Abstract
Pancreatic ductal adenocarcinoma (PDAC) is a disease of aging. The TP53 gene product regulates cell growth, aging, and cancer. To determine the important targets of TP53 in PDAC, we examined the expression of 440 proteins on a reverse phase protein array (RPPA) in PDAC-derived MIA-PaCa-2 cells which either had WT-TP53 or lacked WT-TP53. MIA-PaCa-2 cells have a TP53 mutation as well as mutant KRAS and represent a good in vitro model to study PDAC. RPPA analysis demonstrated expression of tumor promoting proteins in cells that lacked WT-TP53; and this feature could be reversed significantly when the cells were transfected with vector encoding WT-TP53 or treated with berberine or a modified berberine (BBR). Expression of miR-34a-associated signaling was elevated in cells expressing WT-TP53 compared to cells expressing mTP53. Results from in vivo studies using human PDAC specimens confirmed the in vitro results as the expression of miR-34a and associated signaling was significantly decreased in PDAC specimens compared to non-cancerous tissues. This study determined SERPINE1 as a miR-34a target with relevance to the biology of PDAC. Thus, we have identified a key target (SERPINE1) of the TP53/miR-34a axis that may serve as a potential biomarker for early detection of pancreatic cancer.
Keywords: PDAC, Aging, cancer, TP53, miR-34a, SERPINE1
INTRODUCTION
The risk of developing cancer of the pancreas increases with age; it was estimated that only 13% of all patients with pancreatic cancer are diagnosed before the age of 60 [1]. The increasing incidence and mortality from pancreatic ductal adenocarcinoma (PDAC) are medical issues of paramount importance [2, 3]. Current treatments combining surgical resection and chemotherapy are only minimally effective [4, 5]. In most cases, by the time PDAC is diagnosed, it has already spread to distant sites, making treatment an impossible task. PDAC is the ninth most common cancer in the USA, has the highest mortality of any cancer, and will soon be the second most common cause of cancer death in USA [6, 7].
Two of the key genes involved in the development of PDAC are KRAS and TP53 [8]. KRAS (activation) mutations occur in about 90% of PDAC while TP53 (inactivation) mutations occur in approximately 75% of pancreatic cancers [9]. Apart from mutations in these genes, host cell microRNAs (miRNAs) also have crucial roles to play in various biological processes, including: inflammation, cell growth, aging, differentiation, proliferation, and metastasis [10, 11]. Increasing evidence in recent years suggests that miRNAs control the development and progression of inflammation and cancer [12–15]. In this study we focused on miR-34a over other miRNAs because of the following reasons: (i) Expression of miR-34a is significantly down-regulated or absent in a variety of cancers including hepatocellular and renal cell carcinomas, colon, breast, lung, prostate, ovarian, and pancreatic cancers [16–22]; (ii) The two major oncogenes that are mutated in PDAC are KRAS and TP53 [23]; (iii) TP53 directly transactivates miR-34a expression [24] while mutated KRAS indirectly lowers expression of miR-34a via the transcription factor, ZEB1 [25, 26]. Therefore, inactivation of TP53 and increases in mutated KRAS expression result in a sharp decline in miR-34a expression during tumorigenesis.
The miR-34 family contains three members and is encoded by two genes located on chromosomes 1 and 11 [27]. The mature miR-34a shares 86% identity (19/22 nt) with miR-34b and 82% identity (18/22 nt) with miR-34c, respectively. The position 2-9 adjacent at the 5' end (8 nt) is considered the “seed region” for all three members [27–29]. Among these members, miR-34a is expressed at higher levels than miR-34b/c, with the exception of the lung [30].
miR-34a is a key regulator of tumor suppression and is considered to have a broad anti-oncogenic activity [30]. We hypothesize miR-34a to play a major role in the development of PDAC. As of this date, there are limited investigations conducted to understand the roles of miR-34a in the biology of PDAC. Therefore, the focus of this study was to decipher a potential role for TP53>miR-34a-associated signaling in pancreatic cancer using in vitro and in vivo models. Our study determined a decrease in the expression of miR-34a in human PDAC specimens. Using in vitro and in vivo approaches, we ascertained SERPINE1 to be a target of miR-34a and their patho-physiological significance is discussed.
RESULTS
Profiling of tumor promoting and suppressor proteins in response to expression of wild-type TP53 in MIA-PaCa-2 cells
RPPA assay was performed to elucidate the effects of expressing WT-TP53 in MIA-PaCa-2 cells. The crucial step prior to performing the RPPA assay was to characterize the MIA-PaCa-2 cells used in this study. This is important as these cells expressing the mTP53 and WT-TP53 form the basis for the in vitro experiments conducted in this study. The MIA-PaCa-2+WT-TP53 cells were more sensitive to the chemotherapeutic drugs compared to MIA-PaCa-2+pLXSN cells (Supplementary Figure 1). Similar results have been reported by earlier studies [23, 31–33]. The above results authenticate the physiological effects of expressing different forms of TP53 and associated cell signaling. RPPA is a high-throughput technology based on the detection of proteins along with their post-translational protein modifications, e.g., cleavage and phosphorylation [34]. To this end, we performed RPPA using a selection of 446 antibodies (Supplementary Table 1). RPPA analysis revealed a mTP53-dependent modulation of multiple cell signaling molecules involved in cell proliferation and survival (Figure 1A). Further, the analysis documented an increase and decrease in the expression of specific proteins that promoted tumor formation (Table 1) in MIA-PaCa-2 cells with mutated TP53 (MIA-PaCa-2+pLXSN) compared to MIA-PaCa-2 cells expressing WT-TP53 (MIA-PaCa-2+WT-TP53). The expression of proteins in parental MIA-PaCa-2 untransfected cells followed a similar pattern as expressed in MIA-PaCa-2+pLXSN cells (data not shown).
Figure 1.
Changes in protein expression profile in MIA-PaCa-2 cells expressing pLXSN compared to WT-TP53. (A) Protein expression was assayed by RPPA. Proteins indicated in red and green denotes increased and decreased expression, respectively. Genes in red and green indicate tumor promoting and suppressor activities, respectively. (B) Schematic demonstrating cell signaling in MIA-PaCa-2+pLXSN cells promoting cell survival (in red) while significantly inhibiting apoptosis (in green).
Table 1. RPPA analysis demonstrating the tumor promoting milieu in MIA-PaCa-2+pLXSN cells compared to MIA-PaCa-2+WT-TP53 cells.
| Protein name, and phosphorylation status | Gene symbol | Function | GenBank accession no. | Fold change in protein expression |
| INCREASE IN EXPRESSION: | ||||
| AKT serine/threonine kinase 2 (AKT2) | AKT2 | Promotes cancer formation | AAI20996.1 | 2.0 |
| Cyclin dependent kinase 1 (CDK1_pT14)) | CDK1 | Promotes cell division | NP_001777.1 | 2.8 |
| Connexin-43 (Cx43) | GJA1 | Correlates with cancer metastasis | AAA52131.1 | 5.0 |
| Cyclin-B1 | CCNB1 | Promotes cell survival | EAW51306.1 | 2.4 |
| Dual specificity phosphatase 6 (DUSP6) | DUSP6 | Drives poor prognosis in cancer | BAA34369.1 | 3.2 |
| Glycogen synthase kinase 3α/β (GSK-3α/β_pS21_S9) | GSK-3α/β | Promotes cell growth & invasion | NP_063937.2 | 2.1 |
| Minor histocompatibility protein HA-1 (HMHA1) | HMHA1 | Induces cell spread | AAH48129.1 | 5.3 |
| mitogen-activated protein kinase kinase kinase 9 (MLK1) | MLK1 | Induces necroptosis | AAB26359.1 | 2.7 |
| Protein kinase-β II (PKC-β-II_pS660) | PRKCB | Promotes signaling to cause cancer | P05771.4 | 2.0 |
| Pyruvate kinase M1/2 (PKM2) | PKM2 | Drives poor prognosis in cancer | AAH94767.1 | 2.1 |
| Polo like kinase 1 (PLK1) | PLK1 | Promotes proliferation and suppress apoptosis | NP_005021.2 | 3.1 |
| Retinoblastoma protein (Rb_pS807_S811) | Rb1 | Phosphorylation of Rb inactivates the protein | AAH40540.1 | 2.7 |
| Ribonucleotide reductase regulatory subunit M2 (RRM2) | RRM2 | Drives poor prognosis in cancer | NP_001025.1 | 2.4 |
| 40S ribosomal protein S6 (S6_pS235_S236) | S6 | Promotes cell survival | NP_001001.2 | 3.4 |
| 40S ribosomal protein S6 (S6_pS240-S244) | S6 | Promotes cell survival | NP_001001.2 | 3.8 |
| SMAD family member 1 (SMAD1) | SMAD1 | A crucial role in development of cancer | AAC50790.1 | 2.0 |
| Vascular endothelial growth factor receptor-2 (VEGFR-2) | VEGFR-2 | Induces angiogenesis | P35968.2 | 2.5 |
| DECREASE IN EXPRESSION: | ||||
| NAD(P)H quinone dehydrogenase 1 | NQO1 | Regulates autophagy | AAI07740.1 | 0.3 |
| p21 | P21 | Tumor suppressor | AAB29246.1 | 0.5 |
| Serum/Glucocorticoid Regulated Kinase 1 (SGK1) | SGK1 | Inhibits cancer cell invasion and migration | AAH01263.1 | 0.4 |
| von Hippel-Lindau tumor suppressor (VHL) | VHL | Tumor suppressor | AAH58831.1 | 0.4 |
Expression of DNMT1, S6 (phosphorylated on serine residues at 240 and 244), and GSK-3α/3β (phosphorylated on serine residue at 21 of GSK3α or serine 9 of GSK-3β) were elevated in MIA-PaCa-2 cells with mTP53 (MIA-PaCa-2+pLXSN) (Table 2) and MIA-PaCa-2 cells (data not shown). On the same lines, expression of Bax, cleaved caspase-3, and cleaved caspase-8 were down-regulated in MIA-PaCa-2 cells expressing WT-TP53 (MIA-PaCa-2+WT-TP53) (Table 2). Thus, the cellular events seem to promote cell survival while actually inhibiting apoptosis in cells expressing mTP53 (Figure 1B). RPPA analysis demonstrated a crucial role for the WT-TP53 in mediating anti-tumor activity via modulating cell signaling.
Table 2. RPPA analysis demonstrating changes in the expression of proteins that promote cell survival while decreasing apoptosis in MIA-PaCa-2+pLXSN cells compared to MIA-PaCa-2+WT-TP53 cells.
| Protein name, and phosphorylation status | Gene symbol | Function | GenBank accession no. | Fold change in protein expression |
| PROMOTING CELL SURVIVAL: | ||||
| DNA methyltransferase 1 (DNMT1) | DNMT1 | Promotes cell survival | AAI26228.1 | 2.4 |
| 40S ribosomal protein S6 (S6_pS240-S244) | S6 | Promotes cell survival | NP_001001.2 | 3.8 |
| Glycogen synthase kinase 3α/β (GSK-3α/β_pS21_S9) | GSK-3α/β | Promotes cell survival | NP_063937.2 | 2.1 |
| DECREASING TUMOR SUPPRESSION: | ||||
| BCL2 associated X, apoptosis regulator (BAX) | BAX | Promotes apoptosis | Q07812.1 | 0.3 |
| Cleaved caspase-3 (Caspase-3) | CASP3 | Promotes apoptosis | CAC88866.1 | 0.4 |
| Cleaved caspase-8 (Caspase-8) | CASP8 | Promotes apoptosis | BAB32555.1 | 0.3 |
Effect of treating MIA-PaCa-2 cells with BBR and MBBR on cell division, proliferation, survival, migration, and apoptosis
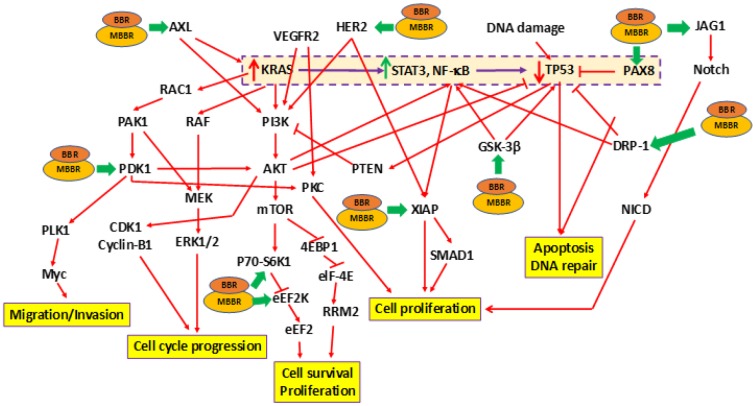
Earlier studies by us determined that BBR and MBBR inhibited proliferation of pancreatic cancer cells [31, 32]. In the current study, we determined the effect of treating MIA-PaCa-2+pLXSN cells with BBR and MBBR (NAX060) on cell signaling using RPPA. Treatment of MIA-PaCa-2+pLXSN cells (carrying mTP3) with BBR and MBBR altered the expression of 11 proteins to varying extents (Table 3). Each of these proteins influence tumorigenesis by regulating cell cycle progression, survival, proliferation, apoptosis and DNA repair. The effects of BBR and MBBR on the proliferation of MIA-PaCa-2+pLXSN cells is presented in the schematic (Figure 2). The schematic also represents the manner by which BBR and MBBR may directly or indirectly alter the expression of mTP53-associated signaling molecules (Figure 1A). RPPA analysis demonstrated the ability of BBR and MBBR to promote anti-tumor activity in MIA-PaCa-2+pLXSN and MIA-PaCa-2 (data not shown) cells by inhibiting cell cycle progression, proliferation, and survival to varying extents.
Table 3. RPPA analysis demonstrating the fold change in activity of proteins in response to treating MIA-PaCa-2+pLXSN cells with BBR and MBBR.
| Protein name, and phosphorylation status | Gene symbol | Function | GenBank accession no. | % drop in expression | |
| BBR | MBBR | ||||
| AXL receptor tyrosine kinase (AXL) | AXL | Promotes proliferation, stem cell phenotype | AAH32229.1 | 34% | 46% |
| Dynamin-related protein 1 (DRP-1) | DRP-1 | Promotes cell survival, migration | O00429.2 | 44% | 43% |
| Eukaryotic elongation factor 2 kinase (eEf2K) | eEf2K | Promotes cell survival, proliferation | AAH32665.1 | 31% | 38% |
| Glycogen synthase kinase 3α/β (GSK-3α/β_pS21_S9) | GSK-3α/β | Promotes cell growth & invasion | NP_063937.2 | 92% | 33% |
| Human epidermal growth factor receptor 2 (HER2) | HER2 | Correlates with worse survival | P04626.1 | 39% | 92% |
| Jagged canonical Notch ligand 1 (JAG1) | JAG1 | Promotes migration and invasion of cells | NP_000205.1 | 38% | 42% |
| Paired box 8 (PAX8) | PAX8 | Promotes cell proliferation | AAB34216.1 | 54% | 44% |
| Pyruvate dehydrogenase kinase 1 (PDK1) | PDK1 | Promotes cell growth and survival | AAH39158.1 | 86% | 35% |
| Ribosomal protein S6 kinase B1 (S6K1) | S6K1 | Promotes cell proliferation | P23443.2 | 52% | 37% |
| X-linked inhibitor of apoptosis (XIAP) | XIAP | Inhibitor of apoptosis | NP_001191330.1 | 70% | 33% |
Figure 2.
Effects of treating MIA-PaCa-2 cells with BBR and NAX060 on cell division, proliferation, survival, migration, and apoptosis. A schematic depicting the effects of BBR and NAX060 on the N-RAS/TP53-associated signaling critical to PDAC development. The model is based on the fact that over-expression of mutated KRAS significantly enhances STAT3, NF-κB signaling which in turn lowers the TP53 expression (highlighted and boxed in dotted purple line). Green bold arrows denote inhibiting effects of BBR/MBBR on the signaling molecule.
WT-TP53 enhances expression of miR-34a in MIA-PaCa2 cells
TP53 directly transactivates miR-34a expression [24]. Therefore, we set out to compare the expression levels of miR-34a in MIA-PaCa-2 cells in vitro. The expression levels of miR-34a were significantly lower in the pancreatic cancer cell lines MIA-PaCa-2 and MIA-PaCa-2+pLXSN than those of MIA-PaCa2 cells that were stably transfected with vector encoding WT-TP53 (MIA-PaCa-2+WT-TP53) (Figure 3A). Mock transfection (data not shown) did not significantly alter the expression profile of miR-34a. These results indicate the following: a) miR-34a levels are inherently lower in cells derived from pancreatic cancer which have a mTP53; and b) There is a direct positive correlation between the expression of WT-TP53 and miR-34a.
Figure 3.
miR-34a expression in MIA-PaCa-2+pLXSN cells. (A) qRT-PCR was conducted to determine the miR-34a expression in MIA-PaCa-2+WT-TP53 and MIA-PaCa-2+pLXSN cells. Briefly, approximately 500 ng of RNA was reverse transcribed in a 25 μl reaction volume using the All-in-one miRNA qRT-PCR detection kit (GeneCopoeia, Rockville, MD). The synthesized cDNAs were used in the PCR reaction. The expression levels of miR-34a were measured employing the SYBR green detection and specific forward primer for the mature miRNA sequence and the universal adaptor reverse primer (GeneCopoeia, USA). Two-tailed P value of 0.05 or less was considered statistically significant; ***p < 0.001. (B) The putative targets of miR-34a that were significantly altered in MIA-PaCa-2+pLXSN and MIA-PaCa-2+WT-TP53 cells when the cells were treated with BBR and MBBR. A select few of the miR-34a target proteins that were significantly altered by treatment of MIA-PaCa-2 cells with BBR and NAX060 are projected. The data represent average of three individual experiments. (C) qRT-PCR was conducted to determine the expression of miR-34a-target genes in MIA-PaCa2+pLXSN and MIA-PaCa-2+WT-TP53 cells. qRT-PCR was performed to monitor expression of the different miR-34a-putative target genes in untreated MIA-PaCa-2+pLXSN cells and MIA-PaCa-2 expressing WT-TP53 or those treated with BBR and MBBR, respectively, using specific primers and SYBR green detection as per standard protocols. Bars represent average ± s.d. of three individual experiments.
One miRNA may target several genes. By using the miRmap and PiCTar tool algorithms [35, 36], we identified potential targets for miR-34a (Supplementary Tables 2 and 3). Analysis of RPPA data identified expression of a few of the miR-34a target proteins was altered in MIA-PaCa-2 cells. We determined a significant decrease in the expression of putative miR-34a targets (ATG4B, AXL, GATA3, JAG1, LDHA, MAP2K1, MYT1, NOTCH1, PEA-15, SERPINE1, and SNAIL) in MIA-PaCa-2+WT-TP53 compared to MIA-PaCa-2+pLXSN (Figure 3B). Expression of putative miR-34a targets (PCD4 and MAPT) were significantly elevated in MIA-PaCa-2+WT-TP53 compared to MIA-PaCa-2+pLXSN (Figure 3B). The effect of expressing WT-TP53 on the miR-34a targets at the level of transcription was monitored in cells by qRT-PCR. qRT-PCR data (Figure 3C) corroborated the RPPA analysis. The study established an inverse correlation between the expression of miR-34a and its target genes.
In vivo expression profile of miR-34a reflects its in vitro expression pattern
To monitor in vivo expression of miR-34a, we used human pancreas samples obtained from PDAC patients with appropriate controls. The expression levels of miR-34a were measured employing qRT-PCR with the SYBR green detection and specific forward primer for the mature miRNA sequence [74] and the universal adaptor reverse primer (GeneCopoeia, USA). Our preliminary results (Figure 4A) demonstrate a significant decrease in the levels of miR-34a in PDAC tumors when compared to healthy pancreas controls. The next obvious question was to understand the expression profiles of the set of putative miR-34a target genes that were significantly altered in vitro (Figure 3B, 3C). The expression profile of the miR-34a target genes (ATG4B, AXL, GATA3, JAG1, LDHA, MAP2K1, MYT1, NOTCH1, PEA-15, SERPINE1, and SNAIL) followed an identical expression pattern (Figure 4B). Expression of PCD4 was at undetectable levels in vivo (Figure 4B). Interestingly, expression of SERPINE1 was significantly greater than any other miR-34a target genes of interest. This along with the fact that little is known about miR-34a>SERPINE1 associated signaling led us to further investigate the biology of this interaction in pancreatic cancer.
Figure 4.
Expression profile of miR-34a in human PDAC samples. (A) miR34-a expression levels are lower in PDAC specimens compared to healthy pancreas controls. We compared the expression of miR-34a in 10 specimens in each group. Student t test was performed to compare groups. Two-tailed P value of 0.05 or less was considered statistically significant. ***p < 0.001. (B) qRT-PCR was conducted to determine the expression of miR-34a-target genes in human PDAC or healthy pancreas control specimens. Expression of miR-34a-target genes in human PDAC and healthy pancreas control specimens were detected by qRT-PCR using specific primers and SYBR green detection as per standard protocols. Bars represent average ± s.d. of three individual experiments. Two-tailed P value of 0.05 or less was considered statistically significant; ***p < 0.001.
miR-34a targets SERPINE1
The secondary structure of the pre-miR-34a was predicted using the RNAstructure software [37] (Supplementary Figure 2). By using the DIANA and MiRmap tool algorithms, we identified a putative miR-34a binding site located in the 3′-UTR of SERPINE1 mRNA (Supplementary Figure 3). To confirm the ability of miR-34a to specifically inhibit SERPINE1 expression, we monitored the expression of SERPINE1 in target cells that were untransfected, transfected with miR-34A mimic, or miR-NC. The range of doses tested in this study is comparable to those reported in the earlier studies [38–40]. The doses of the mimic and inhibitor used in the study did not significantly induce cell death in MIApaCa-2+pLXSN cells (Figure 5A, 5B). Transfection of MIA-PaCa-2+pLXSN cells with the miR-34a mimic significantly lowered the expression of SERPINE1 and SERPINE1 encoded protein, plasminogen activator inhibitor (PAI-1) levels at 24h post transfection compared to untransfected cells and cells transfected with miR-NC (Figure 5C, 5D). There was an inverse correlation observed in the expression of miR-34a and SERPINE1 and PAI-1 levels in MIA-PaCa-2+pLXSN cells (Figure 5D, 5E). These results authenticate the fact that SERPINE1 expression may well be regulated by miR-34a.
Figure 5.
miR-34a targets SERPINE1. (A, B) To determine the cytotoxic effect of miR-34a mimic and inhibitor, MIA-PaCa2+pLXSN cells were transfected with different concentrations of miR-34a mimic and inhibitor. At 24 h post transfection, MTT was added to each well and the absorption was measured. Percentage of cell death was monitored for miR-34a mimic (miR-mimic) (A) and miR-inhibitor (B) compared with 0.01% DMSO as control. (C, D) miR-34a mimic significantly decreased expression of SERPINE1 and PAI-1 in MIA-PaCa-2+pLXSN cells. MIA-PaCa-2+pLXSN cells were untransfected, mock transfected, or transfected with miR-34a mimic or miR-NC. At the end of 24h of incubation at 37°C, the cells were lysed, RNA extracted (panel C), cDNA synthesized, and SERPINE1 expression monitored by qRT-PCR. In another set of experiments, the cells were lysed were probed for PAI-1 expression by Western blotting (panel D). (E) The relative expression of SERPINE1 and miR-34a in MIA-PaCa-2 target cells was monitored by qRT-PCR. The expression was measured in terms of cycle threshold value (Ct) and normalized to expression of β-actin and snRNA RNU6B, respectively. The x-axis denotes the cell type and y-axis denotes fold change in expression of SERPINE1 and miR-34a. The R2 values for the miRNA expression are provided. (F) In another set of experiments, the above cells were lysed and probed for PAI-1 expression by Western blotting (panel F). Bars (A–C, E) represent average ± s.d. of five individual experiments. Student t test was performed to compare groups. Two-tailed P value of 0.05 or less was considered statistically significant. **p,0.01; ***p < 0.001; NS-not significant.
In order to determine the bona fide target of miR-34a, a luciferase reporter assay was performed. In this assay, two quantifiable genes encoding luciferase proteins were cloned in a vector. The SERPINE1 3′ UTR with the target region was placed downstream GLuc to regulate its translation, and SEAP was placed under no regulation for normalization. 293 cells were co-transfected with the SERPINE1 3′ UTR vector plasmid and miR-34a mimic. miR-34a mimic significantly decreased the relative luciferase activity compared to the cells that were transfected with miR-NC (Figure 6). In contrast, transfection of cells with miR-inhibitor reversed the ability of miR-34a mimic from lowering the luciferase activity (Figure 6). These results suggest that miR-34a directly targets SERPINE1 and thereby downregulates its expression.
Figure 6.
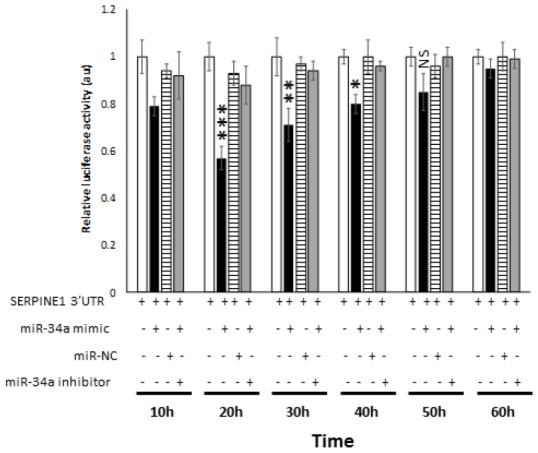
miR-34a specifically binds and interact with SERPINE1. Luciferase activity in 293 cells transfected with Dual-luciferase vector encoding Gaussia Luciferase (GLuc) and secreted alkaline phosphatase (SEAP) with 3′UR of SERPINE1 placed downstream of Glu luciferase reporter (SERPINE1 3′UTR). 293 cells were either transfected with SERPINE1 3′UTR, co-transfected with SERPINE1 3′UTR and miR-34a mimic, co-transfected with SERPINE1 3′UTR and control mimic (miR-NC), or co-transfected with SERPINE1 3′UTR, miR-34a mimic and miR-34a inhibitor. GLuc activity was monitored at 10 h, 22 h, 30 h, 40h, 50 h, and 60 h post-transfection and was normalized to SEAP. Data is plotted as GLuc/SEAP ratio where the x-axis indicates the transfection and time points, and y-axis indicates the relative luciferase activity. Bars represent average ± s.d. of five individual experiments. Student t test was performed to compare groups. Two-tailed P value of 0.05 or less was considered statistically significant. *p < 0.05; **p,0.01; ***p < 0.001; NS-not significant.
DISCUSSION
The TP53 tumor suppressor gene is also known as the “guardian of the genome” as it serves to identify DNA damage, pause cell cycle progression to allow for repair, and when repair is not possible, to induce apoptosis [41, 42]. The multiplatform molecular analysis of the PDAC-derived target cells exhibits a range of neoplastic cellularity representative of the clinico-pathologic spectrum of this disease (Figure 1). The RPPA analysis demonstrated the following: (i) expression of mTP53-associated signaling promoted cell survival and proliferation while inhibiting apoptosis (Figure 1; Tables 1, 2). Cells with mTP53 alone (MIA-PaCa-2+pLXSN) had an increase in the expression DUSP6 (Figure 1). The role of DUSP6 in tumor formation depends on the micro-environment [43]. Recent studies demonstrated over-expression of DUSP6 to induce tumor formation [44]; (ii) expression of WT-TP53 had an opposing effect on mTP53-associated signaling (Figure 1); (iii) Treatment of cells expressing mTP53 with BBR and MBBR can reverse cell signaling critical to tumor formation (Figure 2; Table 3). Inactivation of TP53 is believed to be a critical step in pancreatic cancer progression. The above results are a crucial piece of evidence to this work on miRNA as this allowed us to establish a cell culture model to study the effects of TP53 on miR-34a and associated signaling.
TP53 mutations frequently occur during the transition from benign pancreatic intra-epithelial neoplasia to the highly-aggressive, invasive and metastatic PDAC [45]. TP53 is a transcription factor that controls the expression of many key genes and miRNAs that are involved in the regulation of cell cycle progression, apoptosis, cellular senescence and other critical biological processes [46–48]. miR-34a expression in PDAC-derived cell lines like MIA-PaCa-2 cells is relatively low [49]. It was demonstrated in this study that miR-34a levels could be significantly increased in the same MIA-PaCa-2 cells when they were transfected with vector expressing WT-TP53 (Figure 3A). RPPA analysis also demonstrated a sharp decline in the expression of miR-34a-associated target genes in MIA-PaCa-2 cells over-expressing WT-TP53 compared to cells expressing mTP53 (Figure 3B, 3C). Overall, this is the first report to demonstrate a direct correlation between the WT-TP53 and miR-34a expression in PDAC-derived cells.
In order to appreciate the clinical relevance of the expression of miR-34a and its cognate targets in vivo, we monitored the expression profiles of miR-34a and associated signaling in vivo using PDAC specimens derived from human participants. miR-34a levels were significantly lower in PDAC specimens compared to healthy pancreatic tissues (Figure 4A). Also, we observed an increase in the expression of majority of the miR-34a targets (Figure 4B) that were analyzed by RPPA using lysates from MIA-PaCa-2 cells (Figure 3B, 3C). The only difference observed was as follows: (i) in vivo expression of PCD4 was at undetectable levels; and (ii) expression of SERPINE1 was significantly elevated compared to the rest of the miR-34a targets (Figure 5B). SERPINE1 levels have been identified to be significantly increased in colorectal cancer [50], lung cancer [51], gastric cancer [52], bladder cancer [53], head and neck squamous cell carcinoma [54], and others. Interestingly, earlier studies demonstrated ability of the SERPINE1 encoded protein, plasminogen activator inhibitor (PAI-1), to mediate proliferation and invasion of PDAC-derived cell lines, including MIA-PaCa-2 cells [55]. A recent study also concluded that the expression of SERPINE1 is negatively-related to the survival of PDAC patients [56]. Nonetheless, there are only three manuscripts that describe the expression of SERPINE1 and its association with PDAC and they were all performed with cell line models [55–57]. This is the first report of that links miR-34a>SERPINE1 expressions to PDAC using an in vivo patient-derived sample model.
It is a known fact that multiple genes may be regulated by one miRNA [58]. On the same note, a single mRNA transcript may be regulated by multiple miRNAs [59]. It is more than likely that the relationships between miRNAs and their targets are not one-to-one but multiple-to-multiple in cancers as reported in gastric carcinogenesis [60]. Earlier studies have demonstrated SERPINE1 as a target of miR-34a in colorectal [61] and non-small cell lung cancer [62]. Using bioinformatics tools, we identified SERPINE1 to be a promising target to miR-34a (Supplementary Figure 3). The results from luciferase reporter assays confirmed SERPINE1 to be a target for miR-34a (Figure 6). Accordingly, there was an inverse correlation between the expression of miR-34a and SERPINE1 (Figure 5E). Taken together, our results for the first time demonstrates a direct link between TP53, miR-34a, and SERPINE1 expression profiles in the pathobiology of PDAC.
The SERPINE1 gene is located at 7q21.2-q22 and encodes a single-chain glycoprotein of about 50kDa. The SERPINE1 gene is one of the main regulators of the plasminogen activator system (PAs). SERPINE1 inhibits the urokinase-type plasminogen (uPA) and tissue-type plasminogen activator (tPA), which in turn, reduce the conversion of plasminogen to the active protease plasmin [21]. Thus, the plasminogen activator inhibitor-1 (PAI-1) encoded by the SERPINE1 gene regulates tumor cell migration and invasion crucial to tissue remodeling and tumorigenesis [63, 64]. PAI-1 protein can exist in two distinct forms: active and inactive forms. This is crucial because depending on the conformation, PAI-1 can activate distinct cell signaling pathways critical to development of tumors [65].
miR-34a expression inhibits components of inflammatory response [66]. miR-34a downregulates expression of NF-κB via APE1/Ref-1 or SEMA4B [67, 68]. Importantly, miR-34a targets more TP53 network genes compared to miR-34b/c [24]. miR-34a is a key regulator of tumor suppression and is considered to have a broad anti-oncogenic activity [30]. Expression of miR-34a is significantly down-regulated or absent in a variety of cancers including hepatocellular and renal cell carcinomas, colon, breast, lung, prostate, ovarian, and pancreatic cancers [16–22]. The focus of this study was on miR-34a; which is the target of TP53 [69]. In the process, we were able to identify a key link between miR-34a, SERPINE1, and PDAC. Just as the age is a risk factor for the development of PDAC [70], PAI-1 is a part of the senescence-associated secretory phenotype (SASP) [71] and its expression is accordingly elevated in the elderly [72, 73]. Future studies are aimed at delineating the interactions between miR-34a and SERPINE1 in the context of PDAC and aging.
MATERIALS AND METHODS
Cells
The MIA-PaCa-2 (ATCC® CRM-CRL-1420™) carcinoma cell line was derived from a 65-year old Caucasian male [74]. MIA-PaCa-2 cells have the R248W TP53 GOF mutation. The R248W TP53 mutation present in MIA-PaCa-2 cells is a missense point mutation in the central DNA binding domain which abrogates its DNA contact [75]. This TP53 mutation results in a TP53 protein that is unable to bind to all TP53 target sequences in TP53-responsive genes and 2results in loss of its tumor suppressor properties [76, 77]. MIA-PaCa-2 cells also have an activating mutation at KRAS (G12C) and an elevated PI3K/AKT pathway activity. MIA-PaCa-2 cells were purchased from the ATCC (Rockville, MD, USA). Cells were cultured in medium containing 5% fetal bovine serum (FBS) purchased from (Atlanta Biologicals, Atlanta, GA, USA) as described in [33]. Tissue culture medium (Dulbecco's modified Eagles medium, DMEM), antibiotics containing l-glutamine and trypsin were obtained from Invitrogen (Carlsbad, CA, USA).
BBR and modified BBR (NAX060)
BBR was purchased from Sigma-Aldrich (Saint Louis, MO, USA). NAX060 compound was synthesized, purified and provided as a gift by Dr. Paolo Lombardi (Naxospharma, Milan, Italy) [78, 79].
Infection of cells with a retroviral vector encoding WT-TP53
The MIA-PaCa-2 cell line was infected with either a retroviral vector encoding WT-TP53 (MIA-PaCa-2+WT-TP53) or the empty pLXSN vector (MIA-PaCa-2+pLXSN) as a control as described [23]. Stably infected cell lines were isolated in the presence of 2 mg/ml G418 (geneticin; Sigma-Aldrich). Pools were established after approximately four weeks in culture as per standard protocols [31].
Reverse phase protein array (RPPA)
Target cells were either untreated or treated with 1,000 nM BBR or 1,000 nM NAX060 for 24h at 37°C. Cells were lysed 24 h later, denatured with 1% SDS and beta-mercaptoethanol, and five 2-fold serial dilutions of the samples were arrayed on nitrocellulose-coated slides (Grace Bio Lab, Bend, OR, USA) by an Aushon 2470 Arrayer (Aushon BioSystems, Bellerica, MA, USA). Each slide was probed with 419 primary antibodies and a biotin-conjugated secondary antibody. The stained samples were precipitated with 3,3' diaminobenzidine tetrahydrochloride (DAB) and quantified for spot intensity by using customized software. The signals were amplified with a Catalyzed Signal Amplification System (DakoCytomation, Glostrup, Denmark). Only target antibodies with a Pearson correlation coefficient (RPPA: western blotting) greater than 0.7 were used in the RPPA analysis. Each dilution curve was fitted with a logistic model (“Supercurve Fitting,” developed by the Department of Bioinformatics and Computational Biology at MD Anderson Cancer Center). R software and the package Ggplot2 were used to visualize the heatmap.
Human PDAC specimens
A total of ten frozen PDAC human specimens were used in this study. We also used a total of ten frozen healthy pancreas specimens as controls. A total of these 20 samples were obtained from the North Carolina Tissue Consortium, Division of Surgical Oncology, Brody Medical Sciences Building, Greenville, NC. All these specimens were preserved in a liquid nitrogen container.
Monitoring expression of miR-34a
RNA was extracted from the cells and the tissues as per standard laboratory procedures using TRIzol (Invitrogen) [38]. The RNA concentrations were measured with a NanoDrop ND-2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA), and then verified for quality using an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). Only the RNA samples with 260/280 ratios of 1.8 to 2.0 were used in the study.
Approximately 500 ng of RNA was reverse transcribed in a 25 μl reaction volume using the All-in-oneTM miRNA qRT-PCR detection kit (GeneCopoeia, Rockville, MD, USA). Briefly, the cDNA was synthesized in a 25 μl reaction mix containing 5 μl of 5x reaction buffer, 2.5U/μl poly A polymerase, 10ng/μl MS2 RNA, and 1μl RTase mix. The reaction was performed at 37°C for 60 min and terminated at 85°C for 5 min. cDNA that was produced in the RT reaction was diluted ten-fold and was used as the template for the PCR reaction in an Applied Biosystems ViiA 7 Real-Time PCR System (Thermo Fisher Scientific). In this system, MS2 RNA was used as an external reference for the quality of the extracted miRNAs, and RNU6B, RNU44, RNU48, and RNU49 were used for normalization. The expression levels of miRNAs were measured employing qRT-PCR with the SYBR green detection and specific forward primer for the mature miRNA sequence and the universal adaptor reverse primer (GeneCopoeia, USA). The specific forward primer to amplify miR-34a was 5’-TGGCAGTGTCTTAGCTGGTTGT-3’.
qRT-PCR to monitor expression of miR-34a putative targets
RNA was extracted from the cells and the tissues as per standard laboratory procedures using TRIzol [38]. Expression of ATG4B, AXL, GATA3, JAG1, LDHA, MAP2K1, MYT1, NOTCH1, PEA-15, SERPINE1, and SNAIL mRNAs by qRT-PCR was conducted as per earlier protocols [58] using appropriate primers (Supplementary Table 4).
Cytotoxicity assay
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) assays were performed to assess the sensitivity of cells to drugs, as previously described [23, 31, 80]. Target cells were treated with different concentrations of miR-34a mimic, inhibitor, or with appropriate controls at 37°C in a V-bottom 96-well plate. After a 24 h incubation, the percentage viable cells were assayed with MTT (Sigma-Aldrich). The optical density (OD) at the wavelength of 570 nm was used to calculate cell viability.
Western blotting
All the buffers used in this project were made with water that was endotoxin and pyrogen free. Western blotting was conducted as per earlier studies using the following primary antibodies: rabbit anti-PAI-1 polyclonal antibody (ThermoFisher Scientific) and mouse anti-actin antibodies (Clone AC-74; Sigma-Aldridge).
Dual-luciferase reporter assay
Luciferase reporter plasmids with wild-type SERPINE1 3′-UTR were purchased from GeneCopoeia. 293 cells were plated in 6-well plates. At 24 h post-plating, 293 cells were co-transfected with SERPINE1 3′-UTR luciferase reporter plasmid and miR-34a mimic, a scramble control (miR-NC), and/or miR-34a inhibitor using FuGene HD (Promega, Madison, WI, USA). At 10, 20, 30, 40, 50, and 60 h post transfection, supernatants were collected from each treatment and the luciferase activity measured using the Secrete-Pair Dual Luminescence Assay Kit (GeneCopoeia) as per the manufacturers’ recommendations.
Supplementary Material
Footnotes
AUTHOR CONTRIBUTIONS: SMA and JAM designed the hypothesis; JAM and PPR designed and conducted the RPPA experiments; SMA designed and conducted miRNA experiments; SMA and JAM contributed equally in analyzing the data and writing the manuscript.
FUNDING: SMA and JAM were supported in part by East Carolina University Grants [grant numbers 111104 and 11110-668715-0000).
CONFLICTS OF INTEREST: The authors declare no conflicts of interest.
REFERENCES
- 1.Higuera O, Ghanem I, Nasimi R, Prieto I, Koren L, Feliu J. Management of pancreatic cancer in the elderly. World J Gastroenterol. 2016; 22:764–75. 10.3748/wjg.v22.i2.764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Idachaba S, Dada O, Abimbola O, Olayinka O, Uma A, Olunu E, Fakoya AO. A Review of Pancreatic Cancer: Epidemiology, Genetics, Screening, and Management. Open Access Maced J Med Sci. 2019; 7:663–71. 10.3889/oamjms.2019.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel N, Khorolsky C, Benipal B. Incidence of Pancreatic Adenocarcinoma in the United States from 2001 to 2015: A United States Cancer Statistics Analysis of 50 States. Cureus. 2018; 10:e3796. 10.7759/cureus.3796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jeune F, Coriat R, Prat F, Dousset B, Vaillant JC, Gaujoux S. Pancreatic cancer surgical management. Presse Med. 2019; 48:e147–58. 10.1016/j.lpm.2019.02.027 [DOI] [PubMed] [Google Scholar]
- 5.Falzone L, Salomone S, Libra M. Evolution of Cancer Pharmacological Treatments at the Turn of the Third Millennium. Front Pharmacol. 2018; 9:1300. 10.3389/fphar.2018.01300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014; 74:2913–21. 10.1158/0008-5472.CAN-14-0155 [DOI] [PubMed] [Google Scholar]
- 7.Saad AM, Turk T, Al-Husseini MJ, Abdel-Rahman O. Trends in pancreatic adenocarcinoma incidence and mortality in the United States in the last four decades; a SEER-based study. BMC Cancer. 2018; 18:688. 10.1186/s12885-018-4610-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waters AM, Der CJ. KRAS: The Critical Driver and Therapeutic Target for Pancreatic Cancer. Cold Spring Harb Perspect Med. 2018; 8:8. 10.1101/cshperspect.a031435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bryant KL, Mancias JD, Kimmelman AC, Der CJ. KRAS: feeding pancreatic cancer proliferation. Trends Biochem Sci. 2014; 39:91–100. 10.1016/j.tibs.2013.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braun T, Gautel M. Transcriptional mechanisms regulating skeletal muscle differentiation, growth and homeostasis. Nat Rev Mol Cell Biol. 2011; 12:349–61. 10.1038/nrm3118 [DOI] [PubMed] [Google Scholar]
- 11.Shenoy A, Blelloch RH. Regulation of microRNA function in somatic stem cell proliferation and differentiation. Nat Rev Mol Cell Biol. 2014; 15:565–76. 10.1038/nrm3854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tahamtan A, Teymoori-Rad M, Nakstad B, Salimi V. Anti-Inflammatory MicroRNAs and Their Potential for Inflammatory Diseases Treatment. Front Immunol. 2018; 9:1377. 10.3389/fimmu.2018.01377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sonkoly E, Pivarcsi A. microRNAs in inflammation. Int Rev Immunol. 2009; 28:535–61. 10.3109/08830180903208303 [DOI] [PubMed] [Google Scholar]
- 14.Sethi S, Li Y, Sarkar FH. Regulating miRNA by natural agents as a new strategy for cancer treatment. Curr Drug Targets. 2013; 14:1167–74. 10.2174/13894501113149990189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peng Y, Croce CM. The role of MicroRNAs in human cancer. Signal Transduct Target Ther. 2016; 1:15004. 10.1038/sigtrans.2015.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bharali D, Jebur HB, Baishya D, Kumar S, Sarma MP, Masroor M, Akhter J, Husain SA, Kar P. Expression Analysis of Serum microRNA-34a and microRNA-183 in Hepatocellular Carcinoma. Asian Pac J Cancer Prev. 2018; 19:2561–68. 10.22034/APJCP.2018.19.9.2561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vogt M, Munding J, Grüner M, Liffers ST, Verdoodt B, Hauk J, Steinstraesser L, Tannapfel A, Hermeking H. Frequent concomitant inactivation of miR-34a and miR-34b/c by CpG methylation in colorectal, pancreatic, mammary, ovarian, urothelial, and renal cell carcinomas and soft tissue sarcomas. Virchows Arch. 2011; 458:313–22. 10.1007/s00428-010-1030-5 [DOI] [PubMed] [Google Scholar]
- 18.Toraih EA, Alghamdi SA, El-Wazir A, Hosny MM, Hussein MH, Khashana MS, Fawzy MS. Dual biomarkers long non-coding RNA GAS5 and microRNA-34a co-expression signature in common solid tumors. PLoS One. 2018; 13:e0198231. 10.1371/journal.pone.0198231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orosz E, Kiss I, Gyöngyi Z, Varjas T. Expression of Circulating miR-155, miR-21, miR-221, miR-30a, miR-34a and miR-29a: Comparison of Colonic and Rectal Cancer. In Vivo. 2018; 32:1333–37. 10.21873/invivo.11383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Imani S, Wu RC, Fu J. MicroRNA-34 family in breast cancer: from research to therapeutic potential. J Cancer. 2018; 9:3765–75. 10.7150/jca.25576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang L, Yu J, Xu J, Zheng C, Li X, Du J. The analysis of microRNA-34 family expression in human cancer studies comparing cancer tissues with corresponding pericarcinous tissues. Gene. 2015; 554:1–8. 10.1016/j.gene.2014.10.032 [DOI] [PubMed] [Google Scholar]
- 22.Zhao K, Cheng J, Chen B, Liu Q, Xu D, Zhang Y. Circulating microRNA-34 family low expression correlates with poor prognosis in patients with non-small cell lung cancer. J Thorac Dis. 2017; 9:3735–46. 10.21037/jtd.2017.09.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abrams SL, Lertpiriyapong K, Yang LV, Martelli AM, Cocco L, Ratti S, Falasca M, Murata RM, Rosalen PL, Lombardi P, Libra M, Candido S, Montalto G, et al. Introduction of WT-TP53 into pancreatic cancer cells alters sensitivity to chemotherapeutic drugs, targeted therapeutics and nutraceuticals. Adv Biol Regul. 2018; 69:16–34. 10.1016/j.jbior.2018.06.002 [DOI] [PubMed] [Google Scholar]
- 24.Navarro F, Lieberman J. miR-34 and p53: New Insights into a Complex Functional Relationship. PLoS One. 2015; 10:e0132767. 10.1371/journal.pone.0132767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Slabáková E, Culig Z, Remšík J, Souček K. Alternative mechanisms of miR-34a regulation in cancer. Cell Death Dis. 2017; 8:e3100. 10.1038/cddis.2017.495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sangrador I, Molero X, Campbell F, Franch-Expósito S, Rovira-Rigau M, Samper E, Domínguez-Fraile M, Fillat C, Castells A, Vaquero EC. Zeb1 in Stromal Myofibroblasts Promotes Kras-Driven Development of Pancreatic Cancer. Cancer Res. 2018; 78:2624–37. 10.1158/0008-5472.CAN-17-1882 [DOI] [PubMed] [Google Scholar]
- 27.Hermeking H. The miR-34 family in cancer and apoptosis. Cell Death Differ. 2010; 17:193–99. 10.1038/cdd.2009.56 [DOI] [PubMed] [Google Scholar]
- 28.Agostini M, Knight RA. miR-34: from bench to bedside. Oncotarget. 2014; 5:872–81. 10.18632/oncotarget.1825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rokavec M, Li H, Jiang L, Hermeking H. The p53/miR-34 axis in development and disease. J Mol Cell Biol. 2014; 6:214–30. 10.1093/jmcb/mju003 [DOI] [PubMed] [Google Scholar]
- 30.Misso G, Di Martino MT, De Rosa G, Farooqi AA, Lombardi A, Campani V, Zarone MR, Gullà A, Tagliaferri P, Tassone P, Caraglia M. Mir-34: a new weapon against cancer? Mol Ther Nucleic Acids. 2014; 3:e194. 10.1038/mtna.2014.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abrams SL, Follo MY, Steelman LS, Lertpiriyapong K, Cocco L, Ratti S, Martelli AM, Candido S, Libra M, Murata RM, Rosalen PL, Montalto G, Cervello M, et al. Abilities of berberine and chemically modified berberines to inhibit proliferation of pancreatic cancer cells. Adv Biol Regul. 2019; 71:172–82. 10.1016/j.jbior.2018.10.003 [DOI] [PubMed] [Google Scholar]
- 32.Akula SM, Candido S, Libra M, Abrams SL, Steelman LS, Lertpiriyapong K, Ramazzotti G, Ratti S, Follo MY, Martelli AM, Murata RM, Rosalen PL, Bueno-Silva B, et al. Abilities of berberine and chemically modified berberines to interact with metformin and inhibit proliferation of pancreatic cancer cells. Adv Biol Regul. 2019; 73:100633. 10.1016/j.jbior.2019.04.003 [DOI] [PubMed] [Google Scholar]
- 33.Candido S, Abrams SL, Steelman LS, Lertpiriyapong K, Martelli AM, Cocco L, Ratti S, Follo MY, Murata RM, Rosalen PL, Bueno-Silva B, de Alencar SM, Lombardi P, et al. Effects of the MDM-2 inhibitor Nutlin-3a on PDAC cells containing and lacking WT-TP53 on sensitivity to chemotherapy, signal transduction inhibitors and nutraceuticals. Adv Biol Regul. 2019; 72:22–40. 10.1016/j.jbior.2019.03.002 [DOI] [PubMed] [Google Scholar]
- 34.Espina V, Wulfkuhle J, Calvert VS, Liotta LA, Petricoin EF 3rd. Reverse phase protein microarrays for theranostics and patient-tailored therapy. Methods Mol Biol. 2008; 441:113–28. 10.1007/978-1-60327-047-2_8 [DOI] [PubMed] [Google Scholar]
- 35.Vejnar CE, Zdobnov EM. MiRmap: comprehensive prediction of microRNA target repression strength. Nucleic Acids Res. 2012; 40:11673–83. 10.1093/nar/gks901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen K, Rajewsky N. Natural selection on human microRNA binding sites inferred from SNP data. Nat Genet. 2006; 38:1452–6. 10.1038/ng1910 [DOI] [PubMed] [Google Scholar]
- 37.Reuter JS, Mathews DH. RNAstructure: software for RNA secondary structure prediction and analysis. BMC Bioinformatics. 2010; 11:129. 10.1186/1471-2105-11-129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hussein HA, Akula SM. miRNA-36 inhibits KSHV, EBV, HSV-2 infection of cells via stifling expression of interferon induced transmembrane protein 1 (IFITM1). Sci Rep. 2017; 7:17972. 10.1038/s41598-017-18225-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goldgraben MA, Russell R, Rueda OM, Caldas C, Git A. Double-stranded microRNA mimics can induce length- and passenger strand-dependent effects in a cell type-specific manner. RNA. 2016; 22:193–203. 10.1261/rna.054072.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kiener M, Chen L, Krebs M, Grosjean J, Klima I, Kalogirou C, Riedmiller H, Kneitz B, Thalmann GN, Snaar-Jagalska E, Spahn M, Kruithof-de Julio M, Zoni E. miR-221-5p regulates proliferation and migration in human prostate cancer cells and reduces tumor growth in vivo. BMC Cancer. 2019; 19:627. 10.1186/s12885-019-5819-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aubrey BJ, Kelly GL, Janic A, Herold MJ, Strasser A. How does p53 induce apoptosis and how does this relate to p53-mediated tumour suppression? Cell Death Differ. 2018; 25:104–13. 10.1038/cdd.2017.169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frum RA, Love IM, Damle PK, Mukhopadhyay ND, Palit Deb S, Deb S, Grossman SR. Constitutive Activation of DNA Damage Checkpoint Signaling Contributes to Mutant p53 Accumulation via Modulation of p53 Ubiquitination. Mol Cancer Res. 2016; 14:423–36. 10.1158/1541-7786.MCR-15-0363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kidger AM, Keyse SM. The regulation of oncogenic Ras/ERK signalling by dual-specificity mitogen activated protein kinase phosphatases (MKPs). Semin Cell Dev Biol. 2016; 50:125–32. 10.1016/j.semcdb.2016.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu QN, Liao YF, Lu YX, Wang Y, Lu JH, Zeng ZL, Huang QT, Sheng H, Yun JP, Xie D, Ju HQ, Xu RH. Pharmacological inhibition of DUSP6 suppresses gastric cancer growth and metastasis and overcomes cisplatin resistance. Cancer Lett. 2018; 412:243–55. 10.1016/j.canlet.2017.10.007 [DOI] [PubMed] [Google Scholar]
- 45.Weissmueller S, Manchado E, Saborowski M, Morris JP 4th, Wagenblast E, Davis CA, Moon SH, Pfister NT, Tschaharganeh DF, Kitzing T, Aust D, Markert EK, Wu J, et al. Mutant p53 drives pancreatic cancer metastasis through cell-autonomous PDGF receptor β signaling. Cell. 2014; 157:382–94. 10.1016/j.cell.2014.01.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Issler MV, Mombach JC. MicroRNA-16 feedback loop with p53 and Wip1 can regulate cell fate determination between apoptosis and senescence in DNA damage response. PLoS One. 2017; 12:e0185794. 10.1371/journal.pone.0185794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yue Z, Zhou Y, Zhao P, Chen Y, Yuan Y, Jing Y, Wang X. p53 Deletion promotes myeloma cells invasion by upregulating miR19a/CXCR5. Leuk Res. 2017; 60:115–22. 10.1016/j.leukres.2017.07.003 [DOI] [PubMed] [Google Scholar]
- 48.Olivier M, Eeles R, Hollstein M, Khan MA, Harris CC, Hainaut P. The IARC TP53 database: new online mutation analysis and recommendations to users. Hum Mutat. 2002; 19:607–14. 10.1002/humu.10081 [DOI] [PubMed] [Google Scholar]
- 49.Kent OA, Mullendore M, Wentzel EA, López-Romero P, Tan AC, Alvarez H, West K, Ochs MF, Hidalgo M, Arking DE, Maitra A, Mendell JT. A resource for analysis of microRNA expression and function in pancreatic ductal adenocarcinoma cells. Cancer Biol Ther. 2009; 8:2013–24. 10.4161/cbt.8.21.9685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zeng C, Chen Y. HTR1D, TIMP1, SERPINE1, MMP3 and CNR2 affect the survival of patients with colon adenocarcinoma. Oncol Lett. 2019; 18:2448–54. 10.3892/ol.2019.10545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arroyo-Solera I, Pavón MA, León X, López M, Gallardo A, Céspedes MV, Casanova I, Pallarès V, López-Pousa A, Mangues MA, Barnadas A, Quer M, Mangues R. Effect of serpinE1 overexpression on the primary tumor and lymph node, and lung metastases in head and neck squamous cell carcinoma. Head Neck. 2019; 41:429–39. 10.1002/hed.25437 [DOI] [PubMed] [Google Scholar]
- 52.Li L, Zhu Z, Zhao Y, Zhang Q, Wu X, Miao B, Cao J, Fei S. FN1, SPARC, and SERPINE1 are highly expressed and significantly related to a poor prognosis of gastric adenocarcinoma revealed by microarray and bioinformatics. Sci Rep. 2019; 9:7827. 10.1038/s41598-019-43924-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li X, Dong P, Wei W, Jiang L, Guo S, Huang C, Liu Z, Chen J, Zhou F, Xie D, Liu Z. Overexpression of CEP72 Promotes Bladder Urothelial Carcinoma Cell Aggressiveness via Epigenetic CREB-Mediated Induction of SERPINE1. Am J Pathol. 2019; 189:1284–97. 10.1016/j.ajpath.2019.02.014 [DOI] [PubMed] [Google Scholar]
- 54.Yang K, Zhang S, Zhang D, Tao Q, Zhang T, Liu G, Liu X, Zhao T. Identification of SERPINE1, PLAU and ACTA1 as biomarkers of head and neck squamous cell carcinoma based on integrated bioinformatics analysis. Int J Clin Oncol. 2019; 24:1030–41. 10.1007/s10147-019-01435-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Botla SK, Savant S, Jandaghi P, Bauer AS, Mücke O, Moskalev EA, Neoptolemos JP, Costello E, Greenhalf W, Scarpa A, Gaida MM, Büchler MW, Strobel O, et al. Early Epigenetic Downregulation of microRNA-192 Expression Promotes Pancreatic Cancer Progression. Cancer Res. 2016; 76:4149–59. 10.1158/0008-5472.CAN-15-0390 [DOI] [PubMed] [Google Scholar]
- 56.Xiao Y. Construction of a circRNA-miRNA-mRNA network to explore the pathogenesis and treatment of pancreatic ductal adenocarcinoma. J Cell Biochem. 2020; 121:394–406. 10.1002/jcb.29194 [DOI] [PubMed] [Google Scholar]
- 57.Radke DI, Ling Q, Häsler R, Alp G, Ungefroren H, Trauzold A. Downregulation of TRAIL-Receptor 1 Increases TGFβ Type II Receptor Expression and TGFβ Signalling Via MicroRNA-370-3p in Pancreatic Cancer Cells. Cancers (Basel). 2018; 10:10. 10.3390/cancers10110399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hussein HA, Akula SM. Profiling of cellular microRNA responses during the early stages of KSHV infection. Arch Virol. 2017; 162:3293–303. 10.1007/s00705-017-3478-y [DOI] [PubMed] [Google Scholar]
- 59.Shivdasani RA. MicroRNAs: regulators of gene expression and cell differentiation. Blood. 2006; 108:3646–53. 10.1182/blood-2006-01-030015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hashimoto Y, Akiyama Y, Yuasa Y. Multiple-to-multiple relationships between microRNAs and target genes in gastric cancer. PLoS One. 2013; 8:e62589. 10.1371/journal.pone.0062589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang T, Xu H, Liu X, Chen S, Zhou Y, Zhang X. Identification of Key Genes in Colorectal Cancer Regulated by miR-34a. Med Sci Monit. 2017; 23:5735–43. 10.12659/MSM.904937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lin X, Lin BW, Chen XL, Zhang BL, Xiao XJ, Shi JS, Lin JD, Chen X. PAI-1/PIAS3/Stat3/miR-34a forms a positive feedback loop to promote EMT-mediated metastasis through Stat3 signaling in Non-small cell lung cancer. Biochem Biophys Res Commun. 2017; 493:1464–70. 10.1016/j.bbrc.2017.10.014 [DOI] [PubMed] [Google Scholar]
- 63.Wan J, Deng D, Wang X, Wang X, Jiang S, Cui R. LINC00491 as a new molecular marker can promote the proliferation, migration and invasion of colon adenocarcinoma cells. Onco Targets Ther. 2019; 12:6471–80. 10.2147/OTT.S201233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Saleh AD, Cheng H, Martin SE, Si H, Ormanoglu P, Carlson S, Clavijo PE, Yang X, Das R, Cornelius S, Couper J, Chepeha D, Danilova L, et al. Integrated Genomic and Functional microRNA Analysis Identifies miR-30-5p as a Tumor Suppressor and Potential Therapeutic Nanomedicine in Head and Neck Cancer. Clin Cancer Res. 2019; 25:2860–73. 10.1158/1078-0432.CCR-18-0716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Declerck PJ, De Mol M, Vaughan DE, Collen D. Identification of a conformationally distinct form of plasminogen activator inhibitor-1, acting as a noninhibitory substrate for tissue-type plasminogen activator. J Biol Chem. 1992; 267:11693–96. [PubMed] [Google Scholar]
- 66.Jiang P, Liu R, Zheng Y, Liu X, Chang L, Xiong S, Chu Y. MiR-34a inhibits lipopolysaccharide-induced inflammatory response through targeting Notch1 in murine macrophages. Exp Cell Res. 2012; 318:1175–84. 10.1016/j.yexcr.2012.03.018 [DOI] [PubMed] [Google Scholar]
- 67.Ando K, Hirao S, Kabe Y, Ogura Y, Sato I, Yamaguchi Y, Wada T, Handa H. A new APE1/Ref-1-dependent pathway leading to reduction of NF-kappaB and AP-1, and activation of their DNA-binding activity. Nucleic Acids Res. 2008; 36:4327–36. 10.1093/nar/gkn416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jian H, Zhao Y, Liu B, Lu S. SEMA4B inhibits growth of non-small cell lung cancer in vitro and in vivo. Cell Signal. 2015; 27:1208–13. 10.1016/j.cellsig.2015.02.027 [DOI] [PubMed] [Google Scholar]
- 69.Chang TC, Wentzel EA, Kent OA, Ramachandran K, Mullendore M, Lee KH, Feldmann G, Yamakuchi M, Ferlito M, Lowenstein CJ, Arking DE, Beer MA, Maitra A, Mendell JT. Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Mol Cell. 2007; 26:745–52. 10.1016/j.molcel.2007.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bekkali NL, Oppong KW. Pancreatic ductal adenocarcinoma epidemiology and risk assessment: could we prevent? Possibility for an early diagnosis. Endosc Ultrasound. 2017. (Suppl 3); 6:S58–61. 10.4103/eus.eus_60_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Özcan S, Alessio N, Acar MB, Mert E, Omerli F, Peluso G, Galderisi U. Unbiased analysis of senescence associated secretory phenotype (SASP) to identify common components following different genotoxic stresses. Aging (Albany NY). 2016; 8:1316–29. 10.18632/aging.100971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Khan SS, Shah SJ, Klyachko E, Baldridge AS, Eren M, Place AT, Aviv A, Puterman E, Lloyd-Jones DM, Heiman M, Miyata T, Gupta S, Shapiro AD, Vaughan DE. A null mutation in SERPINE1 protects against biological aging in humans. Sci Adv. 2017; 3:eaao1617. 10.1126/sciadv.aao1617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yamamoto K, Takeshita K, Kojima T, Takamatsu J, Saito H. Aging and plasminogen activator inhibitor-1 (PAI-1) regulation: implication in the pathogenesis of thrombotic disorders in the elderly. Cardiovasc Res. 2005; 66:276–85. 10.1016/j.cardiores.2004.11.013 [DOI] [PubMed] [Google Scholar]
- 74.Deer EL, González-Hernández J, Coursen JD, Shea JE, Ngatia J, Scaife CL, Firpo MA, Mulvihill SJ. Phenotype and genotype of pancreatic cancer cell lines. Pancreas. 2010; 39:425–35. 10.1097/MPA.0b013e3181c15963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu DP, Song H, Xu Y. A common gain of function of p53 cancer mutants in inducing genetic instability. Oncogene. 2010; 29:949–56. 10.1038/onc.2009.376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brosh R, Rotter V. When mutants gain new powers: news from the mutant p53 field. Nat Rev Cancer. 2009; 9:701–13. 10.1038/nrc2693 [DOI] [PubMed] [Google Scholar]
- 77.Solomon H, Buganim Y, Kogan-Sakin I, Pomeraniec L, Assia Y, Madar S, Goldstein I, Brosh R, Kalo E, Beatus T, Goldfinger N, Rotter V. Various p53 mutant proteins differently regulate the Ras circuit to induce a cancer-related gene signature. J Cell Sci. 2012; 125:3144–52. 10.1242/jcs.099663 [DOI] [PubMed] [Google Scholar]
- 78.Pierpaoli E, Arcamone AG, Buzzetti F, Lombardi P, Salvatore C, Provinciali M. Antitumor effect of novel berberine derivatives in breast cancer cells. Biofactors. 2013; 39:672–79. 10.1002/biof.1131 [DOI] [PubMed] [Google Scholar]
- 79.Pierpaoli E, Fiorillo G, Lombardi P, Salvatore C, Geroni C, Piacenza F, Provinciali M. Antitumor activity of NAX060: A novel semisynthetic berberine derivative in breast cancer cells. Biofactors. 2018; 44:443–52. 10.1002/biof.1440 [DOI] [PubMed] [Google Scholar]
- 80.Tang XJ, Huang KM, Gui H, Wang JJ, Lu JT, Dai LJ, Zhang L, Wang G. Pluronic-based micelle encapsulation potentiates myricetin-induced cytotoxicity in human glioblastoma cells. Int J Nanomedicine. 2016; 11:4991–5002. 10.2147/IJN.S114302 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.