Abstract
Introduction
Curcumin (Cur) is a natural extract of Asian spice Curcumin longa, showing multi-targeting capability and low toxicity in anti-tumor activities. The low bioavailability restricts its application as a therapeutic agent. Folate (FA) receptors are highly expressed in many malignant tumors while low expressed in normal tissue. Herein, we developed a self-assembled FA modified MPEG-PCL micelle to incorporate Cur (FA/Nano-Cur) and applied it for colorectal cancer therapy.
Methods
We prepared FA/Nano-Cur micelles and identified their characteristics. The drug release behavior, pharmacokinetics and in vitro anti-tumor activities of FA/Nano-Cur were studied. Furthermore, the in vivo anti-tumor ability assessment and anti-tumor mechanisms investigation were carried out in murine colorectal cancer model.
Results
FA/Nano-Cur micelles had an average particle size of 30.47 nm. Elongated T1/2 and larger AUC were found in FA/Nano-Cur group than that in the Free Cur group. MTT assay and apoptotic study indicated the growth inhibitory effect and pro-apoptotic effect of FA/Nano-Cur were the most significant among all treatments. Moreover, the in vivo study demonstrated that FA/Nano-Cur micelles exhibited a much stronger effect to suppress tumor growth, promote tumor apoptosis and attenuate tumor angiogenesis than Free Cur and Nano-Cur micelles.
Conclusion
The present study demonstrated FA/Nano-Cur micelles might be a promising therapeutic agent in colorectal cancer treatment with distinctive advantages of improved bioavailability, sustained drug release, tumor-targeted delivery and low toxicity.
Keywords: curcumin, colorectal cancer, folate, nanoformulation, apoptosis, angiogenesis
Introduction
Colorectal cancer (CRC) is the third most common tumor and one of the main causes of cancer-related death among both men and women worldwide.1 The highest incidence of CRC is reported in Alaska natives and blacks, while the lowest is in Asian Pacific Islanders, which is closely related to age and so-called western lifestyle.2–4 In recent years, the morbidity and mortality rates of CRC have begun to decrease as a result of lifestyle changes in pathogenetic reasons (e.g., decreased smoking and alcohol consumption, body shaping and reduced red meat intake), the application of sigmoidoscopy and colonoscopy, as well as improved treatment.4–9 Unfortunately, the patients who developed distant metastasis had a five-year survival of only about 11.7%.10 The treatment options for CRC included surgery, chemotherapy, targeted therapy, radiotherapy and immunotherapy. Chemotherapy as the principal treatment approach has some adverse effects involving gastrointestinal tract toxicity, bone marrow suppression, neurotoxicity, liver and kidney damage and other tissue damages.11 Moreover, current treatment exhibited high recurrence and poor prognosis. Therefore, it is increasingly important to explore more efficient and less toxic treatment approach for CRC.
Phytotherapy has received more and more attention in the field of medicine, focusing mainly on herbal active pharmaceutical ingredients.12 Curcumin (Cur), an active polyphenolic compound extracted from turmeric (Curcuma longa L.), was found to possess beneficial properties including anti-oxidant, anti-inflammatory, anti-hyperlipidemic, anti-diabetic, anti-microbial and powerful anti-tumor effects.13–18 Among these, the anti-tumor effect of Cur is the most popular at present. It is reported that Cur had inspiring anti-tumor abilities in melanoma, gastrointestinal, genitourinary, pancreatic, breast, lung, haematological, head and neck, neurological carcinomas and sarcoma.15,19–23 What’s more, Cur proved to be non-toxic and safe in animal studies, making it a promising drug agent.24 Despite these advantages, Cur has not yet been approved as a therapeutic agent for cancer. The main drawback is poor bioavailability.25–27 Some pharmacokinetic researches have indicated that the poor bioavailability of unformulated Cur is mainly attributed to low water solubility, fast metabolism and rapid systemic clearance, which limit its therapeutic efficiency.27 Thereupon, we aim to develop a new dosage form of Cur to overcome the poor bioavailability and improve therapeutic efficacy in cancer treatment.
Nanotechnology is playing a significant role in drug delivery systems nowadays. Nanoparticles, such as liposomes, polymeric micelles, can encapsulate water-insoluble drugs as vectors and protect them from enzymatic and hydrolytic degradation.28–30 Methoxypoly (ethylene glycol)-poly (ɛ-caprolactone) (MPEG–PCL) are biodegradable, amphiphilic diblock copolymers.31 When the copolymers are in aqueous solution, the hydrophobic PCL segments can spontaneously assemble to form a core enclosing water-insoluble drug; meanwhile, the hydrophilic PEG segments are encircled by water to form a shell-like structure.32 The hydrophobic drugs loaded MPEG-PCL copolymers are capable of sustaining drug release and improving drug bioavailability.33,34 Moreover, the amphiphilic MPEG-PCL micelles have biodegradability and nanoscale properties, making them ideal drug carriers for systemic administration.
Usually, the activity of pharmaceutical agent is non-selective, and the agent can be inactivated or induce adverse effects on normal tissues and organs.35 Folate (FA) is a member of water-soluble vitamin B family, which has low immunogenicity. FA receptors are glycoproteins fixed to the cell membrane by glycosyl phosphatidyl inositol (GPI), showing high affinity to FA and FA modified drug formulation, so the drug can be transferred into cells through endocytosis.36 FA exhibits distinguished advantage in increasing the specificity of anti-cancer drug vehicle because the FA receptors are highly expressed in various malignant tumors including colon, brain, lung and breast carcinomas, but low expressed in normal tissues.37–40 Therefore, FA conjugated nanoformulation might be useful to realize active tumor-targeted drug delivery, sustain drug release, as well as reduce adverse effect. In this research, we prepared the FA/Nano-Cur formulation and compared its therapeutic effect with Free Cur and Nano-Cur formulation in the treatment of CRC. Our in vitro and in vivo results demonstrated that the Cur-loaded FA/MPEG-PCL micelles might be a promising therapeutic approach for CRC.
Materials and Methods
Materials and Reagent
Curcumin, FA, MPEG (molecular weight=2000D), ε-caprolactone (ε-CL) (molecular weight=2000D), 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) were purchased from Sigma, USA. Roswell Park Memorial Institute (RPMI) 1640 and fetal bovine serum (FBS) were purchased from Gibco, USA. Methanol and acetic acid (HPLC grade) were purchased from Fisher Scientific, UK. 4ʹ, 6-diamidino-2-phenylindole (DAPI) and hematoxylin and eosin (HE) dye from Beyotime Biotechnology, China. Dimethyl sulfoxide (DMSO) and acetone were purchased from KeLong Chemicals, China. Antibodies used include: rabbit anti-mouse cluster of differentiation 31 (CD31) polyclonal antibody, rabbit anti-mouse proliferating cell nuclear antigen (PCNA) antibody, and horseradish peroxidase (HRP)-conjugated secondary antibody (Servicebio, China).
Murine CT26 colon carcinoma cells were purchased from American Type Culture Collection (ATCC, USA) and cultured in RPMI 1640 culture medium containing 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin. Cells were maintained in a humidified chamber at 37°C in an atmosphere containing 5% CO2. 6–8 weeks female BALB/C mice were obtained from the Animal Center Laboratory of Beijing HFK Bioscience (China) and provided with sterilized water and chow. All animal experimental procedures were approved by the Institutional Animal Care and Treatment Committee of Sichuan University and conducted according to the Institutional Animal Care and Use Guidelines.
Preparation and Characterization of FA/Nano-Cur Micelles
According to the previous reports,33 MPEG-PCL diblock copolymer was synthesized by ring-opening polymerization of MPEG and ε-CL catalyzed by Sn(Oct)2. Briefly, an equal amount of MPEG, ε-CL were introduced into a glass ampoule with the addition of Sn(Oct)2 (0.5% w/w) under nitrogen protection. The mixture reactant was maintained at 130°C for 6 h. The crude product was then dissolved in dichloromethane and purified by precipitation in ice-cooled diethyl ether followed by vacuum drying for further use. FA (100 mg) and NH2-PEG-PCL (200 mg) dissolved in 4 mL of dimethylsulfoxide (DMSO) were introduced into a flask, with the addition of DMAP (150 mg) and DCC (130 mg). After agitation at 37°C under nitrogen for 10 hrs, the synthesized copolymer solution was dialyzed and dried under vacuum. All obtained products were stored in a desiccator.
Cur (10 mg), MPEG-PCL (80 mg) and FA/MPEG-PCL (10 mg) were dissolved in 3 mL acetone in a round bottom flask. Next, the acetone was removed by vacuum evaporation under 55°C water bath and a yellow film was formed on the bottom of the flask. PBS was added to hydrate the film and slowly stirred. The un-encapsulated drug and polymerized copolymer were filtered and removed with a 0.22-μm filter. Blank micelles were prepared following the same procedure. The particle size and zeta potential of FA/Nano-Cur micelles were determined by dynamic light scattering (Malvern Nano-ZS 90, UK). The morphology of FA/Nano-Cur was observed under a transmission electron microscope (TEM) after phosphotungstic acid staining (Hitachi H-6009IV, Japan). The content of Cur in FA/Nano-Cur was determined by high-performance liquid chromatography (HPLC). The encapsulation efficiency (EE) and drug loading (DL) of FA/Nano-Cur micelles were calculated according to the equations: drug loading (DL) = experimental Cur loading/total micelle mass × 100% (a), encapsulation efficiency (EE) = experimental Cur loading/theoretical Cur loading ×100% (b).
The in vitro drug release behavior of Cur from Free Cur, Nano-Cur and FA/Nano-Cur were conducted using the dialysis method. 0.5 mL of Free Cur in DMSO solution, Nano-Cur solution and FA/Nano-Cur solution was placed in the dialysis bag (molecular weight cut-off, 3.5 kDa), respectively. Dialysis bags were incubated in 30 mL of phosphate-buffered solution (pH=7.4) containing Tween-80 (0.5 wt%) at 37°C with mild shaking. The incubation medium was replaced with fresh incubation medium at different time points, respectively. The drug release profiles of Cur from different formulations were studied by HPLC assay.
Pharmacokinetic Study
Firstly, a catheter was introduced into the jugular vein of rats 24 hrs before drug administration. The rats were then assigned into three groups and received different drugs: Free Cur group (50 mg/kg Cur), Nano-Cur group (50 mg/kg Cur) and FA/Nano-Cur (50 mg/kg Cur) group. At the indicated interval, the blood was collected from the catheter in the jugular vein. The plasma of each blood sample was extracted and lyophilized. The dried plasma was dissolved in methanol and analyzed by HPLC.
Cell Cytotoxicity Assay
CT26 cells at the logarithmic phase were seeded in 96-well culture plates at a density of 3×103 or 5×103 cells/well and incubated for 24 hrs at 37°C for cell attachment. Cells were treated with different concentrations of Free Cur, Nano-Cur and FA/Nano-Cur for 24 hrs or 48 hrs, and then incubated with the addition of 20 μL MTT solution (5 mg/mL) for 4 hrs. Next, the culture medium was aspirated carefully and 150 μL DMSO was added. After gently shaking for 10 mins to dissolve the formazan, the absorbance of each well at 570 nm (OD570) was measured by a microplate reader (OPTImax, Molecular Dynamics, USA).
Cell Necrosis and Apoptosis Assay
Cell necrosis and apoptosis were analyzed using a commercial Propidium (PI)/fluorescein isothiocyanate (FITC) conjugated Annexin-V apoptosis detection kit (BD Pharmingen, USA). CT26 cells (2×105 cells/well) were seeded in six-well plates for cell attachment and then incubated in culture medium containing different concentrations of Free Cur, Nano-Cur or FA/Nano-Cur. After incubated for 48 hrs, cells were collected and washed with PBS. Next, cells were re-suspended with 100 μL binding buffer containing 3 μL PI and 3 μL Annexin-V for 15 mins. The percentages of cell necrosis and apoptosis were determined by a flow cytometer (Novocyte, USA).
Mice Model and Treatment
In this work, the mice model of heterotopic CRC was established to evaluate the anti-tumor ability of different treatments by inoculating CT26 cells (1×106) in the right flank of mice. When the tumor volume reached about 100 mm3, tumor-bearing mice were randomly allocated into five treatment groups (Control, FA/Nano, Free Cur, Nano-Cur and FA/Nano-Cur) and received the treatment by intravenous injections every 2 days. Control group: normal saline (NS), FA/Nano group: equal amount of FA/Nano as FA/Nano-Cur, Free Cur group: 50 mg/kg Cur; Nano-Cur group: 50 mg/kg Cur; FA/Nano-Cur: 50 mg/kg Cur. The tumor volume and the body weight of mice were measured on indicated days during the experimental period. The calculation equation of tumor volume: V = π×A×B2/6 (A: longest diameter, B: shortest diameter). All experimental mice were sacrificed on the 18th days after inoculation and the tumor, peripheral blood and vital organs of mice were collected for further analysis.
Immunohistochemical Analysis
Tumor sections (4 μm) were prepared and stained with PCNA and CD31 antibodies for the purpose of evaluating tumor proliferation and tumor angiogenesis in different treatment groups. Briefly, the paraffin-embedded tumor tissue sections underwent de-paraffinage and rehydration. The antigen retrieval process was conducted, followed by endogenous peroxidase inactivation with a 3% hydrogen peroxide solution. Then, the tumor sections were punched with Triton X-100 (for PCNA staining), blocked with goat serum, and sequentially incubated with PCNA antibody or CD31 antibody (Servicebio, China) and corresponding HRP conjugated secondary antibody (Servicebio, China). Finally, the labeled sections were colorated by DAB and counterstained with hematoxylin. All stained sections were observed under a light microscope at 200× magnification. Cells with positive expression of PCNA or CD31 were stained in yellow brown. The PCNA index was calculated as the amount of PCNA staining positive cells divided by the amount of total cells in five randomly selected fields. The microvascular density was calculated as the amount of CD31 staining positive cells in five randomly selected fields.
Immunofluorescent Staining
Paraffin-embedded tumor sections (4 μm) were deparaffinized and rehydrated as above. The terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) staining was carried out according to the manufacturer's description of a commercially available TUNEL staining kit from Promega, USA. DNA fragmentation of apoptotic cells in tumor tissue sections from different treatment group would be labelled with FITC green fluorescence. Finally, all stained sections were examined under a fluorescence microscope at 200× magnification. The apoptotic cell percentage was calculated as the amount of TUNEL staining positive cells divided by the amount of total cells in five randomly selected fields.
Safety Assessment
The important organs including heart, liver, spleen, lung and kidney of the mice were harvested and prepared as paraffin-embedded sections (4 μm). These tissue sections were stained with H&E dye and examined under a light microscope by two independent pathologists for morphology identification. What is more, the serums of peripheral blood samples were obtained for biochemical analysis to further evaluate the toxicity of different treatments in vivo.
Statistical Analyses
Statistical analysis was performed using GraphPad Prism 6.0 software (GraphPad, USA). Data were expressed as mean ± standard error (SD). Student’s t-test was utilized to compare two experimental groups. P<0.05 was considered to be statistically significant.
Results
Characterization and Drug Release Behavior of FA/Nano-Cur
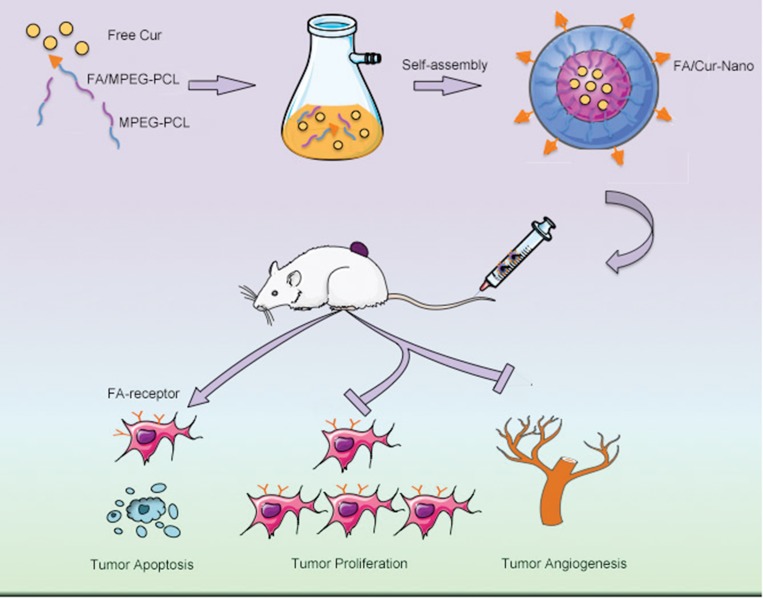
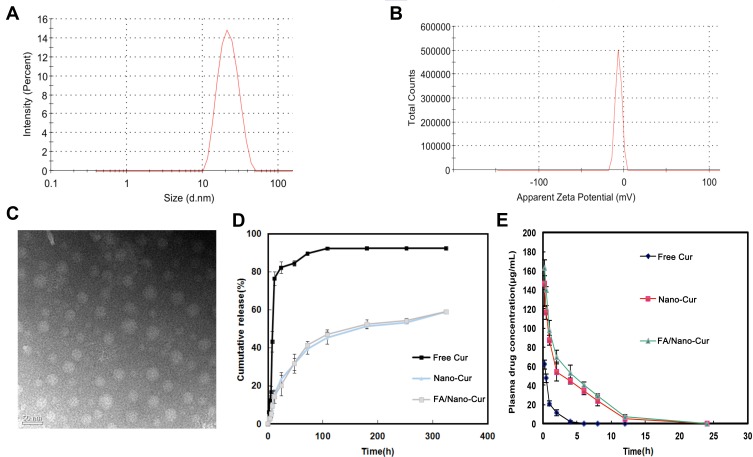
A graphic scheme was shown to introduce the preparation process of FA/Nano-Cur micelles in the study (Figure 1). The amphiphilic FA/MPEG-PCL copolymers and MPEG-PCL copolymers could spontaneously gather to form a spherical like structure in aqueous solution. As is shown, water-insoluble Cur was entrapped in the hydrophobic core of the self-assembled FA/MPEG-PCL micelle to form FA/Nano-Cur micelles. FA/Nano-Cur was suggested to have the capability to target the FA receptors on tumor cells, thus to enhance tumor-targeted drug delivery. The characteristics of the FA/Nano-Cur micelles were identified by the dynamic light scattering (DLS) method. The particle size of FA/Nano-Cur micelles was found to be 30.47±0.65 nm with PDI being 0.17, indicating a very narrow particle size distribution (Figure 2A). The zeta potential is −3.55 mV, which was slightly negative (Figure 2B). According to the observation under TEM, FA/Nano-Cur micelles were well dispersed with a nearly spherical morphology, indicating successful self-assembly of the polymers (Figure 2C). Furthermore, the DL and EE of FA/Cur-Nano were determined to be 10% and 98%, respectively, by HPLC.
Figure 1.
Schematic diagram of the study. The self-assembled FA/Nano-Cur micelles composed of MPEG-PCL polymers, FA/MPEG-PCL polymers and Free Cur were administrated intravenously in the mice heterotopic colorectal cancer model, showing the capability of promoting tumor apoptosis, inhibiting tumor proliferation and attenuating tumor angiogenesis.
Figure 2.
Characterization of FA/Nano-Cur micelles and drug release study. The particle size and the zeta-potential of FA/Nano-Cur micelles measured by the dynamic light scattering (DLS) method (A and B). Morphological identification of FA/Nano-Cur micelles under transmission electron microscopy (TEM) (C). Cur release from Free Cur, Nano-Cur micelles and FA/Nano-Cur micelles (D). Plasma drug concentration of Cur in Free Cur group, Nano-Cur group and FA/Nano-Cur group at different time point (E).
The drug release study is necessary for the evaluation of a drug delivery system. Thereupon, the release behavior of Cur from Free Cur, Nano-Cur and FA/Nano-Cur were investigated in our study. According to the in vitro drug release study, 91.60%, 44.53%, 46.32% of Cur has been released at 100 hrs in Free Cur group, Nano-Cur group and FA/Nano-Cur group, respectively (Figure 2D). Compared with Free Cur group, a significantly slower release of Cur was observed in the Nano-Cur group and FA/Nano-Cur group, suggesting a more sustainable drug release. Moreover, the in vivo drug release study results showed elongated T1/2 and increased Cmax as well as enlarged area under curve (AUC) by Nano-Cur and FA/Nano-Cur micelles. The Tmax, Cmax, T1/2 and AUC of Free Cur were 0.25 hrs, 61.67 μg/mL, 0.81 hrs and 62.28 μg mL−1 hr. The Tmax, Cmax, T1/2 and AUC of Nano-Cur were 0.25 hrs, 146.67 μg/mL, 1.42 hrs and 478.6 μg mL−1 hr. The Tmax, Cmax, T1/2 and AUC of FA/Nano-Cur were 0.25 hrs, 163.00 μg/mL, 1.58 hrs and 579.1 μg mL−1 hr (Figure 2E). The results showed that the nanoformulation of Cur significantly extended the drug duration of Cur agent.
Cytotoxicity Assay
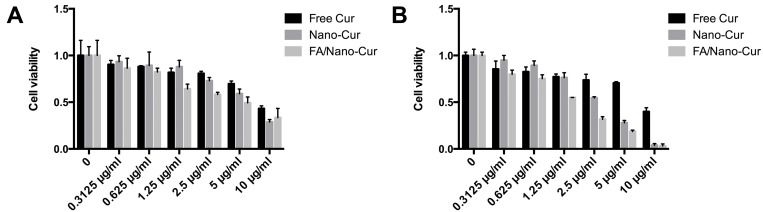
We then investigated the cytotoxic effect of Free Cur, Nano-Cur and FA/Nano-Cur on CT26 cells using MTT assay. The results demonstrated that the cytotoxicity of Free Cur, Nano-Cur and FA/Nano-Cur were in dose-dependent and time-dependent manners. At 24 hrs, the half-maximal inhibitory concentration (IC50) of the Free Cur, Nano-Cur and FA/Nano-Cur were 9.614 μg/mL, 5.683 μg/mL, 5.777 μg/mL, respectively (Figure 3A). At 48 hrs, the IC50 of the Free Cur, Cur-Nano, FA/Cur-Nano was 9.490 μg/mL, 2.642 μg/mL, 1.373 μg/mL, respectively (Figure 3B), which illustrated that FA/Nano-Cur exhibited the strongest cytotoxic effect compared with Free Cur and Nano-Cur in vitro study.
Figure 3.
Cell cytotoxicity analysis. CT26 cells viability detected by MTT assay after treatment with different concentrations of Free Cur, Nano-Cur and FA/Nano-Cur at 24 hrs (A) and 48 hrs (B).
Cell Necrosis and Apoptosis Assay
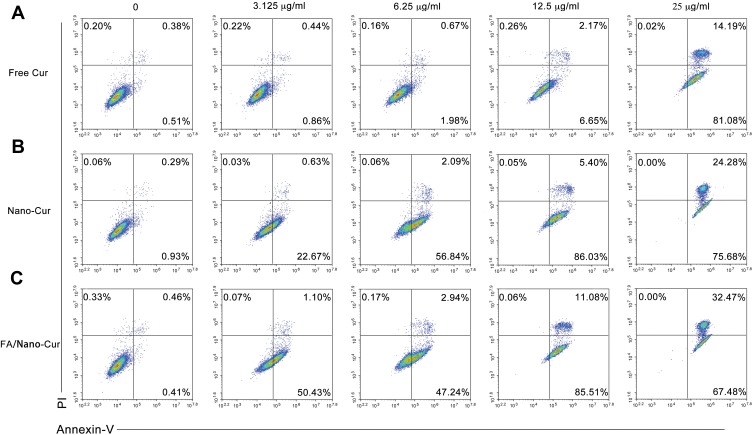
Many studies have already reported the pro-apoptotic effects of Cur on tumor cells. We performed PI/Annexin-V staining flow cytometry to determine the effects of Free Cur, Nano-Cur and FA/Nano-Cur on CT26 cells. The results indicated that CT26 cell death caused by Free Cur and Cur-loaded nanomicelles were mainly apoptosis. The percentage of Annexin-V+ cells was 0.89%, 1.3%, 2.65%, 8.82% and 95.27% in the Free Cur treated group (Figure 4A), 1.22%, 23.3%, 58.93%, 91.43% and 99.96% in the Nano-Cur treated group (Figure 4B), versus 0.87%, 51.53%, 50.18%, 96.59% and 99.95% in the FA/Nano-Cur treated group (Figure 4C) for concentrations of Cur at 0, 3.125 μg/mL, 6.25 μg/mL, 12.5 μg/mL and 25 μg/mL, respectively. Hence, the above results confirmed that FA/Nano-Cur and Nano-Cur induced more cell apoptosis than Free Cur at the same concentration. Besides, there was an upper-shift of cell population with the increase of drug concentration, which might be attributed to the auto-fluorescence of Cur. It is noteworthy that the FA modification further enhanced the cytotoxicity of Nano-Cur, demonstrated by more apoptotic cells in the FA/Nano-Cur treated group than that in the Nano-Cur treated group.
Figure 4.
Cell apoptosis and necrosis analysis. Flow cytometry results of PI/Annexin-V staining of CT26 cells treated with different concentrations of Free Cur (A), Nano-Cur (B) and FA/Nano-Cur (C) for 48 hrs.
In vivo Anti-Tumor Activity
Therapeutic Efficacy in Mice Model of Heterotopic CRC
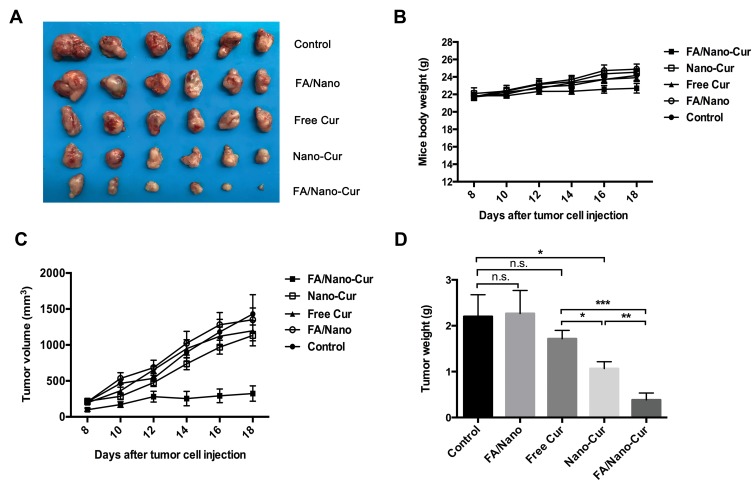
The in vitro study results had found that FA/Nano-Cur and Nano-Cur showed enhanced ability to inhibit tumor proliferation and promote tumor apoptosis. Thereafter, a mice model of heterotopic CRC was established to evaluate the anti-tumor efficacy of different treatments in vivo. Figure 5A shows the subcutaneous tumors in each treatment group on the 18th day after tumor inoculation. The body weight of mice in each group increased gradually (Figure 5B). The tumor growth curves were plotted by dynamic monitoring of subcutaneous tumor volume, showing that the tumors in the control, FA/Nano, Free Cur, and Nano-Cur groups grew rapidly, compared with slowly increase in the FA/Nano-Cur group from Day 12 to Day 18 (Figure 5C). On the 18th day, the tumor-inhibition rate was 77.32% in the FA/Cur-Nano group, 20.77% in the Nano-Cur group and 16.25% in the Free Cur group. No significant difference was shown between the FA/Nano group and control group. In addition, the comparison of tumor weight among different treatment groups validated the above conclusion (Figure 5D). Thus, our results demonstrated that Cur-loaded polymeric micelles were more effective than Free Cur, and in which FA/Nano-Cur exerted the strongest anti-tumor effect in the treatment of CRC.
Figure 5.
In vivo anti-tumor ability assessment of different treatments. The photograph of subcutaneous tumors from different treatment groups (A), the boy weight change curve of mice in different groups (B), the tumor growth curve of mice from different treatment groups (C) and the tumor weight of mice from different treatment groups (D) (*P<0.05, **P<0.01, ***P<0.001).
Effect of Inhibiting Tumor Proliferation
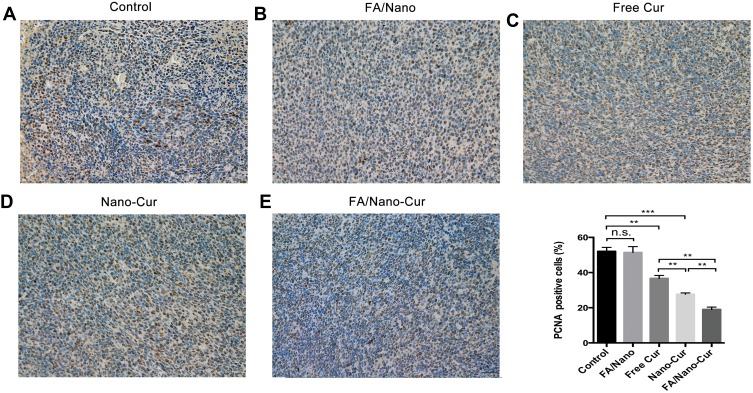
In an anti-tumor drug assessment, it is essential to evaluate the ability of inhibiting tumor proliferation. In the study, the PCNA immunohistochemical staining was performed to compare the effect of different treatments on tumor proliferation. Our results showed that the PCNA index of Free Cur, Nano-Cur and FA/Nano-Cur were significantly lower than that of the control group and FA/Nano group, suggesting decreased tumor proliferation by Cur treatment (Figure 6A–E). Among all groups, the FA/Nano-Cur group had the fewest cells in proliferation status. Therefore, the results indicated that FA/Nano-Cur had a better effect on inhibiting tumor proliferation than other treatment groups.
Figure 6.
Effect on tumor proliferation. PCNA staining positive cells percentage in Control group (A), FA/Nano group (B), Free Cur group (C), Nano-Cur group (D) and FA/Nano-Cur group (E) (**P<0.01, ***P<0.001).
Effect of Inducing Tumor Cells Apoptosis
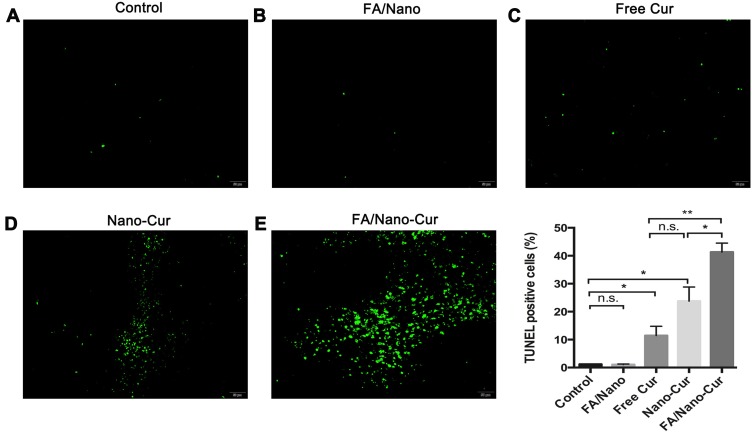
Flow cytometry with PI/Annexin-V staining found that Free Cur, Nano-Cur and FA/Nano-Cur could induce tumor apoptosis in vitro. Hence, the TUNEL staining method was employed to study the effect of different treatments on tumor apoptosis in vivo. As a result, only few apoptotic cells were observed in the control group and the FA/Nano group whereas the apoptotic cells increased slightly in the Free Cur group (Figure 7A–E). There was a large number of apoptotic cells observed in the Nano-Cur treated group and the FA/Nano-Cur treated group, and the proportion of apoptotic cells in the FA/Nano-Cur group was higher than that of the Nano-Cur group. Taken together, the results confirmed that FA/Nano-Cur could induce more tumor cells apoptosis, thereby enhancing the anti-tumor effect.
Figure 7.
Effect on tumor apoptosis. The results of TUNEL staining in Control group (A), FA/Nano-Cur group (B), Free Cur group (C), Nano-Cur group (D) and FA/Nano-Cur group (E) (*P<0.05, **P<0.01).
Effect of Attenuating Tumor Angiogenesis
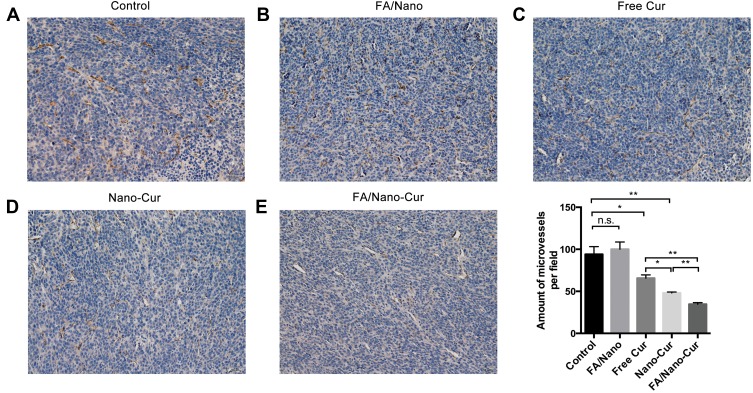
Tumor angiogenesis is crucial for the development and metastasis of malignant tumor. To further explore the anti-angiogenesis capability of different treatments, we labeled the vascular endothelial cells in tumor tissues using immunohistochemical staining method. The number of CD31 positive cells in the randomly selected microscopic field was defined as microvessel density (MVD). Results showed that the MVD in all but the FA/Nano group was significantly lower than the control group, and the FA/Nano-Cur group had the minimum MVD (Figure 8A–E). In addition to inhibiting proliferation and promoting apoptosis, it was demonstrated that Free Cur, Nano-Cur and FA/Nano-Cur exhibited the capability of anti-angiogenesis, among which FA/Nano-Cur was the most effective one.
Figure 8.
Effect on tumor vascularization. Microvessel densities in Control group (A), FA/Nano group (B), Free Cur group (C), Nano-Cur group (D) and FA/Nano-Cur group (E) (*P<0.05, **P<0.01).
Systemic Toxicity Assessment
In order to investigate the systemic toxicity of different treatments, the vital organs including heart, liver, spleen, lung and kidney of mice from each treatment group were sliced and examined by HE staining. No apparent morphological abnormalities were found in all treatment groups (Figure 9A–E). Furthermore, the appetite change, diarrhea and abnormal behaviors of mice were monitored to evaluate the adverse effects. No obvious appetite and behavioral abnormalities appeared in the groups throughout the treatment period. Besides, there was no significant difference in serological biochemical parameters among the groups, suggesting that all treatments were low toxic to the mice (Figure 10A–D). The FA/Nano-Cur and Nano-Cur prepared in the study showed good safety, which indicated their great potential for future application.
Figure 9.
Safety assessment on vital organs. HE staining results of heart (A), Liver (B), spleen (C), lung (D) and kidney (E) sections from different treatment groups were presented.
Figure 10.
Serological biochemical parameters detection. A series of biochemical parameters in mice serum from different groups were detected including ALB, ALP, ALT, AST (A), CHOL, CK, CREA, GLU (B), HDL, LDH, LDL, TG (C) and TP, UA, UREA, AMY (D).
Discussion
As the natural product extracted from traditional Asian spice Curcuma longa L., Cur is easily available, low toxic, and mass-produced. Cur has received extensive attention for its versatile role in many pharmacological activities, which makes it a promising drug candidate in the treatment of various diseases.13,14,16–18 The anti-tumor mechanisms of Cur were associated with the development and progression of cancer. Many signal pathways were involved in the processes including mitogen-activated protein kinase (MAPK), peroxisome proliferator-activated receptor (PPAR), WNT/beta-catenin, sonic hedgehog (sHh), NOTCH and PI3K/PTEN/Akt/mTORC, protein 53 (P53), activating protein 1 (AP-1), signal transducers and activators of transcription 3 (STAT3), B-cell lymphoma-2 (Bcl-2), epidermal growth response-1 (Egr-1), showing the multi-target capability of Cur in cancer treatment.41–47 In CRC, Cur was also able to suppress cyclooxygenase-2 (COX-2) and reduce prostaglandin synthesis in the colon carcinogenesis process.48,49
One previous study has demonstrated that consuming 8 g of Cur per day for 3 months caused no significant toxic effect in patients with high-risk or precancerous lesions.50 However, the solubility of Cur was reported to be less than 0.125 mg/L in water.51 What is more, the content of Cur was undetectable in mice plasma and brain at 0.5 h after oral administration of Cur (50 mg/kg), indicating poor bioavailability.52 Research has shown that the content of Cur was merely 50 ng/mL after oral administration of Cur at the dosage of 10–12 mg/kg in human.53 Some researches reported FA modified MPEG-PCL polymers encapsulating chemotherapeutic or hormone-therapeutic drugs such as doxorubicin, paclitaxel and tamoxifen in cancer therapy, which showed a good anti-tumor effect.54–56 Another study introduced a Y-shaped FA-MPEG-PCL2 copolymer with a particle size of 48.94 nm to improve the uptake of Cur in Hela and HepG2 cells.57 Herein, we synthesized Cur-loading FA/MPEG-PCL micelles (FA/Nano-Cur) with an average size of 30.47 nm for vein injection, which was expected to improve the bioavailability of Cur. Gou et al applied MPEG-PCL micelles in the Cur drug delivery system for CRC treatment, which showed an improved anti-tumor effect than free Cur in mice model.34 In the research, we used FA conjugated MPEG-PCL micelle to encapsulate hydrophobic Cur. The drug release study showed the nanoformulation of Cur significantly extended drug duration of Cur and had a prominent delayed drug release effect. Improved T1/2 and increased Cmax as well as the enlarged area under curve (AUC) by Nano-Cur and FA/Nano-Cur micelles were observed. FA/Nano-Cur not only possessed the outstanding properties of nanoscale MPEG-PCL micelles, such as improving water solubility, sustaining release, reducing systemic elimination, enhanced permeability and retention (EPR) effect, but also exhibited enhanced tumor-targeting capability, which further augmented the therapeutic efficacy. Taken together, the low toxicity of Cur and the selective delivery by FA conjugated nanoformulation would reduce adverse effects, compared with the systemic diffusion of chemotherapeutic drug.
Our results demonstrated Free Cur, Nano-Cur and FA/Nano-Cur were capable of inducing colon tumor cell apoptosis both in vitro and in vivo, and FA/Nano-Cur exhibited the most powerful effect. Cur has been disclosed to induce cell apoptosis via suppressing NF-κB and its associated pathways.44 Cur could also promote apoptosis through activating cancer suppressor gene p53 and down-regulating anti-apoptotic genes Bcl- 2/Bcl-xL as well as upregulating pro-apoptotic genes Bax.47 Furthermore, Cur was found to induce growth arrest and apoptosis by affecting Wnt and cell-cell adhesion pathway.43 In addition, we found the anti-proliferation effect of Free Cur and Cur nanoformulation by MTT assay and PCNA staining method. Research has reported Cur might suppress the expression of epidermal growth factor receptor (EGFR) by reducing Egr-1 activity in colon cancer cells, thus to restrain colon cancer cell growth.41 The tumor angiogenesis in FA/Nano-Cur treated group was significantly mitigated as well. Cur was reported to potentiate the anti-tumor effect of gemcitabine in an orthotopic model of pancreatic cancer by reducing CD31+ microvessel density.45 In summary, the results illustrated that FA/Nano-Cur treatment exhibited stronger anti-tumor ability in terms of promoting tumor cell apoptosis, inhibiting tumor cell growth and reducing tumor angiogenesis, compared with Nano-Cur treatment. Besides, no obvious systemic toxicity induced by FA/Nano-Cur injection was found through serological detection and pathomorphological observation.
So far, there are three ongoing clinical trials on Cur nanoformulation in the treatment of CRC and colorectal adenomatous polyps, indicating promising prospects of FA/Nano-Cur application in future.58 Still, great efforts are required to ascertain the therapeutic effect and toxicity of FA/Nano-Cur for human CRC therapy. Research has reported that Cur showed a synergistic effect with 5-fluorouracil in gastric cancer.59 Cur could also sensitize human CRC xenograft to gamma-radiation.60 We hope our study will provide a new aspect for CRC treatment and more research are warranted to investigate the therapeutic effect of FA/Nano-Cur on other malignant cancer types. The combination therapy of FA/Nano-Cur and other treatment is also an interesting and meaningful topic for future study.
Conclusion
We prepared a new injectable dosage form of Cur formulation by encapsulating Cur in FA modified MPEG-PCL micelles. Our preclinical study in the CRC mice model has shown that FA/Nano-Cur exhibited a significantly better therapeutic effect than Free Cur and Nano-Cur. FA/Nano-Cur treatment induced tumor cell apoptosis while significantly reduced the tumor proliferation and tumor angiogenesis, which might be attributed to improved bioavailability and enhanced tumor-targeting effect. Taken together, FA/Nano-Cur is a promising therapeutic agent for CRC treatment with low toxicity and better efficacy as well as good biodegradability.
Acknowledgment
This research was supported by the General Research Program of the Science and Technology Department of Sichuan Province (2018FZ0094) and the National Natural Science Foundation of China (81972347).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383(9927):1490–1502. doi: 10.1016/S0140-6736(13)61649-9 [DOI] [PubMed] [Google Scholar]
- 2.Center MM, Jemal A, Smith RA, Ward E. Worldwide variations in colorectal cancer. CA Cancer J Clin. 2009;59(6):366–378. doi: 10.3322/caac.20038 [DOI] [PubMed] [Google Scholar]
- 3.Center MM, Jemal A, Ward E. International trends in colorectal cancer incidence rates. Cancer Epidemiol Biomarkers Prev. 2009;18(6):1688–1694. doi: 10.1158/1055-9965.EPI-09-0090 [DOI] [PubMed] [Google Scholar]
- 4.Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67(3):177–193. doi: 10.3322/caac.21395 [DOI] [PubMed] [Google Scholar]
- 5.Chan DS, Aune D, Vieira R, Greenwood DC, Kampman E, Norat T. Red and processed meat and colorectal cancer incidence: meta-analysis of prospective studies. PLoS One. 2011;6(6):e20456. doi: 10.1371/journal.pone.0020456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116(3):544–573. doi: 10.1002/cncr.v116:3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fedirko V, Tramacere I, Bagnardi V, et al. Alcohol drinking and colorectal cancer risk: an overall and dose-response meta-analysis of published studies. Ann Oncol. 2011;22(9):1958–1972. doi: 10.1093/annonc/mdq653 [DOI] [PubMed] [Google Scholar]
- 8.Liang PS, Chen TY, Giovannucci E. Cigarette smoking and colorectal cancer incidence and mortality: systematic review and meta-analysis. Int J Cancer. 2009;124(10):2406–2415. doi: 10.1002/ijc.24191 [DOI] [PubMed] [Google Scholar]
- 9.Ma Y, Yang Y, Wang F, et al. Obesity and risk of colorectal cancer: a systematic review of prospective studies. PLoS One. 2013;8(1):e53916. doi: 10.1371/journal.pone.0053916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62(4):220–241. doi: 10.3322/caac.v62:4 [DOI] [PubMed] [Google Scholar]
- 11.Di Maio M, Basch E, Bryce J, Perrone F. Patient-reported outcomes in the evaluation of toxicity of anticancer treatments. Nat Rev Clin Oncol. 2016;13(5):319–325. doi: 10.1038/nrclinonc.2015.222 [DOI] [PubMed] [Google Scholar]
- 12.Ajazuddin, Alexander A, Qureshi A. Role of herbal bioactives as a potential bioavailability enhancer for active pharmaceutical ingredients. Fitoterapia. 2014;97:1–14. doi: 10.1016/j.fitote.2014.05.005 [DOI] [PubMed] [Google Scholar]
- 13.Aggarwal BB. Targeting inflammation-induced obesity and metabolic diseases by curcumin and other nutraceuticals. Annu Rev Nutr. 2010;30:173–199. doi: 10.1146/annurev.nutr.012809.104755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allegra A, Innao V, Russo S, Gerace D, Alonci A, Musolino C. Anticancer activity of curcumin and its analogues: preclinical and clinical studies. Cancer Invest. 2017;35(1):1–22. doi: 10.1080/07357907.2016.1247166 [DOI] [PubMed] [Google Scholar]
- 15.Anand P, Sundaram C, Jhurani S, Kunnumakkara AB, Aggarwal BB. Curcumin and cancer: an “old-age” disease with an “age-old” solution. Cancer Lett. 2008;267(1):133–164. doi: 10.1016/j.canlet.2008.03.025 [DOI] [PubMed] [Google Scholar]
- 16.Jagetia GC, Aggarwal BB. “Spicing up” of the immune system by curcumin. J Clin Immunol. 2007;27(1):19–35. doi: 10.1007/s10875-006-9066-7 [DOI] [PubMed] [Google Scholar]
- 17.Maheshwari RK, Singh AK, Gaddipati J, Srimal RC. Multiple biological activities of curcumin: a short review. Life Sci. 2006;78(18):2081–2087. doi: 10.1016/j.lfs.2005.12.007 [DOI] [PubMed] [Google Scholar]
- 18.Shin SK, Ha TY, McGregor RA, Choi MS. Long-term curcumin administration protects against atherosclerosis via hepatic regulation of lipoprotein cholesterol metabolism. Mol Nutr Food Res. 2011;55(12):1829–1840. doi: 10.1002/mnfr.201100440 [DOI] [PubMed] [Google Scholar]
- 19.Bar-Sela G, Epelbaum R, Schaffer M. Curcumin as an anti-cancer agent: review of the gap between basic and clinical applications. Curr Med Chem. 2010;17(3):190–197. doi: 10.2174/092986710790149738 [DOI] [PubMed] [Google Scholar]
- 20.Duvoix A, Blasius R, Delhalle S, et al. Chemopreventive and therapeutic effects of curcumin. Cancer Lett. 2005;223(2):181–190. doi: 10.1016/j.canlet.2004.09.041 [DOI] [PubMed] [Google Scholar]
- 21.Ide H, Tokiwa S, Sakamaki K, et al. Combined inhibitory effects of soy isoflavones and curcumin on the production of prostate-specific antigen. Prostate. 2010;70(10):1127–1133. doi: 10.1002/pros.v70:10 [DOI] [PubMed] [Google Scholar]
- 22.Kakarala M, Brenner DE, Korkaya H. Targeting breast stem cells with the cancer preventive compounds curcumin and piperine. Breast Cancer Res Treat. 2010;122(3):777–785. doi: 10.1007/s10549-009-0612-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ravindran J, Prasad S, Aggarwal BB. Curcumin and cancer cells: how many ways can curry kill tumor cells selectively? AAPS J. 2009;11(3):495–510. doi: 10.1208/s12248-009-9128-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soleimani V, Sahebkar A, Hosseinzadeh H. Turmeric (Curcuma longa) and its major constituent (curcumin) as nontoxic and safe substances: review. Phytother Res. 2018;32(6):985–995. doi: 10.1002/ptr.v32.6 [DOI] [PubMed] [Google Scholar]
- 25.Hewlings SJ, Kalman DS. Curcumin: a review of its’ effects on human health. Foods. 2017;6(10):E92. doi: 10.3390/foods6100092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu W, Zhai Y, Heng X, et al. Oral bioavailability of curcumin: problems and advancements. J Drug Target. 2016;24(8):694–702. doi: 10.3109/1061186X.2016.1157883 [DOI] [PubMed] [Google Scholar]
- 27.Yang KY, Lin LC, Tseng TY, Wang SC, Tsai TH. Oral bioavailability of curcumin in rat and the herbal analysis from Curcuma longa by LC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;853(1–2):183–189. doi: 10.1016/j.jchromb.2007.03.010 [DOI] [PubMed] [Google Scholar]
- 28.Hu Y, Wu C, Zhu C, et al. Enhanced uptake and improved anti-tumor efficacy of doxorubicin loaded fibrin gel with liposomal apatinib in colorectal cancer. Int J Pharm. 2018;552(1–2):319–327. doi: 10.1016/j.ijpharm.2018.10.013 [DOI] [PubMed] [Google Scholar]
- 29.Sinha R, Kim G, Nie S, Shin D. Nanotechnology in cancer therapeutics: bioconjugated nanoparticles for drug delivery. Mol Cancer Ther. 2006;5(8):1909–1917. doi: 10.1158/1535-7163.MCT-06-0141 [DOI] [PubMed] [Google Scholar]
- 30.Liu X, Li Y, Sun X, et al. Powerful anti-colon cancer effect of modified nanoparticle-mediated IL-15 immunogene therapy through activation of the host immune system. Theranostics. 2018;8(13):3490–3503. doi: 10.7150/thno.24157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Danafar H, Sharafi A, Kheiri Manjili H, Andalib S. Sulforaphane delivery using mPEG-PCL co-polymer nanoparticles to breast cancer cells. Pharm Dev Technol. 2017;22(5):642–651. doi: 10.3109/10837450.2016.1146296 [DOI] [PubMed] [Google Scholar]
- 32.Zamani M, Shirinzadeh A, Aghajanzadeh M, Andalib S, Danafar H. In vivo study of mPEG-PCL as a nanocarriers for anti-inflammatory drug delivery of simvastatin. Pharm Dev Technol. 2019;24(6):663–670. doi: 10.1080/10837450.2018.1556689 [DOI] [PubMed] [Google Scholar]
- 33.Gou M, Men K, Shi H, et al. Curcumin-loaded biodegradable polymeric micelles for colon cancer therapy in vitro and in vivo. Nanoscale. 2011;3(4):1558–1567. doi: 10.1039/c0nr00758g [DOI] [PubMed] [Google Scholar]
- 34.Gou M, Wei X, Men K, et al. PCL/PEG copolymeric nanoparticles: potential nanoplatforms for anticancer agent delivery. Curr Drug Targets. 2011;12(8):1131–1150. doi: 10.2174/138945011795906642 [DOI] [PubMed] [Google Scholar]
- 35.Lammers T, Kiessling F, Hennink WE, Storm G. Drug targeting to tumors: principles, pitfalls and (pre-) clinical progress. J Control Release. 2012;161(2):175–187. doi: 10.1016/j.jconrel.2011.09.063 [DOI] [PubMed] [Google Scholar]
- 36.Leamon CP, Reddy JA. Folate-targeted chemotherapy. Adv Drug Deliv Rev. 2004;56(8):1127–1141. doi: 10.1016/j.addr.2004.01.008 [DOI] [PubMed] [Google Scholar]
- 37.Antony AC. Folate receptors: reflections on a personal odyssey and a perspective on unfolding truth. Adv Drug Deliv Rev. 2004;56(8):1059–1066. doi: 10.1016/j.addr.2004.01.007 [DOI] [PubMed] [Google Scholar]
- 38.Elnakat H, Ratnam M. Distribution, functionality and gene regulation of folate receptor isoforms: implications in targeted therapy. Adv Drug Deliv Rev. 2004;56(8):1067–1084. doi: 10.1016/j.addr.2004.01.001 [DOI] [PubMed] [Google Scholar]
- 39.Liu X, Wang B, Li Y, et al. Powerful anticolon tumor effect of targeted gene immunotherapy using folate-modified nanoparticle delivery of CCL19 to activate the immune system. ACS Cent Sci. 2019;5(2):277–289. doi: 10.1021/acscentsci.8b00688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parker N, Turk MJ, Westrick E, Lewis JD, Low PS, Leamon CP. Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay. Anal Biochem. 2005;338(2):284–293. doi: 10.1016/j.ab.2004.12.026 [DOI] [PubMed] [Google Scholar]
- 41.Chen A, Xu J, Johnson AC. Curcumin inhibits human colon cancer cell growth by suppressing gene expression of epidermal growth factor receptor through reducing the activity of the transcription factor Egr-1. Oncogene. 2006;25(2):278–287. doi: 10.1038/sj.onc.1209019 [DOI] [PubMed] [Google Scholar]
- 42.Ghosh AK, Kay NE, Secreto CR, Shanafelt TD. Curcumin inhibits prosurvival pathways in chronic lymphocytic leukemia B cells and may overcome their stromal protection in combination with EGCG. Clin Cancer Res. 2009;15(4):1250–1258. doi: 10.1158/1078-0432.CCR-08-1511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jaiswal AS, Marlow BP, Gupta N, Narayan S. Beta-catenin-mediated transactivation and cell-cell adhesion pathways are important in curcumin (diferuylmethane)-induced growth arrest and apoptosis in colon cancer cells. Oncogene. 2002;21(55):8414–8427. doi: 10.1038/sj.onc.1205947 [DOI] [PubMed] [Google Scholar]
- 44.Kunnumakkara AB, Bordoloi D, Harsha C, Banik K, Gupta SC, Aggarwal BB. Curcumin mediates anticancer effects by modulating multiple cell signaling pathways. Clin Sci. 2017;131(15):1781–1799. doi: 10.1042/CS20160935 [DOI] [PubMed] [Google Scholar]
- 45.Kunnumakkara AB, Guha S, Krishnan S, Diagaradjane P, Gelovani J, Aggarwal BB. Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-kappaB-regulated gene products. Cancer Res. 2007;67(8):3853–3861. doi: 10.1158/0008-5472.CAN-06-4257 [DOI] [PubMed] [Google Scholar]
- 46.Patel BB, Sengupta R, Qazi S, et al. Curcumin enhances the effects of 5-fluorouracil and oxaliplatin in mediating growth inhibition of colon cancer cells by modulating EGFR and IGF-1R. Int J Cancer. 2008;122(2):267–273. doi: 10.1002/ijc.23097 [DOI] [PubMed] [Google Scholar]
- 47.Sa G, Das T. Anti cancer effects of curcumin: cycle of life and death. Cell Div. 2008;3:14. doi: 10.1186/1747-1028-3-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goel A, Boland C, Chauhan DP. Specific inhibition of cyclooxygenase-2 (COX-2) expression by dietary curcumin in HT-29 human colon cancer cells. Cancer Lett. 2001;172(2):111–118. doi: 10.1016/S0304-3835(01)00655-3 [DOI] [PubMed] [Google Scholar]
- 49.Zhang F, Altorki NK, Mestre JR, Subbaramaiah K, Dannenberg AJ. Curcumin inhibits cyclooxygenase-2 transcription in bile acid- and phorbol ester-treated human gastrointestinal epithelial cells. Carcinogenesis. 1999;20(3):445–451. doi: 10.1093/carcin/20.3.445 [DOI] [PubMed] [Google Scholar]
- 50.Cheng AL, Hsu CH, Lin JK, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21(4B):2895–2900. [PubMed] [Google Scholar]
- 51.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4(6):807–818. doi: 10.1021/mp700113r [DOI] [PubMed] [Google Scholar]
- 52.Schiborr C, Rimbach G, Frank J. A validated method for the quantification of curcumin in plasma and brain tissue by fast narrow-bore high-performance liquid chromatography with fluorescence detection. Anal Bioanal Chem. 2010;397(5):1917–1925. doi: 10.1007/s00216-010-3719-3 [DOI] [PubMed] [Google Scholar]
- 53.Kaminaga Y, Nagatsu A, Akiyama T, et al. Production of unnatural glucosides of curcumin with drastically enhanced water solubility by cell suspension cultures of Catharanthus roseus. FEBS Lett. 2003;555(2):311–316. doi: 10.1016/S0014-5793(03)01265-1 [DOI] [PubMed] [Google Scholar]
- 54.Zhang L, Zhu D, Dong X, et al. Folate-modified lipid-polymer hybrid nanoparticles for targeted paclitaxel delivery. Int J Nanomedicine. 2015;10:2101–2114. doi: 10.2147/IJN.S77667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zamani M, Rostamizadeh K, Manjili H, Danafar H. In vitro and in vivo biocompatibility study of folate-lysine-PEG-PCL as nanocarrier for targeted breast cancer drug delivery. Eur Polym J. 2018;103:260–270. doi: 10.1016/j.eurpolymj.2018.04.020 [DOI] [Google Scholar]
- 56.Cuong NV, Li YL, Hsieh MF. Targeted delivery of doxorubicin to human breast cancers by folate-decorated star-shaped PEG-PCL micelle. J Mater Chem. 2011;22(3):1006–1020. doi: 10.1039/C1JM13588K [DOI] [Google Scholar]
- 57.Feng R, Zhu W, Chu W, et al. Y-shaped folic acid-conjugated PEG-PCL copolymeric micelles for delivery of curcumin. Anticancer Agents Med Chem. 2017;17(4):599–607. doi: 10.2174/1871520616666160815124014 [DOI] [PubMed] [Google Scholar]
- 58.Wong KE, Ngai SC, Chan KG, Lee LH, Goh BH, Chuah LH. Curcumin nanoformulations for colorectal cancer: a review. Front Pharmacol. 2019;10:152. doi: 10.3389/fphar.2019.00152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koo JY, Kim HJ, Jung KO, Park KY. Curcumin inhibits the growth of AGS human gastric carcinoma cells in vitro and shows synergism with 5-fluorouracil. J Meds Food. 2004;7(2):117–121. doi: 10.1089/1096620041224229 [DOI] [PubMed] [Google Scholar]
- 60.Kunnumakkara AB, Diagaradjane P, Guha S, et al. Curcumin sensitizes human colorectal cancer xenografts in nude mice to gamma-radiation by targeting nuclear factor-kappaB-regulated gene products. Clin Cancer Res. 2008;14(7):2128–2136. doi: 10.1158/1078-0432.CCR-07-4722 [DOI] [PubMed] [Google Scholar]