Abstract
Background
miR-26b-5p is reported to be involved in the progression of multiple cancers, but its function and mechanism in human papillary thyroid cancer (PTC) remain unknown. We aimed to uncover the function and mechanism of miR-26b-5p in PTC.
Methods
We performed qRT-PCR to detect the differences in miR-26b-5p expression between normal tissue and PTC. In vitro, we established cell lines stably overexpressing miR-26b-5p and investigated the function and underlying mechanism of miR-26b-5p in PTC.
Results
miR-26b-5p was downregulated in PTC compared with normal tissue. miR-26b-5p was correlated with the clinical stage. miR-26b-5p inhibited the proliferation, invasion and migration of PTC cell lines. We next detected EMT and proliferation markers. miR-26b-5p was shown to exert its function in a β-catenin-dependent manner.
Conclusion
Taken together, our results showed that miR-26b-5p inhibits proliferation, migration, invasion and EMT by degrading β-catenin.
Keywords: miR-26b-5p, PTC, migration and invasion, epithelial-mesenchymal transition, β-catenin
Background
Papillary thyroid cancer (PTC) is the most common endocrine carcinoma.1 Through many advancements in therapy the overall survival of PTC patients is satisfactory. The exact mechanism of tumourigenesis is still unknown, and more biomarkers predicting prognosis and therapeutic targets are still needed.
MicroRNAs are long noncoding RNAs that are involved in vital cellular processes, such as cell division and differentiation.2,3 MicroRNAs have also been reported to participate in the tumourigenesis and progression of multiple cancers.4–6 However, the function of the same microRNA varies in different kinds of cancers. miR-26b-5p was reported to be involved in the proliferation, migration and invasion of hepatology cancers.7 The potential function of miR-26b-5p in PTC is still unknown.
We performed qRT-PCR to detect the differences in expression between normal tissue and PTC. After examining the function of miR-26b-5p in PTC cell lines, we found that miR-26b-5p inhibited the proliferation, migration and invasion of PTC through the β-catenin pathway.
Materials and Methods
Tissue Samples
All human tissues were obtained from the Department of Thyroid and Breast Surgery, Shandong Provincial Hospital affiliated with Shandong University after confirmation by a pathologist. Tissues were obtained with the patients’ written and informed consent approved by the Institutional Review Board of Shandong Provincial Hospital of Shandong University.
Cell Culture
The TPC-1 and K1 cell lines were gifts from Professor Tian of Shandong Provincial Hospital of Shandong University which was approved by the Institutional Review Board of Shandong Provincial Hospital of Shandong University and the cells were cultured in DMEM (Sigma-Aldrich, St. Louis, MO, USA) with 10% foetal bovine serum (FBS; Invitrogen, Carlsbad, CA, USA) and penicillin–streptomycin.
Colony Formation Assay
To measure the proliferation of cells, we applied colony formation, 200 cells per well were seeded and incubated for 2 weeks. The colonies were fixed using methanol and stained with 0.1% crystal violet for 10 mins.
Cell Counting Kit-8 (CCK-8) Assay
Certain cells of 500 cells were seeded into per well of 96-well plates. The viability of cells was determined using a CCK-8 assay (Dojindo, Kumamoto, Japan) every 24 hrs and by measuring absorbance at 450 nm (BioTek, Winooski, VT, USA) following the manufacturer’s instructions.
Transfection
Cells were seeded into a 6-well plate after incubating for 24 h in the incubator. Plasmid or shRNA were transfected into the cells according to the protocol for Lipofectamine 3000®. For the rescue assay, plasmids containing the whole ORF of β-catenin or specific shRNA were transfected into the cells.
Luciferase Activity Assays
Cells were transfected with different plasmid described above and the luciferase activity was gained through the ration of GFP/RFP. The relative level was normalize to the negative control.
Real-Time (RT) qPCR
Total RNA from cells or tissues was extracted using TRIzol reagent (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s protocol. Quantitative RT (qRT)-PCR was conducted using SYBR Green PCR Master Mix (Takara Bio) according to the protocol. The quantification was performed on a CFX96 Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA). Relative expression was normalized to that of actin. The 2−ΔΔCt method was used to calculate relative RNA expression.
Western Blot Assay
Equal amounts of protein extracts were run on a 12% SDS-PAGE gel, and after transfer onto a membrane and blocking, the membrane was incubated with a primary antibody such as PCNA (Abcam ab92552 1:1000),N-cadherin (Abcam ab182031:1000),E-cadherin(Abcam ab760551:1000),Vimentin(Abcam ab1935551:1000),Snail(Abcam ab1807141:1000) and β-actin(Abcam ab82261:1000) overnight at 4°C. Finally, the membrane was incubated with secondary antibodies for 1 h at room temperature. The protein bands of interest were visualized using enhanced chemiluminescence reagents. (Millipore, Burlington, MA, USA)
Transwell Assay and Invasion Chamber
A transwell assay was performed to measure the migration and invasion ability. A total of 4×104 cells/well were resuspended in 250 μL of plain medium without any FBS in the upper chamber (8-μm pore size, Costar, Corning, NY, USA), and the lower chamber was filled with 0.75 mL of medium supplemented with 10% FBS. The cells were incubated for 24 h at 37°C. For the invasion assay, the upper chamber was coated with Matrix gel. The invasive and migrated cells were fixed with 100% methanol and stained with 0.5% crystal violet for 20 mins before counting under an inverted microscope.
Wound Healing Assay
We performed a wound healing assay to detect the migration ability of cells. We seeded 2×105 cells per well into a 6-well plate. Cells were incubated with complete medium (10% FBS and 1% PS with the corresponding medium mentioned above). After the cells reached 100% confluence, equal wounds were made with a 1 mL pipette, and the cells were incubated for 24 h. The relative width was measured by the ratio of area and length. The area was calculated by ImageJ.
Statistical Analysis
Statistical analyses were performed using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA). Data are presented as the mean ± SD. The chi-squared test was used to analyse clinicopathological characteristics. Comparisons between two groups were evaluated using Student’s t-test. Differences among groups were tested using a one-way ANOVA followed by a Tukey post-hoc test. All experiments were repeated at least three times. A P value < 0.05 was considered statistically significant.
Results
miR-26b-5p Is Downregulated in Papillary Thyroid Cancer
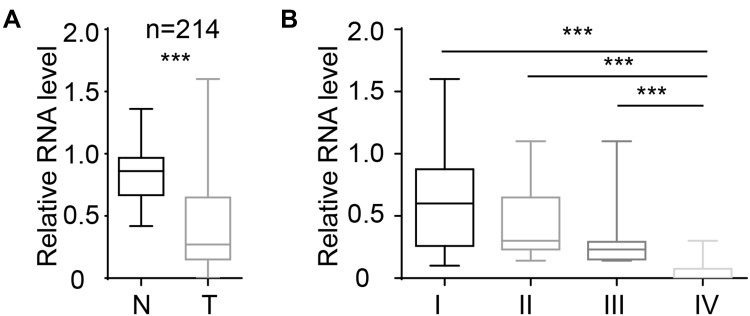
We collected 214 samples and analysed the level of miR-26b-5p. The results are shown in Figure 1A. miR-26b-5p was downregulated in cancer tissues compared with normal tissue. We next analysed the correlation of miR-26b-5p and the clinical stage, and the level of miR-26b-5p decreased with the clinical stage. A total of 214 fresh PTCs and paired normal tissues Patients with higher stage (III, IV) harboured lower miR-26b-5p levels compared with those with lower stage (I, II) (Figure 1B, P<0.001). The subtype and the TNM classification of the whole cohort was shown in Tables 1 and 2.
Figure 1.
miR-26b-5p was downregulated in PTC tissues. (A) The relative level of miR-26b-5p of PTC and paired normal tissue, n=214, ***P<0.001. (B) The relative level of miR-26b-5p in different stages of PTC, ***P<0.001.
Table 1.
Subtype Classification of the Whole Cohort
| Subtype | Number |
|---|---|
| Follicular subtype | 157 |
| Solid subtype | 32 |
| Tall cell subtype | 21 |
| Diffuse sclerosing subtype | 4 |
| Total | 214 |
Table 2.
TNM Classification of the Whole Cohort
| TNM Stage | Number |
|---|---|
| I/II | 164 |
| III/IV | 50 |
| Total | 214 |
miR-26b-5p Inhibits the Proliferation of PTC Cells
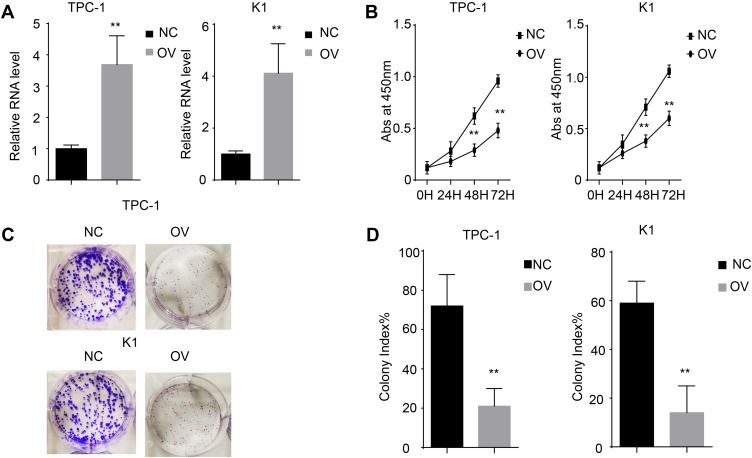
We demonstrated that miR-26b-5p was downregulated in PTC. However, the potential function was still unknown. To uncover the biological function of miR-26b-5p on PTC cells, we next established a miR-26b-5p stably overexpressing cell line (Figure 2A). We next performed the CCK-8 assay and colony formation assay. Cells with stable overexpression of miR-26b-5p (TPC-1-OV and K1-OV) showed a limited proliferation rate in the short term compared with the control (Figure 2B, P<0.01). We next used a colony formation assay to examine the function of miR-26b-5p on long-term proliferation. The results are shown in Figure 2C and D. The colony index decreased with the overexpression of miR-26b-5p (P<0.01). Overall, miR-26b-5p inhibits both the short-term and the long-term proliferation of PTC.
Figure 2.
miR-26b-5p inhibits the proliferation of PTC. (A) The relative RNA level of miR-26b-5p in stable cell line. **P<0.01. (B) The abs at 450nm of different cell lines. **P<0.01. (C) The representative image of colony formation assay. (D) The statistical analysis of colony index. **P<0.01.
Abbreviations: NC, negative control; OV, overexpression.
miR-26b-5p Inhibits the Migration and Invasion of PTC
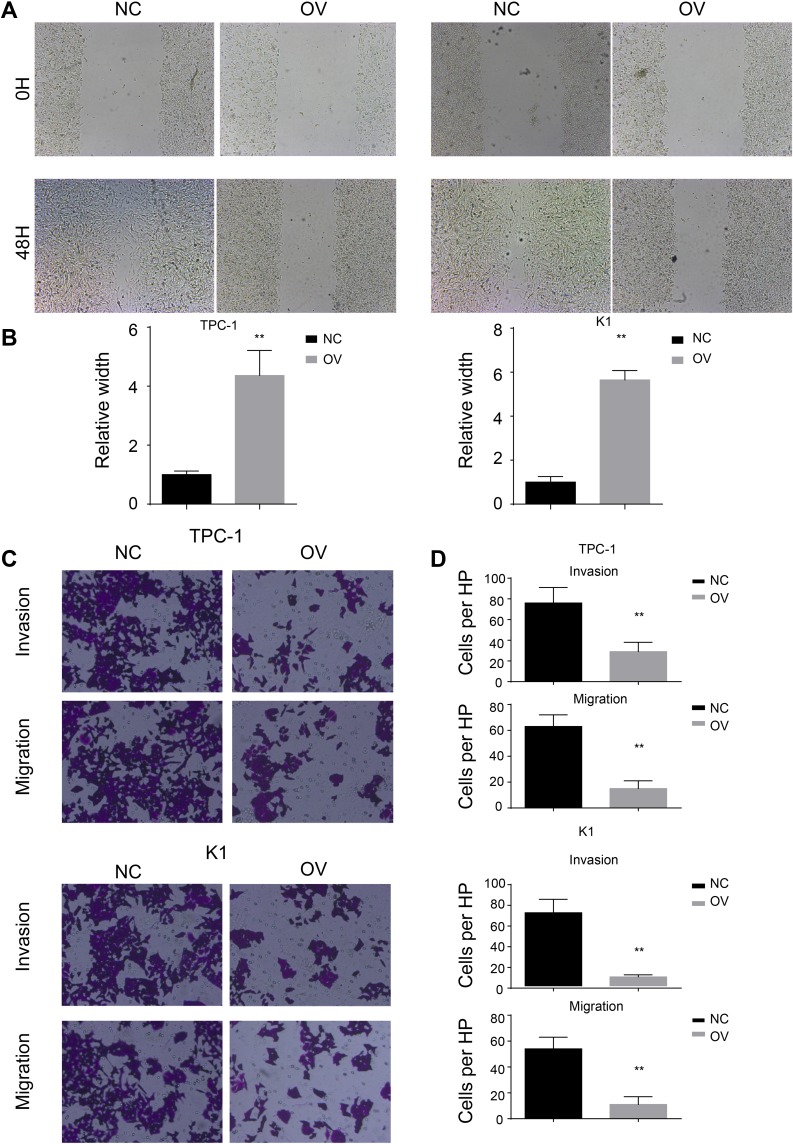
We have shown that miR-26b-5p inhibits the proliferation of PTC. We next examined the function of miR-26b-5p on migration and invasion. We performed a wound healing assay and a transwell invasion assay as previously described. Cells with higher levels of miR-26b-5p had decreased migration ability in both wound healing assays (Figure 3A and B, P<0.01) and transwell invasion assays (Figure 3C and D, P<0.01). These data suggest that miR-26b-5p inhibits the migration and invasion of PTC.
Figure 3.
miR-26b-5p inhibits the migration and invasion of PTC. (A) The representative image of wound healing, 200x magnification. (B) The statistical analysis of wound healing. **P<0.01. (C) The representative image of trans-well and invasion chamber, 200x magnification. (D) The statistical analysis of trans-well and invasion chamber. **P<0.01.
miR-26b-5p Inhibits the EMT Pathway
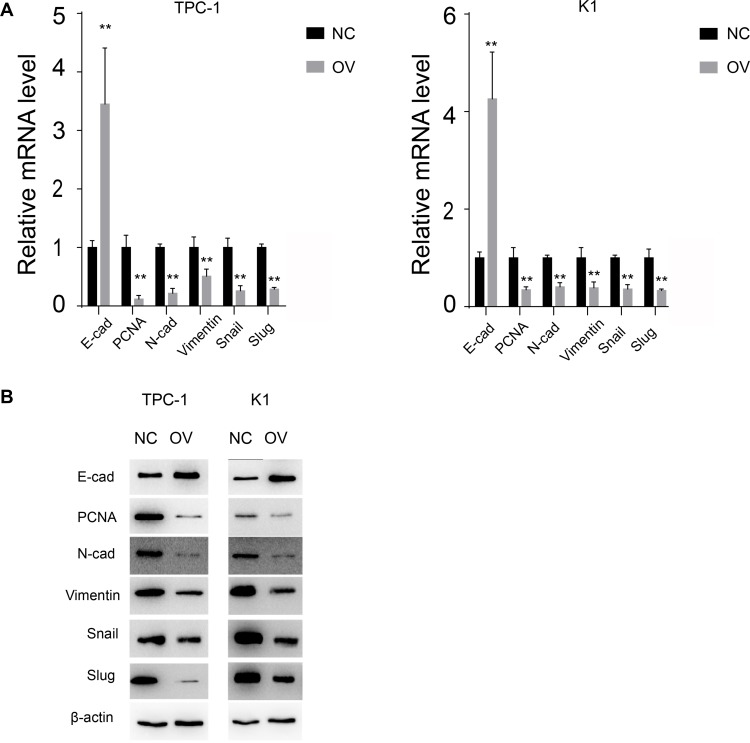
The EMT pathway is a common pathway reflecting the cell mesenchymal and epithelial status. We next detected the RNA and protein levels of EMT markers. Consistent with the previous results, the mesenchymal markers N-cadherin and vimentin decreased at both the RNA and protein levels, while the epithelial markers increased, indicating that the cells with miR-26b-5p stable overexpression transition to an epithelial phenotype from a mesenchymal phenotype (Figure 4A and B, P<0.01). The proliferation marker PCNA also decreased with the overexpression of miR-26b-5p.
Figure 4.
miR-26b-5p inhibits EMT of PTC. (A) The relative mRNA level of EMT markers and PCNA, **P<0.01. (B) The WB of EMT markers and PCNA.
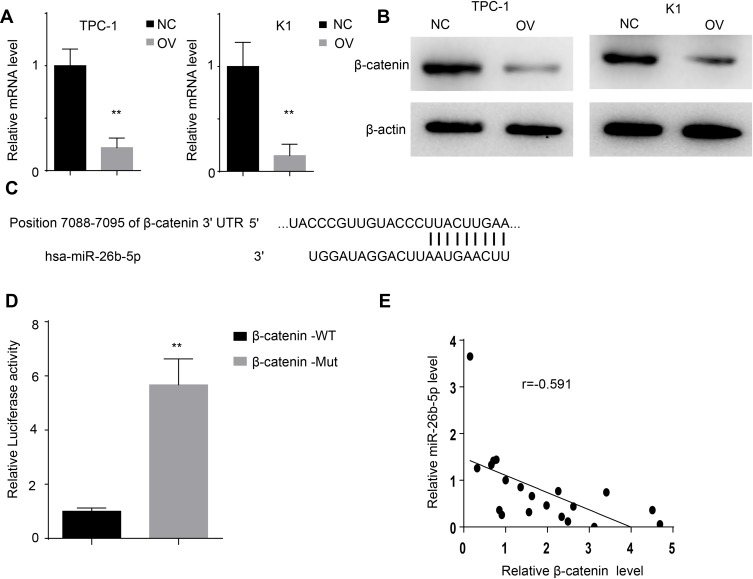
miR-26b-5p Leads to the Degradation of β-Catenin by Binding to the UTR
miRNAs mostly bind to the UTR of the target genes to exert their post-transcriptional regulation. We searched TargetScan to identify the potential target genes of miR-26b-5p. We selected β-catenin for its vital role in the tumourigenesis of cancers. We next detected the RNA level and protein level of β-catenin. The RNA level and protein level of β-catenin decreased in the TPC-1-OV and K1-OV cell lines (Figure 5A and B, P<0.01). We next characterized the interaction between β-catenin and miR-26b-5p as shown in Figure 5C. We next generated β-catenin-WT and β-catenin-Mut luciferase reporters. The luciferase activity decreased in β-catenin-WT-transfected cells compared with Mut-transfected cells, indicating that β-catenin could be degraded by miR-26b-5p (Figure 5D, P<0.01). Then, we detected the levels of β-catenin and miR-26b-5p in papillary thyroid cancer tissues. The regression analysis is shown in Figure 5E.
Figure 5.
miR-26b-5p directly targets β-catenin. (A) The relative mRNA level of β-catenin of the different cell lines, **P<0.01. (B) The protein level of β-catenin of the different cell lines. (C) The interaction of miR-26b-5p and β-catenin scanned by TargetScan. (D) The luciferase activity of β-catenin in 293T transfected with different plasmid, **P<0.01. (E) The regression analysis of β-catenin and miR-26b-5p in PTC.
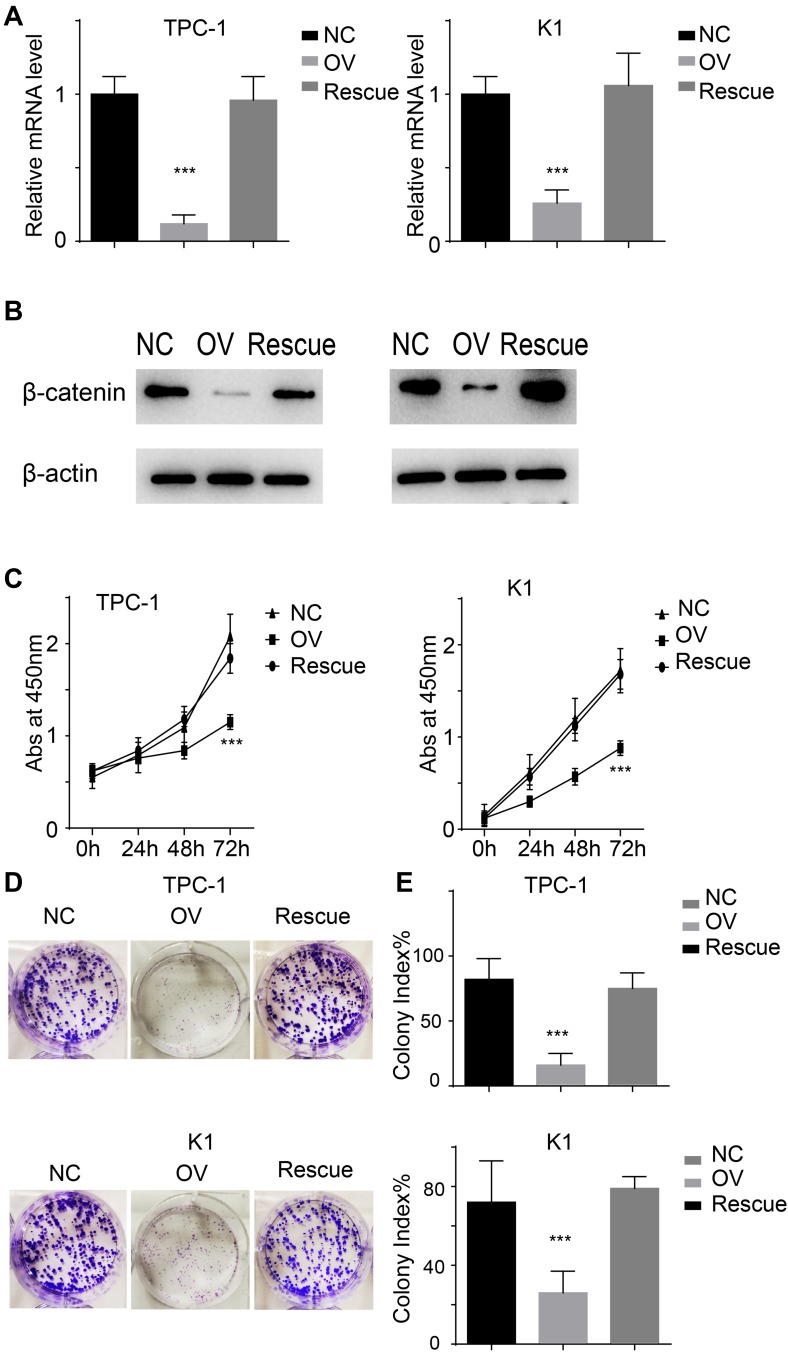
miR-26b-5p Inhibits the Proliferation of PTC Cells in a β-Catenin-Dependent Manner
We previously demonstrated that miR-26b-5p exerts its function through the β-catenin pathway. We next re-expressed β-catenin in miR-26b-5p stable overexpressing cells (named Rescue) (Figure 6A and B). We next applied the CCK-8 assay and colony formation assay. The results show that the proliferation ability was completely restored in the Rescue cell line compared with the OV and NC (Figure 6C–E, P<0.001). These data suggest that miR-26b-5p inhibits the proliferation of PTC cells in a β-catenin-dependent manner.
Figure 6.
miR-26b-5p inhibits the proliferation in β-catenin dependent manner. (A) The relative mRNA level of β-catenin of the different cell lines, ***P<0.001. (B) The protein level of β-catenin of the different cell lines. (C) The abs at 450nm of different cell lines. ***P<0.001. (D) The representative image of colony formation assay. (E) The statistical analysis of colony index. ***P<0.001.
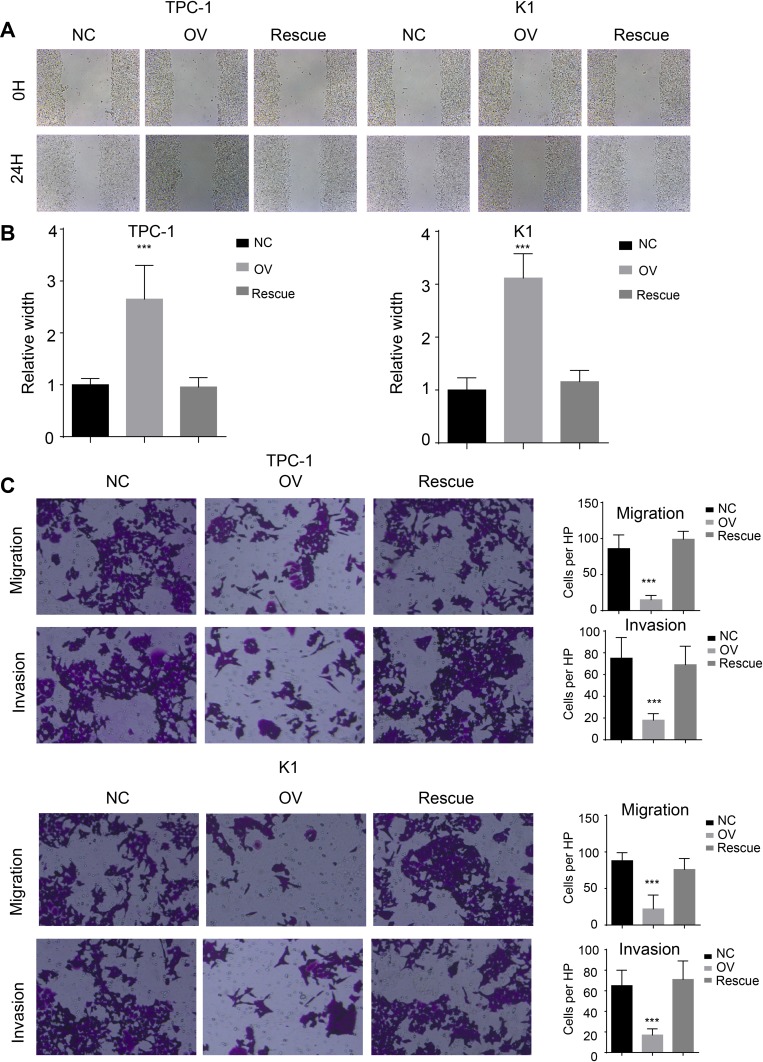
miR-26b-5p Inhibits the Migration, Invasion and EMT of PTC Cells in a β-Catenin-Dependent Manner
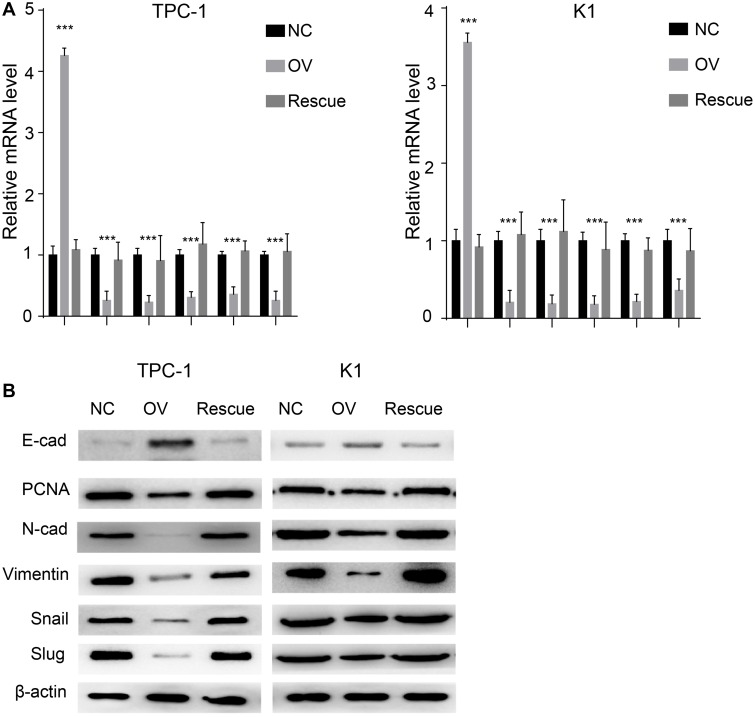
We used a wound healing assay and a transwell invasion assay. The re-expression of β-catenin restored the migration and invasion ability as shown in both wound healing (Figure 7A and B, P<0.001) and transwell invasion assays (Figure 7C, P<0.001). We next analysed the EMT markers and proliferation markers. The mesenchymal marker and PCNA were restored while the epithelial marker decreased at both the RNA and protein levels (Figure 8A and B). Overall, miR-26b-5p inhibits the migration, invasion and EMT of PTC cells in a β-catenin-dependent manner.
Figure 7.
miR-26b-5p inhibits the migration and invasion of PTC in β-catenin dependent manner. (A) The representative image of wound healing, 200× magnification. (B) The statistical analysis of wound healing. ***P<0.001. (C) Left: The representative image of trans-well and invasion chamber, 200× magnification. Right: The statistical analysis of trans-well and invasion chamber. ***P<0.001.
Figure 8.
miR-26b-5p inhibits EMT of PTC in β-catenin dependent manner. (A) The relative mRNA level of EMT markers and PCNA, ***P<0.001. (B) The WB of EMT markers and PCNA.
Discussion
Human papillary thyroid cancer is the most common endocrine malignancy worldwide. Despite advancements in radiotherapy and immunotherapy, patients with late-stage cervical cancer still suffer from recurrence and metastasis. Therefore, more biomarkers and therapeutic targets are needed.
miRNAs are reported to be involved in the proliferation and migration of normal cells and the tumourigenesis of multiple cancers.8,9 miRNAs exert their function mostly through post-transcriptional regulation. miRNA-708 was reported to target ZEB1 and thus regulate the mTOR pathway.10 miRNA 217 was shown to inhibit proliferation in hepatology cancers by degrading KLH5.11 However, the role of miR-26b-5p in thyroid cancers has rarely been studied. Studies have shown that miR-26b-5p is downregulated in multiple cancers.12–14 The potential function and mechanism of miR-26b-5p in PTC is still unknown and some papers did not classified miR-26b-5p as one of the dysregulated miRNAs.15 Hence, we examined the level of miR-26b-5p in papillary thyroid cancer and its correlation with clinical stage. To uncover the biological function of miR-26b-5p, we established stably overexpressing cell lines. Cells with higher levels of miR-26b-5p had decreased proliferation ability and migration ability. We next detected the mesenchymal markers and epithelial markers in the cells above. miR-26b-5p was demonstrated to promote the transition from a mesenchymal phenotype to an epithelial phenotype. We next performed luciferase reporter assays and found that miR-26b-5p targets β-catenin. The rescue cell line re-expressing β-catenin restored the proliferation, migration and invasion ability.
Taken together, our results show that miR-26b-5p inhibits proliferation, migration and invasion of human papillary thyroid cancer in a β-catenin pathway-dependent manner.
Conclusion
The findings of this study demonstrate that miR-26b-5p is downregulated in PTC tissues. miR-26b-5p inhibits proliferation, migration and invasion through the β-catenin pathway.
Acknowledgments
We thank Professor Tian for the technical assistance.
Ethics Statement
This study was approved by the Medical Ethics and Human Clinical Trial Committee and Animal Use and Management Committee at Shandong Provincial Hospital affiliated with Shandong University.
Author Contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.v68.6 [DOI] [PubMed] [Google Scholar]
- 2.Yu FQ, Wang Z, Wang X-W, et al. MicroRNA-885-5p promotes osteosarcoma proliferation and migration by downregulation of cell division cycle protein 73 homolog expression. Oncol Lett. 2019;17(2):1565–1572. doi: 10.3892/ol.2018.9802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang WJ, Peng K-Y, Tang Z-F, et al. MicroRNA-15a - cell division cycle 42 signaling pathway in pathogenesis of pediatric inflammatory bowel disease. World J Gastroenterol. 2018;24(46):5234–5245. doi: 10.3748/wjg.v24.i46.5234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang G, Wang M, Li X, et al. TUSC7 suppression of Notch activation through sponging MiR-146 recapitulated the asymmetric cell division in lung adenocarcinoma stem cells. Life Sci. 2019;232:116630. doi: 10.1016/j.lfs.2019.116630 [DOI] [PubMed] [Google Scholar]
- 5.Kong XX, Lv Y-R, Shao L-P, et al. HBx-induced MiR-1269b in NF-kappaB dependent manner upregulates cell division cycle 40 homolog (CDC40) to promote proliferation and migration in hepatoma cells. J Transl Med. 2016;14(1):189. doi: 10.1186/s12967-016-0949-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bu P, Wang L, Chen K-Y, et al. A miR-34a-numb feedforward loop triggered by inflammation regulates asymmetric stem cell division in intestine and colon cancer. Cell Stem Cell. 2016;18(2):189–202. doi: 10.1016/j.stem.2016.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang R, Niu Z, Pei H et al. Long noncoding RNA LINC00657 induced by SP1 contributes to the non-small cell lung cancer progression through targeting miR-26b-5p/COMMD8 axis. J Cell Physiol. 2019; 235(4): 3340–3349. [DOI] [PubMed] [Google Scholar]
- 8.Dejene SB, Ohman AW, Du W, et al. Defining fallopian tube-derived miRNA cancer signatures. Cancer Med. 2019;8(15):6709–6716. doi: 10.1002/cam4.v8.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Y, Tang D, Zhu L, et al. Label-free detection of miRNA cancer markers based on terminal deoxynucleotidyl transferase-induced copper nanoclusters. Anal Biochem. 2019;585:113346. doi: 10.1016/j.ab.2019.113346 [DOI] [PubMed] [Google Scholar]
- 10.Sun S, Hang T, Zhang B, et al. miRNA-708 functions as a tumor suppressor in colorectal cancer by targeting ZEB1 through Akt/mTOR signaling pathway. Am J Transl Res. 2019;11(9):5338–5356. [PMC free article] [PubMed] [Google Scholar]
- 11.Gao W, Lu Y-X, Wang F, et al. miRNA-217 inhibits proliferation of hepatocellular carcinoma cells by regulating KLF5. Eur Rev Med Pharmacol Sci. 2019;23(18):7874–7883. doi: 10.26355/eurrev_201909_18997 [DOI] [PubMed] [Google Scholar]
- 12.Khosla R, Hemati H, Rastogi A, et al. miR-26b-5p helps in EpCAM+cancer stem cells maintenance via HSC71/HSPA8 and augments malignant features in HCC. Liver Int. 2019;39(9):1692–1703. doi: 10.1111/liv.v39.9 [DOI] [PubMed] [Google Scholar]
- 13.Wilke CM, Hess J, Klymenko SV, et al. Expression of miRNA-26b-5p and its target TRPS1 is associated with radiation exposure in post-Chernobyl breast cancer. Int J Cancer. 2018;142(3):573–583. doi: 10.1002/ijc.v142.3 [DOI] [PubMed] [Google Scholar]
- 14.Cochetti G, Poli G, Guelfi G, et al. Different levels of serum microRNAs in prostate cancer and benign prostatic hyperplasia: evaluation of potential diagnostic and prognostic role. Onco Targets Ther. 2016;9:7545–7553. doi: 10.2147/OTT [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosignolo F, Memeo L, Monzani F, et al. MicroRNA-based molecular classification of papillary thyroid carcinoma. Int J Oncol. 2017;50(5):1767–1777. doi: 10.3892/ijo.2017.3960 [DOI] [PubMed] [Google Scholar]