Abstract
Background
Corneal refractive surgery is widely used worldwide. Myopia is the most common reason for laser-assisted corneal refractive surgery (LASIK) and one of the risk factors for glaucoma. Intraocular pressure (IOP) measurement becomes variable postoperatively as the results are affected by the decrease in corneal thickness and biomechanics. This prospective clinical case study attempted to establish a simple correction formula for the calculation of IOP in post-LASIK myopic patients.
Methods
This study included 300 eyes of 150 patients with myopia and myopic astigmatism as a refractive error who underwent LASIK. IOP was measured preoperatively and 6 months postoperatively. Preoperative and postoperative corneal thickness as well as ablation depth were measured. Statistical analysis was performed to detect the relationship between ablation depth and change in IOP. An attempt was made to construct a correction formula for the calculation of post-LASIK IOP.
Results
The age of the patients ranged between 18 and 50 (mean ± SD 34.78±8.8) years. The spherical equivalent of refractive error ranged between −1.5 and −10 diopters. The mean IOP decreased significantly from 15.72±2.37 mmHg preoperatively to 11.71±2.24 mmHg postoperatively, with a mean difference of 4±1.75 mmHg (p˂0.001). A positive correlation was detected between corneal thickness and IOP difference among patients both preoperatively and postoperatively (p˂0.001). A positive correlation was identified between ablation depth and IOP change (p˂0.001). The correction formula for IOP was established: Real IOP=4+0.7(preoperative IOP)−0.3(ablation depth).
Conclusion
IOP measurements change after corneal refractive surgery with LASIK. A corrected formula may be a good option for the proper calculation of post-LASIK IOP.
Keywords: intraocular pressure calculation, post-LASIK glaucoma, Goldmann applanation tonometry, glaucoma, laser in situ keratomileusis
Introduction
Corneal refractive surgery is a valuable tool used worldwide.1 A technique known as laser-assisted corneal refractive surgery (laser-assisted in situ keratomileusis [LASIK]) is most commonly used for the treatment of myopia.2 Myopia is a key risk factor for glaucoma, and necessitates regular monitoring of intraocular pressure (IOP) to mitigate this risk.3 Indeed, IOP is a major consideration in both the detection and follow-up of glaucoma, with elevated IOP being the most modifiable risk factor for both glaucoma development and progression.4 Accurate measurement of estimated and corrected IOP is vital for the diagnosis, treatment, and follow-up of glaucoma.5,6 Currently, the Goldmann applanation tonometer (GAT) is considered the gold-standard tool for IOP measurement.4
According to the literature, the GAT measures the force needed to flatten a given area of the cornea, which is affected by the central corneal thickness (CCT).5 However, following LASIK, scleral rigidity and hysteresis decrease, particularly in patients with myopia. These changes in corneal biomechanics after corneal refractive surgery tend to result in applanation and indentation-based tonometer readings that incorrectly underestimate IOP.7 Further research is required to study this phenomenon;8–10 however, the relevant studies have not so far been possible because of the lack of an appropriate tool for the reliable assessment of IOP following a LASIK procedure. Although correction formulae have previously been proposed to correct for the effects of surgery, these have not been sufficiently well documented to allow a valid assessment of the true IOP reading after corneal refractive surgery. Therefore, it seems appropriate to attempt to establish a correction formula to take into account the effect of surgery on GAT measurements.11
Patients and Methods
The study was conducted in the Research Institute of Ophthalmology (RIO), Giza, Egypt. This study was performed under the tenets of the Declaration of Helsinki of 1975 (the 1983 revision). The research committee of the RIO approved the protocol of the study. All patients received a thorough explanation of the procedures entailed in the study and signed an informed consent form prior to enrollment. Preoperative IOP had to be less than 21 mmHg without any anti-glaucoma treatment either preoperatively or postoperatively.
The study included 300 eyes of 150 patients. Inclusion criteria were patients who were fit for corneal refractive surgery, older than 18 years, with stability of refraction for the past year (change ±0.5 diopter) and no family history of glaucoma. Patients who appeared to have an elevation of IOP at any stage of follow-up, or had received any type of anti-glaucoma treatment, had undergone previous ocular surgery, or had corneal diseases, were excluded from the study.
Preoperative and postoperative IOP was measured with a GAT (Haag-Streit, Koeniz, Switzerland). Best corrected visual acuity, manifest and cycloplegic refraction were detected. Corneal topography was assessed and pachymetry was performed using a Pentacam (Oculus, Wetzlar, Germany). CCT was measured with ultrasonic pachymetry, and the average of three consecutive readings was recorded. Corneal ablation depth was documented.
Patients were followed up at 1, 3, and 6 months postoperatively, and the 6-month reading was introduced along with the preoperative IOP reading for statistical analysis.
IOP was statistically described in terms of mean±SD and compared using the paired t-test. Correlations between variables were assessed using the Pearson moment correlation equation for linear relationships between normally distributed variables and the Spearman rank correlation equation for non-normal variables and non-linear monotonic relationships. Linear regression analysis was used to generate an equation to predict postoperative IOP (IOP post) using preoperative IOP (IOP pre) and ablation depth. Two-sided p values <0.05 were considered statistically significant. All statistical calculations were done using IBM SPSS (IBM Corp, Armonk, NY, USA) release 22 for Microsoft Windows.
Results
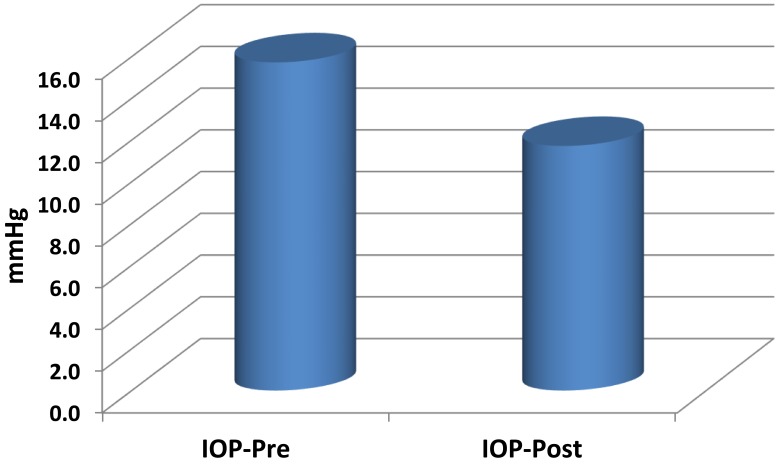
Data from 150 patients (300 eyes) were composed and studied preoperatively and postoperatively. The average age of patients (mean±SD) was 34.8±8.8 years (range 18–50 years); 45% of patients were male while 55% were female. All patients underwent LASIK in both eyes. No family history of glaucoma was declared in any of the patients. All patients included in this study were Egyptian. The spherical equivalent (SE) of the 300 eyes ranged between 1.5 and 10 diopters. The corneal ablation depth for correction of refractive error fluctuated between 41 and 122 µm, with a mean of 59.2±22 µm. The mean preoperative pachymetry measurement was 546.9±31.8 µm preoperatively and 480.4±29.3 µm postoperatively. IOP decreased from 15.72±2.37 mmHg preoperatively to 11.71±2.24 mmHg postoperatively (p˂0.001) (Figure 1).
Figure 1.
Mean preoperative and postoperative intraocular pressure (IOP pre and IOP post) in the study sample.
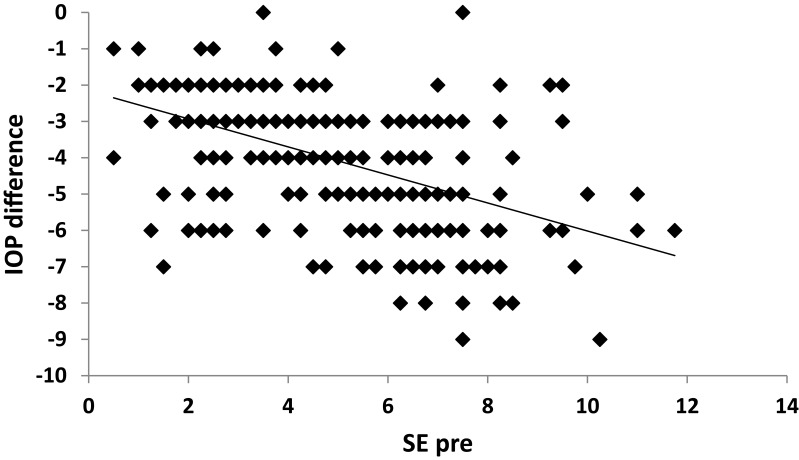
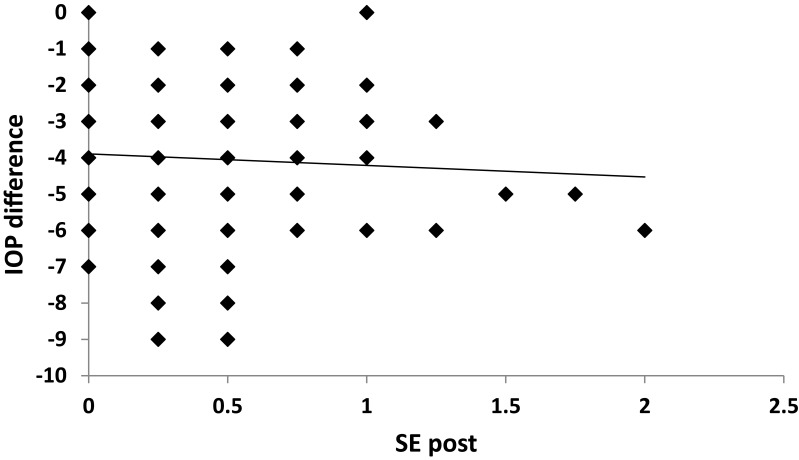
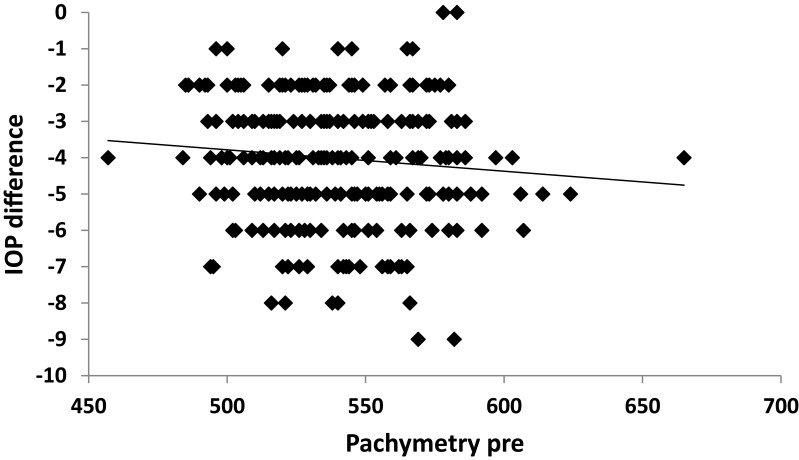
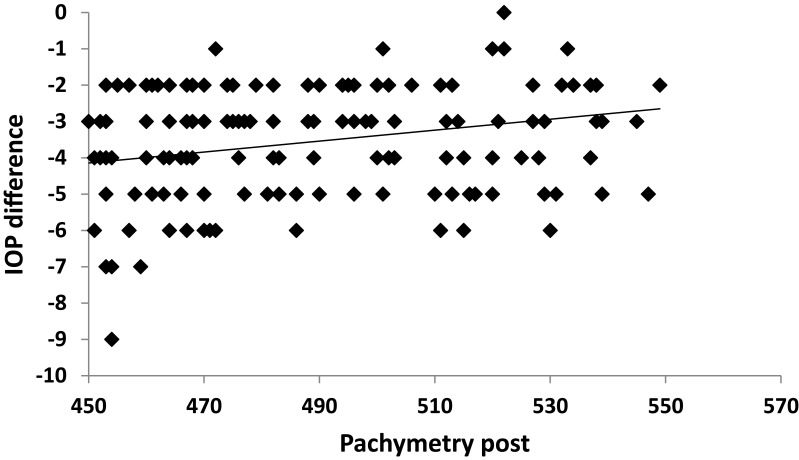
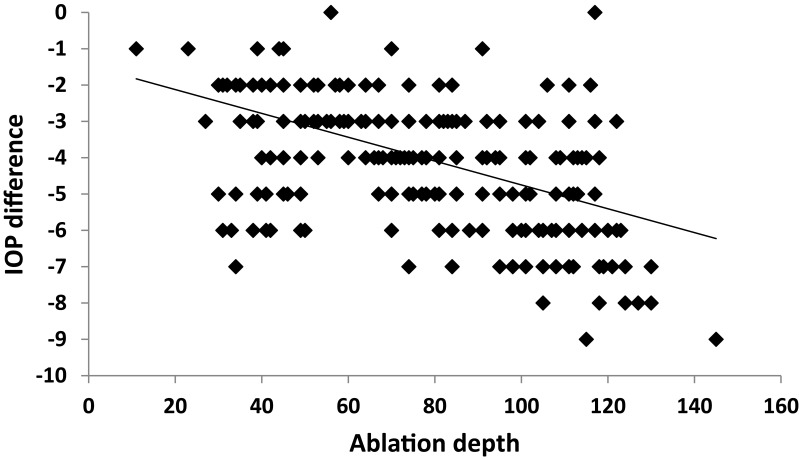
A strong positive correlation was noticed between SE and IOP both preoperatively and postoperatively (p˂0.001) (Figures 2 and 3). A positive correlation between both preoperative and postoperative pachymetry and IOP was detected (p˂0.001) (Figures 4 and 5, Tables 1 and 2). A positive correlation was identified between ablation depth and IOP changes (p˂0.001) (Figure 6, Tables 1–3). A strong positive correlation was detected between both preoperative IOP and ablation depth and postoperative IOP (Table 1). The greater the preoperative pachymetry, refractive error, and ablation corneal depth, the higher the IOP change (Table 1).
Figure 2.
Correlation between preoperative spherical equivalent (SE pre) and intraocular pressure (IOP) difference in the study sample.
Figure 3.
Correlation between postoperative spherical equivalent (SE post) and intraocular pressure (IOP) difference in the study sample.
Figure 4.
Correlation between preoperative pachymetry (pachymetry pre) and intraocular pressure (IOP) difference in the study sample.
Figure 5.
Correlation between postoperative pachymetry (pachymetry post) and intraocular pressure (IOP) difference in the study sample.
Table 1.
Correlation Between Preoperative Intraocular Pressure (IOP pre) and Ablation Depth)
| Model | Unstandardized Coefficients | Standardized Coefficients | t | p | 95% CI for B | |||
|---|---|---|---|---|---|---|---|---|
| B | Std. Error | Beta | Lower | Upper | ||||
| 1 | (Constant) | 4.024 | 0.539 | 7.469 | 0.000 | 2.964 | 5.084 | |
| IOP pre | 0.658 | 0.031 | 0.694 | 21.378 | 0.000 | 0.597 | 0.718 | |
| Ablation depth | −0.034 | 0.003 | −0.422 | −13.000 | 0.000 | −0.039 | −0.029 | |
Notes: aPredictors: (constant), ablation depth, IOP pre. bDependent variable: IOP post.
Table 2.
Correlation Between Postoperative Intraocular Pressure (IOP Post) and Ablation Depth
| ANOVAb | ||||||
|---|---|---|---|---|---|---|
| Model | Sum of Squares | df | Mean Square | F | p | |
| 1 | Regression | 1,039.475 | 2 | 519.737 | 327.127 | 0.000a |
| Residual | 471.872 | 297 | 1.589 | |||
| Total | 1,511.347 | 299 | ||||
Notes: aPredictors: (constant), ablation depth, IOP pre. bDependent variable: IOP post.
Figure 6.
Correlation between ablation depth and intraocular pressure (IOP) difference in the study sample.
Table 3.
Correlations Between Intraocular Pressure Difference (IOP Diff.) and Preoperative Spherical Equivalent (SE Pre), Postoperative Spherical Equivalent (SE Post), Preoperative Pachymetry (Pachy Pre), Postoperative Pachymetry (Pachy Post), and Ablation Depth
| IOP Diff. | ||
|---|---|---|
| SE pre | Pearson correlation | −0.499 |
| pvalue | 0.000 | |
| N | 300 | |
| SE post | Pearson correlation | −0.049 |
| p value | 0.403 | |
| N | 300 | |
| Pachy pre | Pearson correlation | −0.098 |
| pvalue | 0.089 | |
| N | 300 | |
| Pachy post | Pearson correlation | 0.318 |
| p value | 0.000 | |
| N | 300 | |
| Ablation depth | Pearson correlation | −0.520 |
| p value | 0.000 | |
| N | 300 | |
A scatterplot with regression line is plotted between preoperative spherical equivalent (SE pre) and IOP in Figure 2, and between postoperative spherical equivalent (SE post) and IOP in Figure 3. Scatterplots with regression lines between pachymetry and either preoperative or postoperative pachymetry are shown in Figures 4 and 5, respectively. A scatterplot with regression line between ablation depth and IOP difference is shown in Figure 6. Correlations between IOP pre and ablation depth and IOP post are presented in Table 1. A regression analysis and correlations between IOP pre and ablation depth and IOP post are presented in Table 2. Correlations between IOP difference and SE pre, SE post, preoperative pachymetry (pachy pre), postoperative pachymetry (pachy post), and ablation depth are presented in Table 3.
After analyzing the consequential changes and correlations, a correction formula was created. The correction formula was: Real post-LASIK IOP=4+0.7(preoperative IOP)–0.3(ablation depth).
Discussion
The validity of GAT in measuring IOP depends upon a number of assumptions, including the notion that the corneal surface is infinitely thin and perfectly flexible and has a uniform thickness. Because these assumptions are fundamentally flawed, the identification of a more accurate technique for the measurement of IOP following laser surgery is necessary.7–10
Multiple suggestions have been proposed to overcome the inherent inaccuracy of GAT measurements among myopic patients following LASIK correction surgery. Some authors have suggested measuring IOP by the application of GAT on the peripheral cornea to correct for alterations in corneal thickness and biomechanics;2 however, another study has refuted the benefit of this approach.12
Newer devices have been introduced for the measurement of IOP that are based on dynamic rather than static applanation, such as the ocular response analyzer (ORA), Pascal dynamic contour tonometer, and indentation tonometer. These devices may be valid alternatives for the measurement of IOP in post-refractive surgery patients, but results have been mixed. The unsatisfactory results of these approaches may be attributed to changes in biomechanics, which are considered basic parameters for these devices.13–20
Because of the inaccuracy of GAT measurements following LASIK, there is an argument for the development of a correction formula to address this issue. The development of a correction formula has been suggested by several authors previously, but none of the proposals has so far gained wide acceptance or been considered suitable for application.
In the development of our correction formula, we studied the changes that may appear with LASIK, and assessed their effects on GAT readings. Our results revealed a reduction in CCT that correlates with ablation depth. Because CCT is a major factor contributing to the reliability of GAT readings, we considered it necessary to study the correlation between ablation depth and change in IOP reading (as shown in Tables 1–3).
In the current study, we observed a correlation between decrease in postoperative IOP and both preoperative IOP and change in corneal thickness, as well as ablation depth (Tables 1 and 2). In accordance with our findings, Rashad and Bahnassy reported a mean change in GAT readings of 1.63–3.69 mmHg, as well as a strong correlation between reduction in IOP and both preoperative IOP and change in corneal thickness.12 The small number of cases (102 eyes) and the restriction of cases to include myopic astigmatism only may be limiting factors in this previous study compared with the current study.12
Our results indicate a clear linear correlation between ablation depth and change in IOP. This is consistent with multiple studies that have also reported a change in IOP reading following LASIK corrective surgery, ranging from a minimal change in some studies to a more marked change of up to 5 mmHg in others2,7,10 (Table 3 and Figures 2–6).
After taking into account the correlation between spherical equivalent and preoperative or postoperative IOP difference and the correlation between pachymetry and preoperative or postoperative IOP difference, our proposed correction formula may be stated as: Real IOP=4+0.7(preoperative IOP)−0.3(ablation depth).
Our formula is not the first correction formula to be developed in this way. Kohlhaas et al also reported the inaccuracy of IOP measurement post-LASIK, and attempted a correction formula for IOP calculation. These authors found a significant correlation between the measured IOP and CCT in addition to a k-reading. However, this formula considered postoperative IOP as a main element, which may be a source of error as it is the most likely factor to be changed. The small number of cases (n=101) in their study may be a further point of weakness, and was corrected in the current study. Furthermore, in our formula, we consider preoperative IOP to be the main element in the equation, which we firmly believe makes our proposal stronger and more robust to the no touched form of the cornea.11
The Kohlhaas formula contains multiple parameters that make it more complex and impractical in application, while our formula is simpler and more applicable, and has one factor (preoperative IOP) that needs to be fitted. Furthermore, our formula is based on processing the effect of ablation depth on the resulting reading of IOP. The Kohlhaas formula also differs from ours in that it must be applied at every patient visit, whereas our formula has a clear role, which is the conclusion of the most nearly accurate IOP of the patient to be post-LASIK. This number can be documented in the patient's file, and any follow-up documentation of IOP may be compared with this number.
One weak point in our formula in comparison with the Kohlhaas formula is the necessity to preserve and document preoperative IOP.
In our study, change in k-readings was not included as this is not considered a sharp parameter and its measurement may be subject to errors. This is consistent with a study performed by Silva et al in 2011, which found a weak correlation between average simulated keratometry and change in IOP readings.21
Our result is also consistent with a study by Yin Lin et al that explored the factors that may alter IOP measurements following LASIK. These authors reported that CCT and central ablation depth are the main parameters to be considered. This fact was confirmed in a femtosecond laser or microkeratome flap created by LASIK.22
In the current study, the IOP calculation started 6 months postoperatively, when all postoperative treatments including steroids had been completed and discontinued to avoid steroid-induced glaucoma. This coincides with the observation of authors that steroid-induced glaucoma and pressure-induced keratopathy may appear as acute or late-onset events, and may disrupt the reliability of readings and subsequent equations.23,24
A study by Corenemberger et al showed that both eyes of the same patient may have different refraction corneal thicknesses, and consequently different ablation depth;25 therefore, we studied both eyes of the same patient to ensure that the eye chosen had no negative effect on the results.
A limitation of this study may be the restriction to and selection of myopias and myopic astigmatism among patients following LASIK. This may be justified by the strong association between myopia and glaucoma, as reported by many authors. Myopia is an independent risk factor for glaucoma incidence and progression, which mandates early diagnosis and treatment. Moreover, laser correction in myopias affects the central cornea, which is the main concern for GAT.26–29
Conclusion
GAT is the gold standard for IOP measurement, but requires a correction formula for proper assessment post-LASIK. A simple and easily applicable correction formula, such as Real IOP=4+0.7(preoperative IOP)–0.3(ablation depth), may be a good option for the proper assessment and follow-up of post-LASIK IOP.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.El-Danasoury MA, El Maghraby A, Coorpender SJ. Change in intraocular pressure in myopic eyes measured with contact and non-contact tonometer after laser in situ keratomileusis. J Refract Surg. 2001;17(2):97–104. [DOI] [PubMed] [Google Scholar]
- 2.Fan Q, Zhang J, Zheng L, Zheng L, Feng H, Wang H. Intraocular pressure change after myopic laser in situ keratomiluesis as measured on the central and peripheral cornea. Clin Exp Ophthalmol. 2012;95:421–426. [DOI] [PubMed] [Google Scholar]
- 3.Salvetat ML, Zeppieri M, Tosoni C, Brusini P. Baseline factors predicting the risk of conversion from ocular hypertension to primary open angle glaucoma during a 10 year follow up. Eye (Lond). 2016;30(6):784–795. doi: 10.1038/eye.2016.86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leske MC, Wu SY, Hennis A, Honkannen R, Nemesure B. Risk factors for incident open angle glaucoma: the Barbados eye studies. Ophthalmology. 2008;115(1):85–93. doi: 10.1016/j.ophtha.2007.03.017 [DOI] [PubMed] [Google Scholar]
- 5.Tsai ASH, Loon SC. Intraocular pressure assessment after laser in situ keratomiluesis: a review. Clin Exp Ophthalmol. 2012;40(3):295–304. doi: 10.1111/j.1442-9071.2011.02641.x [DOI] [PubMed] [Google Scholar]
- 6.Kwon J, Sung KR, Jo J, Yang SH. Glaucoma progression and its relationship with corrected and uncorrected intraocular pressure in eyes with history of refractive corneal surgery. Curr Eye Res. 2018;43(9):1136–1144. doi: 10.1080/02713683.2018.1467930 [DOI] [PubMed] [Google Scholar]
- 7.Chang DH, Stulting D. Change in intraocular pressure measurements after LASIK the effect of refractive correction and lamellar flap. Ophthalmology. 2005;112(6):1009–1016. doi: 10.1016/j.ophtha.2004.12.033 [DOI] [PubMed] [Google Scholar]
- 8.Cook JA, Botello AP, Elders A, et al.; Surveillance of ocular hypertension study group. Systemic review of the agreement of tonometer with Goldmann applanation tonometry. Ophthalmology. 2012;119(8):1552–1557. doi: 10.1016/j.ophtha.2012.02.030 [DOI] [PubMed] [Google Scholar]
- 9.Sanchez–Naves J, Furfaro L, Piro O, Balle S. Impact and permanence of LASIK–induced structural changes in the cornea on pneumotonometric measurements: contributions of flap cutting and stromal ablation. J Glaucoma. 2008;17(8):611–618. doi: 10.1097/IJG.0b013e3181639ae2 [DOI] [PubMed] [Google Scholar]
- 10.Schalhorn JM, Shalhorn SC, Ou Y. Factors that influence intraocular pressure changes after myopic and hyperopic LASIK and photorefractive keratectomy. A large population studies. Ophthalmology. 2015;122(3):471–479. doi: 10.1016/j.ophtha.2014.09.033 [DOI] [PubMed] [Google Scholar]
- 11.Kohlhaas M, Spoerl E, Boehm AG, Pollack K. A correction formula for real intraocular pressure after LASIK for the correction of myopic astigmatism. J Refract Surg. 2006;22(30):263–267. doi: 10.3928/1081-597X-20060301-11 [DOI] [PubMed] [Google Scholar]
- 12.Rashad KM, Bahnassy AA. Changes in intraocular pressure after laser in situ keratomileusis. J Refract Surg. 2001;17(4):420–427. [DOI] [PubMed] [Google Scholar]
- 13.Zadok D, Tran DB, Twa M, Carpenter M, Schanzlin DJ. Pneumotonometer after laser in situ keratomiluesis for myopia. J Cataract Refract Surg. 1999;25(10):1344–1348. doi: 10.1016/S0886-3350(99)00202-3 [DOI] [PubMed] [Google Scholar]
- 14.Hjortdal JO, Moller-Pedersen T, Ivarsen A. Biomechanical changes in the human cornea after PRK, and LASIK. Invest Ophthalmol Vis Sci. 2005;46(13):5727–5730. [Google Scholar]
- 15.Han KE, Kim H, Kim NR, Jun I, Kim EK, Kim TI. Comparison of intraocular pressures after myopic laser assisted sub epithelial keratectomy: tonometry, Tonometry-pachymetry, Goldman applanation tonometry, dynamic contour tonometry and non-contact tonometry. J Cataract Refract Surg. 2013;39(6):888–897. doi: 10.1016/j.jcrs.2013.01.035 [DOI] [PubMed] [Google Scholar]
- 16.Schneider E, Grehn F. Intraocular pressure measurement –comparison of dynamic contour tonometry and Goldman applanation tonometry. J Glaucoma. 2006;15(1):2–6. doi: 10.1097/01.ijg.0000196655.85460.d6 [DOI] [PubMed] [Google Scholar]
- 17.Cacho I, Sanchez–Naves J, Batres L, Pintor J, Carracedo G. Comparison of intraocular pressure before and after laser in situ keratomiluesis refractive surgery measured with Perkin’s tonometry, non-contact tonometry and trans palpebral tonometry. 2015. J Ophthalmol. 6 article ID 683895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirwan C, O’Keefe M. Measurement of intraocular pressure in LASIK and LASEK patients using the Richter ocular response analyzer and Goldmann applanation tonometry. J Refract Surg. 2008;24(4):366–370. doi: 10.3928/1081597X-20080401-09 [DOI] [PubMed] [Google Scholar]
- 19.Aristeidou AP, Labiris G, Katsanos A, Fanariotis M, Foudoulakis NC, Kozobolis VP. Comparison between Pascal dynamic contour tonometer and Goldman applanation tonometry after different types of refractive surgery. Graefes Arch Clin Exp Ophthalmol. 2011;249(5):767–773. doi: 10.1007/s00417-010-1431-9 [DOI] [PubMed] [Google Scholar]
- 20.Sadigh AL, Fouladi RF, Hashemi H, Beheshtnejad AH. A comparison between goldmann applanation tonometry and dynamic contour tonometry after photorefractive keratectomy. Graefes Arch Clin Exp Ophthalmol. 2013;251(2):603–608. doi: 10.1007/s00417-012-2142-1 [DOI] [PubMed] [Google Scholar]
- 21.Silva TG, Polido JG, Pinheiro MV, et al. Application of corrective formula for intraocular pressure changes in patients that underwent LASIK. Arq Bras Oftalmol. 2011;74(2):102–105. doi: 10.1590/S0004-27492011000200006 [DOI] [PubMed] [Google Scholar]
- 22.Yin Lin M, Chang DCK, Shen YD, Lin Y-K, Lin CP, Wang IJ. Factors influencing intraocular pressure changes after Laser in situ keratomiluesis with flaps created by femtosecond laser or mechanical microkeratome. PLoS One. 2016;11(1):e0147699. doi: 10.1371/journal.pone.0147699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cabral –macias J, Garcia De la Rosa G, Rodriguez –matilde DF, et al. Pressure induced stromal keratopathy after laser in situ keratomiluesis; acute and late onset presentations. J Cataract Refract Surg. 2018;44(10):06053. doi: 10.1016/j.jcrs.2018.06.053 [DOI] [PubMed] [Google Scholar]
- 24.Miyai T, Yonemura T, Nejima R, Otani S, Miyata K, Amano S. Inter-lamellar flap edema due to steroid –induced ocular hypertension after laser in situ keratomiluesis. Jpn J Ophthalmol. 2007;51(3):228–230. doi: 10.1007/s10384-006-0441-y [DOI] [PubMed] [Google Scholar]
- 25.Corenemberger S, Guimaraes CS, Calixto N, Calixto JMF. intraocular pressure and ocular rigidity after LASIK. Arq Bras Oftalmol. 2009;72(4):439–443. doi: 10.1590/S0004-27492009000400003 [DOI] [PubMed] [Google Scholar]
- 26.Tonnu PA, Ho T, Newson T, et al. The influence of central corneal thickness and age on intraocular pressure measured by pneumotonometer, contact tonometry, the Tono pen and Goldman applanation tonometry. Br J Ophthalmol. 2005;89(7):851–854. doi: 10.1136/bjo.2004.056622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perera SA, Wong TY, Tay W-T, Foster PJ, Saw SM, Aung T. Refractive error, axial dimensions and primary open angle glaucoma; The Singapore malay eye study. Arch Ophthalmol. 2010;128(7):900–905. doi: 10.1001/archophthalmol.2010.125 [DOI] [PubMed] [Google Scholar]
- 28.Yang CC, Wang IJ, Chang YC, Lin LKL, Chen THH. A predictive model for postoperative intraocular pressure among patients undergoing laser in situ keratomiluesis (LASIK). Am J Ophthalmol. 2006;141:530–536. doi: 10.1016/j.ajo.2005.10.022 [DOI] [PubMed] [Google Scholar]
- 29.Saw SM, Gazzard G, Shih-Yen EC, Chua WH. Myopia and associated pathological complications. Ophthalmic Physiol Opt. 2005;25(5):381–391. doi: 10.1111/j.1475-1313.2005.00298.x [DOI] [PubMed] [Google Scholar]