Abstract
Background:
An exponential rise in the prevalence of obesity and the associated type 2 diabetes mellitus (T2DM) has led to an explosion in the field of bariatric surgery worldwide. It has been proposed that laparoscopic sleeve gastrectomy (LSG) not only results in excess weight loss (EWL) but also leads to excellent glycemic control.
Aims:
However, not every patient benefits from the bariatric surgery. Furthermore, bariatric surgery is currently indicated based on body mass index (BMI), but BMI solely does not predict diabetes remission after the surgery. We aimed to study the outcome of LSG on the diabetic status and the factors predicting the disease remission.
Subjects and Methods:
This prospective study was conducted on 104 obese patients having T2DM who underwent LSG. Following surgery, the clinical outcome on weight loss, BMI, and glycemic control was studied for 6 months. Various positive and negative predictors of diabetic remission after the surgery were also determined. Student's t-test and Chi-square tests were applied.
Results:
LSG resulted in significant weight loss (P < 0.05); the percentage of EWL was 60.75 ± 6.30 at 6 months. Furthermore, surgery resulted in 78.9% remission of diabetes with fasting blood glucose and glycated hemoglobin values at 6 months being 121.13 ± 15.25 mg/dl and 6.19% ± 0.31%, respectively. Younger and heavier patients, those with lesser disease severity and shorter duration had better chances of disease remission. Gender had no correlation with disease remission.
Conclusion:
LSG is a successful treatment option for T2DM and is more beneficial if offered, not as a last option, but to younger, obese patients with mild disease severity and shorter disease duration after the failure of medical treatment.
KEYWORDS: Bariatric surgery, diabetes remission, laparoscopic sleeve gastrectomy, predictor, type 2 diabetes mellitus
INTRODUCTION
The epidemic rise in obesity prevalence in the late 1970s fuelled the debate to classify it as a disease; and in 1985, obesity was officially classified as a disease. The World Health Organization has described obesity as one of the today's most neglected public health problems, affecting every region of the globe.[1] The problem of obesity has been observed to have a rising trend in the entire Indian subcontinent.[2] Obesity is calculated as body mass index (BMI), and persons with BMI >30 kg/m2 are considered as obese. Morbid obesity (BMI >40 kg/m2) is the harbinger of many diseases that affect essentially every organ systems such as cardiovascular, respiratory, metabolic, musculoskeletal, endocrinal, reproductive, dermatological, neurological, and many more.[3]
There are both nonsurgical and surgical interventions available at our disposal aimed at countering this ever-increasing global epidemic. However, surgical procedures have now superseded nonsurgical measures such as pharmacotherapy, dietary, and behavioral modifications. In 1991, the National Institute of Health Consensus stated that bariatric surgery is the most effective treatment for morbid obesity leading to excellent long-term sustained weight loss along with the reduction of comorbidities and recommended surgery for obesity at a BMI of 40 kg/m2 or a BMI >35 kg/m2 with any comorbid conditions.[4]
Obesity and type 2 diabetes mellitus (T2DM), both highly prevalent diseases, are very closely related and pose a serious threat to the health of an individual. There is seen an improvement in the clinical status of patients as various comorbid conditions respond to bariatric procedures such as hypertension, DM, and dyslipidemia. There is strong evidence that bariatric surgeries can treat most of the associated T2DM in morbidly obese patients.[5] The reduction of mortality by bariatric surgery is mostly attributed to the reduction in diabetes-related death.[6]
The remission of diabetes has been found to be associated with a range of excess weight loss (EWL) after surgery.[7] However, optimal outcomes for diabetes remission after bariatric surgery will occur if patients who are best suited to the surgery are selected, and those who are not likely to benefit much are spared the procedure. Factors, such as old age, a longer T2DM history, and the use of insulin, reflect a destroyed beta-cell mass. These were the negative predictors of disease remission in the previous studies concerning bariatric surgery on morbidly obese patients.[5,8] Using this type of predictor, it is possible to select patients that are best suited for the surgery and exclude those who would give poor results.[9]
With the increasing burden of obesity and a paralleled increase in bariatric procedures worldwide, it is crucial to study the clinical outcome of bariatric surgery such as diabetes remission. Furthermore, it is necessary to determine the factors that are associated with diabetes remission following bariatric surgery, so as to be able to choose patients that are most likely to benefit from bariatric surgery.
Our study aimed at determining the status of T2DM following laparoscopic sleeve gastrectomy (LSG) and also, the factors that predetermine the remission of diabetes after surgery.
SUBJECTS AND METHODS
This prospective study was conducted between July 2013 and October 2017 on 104 obese patients who underwent LSG, and these patients were followed up for 6 months postoperatively. Approval was obtained from the institutional ethics committee. All the patients with BMI >35 kg/m2 having T2DM were included in the study. Patients with a history of bariatric surgery, those who suffering from terminal illness such as advanced-stage cancer or end-stage cardiac/renal disease and patients with psychiatric illness and active substance abuse were excluded from the study.
After obtaining written informed consent, particulars of the patient were duly noted, and a detailed history including symptoms, coexisting comorbid conditions, personal habits such as smoking or alcohol consumption, and treatment history was taken. The patient's history with respect to DM included type, duration, insulin dose and frequency, and dose of oral hypoglycemic agents (OHA). All patients underwent a comprehensive multidisciplinary bariatric evaluation by a dedicated bariatric team which included a bariatric surgeon, dietician, endocrinologist, gastroenterologist, psychiatrist, chest physician, anesthesiologist, and the cardiologist. The various sociodemographic characteristics and clinical parameters noted were age, gender, height, weight, fasting blood sugar (FBS) levels, and glycated hemoglobin (HbA1c) values. BMI was calculated from weight and height of the patient using the formula: BMI = weight/(height)2.
After a detailed preanesthetic checkup and a written informed consent, patients were taken up for LSG. LSG was chosen as the procedure of choice here, given the fact that it has the least complication rate along with the easiness and shorter duration of surgery. The patients underwent a standard LSG using 2–12 mm ports, 1–5 mm ports, and a Nathanson liver retractor. Starting at a point on the greater curvature, 4–8 cm from the pylorus, 75%–80% of the greater curvature was excised using laparoscopic staplers leaving a narrow stomach tube. Postoperatively, patients were encouraged early ambulation, preferably on the same day of the surgery. Patients were started on liquid diet orally the next day of surgery after ruling out any leak on oral gastrografin study done on postoperative day 1.
Postoperatively, trends of the following parameters were studied at various intervals till 6 months: weight, BMI, FBS levels, HbA1c levels, and dose of insulin/OHAs.
Diagnostic, resolution, and improvement criteria for type 2 diabetes mellitus
Definition of diabetes: Treatment with OHA or insulin or HbA1C >6.5%.[10]
Resolution criteria: HbA1c <6.5% without drug therapy.
Improvement criteria: Decrease in medication or HbA1c decrease >1% with no change in medication.
Depending on diabetes status in the postoperative period, patients were categorized into remission and nonremission groups.
Statistical analysis
Data analysis was done using SPSS software (IBM SPSS statistics for windows, version 20.0 Armonk, NY: IBM Corp). Student's t-test and Chi-square tests were applied.
RESULTS
Of 104 patients, 62 (60%) patients were male and rest 42 (40%) were female. The mean age of patients was 47.02 ± 3.59 years. Mean weight, height, and BMI were 129.1 ± 10.2 kg, 1.60 ± 0.07 m, and 50.38 ± 4.95 kg/m2, respectively. Mean FBS and HbA1c levels were 184.77 ± 17.7 mg/dl and 9.26% ± 0.92%, respectively. The mean duration of T2DM was 5.56 ± 3.27 years [Table 1].
Table 1.
Patient’s preoperative characteristics
| n | 104 |
| Male, n (%) | 62 (60) |
| Females, n (%) | 42 (40) |
| Mean age (years) | 47.02±3.59 |
| Mean height (m2) | 1.60±0.07 |
| Mean weight (kg) | 129.1±10.2 |
| Mean BMI (kg/m2) | 50.38±4.95 |
| Mean fasting glucose (mg/dl) | 184.77±17.7 |
| Mean DM duration (years) | 5.56±3.27 |
| Mean HbA1c (%) | 9.26±0.92 |
BMI: Body mass index, DM: Diabetes mellitus, HbA1c: Glycated hemoglobin
Clinical outcome
A significant difference was noticed between the preoperative and postoperative values of mean weight and mean BMI at 3 months and 6 months after the surgery [Table 2]. Reduction in the mean weight from a preoperative value of 129.1 ± 10.2 kg to postoperative value of 105.85 ± 10.30 kg at 3 months and 85.72 ± 9.65 kg at 6 months was found to be statistically significant. Furthermore, the values of mean BMI decreased significantly to 44.35 ± 4.56 kg/m2 at 3 months and 36.58 ± 3.82 kg/m2 at 6 months from a preoperative value of 50.38 ± 4.95 kg/m2. LSG resulted in significant weight loss (P < 0.05); the percentage of EWL was 60.75 ± 6.30 at 6 months. This signifies the role of LSG as bariatric surgery.
Table 2.
Clinical outcomes
| Clinical parameter | Mean | P | |||
|---|---|---|---|---|---|
| Preoperative | Postoperative at 3 months | Postoperative at 6 months | Preoperative versus 3 months | Preoperative versus 6 months | |
| Weight (kg) | 129.1±10.2 | 105.85±10.30 | 85.72±9.65 | <0.0001 | <0.0001 |
| BMI (kg/m2) | 50.38±4.95 | 44.35±4.56 | 36.58±3.82 | <0.0001 | <0.0001 |
| HbA1c (%) | 9.26±0.92 | 7.26±0.57 | 6.19±0.31 | <0.0001 | <0.0001 |
| Fasting glucose levels (mg/dl) | 184.77±17.7 | 150.80±14.35 | 121.13±15.25 | <0.0001 | <0.0001 |
BMI: Body mass index, HbA1c: Glycated hemoglobin
Subsequent to surgery, fall in HbA1C and FBS levels were significant both at 3 months (P < 0.0001) and 6 months (P < 0.0001) postoperatively [Table 2]. HbA1C values decreased from a preoperative value of 9.26% ± 0.92% to 7.26% ± 0.57% and 6.19% ± 0.31% at 3 and 6 months, respectively. A significant decline in the FBS levels at 3 months (150.80 ± 14.35 mg/dl) and at 6 months (121.13 ± 15.25 mg/dl) was also seen from a value of 184.77 ± 17.7 mg/dl prior to surgery. This signifies that LSG surgery resulted in the resolution of diabetes at 6 months after surgery.
Diabetic status
Of 104 patients, diabetes was completely resolved in 82 patients (78.9%) at 6 months after surgery and in the remaining 22 patients (21.1%), diabetic status was improved, out of which 12 patients had the dose of insulin decreased, and ten patients were switched over to OHAs [Figure 1].
Figure 1.
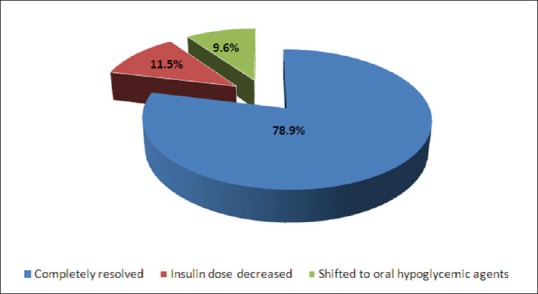
Diabetic status
Predictor analysis for diabetes remission
Five preoperative factors were found to likely predict diabetes remission after LSG. These included age, BMI, HbA1c, FBS levels, and duration of T2DM. Table 3 shows their mean preoperative values in both the remission and nonremission groups and the difference in them being significant.
Table 3.
Predictor analysis of diabetes remission
| Factor | Mean | P, χ2 | |
|---|---|---|---|
| Remission (n=82) | Nonremission (n=22) | ||
| Age ( years) | 45.54±2.03 | 52.54±2.57 | <0.0001 |
| Gender | |||
| Male | 25 | 6 | 0.699, 0.149 |
| Female | 16 | 5 | |
| Diabetes duration (years) | 4.01±1.11 | 11.26±2.01 | <0.0001 |
| BMI (kg/m2) | 51.95±4.18 | 44.47±2.57 | <0.0008 |
| FBS (mg/dl) | 179.94±16.62 | 202.73±6.47 | <0.0005 |
| HbA1c (%) | 9.08±0.81 | 9.945±1.05 | 0.004 |
BMI: Body mass index, FBS: Fasting blood sugar, HbA1c: Glycated hemoglobin
Results showed that younger patients and patients with higher BMI values had better remission rates of diabetes than their older and lower BMI counterparts. Other positive predictors of diabetes remission after LSG included preoperative HbA1c, FBS levels, and the duration of DM. Patients with higher values of FBS and/or HbA1c had lesser chances of diabetes remission than those with lower values. Furthermore, the results showed that patients with a shorter history of diabetes received more benefits of the bariatric surgery in terms of resolution of the disease. However, no association was found between gender and postoperative outcome of diabetes remission after LSG.
DISCUSSION
Speculations do not exist regarding the emerging role of bariatric surgery in the treatment of DM. The most widely performed bariatric procedures are Roux-en-Y gastric bypass, adjustable gastric banding, biliopancreatic diversion (with or without duodenal switch), and sleeve gastrectomy.[11] LSG, a bariatric procedure is being performed since 1999.[12] Earlier LSG was performed for high-risk patients or as an adjunctive surgical procedure to biliopancreatic diversion in high-risk cases. Now, it is a preferred procedure for common morbid obesity even in low-risk patients due to its cost, safety, and effectiveness in weight reduction.
The most conspicuous outcome of LSG is the reduction in body weight. Weight loss is the most important factor that determines whether a procedure succeeds as bariatric surgery or not. Our study shows a significant reduction of both mean weight and BMI postoperatively, which ascertain the relevance of LSG as a bariatric procedure. Similar results were also seen in studies by Gluck et al.,[13] and Boza et al.[3] A reduction of BMI by 5% corresponds to a 33% reduction of T2DM.[14] In addition to a significant reduction in FBS levels, a significant reduction in HbA1c values was also seen, both at 3 months’ and 6 months’ postoperatively. The diabetic cure rate at 6 months was 78.9%, whereas improvement in the diabetic status was seen in all the 104 patients. This validates LSG as a treatment option for diabetes. These results are consistent with a study by Abbatini et al.,[15] who observed a diabetic remission rate of 80.9% after LSG. Furthermore, 100% diabetes resolution rate was seen in a study by Omana et al. after LSG in patients with diabetes.[16] Many other authors also noted a significant reduction in HbA1c and FBS values after LSG.[8,17,18]
Weight loss after LSG is attributed to reduced size and decreased distensibility of the stomach. Furthermore, LSG leads to accelerated gastric emptying, which may alter the regulatory neurohormonal mechanisms such as GLP- 1 hormone production from the distal gut and thus contributing to weight loss and better glucose metabolism. LSG patients experience early satiety and less hunger drive due to decreased levels of ghrelin, a hunger stimulatory hormone produced by P/D1 cells in the fundus of stomach and epsilon cells in pancreas.[19] Alteration in the composition of gut microbiota after bariatric surgery may also play a role in the diabetic remission and weight loss.[20]
Predictor analysis done in our study showed that age, BMI, duration of T2DM, HbA1c, and FBS levels to be positive predictors of diabetic remission after LSG, whereas gender had no correlation with remission. In a recent study, Dixon et al. revealed glycemic response after bariatric surgery to be associated with BMI, disease duration, fasting C-peptide levels, and degree of weight loss.[21] The age was found to be a significant predictor of diabetes remission in a study on the Asian population.[6] Like in our study, other studies also confirm higher remission rates with younger age.[22,23] Remission rates of DM dropped by 12% on addition of 12 years of age in a study by Hamza et al.,[24] the rationale being higher beta-cell reserve in younger patients. However, Hamza et al. also showed female gender to be a positive predictor of diabetes remission; this was not the case in our study. Jurowich et al. also concluded that the gender was a negative predictor of disease remission.[25]
We also noticed in our study, that patients with lower BMI had lower remission rates than those with higher BMI. Similar findings were observed for the 1st time by Lee et al.[26] Higher BMI is usually associated with greater weight loss and hence, the higher remission rates. However, controversy exists, where Mingrone et al. found remission and BMI to be inversely related.[27] Robert et al. found that super morbid obesity (BMI > 50) to be a negative predictor of DM remission.[28] This may be explained by the fact that higher BMI means severe insulin resistance and/or a greater destroyed beta-cell mass.
Higher FBS and HbA1c values indicate severe disease status and thus, a higher insulin resistance state along with a lower beta-cell functional reserve. This decreases the likelihood of disease remission, as was found in our study. This is in accordance with many authors who report higher remission rates with lower HbA1c values.[8,29,30]
Like us, many authors also supported in their studies that the duration of T2DM is an important predictor of disease remission.[5,8,31] The most important finding in a study by Lee et al. included T2DM duration to be the most important predictor of diabetes remission.[6] In a recent study, the remission rate dropped down to 13% if disease duration was >5 years.[31] Shorter disease duration means a greater preserved beta-cell mass and therefore better the chances of disease remission after LSG.
CONCLUSION
In addition to sustained and efficacious weight loss, LSG shows promising results as a treatment option for T2DM. More favorable outcomes are seen in younger patients, patients with higher BMI, lower HbA1c, and relatively shorter duration of diabetes. Therefore, LSG should be offered early in the course of DM, particularly in morbidly obese patients when the disease is not so severe so that remission from diabetes could be achieved. Long-term remission and not just cure of the disease should be the desired goal.
However, ours is an observational study with a small sample size which surely does lack a control group. Unlike ours, a longer follow-up period may reveal some information regarding sustained and long-term effects of bariatric surgery. Nonetheless, RCTs comparing outcomes of various bariatric surgeries may add further insight into the patient.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Misra A, Chowbey P, Makkar BM, Vikram NK, Wasir JS, Chadha D, et al. Consensus statement for diagnosis of obesity, abdominal obesity and the metabolic syndrome for Asian Indians and recommendations for physical activity, medical and surgical management. J Assoc Physicians India. 2009;57:163–70. [PubMed] [Google Scholar]
- 2.Sanjay S, Unnikrishnan AG. Obesity in India: The weight of the nation. J Med Nutr. 2012:1:37–41. [Google Scholar]
- 3.Boza C, Salinas J, Salgado N, Pérez G, Raddatz A, Funke R, et al. Laparoscopic sleeve gastrectomy as a stand-alone procedure for morbid obesity: Report of 1,000 cases and 3-year follow-up. Obes Surg. 2012;22:866–71. doi: 10.1007/s11695-012-0591-6. [DOI] [PubMed] [Google Scholar]
- 4.NIH consensus statement covers treatment of obesity. Am Fam Physician. 1991;44:305–6. [PubMed] [Google Scholar]
- 5.Dixon JB, O’Brien PE. Health outcomes of severely obese type 2 diabetic subjects 1 year after laparoscopic adjustable gastric banding. Diabetes Care. 2002;25:358–63. doi: 10.2337/diacare.25.2.358. [DOI] [PubMed] [Google Scholar]
- 6.Lee WJ, Chong K, Chen JC, Ser KH, Lee YC, Tsou JJ, et al. Predictors of diabetes remission after bariatric surgery in Asia. Asian J Surg. 2012;35:67–73. doi: 10.1016/j.asjsur.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 7.Buchwald H, Estok R, Fahrbach K, Banel D, Jensen MD, Pories WJ, et al. Weight and type 2 diabetes after bariatric surgery: Systematic review and meta-analysis. Am J Med. 2009;122:248–56. doi: 10.1016/j.amjmed.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 8.Schauer PR, Burguera B, Ikramuddin S, Cottam D, Gourash W, Hamad G, et al. Effect of laparoscopic Roux-En Y gastric bypass on type 2 diabetes mellitus. Ann Surg. 2003;238:467–84. doi: 10.1097/01.sla.0000089851.41115.1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rubino F. Is type 2 diabetes an operable intestinal disease? A provocative yet reasonable hypothesis. Diabetes Care. 2008;31(Suppl 2):S290–6. doi: 10.2337/dc08-s271. [DOI] [PubMed] [Google Scholar]
- 10.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26(Suppl 1):S5–20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- 11.Raffaelli M, Sessa L, Mingrone G, Bellantone R. Assessing the obese diabetic patient for bariatric surgery: Which candidate do I choose? Diabetes Metab Syndr Obes. 2015;8:255–62. doi: 10.2147/DMSO.S50659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jossart GH, Anthone G. The history of sleeve gastrectomy. Bariatr Times. 2010;7:9–10. [Google Scholar]
- 13.Gluck B, Movitz B, Jansma S, Gluck J, Laskowski K. Laparoscopic sleeve gastrectomy is a safe and effective bariatric procedure for the lower BMI (35.0-43.0 kg/m2) population. Obes Surg. 2011;21:1168–71. doi: 10.1007/s11695-010-0332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peluso L, Vanek VW. Efficacy of gastric bypass in the treatment of obesity-related comorbidities. Nutr Clin Pract. 2007;22:22–8. doi: 10.1177/011542650702200122. [DOI] [PubMed] [Google Scholar]
- 15.Abbatini F, Rizzello M, Casella G, Alessandri G, Capoccia D, Leonetti F, et al. Long-term effects of laparoscopic sleeve gastrectomy, gastric bypass, and adjustable gastric banding on type 2 diabetes. Surg Endosc. 2010;24:1005–10. doi: 10.1007/s00464-009-0715-9. [DOI] [PubMed] [Google Scholar]
- 16.Omana JJ, Nguyen SQ, Herron D, Kini S. Comparison of comorbidity resolution and improvement between laparoscopic sleeve gastrectomy and laparoscopic adjustable gastric banding. Surg Endosc. 2010;24:2513–7. doi: 10.1007/s00464-010-0995-0. [DOI] [PubMed] [Google Scholar]
- 17.Shah S, Todkar J, Shah P. Effect of laparoscopic sleeve gastrectomy (LSG) on HbA1c levels in T2DM patients: Results at one year. Obes Surg IFSO [internet] 2009 Abstract P-071. [Last accessed on 2019 Jan 10]. Available from: http://sitesgooglecom/a/closnet/mini/ifso 2009 abstr .
- 18.Todkar JS, Shah SS, Shah PS, Gangwani J. Long-term effects of laparoscopic sleeve gastrectomy in morbidly obese subjects with type 2 diabetes mellitus. Surg Obes Relat Dis. 2010;6:142–5. doi: 10.1016/j.soard.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 19.Milone L, Strong V, Gagner M. Laparoscopic sleeve gastrectomy is superior to endoscopic intragastric balloon as a first stage procedure for super-obese patients (BMI>or=50) Obes Surg. 2005;15:612–7. doi: 10.1381/0960892053923833. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen KT, Korner J. The sum of many parts: Potential mechanisms for improvement in glucose homeostasis after bariatric surgery. Curr Diab Rep. 2014;14:481. doi: 10.1007/s11892-014-0481-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dixon JB, Chuang LM, Chong K, Chen SC, Lambert GW, Straznicky NE, et al. Predicting the glycemic response to gastric bypass surgery in patients with type 2 diabetes. Diabetes Care. 2013;36:20–6. doi: 10.2337/dc12-0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee WJ, Hur KY, Lakadawala M, Kasama K, Wong SK, Chen SC, et al. Predicting success of metabolic surgery: Age, body mass index, C-peptide, and duration score. Surg Obes Relat Dis. 2013;9:379–84. doi: 10.1016/j.soard.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 23.Kim S, Richards WO. Long-term follow-up of the metabolic profiles in obese patients with type 2 diabetes mellitus after Roux-En-Y gastric bypass. Ann Surg. 2010;251:1049–55. doi: 10.1097/SLA.0b013e3181d9769b. [DOI] [PubMed] [Google Scholar]
- 24.Hamza N, Abbas MH, Darwish A, Shafeek Z, New J, Ammori BJ. Predictors of remission of type 2 diabetes mellitus after laparoscopic gastric banding and bypass. Surg Obes Relat Dis. 2011;7:691–6. doi: 10.1016/j.soard.2010.03.292. [DOI] [PubMed] [Google Scholar]
- 25.Jurowich C, Thalheimer A, Hartmann D, Bender G, Seyfried F, Germer CT, et al. Improvement of type 2 diabetes mellitus (T2DM) after bariatric surgery – who fails in the early postoperative course? Obes Surg. 2012;22:1521–6. doi: 10.1007/s11695-012-0676-2. [DOI] [PubMed] [Google Scholar]
- 26.Lee WJ, Wang W, Lee YC, Huang MT, Ser KH, Chen JC. Effect of laparoscopic mini-gastric bypass for type 2 diabetes mellitus: Comparison of BMI>35 and<35 kg/m2. J Gastrointest Surg. 2008;12:945–52. doi: 10.1007/s11605-007-0319-4. [DOI] [PubMed] [Google Scholar]
- 27.Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Nanni G, et al. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet. 2015;386:964–73. doi: 10.1016/S0140-6736(15)00075-6. [DOI] [PubMed] [Google Scholar]
- 28.Robert M, Ferrand-Gaillard C, Disse E, Espalieu P, Simon C, Laville M, et al. Predictive factors of type 2 diabetes remission 1 year after bariatric surgery: Impact of surgical techniques. Obes Surg. 2013;23:770–5. doi: 10.1007/s11695-013-0868-4. [DOI] [PubMed] [Google Scholar]
- 29.Yan H, Tang L, Chen T, Kral JG, Jiang L, Li Y, et al. Defining and predicting complete remission of type 2 diabetes: A short-term efficacy study of open gastric bypass. Obes Facts. 2013;6:176–84. doi: 10.1159/000351018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hall TC, Pellen MG, Sedman PC, Jain PK. Preoperative factors predicting remission of type 2 diabetes mellitus after Roux-En-Y gastric bypass surgery for obesity. Obes Surg. 2010;20:1245–50. doi: 10.1007/s11695-010-0198-8. [DOI] [PubMed] [Google Scholar]
- 31.Rosenthal R, Li X, Samuel S, Martinez P, Zheng C. Effect of sleeve gastrectomy on patients with diabetes mellitus. Surg Obes Relat Dis. 2009;5:429–34. doi: 10.1016/j.soard.2008.11.006. [DOI] [PubMed] [Google Scholar]


