Abstract
Background:
The diagnosis of acute myocardial infarction (AMI) is missed more frequently in young women than men, which may be related to the cognitive psychology of the diagnostic process. Physicians start the diagnostic process by intuitively recognizing familiar symptom phenotypes, but little is known about how symptoms combine in individuals as unique symptom phenotypes. We examined how symptoms of AMI combine as unique symptom phenotypes in individual patients to compare the distribution of symptom phenotypes in women versus men.
Methods and Results:
The VIRGO Study was a multicenter, observational cohort study of 3501 young adults hospitalized with AMI. Data were collected on presenting symptoms with standardized interviews and from medical record abstraction. The number and distribution of unique symptom phenotypes were compared between women and men. Because of the 2:1 female-to-male enrollment ratio, women and men were compared with permutation testing and repeated subsampling. There were 426 interview-symptom phenotypes in women and 280 in men. The observed difference between women and men of 146 phenotypes was significant even allowing for the greater enrollment of women (permutation p=.004, median difference 110 under the null hypothesis of no association between sex and phenotype). The repeated subsample analysis also showed significantly more interview-symptom phenotypes in women than men (206.8±7.3 vs. 188.6±6.0, p< .001). Women were more broadly distributed among symptom phenotype subgroups than men (p<.001). Similar findings were observed in the analysis of symptoms abstracted from the medical record.
Conclusions:
Women exhibited substantially more variation in unique symptom phenotypes than men, regardless of whether the symptoms were derived from structured interviews or abstracted from the medical record. These findings may provide an explanation for the higher missed diagnosis rate in young women with AMI and may have important implications for teaching and improving clinicians’ ability to recognize the diagnosis of AMI in women.
The diagnosis of acute myocardial infarction (AMI) is missed more frequently in women than men, particularly in women less than 55 years of age,1 and the explanation for this sex or gender disparity is unknown. A potential explanation might be related to the cognitive psychology of how clinicians make a clinical diagnosis.2–8
Cognitive psychology studies have shown that experienced physicians begin any diagnostic process by generating short lists of diagnostic possibilities.5–8 This step of early hypothesis generation is dependent upon experiential knowledge, where memories of past experiences are stored in long-term memory as exemplars.9,10 Humans use exemplars to categorize and recognize everyday objects, and clinicians use a similar process to intuitively recognize a patient with a medical diagnosis.9,10 Because exemplars are memories of individual phenotypes, greater knowledge about individual symptom phenotypes may be important, and if symptom phenotypes are different in women and men, this could help explain the higher rate of missed diagnosis of AMI in women.
Prior studies have reported that the frequencies of several AMI symptoms are different in women and men at the population level.11–19 However, no prior study has examined how symptoms combine at the individual level as unique symptom phenotypes and how symptom phenotypes are distributed in women and men. The VIRGO Study prospectively collected detailed information on presenting symptoms using structured interviews, which provided an opportunity to study how symptoms combine in individuals as symptom phenotypes.20 The aim of this study was to examine the variation and sex differences in symptom phenotypes at the individual level in patients with AMI.
METHODS
Study Population.
We performed a secondary analysis of the VIRGO Study, which is the largest prospective observational study to date of young women and men with AMI (N=3,501). The VIRGO Study included young women and men (18–55 years) hospitalized for AMI in 103 hospitals in the United States and 24 hospitals in Spain between August 2008 and January 2012.20 Patients were enrolled using a 2:1 female-to-male enrollment ratio (n=2349 women; n=1152 men). Details of the study design and methodology have been reported previously.20 The VIRGO Study obtained institutional review board approval at each participating institution, and patients provided written informed consent for their study participation. The VIRGO investigators intend to share study data and are investigating mechanisms and funding to make this possible. The investigators are currently working on several pilot data-sharing efforts.
Presenting symptoms.
All patients had standardized in-person interviews administered by trained personnel during the index AMI admission. Patients were specifically asked if they had chest pain, pain in the jaw, neck, arm, or back, dizziness, indigestion, nausea, palpitations, shortness of breath, sweating, weakness or fatigue, or confusion. Thus, there were 10 interview symptoms that could combine in individual patients as unique interview-symptom phenotypes.
Information regarding presenting symptoms was also abstracted from the medical record, including whether there was typical chest pain, atypical chest pain, back pain, abdominal pain, other pain, nausea, shortness of breath, fatigue, or other symptoms. Patients were labeled with typical chest pain or atypical chest pain if those words were recorded in the medical record by the treating provider. Under the category of other symptoms, diaphoresis or sweating was specifically examined as a particular symptom for this analysis. Thus, there were 9 abstracted symptoms that could combine in an individual patients as unique abstracted-symptom phenotypes.
Statistical Analysis.
Symptoms obtained from the standardized interviews and from medical record abstraction were analyzed by creating subsets of patients with unique combinations of symptoms (interview-symptom phenotypes and abstracted-symptom phenotypes). Symptom phenotype subsets were further analyzed in subgroups categorized by AMI type (ST-elevation MI [STEMI] and non-ST-elevation MI [NSTEMI]), percutaneous coronary intervention (PCI) status (emergent/urgent PCI and no PCI), by chest pain type in the abstracted record (atypical and not atypical), and by presentation time (<=6 hours from the onset of symptoms and >6 hours). The variation and distributions of the interview-symptom phenotypes and the abstracted-symptom phenotypes were analyzed using SAS/STAT® software, Version 14.3 (SAS Institute, Cary, NC). Because the 2:1 female to male enrollment ratio in the VIRGO study makes rare phenotypes more detectable among the female patients, we used two analytical approaches to compare women and men: Monte Carlo permutation tests21 and repeated subsampling.22
For the Monte Carlo permutation analyses, we randomly permuted the patients’ sexes to generate 99,999 data sets reflecting the null hypothesis that any differences in the number and distribution of phenotypes were due to the enrollment ratio rather than the effect of sex. Two test statistics, the difference between the numbers of phenotypes appearing in women and in men and the Pearson chi-square, were calculated for each dataset in the ensemble (i.e., the original and all the permutations for a total of 100,000), creating empirical distributions for significance testing. The median of the empirical distribution was interpreted as an estimate of the difference in the number of phenotypes between women and men that would have been expected due to the enrollment ratio alone, and the p value was the proportion of the empirical distribution showing a difference as large or larger than the difference in the original data (see Supplementary Material for further explanation of the Monte Carlo permutation analysis).
For the repeated subsampling analyses, 100,000 subsamples of 500 women and 500 men were randomly generated. Subsamples of 400 were used to analyze the smaller subgroups. The numbers of distinct phenotypes in each subsample of women and men were compared with Student’s t-test (with Satterthwaite correction for unequal variances).
Logistic regression was performed on the repeated subsamples to determine if the number of symptoms in a phenotype predicted whether a phenotype was unique to either women or men.
Patient distributions across the subgroups were compared in women and men using chi-square analysis. The symptom counts in women and men were compared using the Wilcoxon-Mann-Whitney rank-sum test.
RESULTS
There were 2349 women and 1152 men in the VIRGO Study. Of the women, 76% were white, 19% were African American, and 6% other, as compared with men, in whom 83% were white, 10% were African American and 7% other (p<.001). Eight percent of both women and men were Hispanic. The average number of interview symptoms per patient was higher in women than men (4.3±2.3, median 4, vs. 3.9±2.1, median 3, p < .001). The average number of abstracted symptoms per patient was also higher in women than men (2.7±1.4, median 3, vs. 2.6±1.4, median 2, p = .038). The frequencies at the population level of the interview symptoms and the abstracted symptoms have been previously reported and are listed in Table 1.15 There were 488 unique interview-symptom phenotypes and 274 unique abstracted-symptom phenotypes among all patients in our analysis.
Table 1.
Frequencies at the population level of interview symptoms and abstracted record symptoms in women and men.
| Interview Symptoms | Women (%) | Men (%) | P value |
|---|---|---|---|
| Chest Pain | 86.8 | 89.7 | 0.015 |
| Jaw/neck/arm/back pain | 60.6 | 53.5 | <0.001 |
| Dizziness | 28.4 | 25.4 | 0.068 |
| Indigestion | 30.0 | 26.6 | 0.034 |
| Nausea | 46.4 | 32.2 | <0.001 |
| Palpitations | 17.8 | 11.5 | <0.001 |
| Shortness of breath | 50.7 | 45.0 | 0.002 |
| Diaphoresis | 53.0 | 55.0 | 0.247 |
| Weakness/fatigue | 43.8 | 38.9 | 0.006 |
| Confusion | 11.4 | 10.4 | 0.400 |
| Abstracted Symptoms | |||
| Typical Chest Pain | 77.2 | 83.3 | <0.001 |
| Atypical Chest Pain | 19.7 | 14.1 | <0.001 |
| Back Pain | 16.5 | 10.2 | <0.001 |
| Abdominal Pain | 4.9 | 3.6 | 0.072 |
| Other Pain | 19.9 | 19.9 | 0.975 |
| Nausea | 44.6 | 34.9 | <0.001 |
| Shortness of Breath | 44.1 | 43.7 | 0.824 |
| Fatigue | 11.4 | 10.0 | 0.201 |
| Diaphoresis | 28.9 | 37.5 | <0.001 |
Sex Differences in Symptom Phenotypes.
There were 426 interview-symptom phenotypes in women and 280 in men. The observed difference between women and men of 146 phenotypes was significant even allowing for the greater enrollment of women (permutation p=.004, median difference of 110 under the null hypothesis of no association between sex and phenotype).
The repeated subsample analysis also showed significantly more interview-symptom phenotypes in women (206.8±7.3) than men (188.6±6.0, p < .001). The distribution of the number of interview-symptom phenotypes in women and men from repeated subsampling is shown in Figure 1.
Figure 1.
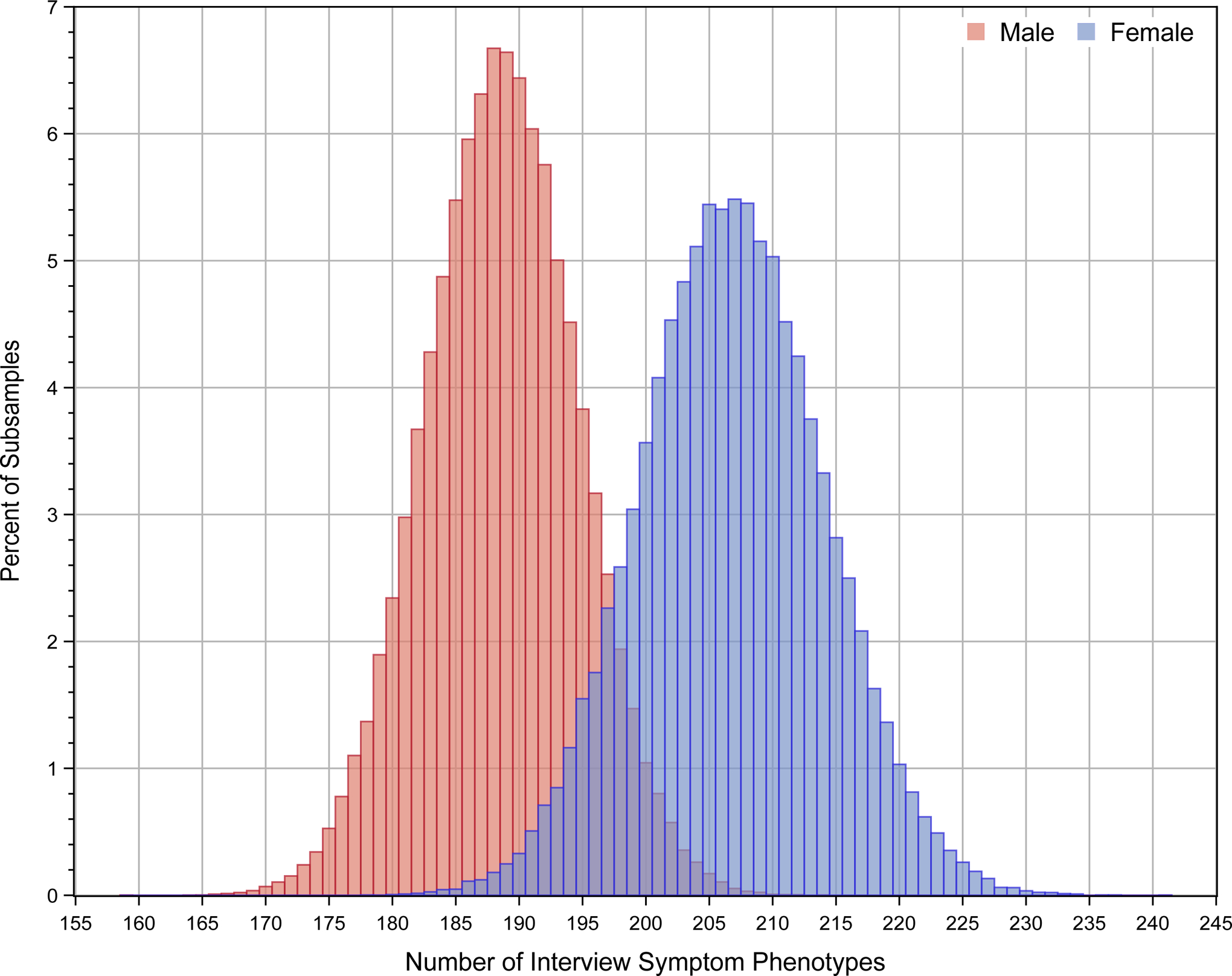
Distribution of the number of interview-symptom phenotypes in women and men from 100,000 sub-samples using a bootstrap sample size of 500.
Women were more broadly distributed in interview-symptom phenotype subgroups than men. Of the 2349 women, 942 (40%) were in one of the 25 most common interview-symptom phenotype subgroups, and of the 1152 men, 535 (46%) were in one of the 25 most common interview-symptom phenotype subgroups (p < .001). The top 25 most frequent interview-symptom phenotypes for women and men are listed in Table 2. The top 2 interview-symptom phenotypes were the same in women and men (chest pain alone and chest pain with jaw, neck, arm, or back pain), 6 were different in women and men, and the remaining 17 were the same in women and men, but in different order of frequency.
Table 2.
Top 25 Interview-Symptom Phenotypes and the Frequency of Each Phenotype among Women and Men.
| Women | Men | |||
|---|---|---|---|---|
| Phenotype | % | Phenotype | % | |
| 1 | Chest Pain | 6.0 | Chest Pain | 7.3 |
| 2 | Chest Pain, Jaw/neck/arm/back Pain | 4.1 | Chest Pain, Jaw/neck/arm/back Pain | 5.4 |
| 3 | Chest Pain, Shortness of breath | 2.6 | Chest Pain, Jaw/neck/arm/back Pain, Diaphoresis | 3.7 |
| 4 | Chest Pain, Jaw/neck/arm/back Pain, Diaphoresis | 1.9 | Chest Pain, Diaphoresis | 3.7 |
| 5 | Chest Pain, Diaphoresis | 1.7 | Chest Pain, Shortness of breath | 2.8 |
| 6 | Chest Pain, Jaw/neck/arm/back Pain, Shortness of breath | 1.6 | Chest Pain, Jaw/neck/arm/back Pain, Shortness of breath | 2.4 |
| 7 | Chest Pain, Dizziness, Nausea, Jaw/neck/arm/back Pain, Shortness of breath, Diaphoresis, Weakness/Fatigue | 1.5 | Chest Pain, Nausea | 1.8 |
| 8 | Chest Pain, Nausea, Jaw/neck/arm/back Pain | 1.5 | Chest Pain, Nausea, Diaphoresis | 1.6 |
| 9 | Chest Pain, Dizziness, Indigestion, Nausea, Jaw/neck/arm/back Pain, Palpitations, Shortness of breath, Diaphoresis, Weakness/Fatigueness/Fatigue | 1.3 | Chest Pain, Nausea, Jaw/neck/arm/back Pain, Diaphoresis | 1.4 |
| 10 | Chest Pain, Nausea, Jaw/neck/arm/back Pain, Shortness of breath, Diaphoresis | 1.3 | Chest Pain, Jaw/neck/arm/back Pain, Shortness of breath, Diaphoresis | 1.4 |
| 11 | Chest Pain, Nausea, Jaw/neck/arm/back Pain, Diaphoresis | 1.3 | Chest Pain, Shortness of breath Diaphoresis | 1.3 |
| 12 | Jaw/neck/arm/back Pain | 1.3 | Chest Pain, Indigestion, Jaw/neck/arm/back Pain, Shortness of breath Diaphoresis, Weakness/Fatigueness/Fatigue | 1.1 |
| 13 | Chest Pain, Nausea, Diaphoresis | 1.2 | Chest Pain, Nausea, Jaw/neck/arm/back Pain | 1.1 |
| 14 | None | 1.2 | Chest Pain, Jaw/neck/arm/back Pain, Shortness of breath, Diaphoresis, Weakness/Fatigueness/Fatigue | 1.1 |
| 15 | Chest Pain, Dizziness, Indigestion, Nausea, Jaw/neck/arm/back Pain, Palpitations, Shortness of breath, Diaphoresis, Weakness/Fatigueness/Fatigue, Confusion | 1.2 | Chest Pain, Diaphoresis, Weakness/Fatigueness/Fatigue | 1.1 |
| 16 | Chest Pain, Shortness of breath, Diaphoresis | 1.2 | Chest Pain, Indigestion, Jaw/neck/arm/back Pain | 1.0 |
| 17 | Chest Pain, Indigestion, Nausea, Jaw/neck/arm/back Pain, Shortness of breath, Diaphoresis, Weakness/Fatigueness/Fatigue | 1.1 | Chest Pain, Dizziness, Indigestion, Nausea, Jaw/neck/arm/back Pain, Shortness of breathDiaphoresis, Weakness/Fatigueness/Fatigue | 1.0 |
| 18 | Chest Pain, Nausea | 1.1 | Chest Pain, Dizziness, Jaw/neck/arm/back Pain, Shortness of breath, Diaphoresis, Weakness/Fatigueness/Fatigue | 1.0 |
| 19 | Chest Pain, Jaw/neck/arm/back Pain, Shortness of breath, Diaphoresis | 1.1 | Chest Pain, Nausea, Jaw/neck/arm/back Pain, Shortness of breath, Diaphoresis, Weakness/Fatigueness/Fatigue | 1.0 |
| 20 | Chest Pain, Jaw/neck/arm/back Pain, Shortness of breath, Weakness/Fatigueness/Fatigue | 1.1 | Chest Pain, Jaw/neck/arm/back Pain, Shortness of breath, Weakness/Fatigueness/Fatigue | 1.0 |
| 21 | Chest Pain, Dizziness, Nausea, Jaw/neck/arm/back Pain, Palpitations, Shortness of breath, Diaphoresis, Weakness/Fatigueness/Fatigue | 1.0 | Chest Pain, Jaw/neck/arm/back Pain, Weakness/Fatigueness/Fatigue | 1.0 |
| 22 | Chest Pain, Indigestion, Jaw/neck/arm/back Pain | 1.0 | Chest Pain, Dizziness, Indigestion, Nausea, Jaw/neck/arm/back Pain, Palpitations, SHORTNESS OF BREATH, Diaphoresis, Weakness/Fatigueness/Fatigue, Confusion | 0.9 |
| 23 | Chest Pain, Nausea, Jaw/neck/arm/back Pain, Shortness of breath, Diaphoresis, Weakness/Fatigueness/Fatigue | 1.0 | Chest Pain, Dizziness, Diaphoresis | 0.9 |
| 24 | Chest Pain, Jaw/neck/arm/back Pain, Weakness/Fatigueness/Fatigue | 1.0 | Chest Pain, Shortness of breath, Diaphoresis, Weakness/Fatigueness/Fatigue | 0.9 |
| 25 | Chest Pain, Nausea, Jaw/neck/arm/back Pain, Diaphoresis, Weakness/Fatigue | 0.9 | None | 0.9 |
Repeated subsampling analysis revealed that 46.9±2.6% of interview-symptom phenotypes constituting 25.8±2.2% of women were only present in women and not present in men, and 41.8±2.7% of interview-symptom phenotypes constituting 21.4±2.1% of men were present in men and not present in women. Logistic regression revealed that the probability of overlap of interview-symptom phenotypes in women and men decreased with the number of symptoms in the phenotypes; the odds ratios accompanying a one-symptom increase were 0.845 (95% CI 0.845–0.846) for women and 0.944 (95% CI 0.944–0.945) for men (both ps < .001).
There were 244 abstracted-symptom phenotypes in women and 154 in men. The observed difference of 90 phenotypes was significant (permutation p=.004, median difference of 62 under the null hypothesis of no association between sex and phenotype). Repeated subsampling analysis also showed more abstracted-symptom phenotypes in women (121.4±5.9) than men (103.8±4.8, p < .001). The distribution of the number of abstracted-symptom phenotypes in women and men from repeated subsampling is shown in Figure 2.
Figure 2.
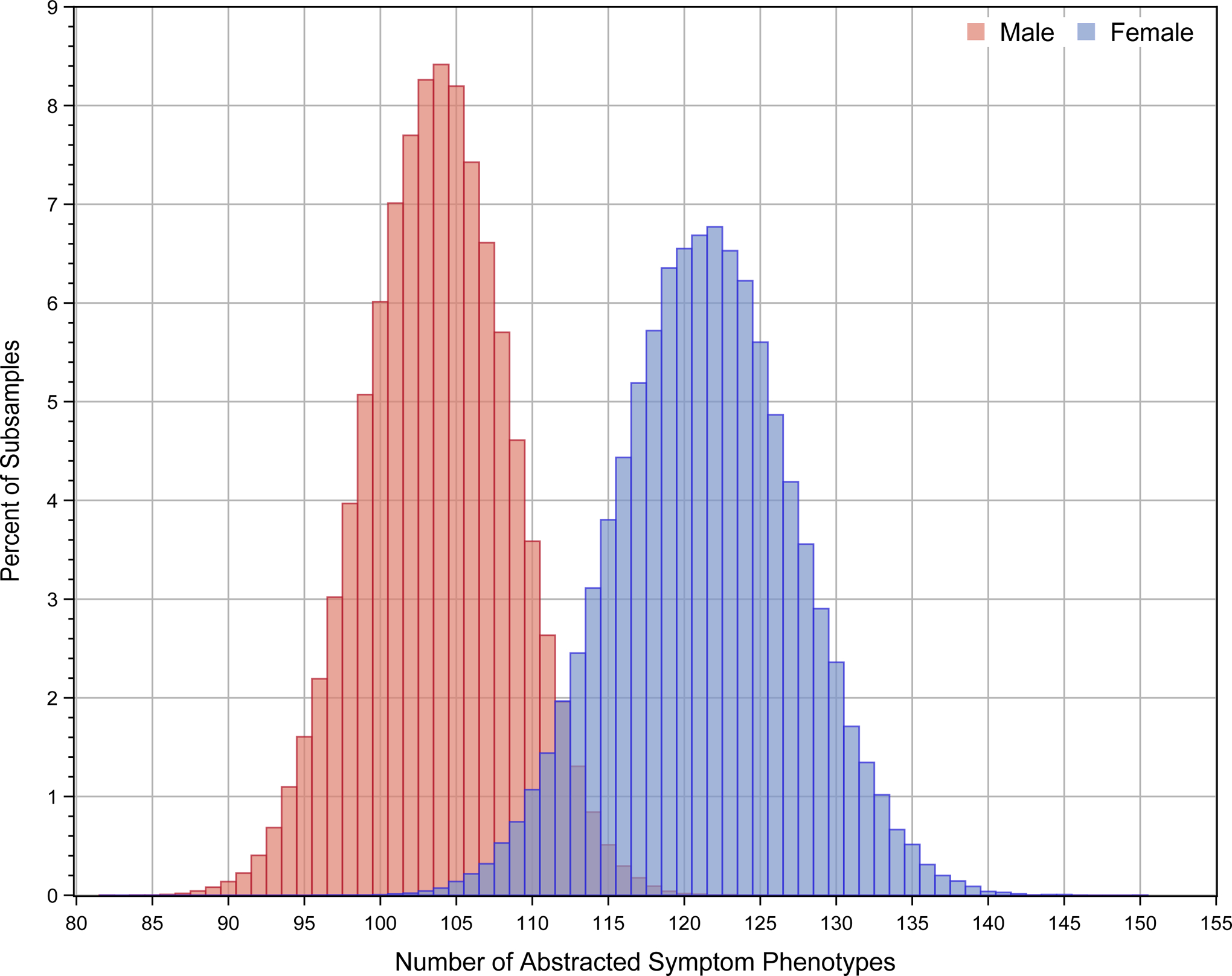
Distribution of the number of abstracted-symptom phenotypes in women and men from 100,000 sub-samples using a bootstrap sample size of 500.
Women were more broadly distributed in the abstracted-symptom phenotype subgroups than men. Of the 2349 women, 1167 (50%) were in one of the 10 most common abstracted-symptom phenotype subgroups, and of the 1152 men, 670 (59%) were in one of the 10 most common abstracted-symptom phenotype subgroups (p < .001). The top 10 most frequent abstracted-symptom phenotypes for women and men are shown in Table 3. The top 2 abstracted-symptom phenotypes were the same in women and men (typical chest pain alone and typical chest pain with shortness of breath), 2 were different in women and men, and the remaining 6 were the same in women and men, but in different order of frequency.
Table 3.
Top 10 Abstracted-Symptom Phenotypes and the Frequency of Each Phenotype among Women and Men.
| Women | Men | |||
|---|---|---|---|---|
| Phenotype | % | Phenotype | % | |
| 1 | Typical chest pain | 16.6 | Typical chest pain | 20.4 |
| 3 | Typical chest pain, shortness of breath | 6.1 | Typical chest pain, shortness of breath | 6.4 |
| 3 | Typical chest pain, nausea | 5.9 | Typical chest pain, diaphoresis | 5.3 |
| 4 | Typical chest pain, nausea, shortness of breath | 4.9 | Typical chest pain, shortness of breath, diaphoresis | 5.3 |
| 5 | Typical chest pain, nausea, shortness of breath, diaphoresis | 4.0 | Typical chest pain, nausea, diaphoresis | 4.6 |
| 6 | Atypical chest pain | 3.5 | Typical chest pain, nausea | 4.2 |
| 7 | Typical chest pain, nausea, diaphoresis | 3.0 | Typical chest pain, nausea, shortness of breath | 3.6 |
| 8 | Typical chest pain, shortness of breath, diaphoresis | 2.4 | Typical chest pain, nausea, shortness of breath, diaphoresis | 3.5 |
| 9 | Typical chest pain, diaphoresis | 2.3 | None | 3.1 |
| 10 | Typical chest pain, back pain | 1.9 | Typical chest pain, other pain | 2.6 |
Repeated sampling analysis revealed that 46.2±3.3% of the abstracted-symptom phenotypes constituting 14.9±1.7% of the women were only present in women and not present in men, and 37.0±3.7% of the abstracted-symptom phenotypes constituting 9.8±1.5% of men were present only in men and not present in women. Logistic regression revealed that the probability of overlap of abstracted-symptom phenotypes in women and men decreased with the number of symptoms in the phenotypes; the odds ratios accompanying a one-symptom increase were 0.661 (95% CI 0.661–0.662) for women and 0.624 (95% CI 0.624–0.625) for men (both ps < .001).
Subgroup Analysis.
The distribution of women and men in subgroups is shown in Table 4. Women were more likely to have a NSTEMI (p<.001), less likely to receive emergent or urgent PCI (p=.006), more likely to have atypical chest pain (p<.001), and more likely to have a delay from the onset of symptoms to presentation of greater than 6 hours (p<.001).
Table 4.
Distribution of women and men in subgroups and permutation analysis of interview-symptom phenotypes and abstracted-symptom phenotypes in subgroups.
| Subgroup | Distribution of Patients | Permutation analysis of Interview-Symptom Phenotypes | Permutation Analysis of Abstracted-Symptom Phenotypes | |||||
|---|---|---|---|---|---|---|---|---|
| Women | Men | Observed Difference | Difference under the Null | p value | Observed Difference | Difference under the Null | p value | |
| STEMI (n=1811, 52%)* | 1126 (48%) | 685 (59%) | 101 | 62 | <.001 | 46 | 36 | 0.15 |
| NSTEMI (n=1690, 48%) | 1223 (52%) | 467 (41%) | 141 | 131 | 0.204 | 98 | 69 | <.001 |
| PCI (n=2650, 79%)** | 1743 (77%) | 907 (81%) | 123 | 91 | 0.007 | 83 | 56 | 0.004 |
| No PCI (n=715, 21%) | 509 (23%) | 206 (19%) | 111 | 98 | 0.097 | 54 | 42 | 0.046 |
| No Atypical Chest Pain (n=2788, 82%)* | 1887 (80%) | 990 (86%) | 130 | 92 | 0.002 | - | - | - |
| Atypical Chest Pain (n=624, 18%) | 462 (20%) | 162 (14%) | 108 | 103 | 0.287 | - | - | - |
| Presentation Delay <= 6 hours (n=2022, 58%)* | 1290 (55%) | 732 (64%) | 106 | 76 | 0.008 | 55 | 41 | 0.072 |
| Presentation Delay > 6 hours (n=1466, 42%) | 1050 (45%) | 416 (36%) | 117 | 112 | 0.343 | 87 | 65 | 0.008 |
Subgroups were not independent of sex (p<.001,
p=.006)
As shown in Table 4, permutation analysis revealed that there were significantly more interview-symptom phenotypes in women than men among STEMI patients (p<.001), among patients who received PCI (p=.007), among patients who did not have atypical chest pain (p=.002), and among patients without a presentation delay of greater than 6 hours (p=.008). There were significantly more abstracted-symptom phenotypes in women than men among NSTEMI patients (p<.001), among patients who received PCI (p=.004) and who did not receive PCI (p=.046), and among patients with a presentation delay of greater than 6 hours (p=.008).
Further subgroup analyses of mortality, race, and ethnicity were unrevealing. The 30-day mortality was 0.6% in women and 0.7% in men (p=.61). Permutation analysis did not reveal a significant difference in the number of phenotypes in women and men in the smaller subsets of those who died, African-Americans, or Hispanic patients.
DISCUSSION
To our knowledge, this is the first study to investigate how specific symptoms of AMI combine in individual patients as unique symptom phenotypes and how symptom phenotypes are distributed in women and men with AMI. Prior studies of patients with AMI have reported how symptoms of AMI are distributed at the population level but have not examined how symptoms combine at the individual level as unique symptom phenotypes.11–19 The VIRGO Study collected detailed information about presenting symptoms, which provided an opportunity to analyze symptom phenotypes and compare the distribution of symptom phenotypes in women and men.
Analysis of both the interview-symptom phenotypes and the abstracted-symptom phenotypes revealed that women had significantly more symptom phenotypes than men. Women were more broadly distributed among a larger number of symptom phenotype subgroups than men, and women had a higher number of symptoms per patient than men. A substantial number of the interview-symptom phenotypes and the abstracted-symptom phenotypes occurred only in one sex or the other. The amount of overlap of phenotypes in women and men decreased as the number of symptoms in those phenotypes increased, showing that patients with a greater number of symptoms were more likely to have a symptom phenotype that was unique to one sex or the other.
Women tended to have a greater number of interview-symptom phenotypes and abstracted-symptom phenotypes than men in subgroup analysis by AMI type, PCI status, type of chest pain, and presentation delay. Differences in some subgroups were not significant, however, which may have been due to a higher number of rare phenotypes in both sexes in those subgroups. A higher number of rare phenotypes would be expected to increase the median difference in phenotypes between women and men under the null, which occurred in the NSTEMI, no PCI, atypical chest pain, and delayed presentation subgroups. Interestingly, the subgroups where the differences were not significant were those where the diagnosis of AMI tends to be clinically ambiguous in both sexes. The subgroup analysis of the abstracted-symptom phenotypes showed a significant or near significant difference between women and men in all subgroups.
Mortality was very low in the VIRGO registry likely because the registry selected patients who were healthy enough to participate in the interviews. The 30-day mortality was not significantly different in women and men and the number of those who died was too small for statistical comparison of phenotype subgroups in women and men. Thus, the study could not address the relationship between the symptom phenotypes and mortality. Similarly, the number of African-Americans and Hispanics in the registry was relatively small and no differences in the number of phenotypes between women and men were noted in these small subgroups, likely due to the statistical limitations.
The greater variability of symptom phenotypes in women as compared with men could provide a potential explanation for why AMI is missed more frequently and why treatments for AMI are under-utilized in young women, as compared with men.1,23 The greater variability in women could provide a source of confusion and distraction for clinicians when trying to discern between diagnostic possibilities. Greater variability also may have contributed to the finding that women were more likely to have a delay from the onset of symptoms to presentation in VIRGO.
Implicit gender bias could be another explanation for why the diagnosis of AMI is missed more often in women.24,25 Also, the lower prevalence of AMI in women, particularly younger women, could be an additional explanation if it causes clinicians to mistakenly disregard AMI as a plausible diagnosis.2,26,27 Implicit bias or distorted probability estimates could play a role, but our findings suggest the additional possibility of a cognitive psychology explanation for why AMI is under-diagnosed in young women.
Among both women and men there was considerable variation in symptom phenotypes and the number of symptom phenotypes obtained from the standardized interviews was substantially higher than the number of symptom phenotypes abstracted from the medical record. It is possible that physicians heuristically work to simplify and combine data elements in the process of taking and recording a patient history.4,28 It is also possible that the interviewers were more diligent and complete than clinicians in recording symptoms. It is also possible that the patients were led to be more expansive in their descriptions of their presenting symptoms during the interview than they were during the initial interview by front-line clinicians. Nevertheless, the differences between women and men in the number of phenotypes persisted, whether taken from the standardized interview or abstracted from the medical record.
It is not possible to determine from our study why women show substantially greater variability in symptom phenotypes than men. It is known that there are differences between women and men in the underlying pathophysiology of AMI.29,30 Women, for example, are more likely than men to have coronary erosion and spontaneous coronary dissection as the pathophysiological mechanism for AMI.30 Patients with STEMI and patients who received PCI would be expected to have a common vascular mechanism for AMI. In these subgroups, women had a higher number of phenotypes, suggesting that the vascular mechanism of AMI was an unlikely explanation for the greater number of phenotypes in women. It is also possible that there are biological differences that determine how symptoms, particularly ancillary symptoms of AMI such as nausea, are manifested in women and men. In addition, societal or psychological differences in gender expectations or expression may cause women to express their symptoms differently than men.31 Finally, it is possible that interviewers perceive and record symptoms differently depending on whether the patient is a woman or a man. The latter possibility is unlikely given the focus of the VIRGO study and the rigorous standards of the standardized interview process.
A strength of our study is that we systematically collected data about symptoms using structured interviews with each patient during the index hospitalization. These data were recorded with consistency using direct as well as open-ended questions. A limitation of this analysis is that the registry does not include a control group of patients without the diagnosis of AMI. It would be interesting to compare symptom phenotypes in patients with and without the established diagnosis of AMI. It should be noted, however, that in practical terms, a control group of patients without AMI could be difficult to discern. To identify a control group without an AMI diagnosis would require defining every diagnostic encounter relevant to the diagnosis of AMI. Identifying all relevant diagnostic encounters has been notoriously difficult in the past for investigators interested in calculating diagnostic error rates.32,33 Even though we are restricted in this analysis to only patients with the established diagnosis of AMI, the comparison of women to men within the class of patients with AMI nonetheless provides novel information about the sex differences in the diagnosis of AMI.
Numerous studies, including prior reports from the VIRGO study, have shown that the frequencies of specific symptoms such as chest pain or nausea are different in women and men with AMI.11–19 These studies have raised awareness regarding the differences in how women and men present with AMI. These studies have reported differences between women and men at the population level. Knowledge of how symptoms combine at the individual patient level should stimulate educators to re-examine how we teach learners to make a clinical diagnosis.34–36 Textbooks often present “classic” or prototypical examples of diseases rather than an array of exemplars,37,38 and prototypes may not give learners and clinicians an appreciation of variation in disease presentation in women and men. Prototypical descriptions of AMI are often images of men, which may be misleading, given that the range of AMI phenotypes (and their exemplars) is different in women and men. Prototypical examples of feature combinations derived from populations where men predominate could actually be stereotypical and misleading for diagnosing AMI in women. Interestingly, the classic prototypical description of AMI that includes the combination of chest pain, radiation, shortness of breath, and diaphoresis occurred as a unique phenotype in only 1% of VIRGO patients, with the remainder of patients in the VIRGO Study having 487 other interview-symptom phenotypes. Clearly, learners should be taught to recognize that there is a great deal more variation in the presentation of symptoms among patients with AMI than is represented by a single prototypical combination of symptoms. Teaching a thorough understanding of the extent of phenotypic variation and sex differences in symptom phenotypes could result in an improvement in the quality of diagnosis and a reduction in gender disparities in the diagnosis and treatment of AMI.
In summary, we have demonstrated marked variation in the symptom phenotypes of patients with AMI. We have also demonstrated that women exhibit substantially more variation than men, regardless of whether the symptoms were derived from structured interviews or abstracted from the medical record. These findings may have important implications for how we teach and could help clinicians improve their ability to recognize the diagnosis of AMI in women.
Supplementary Material
What is known:
The diagnosis of acute myocardial infarction (AMI) is missed more often in young women, which may be related to how clinicians intuitively recognize a diagnosis.
Possible diagnoses come to mind when experienced clinicians recognize familiar symptom phenotypes, yet how symptoms combine as unique symptom phenotypes in individual patients is unknown.
What the study adds:
Using the VIRGO study of 3501 young patients with AMI, we examined how symptoms of AMI combine as unique symptom phenotypes in individual patients and compared the distribution of symptom phenotypes in women and men.
We found that women had significantly more symptom phenotypes than men and symptom phenotypes were distributed differently in women and men.
This finding may have important implications for teaching and improving clinicians’ ability to recognize the diagnosis of AMI in women.
Sources of Funding:
The VIRGO Study was supported by a 4-year National Heart, Lung, and Blood Institute grant (No. 5R01HL081153). IMJOVEN (the Spanish component of VIRGO) was supported in Spain by grant PI 081614 from the Fondo deInvestigaciones Sanitarias del Instituto Carlos III, Ministry of Science and Technology, and additional funds from the Centro Nacional de Investigaciones Cardiovasculares. The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Conflict of Interest Disclosures: Dr. Krumholz works under contract with the Centers for Medicare & Medicaid Services to support quality measurement programs; was a recipient of a research grant, through Yale, from Medtronic and the U.S. Food and Drug Administration to develop methods for post-market surveillance of medical devices; was a recipient of a research grant with Medtronic and is the recipient of a research grant from Johnson & Johnson, through Yale University, to support clinical trial data sharing; was a recipient of a research agreement, through Yale University, from the Shenzhen Center for Health Information for work to advance intelligent disease prevention and health promotion; collaborates with the National Center for Cardiovascular Diseases in Beijing; receives payment from the Arnold & Porter Law Firm for work related to the Sanofi clopidogrel litigation, from the Ben C. Martin Law Firm for work related to the Cook Celect IVC filter litigation, and from the Siegfried and Jensen Law Firm for work related to Vioxx litigation; chairs a Cardiac Scientific Advisory Board for UnitedHealth; was a participant/participant representative of the IBM Watson Health Life Sciences Board; is a member of the Advisory Board for Element Science, the Advisory Board for Facebook, and the Physician Advisory Board for Aetna; and is the co-founder of HugoHealth, a personal health information platform, and co-founder of Refactor Health, an enterprise healthcare AI-augmented data management company.
REFERENCES
- 1.Pope JH, Aufderheide TP, Ruthazer R, Woolard RH, Feldman JA, Beshansky JR, Griffith JL and Selker HP. Missed diagnoses of acute cardiac ischemia in the emergency department. The New England journal of medicine. 2000;342:1163–70. [DOI] [PubMed] [Google Scholar]
- 2.Weber EU, Bockenholt U, Hilton DJ and Wallace B. Determinants of diagnostic hypothesis generation: effects of information, base rates, and experience. J Exp Psychol Learn Mem Cogn. 1993;19:1151–64. [DOI] [PubMed] [Google Scholar]
- 3.Brush JE, Sherbino J, Norman GR. How expert clinicians intuitively recognize a medical diagnosis. Am J Med 2017. June;130(6):629–634. [DOI] [PubMed] [Google Scholar]
- 4.Brush JE. The Science of the Art of Medicine: A Guide to Medical Reasoning. Manakin-Sabot, VA: Dementi Publishing; 2015. [Google Scholar]
- 5.Elstein AS, Shulman L.Sl, Sprafka SA Medical Problem Solving: An Analysis of Clinical Reasoning. Cambridge, MA: Harvard University Press; 1978. [Google Scholar]
- 6.Kassirer JP, Gorry GA Clinical problem solving: A behavioral analysis. Ann Intern Med. 1978; 89:245–255. [DOI] [PubMed] [Google Scholar]
- 7.Barrows HS, Norman GR, Neufeld VR and Feightner JW. The clinical reasoning of randomly selected physicians in general medical practice. Clin Invest Med. 1982;5:49–55. [PubMed] [Google Scholar]
- 8.Brooks LR, Norman GR and Allen SW. Role of specific similarity in a medical diagnostic task. J Exp Psychol Gen. 1991;120:278–87. [DOI] [PubMed] [Google Scholar]
- 9.Medin DL. Concepts and conceptual structure. Am Psychol. 1989;44:1469–81. [DOI] [PubMed] [Google Scholar]
- 10.Custers EJ, Regehr G and Norman GR. Mental representations of medical diagnostic knowledge: a review. Acad Med. 1996;71:S55–61. [DOI] [PubMed] [Google Scholar]
- 11.Arslanian-Engoren C, Patel A, Fang J, Armstrong D, Kline-Rogers E, Duvernoy CS and Eagle KA. Symptoms of men and women presenting with acute coronary syndromes. Am J Cardiol. 2006;98:1177–81. [DOI] [PubMed] [Google Scholar]
- 12.Canto JG, Rogers WJ, Goldberg RJ, Peterson ED, Wenger NK, Vaccarino V, Kiefe CI, Frederick PD, Sopko G, Zheng ZJ and Investigators N. Association of age and sex with myocardial infarction symptom presentation and in-hospital mortality. JAMA. 2012;307:813–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lovlien M, Schei B and Hole T. Women with myocardial infarction are less likely than men to experience chest symptoms. Scand Cardiovasc J. 2006;40:342–7. [DOI] [PubMed] [Google Scholar]
- 14.Khan NA, Daskalopoulou SS, Karp I, Eisenberg MJ, Pelletier R, Tsadok MA, Dasgupta K, Norris CM, Pilote L and Team GP. Sex differences in acute coronary syndrome symptom presentation in young patients. JAMA Intern Med. 2013;173:1863–71. [DOI] [PubMed] [Google Scholar]
- 15.Lichtman JH, Leifheit EC, Safdar B, Bao H, Krumholz HM, Lorenze NP, Daneshvar M, Spertus JA and D’Onofrio G. Sex Differences in the Presentation and Perception of Symptoms Among Young Patients With Myocardial Infarction: Evidence from the VIRGO Study (Variation in Recovery: Role of Gender on Outcomes of Young AMI Patients). Circulation. 2018;137:781–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosengren A, Wallentin L, Simoons M, Gitt AK, Behar S, Battler A and Hasdai D. Age, clinical presentation, and outcome of acute coronary syndromes in the Euroheart acute coronary syndrome survey. Eur Heart J. 2006;27:789–95. [DOI] [PubMed] [Google Scholar]
- 17.Rubini Gimenez M, Reiter M, Twerenbold R, Reichlin T, Wildi K, Haaf P, Wicki K, Zellweger C, Hoeller R, Moehring B, Sou SM, Mueller M, Denhaerynck K, Meller B, Stallone F, Henseler S, Bassetti S, Geigy N, Osswald S and Mueller C. Sex-specific chest pain characteristics in the early diagnosis of acute myocardial infarction. JAMA Intern Med. 2014;174:241–9. [DOI] [PubMed] [Google Scholar]
- 18.Dreyer RP, Sciria C, Spatz ES, Safdar B, D’Onofrio G and Krumholz HM. Young Women with Acute Myocardial Infarction: Current Perspectives. Circ Cardiovasc Qual Outcomes. 2017;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bucholz EM, Strait KM, Dreyer RP, Lindau ST, D’Onofrio G, Geda M, Spatz ES, Beltrame JF, Lichtman JH, Lorenze NP, Bueno H, Krumholz HM. Sex differences in young patients with acute myocardial infarction: A VIRGO study analysis. Eur Heart J Acute Cardiovasc Care 2017. October;6(7):610–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lichtman JH, Lorenze NP, D’Onofrio G, Spertus JA, Lindau ST, Morgan TM, Herrin J, Bueno H, Mattera JA, Ridker PM and Krumholz HM. Variation in recovery: Role of gender on outcomes of young AMI patients (VIRGO) study design. Circ Cardiovasc Qual Outcomes. 2010;3:684–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Upton G and Cook I. A Dictionary of Statistics (3 ed.) Oxford, UK: Oxford University Press; 2014, page 323. [Google Scholar]
- 22.Politis DM, Romano JP, and Wolf M. Subsampling. New York: Springer; 1999. [Google Scholar]
- 23.Vaccarino V, Rathore SS, Wenger NK, Frederick PD, Abramson JL, Barron HV, Manhapra A, Mallik S, Krumholz HM and National Registry of Myocardial Infarction I. Sex and racial differences in the management of acute myocardial infarction, 1994 through 2002. The New England journal of medicine. 2005;353:671–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.FitzGerald C and Hurst S. Implicit bias in healthcare professionals: a systematic review. BMC Med Ethics. 2017;18:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daugherty SL, Blair IV, Havranek EP, Furniss A, Dickinson LM, Karimkhani E, Main DS and Masoudi FA. Implicit Gender Bias and the Use of Cardiovascular Tests Among Cardiologists. J Am Heart Assoc. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vaccarino V, Parsons L, Every NR, Barron HV and Krumholz HM. Sex-based differences in early mortality after myocardial infarction. National Registry of Myocardial Infarction 2 Participants. N Engl J Med. 1999;341:217–25. [DOI] [PubMed] [Google Scholar]
- 27.Mehta LS, Beckie TM, DeVon HA, Grines CL, Krumholz HM, Johnson MN, Lindley KJ, Vaccarino V, Wang TY, Watson KE, Wenger NK. American Heart Association Cardiovascular Disease in Women, Special Populations Committee of the Council on Clinical Cardiology, Council on Epidemiology and Prevention, Council on Cardiovascular and Stroke Nursing and Council on Quality of Care and Outcomes Research. Acute Myocardial Infarction in Women: A Scientific Statement from the American Heart Association. Circulation. 2016;133:916–47. [DOI] [PubMed] [Google Scholar]
- 28.Simon H. Invariants of human behavior. Annual Review of Psychology. 1990; 41:1–20. [DOI] [PubMed] [Google Scholar]
- 29.Shaw LJ, Bairey Merz CN, Pepine CJ, Reis SE, Bittner V, Kelsey SF, Olson M, Johnson BD, Mankad S, Sharaf BL, Rogers WJ, Wessel TR, Arant CB, Pohost GM, Lerman A, Quyyumi AA, Sopko G and Investigators W. Insights from the NHLBI-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Study: Part I: gender differences in traditional and novel risk factors, symptom evaluation, and gender-optimized diagnostic strategies. J Am Coll Cardiol. 2006;47:S4–S20. [DOI] [PubMed] [Google Scholar]
- 30.Bairey Merz CN, Shaw LJ, Reis SE, Bittner V, Kelsey SF, Olson M, Johnson BD, Pepine CJ, Mankad S, Sharaf BL, Rogers WJ, Pohost GM, Lerman A, Quyyumi AA, Sopko G and Investigators W. Insights from the NHLBI-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Study: Part II: gender differences in presentation, diagnosis, and outcome with regard to gender-based pathophysiology of atherosclerosis and macrovascular and microvascular coronary disease. Journal of the American College of Cardiology. 2006;47:S21–9. [DOI] [PubMed] [Google Scholar]
- 31.Kreatsoulas C, Crea-Arsenio M, Shannon HS, Velianou JL, Giocomini M. Interpreting angina: symptoms along a gender continuum. Open Heart 2016; 3:e000376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.National Academies of Science, Engineering, and Medicine. 2015. Improving Diagnosis in Health Care. Washington, DC: The National Academies Press; 10.17226/2194 [DOI] [Google Scholar]
- 33.Graber ML. The incidence of diagnostic error in medicine. BMJ Qual Saf. 2013;22 Suppl 2:ii21–ii27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bowen JL. Educational strategies to promote clinical diagnostic reasoning. N Engl J Med. 2006;355:2217–25. [DOI] [PubMed] [Google Scholar]
- 35.Schmidt HG and Mamede S. How to improve the teaching of clinical reasoning: a narrative review and a proposal. Med Educ. 2015;49:961–73. [DOI] [PubMed] [Google Scholar]
- 36.Eva KW. What every teacher needs to know about clinical reasoning. Med Educ 2004; 39:98–106. [DOI] [PubMed] [Google Scholar]
- 37.Minda JP and Smith JD. Prototypes in category learning: the effects of category size, category structure, and stimulus complexity. J Exp Psychol Learn Mem Cogn. 2001;27:775–99. [PubMed] [Google Scholar]
- 38.Bordage G. Prototypes and semantic qualifiers: from past to present. Med Educ. 2007;41:1117–21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


