diagnostic criteria for intrauterine growth restriction (IUGR)
IUGR was defined as an estimated fetal weight < 10th percentile (adjusted for gender and ethnicity according to charts routinely used by both sites [20]) plus pathological finding(s) in Doppler indices (cerebro-placental ratio < 5th percentile and/or a uterine artery pulsatility index >95th percentile in the second trimester) or a birth weight < 3rd percentile [21].
Assessment of GlyFn, PAPPA2, PlGF, and sFlt-1
All maternal serum samples were aliquoted and stored at − 80 °C until analysis. Commercial immunoassay kits for sFlt-1 and PlGF (R&D systems, Minneapolis, MN, USA), PAPPA2 (Ansh Labs, Webster, TX, USA), and GlyFn (DiabetOmics, Inc., Hillsboro, OR, USA) were used according to manufacturer’s instructions. Inter-assay coefficients of variation for these commercial kits ranged from 1.89–6.65% and the intra-assay coefficients ranged from 2.1–4.5%. Biomarker thresholds for PlGF and sFlt-1 were chosen based on published literature using R&D immunoassays [22]; abnormal PlGF levels are those < 100 pg/ml and abnormal sFlt-1 levels are those > 7000 ng/ml. The threshold for PAPPA2 > 200 ng/ml was determined from prior biomarker studies (unpublished data). GlyFn threshold > 315 μg/ml were derived from the current dataset which best discriminated cases from non-cases and require additional validation in future studies.
Point-of-care test (Lumella™ test system)
A prototype GlyFn POC test strip was previously described that employed a fluorescently labeled fibronectin polyclonal antibody as both the detection and capture antibody, with the signal from maternal serum measured using a commercial automated cassette reader [18]. In the current study, serum samples were analyzed for GlyFn using the second-generation Lumella™ PE test (DiabetOmics, Inc.) according to the manufacturer’s instructions. Test strips were configured with monoclonal antibodies against GlyFn labeled with gold particles for quantification using a hand-held Lumella™ reader system. Briefly, 5 μl of serum is diluted 1:350 in running buffer and 120 μl of diluted serum is added to the test strip and inserted into the reader. The GlyFn concentration is displayed on the reader at the end of 10 min. Calibration information is supplied by the manufacturer as a lot-specific radiofrequency identification (RFID) tag on each test kit. The measurable range of the Lumella™ assay is 100 ng/mL to 800 μg/mL vs 10–2000 μg/mL for the prototype version [16]. The intra-/interassay coefficients of variation at mean concentrations of 50–800 μg/mL were 8.6/10.4 and 9.2/10.2%, respectively.
Participant/sample selection
From a prospective cohort, 226 unique samples were collected. Fifty-seven samples were excluded as we restricted the current investigation to samples derived > 20 and < 37 weeks of gestation and to women who developed clinical PE within 40 days of sample collection or did not develop PE but had a sample collected at similar gestational age. High-risk samples were chosen based on matching for gestational age (within 1 week). No exclusions were needed because of the matching of high-risk women. Women with a diagnosis of PE prior to sample collection were excluded from the analysis. Analyses were restricted to one sample per woman and the earliest sample were chosen from women in the PE group who had multiple samples collected to better represent early prediction. Thereby another 18 samples were excluded because of multiple measurement within 40 days period. Finally, 151 women with samples were included in the current analysis.
Statistical analyses
Baseline maternal characteristics were stratified for women within these groups. The nonparametric, two-sided Wilcoxon rank sum test was used to compare differences between groups for continuous variables, as they are more robust than non-normal distributions, as well as outlying observations. The χ2 test was used for categorical variables. We also compared co-morbidities, pre-existing renal disease, pre-existing diabetes, pre-existing hypertension, and gestational hypertension. Biomarker distributions for women with and without development of clinical PE were calculated and compared, and medians and interquartile ranges (IQR) of the original scales are reported. Non-parametric test equivalent to receiver-operating characteristic (ROC) curve were used to inferentially compare biomarker distributions. Confirmed delivery outcomes were also compared between groups, including gestational age at delivery, neonatal birth weight, Apgar scores, cesarean sections, preterm births, IUGR, and SGA.
ROC curves, the area under the curve (AUC), along with corresponding 95% confidence intervals (CIs) for PE diagnosis were generated using predicted probabilities from simple logistic regression models [23]. We estimated and compared the operating characteristics (sensitivity, specificity) using thresholds described previously (> 315 U/mL for GlyFn, > 200 ng/mL for PAPPA-2, < 100 pg/mL for PlGF, and ≥ 7000 ng/mL for sFlt-1) for detection of PE. We evaluated the ability of the various biomarkers to predict the onset of PE within four weeks of sample collection. Predicted probabilities from simple logistic regression were used to create ROC curves, AUCs, and 95% CI’s [23]. A comparison of the GlyFn plate immunoassay to the GlyFn POC test was performed on samples assayed by both methods. Pearson correlation coefficient was calculated to compare the methods. ROC curves were generated for each method to ascertain classification accuracy. All statistical analyses were performed using R (3.2.2) via Rstudio software version 1.0.136 (https://www.rstudio.com/products/RStudio/). ROC curves were created using the pROC package [24].
Results
Baseline characteristics
Between September 2011 and July 2015, a total of 151 women meeting the inclusion criteria were enrolled in the final study, 32 (21%) of whom received a clinical diagnosis of PE in 4 weeks from sample collection. The maternal and pregnancy characteristics of both groups are summarized in Table 1. The PE group had a shorter interval between blood sampling and delivery (PE 8 d (±9.7 d) vs. without PE 60 d (±42.9 d), P < 0.0001) and delivered earlier in comparison to the without PE group (PE at 31 weeks of gestation (±4.6) vs. without PE at 37 weeks of gestation (±3.5), P < 0.0001). Both groups had notable differences in pregnancy outcome parameters, with lower Apgar scores, lower neonatal birth weight, and higher preterm and higher IUGR rates in the PE group (Table 1).
Table 1.
Clinical characteristics of the study groups
| At-risk women without PE | At-risk women with PE | p-value | |
|---|---|---|---|
| n | 119 | 32 | |
| Maternal Characteristics | |||
| Gestational age at sample collection (wk) | 29 (8.5) | 30 (7.3) | 0.14 |
| Maternal age (yr) | 33 (7.5) | 33 (6.5) | 0.74 |
| Maternal weight pre-pregnancy (kg) | 68 (23.8) | 65.5 (16.5.) | 0.7 |
| Maternal weight at sample collection (kg) | 78 (22.8) | 80 (15.5) | 0.71 |
| Maternal BMI (kg/m2) | 24.16 (7.8) | 25.67 (5.5) | 0.96 |
| Systolic BP (mmHg) | 124 (19) | 160 (39) | < 0.001 |
| Days before delivery | 54 (72) | 4 (10.8) | < 0.001 |
| Ethnicity: Caucasian | 100 (84%) | 23 (72%) | 0.08 |
| Nulliparity | 64 (54%) | 20 (63%) | 0.5 |
| Co-morbidities | |||
| Pre-existing chronic renal disease | 7 (6%) | 3 (9%) | 0.69 |
| Pre-existing diabetes | 4 (3%) | 0 (0%) | 0.67 |
| Pre-existing hypertension | 21 (18%) | 9 (28%) | 0.5 |
| Gestational diabetes | 28 (24%) | 3 (9%) | 0.13 |
| Gestational hypertension | 15 (13%) | 6 (19%) | 0.03 |
| Delivery Outcomes | |||
| Gestational age at delivery (wk) | 38.71 (2.9) | 30.64 (8) | < 0.001 |
| Birth weight (g) | 2980 (12.1) | 1270 (1387.5) | < 0.001 |
| Apgar score 5 min | 9 (2) | 8 (2) | < 0.001 |
| Mode of delivery: C-section | 72 (61%) | 26 (81%) | 0.14 |
| Preterm < 34 weeks | 33 (28%) | 28 (88%) | < 0.001 |
| Small for Gestational Age (SGA) | 10 (8%) | 1 (3%) | 0.31 |
| Intrauterine growth restriction (IUGR) | 27 (23%) | 15 (47%) | 0.01 |
Data are displayed as median (IQR) or n (%). Continuous variables were compared via Wilcoxon rank sum test, categorical variables were compared using χ2 test
BMI body mass index, kg kilogram, PE preeclampsia, wk week, yr year
Biomarker performance
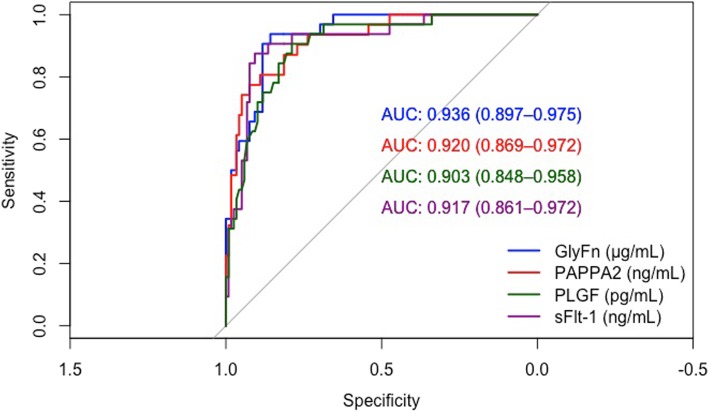
All analytes exhibited concentration differences between groups as shown in Table 2. The performance characteristics for prediction of PE within 4 weeks for all biomarkers are shown in Table 3. All biomarkers tested exhibited a high performance to rule-in or rule-out PE within 4 weeks of sampling [GlyFn AUC = 0.94 (95% CI, 0.90–0.97), PAPPA2 0.92 (95% CI, 0·88–0·96), PlGF 0.90 (95% CI, 0.84–0.95), and sFlt-1 0.93 (95% CI, 0.88–0.97)]. Figure 1 shows the ROC curves and associated AUCs of the biomarkers.
Table 2.
Biomarker serum levels
| At-risk women without PE | At-risk women with PE | p-value | |
|---|---|---|---|
| n | 119 | 32 | |
| Biomarker Levels | |||
| GlyFn (μg/mL) | 233 (92) | 457 (206) | < 0.001 |
| PAPPA2 (ng/mL) | 72 (130) | 505 (330) | < 0.001 |
| PlGF (pg/mL) | 275 (308) | 40 (57) | < 0.001 |
| sFlt-1 (ng/mL) | 2186 (2170) | 9869 (6613) | < 0.001 |
Data are displayed as median (IQR). Biomarker distributions are compared via Wilcoxon rank sum test
GlyFn glycosylated fibronectin, PAPPA2 pregnancy-associated plasma protein-A2, PlGF placental growth factor, sFlt-1 soluble fms-like tyrosine kinase-1
Table 3.
Performance characteristics of biomarkers for short-term prediction of PE
| Biomarker | AUC (95% CI) | Threshold | Sensitivity | Specificity |
|---|---|---|---|---|
| GlyFn (μg/mL) | 0.94 (0.90–0.98) | 315 | 91% | 86% |
| PAPP-A2 (ng/mL) | 0.92 (0.87–0.97) | 200 | 87% | 77% |
| PlGF (pg/mL) | 0.90 (0.85–0.96) | 100 | 81% | 83% |
| sFlt-1 (ng/mL) | 0.92 (0.86–0.97) | 7000 | 84% | 91% |
GlyFn glycosylated fibronectin, PAPPA2 pregnancy-associated plasma protein-A2, PlGF placental growth factor, sFlt-1 soluble fms-like tyrosine kinase-1
Fig. 1.
Receiver-operating characteristic curves showing the classification performance for each biomarker. AUC, area under the curve; GlyFn, glycosylated fibronectin; PAPPA2, pregnancy-associated plasma protein A2; PlGF, placental growth factor; sFlt-1, soluble fms-like tyrosine kinase-1
Performance of the Lumella™ POC test
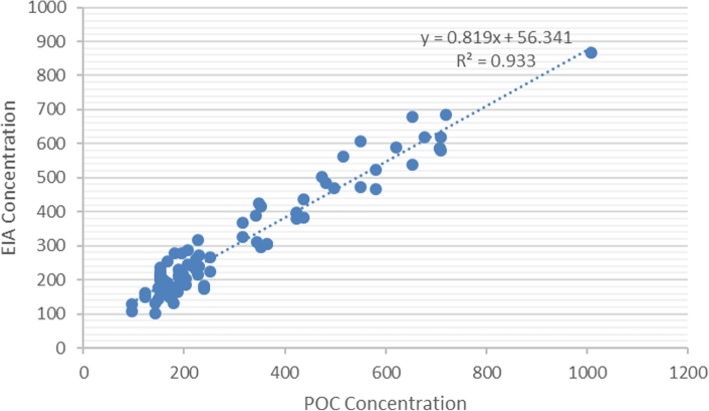
The GlyFn plate immunoassay and the Lumella™ POC test were compared with a subset of randomly selected samples (n = 25 controls and 25 cases) that ranged from 100 to 900 μg/mL (the dynamic range of the Lumella™ reader system). There was a correlation of r = 0.966 between the two assay formats (Fig. 2). The ROC curves generated for both methods were in similar range between the plate (AUC = 0.94, 95% CI = 0.90–0·97) and POC (AUC = 0.99, 95% CI = 0.96–1.0) assays.
Fig. 2.
Correlation between GlyFn plate-based immunoassay (EIA) and Lumella™ POC test
Discussion
Principal findings
The continuing revision of guidelines for prediction of PE [25] reflects the variation in clinical presentation, which makes accurate diagnosis based on a set of maternal signs and symptoms difficult in many cases. This uncertainty has focused attention in the last several years on maternal serum biomarkers as a potentially more consistent parameter for determining disease risk [26–30].Since 2010, the central focus of biomarker research has been on the diagnostic accuracy of commercially available immunoassays of the anti-angiogenic factor sFlt-1 and the pro-angiogenic factor PlGF and the sFlt-1/PlGF ratio. This study focused on the predictive value of a collection of previously described biomarkers in a large prospective observational cohort.
The biomarkers tested, GlyFn, PAPPA2, PlGF, and sFlt-1, all displayed good diagnostic performance for short-term (within 4 weeks) prediction of PE (AUROC of 0.90–0.94). Recent studies have focused on the investigation of pregnancies with signs and symptoms suggestive of PE, with the aim of identifying the development of PE within the subsequent 1–4 weeks. The Prediction of Short-Term Outcome in Pregnant Women with Suspected Preeclampsia study (PROGNOSIS) demonstrated that an sFlt-1/PlGF ratio < 38 exhibited a good NPV of 99.3% to rule out PE or HELLP within 1 week and that a ratio > 38 exhibited a PPV of 36.7% to rule in PE within 4 weeks [31]. Another prospective multicenter study reported an AUC of 0.87 for PlGF <5th percentile for the prediction of PE within 2 weeks [32]. The addition of systolic and diastolic blood pressure, uric acid, or alanine transaminase did not improve the diagnostic accuracy of PlGF alone. In comparison, GlyFn exhibited the best performance of the biomarkers tested in this study for prediction of PE within 4 weeks, with an AUC of 0.94, sensitivity of 91%, specificity of 86%. Additionally, the rapid GlyFn POC test, Lumella™, showed correlation of r = 0.966 with the standard plate assay in our study. The higher correlation and the AUC (0.99) for the Lumella™ assay is an improvement over these values for the earlier prototype (0.76 and 0.78, respectively) [18]. The GlyFn POC test may be of significant clinical utility for triage and intervention in low-resource settings or when the clinical diagnosis should be accurately and timely confirmed or excluded.
Strengths and weaknesses
This is the largest and the first prospective study to evaluate the recently identified biomarkers GlyFn and PAPPA2 and the previous biomarkers sFlt-1 and PlGF in the prediction of PE. We also describe an improved version of a POC test for GlyFn (Lumella™).
A potential weakness of this study is that the proposed thresholds for GlyFn, PAPPA2, PlGF, and sFlt-1 are only initial suggestions for the use of these biomarkers as a simple combined biomarker test. All biomarker levels may vary with gestational age [33] and ethnicity, and may depend on maternal weight, smoker status, fetal growth [34] and parity [35]. These simplified cut-off values should be validated in a different study population before the panel could be integrated into clinical practice.
Because of a limited sample size, we were not able to test the diagnostic accuracy of the biomarkers in pre-existing proteinuria without hypertension. However, recently published studies have shown that PE can be accurately assed in women with chronic renal disease or lupus nephritis using PlGF and sFlt-1 [36–38].
Additionally, the set of biomarkers evaluated at less than 37 weeks of gestation may be restricted to the subset of early-onset potential placental PE. Late-onset PE is more likely to have maternal predisposing risk factors like obesity, diabetes mellitus, hypertension, or metabolic syndrome and varying levels of placental dysfunction [39, 40]. The performance of these biomarkers in late-onset PE was not evaluated as part of this study but might be improved with addition of maternal characteristics.
Conclusion
Our results demonstrate that multiple biomarkers exhibit high performance in prediction of PE in the short term, and that GlyFn is adaptable to a POC format, joining the previously described POC test for PlGF [41].Therefore, we share the opinion of other researchers [26–30] that biomarkers should be incorporated into the definition of placental PE. A revised definition may reduce maternal and fetal mortality and morbidity as well as unnecessary healthcare usage. Additionally, the development of the GlyFn POC test may enable the extension of accurate, rapid, and inexpensive prediction of PE. It will be important to validate the performance of the GlyFn POC test in low and middle-income country settings and to evaluate its potential for detection of PE in early pregnancy and after 37 weeks of gestation.
Acknowledgements
The authors would like to thank our study midwives Cristina Granado and Doris Müller Borer in Basel and Antonina Chilin and Véronique Othenin-Girard in Geneva for data acquisition and management. Special thanks go to all women who participated in this study.
Ethical approval and consent to participate
The study protocol was approved by the Competent Ethics Committee of Northwestern Switzerland and Geneva (IRB approval numbers EKNZ PB_2016_02490 and GE 14–216) and conducted according to the principles of the Declaration of Helsinki. Written informed consent was obtained from all participants.
Abbreviations
- ACOG
American College of Obstetricians and Gynecologists
- AUC
Area under the (receiver-operating) curve
- BMI
Body mass index
- BP
Blood pressure
- CI
Confidence intervals
- c-section
Caesarean section
- DNA
Desoxyribonuclein acid
- EIA
Enzyme immunoassay
- GlyFn
Glycosylated fibronectin
- HELLP
Hemolysis, elevated liver enzymes and low platelets
- ISSHP
International Society for the Study of Hypertension in Pregnancy
- IUGR
Intrauterine growth restriction
- PAPPA2
Pregnancy-associated plasma protein-A2
- PE
Preeclampsia
- PlGF
Placental growth factor
- POC
Point of care
- PROGNOSIS
Pregnant Women with Suspected Preeclampsia study
- ROC
Receiver-operating characteristic
- SD
Standard deviation
- sFlt-1
Soluble fms-like tyrosine kinase-1
- SGA
Small for gestational age
- VEGF
Vascular endothelial growth factor
Authors’ contributions
EH drafted this manuscript together with SN. OL was principal investigator in Basel, study protocol author, obtained ethical approval, and revised the manuscript. BM was principal investigator in Geneva and revised the manuscript. IH and SL helped with recruitment, data acquisition and revised the manuscript. KS performed the statistical analysis. MG and CR made important contributions and critically reviewed the content. All authors have given final approval of the version to be published.
Funding
This study was supported in part by DiabetOmics, Inc. by performing the GlyFn analysis, providing the statistical assistance and drafting of the manuscript together with all other authors. The funding body did not influence the design of the study, recruitment and collection.
Availability of data and materials
The anonymised data supporting our results can be obtained on request to the corresponding author Dr. Huhn.
Consent for publication
Not applicable.
Competing interests
CR and SN are shareholders in, and KS and SN are employees of, DiabetOmics, Inc. OL has acted as a consultant for Roche Diagnostics and has a pending patent related to the dynamics of the sFlt-1 or endoglin: PlGF ratio as an indicator for the prediction of postpartum HELLP syndrome, postpartum eclampsia, or postpartum preeclampsia (PCT/EP2015/051457). The other authors report no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Evelyn A. Huhn and Ina Hoffmann contributed equally to this work.
Contributor Information
Evelyn A. Huhn, Email: evelyn.huhn@usb.ch
Ina Hoffmann, Email: inahoffmann83@gmail.com.
Begoña Martinez De Tejada, Email: Begona.MartinezDeTejada@hcuge.ch.
Soeren Lange, Email: soerenlange@me.com.
Kylie M. Sage, Email: sagky@ohsu.edu
Charles T. Roberts, Email: robertsc@diabetomics.com
Michael G. Gravett, Email: gravettm@uw.edu
Srinivasa R. Nagalla, Email: nagallas@diabetomics.com
Olav Lapaire, Email: olav.lapaire@usb.ch.
References
- 1.Duley L. The global impact of pre-eclampsia and Eclampsia. Semin Perinatol. 2009;33(3):130–137. doi: 10.1053/j.semperi.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 2.Lisonkova S, Sabr Y, Mayer C, Young C, Skoll A, Joseph KS. Maternal morbidity associated with early-onset and late-onset preeclampsia. Obstet Gynecol. 2014;124(4):771–781. doi: 10.1097/AOG.0000000000000472. [DOI] [PubMed] [Google Scholar]
- 3.Khan KS, Wojdyla D, Say L, Gülmezoglu AM, Van Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet. 2006;367(9516):1066–1074. doi: 10.1016/S0140-6736(06)68397-9. [DOI] [PubMed] [Google Scholar]
- 4.Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Heal. 2014;2(6):e323–e333. doi: 10.1016/S2214-109X(14)70227-X. [DOI] [PubMed] [Google Scholar]
- 5.Firoz T, Sanghvi H, Merialdi M, Von Dadelszen P. Pre-eclampsia in low and middle income countries. Best Pract Res Clin Obstet Gynaecol. 2011;25(4):537–548. doi: 10.1016/j.bpobgyn.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Hodgins S. Pre-eclampsia as underlying cause for perinatal deaths: time for action. Glob Heal Sci Pract. 2015;3(4):525–527. doi: 10.9745/GHSP-D-15-00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bramham K., Parnell B., Nelson-Piercy C., Seed P. T., Poston L., Chappell L. C. Chronic hypertension and pregnancy outcomes: systematic review and meta-analysis. BMJ. 2014;348(apr15 7):g2301–g2301. doi: 10.1136/bmj.g2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American College of Obstetricians and Gynecologists, Task Force on Hypertension in Pregnancy Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122–1131. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 9.Tranquilli AL, Dekker G, Magee L, et al. The classification, diagnosis and management of the hypertensive disorders of pregnancy: a revised statement from the ISSHP. Pregnancy Hypertens. 2014;4(2):97–104. doi: 10.1016/j.preghy.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Magee LA, Pels A, Helewa M, Rey E, von Dadelszen P, SOGC Hypertension guideline committee. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy: executive summary. J Obstet Gynaecol Can. 2014;36(7):575–576. doi: 10.1016/S1701-2163(15)30533-8. [DOI] [PubMed] [Google Scholar]
- 11.Brown MA, Hague WM, Higgins J, et al. The detection, investigation and management of hypertension in pregnancy: executive summary. Aust New Zeal J Obstet Gynaecol. 2000;40(2):133–138. doi: 10.1111/j.1479-828X.2000.tb01136.x. [DOI] [PubMed] [Google Scholar]
- 12.Redman C.W.G., Denson K.W.E., Beilin L.J., Bolton F.G., Stirrat G.M. FACTOR-VIII CONSUMPTION IN PRE-ECLAMPSIA. The Lancet. 1977;310(8051):1249–1252. doi: 10.1016/S0140-6736(77)92661-7. [DOI] [PubMed] [Google Scholar]
- 13.Mol BWJ, Roberts CT, Thangaratinam S, Magee LA, de Groot CJM, Hofmeyr GJ. Pre-eclampsia. Lancet (London, England) 2016;387(10022):999–1011. doi: 10.1016/S0140-6736(15)00070-7. [DOI] [PubMed] [Google Scholar]
- 14.Levine RJ, Maynard SE, Qian C, et al. Circulating Angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350(7):672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 15.Macintire K, Tuohey L, Ye L, et al. PAPPA2 is increased in severe early onset pre-eclampsia and upregulated with hypoxia. Reprod Fertil Dev. 2014;26(2):351–357. doi: 10.1071/RD12384. [DOI] [PubMed] [Google Scholar]
- 16.Santillan Mark K., Santillan Donna A., Scroggins Sabrina M., Min James Y., Sandgren Jeremy A., Pearson Nicole A., Leslie Kimberly K., Hunter Stephen K., Zamba Gideon K.D., Gibson-Corley Katherine N., Grobe Justin L. Vasopressin in Preeclampsia. Hypertension. 2014;64(4):852–859. doi: 10.1161/HYPERTENSIONAHA.114.03848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yeung Edwina H., Liu Aiyi, Mills James L., Zhang Cuilin, Männistö Tuija, Lu Zhaohui, Tsai Michael Y., Mendola Pauline. Increased Levels of Copeptin Before Clinical Diagnosis of Preeclampsia. Hypertension. 2014;64(6):1362–1367. doi: 10.1161/HYPERTENSIONAHA.114.03762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rasanen JP, Snyder CK, Rao PV, et al. Glycosylated Fibronectin as a first-trimester biomarker for prediction of gestational diabetes. Obstet Gynecol. 2013;122(3):586–594. doi: 10.1097/AOG.0b013e3182a0c88b. [DOI] [PubMed] [Google Scholar]
- 19.Huhn EA, Kreienbühl A, Hoffmann I, et al. Diagnostic accuracy of different soluble fms-like tyrosine Kinase 1 and placental growth factor cut-off values in the assessment of preterm and term preeclampsia: A gestational age matched case-control study. Front Med. 2018;5(NOV):325. doi: 10.3389/fmed.2018.00325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Voigt M, Rochow N, Guthmann F, Hesse V, Schneider KTM, Schnabel D. Birth weight percentile values for girls and boys under consideration of maternal height. Z Geburtshilfe Neonatol. 2012;216(5):212–219. doi: 10.1055/s-0032-1316324. [DOI] [PubMed] [Google Scholar]
- 21.Gratacós E, Figueras F. Fetal growth restriction as a perinatal and long-term health problem: clinical challenges and opportunities for future (4P) fetal medicine. Fetal Diagn Ther. 2014;36(2):85. doi: 10.1159/000365556. [DOI] [PubMed] [Google Scholar]
- 22.Rasanen J, Quinn MJ, Laurie A, et al. Maternal serum glycosylated fibronectin as a point-of-care biomarker for assessment of preeclampsia. Am J Obstet Gynecol. 2015;212(1):e1–e9. doi: 10.1016/j.ajog.2014.07.052. [DOI] [PubMed] [Google Scholar]
- 23.Dunn OJ. Multiple comparisons using rank sums. Technometrics. 1964;6:241–252. doi: 10.1080/00401706.1964.10490181. [DOI] [Google Scholar]
- 24.Robin X, Turck N, Hainard A, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011;12. 10.1186/1471-2105-12-77. [DOI] [PMC free article] [PubMed]
- 25.Phipps E, Prasanna D, Brima W, Jim B. Preeclampsia: updates in pathogenesis, definitions, and guidelines. Clin J Am Soc Nephrol. 2016;11(6):1102–1113. doi: 10.2215/CJN.12081115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monte S. Biochemical markers for prediction of preclampsia: review of the literature. J Prenat Med 2011;5(3):69–77. http://www.ncbi.nlm.nih.gov/pubmed/22439080. Accessed October 25, 2019. [PMC free article] [PubMed]
- 27.Staff Anne Cathrine, Benton Samantha J., von Dadelszen Peter, Roberts James M., Taylor Robert N., Powers Robert W., Charnock-Jones D. Stephen, Redman Christopher W.G. Redefining Preeclampsia Using Placenta-Derived Biomarkers. Hypertension. 2013;61(5):932–942. doi: 10.1161/HYPERTENSIONAHA.111.00250. [DOI] [PubMed] [Google Scholar]
- 28.Poon LC, Nicolaides KH. First-trimester maternal factors and biomarker screening for preeclampsia. Prenat Diagn. 2014;34(7):618–627. doi: 10.1002/pd.4397. [DOI] [PubMed] [Google Scholar]
- 29.Kar M. Role of biomarkers in early detection of preeclampsia. J Clin Diagn Res. 2014;8(4):BE01–BE04. 10.7860/JCDR/2014/7969.4261. [DOI] [PMC free article] [PubMed]
- 30.Wu P, Van Den Berg C, Alfirevic Z, et al. Early pregnancy biomarkers in pre-eclampsia: a systematic review and meta-analysis. Int J Mol Sci. 2015;16(9):23035–23056. doi: 10.3390/ijms160923035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zeisler H, Llurba E, Chantraine F, et al. Predictive value of the sFlt-1:PlGF ratio in women with suspected preeclampsia. N Engl J Med. 2016;374(1):13–22. doi: 10.1056/NEJMoa1414838. [DOI] [PubMed] [Google Scholar]
- 32.Chappell Lucy C., Duckworth Suzy, Seed Paul T., Griffin Melanie, Myers Jenny, Mackillop Lucy, Simpson Nigel, Waugh Jason, Anumba Dilly, Kenny Louise C., Redman Christopher W.G., Shennan Andrew H. Diagnostic Accuracy of Placental Growth Factor in Women With Suspected Preeclampsia. Circulation. 2013;128(19):2121–2131. doi: 10.1161/CIRCULATIONAHA.113.003215. [DOI] [PubMed] [Google Scholar]
- 33.Sovio Ulla, Gaccioli Francesca, Cook Emma, Hund Martin, Charnock-Jones D. Stephen, Smith Gordon C.S. Prediction of Preeclampsia Using the Soluble fms-Like Tyrosine Kinase 1 to Placental Growth Factor Ratio. Hypertension. 2017;69(4):731–738. doi: 10.1161/HYPERTENSIONAHA.116.08620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gaccioli F, Sovio U, Cook E, Hund M, Charnock-Jones DS, Smith GCS. Screening for fetal growth restriction using ultrasound and the sFLT1/PlGF ratio in nulliparous women: a prospective cohort study. Lancet Child Adolesc Heal. 2018;2(8):569–581. doi: 10.1016/S2352-4642(18)30129-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dragan I, Georgiou T, Prodan N, Akolekar R, Nicolaides KH. Screening for pre-eclampsia using sFlt-1/PlGF ratio cut-off of 38 at 30–37 weeks’ gestation. Ultrasound Obstet Gynecol. 2017;49(1):73–77. doi: 10.1002/uog.17301. [DOI] [PubMed] [Google Scholar]
- 36.Rolfo A, Attini R, Nuzzo AM, et al. Chronic kidney disease may be differentially diagnosed from preeclampsia by serum biomarkers. Kidney Int. 2013;83(1):177–181. doi: 10.1038/ki.2012.348. [DOI] [PubMed] [Google Scholar]
- 37.Masuyama H, Nobumoto E, Okimoto N, Inoue S, Segawa T, Hiramatsu Y. Superimposed preeclampsia in women with chronic kidney disease. Gynecol Obstet Investig. 2012;74(4):274–281. doi: 10.1159/000339935. [DOI] [PubMed] [Google Scholar]
- 38.de Jesus GR, de Jesus NR, Levy RA, Klumb EM. The use of angiogenic and antiangiogenic factors in the differential diagnosis of pre-eclampsia, antiphospholipid syndrome nephropathy and lupus nephritis. Lupus. 2014;23(12):1299–1301. doi: 10.1177/0961203314529172. [DOI] [PubMed] [Google Scholar]
- 39.Van Der Merwe JL, Hall DR, Wright C, Schubert P, Grové D. Are early and late preeclampsia distinct subclasses of the diseasewhat does the placenta reveal. Hypertens Pregnancy. 2010;29(4):457–467. doi: 10.3109/10641950903572282. [DOI] [PubMed] [Google Scholar]
- 40.von Dadelszen P, Magee LA, Roberts JM. Subclassification of preeclampsia. Hypertens Pregnancy. 2003;22(2):143–148. doi: 10.1081/PRG-120021060. [DOI] [PubMed] [Google Scholar]
- 41.Knudsen UB, Kronborg CS, von Dadelszen P, et al. A single rapid point-of-care placental growth factor determination as an aid in the diagnosis of preeclampsia. Pregnancy Hypertens. 2012;2(1):8–15. doi: 10.1016/j.preghy.2011.08.117. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The anonymised data supporting our results can be obtained on request to the corresponding author Dr. Huhn.