The aim of this work was to evaluate an easy-to-perform assay based upon inhibition of mobile colistin resistance (MCR) activity by EDTA. We included 92 nonrelated isolates of Enterobacteriaceae (74 Escherichia coli, 17 Klebsiella pneumoniae, and 1 Serratia marcescens). Our proposed method is based on a modification of the colistin agar-spot screening test (CAST), a plate containing 3 μg/ml colistin, by adding an extra plate of colistin agar-spot supplemented with EDTA (eCAST).
KEYWORDS: MCR, Enterobacteriaceae, colistin, EDTA
ABSTRACT
The aim of this work was to evaluate an easy-to-perform assay based upon inhibition of mobile colistin resistance (MCR) activity by EDTA. We included 92 nonrelated isolates of Enterobacteriaceae (74 Escherichia coli, 17 Klebsiella pneumoniae, and 1 Serratia marcescens). Our proposed method is based on a modification of the colistin agar-spot screening test (CAST), a plate containing 3 μg/ml colistin, by adding an extra plate of colistin agar-spot supplemented with EDTA (eCAST). Bacterial growth was evaluated after 24 h of incubation at 35°C. All the colistin-resistant isolates showed development on the CAST plates. Colistin-resistant K. pneumoniae without mcr-1 and S. marcescens also grew on the eCAST plates. In contrast, colistin-resistant MCR-producing E. coli was not able to grow in eCAST plates. The combined CAST/eCAST test could provide a simple and easy-to-perform method to differentiate MCR-producing Enterobacteriaceae from those in which colistin resistance is mediated by chromosomal mechanisms.
INTRODUCTION
Worldwide dissemination of multidrug-resistant and extremely drug-resistant Gram-negative bacteria, including carbapenemase-producing Enterobacteriaceae led to reviving colistin (COL) as a last-resort therapy (1); this antibiotic interacts directly with the outer membrane lipopolysaccharide (2). The main resistance mechanisms involve modification of lipid A by more basic substituents; chromosome-encoded mechanisms have been known to emerge, even intratreatment, in clinically relevant microorganisms such as Klebsiella pneumoniae by different mutations in regulatory system genes (3–5). Since the first electronic report on the emergence of plasmid-mediated colistin resistance, including the description of the mcr-1 (mobile colistin resistance) gene published in 2016 (6), the presence of this plasmid-dependent mechanism has been found in almost every country where it was searched for. The mcr-1 gene encodes a phosphoethanolamine (PEtN) transferase family member, a zinc-containing metalloprotein that catalyzes the addition of PEtN to lipid A in Escherichia coli, conferring resistance to COL (7, 8). Even though several variants of this metalloenzyme have been described (mcr-2 to -9) (9–15), mcr-1 is by far the most prevalent marker worldwide, where it has been disseminating unnoticed for decades.
Broth microdilution assays and the polymyxin NP test have been demonstrated to be accurate in detecting COL resistance (16, 17). However, they are not able to distinguish the COL-resistant mcr-producing isolates from those expressing chromosomal mechanisms (e.g., those affecting regulatory genes) (3–5). In this regard, zinc-limiting conditions have been proposed as an alternative for phenotypic identification of MCR-1-producing E. coli (16–19). Here, we describe an easy-to-perform phenotypic assay based upon inhibition of MCR activity by EDTA, which may enable the efficient detection of MCR-producing Enterobacteriaceae even in resource-limited health care settings.
MATERIALS AND METHODS
A total of 92 nonrelated isolates of Enterobacteriaceae recovered from human (n = 62) and animal (n = 30) samples were evaluated. These included mcr-1-like-positive COL-resistant (COLR) E. coli (n = 45), mcr-2-positive COLR E. coli (n = 1), mcr-4-positive COLR E. coli (n = 1), mcr-5-positive COLR E. coli (n = 1), mcr-1-positive COLR K. pneumoniae (n = 1), mcr-negative COLR K. pneumoniae (n = 8), COL-susceptible (COLS) E. coli (n = 25), COLS K. pneumoniae (n = 8), and Serratia marcescens (n = 1), which belong to the culture collection of the Laboratorio de Resistencia Bacteriana. E. coli ATCC 25922 was also included. Some of the COLR and COLS strains are carbapenemase producers (Table 1). All isolates were previously characterized for mcr-1 to mcr-5 (20) and the presence of carbapenemases (21) by PCR multiplex and DNA sequencing. The mgrB architecture (gene encoding a negative feedback regulator of the PhoQ-PhoP signaling system) was analyzed with different PCRs using specific primers (22). Susceptibility to COL was determined by broth microdilution and interpreted following EUCAST guidelines (16).
TABLE 1.
Results summarizing the assays of the colistin agar-spot screening test (CAST) and EDTA colistin agar-spot screening test (eCAST)
| Isolate | No. of isolates | MIC50 and MIC range (mg/liter) | CASTd | eCASTd |
|---|---|---|---|---|
| COLR mcr positivea | 49 | 8 (4–32) | G | NG |
| COLR mcr negativeb | 9 | 16 (16–64) | G | G |
| COLSc | 34 | 0.5 (0.25–2) | NG | NG |
The 49 MCR-producing isolates included 48 E. coli (45 mcr-1, 1 mcr-2, 1 mcr-4, and 1 mcr-5) and 1 K. pneumoniae (mcr-1); 4 out of 46 mcr-1-positive strains were carbapenemase producers (2 NDM-1 and 2 OXA-163).
The nine colistin-resistant isolates included one S. marcescens and eight K. pneumoniae; six of them were carbapenemase producers (five KPC-2 and one NDM-1). Five out of eight K. pneumoniae isolates showed the ΔmgrB locus.
The colistin-susceptible isolates included 26 E. coli and 8 K. pneumoniae (all of them mcr negative); 10 out of 34 were carbapenemase producers (9 NDM-1 and 1 OXA-181).
G, growth; NG, no growth.
The proposed method is based on a modification of the colistin agar-spot screening test (CAST) proposed by Servicio de Antimicrobianos, INEI ANLIS “Dr. Carlos G. Malbrán” (http://antimicrobianos.com.ar/ATB/wp-content/uploads/2017/09/Protocolo-Agar-spot-COL-2017-version2-Agosto2017.pdf), already distributed by a diagnostics company (https://www.britanialab.com/back/public/upload/productos/upl_5bd08fc36c844.pdf). In this method, a spot of approximately 10 to 15 mm is inoculated using a swab (from a 0.5 McFarland suspension) on the surface of a Mueller-Hinton agar (Britania, Argentina) plate containing 3 μg/ml COL (colistin sulfate salt; Sigma-Aldrich) (plate A). In our case, we also included an extra plate of colistin agar-spot in which EDTA (Sigma-Aldrich) was added (eCAST) (plate B: 3 μg/ml colistin Mueller-Hinton agar plus 1 mM EDTA). As a growth control, Mueller-Hinton plates with EDTA were used to show any inhibition of colony growth by EDTA itself (plate C: 1mM EDTA Mueller-Hinton agar), inoculated in the same way. The presence of colonies was evaluated after 24 h of incubation at 35°C. All assays were performed in triplicate on different dates.
In the CAST (plate A), visualization of at least 3 colonies (according to Britania’s recommendations) was interpreted as COL resistance. The combination of resistance detection in plate A and lack of bacterial growth in eCAST (plate B) was interpreted as resistance to COL by MCR producers. On the other hand, bacterial growth in eCAST (≥3 colonies) was considered COL resistance without MCR production. Growth of all the tested isolates was checked in plate C for discarding inhibitory effects by EDTA alone.
The sensitivity and specificity of the combined CAST/eCAST test for detection of MCR-producing isolates was determined in comparison to the presence/absence of the mcr gene based on the molecular characterization of the isolates and their susceptibility profiles to COL.
Data availability.
A list of the isolates tested, along with the test results, can be found at https://datadryad.org/stash/share/_g44_XaKNaudK4CMebGy1thaecK-9LRe7TNoQzST7PE.
RESULTS
We first defined the best concentration of EDTA to be incorporated into the final eCAST plates by the ability to inhibit bacterial growth only when COL resistance was due to MCR expression but not when resistance was due to chromosomal mechanisms. For these studies, seven COLR isolates (four of them MCR producers) and three COLS isolates were tested at 0.5 mM, 1mM, 2mM, and 5mM EDTA. As 5 mM EDTA inhibited all isolates’ growth and 0.5 mM EDTA was not able to inhibit the growth of some mcr-1-producing isolates, a final concentration of 1 mM EDTA was chosen to prepare the B plates. These plates were used within a period of 2 months preserved at 4°C.
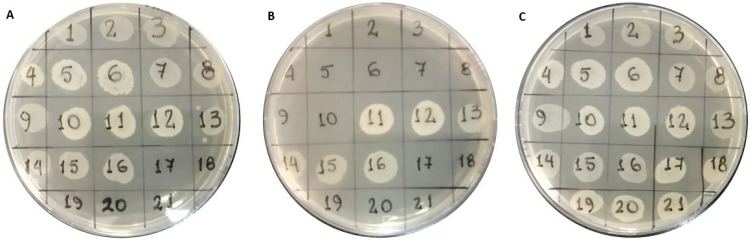
All COLR isolates grew on plates of CAST (plate A); resistant K. pneumoniae without mcr-1 and S. marcescens also displayed growth in eCAST (plate B), whereas not even a single colistin-resistant MCR-producing Enterobacteriaceae isolate was able to grow in these plates. As expected, COLS strains (E. coli and K. pneumoniae) exhibited no bacterial growth on both COL-containing plates. All the isolates analyzed grew in the Mueller-Hinton plates with EDTA medium (plate C). These results are exemplified in Fig. 1 and summarized in Table 1. This combined assay (plate A + plate B) showed 100% sensitivity (95% confidence interval [CI95] = 92.7% to 100%) and specificity (CI95 = 91.8% to 100%) for the detection of MCR-producing Enterobacteriaceae (mostly represented by MCR-1-producing E. coli).
FIG 1.
Differential growth in the combined CAST/eCAST test. Colistin-resistant isolates showed growth in the colistin agar-spot screening test (CAST) (plate A: Mueller-Hinton agar with 3 μg/ml COL). Of these isolates, only MCR producers did not grow in 1 mM EDTA colistin agar-spot screening test (eCAST) (plate B: Mueller-Hinton agar with 3 μg/ml COL + 1 mM EDTA). In contrast, mcr-negative strains harboring other resistance mechanisms also grew in these plates. A control plate (plate C: Mueller-Hinton agar with 1 mM EDTA) was used as a growth control for each isolate. Sections 1 to 10, mcr-positive COL-resistant isolates; 11 to 16, mcr-negative COL-resistant isolates; 17 to 21, mcr-negative COL-susceptible isolates.
DISCUSSION
Resistance to COL, especially by plasmid-borne mcr genes, is being increasingly reported in bacterial isolates from humans, animals, farms, foods, and the environment. To mitigate this rapidly spreading threat, efficient and easy-to-perform diagnostic tests that allow identification of these COLR bacteria have become indispensable and urgently necessary (23).
In this study, we evaluated a phenotypic combined CAST/eCAST test for the detection of COL-resistant MCR-positive enterobacteria recovered from human and animal samples based on the inhibition of the PEtN transferase enzyme using a chelator (EDTA). It must be noted that under the conditions described herein, standard 90-mm plates are sufficient for testing 21 isolates simultaneously, and by using the Société Française de Microbiologie 120-mm square plates, up to at least 36, which would be a clear advantage when testing large isolate collections.
The COL concentration used for the combined CAST/eCAST test was 3 μg/ml. This feature could be considered a limitation to detect the reduced number of mcr-harboring isolates with a COL MIC of ≤2 μg/ml (19), which were absent in our collection.
Previous studies for detecting MCR-harboring strains utilizing chelators such as EDTA or dipicolonic acid (DPA) have been already published. Inhibition of MCR-1 by dipicolinic acid (another metalloenzyme chelator) was reported as a useful method (called the colistin-MAC test) for the phenotypic detection of COL-resistant E. coli; it is a broth microdilution method displaying promising results (96.7% sensitivity and 100% specificity) for predicting mcr-1-positive isolates (18). Similarly, among other proposed methods that include EDTA as an inhibitor, in the colistin MIC reduction test, a COL MIC reduction in EDTA-containing wells is interpreted as MCR-1 positive, with 96.7% sensitivity and 83.3% specificity (19). In a recently modified colistin broth-disk elution test, any reduction of colistin MIC in the presence of EDTA displayed 100% and 95.8% sensitivity and specificity, respectively (24).
Finally, an EDTA-based combined disk diffusion test comparing the inhibition zones of COL and COL plus EDTA on Mueller-Hinton agar initially proved to be useful for the detection of mcr-bearing E. coli, but further analysis showed that it produces unreliable results (25). Similarly, a DPA-based disk diffusion test was attempted with poor results. This phenomenon has been ascribed to the low diffusion of COL into the agar medium (18, 19). In this direction, we have already proposed a phenotypic assay based on COL prediffusion disks and differential inhibition with EDTA (CPD-E test) (26). In this case, however, its potential use can be foreseen as for single-isolate testing.
In conclusion, our results show that the use of the combined CAST/eCAST test could provide a simple and easy-to-perform method to differentiate colistin-resistant MCR-producing Enterobacteriaceae from colistin-resistant microorganisms by chromosomal mechanisms with excellent discriminatory power. It must be noted that a discrete number of different isolates can be tested in the same plates, making it more convenient for evaluating the presence of MCR in epidemiological or surveillance screenings (even in resource-limited settings) in which several strains need to be tested simultaneously without any extra (or nonconventional) equipment.
The ability to differentiate resistance mediated by mcr genes other than mcr-1 opens the possibility to test natural isolates carrying these genes. This should not be taken for granted, as only one strain of each was assayed here. In any case, the tested bacteria represent the current scenario in which mcr-1 is highly prevalent. A possibility exists that in other settings, our test may display different sensitivity and discrimination power, a general consideration that is also true for all available and newly developed methods.
ACKNOWLEDGMENTS
This research was supported by Agencia Nacional de Promoción Científica y Tecnológica (grant PICT 2015-1925 to G.O.G.). We also acknowledge grant 20020170100473BA from UBACyT, which allowed us to collect and characterize the resistance data in previous studies for the microorganisms used here.
E.G.E. is Ph.D. student at Universidad de Buenos Aires under a fellowship from the PRONABEC, Ministerio de Educación, Perú (Resolución Jefatural no. 142-2017-MINEDU-VMGI-PRONABEC-OBPOST).
Grateful acknowledgment is made to Nilton Lincopan, Rafael Vignoli, and Rafael Cantón for the selfless contribution of mcr-2-, mcr-4-, or mcr-5-harboring strains.
REFERENCES
- 1.Falagas ME, Kasiakou SK. 2005. Colistin: the revival of polymyxins for the management of multidrug-resistant Gram-negative bacterial infections. Clin Infect Dis 40:1333–1341. doi: 10.1086/429323. [DOI] [PubMed] [Google Scholar]
- 2.Hancock REW, Chapple DS. 1999. Peptide antibiotics. Antimicrob Agents Chemother 43:1317–1323. doi: 10.1128/AAC.43.6.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gao R, Hu Y, Li Z, Sun J, Wang Q, Lin J, Ye H, Liu F, Srinivas S, Li D, Zhu B, Liu YH, Tian GB, Feng Y. 2016. Dissemination and mechanism for the MCR-1 colistin resistance. PLoS Pathog 12:e1005957. doi: 10.1371/journal.ppat.1005957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giske CG. 2015. Contemporary resistance trends and mechanisms for the old antibiotics colistin, temocillin, fosfomycin, mecillinam and nitrofurantoin. Clin Microbiol Infect 21:899–905. doi: 10.1016/j.cmi.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 5.Blair JM, Webber MA, Baylay AJ, Aqbolu DO, Piddock LJ. 2015. Molecular mechanisms of antibiotic resistance. Nat Rev Microbiol 13:42–51. doi: 10.1038/nrmicro3380. [DOI] [PubMed] [Google Scholar]
- 6.Liu YY, Wang Y, Walsh TR, Yi LX, Zhang R, Spencer J, Doi Y, Tian G, Dong B, Huang X, Yu LF, Gu D, Ren H, Chen X, Lv L, He D, Zhou H, Liang Z, Liu JH, Shen J. 2016. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis 16:161–168. doi: 10.1016/S1473-3099(15)00424-7. [DOI] [PubMed] [Google Scholar]
- 7.Hinchliffe P, Yang QE, Portal E, Young T, Li H, Tooke CL, Carvalho MJ, Paterson NG, Brem J, Niumsup PR, Tansawai U, Lei L, Li M, Shen Z, Wang Y, Schofield CJ, Mulholland AJ, Shen J, Fey N, Walsh TR, Spencer J. 2017. Insights into the mechanistic basis of plasmid-mediated colistin resistance from crystal structures of the catalytic domain of MCR-1. Sci Rep 7:39392. doi: 10.1038/srep39392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stojanoski V, Sankaran B, Prasad BV, Poirel L, Nordmann P, Palzkill T. 2016. Structure of the catalytic domain of the colistin resistance enzyme MCR-1. BMC Biol 14:81. doi: 10.1186/s12915-016-0303-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xavier BB, Lammens C, Ruhal R, Kumar-Singh S, Butaye P, Goossens H, Malhotra-Kumar S. 2016. Identification of a novel plasmid-mediated colistin-resistance gene, mcr-2, in Escherichia coli, Belgium, June 2016. Euro Surveill 21:30280 https://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2016.21.27.30280. [DOI] [PubMed] [Google Scholar]
- 10.Yin W, Li H, Shen Y, Liu Z, Wang S, Shen Z, Zhang R, Walsh TR, Shen J, Wang Y. 2017. Novel plasmid-mediated colistin resistance gene mcr-3 in Escherichia coli. mBio 8:e00543-17. doi: 10.1128/mBio.00543-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carattoli A, Villa L, Feudi C, Curcio L, Orsini S, Luppi A, Pezzotti G, Magistrali CF. 2017. Novel plasmid-mediated colistin resistance mcr-4 gene in Salmonella and Escherichia coli, Italy 2013, Spain and Belgium, 2015 to 2016. Euro Surveill 22:30589 https://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2017.22.31.30589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borowiak M, Fischer J, Hammerl JA, Hendriksen RS, Szabo I, Malorny B. 2017. Identification of a novel transposon-associated phosphoethanolamine transferase gene, mcr-5, conferring colistin resistance in d-tartrate fermenting Salmonella enterica subsp. enterica serovar Paratyphi B. J Antimicrob Chemother 72:3317–3324. doi: 10.1093/jac/dkx327. [DOI] [PubMed] [Google Scholar]
- 13.Yang YQ, Li YX, Lei CW, Zhang AY, Wang HN. 2018. Novel plasmid-mediated colistin resistance gene mcr-7.1 in Klebsiella pneumoniae. J Antimicrob Chemother 73:1791–1795. doi: 10.1093/jac/dky111. [DOI] [PubMed] [Google Scholar]
- 14.Wang X, Wang Y, Zhou Y, Li J, Yin W, Wang S, Zhang S, Shen J, Shen Z, Wang Y. 2018. Emergence of a novel mobile colistin resistance gene, mcr-8, in NDM-producing Klebsiella pneumoniae. Emerg Microbes Infect 7:122. doi: 10.1038/s41426-018-0124-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carroll LM, Gaballa A, Guldimann C, Sullivan G, Henderson LO, Wiedmann M. 2019. Identification of novel mobilized colistin resistance gene mcr-9 in a multidrug-resistant, colistin-susceptible Salmonella enterica serotype Typhimurium isolate. mBio 10:e00853-19. doi: 10.1128/mBio.00853-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.European Committee on Antimicrobial Susceptibility Testing. 2019. Breakpoint tables for interpretation of MICs and zone diameters (Version 9.0). http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_9.0_Breakpoint_Tables.pdf.
- 17.Poirel L, Larpin Y, Dobias J, Stephan R, Decousser JW, Madec JY, Nordmann P. 2018. Rapid polymyxin NP test for the detection of polymyxin resistance mediated by the mcr-1/mcr-2 genes. Diagn Microbiol Infect Dis 90:7–10. doi: 10.1016/j.diagmicrobio.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 18.Coppi M, Cannatelli A, Antonelli A, Baccani I, Di Pilato V, Sennati S, Giani T, Rossolini GM. 2018. A simple phenotypic method for screening of MCR-1-mediated colistin resistance. Clin Microbiol Infect 24:201.e1–201.e3. doi: 10.1016/j.cmi.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 19.Esposito F, Fernandes MR, Lopes R, Muñoz M, Sabino CP, Cunha MP, Silva KC, Cayô R, Martins WMBS, Moreno AM, Knöbl T, Gales AC, Lincopan N. 2017. Detection of colistin-resistant MCR-1-positive Escherichia coli by use of assays based on inhibition by EDTA and zeta potential. J Clin Microbiol 55:3454–3465. doi: 10.1128/JCM.00835-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lescat M, Poirel L, Nordmann P. 2018. Rapid multiplex polymerase chain reaction for detection of mcr-1 to mcr-5 genes. Diagn Microbiol Infect Dis 92:267–269. doi: 10.1016/j.diagmicrobio.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 21.Poirel L, Walsh TR, Cuvillier V, Nordmann P. Multiplex PCR for detection of acquired carbapenemase genes. 2011. Diagn Microbiol Infect Dis 70:119–23. doi: 10.1016/j.diagmicrobio.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Cannatelli A, Giani T, D’Andrea MM, Di Pilato V, Arena F, Conte V, Tryfinopoulou K, Vatopoulos A, Rossolini GM. 2014. MgrB inactivation is a common mechanism of colistin resistance in KPC-producing Klebsiella pneumoniae of clinical origin. Antimicrob Agents Chemother 58:5696–703. doi: 10.1128/AAC.03110-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Osei J. 2019. Mcr colistin resistance gene: a systematic review of current diagnostics and detection methods. Microbiologyopen 8:e00682. doi: 10.1002/mbo3.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bell DT, Bergman Y, Kazmi AQ, Lewis S, Tamma PD, Simner PJ. 2019. A novel phenotypic method to screen for plasmid-mediated colistin resistance among Enterobacteriales. J Clin Microbiol 57:e00040-19. doi: 10.1128/JCM.00040-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clément M, Büdel T, Bernasconi OJ, Principe L, Perreten V, Luzzaro F, Endimiani A. 2018. The EDTA-based disk-combination tests are unreliable for the detection of MCR-mediated colistin resistance in Enterobacteriaceae. J Microbiol Methods 153:31–34. doi: 10.1016/j.mimet.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 26.Yauri K, Gonzales E, Di Conza J, Gutkind G. 2019. Detection of plasmid-mediated colistin resistance by colistin pre-diffusion and inhibition with EDTA test (CPD-E) in Enterobactereaceae. J Microbiol Methods 167:105759. doi: 10.1016/j.mimet.2019.105759. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
A list of the isolates tested, along with the test results, can be found at https://datadryad.org/stash/share/_g44_XaKNaudK4CMebGy1thaecK-9LRe7TNoQzST7PE.