Abstract
Background
The optimal treatment for patients with high-risk prostate cancer (PCa) remains a debate and selection of patients to receive proper therapy is still an unsettled question. This systematic review was conducted to compare the effectiveness of prostatectomy (RP) and radiotherapy (RT) in patients with high-risk PCa and to select candidates for optimal treatment.
Methods
PubMed, EMBASE, and Cochrane Central Register of Controlled Trials were searched for eligible studies. We extracted hazard ratios (HRs) and 95% confidence interval (CI) of all included studies. The primary outcomes were overall survival (OS) and cancer-specific survival (CSS); the secondary outcomes were biochemical recurrence-free survival (BRFS), metastasis-free survival (MFS) and clinical recurrence-free survival (CRFS). The meta-analysis was performed using Review Manager 5.3. Subgroup analyses were conducted according to Gleason score (GS), T stage and RT types. Quality of life (QoL) was compared with these two treatments.
Results
A total of 25 studies were included in this meta-analysis. Overall, RP showed more survival benefits than RT on CSS (P = 0.003) and OS (P = 0.002); while RT was associated with better BRFS (P = 0.002) and MFS (P = 0.004). Subgroup analyses showed RT was associated with similar or even better survival outcomes compared to RP in patients with high GS, high T stage or received external beam radiotherapy plus brachytherapy (EBRT + BT). As for QoL, RP was associated with poorer urinary and sexual function but better performance in the bowel domain.
Conclusion
RP could prolong the survival time of patients with high-risk PCa; however, RT could delay the disease progression, and combined RT (EBRT + BT) even brought preferable CSS and similar OS compared to RP. RT might be the prior choice for patients with high T stage or high GS. RP could lead to poorer urinary and sexual function, while bringing better performance in the bowel domain.
Keywords: High-risk prostate cancer, Radical prostatectomy, Radiotherapy
Background
About 127,106 patients worldwide are diagnosed with prostate cancer (PCa) annually, accounting for 7.1% of all cancers diagnosed [1]; and it is the most common malignant tumor in the USA [2]. Among men diagnosed with PCa, approximately 20%–30% of patients are grouped as high-risk PCa [3], which is more likely to progress and relapse [4]. To date, radiotherapy (RT) plus androgen deprivation therapy (ADT) has still been the standard treatment for high-risk PCa. In several randomized controlled trials (RCT), RT plus ADT showed better survival benefit than single treatment (RT or ADT alone) [5–10]. Although the level of evidence is low, increasing population-based evidence in recent years has suggested that radical prostatectomy (RP) could provide similar or better survival benefit than RT-based systemic therapy [11–15].
Now, both RT and RP are recommended by current guidelines for patients with high-risk PCa [16]. However, as no large RCT has directly compared the two treatments in high-risk PCa settings, the optimal treatment for this population remains a debate, and selection of patients to receive proper therapy is still an unsettled question. Previous meta-analyses have tried to compare the efficacy of RP and RT in patients with high-risk PCa [17, 18]; however, they failed to perform detailed subgroup analyses for patients with high-risk PCa, such as when patients had different levels of Gleason score (GS) and T stage, or when patients received different types of RT. In fact, it is also unclear whether these differences would affect the comparison between RP and RT. Additionally, limited information was provided by these previous meta-analyses due to inappropriate statistical methods and rough analyses.
Thus, with increasing literature on this topic, we updated this systematic review and meta-analysis to compare the effectiveness of RP and RT in patients with high-risk PCa and select candidates for optimal treatment.
Materials and methods
Protocol and searching strategy
This meta-analysis was conducted according to the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines [19]. A protocol was developed to define the search strategy and the review was registered on the PROSPERO of the Centre for Review and Dissemination (CRD42019132967). EMBASE (1947 to July 2019), PubMed (1966 to July 2019), and the Cochrane Library database (1948 to July 2019) were searched for relevant studies. We also searched relevant journals and reviews for additional articles. Detailed search strategies and keywords can be found in the protocol.
Inclusion and exclusion criteria
Inclusion criteria included (a) patients with high-risk PCa: National Comprehensive Cancer Network (NCCN) criteria (≥ T3 or GS 8–10 or PSA > 20), D’Amico criteria (≥ T2c or GS 8–10 or PSA > 20) or the other criteria; (b) patients who received RP or RT as primary treatment, and RT including external beam radiotherapy (EBRT), brachytherapy (BT) or combined RT (EBRT+BT); (c) articles which reported survival outcomes or disease control using hazard ratios (HRs) to present the results of comparison, or articles which reported quality of life (QoL); and (d) studies published in English.
Exclusion criteria include (a) patients with metastatic disease; (b) patients with any disease incompatible with the planned treatment; (c) review, editorial or case report; and (d) studies published not in English.
Study selection and data extraction
Two researchers (W.Z.P. and N.Y.C.) reviewed titles, abstracts, and then full texts to determine the final included studies. The two reviewers independently collected and checked the data from those included studies. For each included study, we extracted information on the first author, publication year, median age, sample size, study design, characteristics of high-risk PCa, comparison of treatments, median follow-up, RT dose, and end-points. Any disagreements or discrepancies were resolved by consultation with a third researcher (C.J.R.).
Quality assessment and publication bias
Two researchers (W.Z.P. and N.Y.C.) independently evaluated the methodological quality of the included cohort studies according to the Newcastle-Ottawa Scale (NOS) [20]; scores ≥ 7 points were considered as high quality. Patient selection, comparability, and outcomes were assessed to evaluate the quality. RCT was evaluated according to the criteria outlined in chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions. Publication bias was evaluated by a funnel plot.
Outcomes
The primary outcomes were survival outcomes, including cancer-specific survival (CSS) and overall survival (OS). CSS was defined as the time from RP/RT until death from PCa. OS was defined as the time from RP/RT until death from any cause.
The secondary outcomes were disease control, including biochemical recurrence-free survival (BRFS), metastasis-free survival (MFS), and clinical recurrence-free survival (CRFS). BRFS was defined as the time from RP/RT until biochemical failure. MFS was defined as the time from RP/RT to metastasis. CRFS was defined as the time from RP/RT until metastasis identified via imaging or biopsy-proven local recurrence. And we chose urinary, sexual, and bowel function as the main evaluation indicators of QoL.
Statistical analysis
The meta-analysis was conducted using Review Manager 5.3 software. The hazard ratio (HR) and corresponding 95% confidence interval (95% CI) were extracted directly from the study reports. If insufficient data were available, supplementary data might be sought directly from the investigators of studies. A fixed-effect model or random-effect model was used for analyses based on heterogeneity among studies. We used the Chi-square and the I-square tests to assess the heterogeneity among the studies. Chi-squared with a P < 0.10 or I-square > 50% was considered as significant heterogeneity. Subgroup analyses were performed according to RT types, GS, and clinical T stage. What we need to pay attention to is that we did not conduct a meta-analysis with secondary outcomes in some subgroups since we were unable to extract enough data from these studies.
Results
Study and patient characteristics
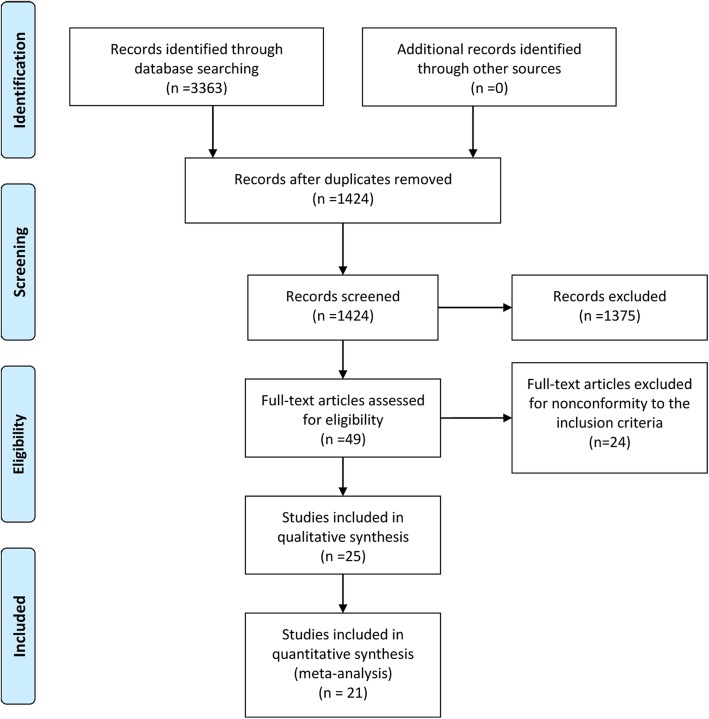
Three thousand three hundred sixty three records were identified, and 25 studies were finally included in this systematic review and meta-analysis [11–15, 21–40]. Due to a lack of data, 4 studies cannot be meta-analyzed, so we only presented the results of QoL in these studies. The flowchart of study inclusion was shown in Fig. 1. In total, there were 21 retrospective studies, 3 prospective studies, 1 RCT study published between 2006 and 2019. The median age was 58.2–71.8 years for the RP group and 58.1–75.0 years for the RT group. The median follow-up ranged from 23.89 months to 15 years. The characteristics of all included studies were shown in Table 1. Although the definitions of “high-risk” varied in each trial, most of them were consistent with the NCCN or D’Amico criteria. Detailed comparison data can be found in Additional file 1: Table S1.
Fig. 1.
Flowchart of literature searches
Table 1.
Characteristics of included studies (N = 25)
| Study ID/date | Study design | Time | Definition of high-risk PCa | Median age (RP vs RT) | Sample (n) | Comparison of treatments | Median follow-up | End-points |
|---|---|---|---|---|---|---|---|---|
| Jayadevappa 2019 | R. cohort | 1996–2003 | GS ≥ 8 or T ≥ T2c | NA | 6296 | RP vs EBRT vs EBRT + BT | 10 years | OS/CSS |
| Reichard 2019 | R. cohort | 2004–2013 | NCCN | 66 vs 61 | 304 | RP vs RT | 82.9 months | OS/MFS |
| Caño-Velasco 2019 | R. cohort | 1996–2008 | EAU | 65 vs 71 | 286 | RP vs EBRT |
RP: 152 months RT: 97 months |
CSS/OS |
| Muralidhar 2019 | R. cohort | 2004–2012 | GS: 9–10 | NA |
4367 2276 |
RP+aRT vs EBRT+BT |
6.0 years (NCDB) 5.8 years (SEER) |
OS |
| Berg 2019 | R. cohort | 2004–2015 | NCCN | 58.15 vs 58.12 | 13985 | RP vs EBRT+BT |
RP: 91 months EBRT+BT: 101 months |
OS |
| Tilki 2019 | R. cohort | 1992–2013 | GS: 9–10 |
MaxRT: 70.34 RP: 66. 40 RP+aRT:66.64 MaxRP: 66.04 RP+ADT: 66.38 |
639 |
RP vs RP+aRT vs MaxRP vs RP+ADT vs MaxRT |
MaxRT: 5.51 years RP: 4.89 years RP + aRT: 3.87 years MaxRP: 4.88 years RP + ADT: 4.65 years |
CSS/OS |
| Jang 2018 | R. cohort | 1992–2009 | T ≥ T3 | NA | 13856 | RP+aRT vs RT | 14.6 years | CSS/OS |
| Tyson 2018 | P. cohort | 2011–2012 | D’Amico | 65 | 2117 | RP vs EBRT | 3 years | QoL |
| Ennis 2018 | R. cohort | 2004–2013 | NCCN |
RP vs EBRT vs EBRT+BT: 62.61 vs 69.66 vs 67.15 |
42765 | RP vs EBRT vs EBRT+BT | 36.34 months | OS |
| Gu 2018 | R. cohort | 2004–2008 | NCCN | 66 | 7656 | RP vs EBRT | NA | CSS/OS |
| Markovina 2018 | R. cohort | 2002–2011 | NCCN | 62.9 vs 64.2 | 246 | RP vs EBRT |
RP: 41 months RT: 51.4 months |
OS/MFS |
| Kishan 2018 | R. cohort | 2000–2013 | GS: 9–10 |
RP vs EBRT vs EBRT+BT: 61 vs 67.7 vs 67.5 |
1809 |
RP vs EBRT vs EBRT+BT 61 vs 67.7 vs 67.5 |
RP: 4.2 years EBRT: 5.1 years EBRT + BT: 6.3 years | CSS/OS/MFS |
| Robinson 2018 | R. cohort | 1998–2012 | NCCN | 63.1 vs 67 | 41953 | RP vs RT |
RP: 7.3 years RT: 6.9 years |
CSS |
| Feldman 2017 | R. cohort | 1992–2009 | T3 | 71.76 vs 71.75 | 2935 | RP vs EBRT |
RP: 11.47 years EBRT: 7.04 years |
OS/CSS/QoL |
| Ciezki 2017 | P. cohort | 1996–2012 | NCCN |
LDRBT vs EBRT vs RP: 70 vs 68.5 vs 62 |
2557 | LDRBT vs EBRT vs RP |
LDRBT: 48.9 months EBRT: 94.6 months RP: 55.6 months |
BRFS/CSS/QoL |
| Yamamoto 2015 | P. cohort | 2006–2010 | NCCN | 67 vs 71 | 150 | RP vs EBRT | 2 years | QoL |
| Sun 2014 | R. cohort | 1992–2005 | T2c | 70 vs 73 | 67087 | RP vs RT vs WW | NA | OS/CSS |
| Hoffman 2013 | R. cohort | 1994–1995 | PSA > 10 or GS 8–10 | NA | 1655 | RP vs EBRT | 15 years | OS/CSS |
| Kibel 2012 | R. cohort | 1995–2005 | D’Amico |
RP vs EBRT vs BT: 60 vs 69 vs 68(Clinic 1) 61 vs 70 vs 69(Clinic 2) |
10429 | RP vs EBRT vs BT | 67 months | OS/CSS |
| Westover 2012 | R. cohort | 1988–2009 | D’Amico | 65 vs 70 | 657 | RP vs EBRT+BT |
RP: 7.6 years EBRT + BT: 3.6 years |
CSS |
| Boorjian 2011 | R. cohort | 1988–2004 | NCCN |
RP: 66 EBRT+ADT: 68.8 EBRT: 69.3 |
1847 | RP vs EBRT+ADT vs EBRT |
RP: 10.2 years EBRT + ADT: 6.0 years EBRT: 7.3 years |
OS/CSS |
| Aizer 2009 | R. cohort | 1997–2005 | MSK/NCCN | NA | 556 | RP vs EBRT |
RP: 46 months EBRT: 40 months |
BRFS |
| Takizawa 2009 | R. cohort | 1998–2004 | NCCN | 64.9 vs 71.1 | 162 | RP vs EBRT | 41 months | OS/BRFS/QoL |
| Arcangeli 2009 | R. cohort | 2003–2007 | NCCN | 65.5 vs 75 | 284 | RP vs EBRT | RP: 33.8 months EBRT: 38.6 months | BRFS |
| Akakura 2006 | RCT | 1989–1993 | T2b-3N0M0 | 68.1 vs 68.7 | 95 | RP vs EBRT | 102 months | BRFS/CSS/OS/QoL |
RP prostatectomy, RT radiotherapy, EBRT external beam radiation therapy, BT brachytherapy, ADT androgen deprivation therapy, aRT adjuvant radiotherapy, WW watchful waiting, MaxRP RP + aRT + ADT, MaxRT EBRT + BT + ADT, LDRBT low-dose-rate brachytherapy, PSA prostate-specific antigen, GS Gleason score, NCCN National Comprehensive Cancer Network, MSK Memorial Sloan Kettering, QoL quality of life, OS overall survival, CSS cancer-specific survival, BRFS biochemical recurrence-free survival, MFS metastasis-free survival, CRF clinical recurrence-free survival, R. cohort retrospective cohort, P. cohort prospective cohort, P. and R. cohort prospective and retrospective cohort, RCT randomized controlled trial, vs versus, NA not available
Quality assessment and publication bias
Quality assessment was shown in Additional file 2: Table S2. The only 1 RCT was evaluated as high-risk of bias. Twenty three cohort studies were evaluated as high quality (scores 7–9) and 1 cohort study was evaluated as median quality (score 6). A funnel plot was used to evaluate publication bias. As shown in Additional file 3: Figure S1, relative symmetry could be found in the plot, which indicated that there was no obvious publication bias.
Effect of RP versus RT in all patients with high-risk PCa
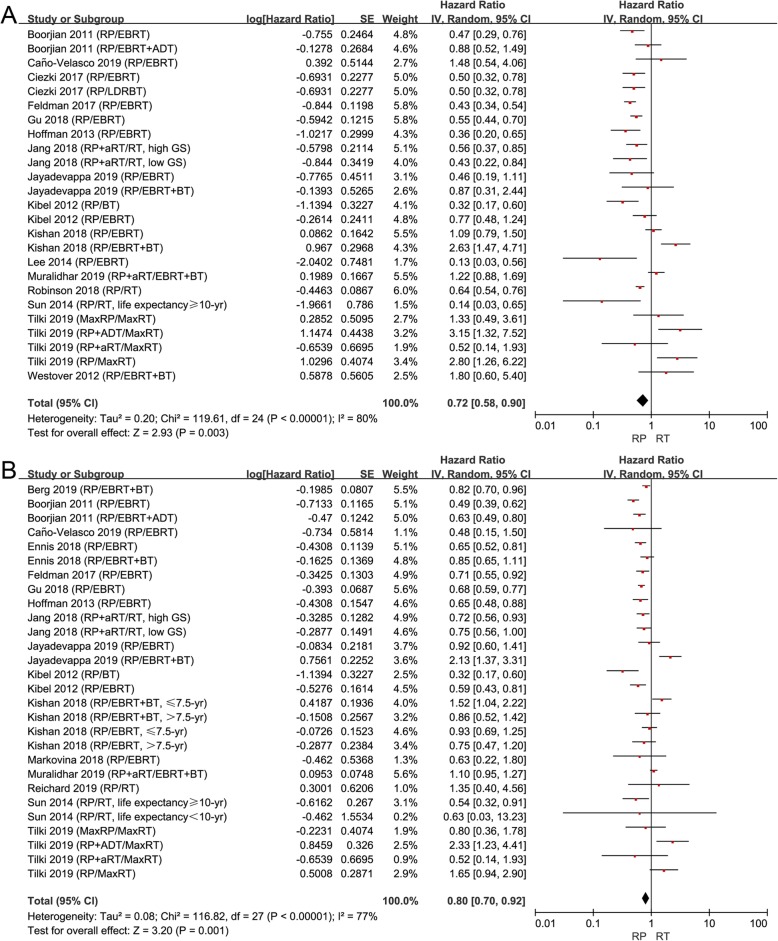
From our results, HRs of CSS and OS were reported in 16 studies, respectively. RP showed more survival benefits than RT on CSS (HR 0.72, 95% CI 0.58–0.90, P = 0.003, I2 = 80%; Fig. 2a) and OS (HR 0.80, 95% CI 0.70–0.92, P = 0.002, I2 = 77%; Fig. 2b) for patients with high-risk PCa.
Fig. 2.
a Forest plot of HR for CSS following RP and RT; (b) forest plot of HR for OS following RP and RT
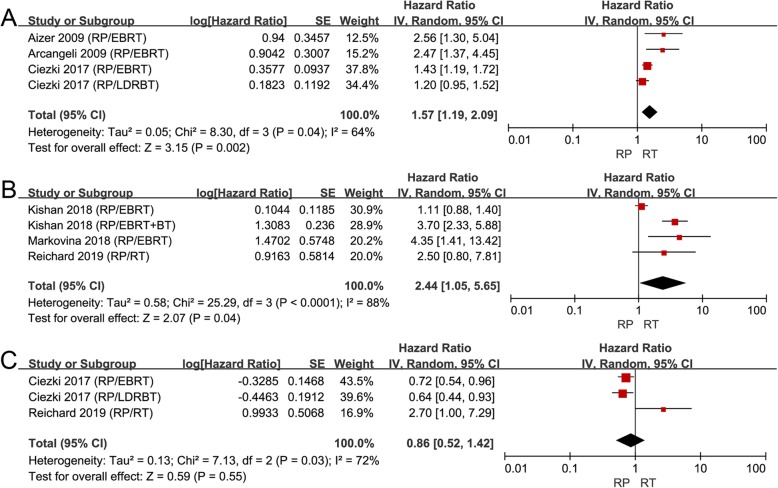
Although no significant difference was found between RP and RT on CRFS (HR 0.86, 95% CI 0.52–1.42, P = 0.55, I2= 72%; Fig. 3c), patients treated with RP had a worse outcome of BRFS (HR 1.57, 95% CI 1.19–2.09, P = 0.002, I2 = 64%; Fig. 3a) and MFS (HR 2.44, 95% CI 1.05–5.65, P = 0.04, I2 = 88%; Fig. 3b). Taken together, these results suggested that RT could bring better biochemical and metastasis control than RP, although RP could prolong the OS and CSS of these patients.
Fig. 3.
a Forest plot of HR for BRFS following RP and RT; (b) forest plot of HR for MFS following RP and RT; (c) forest plot of HR for CRFS following RP and RT
Effect of RP versus RT in high GS subgroup
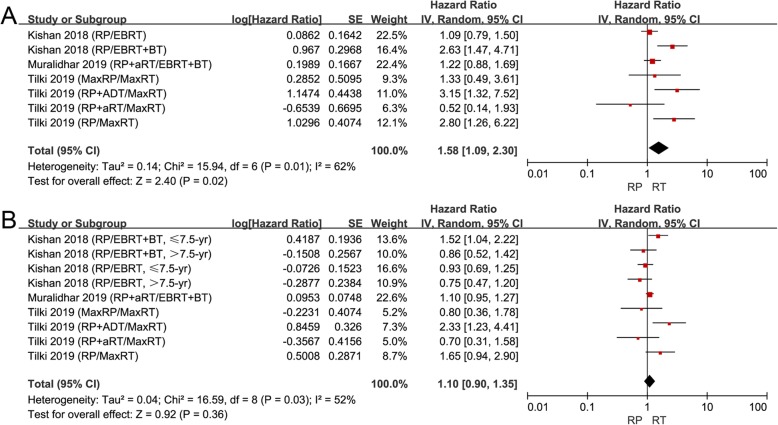
Some studies reported that PCa patients with GS 9–10 had a particularly aggressive disease [41, 42]. So, we conducted a subgroup analysis for patients with high GS 9–10. Only 3 articles separately compared RP to RT for patients with high GS 9–10 [34, 36, 40]. RT was associated with improved CSS (HR 1.58, 95% CI 1.09–2.30, P = 0.02, I2 = 62%; Fig. 4a) and similar OS (HR 1.10, 95% CI 0.90–1.35, P = 0.36, I2 = 52%; Fig. 4b) compared to RP in patients with high GS. RT seemed to have similar or even better survival benefit than RP for these patients. As for other outcomes, only Kishan and colleagues reported that no significant difference was found between RP and EBRT on MFS, while EBRT + BT was associated with longer time to distant metastasis compared to RP [36].
Fig. 4.
a Forest plot of HR for CSS following RP and RT in the “high GS” subgroup; (b) Forest plot of HR for OS following RP and RT in the “high GS” subgroup. High GS was defined as GS: 9-10
Effect of RP versus RT in different T stage subgroups
Subgroup analysis was conducted according to the T stage. Due to data limitations, we cannot directly compare the data with complete high T stage and data with complete low T stage. We can only separate two relatively high and low T stage subgroups according to the ratio of different T stages. Then we selected 60% as the cut-off point based on the characteristics of the included studies. Low T stage subgroup was defined as studies that included > 60% patients with ≤ T2 stage and high T stage subgroup was defined as studies that included < 60% patients with ≤ T2 stage.
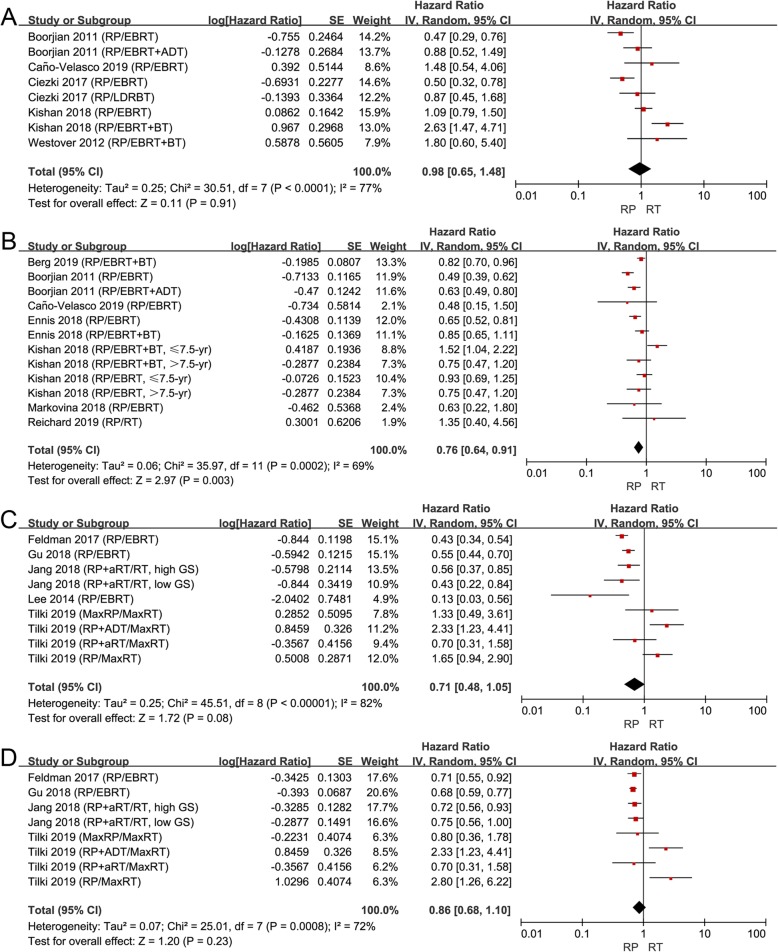
Finally, 9 and 5 studies were respectively grouped into the “low T stage” subgroup and "high T stage” subgroup. In the “low T stage” subgroup, no significant difference was found among patients treated with RP or RT on CSS (Fig. 5a), while RP did extend the OS (HR 0.76, 95% CI 0.64–0.91, P = 0.003, I2 = 69%; Fig. 5b). However, we found that RT brought a similar survival benefit compared to RP on CSS (Fig. 5c) and OS (Fig. 5d) in the “high T stage” subgroup. With the increase in the T stage, it seemed that RT had better survival benefits.
Fig. 5.
a Forest plot of HR for CSS following RP and RT in the “low T stage” subgroup; (b) forest plot of HR for CSS following RP and RT in the “low T stage” subgroup; (c) forest plot of HR for CSS following RP and RT in “high T stage” subgroup; (d) forest plot of HR for OS following RP and RT in the “high T stage” subgroup. “Low T stage” group was defined as studies that included >60% patients with ≤T2 stage; “high T stage” group was defined as studies that included <60% patients with ≤T2 stage
Subgroup analysis according to RT types
Patients might receive different types of RT (EBRT or EBRT+BT) in different centers, so we performed a subgroup analysis according to the types of RT. Since the types of RT were not described in detail in some studies, we only included these studies which exactly reported that patients received EBRT or EBRT+BT in different subgroups.
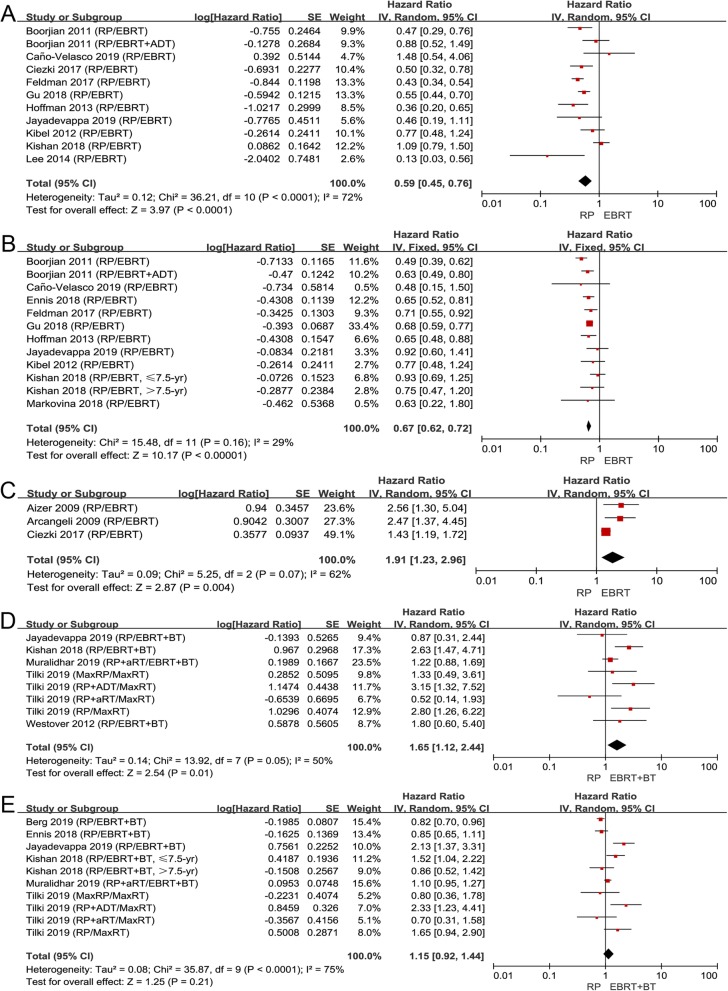
There were 14 articles comparing RP to EBRT and 7 articles comparing RP to EBRT + BT. We separately analyzed the data comparing patients who received EBRT or EBRT + BT to those who received RP. Patients treated with RP had better survival outcomes than EBRT on CSS (HR 0.59, 95% CI 0.45–0.76, P < 0.0001, I2 = 72%; Fig. 6a) and OS (HR 0.67, 95% CI 0.62–0.72, P < 0.00001, I2 = 29%; Fig. 6b). Although RP brought better survival benefits, EBRT was associated with better biochemical control than RP (HR 1.91, 95% CI 1.23–2.96, P = 0.004, I2 = 62%; Fig. 6c), which was consistent with the overall comparison between RP and RT.
Fig. 6.
a Forest plot of HR for CSS following RP and EBRT; (b) forest plot of HR for OS following RP and EBRT; (c) forest plot of HR for BRFS following RP and EBRT; (d) forest plot of HR for CSS following RP and EBRT+BT; (e) forest plot of HR for OS following RP and EBRT+BT
It seemed that EBRT+BT had similar or even better survival benefit than RP. RP showed obvious inferiority on CSS (HR 1.65, 95% CI 1.12–2.44, P = 0.01, I2 = 50%; Fig. 6d) compared to EBRT + BT, and no significant difference between the two subgroups was observed on OS (HR 1.15, 95% CI 0.92–1.44, P = 0.21, I2 = 75%; Fig. 6e). As for other outcomes, Kishan and colleagues reported EBRT + BT was associated with longer MFS than RP [36].
Overall, most patients received EBRT in the included studies. Although RP was more beneficial to survival than EBRT, it was not as good as EBRT in PSA control, and the survival benefit of RP would no longer exist once EBRT was combined with BT.
Health-related quality of life
Owing to the limited information available from studies, meta-analysis about the QoL of high-risk PCa patients who received RP/RT was not performed. The QoL was described in 6 studies. Additional file 2: Table S2 showed the characteristics of the included studies which reported QoL. Five studies demonstrated that RT performed better in urinary function. Only Takizawa reported there was no difference between RP and RT on urinary function in the high-risk group (P = 0.05) [23]. Three studies showed patients treated with RT had better sexual function than those treated with RP. Two studies reported no significant difference between RP and RT groups about sexual function. Four studies reported no difference between RP and RT on bowel function; the other 2 studies reported that patients in the RP group had a significantly lower risk of suffering from bowel toxicities. It seemed that RP had better performance on the bowel domain, while RT was associated with better QoL in urinary and sexual domains.
Discussion
Currently, both RT and RP are first-line treatments for clinically high-risk PCa patients, and the optimal treatment remains a debate. One small RCT has compared the survival outcomes for patients with T2b-3N0M0 PCa treated with surgery or radiotherapy [21]. Except for 2 reviews and meta-analysis focused on localized PCa [43, 44], there were two meta-analyses about high-risk prostate cancer published in 2014 and 2015 [17, 18], while limited information was provided due to inappropriate statistical methods and rough analyses. Petrelli and colleagues reported a meta-analysis comparing the efficacy of RP and RT in patients with high-risk PCa and demonstrated the superiority of RP [17]. However, Petrelli and colleagues used odds ratios to present the comparison results, which inevitably ignored the time-to-event outcomes. More recently, a meta-analysis was conducted by Lei and colleagues; they reported that RP brought lower CSM than RT [18], while this meta-analysis was conducted only based on 3 studies. It was particularly noteworthy that none of these previous meta-analyses did a subgroup analysis according to T stage, GS, or RT types, and thus, no detailed data were available for clinicians to optimize treatment strategies.
In the present study, the most up-to-date data were comprehensively analyzed and we found better survival outcomes for patients treated with RP compared to those who received RT. However, RT was associated with better disease control. Subgroup analyses furtherly showed that similar or even better survival outcomes were associated with RT in patients with high GS, high T stage, or received EBRT + BT.
The better disease control for patients treated with RT was likely due to the wider scope of radiotherapy than that of surgery, which made it possible to eliminate micro-metastases outside the prostate and resulted in improved BRFS and MFS. Moreover, adjuvant ADT in addition to RT could further help control the micro-metastases and delay biochemical relapse. However, the improvement of BRFS and MFS by RT did not convert into superior survival benefit compared to RP. Several potential reasons might explain this phenomenon. First, patients treated with RT were older and harbored more adverse clinicopathological features than those with RP. Thus, it was not surprising that patients with RT had a worse prognosis than men with RP. Secondly, to a large extent, the efficacy of RT was determined by the type and dosage. The modality of RT varied in the included studies and the dosage of RT in several studies was lower than what was recommended by current guidelines. Then, patients could choose salvage RT after receiving RP firstly but those patients who chose RT firstly rarely received salvage RP. Last, RT and ADT have greater toxicity than RP, which might lead to the worse OS.
According to our analysis, the types of RT could affect the survival and progression of patients. In fact, several RCT demonstrated a BRFS benefit to EBRT + BT over EBRT [45–47] and several retrospective studies have reported that EBRT + BT brought better outcome than RP on BRFS [39, 48, 49] and MFS [35, 36, 38, 50]. It was not difficult to find that EBRT + BT had strong control over disease progression, which might lead to better CSS for patients treated with EBRT + BT than patients treated with RP. Although EBRT+BT had a better benefit of CSS than RP, this benefit might be neutralized by the increasing other-causes mortality caused by radiotherapy. From our data, we should believe that both RP and EBRT + BT were prior choice than EBRT for patients with high-risk PCa.
As is known, GS is one of the most important prognostic factors [51] and some researches had shown that patients with GS 9–10 had a particularly aggressive disease [41, 42]. Patients with GS 9–10 were at higher risk of disease progression. As mentioned above, RT might have superiority over RP on eliminating micro-metastases and ultimately resulted in better disease control. So these results might be combined to explain the improvement of CSS in patients treated with RT. Similar outcomes were also observed in subgroup analyses according to the T stage. Due to data limitations, we can only use ratios to separate two relatively high and low T stage groups. While we might infer that RT might bring better survival benefits than RP in patients with higher T stage. The inspiration for our clinicians was that RP might be less appropriate and RT might be the first choice for patients with high T stage or high GS.
With the development of treatment modalities, more patients with high-risk PCa can maintain a stable condition for a long term or even be cured. However, making an optimal treatment decision is not only pursuing maximal survival benefit but also a better health-related quality of life. Thus, the assessment of treatments on QoL is also critical for decision making. RP had better performance when considering QoL in the bowel domain, while RT was associated with better QoL in urinary and sexual domains. In clinical practice, younger patients with high-risk prostate cancer who have a greater need for retention of sexual and urinary function after treatment could be recommended with RT. Moreover, RP might be more suitable for patients who need a better bowel function.
Although our research was the most up to date and we did a lot of subgroup analysis, this study still had some limitations. First, heterogeneity was relatively high due to inconsistent inclusion criteria and different treatment modalities. Second, because of the limited data, the population ratio was used to subdivide studies for analysis. Third, in some subgroups, the number of studies and patients is relatively small. Fourth, we failed to perform a meta-analysis on QoL and only presented the results of these studies. This area requires more research and data. Finally, some people included in the studies would inevitably be partially duplicated as the same database was used. Therefore, those results obtained in these subgroups cannot be strong evidence, but can only be used as a reference for interpreting the results.
Conclusions
In conclusion, RP could prolong the survival time of patients with high-risk PCa; however, RT could delay disease progression, and combined RT (EBRT + BT) even brought similar OS and better CSS than RP. RT might be the prior choice for patients with high T stage or high GS. RP could lead to poorer urinary and sexual function, while bringing better performance in the bowel domain. For clinicians, we should fully consider the patient's characteristics and balance the effectiveness and safety of different treatments when making decisions.
Supplementary information
Additional file 1: Table S1. Results of high-risk group of included studies (N = 25).
Additional file 2: Table S2. Quality assessment of the included studies.
Additional file 3: Figure S1. Funnel plot of radical prostatectomy versus radiotherapy using outcome of overall survival.
Acknowledgements
Not applicable
Abbreviations
- ADT
Androgen deprivation therapy
- aRT
Adjuvant radiotherapy
- BRFS
Biochemical recurrence-free survival
- BT
Brachytherapy
- CI
Confidence interval
- CRFS
Clinical recurrence-free survival
- CSS
Cancer-specific survival
- EBRT
External beam radiation therapy
- GS
Gleason score
- HRs
Hazard ratios
- MFS
Metastasis-free survival
- NCCN
National Comprehensive Cancer Network
- NOS
Newcastle-Ottawa Scale
- OS
Overall survival
- PCa
Prostate cancer
- PSA
Prostate-specific antigen
- QoL
Quality of life
- RCT
Randomized controlled trial
- RP
Prostatectomy
- RT
Radiotherapy
Authors’ contributions
HZ and PFS conceived and designed the experiments. ZPW, YCN, and JRC performed the experiments. ZPW, YCN, and JRC analyzed the data. ZPW and YCN wrote the main manuscript text and prepared all figures and tables. All authors revised the manuscript. The author(s) read and approved the final manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (NSFC 81974398 and 81672547), the National Clinical Research Center for Geriatrics (Z2018A01), and 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University.
Availability of data and materials
The studies included were all retrieved from PubMed, EMBASE, and Cochrane databases.
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zhipeng Wang and Yuchao Ni contributed equally to this work.
Contributor Information
Pengfei Shen, Email: cdhx510@foxmail.com.
Hao Zeng, Email: kucaizeng@163.com.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12957-020-01824-9.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 3.Sanoj P, Cooperberg MR. The epidemiology of high-risk prostate cancer. Curr Opin Urol. 2013;23(4):331–336. doi: 10.1097/MOU.0b013e328361d48e. [DOI] [PubMed] [Google Scholar]
- 4.Chang AJ, Autio KA, Iii MR, Scher HI. High-risk prostate cancer—classification and therapy. Nat Rev Clin Oncol. 2014;11(6):308–323. doi: 10.1038/nrclinonc.2014.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pilepich MV, Winter K, Lawton CA, Krisch RE, Wolkov HB, Movsas B, et al. Androgen suppression adjuvant to definitive radiotherapy in prostate carcinoma--long-term results of phase III RTOG 85-31. Int J Radiat Oncol Biol Phys. 2005;61(5):1285–1290. doi: 10.1016/j.ijrobp.2004.08.047. [DOI] [PubMed] [Google Scholar]
- 6.Widmark A, Klepp O, Solberg A, Damber JE, Angelsen A, Fransson P, et al. Endocrine treatment, with or without radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3): an open randomised phase III trial. Lancet. 2009;373(9660):301–308. doi: 10.1016/S0140-6736(08)61815-2. [DOI] [PubMed] [Google Scholar]
- 7.Bolla M, Tienhoven GV, Warde P, Dubois JB, Mirimanoff RO, Storme G, et al. External irradiation with or without long-term androgen suppression for prostate cancer with high metastatic risk: 10-year results of an EORTC randomised study. Lancet Oncol. 2010;11(11):1016–1017. doi: 10.1016/S1470-2045(10)70223-0. [DOI] [PubMed] [Google Scholar]
- 8.Padraig W, Malcolm M, Keyue D, Peter K, Michael B, Richard C, et al. Combined androgen deprivation therapy and radiation therapy for locally advanced prostate cancer: a randomised, phase 3 trial. Lancet. 2011;378(9809):2104–2111. doi: 10.1016/S0140-6736(11)61095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mason MD, Parulekar WR, Sydes MR, Brundage M, Kirkbride P, Gospodarowicz M, et al. Final report of the intergroup randomized study of combined androgen deprivation therapy plus radiotherapy versus androgen deprivation therapy alone in locally advanced prostate cancer. J Clin Oncol. 2015;33(19):2143–2150. doi: 10.1200/JCO.2014.57.7510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fosså SD, Wiklund F, Klepp O, Angelsen A, Solberg A, Damber J-E, et al. Ten- and 15-yr prostate cancer-specific mortality in patients with nonmetastatic locally advanced or aggressive intermediate prostate cancer, randomized to lifelong endocrine treatment alone or combined with radiotherapy: final results of the Scandinavian prostate cancer group-7. Eur Urol. 2016;70(4):684–691. doi: 10.1016/j.eururo.2016.03.021. [DOI] [PubMed] [Google Scholar]
- 11.Boorjian SA, Karnes RJ, Viterbo R, Rangel LJ, Bergstralh EJ, Horwitz EM, et al. Long-term survival after radical prostatectomy versus external-beam radiotherapy for patients with high-risk prostate cancer. Cancer. 2011;117(13):2883–2891. doi: 10.1002/cncr.25900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feldman AS, Meyer CP, Sanchez A, Krasnova A, Reznor G, Menon M, et al. Morbidity and mortality of locally advanced prostate cancer: a population based analysis comparing radical prostatectomy versus external beam radiation. J Urol. 2017;198(5):1061–1068. doi: 10.1016/j.juro.2017.05.073. [DOI] [PubMed] [Google Scholar]
- 13.Ennis RD, Hu L, Ryemon SN, Lin J, Mazumdar M. Brachytherapy-based radiotherapy and radical prostatectomy are associated with similar survival in high-risk localized prostate cancer. Journal of Clinical Oncology Official Journal of the American Society of Clinical Oncology. 2018;36(12):1192. doi: 10.1200/JCO.2017.75.9134. [DOI] [PubMed] [Google Scholar]
- 14.Gu X, Gao X, Cui M, Xie M, Ma M, Qin S, et al. Survival outcomes of radical prostatectomy and external beam radiotherapy in clinically localized high-risk prostate cancer: a population-based, propensity score matched study. Cancer Manag Res. 2018;10:1061–1067. doi: 10.2147/CMAR.S157442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berg S, Cole AP, Krimphove MJ, Nabi J, Marchese M, Lipsitz SR, et al. Comparative effectiveness of radical prostatectomy versus external beam radiation therapy plus brachytherapy in patients with high-risk localized prostate cancer. Eur Urol. 2019;75(4):552–555. doi: 10.1016/j.eururo.2018.10.032. [DOI] [PubMed] [Google Scholar]
- 16.Mohler JL, Antonarakis ES, Armstrong AJ, D'Amico AV, Davis BJ, Dorff T, et al. Prostate Cancer, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network : JNCCN. 2019;17(5):479–505. doi: 10.6004/jnccn.2019.0023. [DOI] [PubMed] [Google Scholar]
- 17.Petrelli F, Vavassori I, Coinu A, Borgonovo K, Sarti E, Barni S. Radical prostatectomy or radiotherapy in high-risk prostate cancer: a systematic review and metaanalysis. Clinical Genitourinary Cancer. 2014;12(4):215–224. doi: 10.1016/j.clgc.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 18.Lei JH, Liu LR, Qiang W, Yan SB, Song TR, Lin FS, et al. Systematic review and meta-analysis of the survival outcomes of first-line treatment options in high-risk prostate cancer. Sci Rep. 2015;5:7713. doi: 10.1038/srep07713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ (Clinical research ed) 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andreas S. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 21.Koichiro A, Hiroyoshi S, Tomohiko I, Hiroyuki F, Osamu M, Michiyuki U, et al. A randomized trial comparing radical prostatectomy plus endocrine therapy versus external beam radiotherapy plus endocrine therapy for locally advanced prostate cancer: results at median follow-up of 102 months. Jpn J Clin Oncol. 2006;36(12):789–793. doi: 10.1093/jjco/hyl115. [DOI] [PubMed] [Google Scholar]
- 22.Giorgio A, Lidia S, Stefano A, Maria Grazia P, Biancamaria S, Sara G, et al. Retrospective comparison of external beam radiotherapy and radical prostatectomy in high-risk, clinically localized prostate cancer. Int J Radiat Oncol Biol Phys. 2009;75(4):975–982. doi: 10.1016/j.ijrobp.2008.12.045. [DOI] [PubMed] [Google Scholar]
- 23.Takizawa I, Hara N, Nishiyama T, Kaneko M, Hoshii T, Tsuchida E, et al. Oncological results, functional outcomes and health-related quality-of-life in men who received a radical prostatectomy or external beam radiation therapy for localized prostate cancer: a study on long-term patient outcome with risk stratification. Asian journal of andrology. 2009;11:283–290. doi: 10.1038/aja.2009.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aizer AA, Yu JJ. Radical prostatectomy vs. intensity-modulated radiation therapy in the management of localized prostate adenocarcinoma. Radiotherapy & Oncology Journal of the European Society for Therapeutic Radiology & Oncology. 2009;93(2):185–191. doi: 10.1016/j.radonc.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 25.Westover K, Chen M-H, Moul J, Robertson C, Polascik T, Dosoretz D, et al. Radical prostatectomy vs radiation therapy and androgen-suppression therapy in high-risk prostate cancer. BJU Int. 2012;110(8):1116–1121. doi: 10.1111/j.1464-410X.2012.11012.x. [DOI] [PubMed] [Google Scholar]
- 26.Kibel AS, Ciezki JP, Klein EA, Reddy CA, Lubahn JD, Haslag-Minoff J, et al. Survival among men with clinically localized prostate cancer treated with radical prostatectomy or radiation therapy in the prostate specific antigen era. J Urol. 2012;187(4):1259–1265. doi: 10.1016/j.juro.2011.11.084. [DOI] [PubMed] [Google Scholar]
- 27.Hoffman RM, Koyama T, Fan KH, Albertsen PC, Barry MJ, Goodman M, et al. Mortality after radical prostatectomy or external beam radiotherapy for localized prostate cancer. Jnci Journal of National Cancer Institute. 2013;105(10):711–718. doi: 10.1093/jnci/djt059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun M, Sammon JD, Becker A, Roghmann F, Tian Z, Kim SP, et al. Radical prostatectomy vs radiotherapy vs observation among older patients with clinically localized prostate cancer: a comparative effectiveness evaluation. BJU Int. 2014;113(2):200–208. doi: 10.1111/bju.12321. [DOI] [PubMed] [Google Scholar]
- 29.Yamamoto S, Masuda H, Urakami S, Fujii Y, Sakamoto K, Kozuka T, et al. Patient-perceived satisfaction after definitive treatment for men with high-risk prostate cancer: radical prostatectomy vs. intensity-modulated radiotherapy with androgen deprivation therapy. Urology. 2015;85(2):407–414. doi: 10.1016/j.urology.2014.09.046. [DOI] [PubMed] [Google Scholar]
- 30.Robinson D, Garmo H, Lissbrant IF, Widmark A, Pettersson A, Gunnlaugsson A, et al. Prostate cancer death after radiotherapy or radical prostatectomy: a nationwide population-based observational study. Eur Urol. 2017;73(4):502–511. doi: 10.1016/j.eururo.2017.11.039. [DOI] [PubMed] [Google Scholar]
- 31.Ciezki JP, Weller M, Reddy CA, Kittel J, Singh H, Tendulkar R, et al. A comparison between low-dose-rate brachytherapy with or without androgen deprivation, external beam radiation therapy with or without androgen deprivation, and radical prostatectomy with or without adjuvant or salvage radiation therapy for high-risk prost. Int J Radiat Oncol Biol Phys. 2017;97(5):962. doi: 10.1016/j.ijrobp.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 32.Jang TL, Patel N, Faiena I, Radadia KD, Moore DF, Elsamra SE, et al. Comparative effectiveness of radical prostatectomy with adjuvant radiotherapy versus radiotherapy plus androgen deprivation therapy for men with advanced prostate cancer. Cancer. 2018;124(20):4010–4022. doi: 10.1002/cncr.31726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ii MDT, Koyama T, Dan L, Hoffman KE, Resnick MJ, Wu XC, et al. Effect of prostate cancer severity on functional outcomes after localized treatment: comparative effectiveness analysis of surgery and radiation study results. Eur Urol. 2018;74(1):26. doi: 10.1016/j.eururo.2018.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tilki D, Chen M-H, Wu J, Huland H, Graefen M, Braccioforte M, et al. Surgery vs radiotherapy in the management of biopsy Gleason score 9-10 prostate cancer and the risk of mortality. JAMA Oncology. 2019;5(2):213–220. doi: 10.1001/jamaoncol.2018.4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Markovina S, Meeks M, Badiyan S, Vetter J, Gay H, Paradis A, et al. Superior metastasis-free survival for patients with high risk prostate cancer treated with definitive radiation therapy compared to radical prostatectomy; a propensity score matched analysis. Advances in Radiation Oncology. 2018;3(2):190–196. doi: 10.1016/j.adro.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kishan AU, Cook RR, Ciezki JP, Ross AE, Pomerantz MM, Nguyen PL, et al. Radical prostatectomy, external beam radiotherapy, or external beam radiotherapy with brachytherapy boost and disease progression and mortality in patients with Gleason score 9-10 prostate cancer. Jama. 2018;319(9):896. doi: 10.1001/jama.2018.0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jayadevappa R, Lee DI, Chhatre S, Guzzo TJ, Malkowicz SB. Comparative effectiveness of treatments for high-risk prostate cancer patients. Urologic Oncology: Seminars and Original Investigations. 2019;37(9):574.e11–574.e18. doi: 10.1016/j.urolonc.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 38.Reichard Chad A., Hoffman Karen E., Tang Chad, Williams Stephen B., Allen Pamela K., Achim Mary F., Kuban Deborah A., Chapin Brian F. Radical prostatectomy or radiotherapy for high- and very high-risk prostate cancer: a multidisciplinary prostate cancer clinic experience of patients eligible for either treatment. BJU International. 2019;124(5):811–819. doi: 10.1111/bju.14780. [DOI] [PubMed] [Google Scholar]
- 39.Caño-Velasco J, Herranz-Amo F, Barbas-Bernardos G, Polanco-Pujol L, Hernández-Cavieres J, Lledó-García E, et al. Diferencias en la supervivencia global y supervivencia cáncer específica en pacientes con cáncer de próstata de alto riesgo según el tratamiento primario aplicado. Actas Urol Esp. 2019;43(2):91–98. doi: 10.1016/j.acuro.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 40.Muralidhar Vinayak, Mahal Brandon A., Butler Santino, Lamba Nayan, Yang David D., Leeman Jonathan, D'Amico Anthony V., Nguyen Paul L., Trinh Quoc-Dien, Orio Peter F., King Martin T. Combined External Beam Radiation Therapy and Brachytherapy versus Radical Prostatectomy with Adjuvant Radiation Therapy for Gleason 9-10 Prostate Cancer. Journal of Urology. 2019;202(5):973–978. doi: 10.1097/JU.0000000000000352. [DOI] [PubMed] [Google Scholar]
- 41.Ham WS, Chalfin HJ, Feng Z, Trock BJ, Epstein JI, Cheung C, et al. New prostate cancer grading system predicts long-term survival following surgery for Gleason score 8-10 prostate cancer. Eur Urol. 2016;71(6):907. doi: 10.1016/j.eururo.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 42.Epstein JI, Zelefsky MJ, Sjoberg DD, Nelson JB, Egevad L, Magi-Galluzzi C, et al. A contemporary prostate cancer grading system: a validated alternative to the Gleason score. Eur Urol. 2016;69(3):428–435. doi: 10.1016/j.eururo.2015.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wallis CJD, Saskin R, Choo R, Herschorn S, Kodama RT, Satkunasivam R, et al. Surgery versus radiotherapy for clinically-localized prostate cancer: a systematic review and meta-analysis. Eur Urol. 2016;70(1):21–30. doi: 10.1016/j.eururo.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 44.Chen L, Li Q, Wang Y, Zhang Y, Ma X. Comparison on efficacy of radical prostatectomy versus external beam radiotherapy for the treatment of localized prostate cancer. Oncotarget. 2017;8(45):79854–79863. doi: 10.18632/oncotarget.20078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoskin PJ, Rojas AM, Bownes PJ, Lowe GJ, Ostler PJ, Bryant L. Randomised trial of external beam radiotherapy alone or combined with high-dose-rate brachytherapy boost for localised prostate cancer. Radiother Oncol. 2012;103(2):217–222. doi: 10.1016/j.radonc.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 46.Morris WJ, Tyldesley S, Rodda S, Halperin R, Pai H, McKenzie M, et al. Androgen suppression combined with elective nodal and dose escalated radiation therapy (the ASCENDE-RT Trial): an analysis of survival endpoints for a randomized trial comparing a low-dose-rate brachytherapy boost to a dose-escalated external beam boost for high- and intermediate-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2017;98(2):275–285. doi: 10.1016/j.ijrobp.2016.11.026. [DOI] [PubMed] [Google Scholar]
- 47.Dayes IS, Parpia S, Gilbert J, Julian JA, Davis IR, Levine MN, et al. Long-term results of a randomized trial comparing iridium implant plus external beam radiation therapy with external beam radiation therapy alone in node-negative locally advanced cancer of the prostate. Int J Radiat Oncol Biol Phys. 2017;99(1):90–93. doi: 10.1016/j.ijrobp.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 48.Merino T, Francisco IFS, Rojas PA, Bettoli P, Zúñiga Á, Besa P. Intensity-modulated radiotherapy versus radical prostatectomy in patients with localized prostate cancer: long-term follow-up. BMC Cancer. 2013;13(1):1–9. doi: 10.1186/1471-2407-13-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Andic F, Izol V, Gokcay S, Arslantas HS, Bayazit Y, Coskun H, et al. Definitive external-beam radiotherapy versus radical prostatectomy in clinically localized high-risk prostate cancer: a retrospective study. BMC Urol. 2019;19(1):3. doi: 10.1186/s12894-018-0432-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baker CB, Mcdonald AM, Yang ES, Jacob R, Rais-Bahrami S, Nix JW, et al. Pelvic radiotherapy versus radical prostatectomy with limited lymph node sampling for high-grade prostate adenocarcinoma. Prostate Cancer. 2016;2016:1–8. doi: 10.1155/2016/2674954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Epstein JI. An update of the Gleason grading system. J Urol. 2010;183(2):433–440. doi: 10.1016/j.juro.2009.10.046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Results of high-risk group of included studies (N = 25).
Additional file 2: Table S2. Quality assessment of the included studies.
Additional file 3: Figure S1. Funnel plot of radical prostatectomy versus radiotherapy using outcome of overall survival.
Data Availability Statement
The studies included were all retrieved from PubMed, EMBASE, and Cochrane databases.