Summary:
Background:
Identifying new antifungals for cryptococcal meningitis remains a priority given the inadequacy of current therapy. Sertraline has previously demonstrated in vitro and in vivo activity against Cryptococcus. We evaluated the efficacy of adjunctive sertraline for cryptococcal meningitis in a double-blind, randomised, placebo-controlled clinical trial.
Methods:
We assessed 18-week survival among HIV-infected Ugandan adults with cryptococcal meningitis enrolled from 09 March 2015 to 29 May 2017. Participants were randomly assigned to receive standard therapy with 7–14 days of amphotericin (0·7–1·0 mg/kg/day) + fluconazole (starting at 800 mg/day) with either adjunctive sertraline or placebo. Sertraline was administered at a dose of 400 mg/day for 2 weeks, followed by 200 mg/day for 12 weeks, then tapered off over 3 weeks. Randomisation in a 1:1 ratio was performed with variable block sizes of 2 and 4, with stratification by site (Kampala or Mbarara) and antiretroviral status (experienced or naïve). Analysis was by intention-to-treat. This study is registered with ClinicalTrials.gov, number .
Findings:
The trial was stopped for futility after enrolling 460 of a planned 550 participants. The 18-week mortality was 52% (120/229) in the sertraline group and 46% (106/231) in the placebo group (hazard ratio for sertraline, 1.21; 95%CI, 0·93–1·57; P=0·15). The rate of fungal clearance from cerebrospinal fluid was similar between groups, as was incidence of grade 4 or 5 adverse events (31% in the sertraline group vs. 33% in those receiving placebo; p=0·98). The incidence of relapse and paradoxical immune reconstitution inflammatory syndrome were low in both groups, and re-hospitalization rates were similar.
Interpretation:
Sertraline did not reduce mortality and should not be used to treat patients with HIV-associated cryptococcal meningitis. The reasons for sertraline inactivity appear to be multifactorial and may be related to insufficient duration of therapeutic sertraline concentrations.
Funding:
National Institutes of Health, Medical Research Council/Wellcome Trust.
Keywords: Cryptococcus, cryptococcal meningitis, HIV, sertraline, antifungal therapy, drug repurposing
INTRODUCTION
Cryptococcal meningitis remains one of the most frequent and deadly opportunistic infections in HIV patients, and is responsible for 15% of AIDS-related deaths.1 Early mortality remains unacceptably high, largely due to prohibitive costs, limited availability, and frequent toxicity associated with an insufficient armamentarium of effective antifungals. Given these challenges, more effective and less toxic antifungal regimens are urgently needed to improve outcomes. However, antifungal development is hindered by the high costs and significant time required to bring new drugs to market, coupled by a reluctance for pharmaceutical companies to invest in drugs that offer little promise for financial return. As a result, there are few new anti-cryptococcal agents in the development pipeline.
Given the inherent challenges involved in de novo antifungal development, drug repurposing is becoming an attractive approach for new antifungal discovery. Insomuch as existing pharmaceutical and safety data already exists, investigation into their clinical use as antifungals is greatly accelerated, offering particular benefit in the development of new therapies for globally neglected fungal diseases such as cryptococcal meningitis. This approach expedites the time required from bench to bedside, especially when it involves a new indication for an existing and widely-used marketed drug. The selective serotonin reuptake inhibitor (SSRI) antidepressant sertraline provides an archetypal example of such repurposing for a potential role in the treatment of cryptococcal meningitis.
Sertraline has been shown in multiple studies to have potent in vitro fungicidal activity against Cryptococcus neoformans that is synergistic to fluconazole.2–7 Sertraline has also been effective in in vivo models of experimentally infected mice, where the inhibitory effect of sertraline was particularly potent in the brain of Cryptococcus-infected mice with efficacy similar to fluconazole.3,5 The most potent anti-fungal effects were observed in mice treated with sertraline and fluconazole combination therapy. In humans, sertraline readily crosses the blood-brain barrier and concentrates in the central nervous system,8 and abundant clinical data supports the safety and long-term use of sertraline as an antidepressant.9
Taken together, these studies demonstrate convincing clinical potential for sertraline in the treatment of cryptococcal meningitis. This potential led to early-phase clinical trials evaluating the safety and efficacy of adjunctive sertraline based on the rate of fungal clearance from cerebrospinal fluid (CSF).7,10 In a previous open-label, dose-ranging pilot study among patients with HIV-associated cryptococcal meningitis that we conducted in Uganda, adjunctive sertraline at doses of 100–400 mg/day was safe, well-tolerated, and appeared to have improved rates of CSF cryptococcal clearance compared to historical controls.7 This was used as justification for the current study to determine whether treatment with adjunctive sertraline would improve survival among adults with HIV-associated cryptococcal meningitis.
METHODS
Study design and participants
We assessed 18-week survival among HIV-infected adults (aged ≥18 years) with first episode cryptococcal meningitis from 09 March 2015 to 29 May 2017 in a double-blind, randomised, placebo-controlled trial. Participants were recruited from two referral hospitals in Kampala and Mbarara, Uganda and randomly assigned in a 1:1 ratio to receive standard therapy for cryptococcal meningitis with either adjunctive sertraline or placebo.
Diagnosis of cryptococcal meningitis was made at bedside via a positive CSF CrAg lateral flow assay (Immy Inc., Norman, Oklahoma, USA). Participants were included in the study if they had concurrent HIV infection and were willing to receive protocol-specified lumbar punctures (LPs). Patients were excluded from participating in the study if they had received more than two doses of amphotericin B, had a previous history of cryptococcal meningitis, were unable to attend scheduled outpatient visits, had jaundice or known liver cirrhosis, were pregnant or were breastfeeding.
Participants provided written informed consent at time of cryptococcal diagnosis. Approval for the clinical trial was obtained from the Uganda National Council for Science and Technology and institutional review boards in Uganda and at the University of Minnesota.
Randomisation and masking
A computer-generated randomisation sequence was accessible only to the study statisticians and the central study pharmacist in Kampala, neither of whom were involved in enrolment or clinical care. This randomisation list was used to prepare blinded treatment boxes (inpatient) and bottles (outpatient) containing sertraline or identical placebo in tablet formulation. Randomisation was performed with variable block sizes of 2 and 4 and was stratified according to antiretroviral therapy (ART) status (ART-experienced or ART-naïve) and by enrolment site. “ART-experienced” was defined as any participant that had received ART within 1 month prior to enrolment. ART status and timing has already been described in detail in a study that combined participants in this and our earlier phase 2 study.11
Treatment and procedures
Participants received standard antifungal therapy plus adjunctive sertraline at 400 mg/day (4 tabs) or placebo for 14 days, followed by a dose of 200 mg/day (2 tabs) for the next 12 weeks prior to discontinuing sertraline or placebo, which were tapered over 3 weeks. Administration of study drug was by directly observed therapy via enteral route (by mouth or nasogastric tube) over the first 14 days of the study, and by self-administration thereafter. All patients and clinicians providing care and assessing outcomes were blinded to study treatment allocation. Standard antifungal therapy included intravenous amphotericin B deoxycholate (0·7–1·0 mg/kg/day) for up to 14 days and oral fluconazole (800 mg/day, dose adjusted to 1200 mg/day if concurrently receiving rifampin) for ~4 weeks, followed by fluconazole 400 mg/day for 8 weeks of consolidation therapy and fluconazole 200 mg/day for secondary prophylaxis. The use of high-dose fluconazole as part of combination induction therapy was based on guideline recommendations in the absence of flucytosine, which remains unavailable in Uganda.
Amphotericin was generally discontinued if the baseline CSF culture was sterile after 7 days of incubation, with continuation of fluconazole and sertraline or placebo. For ART-naive participants or those on failing regimens, ART was initiated or switched 4–6 weeks after cryptococcal diagnosis. Participants were monitored for signs of depression throughout the study. A CES-D score of ≥ 16 was interpreted as suggestive of depressive symptomatology though a diagnosis of depression required complete clinical assessment. In cases of severe depression, the protocol dictated a switch to open-label sertraline and referral to a psychiatrist for further management.
Therapeutic LPs were routinely performed using manometers on day 3, day 7, at the end of amphotericin therapy to determine the CSF pressure and response to treatment. CSF pressure was measured using spinal manometers and CSF drained as necessary. Additional LPs were performed as indicated for symptoms of elevated intracranial pressure and when opening pressure was ≥250 mmH2O during the previous days’ lumbar puncture. Quantitative CSF cultures were performed with five serial 1:10 dilutions of 100 μL of CSF, as described previously.12,13 CSF culture sterility was defined as no growth of Cryptococcus after 10 days of incubation, with a limit of detection of 10 colony-forming units (CFU) per mL.
Clinical and laboratory data were collected over an 18-week period. We used the National Institute of Allergy and Infectious Diseases Division of AIDS toxicity scale, version 2·0, to assess adverse events in all participants. Because of an expected 80% incidence of grade 3–5 adverse events with amphotericin B deoxycholate, we only reported grade 4–5 adverse events, which were expected to have an incidence of 35–40% and to be dominated by amphotericin-related toxic effects.7,14,15 Assessment of neurocognitive function and depression screening were performed after 3 months, the timing of which was intended to be as close to the end of the 18-week follow-up period while still allowing scheduling flexibility given the significant demands required of neurocognitive evaluation.
The sertraline dosing regimen used in this study was based on previously published sertraline distribution into human brain (median 16·5-fold; interquartile range (IQR), 13·0 to 21·3-fold increase over plasma)8, the minimum inhibitory concentration (MIC) of sertraline in Ugandan isolates,4,7 and the plasma concentrations observed in our earlier pilot study to determine the probability of reaching a target concentration in brain tissue using 25,000 replicates (Microsoft Excel). Additional sertraline plasma concentrations were measured after completion of this trial on a sub-sample of 106 randomly chosen participants at 7–14 days (n=106) and at 4 weeks (n=77), as described.7
Outcomes
The primary outcome of the study was 18-week survival. Secondary outcomes included early fungicidal activity (EFA) to demonstrate the Cryptococcus clearance rate in CSF, incidence of adverse events, 2-week CSF culture sterility, incidence of relapse or paradoxical immune-reconstitution inflammatory syndrome (IRIS), quantitative neurocognitive performance z-score (QNPZ-8),16,17 center for epidemiological studies depression score (CES-D), and proportion of participants diagnosed with severe depression.
Monitoring Plan
An independent data and safety monitoring committee oversaw trial safety. The study was designed for 3 interim analyses (after 25%, 50%, and 75% of participants had at least 18 weeks of potential follow-up time) and one final analysis (Supplementary Appendix). The study statisticians were unblinded to treatment allocation and prepared the safety reports. A trial steering committee consisting of three independent members, two study investigators, and an observer provided advice on the conduct of the trial.
Statistical Analysis
With a planned enrolment of 550 participants, the study was powered to detect a hazard ratio (sertraline compared to placebo) of 0·65, assuming a two-sided alpha = 0·05, and a 40% 18-week mortality rate in the placebo arm. Under these assumptions, the power was approximately 80%, the expected mortality rate in the sertraline arm was 28% (for a 12% absolute difference), and a total of 188 deaths were expected.
All analyses were by intent-to-treat, with comparison of sertraline vs. placebo groups. All participants randomised were included in the final analyses using all available study data. Time-to-event analyses, including Kaplan-Meier curves and proportional hazards regression models, were used to summarize the pattern of mortality over 18-weeks. Preplanned subgroup analyses for mortality were done by randomisation strata (clinical site and ART use at baseline) and normal vs abnormal Glasgow Coma Score (GCS). A priori subgroup analyses for mortality included gender, presence of CSF pleocytosis (WBC ≤ 5 cells/mm3 vs WBC > 5 cells/mm3), and baseline fungal burden.
The secondary outcome of early fungicidal activity was calculated for all participants with at least two quantitative CSF cultures in the first 18 days of the study via 1) linear regression models as previously reported in multiple cohorts,18,19 and by 2) using a mixed effects regression model with a random intercept for individual measurements to account for the intra-subject correlation induced by repeated measures over time. All CSF culture time points were used in the calculation of EFA. Additional EFA models were calculated limiting the CSF cultures to three time points (generally days 1, 7 and 14), to compare the EFA to other studies with more limited quantitative culture collection and provide context for discussion. To determine if fungal clearance differed by the amount of CSF removed through serial lumbar punctures, we compared EFA by tertiles of total volume of CSF removed over the first 18 days of the study.
Other secondary endpoints were summarized with cumulative incidence functions to account for the competing risk of mortality, by using logistic regression and Fisher’s Exact methods, or were compared with general linear models or Wilcoxon rank-sum tests, as appropriate. All analyses were conducted with SAS version 9·3 (SAS Institute, Cary, NC, USA).
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
RESULTS
After screening 842 patients with suspected meningitis and enrolling 460 participants of an intended 550 with cryptococcal meningitis, the data and safety monitoring board recommended that the trial be stopped for futility at the third interim analysis based on the failure of adjunctive sertraline to demonstrate a benefit across key outcomes (Figure 1). No unplanned interim analyses were conducted. At the time of trial suspension, 229 patients had been randomised to the sertraline group and 231 randomised to the placebo group. Most participants were recruited from Kampala (77%; 353/460), and slightly less than half of the participants in each treatment group were already receiving ART at time of enrolment (Supplemental Table 1). The proportion of participants presenting with baseline CSF pleocytosis (white-cell count >5 cells/μL) was higher in the sertraline group (p=0·02; Table 1). All other baseline characteristics were well balanced (p≥0·10) between the two study groups. High baseline fungal burdens were common, and 8% (37/460) had sterile CSF with a positive CSF cryptococcal capsular polysaccharide antigen.
Figure 1: Modified CONSORT diagram of study participants.
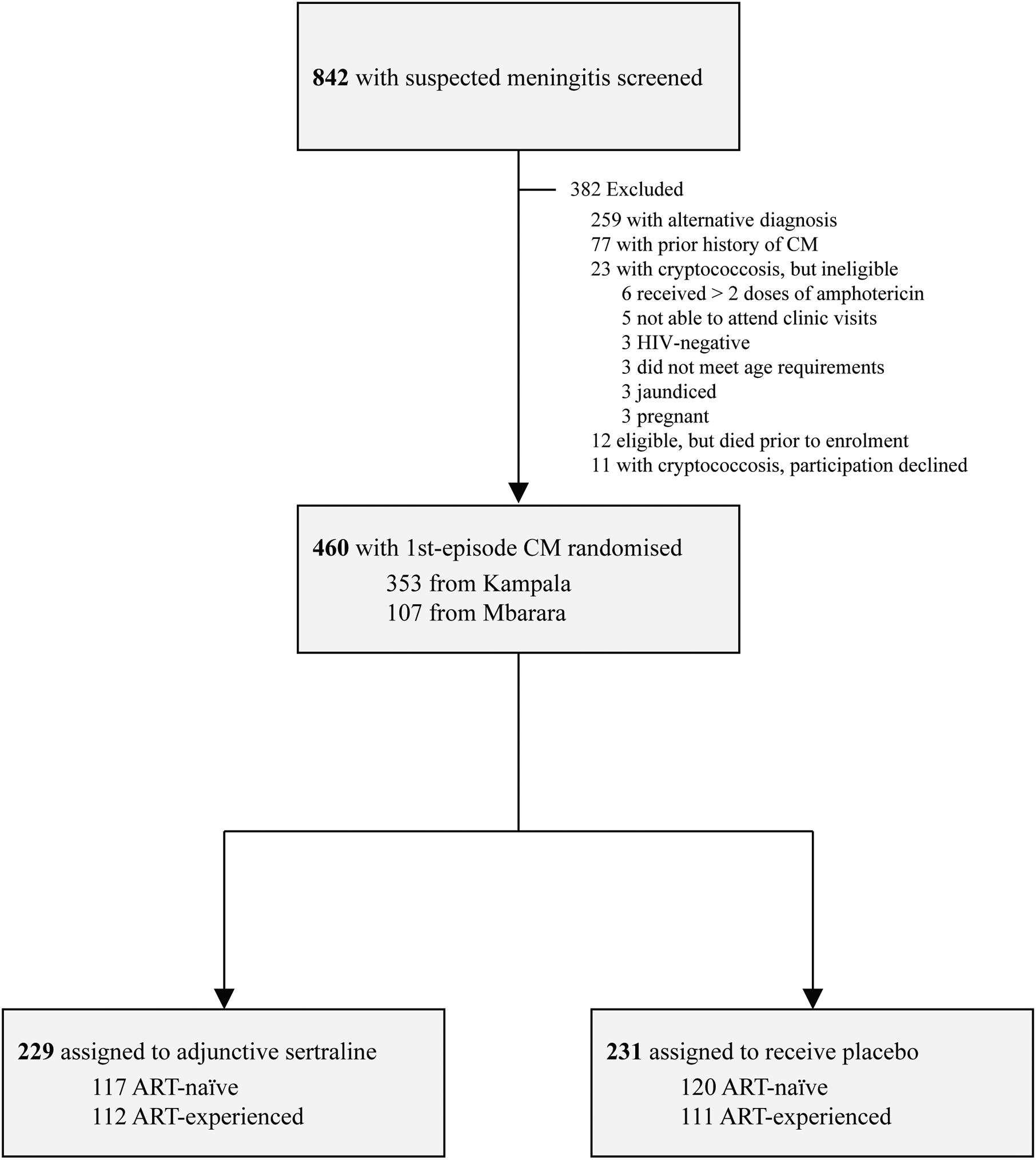
Persons with suspected were screened for the study. Those with cryptococcal meningitis meeting enrolment criteria and providing consent were randomised in a 1:1 distribution to receive adjunctive sertraline or matched placebo. Even proportions of participants were ART-experienced in each treatment group, and constituted nearly half of the patients enrolled. All 460 randomized participants were included in the primary analysis using all available data.
Table 1: Baseline characteristics by treatment group.
| Baseline Characteristic | Sertraline | Placebo | |
|---|---|---|---|
| (N=229) | (N=231) | ||
| Demographics | |||
| Age, years | 35 [29, 40] | 35 [30, 41] | |
| Women | 97 (42%) | 87 (38%) | |
| Clinical characteristics | |||
| Weight, kg | 51·5 [49·0, 60.0] | 54·0 [49·0, 60·0] | |
| Glasgow Coma Score < 15 | 116 (51%) | 105 (46%) | |
| Hemoglobin, g/dL | 11·3 [9·7, 12·9] | 11·7 [10·0, 12·9] | |
| Creatinine, mg/dL | 0·7 [0·6, 0·9] | 0·7 [0·6, 0·9] | |
| CD4 cells/μL | 17 [6, 46] | 13 [6, 41] | |
| Receiving antiretroviral therapy | 112 (49%) | 111 (48%) | |
| Receiving TB therapy | 18 (8%) | 20 (9%) | |
| Baseline CSF Analysis | |||
| CSF opening pressure, mm H2O | 248 [170, 380] | 270 [185, 400] | |
| CSF quantitative culture, log10 CFU/mL | 4·7 [2·8, 5·6] | 4·8 [3·3, 5·5] | |
| Sterile CSF cryptococcal culture | 25 (11%) | 15 (7%) | |
| CSF white-cell count >5 cells/μL | 90 (41%) | 67 (30%) | |
| CSF protein, mg/dL | 47 [23, 106] | 42 [22, 100] | |
Data are median (IQR) or N (%). Abbreviations: TB, tuberculosis; CSF, cerebrospinal fluid.
P-value from Kruskall-Wallis test for medians, Chi-squared test for proportions.
The primary outcome of 18-week mortality was 52% (120/229) in the sertraline group and 46% (106/231) in the placebo group (hazard ratio for sertraline, 1·21; 95% CI, 0·93–1·57; p=0·15) (Figure 2). In subgroup analysis, no differences in 18-week mortality were observed between treatment groups by enrolment site, ART status and timing, baseline Glasgow Coma Scale (GCS), gender, the presence of CSF pleocytosis, or initial fungal burden (Supplemental Figure 1). Despite the imbalance in the proportion of participants presenting with baseline CSF pleocytosis, there was no significant interaction with treatment group in a post hoc proportional hazards regression model (p=0·76).
Figure 2: Kaplan-Meier survival plot for sertraline and placebo.
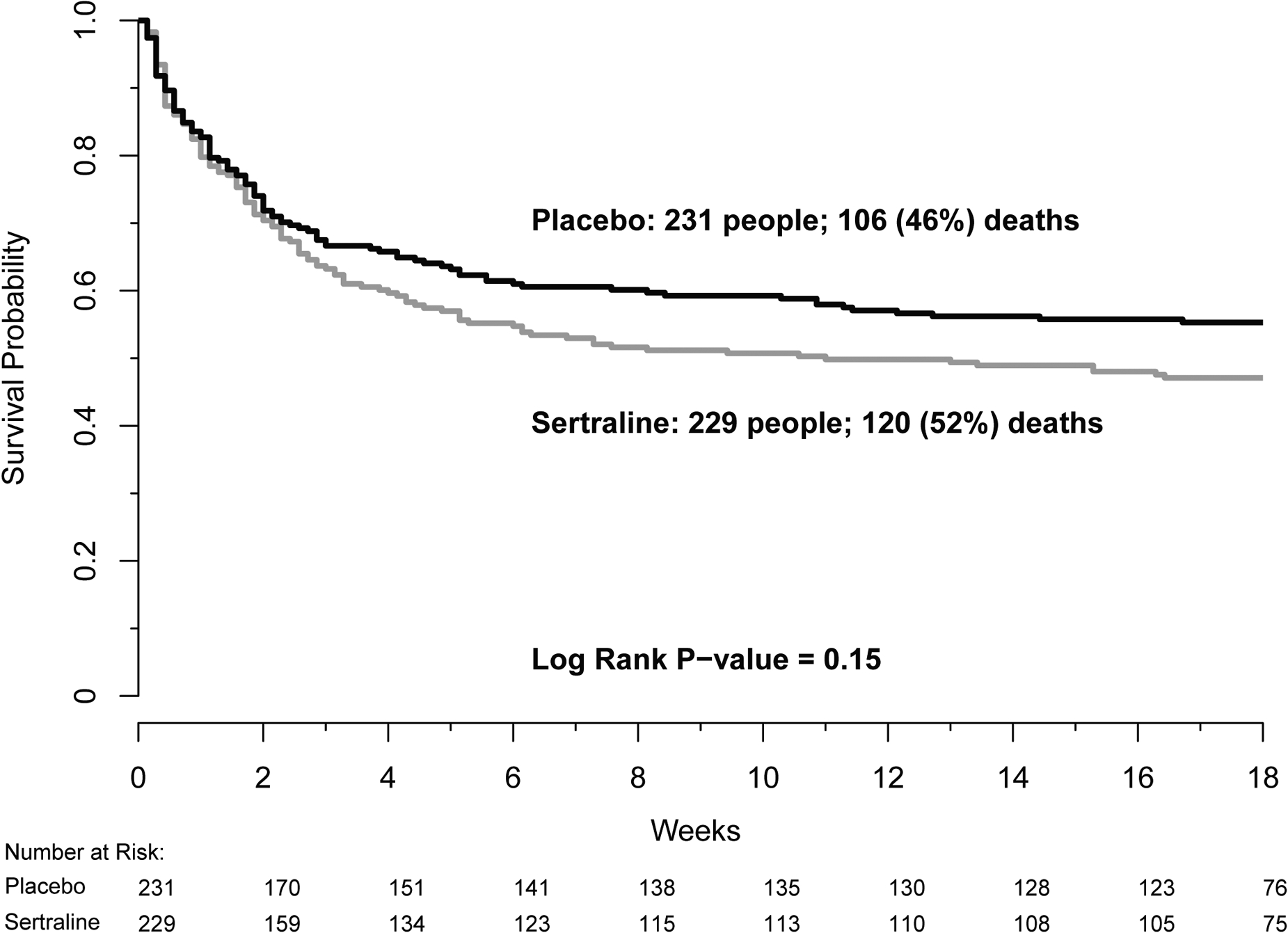
Kaplan-Meier survival plot for sertraline (grey line) and placebo (black line). Overall 18-week mortality was 52% in the sertraline group compared to 46% in the placebo group, resulting in a hazard ratio of 1·21 (95% CI, 0·93–1·57; p=0·15).
When using all available CSF culture time points collected by lumbar punctures through the first 18 days of study participation, the rate of fungal clearance from CSF was similar between groups using either method of calculation (Table 2). CSF culture sterility was obtained in 45% of participants by 2 weeks, and this did not differ by treatment group. Participants received a median of 3 (IQR 2–4, maximum=15) lumbar punctures over the first 18 days. Early fungicidal activity varied little by ART status or by total volume of CSF removed (Supplemental Figure 2). When the CSF quantitative cultures used for EFA estimations was limited to those collected by lumbar punctures on days 1, 7 and 14, the rate of fungal clearance decreased (Supplemental Table 2).
Table 2: Secondary outcomes.
| Sertraline | Placebo | |||
|---|---|---|---|---|
| Event | (N=229) | (N=231) | P-value* | |
| EFA†, general linear regression | 0·43 (0.37 to 0.50) | 0·47 (0.40 to 0.54) | 0·49 | |
| EFA†, mixed-effects regression | 0·33 (0·30 to 0·36) | 0·33 (0·30 to 0·35) | 0·56 | |
| 2-week CSF sterility‡ | 90 (44%) | 101 (47%) | 0·59 | |
| Grade 4 or 5 adverse events§ | 72 (31%) | 75 (33%) | 0·98 | |
| Re-hospitalization | 30 (13%) | 29 (13%) | 0·53 | |
| Serotonin syndrome | 0 (0%) | 0 (0%) | ||
| Culture-positive relapse | 2 (1%) | 2 (1%) | ||
| Paradoxical CM-IRIS‖ | 3 (3%) | 7 (6%) | ||
| Lost to follow-up¶ | 3 (1%) | 3 (1%) | ||
Data are mean (95% CI) or N (%). Abbreviations: EFA, early fungicidal activity; CM-IRIS, cryptococcal meningitis-immune reconstitution inflammatory syndrome.
P-value from Chi-squared test for proportions.
Among participants with at least two quantitative CSF cultures in the first 18 days; N=173 for sertraline and N=180 for placebo. Expressed in −log10 CFU/mL/day.
Documented sterile CSF culture within first 18 days of study, excluding those with no growth at baseline; N=204 for sertraline and N=216 for placebo.
Combined clinical and laboratory adverse events. Reported as number of patients, see Table 3 for further details including total number and types of adverse events.
Among participants that were ART-naïve at randomisation; N=118 for sertraline and N=122 for placebo.
Lost to follow-up for at least 6 weeks (no data after 12 weeks).
The numbers of participants with culture-positive relapse (1% in each group) or re-hospitalization (13% in each group) within 18 weeks were also similar in the two groups. The overall incidence of paradoxical CM-IRIS was low, with three cases observed in the sertraline group and seven cases observed in the placebo group.
The incidence of grade four or grade five adverse events (laboratory or clinical) in this critically ill population over the 18-week study period was 31% (72 participants experiencing 141 events) in the sertraline group, compared to 33% (75 participants experiencing 121 events) in the placebo group (p=0·98). Most grade 4–5 adverse events were related to amphotericin toxicity and rates of individual adverse events were similar between groups (Table 3). Anemia, acute kidney injury and electrolyte abnormalities (hypokalemia, hyponatremia) were the most common AEs observed in both treatment groups. The causes of death by clinician determination was also similar between groups, with most deaths attributed to cryptococcal meningitis, sepsis, or tuberculosis (Supplemental Table 3). No cases of serotonin syndrome were observed in the trial. We observed no difference in overall neurocognitive performance between groups at 3 months (mean QNPZ-8 score −1·3 for sertraline compared to −1·4 for placebo; p=0·43). Depression scores after 3 months were mildly improved among those receiving sertraline (median CES-D score of 13·2, 95%CI 11·0–15·5) compared to those receiving placebo (median CES-D score of 16·6, 95%CI 14·3–18·8; p=0·04). No participants required a switch from blinded study drug to open-label sertraline for severe depression.
Table 3: Adverse events by treatment group.
| Sertraline | Placebo | |||
|---|---|---|---|---|
| Event | N (%) | N (%) | ||
| Total grade 4–5 AEs reported, by event | 141 | 121 | ||
| Laboratory AEs, by participant | ||||
| Grade 3–4 | 65 (28·4%) | 66 (28·6%) | ||
| Grade 4–5 | ||||
| 1 event | 40 (17·5%) | 48 (20·8%) | ||
| 2 events | 12 (5·2%) | 8 (3·5%) | ||
| 3–5 events | 5 (2·2%) | 3 (1·3%) | ||
| Grade 4–5, types* | ||||
| Elevated creatinine | 7 (3·1%) | 6 (2·6%) | ||
| Hypokalemia | 8 (3·5%) | 2 (0·9%) | ||
| Hyponatremia | 10 (4·4%) | 11 (4·8%) | ||
| Hypernatremia | 2 (0·9%) | 0 (0·0%) | ||
| Hypomagnesemia | 0 (0·0%) | 2 (0·9%) | ||
| Anemia | 30 (13·1%) | 30 (13·0%) | ||
| Leukopenia | 1 (0·4%) | 0 (0·0%) | ||
| Neutropenia | 4 (1·7%) | 0 (0·0%) | ||
| Thrombocytopenia | 1 (0·4%) | 0 (0·0%) | ||
| Lymphopenia | 10 (4·4%) | 9 (3·9%) | ||
| Elevated ALT | 0 (0·0%) | 1 (0·4%) | ||
| Elevated AST | 0 (0·0%) | 3 (1·3%) | ||
| Elevated bilirubin | 9 (3·9%) | 9 (3·9%) | ||
| Clinical AEs, by participant† | ||||
| 1 event | 42 (18·3%) | 35 (15·2%) | ||
| 2–3 events | 8 (3·5%) | 6 (2·6%) | ||
Percentages expressed from total enrolled in each group, N=229 for sertraline and N=231 for placebo. Abbreviations: AE, adverse event; ALT, alanine aminotransferase, AST, aspartate aminotransferase.
Not mutually exclusive.
Grade 4–5 clinical AEs (clinical AEs < grade 4 were not captured).
Sertraline concentrations in plasma were quantified among 106 participants between 7–14 days of therapy, when steady state was reached. The overall median sertraline plasma steady state concentration was 390 ng/mL (IQR 220–610) when receiving 400 mg/day; however, the presence of ART resulted in 45% lower median sertraline concentrations (Figure 3). In those receiving 400mg/day without ART (n=53), sertraline concentrations were 540 ng/mL (IQR 310–680) compared with 300 ng/mL (IQR 130–500) in those receiving concurrent ART (n=53) at presentation (P<0·0001). Sertraline concentrations were lowest for regimens that included nevirapine (median 150 ng/mL; n=10) compared to those with efavirenz (median 285 ng/mL; n=26) or atazanavir/ritonavir (median 435 ng/mL; n=6). After 2 weeks, participants received sertraline at 200mg/day through 12 weeks. In ART-naïve participants receiving 200mg/day at 4 weeks (n=42), median sertraline concentrations were 177 ng/mL (IQR 80–335) compared with 179 ng/mL (IQR 104–344) in those receiving concurrent ART (n=35) at 4 weeks, without a statistical difference (P=0·95). Among nine participants with specimens measured between 8–12 weeks, the median sertraline level was 70 ng/mL (IQR 52 to 221).
Figure 3: Plasma concentrations of sertraline among trial participants.
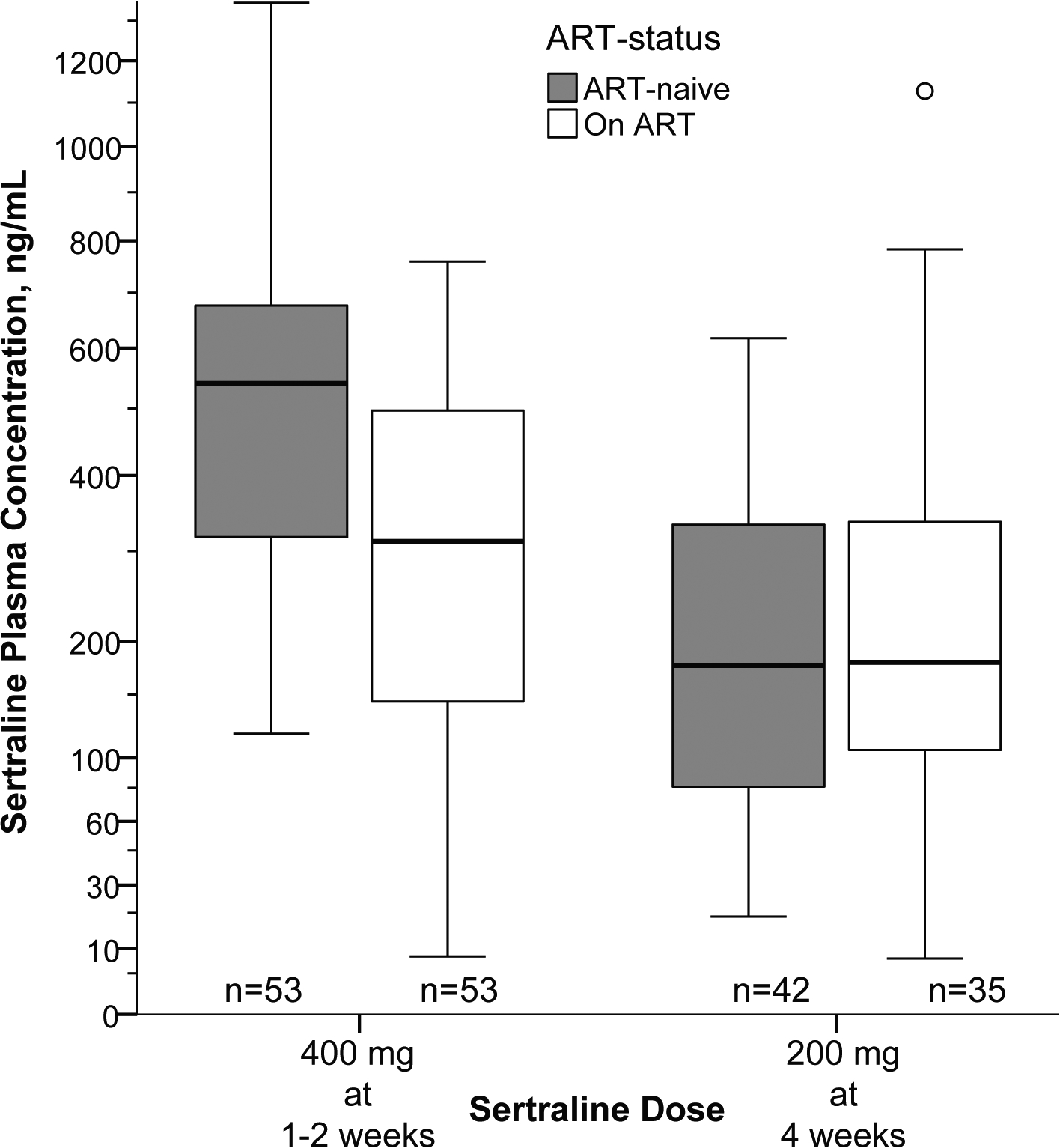
Steady state plasma concentrations while receiving 400mg/day were measured between 7 and 14 days of sertraline therapy and averaged. Participants receiving ART had ~45% lower median plasma sertraline concentrations (P<·001). Sertraline concentrations were similar at 4 weeks when receiving 200mg/day of sertraline, regardless of ART-status.
Based on the MIC of sertraline on clinical isolates of Cryptococcus from Uganda, observed plasma exposure, and published distribution in the brain, the probability of achieving therapeutic sertraline concentrations in brain at steady state was 92% for those receiving 400mg/day without ART, 72% with 400mg/day and ART, and only 54% when receiving 200mg/day with ART (Supplemental Figure 3).
DISCUSSION
In this phase 3 randomised trial, we found that sertraline did not reduce mortality when added to standard therapy among patients with HIV-associated cryptococcal meningitis. The consistency of hazard ratios for survival across sites and ART strata strengthen our conclusion that sertraline provides no survival benefit at the doses and duration tested. Based on the results of this trial, sertraline cannot be recommended in the treatment of cryptococcal meningitis.
We hypothesized that sertraline would improve outcomes by improving the rate of fungal clearance from the central nervous system. We did not observe differences in fungal clearance with the addition of sertraline to standard combination therapy with amphotericin and fluconazole. The reasons for in vivo sertraline inactivity are likely multifactorial and possibly related to inadequate drug concentrations or drug-drug interactions. Based on predicted sertraline distribution, known MICs of sertraline in Cryptococcus, and the time required for sertraline to reach steady state, therapeutic concentrations should have been attained but may have only been sustained in the brain over a relatively short period. For patients receiving 400mg/day, we estimate that therapeutic concentrations would have been reached only between days 7 and 14 of treatment. This short time frame of therapeutic exposure, during which time amphotericin was being received, would have been unlikely to substantially effect positive outcomes.
Our findings are consistent with a recent small Mexican study in individuals with HIV-associated cryptococcal meningitis.10 This 12-person study, which utilized the same antifungal backbone (amphotericin plus high dose fluconazole) for induction therapy, failed to demonstrate a difference in rate of fungal clearance with adjunctive sertraline at 200mg daily when compared to placebo given over 14 days. Although the higher doses and longer duration of sertraline administered in our trial might be expected to have improved fungal clearance, this did not occur in our study in the presence of amphotericin B.
One possible reason for the failure to reach therapeutic concentrations was an under-appreciation of the interaction between sertraline and ART. While unable to make any conclusions about interactions between individual ART regimens, we observed substantially lower sertraline concentrations in the presence of ART, particularly at higher sertraline doses. We also observed lower than expected plasma concentrations of sertraline in those collected at later time points (8–12 weeks). Possible explanations for low plasma concentrations at later time points include poor sertraline adherence in the absence of directly observed therapy or increased induction of metabolism by ART. Differences observed in plasma sertraline concentrations by ART status did not translate to differences in fungal clearance, suggesting that sertraline had little effect on fungal clearance even in ART-naïve individuals.
Although we failed to demonstrate a survival benefit for sertraline, we observed good safety and tolerability of sertraline despite the high doses used during the induction phase. In addition, sertraline appears to have been beneficial for treating depression, with a CES-D score 3.4 points lower in the sertraline group at week 14 than the placebo group. Consistent with other HIV trials in sub-Saharan Africa,20,21 we found depression to be prevalent in our population. This study further underscores the value of early depression screening and treatment when indicated among HIV-infected patients presenting with opportunistic infections.
Because of toxicity and difficulties with the availability and administration of amphotericin, there is a critical need for new, well-tolerated, low-cost, orally-administered antifungals. Flucytosine is still not available in low and middle income countries. Agents with lower stand-alone fungicidal activity, while not intended to be a substitute for amphotericin B, may still be suitable for adjunctive use in combination induction therapy or for use outside the induction period provided they can be administered orally for extended durations with low toxicity. The potential of sertraline, like with fluconazole, is as an adjunct to more active induction therapy. However, evaluating the antifungal efficacy of candidate antifungal agents for this purpose is difficult in the presence of more potent amphotericin-based combination therapy and presents a dilemma in study design. It could be possible that amphotericin provides an “efficacy ceiling” that masks any potential activity of lower-potency antifungals such as sertraline. Few would argue the important role that fluconazole plays in the treatment of cryptococcal meningitis, in consolidation or maintenance therapy for example, despite the lack of a demonstrated mortality benefit as part of combination induction therapy.22 In other words, demonstrating efficacy of sertraline might have been difficult in this trial notwithstanding the limitations outlined above. Following this line of reason, it may be more appropriate to evaluate lower-potency antifungals in trials designed to measure outcomes outside the induction period, while trials evaluating outcomes in the induction period be reserved for drugs with perceived efficacy comparable to amphotericin.
A perceived improvement in fungal clearance compared to historical controls provided partial justification to advance our phase 2 trial,7 though results from this randomised trial now clearly refute that adjunctive sertraline changes CSF fungal clearance. Of note, the EFA observed in both the sertraline and placebo arms were similar to that in our earlier dose-ranging pilot study and were both also improved compared to historical controls receiving the same standard antifungal therapy at the same sites from 2010–2012 (Supplemental Figure 4).14 The apparent improved fungal clearance compared to historical controls appears to have resulted from a statistical anomaly due to changes in the quantity and timing of CSF culture data collected. While the study population and standard antifungal regimen remained similar over time, the number of lumbar punctures administered per patient has increased, due to the known survival benefits of therapeutic lumbar punctures.23 Specifically, in our current studies we performed more systematic lumbar punctures, particularly on days 3 and 10 in addition to days 1, 7, and 14. While total volume of CSF removed from each patient over the 14-day induction period did not directly affect EFA, an increased number of data points resulted in more accurate EFA estimations and contributed to a false perception of improved fungal clearance in the phase 2 pilot study over historical controls,7,14 when in fact there was no change. When limiting EFA calculations to only using day 1, 7, and 14 culture data, for example, this trial’s EFA was 0·34 log10 CFU/mL/day, which is similar to the EFA calculated in historical controls used in the comparison.14
The trial was stopped early based on the failure for adjunctive sertraline to demonstrate a clinical benefit. As a result, the treatment groups did not reach the proposed cohort size. While the smaller sample size limits the power for subgroup analysis, we believe the main findings of this multisite treatment trial are valid and generalisable across clinical settings in resource-limited and high income countries. While the standard of care antifungal regimen in Uganda remains suboptimal due to the absence of flucytosine, participants in this trial underwent prompt lumbar puncture with comprehensive, state-of-the-art diagnostics on CSF, and received a high level of care from experienced clinicians and nurses.24 A strength of the trial was the extensive monitoring and aggressive control of elevated intracranial pressure with serial therapeutic lumbar punctures. This trial provides further evidence that high levels of care for cryptococcal meningitis can be delivered in resource-limited settings leading to improved survival in these settings.15,25,26
In conclusion, we have evaluated a repurposed, off-label drug for the treatment of cryptococcal meningitis because of the inadequacy of currently available regimens and the lack of new antifungal drug development and discovery. This practical trial was designed to determine whether treatment with adjunctive high-dose sertraline, started at the time of diagnosis, would improve survival among patients with cryptococcal meningitis. We found that sertraline was safe and had possible benefit for depression, but that it did not improve survival or microbiologic outcomes as adjunctive antifungal therapy. Reasons for sertraline inactivity were thought to be multifactorial and related to insufficient drug concentrations and underappreciated interactions with ART. Our trial has illustrated some of the limitations of using surrogate endpoints and retrospective comparisons in advancing clinical trials, particularly when combinations of antifungals are used. Despite the findings of this trial, we remain proponents of repurposing drugs as a means of fast-tracking promising therapeutic agents for cryptococcal meningitis. Until more effective adjuvants are identified, improving access to the most effective regimens containing amphotericin and flucytosine must remain a global priority for the treatment of cryptococcosis.
Supplementary Material
Sources of funding
This research was supported by the National Institute of Neurologic Diseases and Stroke (R01NS086312), the Fogarty International Center (K01TW010268, R25TW009345), the National Institute of Allergy and Infectious Diseases (T32AI055433), United Kingdom Medical Research Council / DfID / Wellcome Trust Global Clinical Trials (M007413/1), and Grand Challenges Canada (S4-0296-01). DBM was also supported by DELTAS Africa Initiative grant # DEL-15-011 to THRiVE-2. The DELTAS Africa Initiative is an independent funding scheme of the African Academy of Sciences (AAS)’s Alliance for Accelerating Excellence in Science in Africa (AESA) and supported by the New Partnership for Africa’s Development Planning and Coordinating Agency (NEPAD Agency) with funding from the Wellcome Trust grant #107742/Z/15/Z and the UK government. The views expressed in this publication are those of the author(s) and not necessarily those of AAS, NEPAD Agency, Wellcome Trust or the UK government. This work was supported in part by the Doris Duke Charitable Foundation through a grant supporting the Doris Duke International Clinical Research Fellows Program at the University of Minnesota.
We would also like to acknowledge and thank the study participants, the trial scientific review board, members of independent review boards at each study site, all manuscript reviewers, members of the trial data safety and monitoring board including Drs Joseph N. Jarvis, Jason V. Baker, Mohammed Lamorde, and Marcel Wolbers, as well as members of our trial steering committee, including Drs. John R. Perfect, Graeme Meintjes, and Edward N. Janoff.
Footnotes
Declarations of interests
All authors report no conflicts of interest to declare.
References
- 1.Rajasingham R, Smith RM, Park BJ, et al. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis 2017; 17(8): 873–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nayak R, Xu J. Effects of sertraline hydrochloride and fluconazole combinations on Cryptococcus neoformans and Cryptococcus gattii. Mycology 2010; 1(2): 99–105. [Google Scholar]
- 3.Zhai B, Wu C, Wang L, Sachs MS, Lin X. The antidepressant sertraline provides a promising therapeutic option for neurotropic cryptococcal infections. Antimicrob Agents Chemother 2012; 56(7): 3758–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith KD, Achan B, Hullsiek KH, et al. Increased Antifungal Drug Resistance in Clinical Isolates of Cryptococcus neoformans in Uganda. Antimicrob Agents Chemother 2015; 59(12): 7197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trevino-Rangel Rde J, Villanueva-Lozano H, Hernandez-Rodriguez P, et al. Activity of sertraline against Cryptococcus neoformans: in vitro and in vivo assays. Med Mycol 2016; 54(3): 280–6. [DOI] [PubMed] [Google Scholar]
- 6.Rossato L, Loreto ES, Zanette RA, Chassot F, Santurio JM, Alves SH. In vitro synergistic effects of chlorpromazine and sertraline in combination with amphotericin B against Cryptococcus neoformans var. grubii. Folia Microbiol (Praha) 2016; 61(5): 399–403. [DOI] [PubMed] [Google Scholar]
- 7.Rhein J, Morawski BM, Hullsiek KH, et al. Efficacy of adjunctive sertraline for the treatment of HIV-associated cryptococcal meningitis: an open-label dose-ranging study. Lancet Infect Dis 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewis RJ, Angier MK, Williamson KS, Johnson RD. Analysis of sertraline in postmortem fluids and tissues in 11 aviation accident victims. J Anal Toxicol 2013; 37(4): 208–16. [DOI] [PubMed] [Google Scholar]
- 9.Hansen RA, Gartlehner G, Lohr KN, Gaynes BN, Carey TS. Efficacy and safety of second-generation antidepressants in the treatment of major depressive disorder. Ann Intern Med 2005; 143(6): 415–26. [DOI] [PubMed] [Google Scholar]
- 10.Villanueva-Lozano H, Trevino-Rangel RJ, Gonzalez GM, et al. Clinical evaluation of the antifungal effect of sertraline in the treatment of cryptococcal meningitis in HIV patients: a single Mexican center experience. Infection 2018; 46(1): 25–30. [DOI] [PubMed] [Google Scholar]
- 11.Rhein J, Hullsiek KH, Evans EE, et al. Detrimental Outcomes of Unmasking Cryptococcal Meningitis With Recent ART Initiation. Open Forum Infect Dis 2018; 5(8): ofy122.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bicanic T, Meintjes G, Wood R, et al. Fungal burden, early fungicidal activity, and outcome in cryptococcal meningitis in antiretroviral-naive or antiretroviral-experienced patients treated with amphotericin B or fluconazole. Clin Infect Dis 2007; 45(1): 76–80. [DOI] [PubMed] [Google Scholar]
- 13.Dyal J, Akampurira A, Rhein J, et al. Reproducibility of CSF quantitative culture methods for estimating rate of clearance in cryptococcal meningitis. Med Mycol 2016; 54(4): 361–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boulware DR, Meya DB, Muzoora C, et al. Timing of antiretroviral therapy after diagnosis of cryptococcal meningitis. N Engl J Med 2014; 370(26): 2487–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Molloy SF, Kanyama C, Heyderman RS, et al. Antifungal Combinations for Treatment of Cryptococcal Meningitis in Africa. N Engl J Med 2018; 378(11): 1004–17. [DOI] [PubMed] [Google Scholar]
- 16.Montgomery MP, Nakasujja N, Morawski BM, et al. Neurocognitive function in HIV-infected persons with asymptomatic cryptococcal antigenemia: a comparison of three prospective cohorts. BMC Neurol 2017; 17(1): 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carlson RD, Rolfes MA, Birkenkamp KE, et al. Predictors of neurocognitive outcomes on antiretroviral therapy after cryptococcal meningitis: a prospective cohort study. Metab Brain Dis 2014; 29(2): 269–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jarvis JN, Bicanic T, Loyse A, et al. Determinants of mortality in a combined cohort of 501 patients with HIV-associated Cryptococcal meningitis: implications for improving outcomes. Clin Infect Dis 2014; 58(5): 736–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bicanic T, Muzoora C, Brouwer AE, et al. Independent association between rate of clearance of infection and clinical outcome of HIV associated cryptococcal meningitis: analysis of a combined cohort of 262 patients. Clin Infect Dis 2009; 49(5): 702–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ayano G, Solomon M, Abraha M. A systematic review and meta-analysis of epidemiology of depression in people living with HIV in east Africa. BMC Psychiatry 2018; 18(1): 254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lofgren SM, Nakasujja N, Boulware DR. Systematic Review of Interventions for Depression for People Living with HIV in Africa. AIDS Behav 2018; 22(1): 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tenforde MW, Shapiro AE, Rouse B, et al. Treatment for HIV-associated cryptococcal meningitis. Cochrane Database Syst Rev 2018; 7: CD005647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rolfes MA, Hullsiek KH, Rhein J, et al. The effect of therapeutic lumbar punctures on acute mortality from cryptococcal meningitis. Clin Infect Dis 2014; 59(11): 1607–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guidelines for The Diagnosis, Prevention and Management of Cryptococcal Disease in HIV-Infected Adults, Adolescents and Children: Supplement to the 2016 Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection. Geneva; 2018. [PubMed] [Google Scholar]
- 25.Flynn AG, Meya DB, Hullsiek KH, et al. Evolving Failures in the Delivery of Human Immunodeficiency Virus Care: Lessons From a Ugandan Meningitis Cohort 2006–2016. Open Forum Infect Dis 2017; 4(2): ofx077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beardsley J, Wolbers M, Kibengo FM, et al. Adjunctive Dexamethasone in HIV-Associated Cryptococcal Meningitis. N Engl J Med 2016; 374(6): 542–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


