Abstract
The advent of chimeric antigen receptor T (CAR-T) and the burgeoning field of cellular therapy has revolutionized the treatment of relapsed/refractory leukemia and lymphoma. This personalized “living therapy” is highly effective against a number of malignancies, but this efficacy is tempered by side effects relatively unique to immunotherapies, including CAR-T. The overwhelming release of cytokines and chemokines by activated CAR-T and other secondarily activated immune effector cells can lead to cytokine release syndrome (CRS), which can have clinical and pathophysiology similarities to systemic inflammatory response syndrome and macrophage activating syndrome/hemophagocytic lymphohistiocytosis. Tocilizumab, an anti-IL6 receptor antibody, was recently FDA approved for treatment of CRS after CAR-T based on its ability to mitigate CRS in many patients. Unfortunately, some patients are refractory and additional therapies are needed. Patients treated with CAR-T can also develop neurotoxicity and, as the biology is poorly understood, current therapeutic interventions are limited to supportive care. Nevertheless, a number of recent studies have shed new light on the pathophysiology of CAR-T-related neurotoxicity, which will hopefully lead to effective treatments. In this review we discuss some of the mechanistic contributions intrinsic to the CAR-T construct, the tumor being treated, and the individual patient that impact the development and severity of CRS and neurotoxicity. As CAR-T and cellular therapy have redefined the concept of personalized medicine, so too will personalization be necessary in managing the unique side effects of these therapies.
Keywords: CAR-T, cell therapy, CRES, cytokine release syndrome, immunotherapy
1. ∣. INTRODUCTION
The introduction of chimeric antigen receptor T (CAR-T) to the clinic has changed the therapeutic landscape for refractory and relapsed B-cell hematologic malignancies, including acute lymphoblastic leukemia (ALL).1,2 CAR-T represent a pinnacle of personalized medicine. A patient's T-cells are collected, modified ex vivo to recognize a specific antigen and re-infused into the patient. Reinfused CAR-T are able to identify and destroy antigen expressing cells, even in sanctuary sites such as the central nervous system (CNS). The flagship trials of Tisagenlecleucel (Kymriah, Novartis) and Axicabtagene ciloleucel (Yescarta, Gilead), the two FDA approved CD19 directed CAR-T showed overall initial response rates in the multiply relapsed/refractory setting of 81% and 82%, respectively.1,3 Clinical use of these new cell-based therapies has been robust though it has been tempered by the related side effect profile.
The most common side effects due to CAR-T infusion are directly related to their mechanism of action.4-7 In recognizing target antigen, the chimeric antigen receptor binds and activates the T-cell with the help of in-line co-activating molecules (CD28, 4-IBB). In the normal course of T-cell activation cytokines and chemokines are released to (a) activate additional components of the immune system, (b) induce propagation of additional T-cells, and (c) kill target cells. This response is predicated on having an exceedingly rare number of T-cells that recognize any one particular epitope. CAR-T are manufactured to all recognize the same epitope and thus activate the system with an overwhelming signal. The abundant production of cytokines and chemokines leads to cytokine release syndrome (CRS), an almost sepsis-like picture in its pathophysiology that must be carefully managed to optimize patient recovery and CAR-T efficacy. The abundance of circulating cytokines and chemokines cause significant activation of monocytes and macrophages which are able to cross the blood-brain barrier. Together with the overall inflammatory milieu and highly activated T-cells, the CNS can enter into a CAR-T-induced encephalopathic state, the duration and severity of which is hard to predict. Thus, a significant amount of current research aims at optimizing supportive care of patients receiving CAR-T therapy and producing CAR-T that produce less toxicity.
2. ∣. EFFICACY OF CAR-T IN HEMATOLOGIC MALIGNANCIES
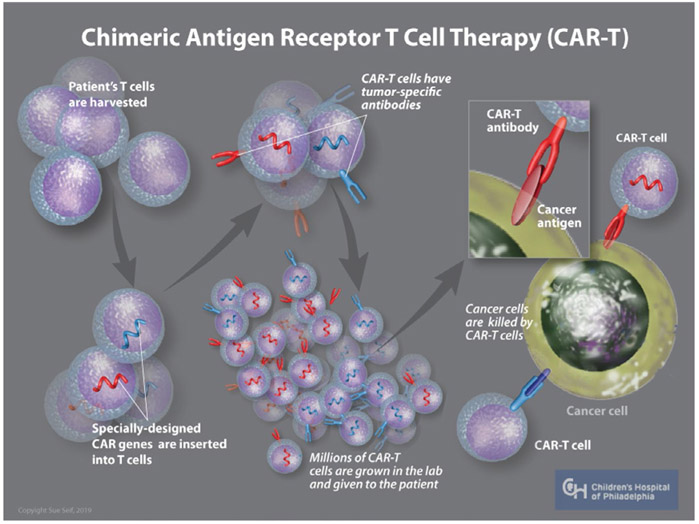
The ability to generate CAR-T relies on repurposing a T-cell population to recognize and target a single tumor antigen. In order to achieve this, T-cells are modified ex vivo by substituting the external, antigen recognition portion of a T-cell receptor (TCR) with a single-chain variable fragment from the antigen recognition portion of an antibody. This receptor is linked to a CD3-zeta internal signal transduction domain of the native TCR. In this way, the antibody receptor recognizes a target antigen and then signals through a TCR pathway to activate T-cell mediated tumor destruction (Figure 1). Initial studies of CAR-T occurred in the 1980s with the production of CAR-T against novel antigens such 2,4,6 Trinitrophenyl.8 In vitro assays measured T-cell activation by measuring IL2 secretion and cytolytic activity. While these first-generation CAR-Ts were active and able to target appropriate antigen, they were unable to mount a robust and persistent response necessary for sustained tumor destruction.9
FIGURE 1.
CAR-T generation. Autologous CAR-Ts are generated by first collecting the patient's T-cells through leukapharesis. T-cells are then transduced with a virus that contains genetic information encoding the receptor of interest. CAR-Ts are then propagated in the lab until a sufficient quantity is generated, at which time they are reinfused back into the patient. The CAR-Ts circulate and survey for antigen of interest and are activated upon engaging their target
Second-generation CAR-T aimed to rectify this problem by the addition of a co-stimulatory molecule, such as CD28 or 4-1BB to the original CAR-T structure. These second-generation CAR-T therapies have been used in the seminal CAR-T trials in CD19 and/or CD22 expressing malignancies.1,3,10 More recently, investigators have been researching methods to leverage current CAR-T and increase persistence and/or cytolytic effect by incorporating various combinations of co-stimulatory molecules including: 4-IBB, CD27, CD134, and ICOS.11,12 The inclusion of suicide genes such as icasp9, surface marker depletions of EGFR and other analogous mechanisms to destroy CAR-T at a clinically indicated time are also being investigated.13-15
Chimeric antigen receptor T directed at CD19 was initially tested in chronic lymphocytic leukemia (CLL) with moderate effect.16 The most mature dataset of these trials was recently published and included 14 patients with multiply relapsed, progressive CLL treated with CD19 directed CAR-T.17 Prior to CAR-T, the only curative approach available to these patients was hematopoietic stem cell transplantation however many patients with CLL are ineligible for HSCT due to co-morbid conditions. On this trial, 27% of patients had a complete response with no detectable MRD at a median time from infusion of 40 months. An additional 27% of patients had a partial response. Studies with other CD19 directed CAR-T have had similar success in CLL.18
In pediatric and adolescent pre-B-cell acute lymphocytic leukemia (ALL) a CD-19 directed CAR-T with 4-IBB showed remarkable effect in relapsed/refractory setting.1 This product, developed at the University of Pennsylvania (UPENN), tisagenlecleucel (Kymriah, previously CTL019), became the first FDA approved CAR-T therapy. Over 200 children have been treated to date with CTL019 at Children's Hospital of Philadelphia (CHOP). Our initial pilot study of 25 pediatric and 5 adult patients showed a 90% initial complete remission (CR) rate including 2 patients that were previously refractory to the CD19-directed bi-specific T-cell engaging (BiTE) antibody, blinatumumab.19 Further follow-up showed a 6-month event-free survival of 67% and an overall survival of 78%. Patients with early B-cell recovery tended to do worse than patients with continued B-cell aplasia.
Cytokine release syndrome developed in all 30 patients in this study with 22/30 having mild to moderate CRS and 8/30 (27%) of patients having severe CRS. Patients that developed severe CRS had a median time to onset of 1 day after CAR-T infusion, while those that developed mild to moderate CRS had a median time to onset of 4 days after CAR-T infusion. In this initial patient cohort, 13/30 patients had transient neurotoxicity that self-resolved in all patients. These data led to the international phase II multi-center registrational ELIANA trial. Of 75 pediatric patients treated in this cohort, 83% had a complete remission at 3 months postinfusion. At 12 months CTL019 produced a 50% event-free survival rate and a 73% overall survival rate at 12 months post-CAR-T infusion. The most recent update to the data indicates a 70% overall survival rate in pediatric patients at 18 months post-transfusion. Relapsed patients either had CD19 negative ALL or early loss of CAR-T persistence.
Similar excellent initial data were seen using 19-28z CAR-T, a CD-19 directed CD28 CAR-T in adult relapsed/refractory ALL.20,21 For patients with CD19-negative ALL, either pre- or post initial infusion with CD-19 directed CAR-T, a CD-22 directed 4-IBB CAR-T showed excellent efficacy in relapsed/refractory ALL with a 57% initial rate of complete remission with an intention to treat analysis. In the patients that received the higher dose of CD-22 directed CAR-T (>1 × 106 CD22 CAR-T cells/kg), there was a 73% initial rate of complete remission.10,20 Updated data from an expanded 43 patients in this trial showed that CD22 directed therapy prior to CD22 CAR-T negatively impacted efficacy of the CAR-T.22
Abxicabtagene ciloleucel, marketed as Yescarta (Gilead), is an FDA approved CD-19 directed CAR-T with a CD28 costimulatory domain that showed similarly excellent results in relapsed/refractory adult Non-Hodgkin's Lymphomas (NHL).3 In the most recent update of the Zuma-1 trial, there was an overall survival of 51% with a CR of 39% in patients with large B-cell lymphomas at a median of 2 years follow-up from infusion.23 Similar excellent results were recently published with tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma.24
In CLL, trials have shown that effectiveness of the CD19 directed CAR-T is dependent on the intrinsic T-cell function itself.25 Similar observations on intrinsic T-cell factors affecting CAR-T efficacy have been recently made in the pediatric population as well.26 Patients with robust T-cell proliferation and activation had improved CAR-T responses compared to patients without good responses. Given the average age of the population with CLL the intrinsic T-cell response is variable and thus effects outcome (Table 1). Investigations using immune modulating agents such as PD1/PDL-1 inhibitors and ibrutinib are underway to try and improve CAR-T function and efficacy in leukemia and other indications.27-29
TABLE 1.
Recent major CAR-T trials in leukemia
| CAR-T | Target | NCT | CR rate | Age | Tocilizumab/CRS ratio |
|---|---|---|---|---|---|
| CTL019 | CD19 (4-IBB) r/r B-ALL |
|
88/105 | Pediatric | 37/88 |
| JCAR015 | CD19 (CD28) r/r B-ALL | 44/53 | Adult | 19/45 | |
| FHCRC | CD19 (4-IBB) (ALL, CLL, NHL) | 31/33 (ALL only) | Adult | 21/92 (from 133 total treated patients) | |
| JCAR018 | CD22 (4-IBB) r/r B-ALL | 12/21 | Pediatric | 1/16 | |
| Axi-Cel | CD19 (CD28) Large B-cell Lymphoma | 59/101 | Adult | 43/101 |
Abbreviations: ALL, acute lymphoblastic leukemia; CAR-T, chimeric antigen receptor T-cell; CR, complete remission; CRS, cytokine release syndrome; r/r, relapse/refractory.
Recent efforts have been aimed at developing CAR-T directed toward other antigens and in other indications. The most developed program is a B-cell maturation antigen (BCMA) directed CAR-T in clinical trials for relapsed/refractory multiple myeloma.30-32 Additional B-cell targeted single antigen therapy CAR-T directed at TSLPR and CD38 are under development, though these are in earlier stages of development as compared with BCMA directed CAR-T.33,34 Allogeneic CD7 directed CAR-T that have been modified by CRISPR/Cas9 to not express CD7 are in development for T-cell malignancies.35 In addition, CAR-T directed at CD2, CD5, and CD38 are under development for T-cell cancers, including T-ALL.34,36,37 CD123 and CD33 directed CAR-T approaches are being explored in acute myelogenous leukemia (AML).38-40 These antigens however are expressed on hematopoietic stem cells and thus likely necessitates hematopoietic stem cell transplant after CAR-T administration.
Other novel approaches to attempt to bypass the marrow aplasia caused by certain CAR-T therapies are active areas of investigation. The ability to include a suicide gene within the intrinsic CAR-T construct so that the CAR-T may be destroyed to allow normal hematopoiesis to repopulate the marrow is one such approach.13,15 Another recently published method relies on CRISPR/Cas9 editing of hematopoietic stem cells to inactivate CD33 expression.41 The authors show that hematopoietic stem cells can proliferate and function normally without CD33. These hematopoietic stem cells can therefore be infused into the patient and be impervious to CD33 directed CAR-T.
Other laboratories are investigating CAR-T in a variety of solid tumors. For instance, a GD2 directed CAR-T is in development for neuroblastoma and brain tumors.42-45 CAR-T recognizing multiple antigens (eg CD19 and CD22, CD19 and CD123) or infusion of both CD19 and CD22 directed CAR-T at the same time are under investigation to limit recurrence of resistant (ie antigen negative) tumor cells.46-48 Each iteration of CAR-T and each tumor type presents specific challenges that research must overcome including anti-inflammatory tumor milieu, antigen expression on healthy cells and side effects of CAR-T administration and activation. The ideal antigens are those that are solely expressed on tumor cells and not on healthy cells to limit unintended side effects of CAR-T infusion. Additional efforts are currently underway to produce allogeneic models of current approved CAR-T structures so that they are available “off-the-shelf” to more patients with less reliance on difficult manufacturing processes.49 Early data using this approach with CD-19 directed allogeneic CAR-T (UCART19) were promising.50
3 ∣. CYTOKINE RELEASE SYNDROME
Cytokine Release Syndrome is the most common side effect of CAR-T therapy.4 At a mechanistic level it evolves from production of high levels of pro-inflammatory cytokines including: IFNγ, IL6, IL1, & IL2RA.51 The high levels of systemic cytokines can activate the prostaglandin system, inducing flu-like symptoms including fevers, myalgias, and fatigue. Hypercytokinemia leads to vasodilation with subsequent hypotension, tachycardia, and capillary leak with edema, culminating in organ damage including hepatic, renal, and cardiopulmonary toxicity. Patients can develop severe shock that is fluid-refractory and requires high dose vasoactive support to maintain tissue perfusion.52 The biology of severe CRS mirrors hemophagocytic lymphohistiocytosis (HLH)/macrophage activation syndrome (MAS) and patients can develop similar clinical and laboratory manifestations including cytopenias, hepatosplenomegaly, coagulopathy with marked hypofibrinogenemia, and hyperferritinemia.53
Cytokine Release Syndrome is not unique to CAR-T, though its manifestations are generally more severe after CAR-T infusion. CRS has previously been seen in other cellular and immunotherapies including haploidentical hematopoietic stem cell transplant, checkpoint inhibitors (PD-1/PD-L1), BiTEs and other therapies that activate the immune response.54-57 The severity of CRS after CAR-T however has necessitated novel and unique approaches to treatment. Even within the realm of CAR-T, CRS manifests with different time courses and severity based on the CAR-T construct, tumor type and patient co-morbidities.
Cytokine Release Syndrome is a known side effect of all current CD-19 directed CAR-T therapies and has occurred in patients with B-ALL, DLCBL and CLL.1,17,23 The onset of CRS is directly related to the properties of the co-stimulatory molecules incorporated in the CAR-T construct. For instance, tisagenlecleucel, which has a 4-IBB co-stimulatory molecule generally expands and activates at a slower rate but has longer persistence compared to CD28 based CAR-T. In patients treated with tisagenlecleucel for B-ALL median onset from time of infusion to beginning of CRS was a median of 3 days (range: 1-22 days) and to resolution was a median of 8 days (range: 1-36 days).1 In comparison to 4-IBB, the inclusion of a CD28 costimulatory domain promotes rapid and immediate CAR-T proliferation with a shortened persistence. In patients with NHL treated with abxicabtagene ciloleucel the median onset from time of infusion to beginning of CRS was a median of 2 days (range: 1-12 days) and to resolution was a median of 7 days (range: 2-58 days).23 The onset of CRS is also dependent on receptor-antigen interaction and the downstream signal transduction from the chimeric TCR. In CD22 directed CAR-T 16/21 patients experienced CRS though it occurred after day +5 in all patients. All patients in this trial had their CRS managed with supportive care only and did not require additional interventions.10
Due to the frequency and variable severity of clinical CRS after CAR-T infusion, investigators have attempted to develop clinical grading criteria to unify treatment decision-making. The different patient populations (ie adult vs pediatric), CAR-T constructs and tumor types have made CRS difficult to predict and to classify. Due to the many input variables involved with CRS, a number of groups have attempted to develop consensus guidelines to grade CRS however none have yet been universally adopted (Table 2).5,58,59 These grading-schema enable a certain degree of uniformity within institutions and cohorts.
TABLE 2.
CRS grading scales
| Institution | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|
| CHOP/PENN | Mild symptoms (supportive care only) | Moderate symptoms with some signs of organ dysfunction (need for hospital admission for some IV antibiotics and monitoring) | Moderate to severe symptoms with increased organ dysfunction (interventions for hypotension, coagulopathy & hypoxia) | Life threatening complications requiring aggressive interventions (high dose vasoactives, mechanical respiratory support) |
| Lee Criteria | Mild flu-like symptoms (supportive care only) | Moderate symptoms requiring intervention: hypotension responsive to fluids or low dose vasoactive, hypoxia responsive to supplemental O2, mild organ dysfunction | Increasing symptomatology: Hypotension necessitating multiple vasoactives, hypoxia necessitating increasing O2 supplementation (>40%), grade 4 transaminitis | Life-threatening symptoms (mechanical respiratory support, grade 4 organ toxicity) |
| MSKCC | Mild symptoms (observation and supportive care) | Hypotension requiring vasoactive <24 h or Hypoxia requiring supplemental O2 <40% | Hypotension requiring vasoactive >24 h. Hypoxia requiring supplemental O2 >40% | Life threatening: Hypotension refractory to vasoactives. Hypoxia necessitating mechanical respiratory support |
| CARTOX | Temperature ≥38°C. Grade 1 organ toxicity | Hypotension responsive to fluids or low dose vasoactives. Hypoxia requiring supplemental O2 <40%. Grade 2 organ toxicity | Hypotension requiring multiple vasoactives. Hypoxia requiring >40% O2. Grade 3 organ toxicity or Grade 4 transaminitis | Life threatening hypotension. Hypoxia requiring mechanical respiratory support. Grade 4 organ dysfunction |
| ASBMT | Temperature ≥38°C | Temperature ≥38°C with hypotension and/or hypoxia (not requiring additional interventions other than supplemental O2 by NC) | Temperature ≥38°C with hypotension requiring 1 vasoactive (±vasopressin) or hypoxia requiring high flow or other non-positive pressure intervention | Temperature ≥38°C with hypotension requiring multiple vasoactives or hypoxia requiring positive pressure |
Abbreviations: ASBMT, American Society for Blood and Marrow Transplantation; CARTOX, CAR-T therapy associated toxicity working group; CHOP, Children's Hospital of Philadelphia; MSKCC, Memorial Sloan Kettering Cancer Center.
Cytokine release syndrome induces a potent, dysregulated inflammatory milieu within a patient akin to the inflammation during HLH/MAS. Thus, the laboratory findings in a patient with CRS mimic those that one would expect in a patient with primary (inherited) HLH/MAS.53 In particular, marked hyperferritinemia greater than 10 000 mg/dL is often found in association with severe CRS. Likewise, the beginning of clinical resolution of CRS generally heralds a drop in the ferritin concentration. CRP generally tracks the ferritin and is an additional inflammatory marker that is non-specific, yet very elevated during severe CRS. Though there are higher peaks in ferritin levels in severe CRS, mild to moderate CRS may also be associated with disproportionately high ferritin levels. The positive predictive value of differentially elevated CRP or ferritin levels to predict CRS severity in the immediate post-CAR-T setting is less than 50%.53
Liver and renal dysfunction, likely secondary to decreased organ perfusion during hypotension, is common in severe CRS and can result in significant transaminitis. Hypofibrinogenemia defined as fibrinogen, less than 150 mg/dL is also associated with the pathophysiology of severe CRS. Of note the coagulopathy seen with CRS often has hypofibrinogenemia out of proportion to prolonged coagulation times. Similar to HLH/MAS, this profound hypofibrinogenemia may be due to IL1b and TNFα inducing macrophages to secrete plasminogen which in turn causes fibrinogen degradation.60 Other general markers of inflammation such as elevated lactate dehydrogenase, elevated sedimentation rate serve as a surrogate for the state of inflammation but like CRP, ferritin and fibrinogen are not in and of themselves good markers of CRS severity.
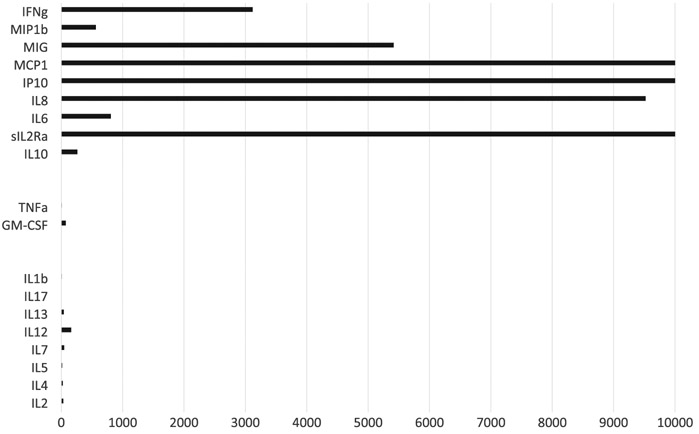
Just as the clinical features of CRS may resemble or mimic HLH/MAS, the underlying biology of CRS shares similar mechanistic components to HLH/MAS (Figure 2). Notably, a slew of macrophage/ monocyte associated cytokines including IL1RA, IL10, IL6, IP10, MIG, IFNα, MIP1a, MIP1b, sIL6R were found to be differentially elevated in patients with severe CRS.61 MCP1 and MIP1b, which are both elevated with severe CRS also act as chemotactic factors for macrophages and serve as navigation for these cell types as well as to further amplify the signal transduction pathways. T-cell factors also play an important role in CRS mediated pathology as evidenced by elevation of sIL2Ra, IFNγ, sIL6r, and GM-CSF in patients with severe CRS as compared to other CAR-T patients. Elevated IL6 in CRS was previously thought to be secreted from T-cells but has recently been shown to be monocyte derived.62-64 Mediators of tissue and vascular damage are also differentially elevated in patients with severe CRS.
FIGURE 2.
Cytokine release syndrome (CRS) hypercytokinemia mirrors HLH. Patients with severe CRS have a cytokine pattern that is similar to patients with hemophagocytic lymphohistiocytosis (HLH). Cytokines were measured serially in 14 patients treated with tisagenlecleucel. Figure depicts mean peak cytokine level in the first 35 d after infusion. Cytokines are allocated into 3 groups. Top group (IFNγ, MIP1β, MIG, MCP1, IP10, IL8, IL6, sIL2Ra, and IL10) are those expected to be elevated in patients with HLH. Middle group (TNFα and GM-CSF) are sometimes elevated in patients with HLH and sometimes normal, depending on the type of HLH. Bottom group (IL1B, IL17, IL13, IL12, IL7, IL5, IL4, IL2) are expected to be normal in patients with HLH. Raw data, additional details, and methods were previously published in Teachey et al. (2016)
Like CAR-T therapy itself, the management of CRS requires a personalized approach. At Children's Hospital of Philadelphia, we infuse CAR-T in an outpatient setting with frequent outpatient clinic visits in first few weeks postinfusion. Fever, oftentimes the heralding sign of impending CRS requires an immediate emergency room or clinic visit at which time broad-spectrum antibiotics are initiated with admission to the inpatient unit. At the minimum a patient must remain afebrile for 24 hours before being eligible to be discharged home. Many times, mild to moderate CRS symptoms spontaneously resolve with supportive care. In severe cases they require more aggressive intervention. During admission, fevers and inflammatory markers including CRP and ferritin are closely monitored. Fevers and tachycardia are managed with antipyretics as needed. If hypotension develops, fluid resuscitation is used judiciously and in general does not exceed 40-60 cc/kg as there is significant fluid extravasation secondary to the capillary leak syndrome, leading to anasarca, ascites, and pulmonary edema. Refractory hypotension necessitates admission to critical care, vasoactives, and cytokine blockade with tocilizumab. If vasoactives are necessary, tocilizumab is administered generally as the infusion is titrated up from baseline. Our dosing strategy for tocilizumab is 12 mg/kg for patients with a weight less than 30 kg or 8 mg/kg for weight greater than 30 kg with a maximum dose of 800 mg. This is in accordance with the FDA guidelines.65,66 This dose may be administered repeatedly every 8 hours over the first 24 hours and for a maximum of four doses total if there is continued decline in clinical status. In general, we do not start steroids unless there is worsening of CRS 24 hours after tocilizumab was infused. Our starting methylprednisolone dose is 1 mg/kg every 12 hours (though clinicians may decide to administer an initial 2 mg/kg bolus). Steroids are maintained at this dose until CRS abates to grade 1 at which point they are tapered over the course of 3 days. If there is minimal or no response to this dose of steroids, clinicians consider higher dose steroids, siltuximab and other investigational interventions discussed below.
Given the individualized risk assessment that must account for CAR-T structure, tumor type and patient comorbidities among other considerations, our outpatient approach at CHOP may not be suitable at other centers or with other CAR-T constructs. Indeed, some centers report providing CAR-T infusion only on an inpatient basis. They further recommend continued hospitalization with cardiac monitoring by telemetry from the time of CAR-T administration and for a minimum of 7 days post-CAR-T infusion.67,68 While we have found this approach to be unwarranted in the pediatric population with B-ALL using a 4-IBB based CD19 directed CAR-T, these may be prudent guidelines for certain adult population with additional co-morbidities and with different CAR-T products.
Tocilizumab is a monoclonal anti-IL6 receptor antibody that received FDA approval for CRS concomitantly with the first FDA approval for CAR-T therapy.65 Tocilizumab was first used at CHOP on the initial pediatric patient that received CTL019 therapy. She became critically ill, but had marked improvement and prompt resolution of CRS with tocilizumab.69 Existing data suggest tocilizumab can ameliorate CRS without effecting CAR-T anti-tumor effect. Nevertheless, most published data detailing the use of tocilizumab detail their use after the development of severe CRS.6 It is unknown if tocilizumab will impact CAR-T efficacy if used earlier or as a preventative. At CHOP, we have an on-going trial whereby tocilizumab is administered to patients predicted to be at higher risk of developing CRS based on pre-CAR-T infusion disease burden, as soon as a second fever is documented (). This early administration of tocilizumab is being evaluated for dampening progression of CRS but also to ensure that there is no detriment in CAR-T directed anti-leukemia activity.
While tocilizumab is the only current FDA approved therapy for CRS, other interventions and therapeutics have been used if CRS is refractory to tocilizumab administrations. Corticosteroids are generally a second line therapeutic used to treat CRS and are often effective.6 Unlike tocilizumab, corticosteroids have the ability to directly inhibit CAR-T anti-leukemic effect and thus should be used only when necessary. Other groups have a lower threshold to initiate corticosteroids, including high dose steroids. We recommend a more tempered approach. Siltuximab, a monoclonal anti-IL6 antibody has also been used at our center and others with limited effect in refractory CRS. The data on siltuximab efficacy is sparse and there is no head to head comparison comparing tocilizumab to siltuximab to evaluate which anti-IL6 intervention is best for CRS. As tocilizumab is the only FDA-approved cytokine blocking drug for CRS, siltuximab should be reserved for tocilizumab-refractory cases.
Given that most CRS directed therapy is supportive with only IL6 directed intervention and corticosteroids currently available for more severe cases, new interventions and therapies must be discovered in order to allow the use of CAR-T therapy reach its full potential. Inhibitors of the Jak/Stat signaling pathway which mediate downstream signal transduction of a number of cytokines including IL6 and INFγ may be effective for CRS. Ruxolitinib (Jakafi, Incyte) is a JAK1 and JAK2 inhibitor that has been FDA approved for polycythemia vera and myelofibrosis and has an sNDA under consideration for use in graft vs host disease. Ruxolitinib has shown promising results in ameliorating CRS and CAR-T associated neurotoxicity while preserving CAR-T function in a murine model that received CAR-T for AML.70 Ruxolitinib has also been effective in a number of case reports of patients with refractory HLH.71,72 As ruxolitinib can inhibit T-cell proliferation, it is plausible it might impact the efficacy of CAR-T.
Dasatinib, an FDA approved tyrosine kinase inhibitor used for treatment of t(9;22) chronic myelogenous leukemia and Philadelphia chromosome positive acute lymphoblastic leukemia has been shown to modulate CAR-T cytokine secretion in vitro.73 Dasatinib can also inhibit T-cell proliferation and thus may impact efficacy of CAR-T. Catecholamine blockade is also being investigated to reduce CRS toxicities.74 Recent data on the use of lenzilumab, a monoclonal anti-granulocyte-macrophage colony-stimulating factor (GM-CSF) has shown that in a murine model it is efficacious in preventing the progression of CRS.75 Importantly, investigators involved in that study showed no detrimental effect on the anti-leukemic effect of the CAR-T in mice. An additional positive finding for this therapy was that unlike tocilizumab and other IL6 directed interventions, there was appreciable effect on moderating CAR-T-induced encephalopathy (discussed below).
Similarly, studies have recently shown that IL-1 directed therapy with anakinra, a monoclonal anti-IL1 antibody, can also ameliorate progression of CRS in mouse models.63,64 As IL1 secretion begets the production of IL6, this therapy is able to moderate two pro-inflammatory cytokines at the same time. In fact, this mechanism has recently been exploited in multicentric Castleman's disease with reports of disease control using IL1 blockade.76 Anakinra is also able to mediate an effect on CAR-T-induced neurotoxicity by lowering systemic IL1 concentrations that are able to cross the blood-brain barrier. Further studies will be necessary of both these novel drugs to show if they are indeed efficacious, safe and do not inhibit antileukemic effects of CAR-T therapy in humans.
Other studies evaluating means to ameliorate CRS have focused on modifying the CAR-T construct itself. CAR-T structures with modifiable activation strategies have been studied in which the clinician can modulate the activity of these cells by the careful addition of exogenous agents. Finally, CAR-T with the addition of suicide genes are also being developed.13,15 These CAR-T structures have the benefit of being able to be destroyed, if needed, by the addition of a specific exogenous agent. The downside however is that the patient loses the continuous surveillance provided by this “living drug” that can persist for years after remission has been achieved.
The fact that CRS is driven by multiple cytokines and the down-stream consequences of hypercytokinemia are complex it behooves us to develop additional therapies to treat and mitigate the effects of this cytokine storm. No one antibody or drug however will likely be able to treat all patients with CRS. Rather, we will require an armamentarium of therapeutic options to treat CRS, particularly as CAR-T therapies expand in additional hematologic malignancies and eventually solid tumor indications. Each cohort of patients and indeed each individual patient may require a personalized approach based on age, comorbidities, tumor burden at time of CAR-T infusion and predictive biomarkers. A combination of cytokine targeted therapies may be necessary for severe cases of CRS. Only in this way will we be able to utilize CAR-T therapy in an expanded cohort of patients and for additional higher risk indications. In addition, correlative biologic studies are needed as different products targeting different antigens are used in different diseases and the biology of CRS may be different, which would require specific treatment protocols for each unique antigen directed CAR-T.
The timing of optimal tocilizumab (or other CRS directed therapy) administration may also be dependent on identifying clinically useful biomarkers that are capable of predicting onset and severity of CRS. Certain parameters indicate higher risk though they are not necessarily biomarkers for CRS. For instance, the pre-CAR-T infusion disease state has been shown to correlate with T-cell activation and CRS in multiple studies. If a patient has a high disease burden or bulky disease, they are more likely to develop severe CRS.77 Disease burden, immediately prior to infusion, has a strong negative predictive value for severe CRS, albeit a relatively poor positive predictive value.53 The addition of fludarabine to lymphodepleting chemotherapy prior to CAR-T infusion has also been associated with increased risk of progression to CRS,77 likely from destruction of Tregs allowing enhanced early enhanced CAR-T proliferation.
Previous data used cytokine signatures sent after CAR-T infusion and before onset of CRS to show that increased levels of IFNγ and sgp130 were strong predictors of progression to severe CRS.53 Furthermore, IFNγ IL6 and sIL2Ra were shown to be differentially elevated in patients with severe CRS compared to those that did not develop severe CRS.53 Cytokine analysis 72 hours post-CAR-T infusion (and prior to development of severe CRS) showed that elevated serum concentrations of IFN, IL13, and MIP1a had greater than 95% positive predictive value (PPV) and negative predictive value (NPV) for predicting severe CRS using logistic regression modeling. Analysis of IL-10 with pre-infusion disease burden had a greater than 90% PPV and NPV for severe CRS. Other groups have done similar cytokine predictive studies for CRS and neurologic complications.61,77 All of these models need additional validation with larger patient cohorts, a variety of CAR-T products, and different tumor types. Development and refinement of these biomarkers are ongoing to make them applicable to clinical situations. It is yet to be seen if these specific signatures will be generalizable with all patients receiving CAR-T, or if each unique CAR-T, patient cohort, and tumor type will require a specific cytokine signature to predict severe CRS.
4 ∣. CELL -RELATED ENCEPHALOPATHY SYNDROME/IMMUNE EFFECTOR CELL-ASSOCIATED NEUROTOXICITY SYNDROME
Chimeric antigen receptor T (CAR-T)-induced neurotoxicity recently termed CAR-T-related encephalopathy syndrome (CRES) or immune effector cell-associated neurotoxicity syndrome (ICANS), is the second major adverse event that has been reported with CD19 directed CAR-T therapy in multiple patient cohorts and different tumor types.59 Similar to CRS, there are multiple proposed grading scales to evaluate CRES59,67,78-80 (Table 3). Neurologic toxicity has also previously been seen in other immunotherapeutic modulators such as Blinatumomab and is also seen after CD22 directed CAR-T as well as preclinical models of GD2-directed CART.43,81,82 The neurological deficits seen in the days and weeks after CAR-T infusion are varied, however encephalopathy is the most common severe side effect. Similar to CRS, CRES can be mild to severe. Additional common neurological manifestations include seizure activity and focal deficits, including aphasia, vision changes, tremors, and facial droop. It is however difficult to ascertain the extent of CRES based on early clinical data as many of the early CAR-T trials did not include detailed neurological exams. Current trials have taken a more comprehensive approach in assessing neurologic deficits.10
TABLE 3.
Modified summary of CRES/ICANS grading scales
| Institution | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|
| CTCAE 5.0 | Mild symptoms (brief partial seizure w/o LOC) | Symptoms limiting instrumental ADL (brief generalized seizure) | Symptoms limiting self-care ADL (multiple seizures despite medical care); New onset cerebral edema | Life threatening symptoms |
| CARTOX | 7-9 CARTOX score (mild impairment) | 3-6 CARTOX score (moderate impairment) | 0-2 CARTOX score (severe impairment); seizures responsive to benzodiazepine; stage 1-2 papilledema | Critical/obtunded condition; Generalized seizures or non-convulsive status epilepticus; stage 3-5 papilledema or cerebral edema |
| ASBMT Adult | 7-9 ICE score; awaken spontaneously | 3-6 ICE score; awaken to voice | 0-2 ICE score; awaken to tactile stimulus; seizure that resolves with intervention; focal edema on neuroimaging | Life threatening symptoms |
| ASBMT Pediatrica | CAPD 1-8 | CAPD 1-8 | CAPD ≥9 | Unable to perform CAPD |
The CTCAE scale incorporates encephalopathy, seizure, dysphagia, tremor, headache, confusion, depressed level of consciousness and cerebral edema; CARTOX criteria incorporates Neurologic Assessment Score (CARTOX-10 score), elevated ICP, seizure or motor weakness. ASBMT criteria are based on ICE score (modified CARTOX-10 evaluation), level of consciousness, seizures, motor findings, and elevated ICP.
Abbreviations: ADL, activities of daily living; LOC, loss of consciousness.
ASBMT pediatric criteria are for children <12 and include all of the adult criteria with the addition of the CAPD score based off the Cornell delirium assessments.
Blinatumomab, a CD19/CD3-bispecific T-cell receptor-engaging (BiTE) antibody has shown a similar side effect profile to that of CAR-T.83 Fever, tachycardia, and hypotension are all reported side effects of blinatumomab infusion. Progression to fulminant CRS and an HLH-like clinical picture have also been reported.54 In the trial of blinatumomab for relapsed ALL in adults, 52% of patients had neurologic deficits during and after the infusion.83,84 Of these neurological findings, 76% were grade 1 and 2 and managed efficaciously with dexamethasone. 20% of patients that had neurologic events were grade 3 and 4% were grade 4. Neurologic deficits were transient, and resolved in these patients. Interestingly, investigators found that patients who had handwriting impairment immediately after infusion were at high risk for developing severe CRES. Since the mechanisms underlying CAR-T and BiTE therapies are similar, the associated toxicities and possible therapeutic interventions are also likely similar and can help inform one another.
Like CRS, CRES onset and progression is variable and depends on CAR-T construct, patient variables, and tumor type. The Fred Hutchinson Cancer Center used a 4-IBB CD19 directed CAR-T construct in adult patients with ALL, CLL, and NHL, approximately 40% of 133 evaluated patients had grade 1 or higher CRES.85 The median time to onset was 4 days after CAR-T infusion with a median time to peak severity of 1 day from time of onset. Seven patients in this cohort developed grade 4 neurotoxicity with 4 patient deaths related to neurological pathology. In this study 93% of patients with CRES also had CRS. In fact, severe and/or early onset CRS, elevated inflammatory markers (ferritin and CRP), coagulopathy, capillary leak, and early peak CD4 and CD8 expansion were all associated with development of CRES. In evaluating the cytokine levels of patients that had CRES, researchers in this study noted that at baseline there was a cytokine gradient between serum and CSF, particularly with inflammatory cytokines such as IFNγ, TNFα, and soluble receptors. In patients with acute neurotoxicity however, there were similarly elevated levels of IFNγ, TNFα, IL6, and TNFR p55 in both the serum and CSF. This underlies the role dysfunction of the BBB can have in the post-CAR-T time period.85
Memorial Sloan Kettering Cancer Center (MSKCC) investigated the use of 19-28z CAR-T in 53 adult patients with ALL. Sixty-two percent of patients had grade 1 or higher CRES and 41.5% of patients had severe (grade 3 or higher) CRES.61 In this cohort, median time to onset was 5 days (range: 2-11 days) after CAR-T infusion and a median time to severe CRS of 9 days (range: 2-11 days) after CAR-T infusion. Importantly, there were no reported deaths secondary to neurologic toxicities in this cohort. Interestingly, seizures and tremors were more common manifestations of CRES in this population and expressive aphasia was noted to herald the onset of severe CRES in 21/22 patients that had severe pathology. The data from this cohort were similar with respect to the first cohort in markers of inflammation and severe or early CRS were associated with severe CRES. Additionally, the investigators reported that age of patient, high pre-CAR-T disease burden and the presence of extramedullary disease were associated with progression to CRES. Furthermore, biological correlates that were undertaken in this trial showed that there was a differential elevation of protein and NMDA receptor agonists (quinolinic acid, glutamine) in the cerebrospinal fluid (CSF) of patients that had severe CRES compared to those that had mild disease. Investigators were able to construct a predictive model using three serum cytokine concentrations on day 3 post-CAR-T infusion. Patients with low IL15 or high EGF were considered low risk for developing severe neurotoxicity. Patients with high IL15, low EGF, and low IL10 were intermediate risk and patients with high IL15, high IL10, and low EGF were high risk for severe neurotoxicity.61
At CHOP, we reported the clinical manifestations of CRES in 51 pediatric ALL patients treated with tisagenlecleucel.7 Forty-five percent of patients experienced neurotoxicity with a median onset of 6 days from CAR-T infusion to neurologic symptoms. This lagged the onset of CRS by approximately 3 days. All patients were treated with supportive care alone for their neurotoxicity. No patients in this cohort died of neurotoxic side effects. In evaluating serum cytokines, there were differential elevated concentrations of IL-12 and soluble tumor necrosis factor receptor-1 at 3 days post-CAR-T infusion in patients that developed neurologic complications compared to those that developed isolated CRS.
A 22 pediatric patient cohort that received 4-IBB CD22-directed CAR-T 8/22 patients was noted to have CAR-T-related neurotoxicity. Neurological findings were assessed prospectively by a battery of exams.10 Importantly, this was the first study to perform and report comprehensive prospectively collected neurologic data. Of note, 82% of these patients previously received CD19 directed CAR-T, 4 of which had neurotoxicity. Interestingly, there was significant discordance in caregiver evaluation of neurotoxicity compared to physician evaluation.82
The mechanism for CAR-T-induced neurotoxicity is still poorly understood though it is likely a conglomerate of several independent pathophysiological processes. A recent mechanistic hypothesis for CRES is centered around the inflammatory mediators released by the activated CAR-T and macrophages. These mediators cause the release of angiopoietin-2 and von Willebrand factor from the Weibel-Palade bodies of the endothelial cells (EC) within the CNS.86 This in turn leads to displacement angiopoietin-1 which leads to inhibition of TIE receptor tyrosine kinase signaling. The EC then loses the integrity of the BBB and becomes more permeable. Further sequestration of high molecular weight von Willebrand factor leads to coagulopathy. This positive feedback loop continues to persist as cytokines and activated inflammatory cells continue to permeate the BBB. This pathophysiology is similar to that of thrombocytopenic thrombotic purpura (TTP) and thus interventions such as plasmapheresis that are utilized in TTP are being considered in CRES.86
An additional mechanism postulated to cause CAR-T-induced neurotoxicity involves the natural killer (NK)-cell population.7 These cells secrete IL2 and IL15 which have both been found to be elevated in patients with CAR-T neurotoxicity. This high level of NK-cell activation would then prime CNS microglial activation and mediate a robust and pathogenic inflammatory milieu within the CNS. Additional studies exploring this mechanism of action are underway in mouse models. The finding that excitatory neurotransmitters are differentially elevated in CSF of patients that have severe CRES has also led to investigation of these molecules as therapeutic targets to ameliorate post-CAR-T neurotoxicity.
Similar to CRS, CAR-T-induced neurotoxicity is dependent not only the host response to CAR-T activation, but also to intrinsic factors of the CAR-T itself, the underlying disease for which the CAR-T is being administered and relevant predisposing factors of the patient being treated. For instance, in pediatric patients with previous treatment-related neurologic insult or concern for bulky CNS disease we begin prophylactic treatment with levetiracetam to prevent seizure activity. Similarly, while we have not had any incidence of posterior reversible encephalopathy syndrome (PRES) or cerebral edema in pediatric patients receiving a 4-IBB CART construct for acute lymphoblastic leukemia, other centers have seen severe PRES and cerebral edema in adult patients receiving a CD28 CART construct for NHL.87 These vast differences underlie the difficulties in elucidating a single mechanism to explain CAR-T neurotoxicity.
Treatment for CAR-T neurotoxicity is mostly supportive. Other than prophylactic levetiracetam at time of CAR-T infusion, there are limited data on other interventions. Though tocilizumab is FDA approved for CRS, it has not been shown to have any positive effect on neurotoxicity.88 This may be in part because it is does cross the BBB. As corticosteroids have potential detrimental effect on CAR-T anti-leukemic efficacy and there are no clear data they help with CRES, we do not routinely use them in pediatric patients with neurotoxicity. In other centers however, corticosteroids are used in cases of severe CRES and they should always be considered in patients suffering from cerebral edema.
Many of the therapeutic targets currently being studied for CRS have shown some efficacy in management of neurotoxicity as well. For instance, anakinra and lenzilumab have both shown impressive data with respect to CRS and neurotoxicity management in mouse models.64,75 While anakinra was shown to prevent toxicity, it has not yet been tested to evaluate if it can reverse neurotoxicity in mice. Trials in humans are needed as well. Further investigations on these agents as well as others (ex: IL2 and IL15 targeted) are needed to identify optimal treatments that will moderate the natural history of CAR-T-induced neurotoxicity while sparing CAR-T mediated leukemia destruction. Recombinant BowANG1 is an attractive therapeutic as it restores the ANG1:ANG2 ratio and the CNS EC integrity. This therapeutic is already in consideration for cerebral malaria, which has similar biology to the proposed mechanisms of CRES.89 Given elevated excitatory neurotransmitters in CSF of patients with CRES, NMDA and AMPA receptor blockade agents may be investigated in the future to ameliorate symptoms. Finally, intrathecal IL6 and IL6R blockade is another possible approach as these monoclonal antibodies do not cross the BBB. Just as in CRS, biomarkers that are predictive of neurotoxicity have preliminarily been identified (elevated sTNFR-1 levels and decreased sCD30 levels) and will be vital for therapeutic intervention.7
5 ∣. OTHER SIDE EFFECTS
An expected, the side effect of CAR-T therapy directed at B-cell antigens is B-cell aplasia and resultant hypogammaglobulinemia.1 Since CD19 is expressed on most B-cells, CAR-T cannot distinguish between healthy B-cell populations and malignant cells. Investigators have used B-cell aplasia in a clinical setting as a surrogate to determine CAR-T persistence. Early B-cell recovery, particularly within 3 months of initial CAR-T infusion has been associated with higher risk of relapse. In these cases, for our patients we will often re-infuse CAR-T. The hypogammaglobulinemia itself, the persistence of which is unknown, is treated with immunoglobulin G replacement according to formulary and institutional practices. Long-term hypogammaglobulinemia does have a potential increased risk of developing autoimmune diseases not directly related to the CAR-T therapy. Other cytopenias may also occur.90 Preclinical data on CD123 directed CAR-T have also shown bone marrow aplasia necessitating hematopoietic stem cell transplant after CAR-T infusion.
In adult patients, cardiac dysrhythmias have been associated with post-CAR-T cytokine release and thus some centers employ pre-emptive cardiac monitoring with telemetry.67 Cardiac toxicity of CAR-T is also under investigation. In a recent analysis of pediatric patients who received CD19 directed CAR-T at CHOP, 10% of patients had systolic dysfunction in the immediate postinfusion period. This dysfunction was transient and persistence was rare. Investigators noted that persistent blast count >25% or pre-existing cardiac dysfunction was associated with need for ionotropic support.91 This however is only one patient cohort with one CAR-T construct and thus further investigation is required to assess if there is persistent cardiac toxicity secondary to CAR-T.
The unique nature of CAR-T and the fact that cells can persist for years and provide continuous surveillance of the marrow and periphery provide a beneficial therapeutic profile against potential relapse. We do not however have any experience of what effects CAR-T persistence will have in the long-term. One instance of potential concern would be the effect of persistent CAR-T in a patient that becomes pregnant as it is unknown what effects they may have on B-cell development in the fetus. Long-term neurological and neurocognitive sequelae are also unknown. Given the multitude of side effects that are possible with CAR-T it will be critical to monitor new CAR-T constructs in new indications for additional and unique toxicities.
6 ∣. CONCLUSION
Chimeric antigen receptor T-cells are uniquely positioned as the first personalized ”living therapy”. By hijacking the body's own natural immune response and unleashing it on a singular antigenic target, the entire might of the immune system is focused on the destruction of tumor cells. Similar to tumor lysis syndrome as a byproduct of cytotoxins released from indiscriminate tumor destruction by conventional chemotherapy, the broad and overwhelming release of cytokines, chemokines, and other immune mediators can lead to serious adverse events in the patient.
Cytokine release syndrome, CRES, and other immune-related complications are directly and indirectly related to the robust T-cell activation in these patients. This is tightly correlated with antigen load as demonstrated by tumor burden at time of CAR-T infusion. While tocilizumab is FDA approved for the treatment of CRS, further studies are indeed needed to evaluate if it is the optimal therapy. Ideally, a therapeutic intervention would be able to ameliorate both CRS and CRES pathophysiology. In order to rationally develop therapies that are better suited for CAR-T-induced cytotoxicities, a better understanding of the mechanisms that mediate these syndromes is necessary. These mechanisms are dependent on a number of individual and cohort characteristics and include: CAR-T construct and co-stimulatory molecules, patient demographics and comorbidities, tumor type, and status at time of therapy. Biomarkers that are predictive of patients that will progress to serious sequelae will also be vital in helping appropriately manage CAR-T sequelae.
Chimeric antigen receptor T and associated cellular therapies have heralded the next phase of personalized medicine and have indeed redefined that very concept. The side effects that are related to the use of these therapies are also unique in that they cannot be categorized as a general entity but rather need to be understood in the context of the individual circumstances and predisposing conditions of each patient. Thus, it is likely that a universal therapy for CRS, CRES, and other toxicities will not be easily attainable. Rather, it is more likely that a set of principles that guide the treatment of these toxicities will be developed that can then further be refined and individualized based on multi-modal therapeutic interventions for certain cohorts of patients with distinct similarities. While this may make the management of post-CAR-T complications more difficult, it will lead to optimal outcomes both from an oncologic remission standpoint as well as a CAR-T side effect profile standpoint. This approach to toxicity management will enable the eventual increased use of CAR-T in additional indications as well as in patients with additional risk factors.
ACKNOWLEDGEMENTS
David T. Teachey receives grant funding from the NIH/NCI (R01CA193776), the Leukemia and Lymphoma Society, Cookies for Kids Cancer, and the American Cancer Society. Joseph H. Oved is funded by National Institute of Health (NIH), National Heart, Lung, and Blood Institute (NHLBI) Grant No. T32 HL715041.
Funding information
NIH/NCI, Grant/Award Number: R01CA193776; Leukemia and Lymphoma Society; Cookies for Kids Cancer; American Cancer Society; National Heart, Lung, and Blood Institute, Grant/Award Number: T32 HL715041
Footnotes
CONFLICT OF INTEREST
David T. Teachey serves on advisory boards for La Roche and Amgen. Though not related to this manuscript, Joseph H. Oved consults for Emendo Bio. JHO owns stock in Gilead and Incyte.
REFERENCES
- 1.Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378(5):439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet (London, England). 2015;385(9967):517–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frey N, Porter D. Cytokine release syndrome with chimeric antigen receptor T cell therapy. Biol Blood Marrow Transplant. 2019;25(4):e123–el27. [DOI] [PubMed] [Google Scholar]
- 5.Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124(2):188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maude SL, Barrett D, Teachey DT, Grupp SA. Managing cytokine release syndrome associated with novel T cell-engaging therapies. Cancer J (Sudbury, Mass). 2014;20(2):119–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gofshteyn JS, Shaw PA, Teachey DT, et al. Neurotoxicity after CTL019 in a pediatric and young adult cohort. Ann Neurol. 2018;84(4):537–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gross G, Waks T, Eshhar Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc Natl Acad Sci USA. 1989;86(24): 10024–10028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abate-Daga D, Davila ML. CAR models: next-generation CAR modifications for enhanced T-cell function. Mol Ther Oncolytics. 2016;3:16014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fry TJ, Shah NN, Orentas RJ, et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med. 2018;24(1):20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Enblad G, Karlsson H, Gammelgård G, et al. A phase I/IIa trial using CD19-targeted third-generation CAR T cells for lymphoma and leukemia. Clin Cancer Res. 2018;24(24):6185–6194. [DOI] [PubMed] [Google Scholar]
- 12.Guedan S, Posey AD Jr, Shaw C, et al. T cell persistence through ICOS and 4-1BB costimulation. JCI Insight. 2018;3(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gargett T Brown MP. The inducible caspase-9 suicide gene system as a “safety switch” to limit on-target, off-tumor toxicities of chimeric antigen receptor T cells. Front Pharmacol. 2014;5:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou X, Di Stasi A, Brenner MK. iCaspase 9 suicide gene system. Methods Mol Biol (Clifton, NJ). 2015;1317:87–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X, Chang W-C, Wong CW, et al. A transgene-encoded cell surface polypeptide for selection, in vivo tracking, and ablation of engineered cells. Blood. 2011;118(5):1255–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365(8):725–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Porter DL, Hwang WT, Frey NV, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med. 2015;7(303):303ra139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turtle CJ, Hay KA, Hanafi L-A, et al. Durable molecular remissions in chronic lymphocytic leukemia treated with CD19-specific chimeric antigen receptor-modified T cells after failure of ibrutinib. J Clin Oncol. 2017;35(26):3010–3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davila ML, Riviere I, Wang X, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014;6(224):224ra225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park JH, Rivière I, Gonen M, et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med. 2018;378(5):449–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yates B, Shalabi H, Salem D, et al. Sequential CD22 targeting impacts CD22 CAR-T cell response. Blood. 2018;132(Suppl 1):282–282. [Google Scholar]
- 23.Locke FL, Ghobadi A, Jacobson CA, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 2019;20(1):31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B cell lymphoma. N Engl J Med. 2019;380(1):45–56. [DOI] [PubMed] [Google Scholar]
- 25.Fraietta JA, Lacey SF, Orlando EJ, et al. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat Med. 2018;24(5):563–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Das RK, Vernau L, Grupp SA, Barrett DM. Naive T-cell deficits at diagnosis and after chemotherapy impair cell therapy potential in pediatric cancers. Cancer Discov. 2019;9(4):492–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fraietta JA, Beckwith KA, Patel PR, et al. Ibrutinib enhances chimeric antigen receptor T-cell engraftment and efficacy in leukemia. Blood. 2016;127(9):1117–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sidaway P Haematological cancer: ibrutinib supercharges CAR T cells. Nat Rev Clin Oncol. 2016;13(4):204. [DOI] [PubMed] [Google Scholar]
- 29.Gargett T, Yu W, Dotti G, et al. GD2-specific CAR T cells undergo potent activation and deletion following antigen encounter but can be protected from activation-induced cell death by PD-1 blockade. Mol Ther. 2016;24(6):1135–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ali SA, Shi V, Marie I, et al. T cells expressing an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of multiple myeloma. Blood. 2016;128(13):1688–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao W-H, Liu J, Wang B-Y, et al. A phase 1, open-label study of LCAR-B38M, a chimeric antigen receptor T cell therapy directed against B cell maturation antigen, in patients with relapsed or refractory multiple myeloma. J Hematol Oncol. 2018;11(1):141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brudno JN, Marie I, Hartman SD, et al. T cells genetically modified to express an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of poor-prognosis relapsed multiple myeloma. J Clin Oncol. 2018;36(22):2267–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qin H, Cho M, Haso W, et al. Eradication of B-ALL using chimeric antigen receptor-expressing T cells targeting the TSLPR oncoprotein. Blood. 2015;126(5):629–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mihara K, Yoshida T, Takei Y, et al. T cells bearing anti-CD19 and/or anti-CD38 chimeric antigen receptors effectively abrogate primary double-hit lymphoma cells. J Hematol Oncol. 2017;10(1):116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cooper ML, Choi J, Staser K, et al. An “off-the-shelf” fratricide-resistant CAR-T for the treatment of T cell hematologic malignancies. Leukemia. 2018;32(9):1970–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mamonkin M, Rouce RH, Tashiro H, Brenner MK. A T-cell-directed chimeric antigen receptor for the selective treatment of T-cell malignancies. Blood. 2015;126(8):983–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raikar SS, Fleischer LC, Moot R, et al. Development of chimeric antigen receptors targeting T-cell malignancies using two structurally different anti-CD5 antigen binding domains in NK and CRISPR-edited T cell lines. Oncoimmunology. 2018;7(3):e1407898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mardiros A, Dos Santos C, McDonald T, et al. T cells expressing CD123-specific chimeric antigen receptors exhibit specific cytolytic effector functions and antitumor effects against human acute myeloid leukemia. Blood. 2013;122(18):3138–3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li S, Tao Z, Xu Y, et al. CD33-specific chimeric antigen receptor T cells with different co-stimulators showed potent anti-leukemia efficacy and different phenotype. Hum Gene Ther. 2018;29(5):626–639. [DOI] [PubMed] [Google Scholar]
- 40.Kenderian SS, Ruella M, Shestova O, et al. CD33-specific chimeric antigen receptor T cells exhibit potent preclinical activity against human acute myeloid leukemia. Leukemia. 2015;29(8):1637–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim MY, Yu KR, Kenderian SS, et al. Genetic inactivation of CD33 in hematopoietic stem cells to enable CAR T cell immunotherapy for acute myeloid leukemia. Cell. 2018;173(6):1439–1453.e1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mount CW, Majzner RG, Sundaresh S, et al. Potent antitumor efficacy of anti-GD2 CAR T cells in H3-K27M(+) diffuse midline gliomas. Nat Med. 2018;24(5):572–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Richman SA, Nunez-Cruz S, Moghimi B, et al. High-affinity GD2-specific CAR T cells induce fatal encephalitis in a preclinical neuroblastoma model. Cancer Immunol Res. 2018;6(1):36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Louis CU, Savoldo B, Dotti G, et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood. 2011;118(23):6050–6056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pule MA, Savoldo B, Myers GD, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med. 2008;14(11):1264–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qin H, Ramakrishna S, Nguyen S, et al. Preclinical development of bivalent chimeric antigen receptors targeting both CD19 and CD22. Mol Ther Oncolytics. 2018;11:127–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ruella M, Barrett DM, Kenderian SS, et al. Dual CD19 and CD123 targeting prevents antigen-loss relapses after CD19-directed immunotherapies. J Clin Investig. 2016;126(10):3814–3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gardner R, Annesley C, Finney O, et al. Early clinical experience of CD19 x CD22 dual specific CAR T cells for enhanced anti-leukemic targeting of acute lymphoblastic leukemia. Blood. 2018;132(Suppl 1):278–278. [Google Scholar]
- 49.Ghosh A, Smith M, James SE, et al. Donor CD19 CAR T cells exert potent graft-versus-lymphoma activity with diminished graft-versus-host activity. Nat Med. 2017;23(2):242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qasim W, Zhan H, Samarasinghe S, et al. Molecular remission of infant B-ALL after infusion of universal TALEN gene-edited CAR T cells. Sci Transl Med. 2017;9(374):eaaj2013. [DOI] [PubMed] [Google Scholar]
- 51.Barrett DM, Teachey DT, Grupp SA. Toxicity management for patients receiving novel T-cell engaging therapies. Curr Opin Pediatr. 2014;26(1):43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fitzgerald JC, Weiss SL, Maude SL, et al. Cytokine release syndrome after chimeric antigen receptor T cell therapy for acute lymphoblastic leukemia. Crit Care Med. 2017;45(2):e124–e131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Teachey DT, Lacey SF, Shaw PA, et al. Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor t-cell therapy for acute lymphoblastic leukemia. Cancer Discov. 2016;6(6):664–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Teachey DT, Rheingold SR, Maude SL, et al. Cytokine release syndrome after blinatumomab treatment related to abnormal macrophage activation and ameliorated with cytokine-directed therapy. Blood. 2013;121(26):5154–5157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abboud R, Keller J, Slade M, et al. Severe cytokine-release syndrome after T cell-replete peripheral blood haploidentical donor transplantation is associated with poor survival and anti-IL-6 therapy is safe and well tolerated. Biol Blood Marrow Transplant. 2016;22(10):1851–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rotz SJ, Leino D, Szabo S, Mangino JL, Turpin BK, Pressey JG. Severe cytokine release syndrome in a patient receiving PD-1-directed therapy. Pediatr Blood Cancer. 2017;64(12):e26642. [DOI] [PubMed] [Google Scholar]
- 57.Teachey DT, Grupp SA. Cytokine release syndrome after haploidentical stem cell transplantation. Biol Blood Marrow Transplant. 2016;22(10):1736–1737. [DOI] [PubMed] [Google Scholar]
- 58.Porter D, Frey N, Wood PA, Weng Y, Grupp SA. Grading of cytokine release syndrome associated with the CAR T cell therapy tisagenlecleucel. J Hematol Oncol. 2018;11(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee DW, Santomasso BD, Locke FL, et al. ASBMT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019;25(4):625–638. [DOI] [PubMed] [Google Scholar]
- 60.Gomez-Salinero JM, Rafii S. Plasmin regulation of acute cytokine storm. Blood. 2017;130(1):5–6. [DOI] [PubMed] [Google Scholar]
- 61.Santomasso BD, Park JH, Salloum D, et al. Clinical and biological correlates of neurotoxicity associated with CAR T-cell therapy in patients with B-cell acute lymphoblastic leukemia. Cancer Discov. 2018;8(8):958–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Singh N, Hofmann TJ, Gershenson Z, et al. Monocyte lineage-derived IL-6 does not affect chimeric antigen receptor T-cell function. Cytotherapy. 2017;19(7):867–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Norelli M, Camisa B, Barbiera G, et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat Med. 2018;24(6):739–748. [DOI] [PubMed] [Google Scholar]
- 64.Giavridis T, van der Stegen S, Eyquem J, Hamieh M, Piersigilli A, Sadelain M. CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat Med. 2018;24(6):731–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Le RQ, Li L, Yuan W, et al. FDA approval summary: tocilizumab for treatment of chimeric antigen receptor T cell-induced severe or life-threatening cytokine release syndrome. Oncologist. 2018;23(8):943–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Research FCfDEa. Tocilizumab drug labeling. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/125276Orig1s114lbl.pdf. Accessed March 10, 2019.
- 67.Neelapu SS, Tummala S, Kebriaei P, et al. Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15(1):47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Teachey DT, Bishop MR, Maloney DG, Grupp SA. Toxicity management after chimeric antigen receptor T cell therapy: one size does not fit 'ALL'. Nat Rev Clin Oncol. 2018;15(4):218. [DOI] [PubMed] [Google Scholar]
- 69.Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. The New England journal of medicine. 2013;368(16):1509–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kenderian SS, Ruella M, Shestova O, et al. Ruxolitinib prevents cytokine release syndrome after CART cell therapy without impairing the anti-tumor effect in a xenograft model. Blood. 2016;128(22):652–652. [Google Scholar]
- 71.Broglie L, Pommert L, Rao S, et al. Ruxolitinib for treatment of refractory hemophagocytic lymphohistiocytosis. Blood Advances. 2017;1(19):1533–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maschalidi S, Sepulveda FE, Garrigue A, Fischer A, de Saint BG. Therapeutic effect of JAK1/2 blockade on the manifestations of hemophagocytic lymphohistiocytosis in mice. Blood. 2016;128(1):60–71. [DOI] [PubMed] [Google Scholar]
- 73.Weber EW, Lynn RC, Sotillo E, Lattin J, Xu P, Mackall CL. Pharmacologic control of CAR-T cell function using dasatinib. Blood Advances. 2019;3(5):711–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Staedtke V, Bai RY, Kim K, et al. Disruption of a self-amplifying catecholamine loop reduces cytokine release syndrome. Nature. 2018;564(7735):273–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sterner RM, Sakemura R, Cox MJ, et al. GM-CSF inhibition reduces cytokine release syndrome and neuroinflammation but enhances CAR-T cell function in xenografts. Blood. 2019;133(7):697–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.El-Osta H, Janku F, Kurzrock R. Successful treatment of Castleman's disease with interleukin-1 receptor antagonist (Anakinra). Mol Cancer Ther. 2010;9(6):1485–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hay KA, Hanafi L-A, Li D, et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T-cell therapy. Blood. 2017;130(21):2295–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Institute NC.Terminology criteria for adverse events (CTCAE) 5.0. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf. Published 2017. Accessed March 5, 2019.
- 79.Mahadeo KM, Khazal SJ, Abdel-Azim H, et al. Management guidelines for paediatric patients receiving chimeric antigen receptor T cell therapy. Nat Rev Clin Oncol. 2019;16(1):45–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Traube C, Silver G, Kearney J, et al. Cornell assessment of pediatric Delirium: a valid, rapid, observational tool for screening delirium in the PICU*. Crit Care Med. 2014;42(3):656–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wilke AC, Gokbuget N. Clinical applications and safety evaluation of the new CD19 specific T-cell engager antibody construct blinatumomab. Expert Opin Drug Saf. 2017;16(10):1191–1202. [DOI] [PubMed] [Google Scholar]
- 82.Shalabi H, Wolters PL, Martin S, et al. Systematic evaluation of neurotoxicity in children and young adults undergoing CD22 chimeric antigen receptor T-cell therapy. J Immunother (Hagerstown, Md: 1997). 2018;41(7):350–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Topp MS, Gökbuget N, Stein AS, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol. 2015;16(1):57–66. [DOI] [PubMed] [Google Scholar]
- 84.Stein AS, Schiller G, Benjamin R, et al. Neurologic adverse events in patients with relapsed/refractory acute lymphoblastic leukemia treated with blinatumomab: management and mitigating factors. Ann Hematol. 2019;98(1):159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gust J, Hay KA, Hanafi L-A, et al. Endothelial activation and blood-brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Cancer Discov. 2017;7(12):1404–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mackall CL, Miklos DB. CNS endothelial cell activation emerges as a driver of CAR T cell-associated neurotoxicity. Cancer Discov. 2017;7(12):1371–1373. [DOI] [PubMed] [Google Scholar]
- 87.Abbasi J, Amid F. Approval filings, another CAR-T therapy patient death. JAMA. 2017;317(22):2271. [DOI] [PubMed] [Google Scholar]
- 88.Karschnia P, Jordan JT, Forst DA, et al. Clinical presentation, management, and biomarkers of neurotoxicity after adoptive immunotherapy with CAR T-cells. Blood. 2019 10.1182/blood-2018-12-893396 [DOI] [PubMed] [Google Scholar]
- 89.Higgins SJ, Purcell LA, Silver KL, et al. Dysregulation of angiopoietin-1 plays a mechanistic role in the pathogenesis of cerebral malaria. Sci Transl Med. 2016;8(358):358ral28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fried S, Avigdor A, Bielorai B, et al. Early and late hematologic toxicity following CD19 CAR-T cells. Bone Marrow Transplant. 2019. 10.1038/s41409-019-0487-3 [DOI] [PubMed] [Google Scholar]
- 91.Burstein DS, Maude S, Grupp S, Griffis H, Rossano J, Lin K. Cardiac profile of chimeric antigen receptor T cell therapy in children: A single-institution experience. Biol Blood Marrow Transplant. 2018;24(8):1590–1595. [DOI] [PubMed] [Google Scholar]