Abstract
Situs anomalies are rare structural defects affecting 0.01% of general population. They present with multisystem structural defects mostly involving cardiovascular, respiratory and GI systems. Situs abnormality with presence of multiple spleen is termed as left atrial isomerism with anatomical and structural differences to its countertype situs ambiguous with asplenia (right atrial isomerism). In this case report, we present an adult case of situs ambiguous anomaly which was diagnosed incidentally during laparoscopic cholecystectomy. The patient had enlarged left lobe of liver, multiple splenules on right side, malrotated small and large gut, interrupted inferior vena cava with azygos continuation, and bilateral bilobed lungs. It is concluded that variations in situs ambiguous cases differ and a single description is not possible. It is crucial to reveal these variations by using imaging modalities and being aware of them prior to surgery and invasive intervention to prevents the possible risks and complications.
KEYWORDS: Heterotaxy, situs ambiguous, visceral malrotation
INTRODUCTION
It is the time inside the uterus when the position of various organs in the chest and the abdomen is defined during the process of development. Normal positioning of the various organs in the chest and abdomen is defined as situs solitus. Situs inversus is inverse of situs solitus, i.e., organs in the chest and abdomen are mirror images of the normal position. The term situs ambiguous is used to describe the arrangement of the vessels and organs in the chest and abdomen in a haphazard and unordered manner. They are arranged in a way that can be neither identified as situs solitus nor as situs inversus (heterotaxy).[1] Situs ambiguous is further divided into two types based on the anatomy of the spleen, i.e., the left isomerism or situs ambiguous with polysplenia and the right isomerism or situs ambiguous with asplenia. We present this case report for the rarity of the condition.
CASE REPORT
A 35-year-old female patient presented to the surgery outpatient department with the complaints of pain and bloating sensation in the abdomen for 6 months. The pain was intermittent, colicky in nature in the right upper abdomen and not associated with vomiting. There was no history of fever, jaundice, and any altered bowel habits. There was no history of any chronic illness, previous surgery, or significant disease history in her family. Physical examination showed soft, nontender abdomen with the absence of any clinical palpable organomegaly. Hemogram, liver function, and renal function tests were within the normal limit. A whole-abdomen ultrasonography (USG) showed the gallbladder sludge suggestive of cholelithiasis. No other abnormality was mentioned in the USG report. With the diagnosis of symptomatic gallstone disease, laparoscopic cholecystectomy under general anesthesia was planned. Thin-walled distended gallbladder was present on laparoscopy. Calot's triangle anatomy was normal. The gallbladder was removed after securing cystic duct and artery. After gallbladder extraction, while placing the drain in the subhepatic region, multiple masses of variable size were noticed there. On laparoscopy, anatomy could not be clearly delineated. With suspicion of malrotation of the gut, the patient was explored by midline incision.
On exploration, multiple splenules (six in numbers) of variable sizes found close to each other in the right hypochondrium with the absence of the spleen at its normal anatomical site [Figure 1]. The liver was centrally placed with its left lobe enlargement. The greater curvature of the stomach was on the right side and the c-loop of the duodenum was extending up to the midline with concavity toward the right side. The head of the pancreas was in the concavity of c-loop of the duodenum with a truncated tail toward the right side [Figure 2]. The duodenojejunal junction was just left of the midline. Small gut loops were appearing normal in shape, size, and color, and no sign of obstruction was there. Cecum and the ascending colon were freely mobile and which was also more or less centrally placed. Distal transverse, descending and sigmoid colon was enclosed in a membranous sac and formed a lumpy cocoon-like structure just left of midline. Beyond the cocoon, the rectosigmoid junction was in midline. The membranous sac enclosing the colon was opened and the colon was freed. The small intestine was placed in the right-side and the large gut on the left side of the abdomen. The patient kept under observation for 7 days postoperatively and was discharged in stable condition. She recovered normally and followed up to 3 months.
Figure 1.
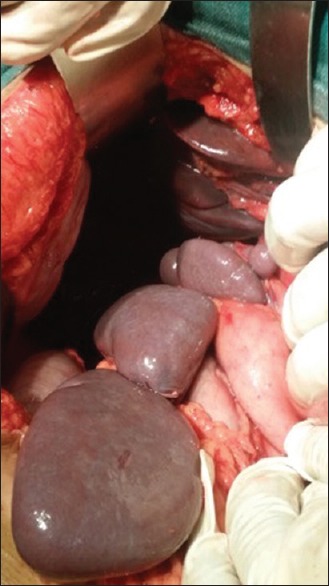
Intra-operative photograph showing multiple splenules in the right upper quadrant of the abdomen
Figure 2.
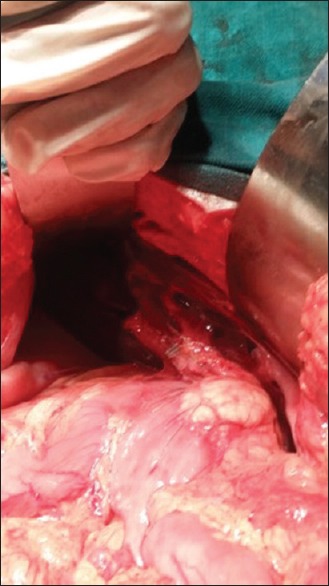
Intra-operative photograph showing malrotated pancreas with the head in midline and tail toward the right side
On the 2nd month of follow-up, computed tomography (CT) of the chest and abdomen was done to rule out other vascular and visceral anomalies. CT scan showed enlarged left lobe of the liver extending toward left hypochondrium. The left lobe of the liver found larger than the right one. The spleen could not be marked in the left hypochondrium instead multiple splenules seen floating on the right side of the abdomen [Figure 3]. The pancreas was also malrotated with head in the midline and tail lying on the right side. The stomach was lying on the right side with its greater curvature lying superolaterally and lesser curvature inferomedially [Figure 4]. There is reversal of the c loop of the duodenum which lies on the left side of the medial plane enclosing the head of the pancreas. Ileal loops also showing malrotation and lie on the left side. The ileocecal junction is on the left side which is medially placed. Ascending colon runs in the midline joined with cecum in the median plane. Hepatic flexure is placed slightly on the left side of the median plane continuing with transverse colon.
Figure 3.
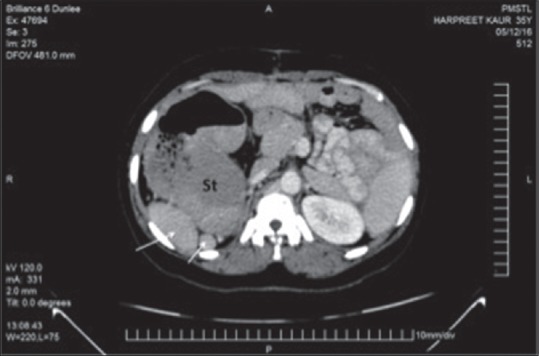
Axial computed tomography scan showing right-sided stomach with multiple spleens (arrows) present in the right upper quadrant
Figure 4.
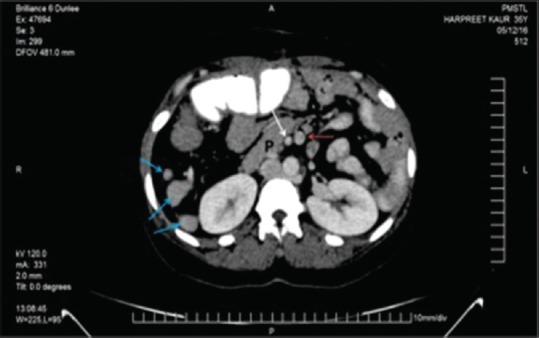
Axial computed tomography image revealing right-sided location of the body and tail of the pancreas and inverted relation of superior mesenteric artery (white arrow) and superior mesenteric vein (red arrow) and multiple spleens on the right side (blue arrows)
In addition to these findings, CT scan of the abdomen showed an interruption of the infrahepatic portion of the inferior vena cava (IVC) which then continues as the azygos vein opening directly into the superior vena cava (SVC). Both the lungs were bilobed with only one fissure visible [Figure 5].
Figure 5.
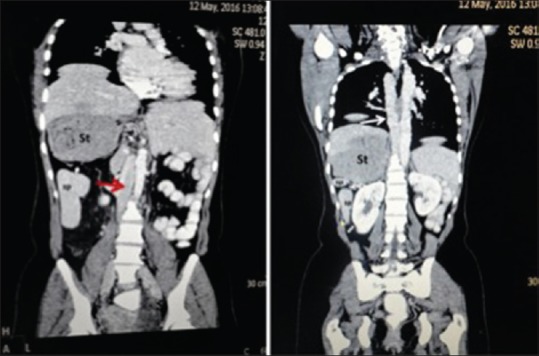
Coronal computed tomography image reveals inferior vena cava (red arrow) of the right lower abdomen continues above with prominent azygos vein (white arrow), right-sided stomach and multiple spleens
DISCUSSION
Appearance of primitive streak during the 3rd week of development helps in identifying the craniocaudal axis and demarcation of the left and right and division of dorsal or ventral surfaces. The first landmark in further development is looping of the heart tube to right in the 4th week and 90° clockwise rotation of the stomach during the 5th week. Afterward, the development progresses through a highly conserved and nonrandom process leading to evident left–right asymmetry.[2]
The normal asymmetrical arrangement of the organs in the chest and abdomen is called the situs solitus. The term situs solitus is described as the positioning of the spleen, stomach, aorta, and cardiac apex with bilobed lung on the left side and liver, gall bladder, IVC, and trilobed lungs on the right side. The anomalous positioning of the organs in the body organs are rotated around the midline and having mirror image arrangement of the normal situs solitus is called as situs inversus. It affects 0.01% of the general population and associated with 5%–10% of structural heart defects.[3] These structural orders can be present with dextroposition (heart in the right) and levoposition (heart in the left). Dextrocardia is dextroposition with cardiac apex to the anatomical right, and levocardia is levoposition with cardiac apex to the anatomical left.[1]
Situs ambiguous is another term defined as defect in the usual left and right distribution of visceral organs in the body cavity. There is no specific pattern of arrangement of organs with a vast number of different types of malformations presenting to clinics.[4] The term “heterotaxy” is also used for it interchangeably. It is a rare disorder that occurs in approximately 1/10,000 births.[5]
It is important to mention here that condition may or may not have fixed set of characteristics, but most of the time sufficient features are present to confirm the diagnosis. The etiology of this condition is not understood yet and is considered to be multifactorial.
Situs ambiguous can be divided into two subcategories depending on the presence or absence of the spleen and number of splenules. Situs ambiguous with absent spleen is called situs ambiguous with asplenia or right atrial isomerism, whereas situs ambiguous with multiple splenules is called situs ambiguous with polysplenia or left atrial isomerism.
Situs ambiguous with polysplenia or left isomerism
Situs abnormality with the presence of multiple spleen is termed as left atrial isomerism with anatomical and structural differences to its countertype situs ambiguous with asplenia. It is associated with multiple discrete spleens in the majority of patients, but some studies report patients with situs ambiguous who have a single, lobulated spleen or even a normal spleen.[6]
Nevertheless, the majority of patients have multiple spleens of variable size and number that may be located in either the left or right side of the abdomen. Left isomerism, in this case report, was associated with multiple variable-sized spleens which are anomalous in location and number. A recent review that included ten pediatric patients with polysplenia reported midline livers in five patients, right-sided livers in four, and a left-sided liver in one.[6] In a larger review of 146 patients with polysplenia, an abnormal arrangement of the abdominal organs was present in only 56%.[7]
Abnormality of bowel can present in different forms with or without features of intestinal obstruction. In a study, abnormalities of bowel rotation were observed in most of the patients with polysplenia. The small bowel was primarily right-sided, and the colon primarily left-sided with rotational abnormalities.[8]
There is a certain difficulty and risk in situs anomalies, especially in terms of diagnostic investigations and surgical operations.[9] Some cases may not meet the criteria conforming a single classification, and it would be necessary to define the situation and the problem for better patient management.
In this literature search, we came across several case presentations related to situs anomalies. Applegate et al. reported a series of ten polysplenic patients with heterotaxy syndrome. They showed that there were midline or left-sided liver in 6/10 patients, right-sided aortic arc and left-sided stomach and IVC in 4/10 patients, right-sided gallbladder and the absence of congenital heart disease (CHD) in 3/10 patients, left-sided polysplenia in 2/10 patients, and dextrocardia in only one of these patients.[6] The majority of patients with situs inversus have no significant clinical problems, with having cardiac abnormalities in 3%–9% and primary ciliary dyskinesia (PCD) in 25% of patients. PCD leads to chronic respiratory infection, sinusitis, and infertility due to ciliary motility disorders.[10] In situs ambiguous, most of the patients presents with congenital heart anomalies such as atrial septal defects (ASDs), ventricular septal defects (VSDs), atrio-VSD, pulmonary stenosis or atresia, and anomalous systemic venous or pulmonary venous return.[11] Asplenia syndrome with cardiac malformation leads to high mortality and morbidity (79% in the 1st year of life) is slightly higher than in polysplenia syndrome (61%) in the 1st year.[12]
In patients with left isomerism, the liver is mostly enlarged and centrally placed with left lobe usually larger than the right lobe; however, it may be malrotated in some cases. Gastrointestinal (GI) system may or may not show malrotation of the stomach, small and large intestine which if present may cause significant risk of volvulus. In left isomerism, most of the small intestine lies on the left side, and most of the large intestine lies on the right side. In addition, she may have truncated pancreas with only head of the pancreas seen lying in the c-loop of the duodenum with agenesis of the tail and body.[1,2]
In left atrial isomerism, many characteristics features related to the thoracic cage are present. Lungs may show only one fissure on the right side leading to bilobed lungs bilaterally. Lungs may show hyparterial bronchi bilaterally, i.e., the main bronchi passes inferior to the main pulmonary artery. Here, the defects of isomerism present with the lungs are bi-lobed, and the main bronchi pass inferior to the ipsilateral main pulmonary artery, that is, bilateral hyparterial bronchi.[1] The most common associated cardiovascular anomalies in polysplenia are noncyanotic congenital heart defects, including ASD and endocardial cushion defects.[13] She may present with interruption of the intra or infra hepatic part of the IVC continuing as azygos or hemiazygos vein which opens directly into the SVC.[7]
Situs ambiguous with asplenia or right isomerism
Right atrial isomerism is situs anomaly characterized by the absence of the spleen. In addition to right atrial isomerism, there are bilateral trilobed lungs with bilateral eparterial bronchi. Concomitant cardiac defects may include transposition of the great arteries, single ventricle physiology, and pulmonary atresia. Almost 50% of cases will have total anomalous pulmonary venous drainage. These abnormalities are more severe than those encountered in polysplenia, and patients often present with severe cyanosis and respiratory distress. The syndrome is associated with a poor prognosis and high mortality rate (80%–90%) in the 1st year of life.[14]
This is a more severe form of situs ambiguous with a patient having severe cardiovascular defect since birth, hence having very poor prognosis and very high mortality ranging 80%–90% during infancy. This is the reason why these patients are less frequently seen or diagnosed during adulthood. The hallmark of this condition is asplenia, i.e., absent of the spleen from both the sides of the abdomen. In addition, the patient will be having bilateral trilobed lungs with epiarterial bronchi, i.e., the main bronchi is passing superior to the main pulmonary artery.[1] About half of these patients suffer from various severe cardiovascular defects such as transposition of the great vessels, total anomalous pulmonary venous drainage, single physiological ventricle leading to serious outcomes such as severe cyanosis and respiratory distress leading to high mortality.[1,2,3] Extracardiac manifestations of asplenic heterotaxy include symmetric or transverse liver, midline stomach with GI malrotation/obstruction, bilateral trilobed lungs, and bilateral eparterial bronchi (upper lobe bronchus is over the second branch of the right pulmonary artery).[15]
Visceral anomalies of these syndromes are not necessarily uniform. These syndromes are incidentally found by multi-detector row CT, which is now widely used for the preoperative evaluation of CHD. The diagnosis of visceral malformations is necessary for better understanding and management of patients. These have been dependent on invasive studies such as angiocardiography, although more recently magnetic resonance imaging (MRI) has been advocated. In pediatric patients, MRI is far from ideal because of the need for sedation and monitoring during the examination. CT is capable of identifying visceral anomalies for each individual.[8] The decision to use CT versus MRI should be based on institutional equipment, scheduling, and availability as well as the patient's ability to cooperate. Furthermore, sonography in cases of situs ambiguus may reveal diagnostic features that can be used with radiographic signs to accurately diagnose the visceroatrial situs.[16]
Patients with complex cardiac lesions and heterotaxy have a poor prognosis, with mortality of over 85% for patients with asplenia and over 50% for patients with polysplenia. Management of patients with heterotaxy syndrome is complex and largely depends on the specific anatomy of both cardiac and noncardiac lesions. Cardiac and noncardiac management must be tailored to individual anatomy, including prophylaxis against encapsulated organisms for asplenic patients.[17]
Malrotation with acute-onset symptoms should prompt emergent open Ladd's operation. Asymptomatic patients with uncertain diagnosis should undergo diagnostic laparoscopy. Laparoscopic Ladd's procedure results in decreased peri-operative complications and faster recovery but may yield an increased risk of postoperative volvulus compared with the open approach. It is reasonable to delay Ladd's procedure in asymptomatic patients with CHD until after palliation of cardiac physiology. The utility of screening upper GI and elective Ladd's procedure in asymptomatic patients with heterotaxy syndrome remains debatable.[18]
Management of “asymptomatic” patients is, however, widely debated. Complications after Ladd's operation include adhesive intestinal obstruction in almost 25% of patients.[19,20,21] Several reports advocate screening for rotational anomalies and their subsequent correction in “asymptomatic” patients with heterotaxy.[22,23,24] Others suggest that a conservative policy “not to operate” is the best approach.[25,26]
In a study in the UK, the author shared the experience of cases for 20 years and concluded that “routine foregut anatomy screening is unnecessary in heterotaxy patients.” We strongly advocate a “watchful waiting” nonoperative policy provided patients remain under close follow-up. Decisions to correct variant foregut anatomy should be carefully individualized taking into account a patient's clinical features to offset the appreciable risks of morbidity and mortality from abdominal surgery.[27] Visceral heterotaxy is present in the developing world at a relatively later age and poses surgical challenges in a resource-scarce situation. A large proportion of patients remain unoperated due to complex manifestations of the syndrome, where surgical mortality is high and the outcome uncertain.[28]
CONCLUSION
Variations in situs ambiguous cases differ. Although the cases are tried to be classified in two main groups, variations are in a broad-spectrum and a single description is not possible. It is crucial to reveal these variations using imaging modalities because being aware of them before surgery and invasive intervention prevents the possible risks and complications.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/ her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Lambert TE, Kuller J, Small M, Rhee E, Barker P. Abnormalities of fetal situs: An overview and literature review. Obstet Gynecol Surv. 2016;71:33–8. doi: 10.1097/OGX.0000000000000262. [DOI] [PubMed] [Google Scholar]
- 2.Abut E, Arman A, Güveli H, Bölükbaş C, Kendir T, Dalay R, et al. Malposition of internal organs: A case of situs ambiguous anomaly in an adult. Turk J Gastroenterol. 2003;14:151–5. [PubMed] [Google Scholar]
- 3.Marta MJ, Falcão LM, Saavedra JA, Ravara L. A case of complete situs inversus. Rev Port Cardiol. 2003;22:91–104. [PubMed] [Google Scholar]
- 4.Brueckner M. Heterotaxia, congenital heart disease, and primary ciliary dyskinesia. Circulation. 2007;115:2793–5. doi: 10.1161/CIRCULATIONAHA.107.699256. [DOI] [PubMed] [Google Scholar]
- 5.Lin AE, Ticho BS, Houde K, Westgate MN, Holmes LB. Heterotaxy: Associated conditions and hospital-based prevalence in newborns. Genet Med. 2000;2:157–72. doi: 10.1097/00125817-200005000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Applegate KE, Goske MJ, Pierce G, Murphy D. Situs revisited: Imaging of the heterotaxy syndrome. Radiographics. 1999;19:837–52. doi: 10.1148/radiographics.19.4.g99jl31837. [DOI] [PubMed] [Google Scholar]
- 7.Peoples WM, Moller JH, Edwards JE. Polysplenia: A review of 146 cases. Pediatr Cardiol. 1983;4:129–37. doi: 10.1007/BF02076338. [DOI] [PubMed] [Google Scholar]
- 8.Fulcher AS, Turner MA. Abdominal manifestations of situs anomalies in adults. Radiographics. 2002;22:1439–56. doi: 10.1148/rg.226025016. [DOI] [PubMed] [Google Scholar]
- 9.Van de Perre S, Vanhoenacker FM, Petré C, Van Doorn J, De Schepper AM. Heterotaxy syndrome. JBR-BTR. 2004;87:158–9. [PubMed] [Google Scholar]
- 10.Winer-Muram HT. Adult presentation of heterotaxic syndromes and related complexes. J Thorac Imaging. 1995;10:43–57. doi: 10.1097/00005382-199501010-00004. [DOI] [PubMed] [Google Scholar]
- 11.Bernstein D. Cyanotic congenital heart disease: Lesions associated with increased pulmonary blood flow. In: Berhman RE, Kliegman RM, Jenson HB, editors. Nelson Textbook of Pediatrics. 16th ed. Vol. 2. Philadelphia: Saunders; 2000. pp. 1395–405. [Google Scholar]
- 12.Rose V, Izukawa T, Moës CA. Syndromes of asplenia and polysplenia. A review of cardiac and non-cardiac malformations in 60 cases withspecial reference to diagnosis and prognosis. Br Heart J. 1975;37:840–52. doi: 10.1136/hrt.37.8.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Mierop LH, Gessner IH, Schiebler GL. Asplenia and polysplenia syndromes. Birth Defects Orig Artic Ser. 1972;8:36–44. [Google Scholar]
- 14.Balan A, Lazoura O, Padley SP, Rubens M, Nicol ED. Atrial isomerism: A pictorial review. J Cardiovasc Comput Tomogr. 2012;6:127–36. doi: 10.1016/j.jcct.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 15.Cohen MS, Anderson RH, Cohen MI, Atz AM, Fogel M, Gruber PJ, et al. Controversies, genetics, diagnostic assessment, and outcomes relating to the heterotaxy syndrome. Cardiol Young. 2007;17(Suppl 2):29–43. doi: 10.1017/S104795110700114X. [DOI] [PubMed] [Google Scholar]
- 16.Oleszczuk-Raschke K, Set PA, von Lengerke HJ, Tröger J. Abdominal sonography in the evaluation of heterotaxy in children. Pediatr Radiol. 1995;25(Suppl 1):S150–6. [PubMed] [Google Scholar]
- 17.Lyimo FR, Pallangyo P, Majani N, Mushi TL, Kubhoja S. Complex congenital cardiac anomalies in the setting of right isomerism in a 31-month-old infant: A case report. J Med Case Rep. 2018;12:324. doi: 10.1186/s13256-018-1835-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lodwick DL, Minneci PC, Deans KJ. Current surgical management of intestinal rotational abnormalities. Curr Opin Pediatr. 2015;27:383–8. doi: 10.1097/MOP.0000000000000215. [DOI] [PubMed] [Google Scholar]
- 19.Murphy FL, Sparnon AL. Long-term complications following intestinal malrotation and the Ladd's procedure: A 15 year review. Pediatr Surg Int. 2006;22:326–9. doi: 10.1007/s00383-006-1653-4. [DOI] [PubMed] [Google Scholar]
- 20.El-Gohary Y, Alagtal M, Gillick J. Long-term complications following operative intervention for intestinal malrotation: A 10-year review. Pediatr Surg Int. 2010;26:203–6. doi: 10.1007/s00383-009-2483-y. [DOI] [PubMed] [Google Scholar]
- 21.Biko DM, Anupindi SA, Hanhan SB, Blinman T, Markowitz RI. Assessment of recurrent abdominal symptoms after Ladd procedure: Clinical and radiographic correlation. J Pediatr Surg. 2011;46:1720–5. doi: 10.1016/j.jpedsurg.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 22.Nakada K, Kawaguchi F, Wakisaka M, Nakada M, Enami T, Yamate N, et al. Digestive tract disorders associated with asplenia/polysplenia syndrome. J Pediatr Surg. 1997;32:91–4. doi: 10.1016/s0022-3468(97)90103-2. [DOI] [PubMed] [Google Scholar]
- 23.Ditchfield MR, Hutson JM. Intestinal rotational abnormalities in polysplenia and asplenia syndromes. Pediatr Radiol. 1998;28:303–6. doi: 10.1007/s002470050358. [DOI] [PubMed] [Google Scholar]
- 24.Ferdman B, States L, Gaynor JW, Hedrick HL, Rychik J. Abnormalities of intestinal rotation in patients with congenital heart disease and the heterotaxy syndrome. Congenit Heart Dis. 2007;2:12–8. doi: 10.1111/j.1747-0803.2007.00066.x. [DOI] [PubMed] [Google Scholar]
- 25.Choi M, Borenstein SH, Hornberger L, Langer JC. Heterotaxia syndrome: The role of screening for intestinal rotation abnormalities. Arch Dis Child. 2005;90:813–5. doi: 10.1136/adc.2004.067504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Papillon S, Goodhue CJ, Zmora O, Sharma SS, Wells WJ, Ford HR, et al. Congenital heart disease and heterotaxy: Upper gastrointestinal fluoroscopy can be misleading and surgery in an asymptomatic patient is not beneficial. J Pediatr Surg. 2013;48:164–9. doi: 10.1016/j.jpedsurg.2012.10.033. [DOI] [PubMed] [Google Scholar]
- 27.Cullis PS, Siminas S, Salim A, Johnson R, Losty PD. Heterotaxy and intestinal rotation anomalies: 20 years experience at a UK regional paediatric surgery centre. Pediatr Surg Int. 2015;31:1127–31. doi: 10.1007/s00383-015-3755-3. [DOI] [PubMed] [Google Scholar]
- 28.Changlani DK, Kotecha M, Changlani TD, Varghese R, Kumar RS. Visceral heterotaxy in the developing world. Heart Lung Circ. 2012;21:598–605. doi: 10.1016/j.hlc.2012.05.739. [DOI] [PubMed] [Google Scholar]


