Abstract
There is a hypothesis that augmentation of the drainage and clearing function of the meningeal lymphatic vessels (MLVs) might be a promising therapeutic target for preventing neurological diseases. Here we investigate mechanisms of photobiomodulation (PBM, 1267 nm) of lymphatic drainage and clearance. Our results obtained at optical coherence tomography (OCT) give strong evidence that low PBM doses (5 and 10 J/cm2) stimulate drainage function of the lymphatic vessels via vasodilation (OCT data on the mesenteric lymphatics) and stimulation of lymphatic clearance (OCT data on clearance of gold nanorods from the brain) that was supported by confocal imaging of clearance of FITC-dextran from the cortex via MLVs. We assume that PBM-mediated relaxation of the lymphatic vessels can be possible mechanisms underlying increasing the permeability of the lymphatic endothelium that allows molecules transported by the lymphatic vessels and explain PBM stimulation of lymphatic drainage and clearance. These findings open new strategies for the stimulation of MLVs functions and non-pharmacological therapy of brain diseases.
1. Introduction
The meningeal lymphatic vessels (MLVs) of rodents [1,2], non-human primates and humans [3] have recently (re)discovered and characterized, although the role of these vessels in the central nervous system (CNS) function and in pathologies remains unclear. Our group [4–7] and others [1,2,8] have shown that meningeal lymphatic vessels play an essential role in maintaining brain homeostasis by draining macromolecules from the CNS (both cerebral spinal fluid, CSF and interstitial fluid, ISF) into the cervical lymph nodes. There is pioneering data suggesting that disruption of MLVs is an aggravating factor in the development of Alzheimer’s disease and promotes amyloid-β deposition in the meninges, which resembles human pathology [8]. Indeed, the amyloid-β protein was initially isolated from the brain meninges of patients with Alzheimer’s disease [9]. Based on these data, it has been assumed that augmentation of drainage and clearing function of MLVs might be a promising therapeutic target for preventing or delaying Alzheimer’s disease [8].
The development of non-pharmacological methods for a therapy of Alzheimer’s disease is a highly actual problem in medicine because there are no pharmacological drugs that provide an effective therapy of Alzheimer’s disease and limit the development of cognitive impairment [10]. Note that pharmaceutical companies such as Biogen, Johnson & Johnson, Pfizer announced the cancellation of funding for the synthesis of antibodies for the treatment of Alzheimer’s disease due to the failure of clinical trials [11]. Obviously, in the next couple of decades, the main strategies for a treatment of AD will be non-invasive methods of stimulation of clearance of the toxic beta-amyloid from the brain tissues.
In our recent pilot study on mice with the injected model of Alzheimer’s disease, we have clearly demonstrated that 9 days course of transcranial photobiomodulation (tPBM, 1267 nm, 32 J/cm2) reduces beta-amyloid plaques in the brain that is associated with improving of the memory and neurocognitive deficit [7]. Using of our original method based on optical coherence tomography (OCT) analysis of clearance of gold nanorods (GNRs) from the brain, we have proposed a possible mechanism underlying tPBM-stimulating effects on clearance of beta-amyloid via the lymphatic system of the brain and the neck. We hypothesized that the tPBM-mediated stimulation of lymphatic drainage might be possible mechanism underlying the tPBM-elimination of beta-amyloid from the brain. These results open breakthrough strategies for a non-pharmacological therapy of Alzheimer’s disease and give strong evidence that tPBM might be a promising therapeutic target for preventing or delaying Alzheimer’s disease.
To test our hypothesis, in this study we analyzed the effects of tPBM on lymphatic pumping and contractility as main physiological mechanisms underlying fluid transport and waste clearance from tissues.
2. Methods
2.1. Subject
Experiments were performed in mongrel male mice (20 to 25 g, n = 90) in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996), and the protocols were approved by the Institutional Review Boards of the Saratov State University (Protocol 7, 07.02.2017). The mice were housed at 25 ± 2°C, 55% humidity, and 12:12 h light – dark cycle. Food and water were given ad libitum.
In our experiments on healthy mice we studied mechanisms underlying the PBM-mediated (1267 nm) stimulation of lymphatic drainage and clearance. There are technical difficulties for visualization and monitoring of the lymph flow (included both ISF and CSF) as well as contractility of MLVs due to an extremely slow lymph flow in these transparent vessels and their very small size [4]. Therefore, for a clear visualization of PBM effects on lymphatic vessels, we analyzed PBM effects on tone and constriction of the mesenteric lymphatics as a classical model for the study of lymphatic functions. Note, that the MLVs express all of the molecular hallmarks of lymphatic endothelium that allows to extrapolate the data obtained from the mesenteric lymphatic vessels to MLVs [5].
2.2. PBM of the mesenteric lymphatic tone and contraction: OCT monitoring
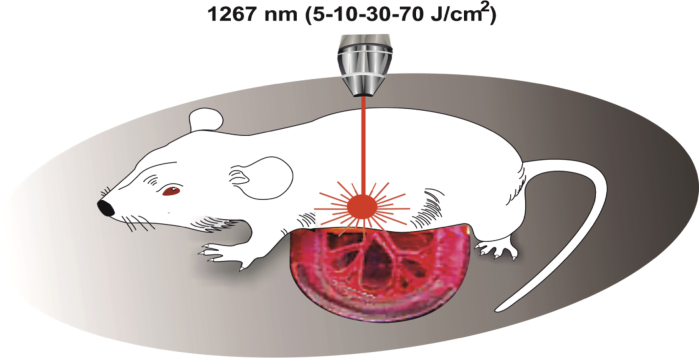
For PBM on the mesenteric lymphatics, the abdomen was opened through a midline incision and the mesentery was gently exteriorized under anesthesia with ketamine (Sigma Chemical Co, 40 mg/kg, i.v.). To eliminate motion artifacts, the mesentery was stabilized by placing it on a Perspex stage. The duration of the recovering process was at least 1 hour after surgical preparation until the mesenteric lymph flow was stabilized. PBM was applied during 61 min using the sequence of: 17 min – irradiation, 5 min – pause. A fiber Bragg grating wavelength locked a high power laser diode (LD-1267-FBG-350, Innolume, Dortmund, Germany) emitting at 1267 nm was used as a source of irradiation. The laser diode was pigtailed with a single mode distal fiber ended by the collimation optics to provide a 5 mm beam diameter at the specimen. Four laser output power densities were used at this study – 50, 100, 150, and 200 mW/cm2 (laser power was measured after the collimation lens). For taken power densities and exposure time of 1020 seconds, the following laser fluences were delivered to the surface of the brain during the experiments: 51 J/cm2, 102 J/cm2, 153 J/cm2, 204 J/cm2, which were converted into 5 J/cm2, 10 J/cm2, 30 J/cm2, 70 J/cm2 at the brain’s surface according to transmission data. The heating of the mesenteric and the brain tissue (in other sets of experiments) caused by exposure to light was monitored by using a thermocouple data logger (Pico Technology, USB TC-08, Cambridge shire, UK).
To study the effect of different PBM-doses (5-10-30-70 J/cm2) on the contractility of the mesenteric lymphatics, we used optical coherence tomography (OCT) for in vivo monitoring of influences of different PBM doses (5-10-30-70 J/cm2) on the diameter of the mesenteric lymphatic vessels and their contractility. The choice of PBM dose is determined by the results of our previous studies, where we showed that the PBM-dose 32 J/cm2 is effective for stimulation of clearance of beta-amyloid from the mouse brain [7]. The transmission analysis revealed that only 10% of the laser energy goes to the brain via the intact mouse skull (the scalp was removed), i.e. 5 J/cm2. Therefore, were selected 5 J/cm2 as a minimal PBM dose for stimulation of the mesenteric contractility. Other PBM dose (10-30-70 J/cm2) was selected arbitrarily.
The OCT monitoring of mesenteric lymphatic functions was performed in 4 groups of mice before and after PBM: 1) 5 J/cm2; 2) 10 J/cm2; 3) 30 J/cm2; 4) 70 J/cm2; n = 10 in each group.
2.3. tPBM of the clearance of GNRs from the mouse brain: OCT monitoring
To study the effects tPBM (via the intact skull, the scalp was removed) on drainage function of MLVs, we analyzed clearance of GNRs from mouse brain before and after tPMB (64 J/cm2). The choice of the PBM dose was due to our results obtained in the first step of the experiments, where we found that the PBM (10 J/cm2) more significantly stimulates contractility and changes in diameter of the mesenteric lymphatic vessels than PBM (5 J/cm2), PBM doses 30 J/cm2 and 70 J/cm2 suppress pumping and contractility of the mesenteric lymphatics. Such high energy doses were delivered onto the surface of the mesenteric lymphatic vessel with the diffraction limited light spot of 10 um. Due to the very low effective absorption inside the thin the mesentery only a small percentage of this energy is effectively contribute to photomodulation of the lymphatic activity. In our previous study on mice with Alzheimer disease, we have shown that tPBM 32 J/cm2 (on surface of the brain) is the minimal laser dose for stimulation of clearance of beta-amyloid from the brain [7]. Considering the results of transmission experiments, tPBM (32 J/cm2) is related to PBM (5 J/cm2) effects on the mesenteric lymphatics. Since we obtained that PBM (10 J/cm2) is most effective for stimulation of contractility of the mesentaric lymphatic, we doubled the tPBM dose.
For PBM mice with shaved head were fixed in stereotaxic frame and irradiated in the area of the frontal cortex using the sequence of: 17 min – irradiation, 5 min – pause during 61min.
We used the commercial spectral domain OCT Thorlabs GANYMEDE (central wavelength 930 nm, spectral band 150 nm, axial resolution 4.4 µm in water, and maximal imaging depth 2.7 mm). To provide a lateral resolution of about 13 µm within the depth of the field, the LSM02 objective was used. A-scan rate of the OCT system was set to 30 kHz. Each B-scan consists of 2048 A-scans to ensure an appropriate spatial sampling.
At each location, 50 B-scans were taken. Logarithmic intensity values were converted into a linear scale via the equation This stack was then stabilized with respect to the reference region (emptiness in the lymphatic node). After stabilization process B-scans were averaged to increase signal-to-noise ratio and accuracy of the measurements.
Since a lymph is optically transparent (low absorption and scattering) in a broad range of wavelengths, “empty” cavities exist in the resulting OCT image of the lymphatic node with a background signal-to-noise ratio inside. In order to image the lymph dynamic accumulation within these cavities, a suspension of GNRs was used as a contrast agent, for which the OCT signal intensity is proportional to the nanoparticle concentration. By tracking the OCT signal temporal alterations inside the node’s cavity, we could confirm the clearance pathways and quantify its relative speed. The OCT recordings were performed under anesthesia with ketamine (100 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.).
GNRs coated with thiolated polyethylene glycol (5 µL at a rate of 0.1 µL/min, the GNRs average diameter and length of 16 ± 3 nm and 92 ± 17 nm) were injected in the cortex (AP - 1.06 mm; DV - 1.5 mm; ML - 1.5 mm), the hippocampus (AP - 2.0 mm; ML - 1.3 mm; DV - 1.9 mm), the cisterna magna, and the right lateral ventricle (AP - 1.06 mm; DV – 2.0 mm; ML – 2.0 mm) 30 min before tPBM. Afterwards, OCT imaging of dcLN was performed during the next 1h for each mouse. The GNR was used with a concentration of 500 µg/ml, and the injected dose of 5 µl containing 2.5 µg Au.
The OCT monitoring of accumulation of GNRs in dcLN was performed in 4 groups of mice before and after PBM (64 J/cm2) and injected of GNRs into: 1) the cortex; 2) the hippocampus; 3) the cisterna magna; and 4) the right lateral ventricle; n = 10 in each group.
2.4. The confocal imaging of the clearance of FITC-dextran 70 kDa from the brain via MLVs
FITC-dextran 70 kDa (5 µL at a rate of 0.1 µL/min Quintessential Stereotaxic injector 53311, Stoelting, US) was injected into the cortex (AP - 1.06 mm; DV – 1., ML - 1.5 mm) in 10 mice. This area of the brain was selected due to stronger PBM effects on clearance of GNRs from the cortex which we obtained in previous steps of experiments. Afterward mice were decapitated; their meninges were removed and fixed as described in Ref. 12. To label MLVs and the cerebral veins, meninges were incubated overnight at + 4°C with goat anti-rabbit LYVE-1 antibody (1:500; PA-16635, Invitrogen, Molecular Probes, Eugene, Oregon, USA) and goat anti-mouse NG2 antibody (1:500; ab 50009, Abcam, Cambridge, United Kingdom), respectively. After several rinses in PBS, the meninges were incubated for 3h at room temperature with fluorescent-labeled secondary antibodies on 1% BSA/0.2% Triton X-100 /PBS (1:500; goat anti-rabbit IgG (H + L) Alexa Four 555 and goat anti-mouse IgG (H + L) Alexa Four 647; Invitrogen, Molecular Probes, Eugene, Oregon, USA) with further confocal analysis (Leica TCS SP 5 (Leica Microsystems Inc., Germany).
2.5. Statistical analysis
The results were reported as a mean value ± standard error of the mean (SEM). Differences from the initial level in the same group were evaluated by the Wilcoxon test. Intergroup differences were evaluated using the Mann-Whitney test and the ANOVA-2 (post hoc analysis with the Duncan’s rank test). The significance levels were set at p <0.05 for all analyses.
3. Results
3.1. PBM-dose related changes in tone of the mesenteric lymphatic vessels
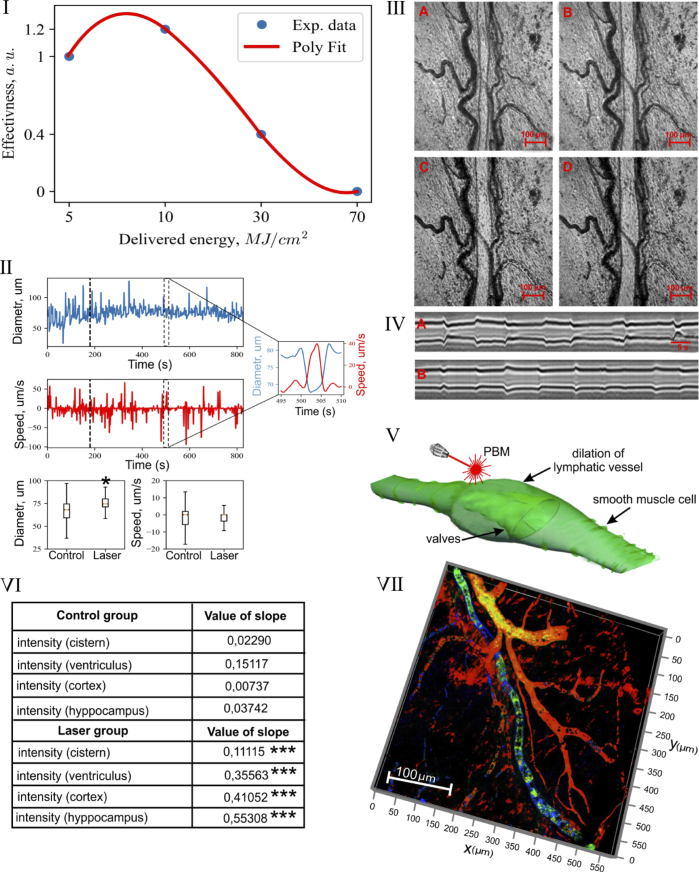
We first evaluated dose-related PBM effects on the mesenteric collecting lymphatics using laser irradiation in different intensities 5-10-30-70 J/cm2. The mesenteric lymphatic vessels are typically found in parallel with arterioles and venules surrounding by adipose cells in mouse mesentery. Figure 2-I illustrate dose-related changes in tone of the mesenteric lymphatic vessels. The low PBM dose (5 J/cm2 and 10 J/cm2) induced a relaxation of the mesenteric lymphatics with maximum response to 10 J/cm2. The higher PBM dose (30 J/cm2 and 70 J/cm2) completely blocked the contractility of these vessels..Based on these finding, we used PBM (10 J/cm2) in further set of experiments as the most effective PBM dose.
Fig. 2.
The PBM of lymphatic tone and contraction: I – PBM-dose related changes in tone of the mesenteric lymphatic vessels; II – The changes in diameter (um) of the mesenteric lymphatic vessels and lymph flow (um/sec) in them before and after tPBM (10 J/cm2); III - The illustration of changes in diameter of the mesenteric lymphatic vessel in systole (A) and diastole (C) before (A and C) after (B and D) PBM (10 J/cm2), scale bars are 100 µm (see also Visualization 1); IV – The time-related changes of diameter of the mesenteric lymphatic vessels before (A) and after (B) PBM (10 J/cm2), scale bars are 5 µm; V – Schematic illustration of PBM-mediated relaxation of the mesenteric lymphatic vessel; VI - The OCT monitoring of the rate of accumulation of GNRs in dcLN in untreated mice and in mice received tPBM (64 J/cm2 via the intact skull and 10 J/cm2 on the brain surface) after GNRs injection into the cisterna magna, the right lateral ventricle, the cortex, the hippocampus; *** - p < 0.001 vs. basal level. n = 10 in each group; VII – The illustration of clearance of FITC-dextran (green color) from the brain via MLVs (blue color), which are located close to the cerebral veins (red color, labeled by NG2).
3.2. The effects of PBM on the contactile function of mesenteric lymphatic vessels
In the next step, we studied PBM effects on contractility of the mesentertic lymphatic vessels in systole and diastole. Figure 2-II demonstrates the fluctuation character in the changes of the diameter of mesenteric lymphatic vessels and lymph flow in them related to systole (constriction and the rise of lymph flow) and diastole (dilation and the fall of lymph flow).
The PBM increased physiological fluctuations in contractility of the mesenteric vessels and lymph flow (75.3 ± 7 um vs. 68.1 ± 11 um, p < 0.05 for the average diameter and 6.0 ± 2.3 um/sec vs. 6.2 ± 2.1 um/sec, for the average speed) (Fig. 2-II). Such high values of deviation of the flow and vessel diameter are related to the constriction cycle (namely its amplitude and frequency) of the lymphatic vessel (Fig. 2-II). Figure 2-III-V illustrate OCT imaging of increase in diameter of the mesenteric lymph vessel in systole and diastole after tPBM (10 J/cm2). The supplementary material (Visualization 1) demonstrates a dilation of the mesenteric lymphatic vessel and an increase a number of macrophages (green points) inside the cavity of vessel after tPBM (10 J/cm2) due to an increasing of interstitial fluid (lymph) uptake.
3.3. The effects of tPBM on the clearance of GNR from the mouse brain
In the next step, we intended to study the effects of tPBM (10 J/cm2) on the drainage function of the meningeal lymphatic system. With this aim, we made injection of GNRs in different areas of the brain and monitored in vivo by OCT accumulation of GNRs in dcLN before and after tPBM. The table in Fig. 2-VI shows that tPBM significantly increased clearance of GNRs from the brain. Indeed, the rate GNR accumulation in dcLN was higher 55.7-fold in the cortex, 14.78-fold in hippocampus, 4.8-fold in the cisterna magna, 2.3-fold in the left ventricle.
3.4. The clearance of FITC-dextran from the brain via MLVs
To analyze the involvement of MLVs in clearance of molecules from the brain, FITC-dextran was injected into the cortex with further confocal analysis of its removing from the brain via MLVs labeled by LYVE-1 (marker of lymphatic endothelium, blood vessels were labeled by NG2). Figure 1-VII clearly shows the presence of FITC-dextran in MLVs 5 min after its injection in the cortex suggesting about lymphatic pathways of removing of tracers from the brain.
Fig. 1.
Schematic illustration of PBM of function of mesenteric lymphatics.
4. Discussion
In these experiments on male mice we studied mechanisms underlying the PBM-mediated stimulation of lymphatic clearance of molecules from the brain, which our group [4–7] and others [1,2,8] discussed in previous studies. The meningeal lymphatic system has prompted an assessment of its role in waste clearance from the brain, including clearance of toxic of beta-amyloid [1,2, 4–9]. Our [7] and other experimental findings [8] clearly show that an augmentation of drainage and clearing function of MLVs might be a promising therapeutic target for preventing Alzheimer disease. In our recent study we demonstrated tPBM (1267 nm, 32 J/cm2) of clearance of beta-amyloid from the mouse brain leading to an improving of neurological and cognitive status of mice with Alzheimer disease. Here we studied mechanisms underlying the PBM-mediated (1267 nm) stimulation of lymphatic drainage and clearance. There are technical difficulties for visualization and monitoring of the lymph flow (included both ISF and CSF) as well as contractility of MLVs due to an extremely slow lymph flow in these transparent vessels. For example, a full clearance of radio-iodinated albumin from the brain requires 25-26 hrs in sheep, cats and rabbits [13]. Therefore, for a clear visualization of PBM effects on lymphatic vessels, we analyzed PBM effects on tone and constriction of the mesenteric lymphatics as a classical model for the study of lymphatic functions. Our results indeed give strong evidence that the low PBM doses (5 J/cm2 and 10 J/cm2 (maximal effect)) induces a relaxation of mesenteric lymphatics in both systole and diastole with a decrease in contraction amplitude (Fig. 2-I-V). We find that a further increase in the PBM dose (30 J/cm2 and 70 J/cm2) completely blocks contractility of these vessels (Fig. 2-I). In our study we used a laser with wavelength 1267 nm, which produces singlet oxygen directly without photosensitizers [15]. In other studies, using lymphatic-specific photodynamic therapy with photosensitizers, which generate singlet oxygen after laser excitation, a suppression of lymphatic contractility also has been shown [16].
The lymphatic vessels run in parallel to the blood vascular system and serve an absorption and clearing role draining a lymph to the lymph nodes/lymphoid organs that facilitates immune control and protection. During the day, approximately 20 L of plasma is filtered through the vascular endothelium of capillaries in the extracellular space in humans and 17 L of the filtered plasma are reabsorbed back into the venous vessels, while three liters remain in the extracellular or interstitial fluid (ISF) [14]. The lymphatic system is not a closed system and the lymphatic vessels are opened in the extracellular scape which provides the return of three liters of ISF to the blood. The contractility of lymphatic vessels is the driving force for active lymph pumping against adverse pressure gradients [17]. The back flow within the lymphatic system is limited by a one-way valves [18]. During systole lymphatic muscles constrict and both inflow and outflow valves are closed that results in a rapid rise in the intraluminal pressure. When the intraluminal pressure exceeds the outflow pressure, the outflow valve opens, ejecting lymph [19]. During diastole lymphatic muscles dilate, intraluminal pressure falls, the outflow valve closes, and the inflow valve then opens to allow filling of the lymphangion. This sequence of events is illustrated by us in the mesenteric lymphatics before and after PBM: consecutive changes in constriction and dilation with an increase and decrease in lymph flow in these vessels, respectively (Fig. 2-III). However, PBM induced relaxation of mesenteric lymphatic vessels with a decrease of constriction amplitude in systole and diastole. At the same time, using the same PBM dose (64 J/cm2 via the intact skull, 10 J/cm2 on the brain surface) and OCT for in vivo monitoring of accumulation of GNRs in dcLN, we demonstrated a significant PBM stimulation of clearance of GNRs from the brain. We also identified the meningeal lymphatic pathway for draining molecules from the brain using confocal imaging of clearance of FITC-dextran 70 kDa from the cortex via MLVs. Thus, we found the relaxing effects of PBM on the mesenteric lymphatics and the stimulating effects of PBM on clearance of GNRs from the brain. We hypothesized that both PBM effects can be related to a PBM-mediated increase in the permeability of lymphatic endothelium that is the key mechanism allowing antigen and toxins transported by the collecting lymphatics to reach local immune cells to mediate immune responses [20]. The basal permeability may therefore serve as the communication between lymph contents and activation of immune cells. This activation may then lead to the production of nitric oxide (NO) or other vasoactive molecules, which would inhibit lymphatic contractions and reduce lymph flow [19]. The increasing of permeability of lymphatic endothelium is possible mechanism underlying lipids diffusion from the tissues to these vessels, which may help to explain why there is always adipose tissue located adjacent to collecting lymphatics and lymph nodes [21]. The enhancement of endothelial NO production is a well-known mechanism of low-level laser therapy [22]. The lymphatic behavior is regulated by NO and other endothelium-derived factors such as prostaglandins and histamine [23,24]. Both lymphatic tone and spontaneous contractions are inhibited by NO as a result of shear stress on the endothelium [25]. NO is a vasodilator that acts via stimulation of soluble guanylate cyclase to form cyclic-GMP (cGMP), which activates protein kinase G causing the opening of calcium-activated potassium channels and re-uptake of Ca2+. The decrease in concentration of Ca2+ prevents myosin light-chain kinase from phosphorylating the myosin molecule, leading to relaxation of lymphatic vessels [26]. There are several other mechanisms by which NO could induce lymphatic dilation: 1) the activation of iron-regulatory factor in macrophages [27], 2) the modulation of proteins such as ribonucleotide reductase [28] and aconitase [29]; the stimulation of the ADP-ribosylation of glyceraldehyde-3-phosphate dehydrogenase [30] and protein-sulfhydryl-group nitrosylation [31].
Thus, a PBM-mediated increasing of relaxation of lymphatic vessels and associated this enhancement of permeability of lymphatic endothelium can be possible mechanism explaining PBM-stimulation of lymphatic clearance of molecules from the brain (GNRs and beta-amyloid [7,8]). Note, that cerebral and peripheral lymphatic vessels express the same molecular hallmarks of lymphatic endothelium [5]. This fact allows us to extrapolate results obtained on PBM-relaxation of the mesenteric lymphatic vessels to MLVs. However, MLVs are proposed to be primitive lymphatic vessels lacking smooth muscles and lymphatic valves [1] suggesting that the increasing of permeability of lymphatic endothelium might be a leading factor in cerebral lymphatic drainage and clearance. Note that the anatomy and functions of cerebral lymphatic are not well understood which significantly limits our understating of mechanisms responsible for PBM effects on the lymphatic drainage and clearance. There are other data, in which it was shown the lymphatic vessels with valves at the base of the skull, but their distribution was relatively scarce and the valves are separated by long stretches of valveless vessel segments [2]. Antila also reported numerous lymphatic vessels with valves in the foramen magnum, cervical verterbrae, in the lymphatics flanking the pterygopalatine and middle meningeal arteries [32]. Lohrberg et al. demonstrated the presence of lymphatic vessels in the pia matter and in the brain tissues [33]. Prineas was first, who reported lymphatic channels in the brain tissues in individuals with neurological disorders [34]. Obviously, it needs more time for a better understating the physiology of cerebral lymphatics. In our recent review, we discussed the important role of MLVs in regenerative mechanisms of the brain and that stimulation of lymphatic drainage and clearance will contribute the progress in an effective therapy of neurological diseases such as stroke, brain trauma, neurodegenerative diseases, glioblastoma [5]. PBM-mediated stimulation of cerebral clearance and drainage might be the promising tool in an augmentation of function of the cerebral lymphatic system and non-pharmacological therapy of brain pathologies.
5. Summary
In this experimental work on male mice, we clearly demonstrate effects of PBM on lymphatic drainage and clearance. The low dose of PBM relaxes of the mesenteric lymphatics with decrease in contraction amplitude and significantly increases clearance of molecules (GNRs) from the brain, partly via MLVs as we showed with clearance of FITC-dextran from the cortex. We believe that PBM-mediated relaxation of lymphatic vessels can be one of mechanisms underlying in increasing of permeability of lymphatic endothelium that allows molecules transported by lymphatic vessels and explain PBM stimulation of lymphatic drainage and clearance.
Acknowledgments
We thank the Laboratory of Nanobiotechnology and the Center for Collective Use “Symbiosis” IBPPM RAS for support in data acquisition (synthesis of GNRs and confocal imaging) in the framework of Research Project no. АААА-А17-117102740097-1.
A typographical correction was made to the author listing.
Funding
Russian Science Foundation10.13039/501100006769 (17-15-01263, 18-15-00172, 19-15-00201).
Disclosures
The authors declare that there are no conflicts of interest related to this article.
References
- 1.Louveau I., Smirnov T., Keyes J., Eccles S., Rouhani J., Peske N., Derecki D., Castle J., Mandell K., Lee T., Harris J., Kipnis, “Structural and functional features of central nervous system lymphatic vessels,” Nature 523(7560), 337–341 (2015). 10.1038/nature14432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aspelund S., Antila S., Proulx T., Karlsen S., Karaman M., Detmar H., Wiig K. A., Alitalo, “A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules,” J. Exp. Med. 212(7), 991–999 (2015). 10.1084/jem.20142290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Absinta M., Ha S.-K., Nair G., Satil P., Luciano N. J., Palisoc M., Louveau A., Zaghloul K. A., Pittaluga S., Kipnis J., Reich D. S., “Human and nonhuman primate meninges harbor lymphatic vessels that can be visualized noninvasively by MRI,” eLife 6, e29738 (2017). 10.7554/eLife.29738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Semyachkina-Glushkovskaya O., Abdurashitov A., Dubrovsky A., Bragin D., Bragina O., Shushunova N., Maslyakova G., Navolokin N., Bucharskaya A., Tuchin V., Kurths J., Shirokov A., “Application of optical coherence tomography for in vivo monitoring of the meningeal lymphatic vessels during opening of blood-brain barrier: mechanisms of brain clearing,” J. Biomed. Opt. 22(12), 1–9 (2017). 10.1117/1.JBO.22.12.121719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Semyachkina-Glushkovskaya O., Postnov D., Kurths J., “Blood−Brain Barrier, Lymphatic Clearance, and Recovery: Ariadne’s Thread in Labyrinths of Hypotheses,” Int. J. Mol. Sci. 19(12), 3818 (2018). 10.3390/ijms19123818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Semyachkina-Glushkovskaya O., Chehonin V., Borisova E., Fedosov I., Namykin A., Abdurashitov A., Shirokov A., Khlebtsov B., Lyubun Y., Navolokin N., Ulanova M., Shushunova N., Khorovodov A., Agranovich I., Bodrova A., Sagatova M., Shareef A. E., Saranceva E., Iskra T., Dvoryatkina M., Zhinchenko E., Sindeeva O., Tuchin V., Kurths J., “Photodynamic opening of the blood-brain barrier and pathways of brain clearing,” J. Biophotonics 11(8), e201700287 (2018). 10.1002/jbio.201700287 [DOI] [PubMed] [Google Scholar]
- 7.Zinchenko E., Navolokin N., Shirokov A., Khlebtsov B., Dunbrovsky A., Saranceva E., Abdurashitov A., Khorovodov A., Terskov A., Mamedova A., Klimova M., Agranovich I., Martinov D., Tuchin V., Semyachkina-Glushkovskaya O., Kurths J., “Pilot study of transcranial photobiomodulation of lymphatic clearance of beta-amyloid from the mouse brain: breakthrough strategies for nonpharmacologic therapy of Alzheimer’s disease,” Biomed. Opt. Express 10(8), 4003–4017 (2019). 10.1364/BOE.10.004003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Da Mesquita S., Louveau A., Vaccari A., Smirnov I., Cornelison R. C., Kingsmore K. M., Contarino C., Onengut-Gumuscu S., Farber E., Raper D., Viar K. E., Powell R. D., Baker W., Dabhi N., Bai R., Cao R., Hu S., Rich S. S., Munson J. M., Lopes M. B., Overall C. C., Acton S. T., Kipnis J., “Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease,” Nature 560(7717), 185–191 (2018). 10.1038/s41586-018-0368-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joachim C., Duffy L., Morris J., Selkoe D., “Protein chemical and immunocytochemical studies of meningovascular β-amyloid protein in Alzheimer’s disease and normal aging,” Brain Res. 474(1), 100–111 (1988). 10.1016/0006-8993(88)90673-7 [DOI] [PubMed] [Google Scholar]
- 10.Dunkel P., Chai C. L., Sperlágh B., Huleatt P. B., Mátyus P., “Clinical utility of neuroprotective agents in neurodegenerative diseases: current status of drug development for Alzheimer’s, Parkinson’s and Huntington’s diseases, and amyotrophic lateral sclerosis,” Expert Opin. Invest. Drugs 21(9), 1267–1308 (2012). 10.1517/13543784.2012.703178 [DOI] [PubMed] [Google Scholar]
- 11.Biogen/Eisai Halt Phase 3 Aducanumab Trials. https://www.alzforum.org/news/research-news/biogeneisai-haltphase-3-aducanumab-trials.
- 12.Louveau I., Kipnis J., “Dissection and immunostaining of mouse whole-mount meninges,” Protocol exchange, doi: 10.1038/protex.2015.047 (2015).
- 13.Cserr H. F., Patlak C. S., “Secretion and bulk flow of interstitial fluid. In Physiology and Pharmacology of the Blood-Brain Barrier,” Bradbury M. W. B., Cuatrecasas P., Herken H., eds.; Handbook of Experimental Pharmacology, 103 (Springer, 1992), pp. 245–261 . [Google Scholar]
- 14.Lauralee S., Human Physiology from Cells to Systems, 8th ed. (Nelson Education, 2015). [Google Scholar]
- 15.Sokolovski S. G., Zolotovskaya S. A., Goltsov A., Pourreyron C., South A. P., Rafailov E. U., “Infrared laser pulse triggers increased singlet oxygen production in tumour cells,” Sci. Rep. 3(1), 3484 (2013). 10.1038/srep03484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kilarski W., Muchowicz A., Wachowska M., Mezyk-Kopec R., Golab J., Swartz M., “Nowak-Sliwinska. Optimization and regeneration kinetics of lymphatic-specific photodynamic therapy in the mouse dermis,” Angiogenesis 17(2), 347–357 (2014). 10.1007/s10456-013-9365-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zweifach B., Prather J., “Micromanipulation of pressure in terminal lymphatics in the rat mesentery,” Am. J. Physiol. 228(5), 1326–1335 (1975). 10.1152/ajplegacy.1975.228.5.1326 [DOI] [PubMed] [Google Scholar]
- 18.Davis M., Rahbar E., Gashev A., Zawieja D., Moore J., “Determinants of valve gating in collecting lymphatic vessels from rat mesentery,” Am J. Physiol. Heart Circ. Physiol. 301(1), H48–H60 (2011). 10.1152/ajpheart.00133.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scallan J., Zawieja S., Castorena-Gonzalez J., Davis M., “Lymphatic pumping: mechanics, mechanisms and malfunction,” J. Physiol. 594(20), 5749–5768 (2016). 10.1113/JP272088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuan E., Ivanov S., Bridenbaugh E., Victora G., Wang W., Childs E., Platt A., Jakubzick C., Mason R., Gashev A., Nussenzweig M., Swartz M., Dustin M., Zawieja D., Randolph G., “Collecting lymphatic vessel permeability facilitates adipose tissue inflammation and distribution of antigen to lymph node – homing adipose tissue dendritic cells,” J. Immunol. 194(11), 5200–5210 (2015). 10.4049/jimmunol.1500221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harvey N. L., “The link between lymphatic function and adipose biology,” Ann. N. Y. Acad. Sci. 1131(1), 82–88 (2008). 10.1196/annals.1413.007 [DOI] [PubMed] [Google Scholar]
- 22.Karu T. I., Pyatibrat L. V., Afanasyeva N. I., “Cellular effects of low power laser therapy can be mediated by nitric oxide,” Lasers Surg. Med. 36(4), 307–314 (2005). 10.1002/lsm.20148 [DOI] [PubMed] [Google Scholar]
- 23.Gasheva O., Gashev A., Zawieja D., “Cyclic guanosine monophosphate and the dependent protein kinase regulate lymphatic contractility in rat thoracic duct,” J. Physiol. 591(18), 4549–4565 (2013). 10.1113/jphysiol.2013.258681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nizamutdinova I., Maejima D., Nagai T., Bridenbaugh E., Thangaswamy S., Chatterjee V., Meininger C., Gashev A., “Involvement of histamine in endothelium-dependent relaxation of mesenteric lymphatic vessels,” Microcirculation 21(7), 640–648 (2014). 10.1111/micc.12143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gashev A., Davis M., Zawieja D., “Inhibition of the active lymph pump by flow in rat mesenteric lymphatics and thoracic duct,” J. Physiol. 540(3), 1023–1037 (2002). 10.1113/jphysiol.2001.016642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murad F., “Discovery of some of the biological effects of nitric oxide and its role in cell signaling,” Biosci. Rep. 24(4-5), 453–474 (2004). 10.1007/s10540-005-2741-8 [DOI] [PubMed] [Google Scholar]
- 27.Drapier J. C., Hirling H., Wietzerbin J., Kaldy P., Kühn L. C., “Biosynthesis of nitric oxide activates iron regulatory factor in macrophages,” EMBO J. 12(9), 3643–3649 (1993). 10.1002/j.1460-2075.1993.tb06038.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lepoivre M., Fieschi F., Coves J., Thelander L., Fontecave M., “Inactivation of ribonucleotide reductase by nitric oxide,” Biochem. Biophys. Res. Commun. 179(1), 442–448 (1991). 10.1016/0006-291X(91)91390-X [DOI] [PubMed] [Google Scholar]
- 29.Drapierand J. C., Hibbs J. B., “Aconitases: a class of metalloproteins highly sensitive to nitric oxide synthesis,” Methods Enzymol. 269, 26–36 (1996). 10.1016/S0076-6879(96)69006-5 [DOI] [PubMed] [Google Scholar]
- 30.Dimmeler S., Lottspeich F., Brune B., “Nitric oxide causes ADPribosylation and inhibition of glyceraldehyde-3- phosphate dehydrogenase,” J. Biol. Chem. 267(24), 16771–16774 (1992). [PubMed] [Google Scholar]
- 31.Stamler J. S., Simon D. I., Osborne J. A., Mullins M. E., Jaraki O., Michel T., Singel D., Loscalzo J., “S-nitrosylation of proteins with nitric oxide: synthesis and characterization of biologically active compounds,” Proc. Natl. Acad. Sci. U. S. A. 89(1), 444–448 (1992). 10.1073/pnas.89.1.444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Antila S., Karaman S., Nurmi H., Airavaara M., Voutilainen M., Mathivet T., Chilov D., Li Z., Koppinen T., Park J., Fang S., Aspelung A., Saarma M., Eichmann A., Thomas J., Alitalo K., “Development and plasticity of meningeal lymphatic vessels,” J. Exp. Med. 214(12), 3645–3667 (2017). 10.1084/jem.20170391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lohrberg M., Wilting J., “The lymphatic vascular system of the mouse head,” Cell Tissue Res. 366(3), 667–677 (2016). 10.1007/s00441-016-2493-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prineas J. W., “Multiple sclerosis: Presence of lymphatic capillaries and lymphoid tissues in the brain and spinal cord,” Science 203(4385), 1123–1125 (1979). 10.1126/science.424741 [DOI] [PubMed] [Google Scholar]