Abstract
Mycophenolate mofetil (MMF) is an immunosuppressive medication used for the management of various autoimmune diseases, and patients with bone marrow and solid organ transplants. Gastrointestinal side effects are seen 45% of the time and they include nausea (29%), vomiting (23%), constipation (38%), diarrhea (50%-92%), and colitis (9%). In 98% of cases, resolution of diarrhea occurs within 20 days upon discontinuation of the MMF. Data is scarce regarding approach in the treatment of MMF-induced colitis. We report a case of MMF-induced colitis diagnosed by colonoscopy and histopathology. This case illustrates the challenges encountered while managing MMF-induced colitis.
Keywords: colitis, mycophenolate induced colitis, crypt cell apoptosis, drug induced colitis, ulcerative colitis, inflammatory bowel disease (ibd)
Introduction
Mycophenolate mofetil (MMF) is an immunosuppressive medication commonly used to prevent rejection in solid organ transplant recipients. Active metabolite of MMF, mycophenolic acid, inhibits inosine monophosphate dehydrogenase which is the rate-limiting enzyme in purine synthesis for T and B-cell proliferation [1]. Enterocytes are 50% dependent on the de novo pathway of purine synthesis which is why they are vulnerable to MMF’s antimetabolic effects; this impedes the growth and replication of small bowel epithelial cells which leads to disruption of fluid absorption and diarrhea [2]. Injurious effects of MMF can be detected in the colon and include mucosal changes ranging from edema, erythema, erosions, and ulcerations. Histopathologic findings of MMF injury include crypt architectural distortion and crypt cell apoptosis [2]. The latency period between initiation of MMF exposure and onset of enterocolitis is between six months to 15 years with the average being around three years [3].
Case presentation
A 68-year-old male with a history of recurrent deep vein thrombosis on warfarin, single lung transplant secondary to idiopathic pulmonary fibrosis on MMF (1000 mg twice a day) for eight months prior to admission, diverticulitis and recurrent diverticular bleed complicated by sigmoidectomy and right colectomy with ileo-colonic anastomosis, respectively, presented with 6-8 episodes of maroon-colored stools daily for the past two months. Over this time, the patient had several admissions at different hospitals requiring blood transfusions and endoscopic evaluations. At an outside hospital, esophagogastroduodenoscopy (EGD) demonstrated esophageal, gastric, and duodenal ulcers. He was treated with oral mesalamine for presumed inflammatory bowel disease. The patient was referred to the hospital for a second opinion after his symptoms did not improve.
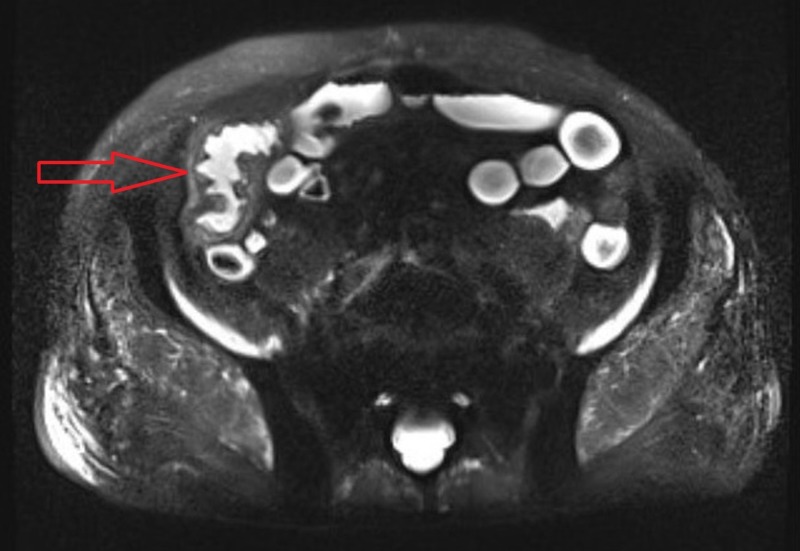
Physical examination was significant for a chronically ill-appearing man with mild tenderness in the right lower quadrant of the abdomen and maroon colored stools on digital rectal exam. The rest of the examination was unremarkable. Laboratory studies revealed a hemoglobin level of 9.4 g/dL (normal range, 13.5-17.5 g/dL), iron 36 ug/dL (normal range, 41-186 ug/dL), ferritin 134.5 ng/mL (normal range, 30 - 565 ng/mL), and creatinine of 1.64 mg/dL (normal range, 0.73 - 1.22 mg/dL). Electrolytes, liver function, blood cytomegalovirus DNA, stool for ova & parasites, interferon-gamma release assay, and stool Clostridium difficile antigen were negative. A magnetic resonance enterography (MRE) of the abdomen and pelvis demonstrated small bowel wall thickening, mural hyper-enhancement, and peri-enteric stranding involving a 10-cm segment of the distal terminal ileum extending to the ileo-colonic anastomosis (Figure 1).
Figure 1. Magnetic resonance enterography depicting small bowel wall thickening, mural hyper enhancement and peri-enteric stranding involving 10-cm segment of the distal terminal ileum (red arrow).
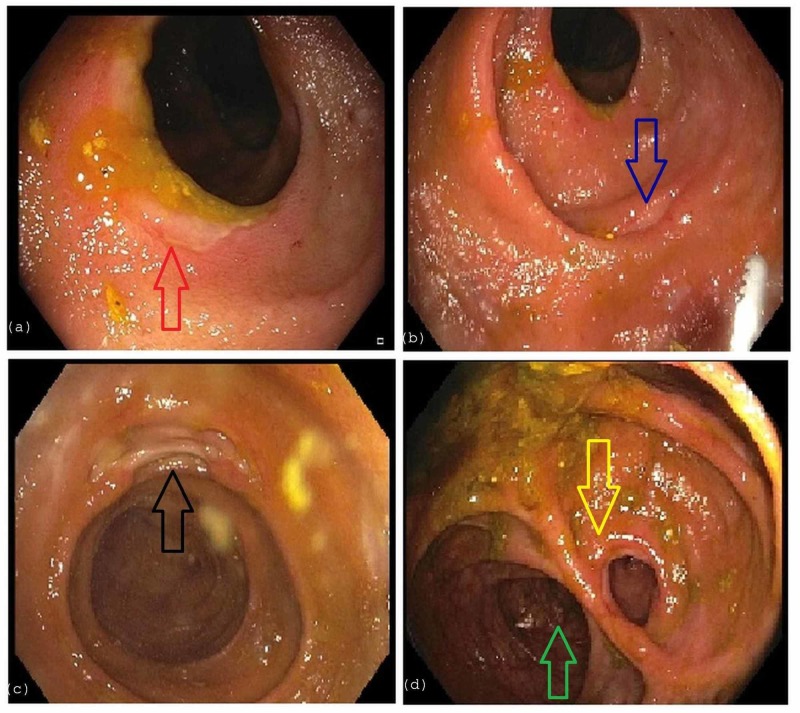
No active inflammation in the colon or rectum was noted. An EGD demonstrated a normal esophagus, stomach, and duodenum. Colonoscopy revealed numerous ulcers in the ascending colon, ileo-colonic anastomosis, and in the distal 15-cm of the neo-terminal ileum with normal-appearing intervening mucosa (Figure 2).
Figure 2. Colonoscopy.
a) depicts ulceration (red arrow) in the ascending colon. b,c) depict ulceration in the distal 15 cm of the neo-terminal ileum (blue and black arrows) with normal-appearing intervening mucosa. d) visualizes anastomosis of the distal ileum (yellow arrow) to the transverse colon (green arrow).
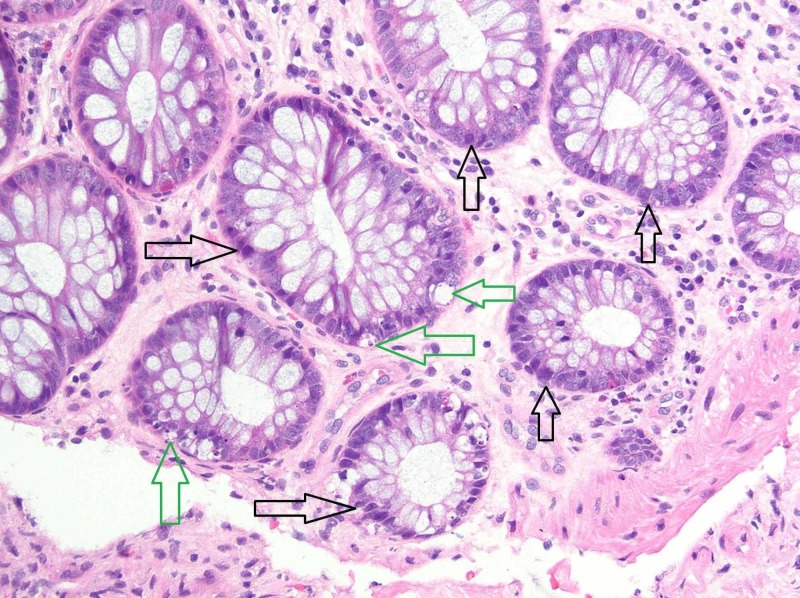
Biopsy of the ulcers with immunohistochemical staining for cytomegalovirus was negative. Histology revealed mild crypt architectural distortion with crypt cell apoptosis from the ascending colonic ulcers and patchy active ileitis from the ileo-colonic anastamotic ulcerations (Figure 3).
Figure 3. Photomicrograph from the colon biopsy showing architectural distortion with unevenly spaced lumen and crypts.
Several damaged crypts (black arrows) are present, scattered throughout the colonic mucosa (hematoxylin and eosin (H&E), original magnification x100). Also present are apoptotic bodies (green arrows) suggestive of cellular injury and turnover (H&E, original magnification x100). There is no evidence of active inflammation or viral cytopathic effect.
In view of MMF exposure and histopathologic findings of crypt architectural distortion and crypt cell apoptosis, our patient was diagnosed with MMF-induced enterocolitis and MMF was promptly discontinued.
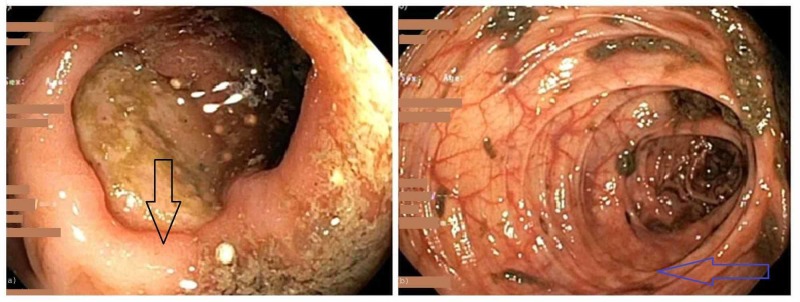
Seventy-two hours after discontinuation of MMF, the patient continued to experience bloody diarrhea requiring RBC transfusions. Intravenous (IV) Solumedrol 20 mg every eight hours was initiated with mild improvement in the frequency of diarrhea. After five days of IV Solumedrol therapy, a repeat colonoscopy demonstrated evidence of complete healing of the numerous small ulcerations and improvement in the appearance of the large ulcerations, suggestive of overall endoscopic improvement (Figure 4).
Figure 4. Repeat colonoscopy after five days of intravenous steroids showing significant mucosal improvement of the ascending colon (4a - black arrow), and transverse colon (4b - blue arrow).
The patient was transitioned to oral steroids and steadily experienced clinical improvement. Unfortunately, the patient’s hospital stay was complicated by hypoxic respiratory failure and he succumbed to death from aspiration pneumonia.
Discussion
MMF is a commonly prescribed adjunct immunosuppressive agent in transplant therapy. Up to 45% of patients report diarrhea, vomiting, and abdominal pain; diarrhea being the most commonly reported side effect [4,5]. As an immunosuppressant, MMF can affect the entire gastrointestinal system leading to clinical complications ranging from esophagitis, gastroesophageal reflux disease, to enteritis and colitis. Development of diarrhea due to enteritis and/or colitis can be difficult to recognize as only 2%-9% of patients on MMF develop these complications [5,6]. Pathology can resemble inflammatory bowel disease, graft-versus-host disease (GVHD), acute colitis or ischemia [6]. There are treatment differences for each condition which is why obtaining biopsies is recommended to differentiate between these etiologies [7]. Specific histologic features of MMF related colitis include: crypt architectural disarray, increased lamina propria inflammation, dilated damaged crypts, increased crypt epithelial apoptosis and GVHD-like changes [8].
No guidelines are available to guide clinicians to treat MMF-induced enterocolitis. Several case reports have demonstrated that diarrhea improves within three to five days of discontinuing MMF [9-10]. One systemic review revealed that in 98% of the cases, diarrhea resolves within 20 days upon discontinuation of the MMF [3]. If symptoms are persistent despite MMF discontinuation, prednisone and/or infliximab has shown improvement.
Table 1 lists the cases of MMF induced colitis and how these patients were managed [10-19]. In 12 of the 13 case reports found, patient's symptoms improved after lowering the offending agent dose or discontinuing the medication. Out of the 13, only two patients were already on steroids [13,14]. Out of the 13 cases, six of them underwent repeat colonoscopy at different times from the intervention to assess for healing [14-19]. Bouhbouh et al. gave a single infusion of 5 mg/kg of infliximab after previous futile attempts with MMF discontinuation, and 50 mg of prednisolone IV daily for two weeks [11]. Within three days, after a single infusion of infliximab, the stool frequency dropped significantly [11].
Table 1. Illustrates reported cases of mycophenolate-induced colitis to date with different management strategies that have been used; the table also indicates the timing of symptom improvement from the intervention.
Important to note that all patients underwent colonoscopy and/or flexible sigmoidoscopy for tissue pathology.
| Mycophenolate mofetil (MMF) dosing | Main symptom | Endoscopic findings | Histologic findings | Steroids given? (dosing) | Infliximab Given? (dosing) | Timing of symptom improvement | |
| Bouhbouh (2010) | 500mg BID | Watery, non-bloody diarrhea, abdominal pain, weight loss | Linear ulcerations throughout colon | Extensive ulceration with transmural mixed-cellular infiltration without granulomata | Yes. 2 weeks of Prednisone 30 mg PO daily, followed by 2 weeks of 25 mg prednisolone IV BID | Yes (5mg/kg) | 72 hours after Infliximab |
| Johal (2014) | 1,500 mg BID | Watery, non-bloody diarrhea, abdominal pain, weight loss | Segmental erythematous mucosa with ulcers in sigmoid, descending, splenic flexure and proximal transverse colon | Dilated crypts, eosinophilic epithelial changes, crypt abscesses with apoptotic bodies | No | No | 5 weeks following MMF cessation |
| Goyal (2016) | Not provided | Watery, non-bloody diarrhea, abdominal tenderness and distention | Normal mucosa | Crypt atrophy, increased crypt apoptosis | No | No | 3 days following MMF cessation |
| Jakes (2012) | 750 mg bid | Abdominal pain and weight loss | Patchy inflammation of ascending colon, ileocecal valve was grossly thickened, stenosed, and ulcerated, consistent with a Crohn’s-like disease process. | Extensive ulceration | No | No | 8 weeks following MMF reduction first to 250 mg bid and eventually discontinuing. Pt also underwent ex-lap s/p right hemicolectomy with no evidence of inflammatory changes within small or large bowel |
| Jakes (2012) | 750 mg bid | Watery, non bloody diarrhea with large mucus | Severe pancolitis | Noncaseating granulomas within the lamina propria consistent with Crohns Disease | No | No | Resolution of colitis after MMF cessation, duration unknown |
| Jakes (2012) | 180 mg bid | Profuse watery, non bloody diarrhea with right lower quadrant abdominal tenderness | Pancolitis with rectal sparing | Focal active colitis, no granulomas. | No | No | 8 months after discontinuation of Myfortic, patient had sigmoidoscopy which showed no active inflammation. Unknown when patient noted improvement in symptoms |
| Moroncini (2018) | Not provided but started 2 months ago | Left sided abdominal pain, nausea, vomiting, and fever | Mucosal hyperemia, multiple serpiginous ulcers involving the transverse and descending colonic mucosa, with rectal sparing | ulceration, granulation tissue and hyalinised appearance of the mucosa and submucosa | No | No | 5 days following MMF discontinuation. Repeat colonoscopy 1 month later showed complete resolution of ulcer |
| Tayyem (2018) | 500 mg bid and Prednisone 15 mg daily | non-bloody diarrhea, dysphagia to solid food, nausea and unintentional weight loss of 2 weeks’ duration. | EGD: normal oesophagus, multiple small antral ulcers and reactive gastropathy. Colonoscopy: mucosal edema and erythema with small mucosal hemorrhages and punctate ulcerations in the ascending colon, patchy colitis in the transverse colon and rectal sparing | Colonic biopsies showed focal crypt abscesses (withered crypts) with occasional apoptosis of epithelial cells, frequent tingible body macrophages and eosinophils within the lamina propria | Patient was already on Prednisone 15 mg daily | No | 5 weeks after MMF discontinuation |
| Gorospe (2012) | 1000 mg bid | 2-week history of profuse, watery diarrhoea that persisted through the night and with fasting | Flexible sigmoidoscopy showed mild erythema | apoptosis, crypt distortion and abscess; consistent with MMF-induced colitis | No | No | Five days later, the patient’s stool frequency decreased to twice daily until complete resolution. At 1 month follow-up, her MMF was restarted at a lower dose (500 mg/day) which was tolerated well without any recurrence of gastrointestinal issues. |
| Hamouda (2012) | Prednisone and MMF. Dosages not known | Profuse watery diarrhea, 6 to 8 times per day and weight loss | ulcerative diffuse colitis from the cecum to the rectum | mild crypt architectural distortion (Figure 1). The lamina propria showed edema and an increased number of inflammatory cells containing many neutrophils. Damaged crypts with mucus depletion and cryptitis. No granuloma | No | No | Symptoms regressed within 5 days after switching from MMF to azathioprine. Control colonoscopy showed reparative changes after 2 months |
| Kim (2000) | Dose not known but between 2 to 3 gm daily. | abdominal pain and watery diarrhea which progressed to bloody diarrhea | multiple ulcers and mucosal hyperemia and edema in the entire colon | Histology did not reveal viral cytopathic changes and immunohistochemical stains for cytomegalovirus infection were negative. | Patient was already on steroids | No | Abdominal pain and hematochezia improved rapidly. Follow-up colonoscopy 1 month later showed complete healing of previous lesions |
| Johal (2014) | 1000 mg bid and increased to 1500 mg bid four months prior to presentation | Abdominal pain, nausea, intermittent bloating and profuse watery non bloody diarrhea. | segmental erythematous mucosa and multiple ulcers in the sigmoid colon, descending colon, splenic flexure and proximal transverse colon | dilated damaged crypts, eosinophilic epithelial changes and crypt abscesses with apoptotic bodies, a pattern of injury highly suggestive of MMF-related colitis | No | No | 5 weeks after MMF discontinuation |
| Sonoda (2017) | 1gm daily | Watery diarrhea which progressed to bloody diarrhea | multiple deep ulcers in the ileum | mild crypt distortion | No | No | Symptoms improved soon after MMF was discontinued. Six months later, the ileal mucosa was healed |
| (Patra) 2012 | Not provided | Significant weight loss, sitophobia for five months, and a recent onset of bleeding per rectum | Colonoscopy demonstrated ileal and cecal ulcers | Histopathology revealed crypt dropout, with focal disarray of the crypt architecture, along with apoptosis of the crypt epithelial cells. The crypt epithelial apoptotic rate was greater than 5 / 100 crypts. The lamina propria was edematous and showed focal collection of mild lymphomononuclear inflammatory cell infiltrate | Patient already on steroids, unknown dose | No | 1 week after MMF cessation. Repeat colonoscopy after 1 month showed healing ulcers |
The mucosal injury from MMF is thought to be related to the formation of immunotoxicologic reactions in the bowel, increased mucosal inflammation, and decreased mucosal protection [20]. The decreased mucosal protection is hypothesized secondary to the upregulation of intracellular phosphatidylcholines, prominent membrane phospholipid that maintains gastrointestinal barrier function, leading to disruption in membrane phospholipids and subsequently decreased mucosal defense [20]. One of the postulated mechanisms for mucosal inflammation is that the byproduct, acyl gluconoride, causes local irritation of the epithelium which then stimulates mononuclear cells to release tumor necrosis factor (TNF)- α, subsequently impacting the development of mucosal inflammation [20].
Conclusions
Since the advent of post-transplant immunosuppression therapy, MMF-induced enterocolitis is uncommon with debilitating complications; limited data is available in the literature regarding the approach to treatment. There continues to be unanswered questions as to why some patients’ have refractory colitis, the benefits of oral or IV steroids, or biologic therapy (i.e. Infliximab), and the need for endoscopic reassessment for mucosal healing. With more cases being reported, we can better understand the natural course of the disease and help identify some of the answers. It is also prudent for physicians to inform the pathologist when a patient is on mycophenolate so the pathologist can be mindful of drug-induced colitis in the differential in addition to inflammatory bowel disease and GVHD.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained by all participants in this study
References
- 1.Mycophenolate mofetil and its mechanisms of action. Allison AC, Eugui EM. Immunopharmacology. 2000;47:85–118. doi: 10.1016/s0162-3109(00)00188-0. [DOI] [PubMed] [Google Scholar]
- 2.Importance of apoptosis in the histopathology of drug related lesions in the large intestine. Lee FD. J Clin Pathol. 1993;46:118–122. doi: 10.1136/jcp.46.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mycophenolate mofetil (MMF)-induced colitis. Dhakal P, Gami R, Giri S, Bhatt VR. Blood. 2016;128:4795. [Google Scholar]
- 4.Adverse gastrointestinal effects of mycophenolate mofetil. Behrend M. https://link.springer.com/article/10.2165/00002018-200124090-00002. Drug. 2001;24:645–663. doi: 10.2165/00002018-200124090-00002. [DOI] [PubMed] [Google Scholar]
- 5.Mycophenolate-induced colitis. Farooqi R, Kamal A, Thind K, Burke C. https://journals.lww.com/ajg/Fulltext/2018/10001/Mycophenolate_Induced_Colitis__1546.1546.aspx Am J Gastroenterol. 2018;113:0. [Google Scholar]
- 6.Endoscopic and histological features of mycophenolate mofetil colitis in patients after solid organ transplantation. Calmet FH, Yarur AJ, Pukazhendhi G, Ahmad J, Bhamidimarri KR. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4480174/ Ann Gastroenterol. 2015;28:366–373. [PMC free article] [PubMed] [Google Scholar]
- 7.Spectrum of histologic changes in colonic biopsies in patients treated with mycophenolate mofetil. Selbst MK, Ahrens WA, Robert ME, Friedman A, Proctor DD, Jain D. https://www.nature.com/articles/modpathol200944. Mod Pathol. 2009;22:737–743. doi: 10.1038/modpathol.2009.44. [DOI] [PubMed] [Google Scholar]
- 8.Mycophenolate mofetil-related gastrointestinal mucosal injury: variable injury patterns, including graft-versus-host disease-like changes. Parfitt JR, Jayakumar S, Driman DK. https://www.ncbi.nlm.nih.gov/pubmed/18763324. Am J Surg Pathol. 2008;32:1367–1372. doi: 10.1097/pas.0b013e31816bf3fe. [DOI] [PubMed] [Google Scholar]
- 9.Mycophenolate mofetil- related colitis: a case report. Kim K, Gardner JM, Schwartz M, Tompson ML, Ro JY. Korean J Pathol. 2010;44:333–337. [Google Scholar]
- 10.A unique case of mycophenolate induced colitis after 10 years of use. Goyal A, Salahuddin M, Govil Y. Case Rep Gastrointest Med. 2016:1–3. doi: 10.1155/2016/3058407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rapid resolution of persistent mycophenolate mofetil-induced diarrhea with a single dose of infliximab. Bouhbouh S, Rookmaaker MB. Nephrol Dial Transplant. 2010;25:3437–3438. doi: 10.1093/ndt/gfq379. [DOI] [PubMed] [Google Scholar]
- 12.Mycophenolate mofetil-induced segmental colitis mimicking ischemic colitis. Johal K, Ratuapli SK, Lam-Himlin DM, Gurudu SR. Case Rep Gastroenterol. 2014;8:95–100. doi: 10.1159/000360847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heart transplant recipient with mycophenolate mofetil-induced colitis: the great imitator. Tayyem O, Saraireh H, Al-Hanayneh M, Stevenson HL. BMJ Case Rep. 2018 doi: 10.1136/bcr-2017-224035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mycophenolate mofetil-induced colitis with graft versus host disease-like features in a liver transplant recipient. Patra S, Vij M, Sukanya B, Kapoor D. Indian J Pathol Microbiol. 2012;55:506–508. doi: 10.4103/0377-4929.107792. [DOI] [PubMed] [Google Scholar]
- 15.Case report: Crohn's-like mycophenolate-induced colitis, a fallout in steroid-free regimens. Jakes AD, Roy A, Veerasamy M, Bhandari S. Transplant Proc. 2013;45:842–844. doi: 10.1016/j.transproceed.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 16.Mycophenolate mofetil-induced colitis in a patient with systemic sclerosis. Moroncini G, Benfaremo D, Mandolesi A, Gabrielli A. BMJ Case Rep. 2018 doi: 10.1136/bcr-2018-224829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chronic diarrhoea from mycophenolate mofetil-induced colitis. Gorospe EC. BMJ Case Rep. 2012 doi: 10.1136/bcr.12.2011.5344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deep ulcers in the ileum associated with mycophenolate mofetil. Sonoda A, Wada K, Mizukami K, et al. Intern Med. 2017;56:2883–2886. doi: 10.2169/internalmedicine.8815-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mycophenolate mofetil-related pancolitis in a kidney transplant recipient. Hamouda M, Mahmoudi H, Skhiri H, Elmay M. Exp Clin Transplant. 2012;10:501–505. doi: 10.6002/ect.2011.0200. [DOI] [PubMed] [Google Scholar]
- 20.Gastrointestinal side effects of mycophenolic acid in renal transplant patients: a reappraisal. Davies NM, Grinyó J, Heading R, Maes B, Meier-Kriesche H, Oellerich M. Nephrol Dial Transplant. 2007;22:2440–2448. doi: 10.1093/ndt/gfm308. [DOI] [PubMed] [Google Scholar]