Abstract
In locally advanced lung cancer, established baseline clinical variables show limited prognostic accuracy and 18F-fluorodeoxyglucose positron emission tomography (FDG PET) radiomics features may increase accuracy for optimal treatment selection. Their robustness and added value relative to current clinical factors are unknown. Hence, we identify robust and independent PET radiomics features that may have complementary value in predicting survival endpoints. A 4D PET dataset (n = 70) was used for assessing the repeatability (Bland-Altman analysis) and independence of PET radiomics features (Spearman rank: |ρ|<0.5). Two 3D PET datasets combined (n = 252) were used for training and validation of an elastic net regularized generalized logistic regression model (GLM) based on a selection of clinical and robust independent PET radiomics features (GLMall). The fitted model performance was externally validated (n = 40). The performance of GLMall (measured with area under the receiver operating characteristic curve, AUC) was highest in predicting 2-year overall survival (0.66±0.07). No significant improvement was observed for GLMall compared to a model containing only PET radiomics features or only clinical variables for any clinical endpoint. External validation of GLMall led to AUC values no higher than 0.55 for any clinical endpoint. In this study, robust independent FDG PET radiomics features did not have complementary value in predicting survival endpoints in lung cancer patients. Improving risk stratification and clinical decision making based on clinical variables and PET radiomics features has still been proven difficult in locally advanced lung cancer patients.
Introduction
Despite the emergence of new technologies and treatment options such as tyrosine kinase inhibitors targeted towards mutations, and immune checkpoint inhibitors, the global survival of lung cancer patients has improved only gradually in the last decades [1–4]. Locally advanced non-small cell lung cancer (NSCLC) is a highly heterogeneous disease where only modest improvements in survival have been observed, with the exception of chemoradiotherapy (CRT) patients treated with the anti-PD-L1 antibody Durvalumab whose overall and progression-free survival significantly improved compared to those receiving CRT alone [5]. New approaches are urgently needed for the selection of treatment strategies for NSCLC patients, which are currently determined mainly by TNM staging [6, 7]. In addition to TNM staging, other well-established, reproducible, independent prognostic factors are used to guide clinicians in making treatment decisions, such as Eastern Cooperative Oncology Group (ECOG) performance status [8, 9], weight loss [10], and gender [11]. Numerous other biomarkers have been investigated, although less reproducible, such as histology [12], age [13], serum blood levels [14, 15], mutation status [16], and protein expression levels [17, 18]. In locally advanced NSCLC, treatment selection based on TNM staging and other clinical variables may not be accurate enough for survival probability prediction [19, 20]. Therefore, the search for more accurate reproducible independent prognostic factors is warranted in the context of personalized medicine.
A current field of interest is the assessment of quantitative image features and its complementary value to well-established clinical prognostic models. Radiomics has been introduced as a sophisticated way to extract and mine a large number of quantitative image features, primarily using anatomical CT information [21]. The basic assumption of radiomics is that underlying tumour biology could be captured [22]. This information may actually be better characterized with functional imaging such as 18F-fluorodeoxyglucose Positron Emission Tomography (FDG PET), the gold standard in NSCLC diagnosis and staging, which is able to characterize molecular heterogeneity in lung cancer [23, 24]. It is therefore worthwhile to investigate the prognostic performance of radiomics features from functional imaging such as PET.
Basic PET radiomics features have provided clinically relevant prognostic information for NSCLC patients. Examples include standardized uptake value (SUV) based metrics like maximum, peak, and mean SUV (SUVmax, SUVpeak, and SUVmean, respectively), metabolic tumour volume (MTV), and total lesion glycolysis (TLG) [25–32]. The more advanced PET texture features employed for quantification of tumour heterogeneity, have also been reported to be of prognostic value [33–41]. However, the variable nature of PET imaging makes it difficult to reproduce these results [42, 43].
Furthermore, PET texture features can also be subject to differences in reconstruction settings and delineation methods [44], SUV binning methods [45, 46], and feature calculation methods [47]. It is not yet clear which PET radiomics features are insensitive to all of these factors, and also to what degree.
Regardless of the issues with variability, complementary PET radiomics features should be independent from well-known prognostic SUV metrics, such as MTV and SUVmax. Some investigators reported specific PET texture features that were associated with MTV [37, 39, 47, 48, 49]. In these cases, prognostic texture features would rather act as a surrogate than as an independent variable. Such an association is also not warranted for clinical variables. Hence, the relationship of PET texture features with well-known prognostic factors has to be thoroughly studied too.
With all the confounding factors described above, in combination with the high number of possible radiomics features, it is not surprising that false discovery rates are high amongst FDG PET and CT studies on texture features [50]. Without proven, robust, and independent prognostic PET texture features, it will be challenging to move further in the field. Therefore, this study aims to investigate the repeatability of PET radiomics features, and also assesses the relationship with well-known prognostic factors in PET, such as MTV and SUVmax. The rationale is to identify a group of radiomics features derived from pre-treatment PET imaging that are robust, independent, and prognostic, with possible additional value to current clinical prognostic variables.
Materials and methods
Patient data
Three NSCLC patient cohorts from the Netherlands Cancer Institute (NKI) and one from the Peter MacCallum Cancer Centre (PMCC) were included in this study to develop and validate a radiomics signature. Peter MacCallum Cancer Centre Ethics and Clinical Research Committees approval was granted and all research was performed in accordance with relevant guidelines/regulations. Patient’s written, informed consent was obtained. An overview of the datasets is given in Table 1. Patients were excluded if the primary tumour was smaller than 10 cc or if the patient had stage IV NSCLC at baseline. To detect brain metastases at baseline, the NKI patients were scanned with MR imaging and the PMCC performed FLT baseline scans before treatment.
Table 1. Overview of the four patient cohorts used in the study.
Unless otherwise stated, values represent the median with the range in parentheses. MTV2.5 = metabolic tumour volume obtained using a SUV threshold of 2.5, MTV40 = metabolic tumour volume obtained using a threshold of 40% of the maximum intensity, SUVmax = maximum SUV uptake, OS = overall survival, PFS = progression-free survival, LRS = local recurrence-free survival, DMS = distant metastases-free survival. Nos = not otherwise specified.
| 4D PET lung | NKI lung 1 | NKI lung 2 | PMCC lung 1 | |
|---|---|---|---|---|
| No. of patients | 70 | 228 | 24 | 40 |
| Age (year) | n/a | 64 (36–87) | 63 (39–82) | 68 (53–86) |
| Gender | n/a | |||
| Male | 142 | 13 | 29 | |
| Female | 86 | 11 | 11 | |
| Disease stage | n/a | IA-IIIC | IIB-IIIC | IB-IIIC |
| IA | 1 | 0 | 0 | |
| IB | 4 | 0 | 1 | |
| IIA | 0 | 0 | 3 | |
| IIB | 16 | 2 | 5 | |
| IIIA | 102 | 7 | 15 | |
| IIIB | 82 | 13 | 12 | |
| IIIC | 23 | 2 | 4 | |
| T stage | n/a | |||
| 1 | 6 | 1 | 5 | |
| 2 | 78 | 4 | 24 | |
| 3 | 63 | 7 | 9 | |
| 4 | 81 | 12 | 2 | |
| N stage | n/a | |||
| 0 | 42 | 4 | 5 | |
| 1 | 20 | 2 | 5 | |
| 2 | 130 | 13 | 17 | |
| 3 | 36 | 5 | 13 | |
| Histology | n/a | |||
| Adeno | 80 | 12 | 16 | |
| Squamous cell | 83 | 7 | 13 | |
| Large cell | 8 | 1 | 5 | |
| Nos or other | 57 | 4 | 6 | |
| GTV (cc) | n/a | 118 (15–906) | 84 (10–351) | 49 (12–544) |
| MTV2.5 (cc) | 62 cc (10–545) | 72 (10–693) | 91 (11–337) | 51 (8–478) |
| MTV40 (cc) | 27 cc (4–169) | 31 (3–394) | 34 (5–289) | 31 (4–378) |
| SUVmax | 11.5 (4.3–55.1) | 14.6 (5.9–44.8) | 15.7 (6.9–28.3) | 16.5 (6.3–33.2) |
| Median follow-up time (months) | n/a | 17 | 22 | 24 |
| 2-year OS | n/a | 40% | 46% | 54% |
| 2-year PFS | n/a | 29% | 21% | 20% |
| 1-year PFS | n/a | 50% | 54% | 35% |
| 1-year LRS | n/a | 58% | 71% | 47% |
| 1-year DMS | n/a | 54% | 54% | 45% |
The repeatability and independence of PET radiomics features was assessed using a 4D PET/CT dataset (4D PET lung) consisting of 70 stage III NSCLC patients. No clinical data was collected for these patients. The second cohort (NKI lung 1) contained 228 patients treated with concurrent chemoradiotherapy (CCRT) for stage IA-IIIC NSCLC in the NKI between 2007 and 2011 as described earlier [51]. The third cohort, also from the NKI (NKI lung 2), consisted of 24 patients with stage IIB-IIIC NSCLC treated between 2013 and 2016, similar as NKI lung 1. The fourth cohort was from the PMCC (PMCC lung 1) and involved 40 stage IB-IIIC NSCLC patients treated with CCRT as previously reported [32].
Clinical endpoints for prognostic model
The primary endpoint used for the prognostic model was two-year overall survival (2-year OS). Overall survival was defined as the time between the start of treatment and date of death. In addition, two-year progression-free survival (2-year PFS), one-year PFS (1-year PFS), one-year local recurrence-free survival (1-year LRS), and one-year distant metastases-free survival (1-year DMS) were also studied. Progression was defined as growth of tumour cells in the primary tumour or involved lymph nodes, or metastases to other organs, or death. LRS was defined as progression in the primary tumour and/or involved lymph nodes as assessed on follow-up scans. DMS was described according to the 8th edition of the TNM classification for NSCLC [52] as evaluated on follow-up scans.
Data acquisition and image reconstruction
Patients from the NKI lung 1 and 2 dataset both underwent a whole-body FDG PET/CT using a Gemini TF or Gemini TF Big Bore scanner (Philips Medical Systems, Cleveland, OH). The reconstruction voxel size of the PET data was 4 × 4 × 4 mm3. Patients fasted for at least 8 h to ensure low levels of serum glucose. Patients with a Body Mass Index (BMI)≤28 were intravenously injected with 190 MBq 18F-FDG, or 240 MBq in case of a BMI>28. Patients were scanned 60 minutes after injection of 18F-FDG. The acquisition time of the PET/CT scanner was 2 minutes per bed position.
In the PMCC lung 1 cohort, whole-body FDG PET/CT scans were acquired on a GE STE (GE Medical Systems, Milwaukee, WI) or Biograph (Siemens Medical Solutions, Erlangen, Germany) scanner. The reconstructed voxel size of the PET data was 4.3 × 4.3 × 3.3 mm3 for the GE STE scanner, and 4.1 × 4.1 × 3.0 mm3 for the Siemens Biograph scanner. Patients fasted for more than 6 hours before 18F-FDG scans. Patients were intravenously injected with 4.2 MBq/kg 18F-FDG. Baseline emission scans were initiated 60 minutes after injection. The acquisition time of the PET/CT scanner was 3 minutes per bed position.
For the 4D PET lung dataset, scans were acquired on a Gemini TF scanner (Philips Medical Systems, Cleveland, OH). The reconstruction voxel size of the PET data was 4 × 4 × 4 mm3. The 4D PET/CT data were reconstructed in 10 phases, and the attenuation in each frame of the 4D PET data was corrected with the corresponding 4D CT frame. The acquisition time of the 4D PET was kept the same as that used for 3D PET [52].
Mid-position scans from 4D PET lung dataset for repeatability testing
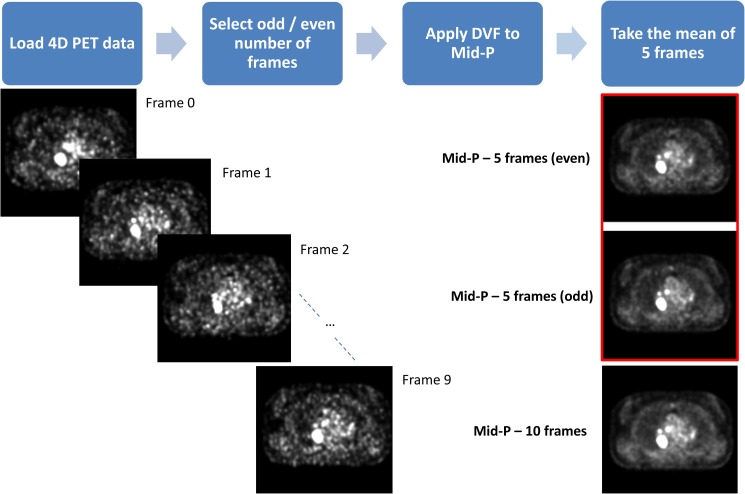
The 4D PET/CT data were reconstructed in 10 phases, and from these phases two new mid-position scans were derived [53]. The first mid-position scan was created from the even phases (0, 2, 4, 6, and 8) and is named ‘Mid-P even’, and the odd phases (1, 3, 5, 7, and 9) were used to create the second mid-position scan ‘Mid-P odd’. The even and odd number of frames were selected to keep the amount of tumour motion balanced in both scans. Fig 1 gives an overview of the workflow.
Fig 1. Workflow of the PET mid-position scans.
A 4D PET scan was loaded for each patient consisting of 10 frames, where the odd or even number of frames were selected. A 4D deformation vector field (DVF) was applied to these frames to deform them to the mid-position. Lastly, the mean of the 5 deformed frames was calculated to obtain the PET mid-position scan. For comparison, the PET mid-position scan obtained from 10 frames has been included in the image too. Mid-P = PET mid-position scan.
The source of variability was different in these two mid-position scans compared to a test–retest setting, since the biological tumour variability has been eliminated. In this case, the variability was mostly caused by minor differences in noise-levels and tumour motion, hence robust quantitative features should not differ substantially in outcome.
Tumour segmentation
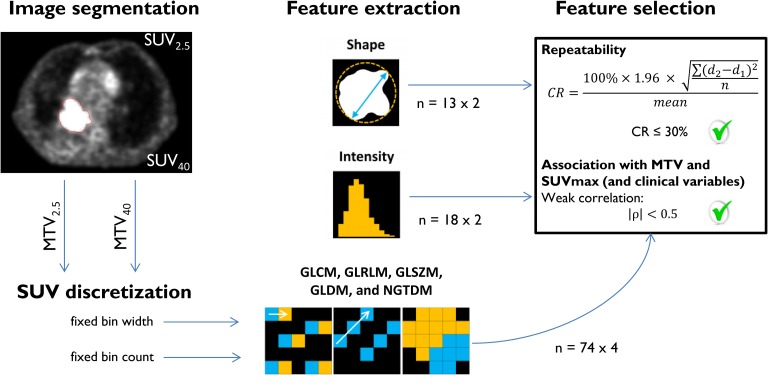
For each patient in the NKI lung 1, NKI lung 2, and PMCC lung 1 cohort, a volume-of-interest (VOI) enveloping the primary tumour was manually drawn by radiation oncologists using information from both PET and CT imaging. From this VOI, the MTV was auto-segmented on the FDG PET scan. Two auto-segmentation methods were applied: a metabolic tumour region delineation that included all voxel intensities above 2.5 (SUV2.5), and a high intensity delineation that included all voxel intensities that were at least 40% of the SUVmax (SUV40). Auto-segmentation was performed with in-house developed software named Match42 (version 1.0.0) using a Python plug-in. The metabolic tumour volume obtained from SUV2.5 and SUV40 were named MTV2.5 and MTV40, respectively. In the 4D PET lung dataset, a VOI was manually drawn around the primary tumour in one PET mid-position scan, and copied to the second PET mid-position scan. The auto-segmentation was performed on both PET mid-position scans independently.
PET radiomics features
The Pyradiomics toolkit was used for radiomics feature extraction [54]. With this toolkit a total of 105 features were available for feature calculations. These were divided into 18 first-order features, 13 shape features (including metabolic tumour volume), and 74 texture features describing the spatial distribution of voxel intensities. The texture features were derived from the gray level co-occurrence matrix (GLCM; 23 features) [55], gray level run-length matrix (GLRLM; 16 features) [56], gray level size-zone matrix (GLSZM; 16 features) [57], gray level dependence matrix (GLDM; 14 features) [58], and neighbourhood gray tone difference matrix (NGTDM; 5 features) [59]. The mathematical definitions of these features were in compliance with feature definitions as described by the Imaging Biomarker Standardization Initiative (IBSI) [60].
SUV discretization and matrix calculation
Before texture features were extracted, pre-processing steps were required in the form of SUV binning and matrix definition. SUV discretization is an intensity-resampling step, before building the texture matrices on which texture features rely. SUV discretization or binning was applied with the fixed bin count method (e.g. 64 bins) and an alternative method using a fixed bin width (e.g. 0.25 SUV). All texture features were calculated from a single matrix taking into account all 13 directions simultaneously. A more detailed description on SUV binning and matrix calculation can be found in S1 File, respectively.
Repeatability
The repeatability assessment was performed within the same patient comparing two different PET mid-position scans. For each patient, the PET mid-position scan obtained from the even numbered frames (Mid-P even) was compared with the PET mid-position scan from the odd numbered frames (Mid-P odd). This resulted in four comparisons: 2 SUV binning methods and 2 thresholding methods were applied.
The repeatability of each PET radiomics feature was assessed with the Coefficient of Repeatability (CR) [61]. See S1 File for more details. The CR was reported as a percentage: , where mean is the average of the PET radiomics feature value within the patient cohort. The threshold for poor repeatability was set to a value of 30%, corresponding to PET Response Criteria in Solid Tumours (PERCIST) [62].
Independence testing
To determine whether the features were correlated with the two commonly reported prognostic PET features MTV and SUVmax, the Spearman’s rank correlation coefficient (ρ) was calculated on one of the Mid-P scans, using the same set-up as for the repeatability testing. PET radiomics features that had a |ρ|≥0.5 were considered to have a correlation with MTV or SUVmax, and were discarded from further analysis. The choice of |ρ|<0.5 as limit for independent features was validated with the ‘elbow method’ using hierarchical clustering [63].
An overview of the radiomics workflow and feature selection procedure is given in Fig 2.
Fig 2. Radiomics feature selection workflow: From PET image segmentation to selected features.
Features from MTV2.5 and MTV40 were seen as a separate set of features, doubling the amount of features in the analysis. This also counts for features calculated with fixed bin width and fixed bin count, except for most intensity and shape features that were not affected by SUV discretization. An exception was observed for first-order features Uniformity and Entropy. A total of 360 PET radiomics features were entered into the analysis, including SUVmax, MTV2.5, and MTV40. PET radiomics features were selected for further analysis when two criteria were met: high repeatability and low association with MTV and SUVmax. SUV2.5 = SUV threshold of 2.5; SUV40 = SUV threshold of 40% of maximum SUV; MTV2.5 = metabolic tumour volume obtained from use of SUV2.5; MTV40 = metabolic tumour volume obtained from use of SUV40. GLCM = gray level co-occurrence matrix; GLRLM = gray level run-length matrix; GLSZM = gray level size-zone matrix; GLDM = gray level dependence matrix; NGTDM = neighbourhood gray tone difference matrix; CR = coefficient of repeatability.
Model training
An elastic net regularized generalized logistic regression model (GLM) was built with PET radiomics features derived from pre-treatment PET imaging (GLMrad). To increase the sample size in the training and test sets, for the purpose of building a GLM, NKI lung 1 and lung 2 were combined. In this study, 80% of the NKI data was used for training the model, and 20% for validation. Different ratios of training/validation were also tested, but were not reported as there was no major differences seen in the results. Elastic net regression analysis using the R package ‘glmnet’ was performed on the training set [64]. With 20-fold cross validation (CV), the most optimal fitted GLMrad with minimal CV error was determined and selected for model validation.
Model validation
To validate the fitted model of the training set, the area under the receiver operating characteristic curve (AUC) was calculated between the predicted outcome and the observed outcome in the validation set. To reduce randomness introduced by selecting a random subset of the complete data for training and validation, the procedure for model training and validation was repeated 100 times. This yields a better estimate of the true validation set performance by randomly simulating many scenarios with varying training and validation set compositions [65]. From the 100-times-repeated training/validation procedure, results were averaged, and the best performing GLMrad was externally validated for each clinical endpoint on PMCC lung 1.
During 100-times-repeated training/validation procedure, per iteration, the fitted model was stored to keep track of the PET radiomics features that were selected by elastic net in the fitted model [66]. PET radiomics features and clinical variables were ranked based on the frequency of inclusion in the fitted model.
Model comparison
Clinical variables such as PET/CT-based GTV, TNM staging, histology, gender, and age were also introduced into the radiomics signature to create a prognostic model containing PET radiomics features and clinical variables (GLMall). In addition, a model based on only the clinical variables was calculated using elastic net regression (GLMclin). To assess the complementary value of PET radiomics features with clinical variables, the mean AUC was calculated from 100 iterations for each model and compared. The Mann Whitney U Test was used to assess any significant differences between the predictive performance of GLMall, GLMclin, and GLMrad, and p-values below 0.05 were seen as significant.
Results
Repeatability
Results of the repeatability test were based on the 4D PET lung dataset and an overview of notable PET radiomics features and their corresponding CR is given in Table 2. All first-order features were repeatable when extracted from MTV2.5 irrespective of SUV binning method. In contrast, 13 out of 18 first-order features were repeatable when extracted from MTV40. Furthermore, around 50 texture features were repeatable when extracted from MTV2.5 regardless of SUV discretization method, versus 28 repeatable texture features extracted from MTV40. With regards to shape features, only MTV40 was not repeatable.
Table 2. An overview of categorized notable PET radiomics features that are commonly reported in literature with their coefficient of repeatability (CR, %).
The asterisk (*) represents features that were repeatable in all four different settings. Per category, the total number of PET radiomics features that met the study repeatability criterion is added.
| CR (%) | Fixed bin width | Fixed bin count | ||
|---|---|---|---|---|
| Notable features | MTV2.5 | MTV40 | MTV2.5 | MTV40 |
| First-order features | 18/18 | 13/18 | 18/18 | 13/18 |
| Entropy* | 3.4 | 5.5 | 3.8 | 6.0 |
| Kurtosis | 26.8 | 34.7 | 26.8 | 34.8 |
| Skewness | 23.1 | 50.4 | 23.1 | 51.3 |
| SUVmax* | 13.2 | 13.2 | 13.2 | 13.2 |
| SUVmean* | 6.0 | 12.9 | 6.0 | 12.7 |
| Uniformity | 17.9 | 41.9 | 21.1 | 37.0 |
| Texture features | 49/74 | 28/74 | 50/74 | 28/74 |
| GLCM Contrast* | 23.2 | 28.1 | 28.8 | 29.9 |
| GLCM Correlation* | 2.6 | 11.9 | 2.7 | 11.2 |
| GLCM DifferenceAverage* | 9.9 | 13.1 | 14.1 | 17.2 |
| GLCM JointEntropy* | 2.8 | 4.5 | 3.3 | 5.7 |
| GLCM SumEntropy* | 2.7 | 4.5 | 2.8 | 4.2 |
| GLRLM GrayLevelNonUniformity | 13.7 | 59.1 | 18.7 | 55.4 |
| NGTDM Busyness | 75.5 | 91.3 | 33.0 | 81.9 |
| NGTDM Coarseness | 12.0 | 41.5 | 16.8 | 35.2 |
| NGTDM Contrast | 23.3 | 68.5 | 31.9 | 64.9 |
| Shape features | 13/13 | 12/13 | 13/13 | 12/13 |
| Elongation* | 4.7 | 10.8 | 4.7 | 10.7 |
| Flatness* | 7.1 | 15.0 | 7.1 | 13.7 |
| MetabolicTumourVolume | 5.9 | 45.5 | 5.9 | 45.3 |
| Sphericity* | 3.2 | 8.9 | 3.2 | 8.7 |
Amongst the four comparisons, 211 out of 360 PET radiomics features were repeatable. An overview of all PET radiomics features and their corresponding CR is given in S1 File. The impact of large delineation inaccuracies on repeatability was studied between contours generated by the two different SUV thresholds, though only reported as supplementary data (S1 File).
Relationship of PET radiomics features with MTV and SUVmax
The Spearman’s Rank correlation coefficient was calculated to assess the relationship of 211 repeatable PET radiomics features with MTV and SUVmax. Four assessments were performed in total on one of the mid-position scans, with groups consisting of a combination of either one of the SUV binning methods and one of the tumour volumes (MTV2.5 or MTV40). Not all repeatable PET radiomics features were found to be independent from MTV and SUVmax. From the first-order features, only Kurtosis and Skewness extracted from MTV2.5 were independent from MTV and SUVmax. There were no independent repeatable first-order features for MTV40. Regarding the fixed bin count method, 17 out of 50 texture features extracted from MTV2.5 were not strongly associated with MTV and SUVmax. This also counted for 5 texture features extracted from MTV40. With regards to the fixed bin width method, there were no texture features independent from either SUVmax or MTV. Elongation, Flatness, and Sphericity were the only independent shape features when extracted from MTV2.5, though only Elongation and Flatness remained independent for MTV40. A complete overview of independence testing for all PET radiomics features is given in S1 File.
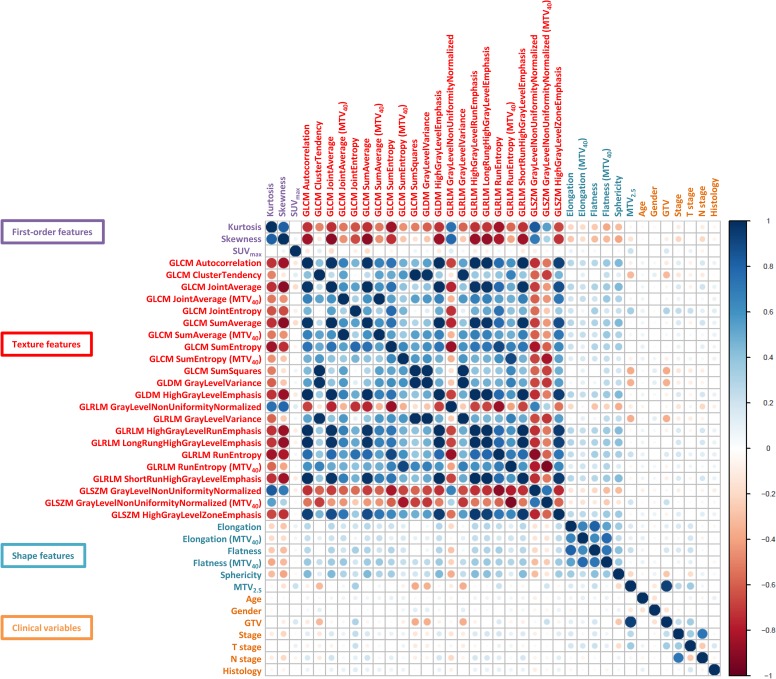
An overview of correlations amongst the selected robust independent PET radiomics features and clinical variables is given in Fig 3. More details on robust and independent PET radiomics features can be viewed in S1 File. The robust independent PET radiomics features did not show any strong correlation with the other clinical variables, such as age, ECOG PS, gender, histology, and TNM stage. However, there were associations present amongst the PET texture features.
Fig 3. Correlation coefficients of the robust independent PET radiomics features and clinical variables.
Positive correlation coefficients are displayed in blue and negative correlation coefficients in red color. Color intensity and the size of the circle are proportional to the correlation coefficients. A distinction was made between features calculated from MTV2.5 and MTV40.
Building the radiomics signature
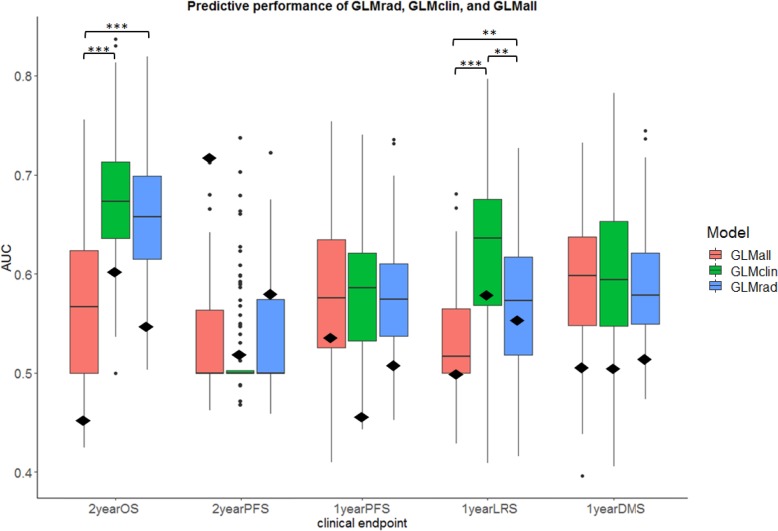
Based on the feature selection criteria, 31 PET radiomics features were selected for the next steps (see Fig 3). Three elastic net regularized GLMs were built per endpoint: GLMrad, GLMclin, and GLMall. Results of the model performances are shown in Fig 4, showing that GLMrad does not significantly outperform GLMclin for any clinical endpoints. The GLMclin has a significantly better predictive performance compared to GLMrad in 2-year OS (p<0.0001), and in 1-year LRS (p<0.001). GLMall did not show a significantly better performance to both GLMrad and GLMclin simultaneously in any endpoint. External validation of GLMall led to AUC values ranging from 0.51 to 0.59 for any clinical endpoint. When GLMclin was externally validated, the highest predictive performance was 0.60 for 2 year OS. For GLMrad, the highest predictive performance was 0.71 for 2-year PFS.
Fig 4. Model performance for the PET radiomics model (GLMrad), the model containing clinical variables (GLMclin), and a combination of radiomics and clinical variables (GLMall).
The median AUC values from 100-times-repeated training/validation are depicted per model, per clinical endpoint. The lower and upper hinges correspond to the 25th and 75th percentiles. The whiskers depict the 1.5*IQR from the lower and upper hinge. Data beyond the end of the whiskers are shown as outlier points. AUC values corresponding to the external validation set are shown as a black diamond. Significance levels, **p<0.001, ***p<0.0001.
Promising features
Table 3 shows selected features for each fitted GLM, and how frequent these features were chosen in the fitted model over 100 iterations. The feature shape Sphericity was present in 100% of the iterations for 2-year OS. From the 100 repetitions, GLCM ClusterTendency was selected in more than 95% for predicting 1-year PFS and 1-year DMS. Clinical variables such as age and GTV were prominent in predicting 2-year OS and 1-year LRS, next to shape Sphericity. As can be seen in Table 3, age, shape Sphericity, and GLCM ClusterTendency are present amongst the most selected features for all clinical endpoints.
Table 3. The most selected features in the model by elastic net, ranked by the number of times selected in the generalized linear model.
Only the top 10 most selected PET radiomics are shown. The features written in italic bold are present in all endpoints.
| Endpoint | GLMall selected features by elastic net | Frequency |
|---|---|---|
| 2-year OS | Age | 100 |
| GTV | 100 | |
| Shape_Sphericity | 100 | |
| MTV2.5 | 78 | |
| glcm_ClusterTendency | 56 | |
| SUVmax | 39 | |
| Gender | 34 | |
| glcm_JointEntropy | 34 | |
| glrlm_GrayLevelNonUniformityNormalized | 33 | |
| glrlm_GrayLevelVariance | 29 | |
| 2-year PFS | Age | 50 |
| SUVmax | 50 | |
| glrlm_GrayLevelNonUniformityNormalized | 49 | |
| shape_Sphericity | 47 | |
| Histology | 42 | |
| MTV2.5 | 38 | |
| glcm_ClusterTendency | 30 | |
| N_status | 28 | |
| T_status | 25 | |
| shape_Elongation_MTV40 | 20 | |
| 1-year PFS | GTV | 99 |
| glcm_ClusterTendency | 95 | |
| shape_Sphericity | 76 | |
| Age | 63 | |
| T_status | 49 | |
| MTV2.5 | 40 | |
| glcm_SumEntropy_MTV40 | 39 | |
| shape_Elongation | 37 | |
| SUVmax | 31 | |
| Histology | 29 | |
| 1-year LRS | GTV | 83 |
| Age | 82 | |
| glcm_ClusterTendency | 65 | |
| glcm_SumEntropy_MTV40 | 63 | |
| shape_Sphericity | 57 | |
| Gender | 48 | |
| gldm_GrayLevelVariance | 47 | |
| N_status | 27 | |
| Stage | 25 | |
| glrlm_GrayLevelVariance | 24 | |
| 1-year DMS | GTV | 99 |
| glcm_ClusterTendency | 96 | |
| shape_Sphericity | 57 | |
| MTV2.5 | 52 | |
| Histology | 34 | |
| T_status | 33 | |
| Age | 32 | |
| glcm_SumEntropy_MTV40 | 32 | |
| shape_Elongation | 29 | |
| SUVmax | 23 |
Discussion
The rationale of this study was to identify a group of FDG PET radiomics features for NSCLC patients that are robust, independent, prognostic, and complementary to well-established clinical variables. We found PET radiomics features that met the study criteria of robustness and independence, and that also exhibited prognostic value. However, results demonstrated that PET radiomics features are not complementary to clinical variables for predicting clinical endpoints in NSCLC patients that were treated with CCRT. This indicates that clinical variables provide more prognostic information than robust independent PET radiomics features, and that the prognostic value in PET radiomics features is minimal. This study did take into account shortcomings of other studies on PET radiomics features [50] with the use of a feature selection method that reduces overfitting and external validation of results.
Feature selection based on the repeatability of PET radiomics features was feasible with the use of different phases from 4D PET imaging, in the absence of test-retest data. Larue et al. showed that in 4D CT, the majority of the features have a high agreement between radiomics feature stability based on 4D CT and test–retest data in lung cancer [67]. It was therefore hypothesized that 4D PET scans could also be used for repeatability testing. To determine robust PET radiomics features, a CR of 30% was chosen as limit for repeatability, based on PERCIST. However, a limitation of using 4D PET for repeatability testing is the absence of biological tumour variability, and PERCIST takes this variability into account. Hence, the use of a 30%-limit could be seen as too tolerant, and 15%, as commonly used in phantom studies, could be more appropriate. Even under these stricter circumstances, 12 first-order features, 24 out of 74 texture features, and all shape features would still meet that criterion as can be seen in S1 File. Besides that, the most prominent PET radiomics features in the fitted GLMs were SUVmax (CR = 13.2%), shape Sphericity (CR = 3.2%), GLCM ClusterTendency (CR = 21.9%), GLRLM GrayLevelNonUniformityNormalized (CR = 18.4%), and MTV2.5 (CR = 5.9%) as seen in Table 3. This shows that repeatable PET radiomics features with a CR>15% are also frequently present in the fitted models. Even though there is literature reporting on stability of PET radiomics features in a test-retest setting [45, 46], there is no objective limit for the level of repeatability for each PET radiomics feature. Determining such an objective limit is only relevant if the studied PET radiomics feature contains clinically useful information. Hence, in the absence of an objective limit for each PET radiomics feature, the 30%-limit of PERCIST was applied to all.
It was observed that the repeatability for features from MTV2.5 is better compared to MTV40 and this is due to two important factors:
From the 13 shape features, only MTV40 had a CR>30% when comparing the MTV40 between two mid-position scans. This variance, of course, has already a great impact on PET radiomics features calculated from MTV40 as it is known that differences in delineation have an impact on feature outcome [44].
Radiomics features are calculated on matrices which dimensions are dependent on the SUV range. With MTV2.5 matrix dimensions are more standardized than MTV40, which is dependent on the maximum SUV (CR = 13.2%).
In this case, the use of MTV2.5 for GTV delineation may be advised over MTV40 in PET radiomics analysis.
Another step of the feature selection procedure was to assess the independence of PET radiomics features, to identify possible prognostic features that could complement basic SUV metrics and volumetric features. In this context, changes in PET radiomics features would be independent from changes in basic SUV metrics and volume, increasing their utility in longitudinal studies. Therefore, the use of a fixed bin width for SUV binning should be avoided as this method resulted in PET radiomics features that were all strongly correlated to either maximum SUV or MTV. While the choice of |ρ|<0.5 for independence testing may seem arbitrary, a |ρ|<0.7 was also studied and did not improve results (see S1 File for more details). Independence testing had the most impact in the feature pre-selection procedure as it resulted in a substantial decrease of PET radiomics features. Unfortunately, results demonstrated that independence testing could not guarantee that remaining robust independent PET radiomics features exhibited complementary value next to clinical variables. Even so, we strongly advise assessing the relationship of radiomics features with current established prognostic factors in any study considering PET radiomics features for prognostication as this is the first important step in showing their potential added value in the clinic.
A final selection of features in the GLM was performed by elastic net regression, robust to collinearity amongst features [66]. More feature selection/classification methods exist [68], though comparing multiple methods was beyond the scope of this study. However, in literature, elastic net regression yielded one of the highest discriminative performances in chemoradiotherapy outcome prediction in 12 patient datasets containing in total 1053 lung cancer patients [65]. Interestingly, elastic net regression could also be used as a standalone feature selection method. A comparison of the feature selection method based on repeatability, independence, and elastic net regression (GLMall), and a method using only elastic net regression (GLMelnet) was performed, see S1 File. Pre-selection of PET radiomics features is worthwhile, because the number of PET radiomics features in GLMelnet was often high (>20 features) and many were highly correlated to volume or SUVmax. In contrast, the average number of features in GLMall was 9. Even so, it was observed that elastic net tends to keep all of the correlated and presumably prognostic features in the fitted model or shrinks all to zero, whereby increasing the number of (correlated) features resulted in a decrease of the predictive performance. This decrease of predictive performance seen in the validation set suggests that overfitting, although reduced, may still be present. This shows the value of dimensionality reduction in order to optimize predictive performance in rather small sample sizes.
The predictive performance of PET texture features in NSCLC has been studied widely, but clear evidence that PET texture features are complementary to clinical variables is lacking [69]. This study has extensively studied PET texture features and did not find any evidence for added value next to current clinical variables. S1 File provides a complete overview of all assessed model performances, including additional investigations with TLG. In literature, typically, only one or two PET texture features have been significantly associated with predicting various survival endpoints [39–41, 47, 70–72]. However, of all the prognostic PET texture features from those studies, such as GLCM Joint Entropy, Correlation, Contrast, Dissimilarity (or Difference Average), NGTDM Coarseness, Busyness, and Contrast, only GLCM Joint Entropy was both repeatable and independent from SUVmax or volume in our dataset. In this study, GLCM Joint Entropy was selected 34 times out of 100 by elastic net regression for predicting 2-year OS, and its value in overall survival was also previously shown [47]. Nonetheless, in our study the average predictive performance for GLMall in all clinical endpoints ranged from 0.50 to 0.66. For comparison, other studies predicting outcome with both PET radiomics and clinical variables in NSCLC found predictive performances of 0.63 for predicting OS [41], 0.72 for local recurrence [71], and 0.71 for distant metastases [72]. Even with those results, neglecting any limitations of those studies, there is still no strong evidence that PET texture features exhibit complementary information.
Results from the external validation demonstrated even lower AUC values in most cases than the internal validation set. Besides the limitation of the use of a small external dataset, differences were observed between institutes regarding patients, treatment, and image acquisition and reconstruction settings, that also can influence outcome [44, 73], and could have resulted in poor generalizability. To overcome the issue of poor generalizability, a prognostic model should be trained on a combination of well-balanced patient cohorts from multiple institutes, and PET acquisition and reconstruction protocols should be harmonized across centers in multi-centre studies. Alternatively, a post-reconstruction harmonization method proposed by Orlhac et al. may also aid in removing the multicenter effect for textural features and SUV [74].
Furthermore, limitations of this paper include the relatively small sample size for machine learning methods that could have affected the predictive performance [75], and the impact of tumour motion on PET radiomics features, especially in lower lobe tumours [76]. Although Grootjans et al. showed that there are specific PET radiomics features whose prognostic accuracy was not affected by respiratory motion and varying noise-levels [29].
To overcome the limitations of this study, and to be certain that there is no complimentary information in PET radiomics features, future studies need to set up large scale multi-centre cohorts to allow for multiple independent validation datasets. To further improve predictive performance, studies could investigate elastic net-Cox proportional hazard models [77], non-linear relationships by applying data transformation on PET radiomics features [21, 78, 79], or assess computer engineered features with neural networks or deep learning networks [80, 81]. Currently, deep learning is under investigation for use in lung nodule detection, tumour segmentation, and tumour classification with histopathology images [82]. Its use in medical image analysis is increasing as algorithms become more sophisticated and more data becomes available, which might lead to new insights in survival prediction. A step further would be to combine radiomics features from multimodal imaging such as PET, CT and MRI [83, 84], where the combination of anatomical and biological features may of added value for providing a personalized treatment strategy.
Conclusion
In this study, robust independent PET radiomics features, identified with 4D PET imaging, did not have complementary value in predicting overall survival and progression-free survival in NSCLC patients treated with concurrent chemoradiotherapy. Improving risk stratification and clinical decision making based on clinical variables and PET radiomics features has still been proven difficult in locally advanced lung cancer patients. New approaches should be investigated in large scale multi-centre studies to deal with current challenges in the field of radiomics before translation to the clinic becomes realistic.
Supporting information
(DOCX)
Acknowledgments
The authors would like to thank Simon van Kranen and Jonas Teuwen for helping out with programming, Michel van den Heuvel for providing NKI lung 1 dataset, Natascha Bruin for providing delineations and clinical data for the NKI lung 2 dataset, and Erik Vegt, Rod Hicks, Nick Hardcastle, David Ball, and Tomas Kron for scientific editing of the manuscript.
Data Availability
Data cannot be shared publicly without approval by our Institutional Review Board, because of institutional policy (legal restrictions). Researchers who meet the criteria for access to confidential data should request approval via IRB@nki.nl. After approval is obtained, data is available from the PET Radiomics Data Access Committee. Contact via t.konert@nki.nl (Chair of the Board).
Funding Statement
One co-author, SE, received the Australian National Health and Medical Research Council (APP1003895) and Victorian Cancer Agency Grant. Sources: http://www.victoriancanceragency.org.au/https://www.nhmrc.gov.au/ The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Aupérin A, Le Péchoux C, Rolland E, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small cell lung cancer. J Clin Oncol 2010; 28: 2181–90. 10.1200/JCO.2009.26.2543 [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J,Soerjomataram I,Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136(5): 359–86. [DOI] [PubMed] [Google Scholar]
- 3.Chan BA, Hughes BGM. Targeted therapy for non-small cell lung cancer: current standards and the promise of the future. Transl Lung Cancer Res 2015; 4(1): 36–54. 10.3978/j.issn.2218-6751.2014.05.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Remon J, Vilariño N, Reguart N. Immune checkpoint inhibitors in non-small cell lung cancer (NSCLC): Approaches on special subgroups and unresolved burning questions. Cancer Treat Rev 2018; 64:21–29. 10.1016/j.ctrv.2018.02.002 [DOI] [PubMed] [Google Scholar]
- 5.Antonia SJ, Villegas A, Daniel D, et al. ; PACIFIC Investigators. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N Engl J Med 2018. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 6.Detterbeck FC, Boffa DJ, Kim AW, et al. The Eighth Edition Lung Cancer Stage Classification. Chest 2017; 151(1): 193–203. 10.1016/j.chest.2016.10.010 [DOI] [PubMed] [Google Scholar]
- 7.Rami-Porta R, Bolejack V, Giroux DJ, et al. , and International Association for the Study of Lung Cancer Staging and Prognostic Factors Committee, Advisory Board Members and Participating Institutions. The IASLC Lung Cancer Staging Project: the new database to inform the eighth edition of the TNM classification of lung cancer. J Thorac Oncol 2014; 9: 1618–1624. 10.1097/JTO.0000000000000334 [DOI] [PubMed] [Google Scholar]
- 8.Paesmans M. Prognostic and predictive factors for lung cancer. Breathe.2012; 9: 112–121. [Google Scholar]
- 9.Berghmans T, Paesmans M, Sculier JP. Prognostic factors in stage III non-small cell lung cancer: a review of conventional, metabolic and new biological variables. Ther Adv Med Oncol 2011; 3: 127–138. 10.1177/1758834011401951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buccheri G, Ferrigno D. Importance of weight loss definition in the prognostic evaluation of non-small-cell lung cancer. Lung Cancer 2001; 34: 433–440. 10.1016/s0169-5002(01)00273-2 [DOI] [PubMed] [Google Scholar]
- 11.Nakamura H, Ando K, Shinmyo T, et al. Female gender is an independent prognostic factor in non-small-cell lung cancer: a meta-analysis. Ann Thorac Cardiovasc Surg 2011; 17: 469–480. 10.5761/atcs.oa.10.01637 [DOI] [PubMed] [Google Scholar]
- 12.Yu KH, Zhang C, Berry GJ, et al. Predicting non-small cell lung cancer prognosis by fully automated microscopic pathology image features. Nat Commun 2016; 7(7): 12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pallis AG, Gridelli C. Is age a negative prognostic factor for the treatment of advanced/metastatic non-small-cell lung cancer? Cancer Treat Rev 2010; 36(5): 436–41. 10.1016/j.ctrv.2009.12.013 [DOI] [PubMed] [Google Scholar]
- 14.Yu Z, Zhang G, Yang M, et al. Systematic review of CYFRA 21–1 as a prognostic indicator and its predictive correlation with clinicopathological features in Non-small Cell Lung Cancer: A meta-analysis. Oncotarget 2017; 8(3): 4043–4050. 10.18632/oncotarget.14022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang AG, Chen HL, Lu HY. The relationship between Glasgow Prognostic Score and serum tumour markers in patients with advanced non-small cell lung cancer. BMC Cancer 2015; 15: 386 10.1186/s12885-015-1403-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steels E, Paesmans M, Berghmans T, et al. Role of p53 as prognostic factor for survival in lung cancer: a systematic review of the literature with a meta-analysis. Eur Respir J 2001; 18: 705–719. 10.1183/09031936.01.00062201 [DOI] [PubMed] [Google Scholar]
- 17.Tong J, Sun X, Cheng H, et al. Expression of p16 in non-small cell lung cancer and its prognostic significance: a meta-analysis of published literatures. Lung Cancer. 2011; 74: 155–163. 10.1016/j.lungcan.2011.04.019 [DOI] [PubMed] [Google Scholar]
- 18.Martin B, Paesmans M, Mascaux C, et al. KI-67 expression and patients survival in lung cancer: systematic review of the literature with meta-analysis. Br J Cancer. 2004; 91: 2018–2025. 10.1038/sj.bjc.6602233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strom HH, Bremnes RM, Sundstrom SH, et al. Poor prognosis patients with inoperable locally advanced NSCLC and large tumours benefit from palliative chemoradiotherapy: a subset analysis from a randomized clinical phase III trial. J Thorac Oncol 2014; 9: 825–33. 10.1097/JTO.0000000000000184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahar AL, Compton C, McShane LM, et al. , on behalf of the Molecular Modellers Working Group of the American Joint Committee on Cancer. Refining prognosis in lung cancer: A report on the quality and relevance of clinical prognostic tools. J Thorac Oncol 2015; 10(11): 1576–1589. 10.1097/JTO.0000000000000652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aerts HJ, Velazquez ER, Leijenaar RT, et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun 2014; 5: 4006 10.1038/ncomms5006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lambin P, Rios-Velazquez E, Leijenaar R, et al. Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer. 2012; 48(4): 441–6. 10.1016/j.ejca.2011.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Szyszko TA, Yip C, Szlosarek P, et al. The role of new PET tracers for lung cancer. Lung Cancer 2016; 94: 7–14. 10.1016/j.lungcan.2016.01.010 [DOI] [PubMed] [Google Scholar]
- 24.Cremonesi M, Gilardi L, Ferrari ME, et al. Role of interim 18F-FDG-PET/CT for the early prediction of clinical outcomes of Non-Small Cell Lung Cancer (NSCLC) during radiotherapy or chemoradiotherapy. A systematic review. Eur J Nucl Med Mol Imaging 2017; 44(11): 1915–1927. 10.1007/s00259-017-3762-9 [DOI] [PubMed] [Google Scholar]
- 25.Dingemans AM, de Langen AJ, van den Boogaart V, et al. First-line erlotinib and bevacizumab in patients with locally advanced and/or metastatic non-small-cell lung cancer: a phase II study including molecular imaging. Ann Oncol 2011; 22(3): 559–66. 10.1093/annonc/mdq391 [DOI] [PubMed] [Google Scholar]
- 26.Mileshkin L, Hicks RJ, Hughes BG, et al. Changes in 18F-fluorodeoxyglucose and 18F-fluorodeoxythymidine positron emission tomography imaging in patients with non-small cell lung cancer treated with erlotinib. Clin Cancer Res 2011; 17(10): 3304–15. 10.1158/1078-0432.CCR-10-2763 [DOI] [PubMed] [Google Scholar]
- 27.Hyun SH, Ahn HK, Kim H, et al. Volume-based assessment by (18)F-FDG PET/CT predicts survival in patients with stage III non-small-cell lung cancer. Eur J Nucl Med Mol Imaging 2014; 41(1): 50–8. 10.1007/s00259-013-2530-8 [DOI] [PubMed] [Google Scholar]
- 28.Moon SH, Cho SH, Park LC, et al. Metabolic response evaluated by 18F-FDG PET/CT as a potential screening tool in identifying a subgroup of patients with advanced non-small cell lung cancer for immediate maintenance therapy after first-line chemotherapy. Eur J Nucl Med Mol Imaging 2013; 40(7): 1005–13. 10.1007/s00259-013-2400-4 [DOI] [PubMed] [Google Scholar]
- 29.Grootjans W, Tixier F, van der Vos CS, et al. The impact of optimal respiratory gating and image noise on evaluation of intra-tumour heterogeneity in 18F-FDG positron emission tomography imaging of lung cancer. J Nucl Med 2016; 57(11): 1692–1698. 10.2967/jnumed.116.173112 [DOI] [PubMed] [Google Scholar]
- 30.Salavati A, Duan F, Snyder BS, et al. Optimal FDG PET/CT volumetric parameters for risk stratification in patients with locally advanced non-small cell lung cancer: results from the ACRIN 6668/RTOG 0235 trial. Eur J Nucl Med Mol Imaging 2017; 44(12): 1969–1983. 10.1007/s00259-017-3753-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paesmans M, Berghmans T, Dusart M, et al. ; European Lung Cancer Working Party, and on behalf of the IASLC Lung Cancer Staging Project. Primary tumour standardized uptake value measured on fluorodeoxyglucose positron emission tomography is of prognostic value for survival in non-small cell lung cancer: update of a systematic review and meta-analysis by the European Lung Cancer Working Party for the International Association for the Study of Lung Cancer Staging Project. J Thorac Oncol. 2010; 5(5): 612–9. 10.1097/JTO.0b013e3181d0a4f5 [DOI] [PubMed] [Google Scholar]
- 32.Everitt S, Ball D, Hicks RJ, Callahan J, Plumridge N, Trinh J, et al. Prospective study of serial imaging comparing fluorodeoxyglucose positron emission tomography (PET) and fluorothymidine PET during radical chemoradiation for non-small cell lung cancer: reduction of detectable proliferation associated with worse survival. Int J Radiat Oncol 2017;99:947–55. [DOI] [PubMed] [Google Scholar]
- 33.Weiss GJ, Ganeshan B, Miles KA, et al. Noninvasive image texture analysis differentiates K-ras mutation from pan-wildtype NSCLC and is prognostic. PLoS ONE. 2014; 9(7): e100244 10.1371/journal.pone.0100244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yip SS, Kim J, Coroller TP, et al. Associations Between Somatic Mutations and Metabolic Imaging Phenotypes in Non-Small Cell Lung Cancer. J Nucl Med. 2017; 58(4): 569–576. 10.2967/jnumed.116.181826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Del Gobbo A, Pellegrinelli A, Gaudioso G, et al. Analysis of NSCLC tumour heterogeneity, proliferative and 18F-FDG PET indices reveals Ki67 prognostic role in adenocarcinomas. Histopathology 2016; 68(5): 746–51. 10.1111/his.12808 [DOI] [PubMed] [Google Scholar]
- 36.van Baardwijk A, Bosmans G, van Suylen RJ, et al. Correlation of intra-tumour heterogeneity on 18F-FDG PET with pathologic features in non-small cell lung cancer: a feasibility study. Radiother Oncol 2008; 87: 55–58. 10.1016/j.radonc.2008.02.002 [DOI] [PubMed] [Google Scholar]
- 37.Cook GJR, O’Brien ME, Siddique M, et al. Non–small cell lung cancer treated with erlotinib: heterogeneity of 18F-FDG uptake at PET—association with treatment response and prognosis. Radiology 2015; 276: 883–893. 10.1148/radiol.2015141309 [DOI] [PubMed] [Google Scholar]
- 38.Fried DV, Tucker SL, Zhou S, et al. Prognostic value and reproducibility of pretreatment CT texture features in stage III non–small cell lung cancer. Int J Radiat Oncol Biol Phys 2014; 90: 834–842. 10.1016/j.ijrobp.2014.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cook GJR, Yip C, Siddique M, et al. Are pre-treatment 18F-FDG PET tumour textural features in non-small cell lung cancer associated with response and survival after chemoradiotherapy? J Nucl Med 2013; 54: 19–26. 10.2967/jnumed.112.107375 [DOI] [PubMed] [Google Scholar]
- 40.Pyka T, Bundschuh RA, Andratschke N, et al. Textural features in pre-treatment [F18]-FDG-PET/CT are correlated with risk of local recurrence and disease-specific survival in early stage NSCLC patients receiving primary stereotactic radiation therapy. Radiat Oncol 2015;10:100 10.1186/s13014-015-0407-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ohri N, Duan F, Snyder BS, et al. Pre-treatment FDG PET Textural Features in Locally Advanced NSCLC Secondary Analysis of ACRIN 6668/RTOG 0235. J Nucl Med. 2016; 57(6): 842–8. 10.2967/jnumed.115.166934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keyes JW Jr. SUV: standard uptake or silly useless value? J Nucl Med 1995; 36(10): 1836–9. [PubMed] [Google Scholar]
- 43.de Jong EEC, van Elmpt W, Hoekstra OS, et al. Quality assessment of positron emission tomography scans: recommendations for future multicenter trials. Acta Oncol 2017; 56(11): 1459–1464. 10.1080/0284186X.2017.1346824 [DOI] [PubMed] [Google Scholar]
- 44.van Velden FHP, Kramer GM, Frings V, et al. Repeatability of Radiomic Features in Non-Small-Cell Lung Cancer [18F]FDG-PET/CT Studies: Impact of Reconstruction and Delineation. Mol Imaging Biol. 2016; 18(5): 788–795. 10.1007/s11307-016-0940-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leijenaar RTH, Nalbantov G, Carvalho S, et al. The effect of SUV discretization in quantitative FDG-PET radiomics: the need for standardized methodology in tumour texture analysis. Sci Rep 2015; 5: 11075 10.1038/srep11075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Desseroit MC, Tixier F, Weber WA, et al. Reliability of PET/CT Shape and Heterogeneity Features in Functional and Morphologic Components of Non-Small Cell Lung Cancer Tumours: A Repeatability Analysis in a Prospective Multicenter Cohort. J Nucl Med 2017; 58(3): 406–411. 10.2967/jnumed.116.180919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hatt M, Majdoub M, Vallieres M, et al. 18F-FDG PET uptake characterization through texture analysis: investigating the complementary nature of heterogeneity and functional tumour volume in a multi-cancer site patient cohort. J Nucl Med 2015; 56: 38–44. 10.2967/jnumed.114.144055 [DOI] [PubMed] [Google Scholar]
- 48.Brooks FJ, Grigsby PW. The effect of small tumour volumes on studies of intratumoural heterogeneity of tracer uptake. J Nucl Med. 2014; 55: 37–42. 10.2967/jnumed.112.116715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Orlhac F, Soussan M, Maisonobe J, et al. Tumour texture analysis in 18F-FDG PET: relationships between texture parameters, histogram indices, standardized uptake values, metabolic volumes, and total lesion glycolysis. J Nucl Med 2014; 55:414–422. 10.2967/jnumed.113.129858 [DOI] [PubMed] [Google Scholar]
- 50.Chalkidou A, O'Doherty MJ, Marsden PK. False Discovery Rates in PET and CT Studies with Texture Features: A Systematic Review. PLoS One. 2015; 10(5): e0124165 10.1371/journal.pone.0124165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Walraven I, van den Heuvel M, van Diessen J, et al. Long-term follow-up of patients with locally advanced non-small cell lung cancer receiving concurrent hypofractioned chemoradiotherapy with or without cetuximab. Radiother Oncol 2016;118:442–446. 10.1016/j.radonc.2016.02.011 [DOI] [PubMed] [Google Scholar]
- 52.Detterbeck FC, Boffa JB, Kim AW, et al. The Eighth Edition Lung Cancer Stage Classification. CHEST 2017; 151(1):193–203. 10.1016/j.chest.2016.10.010 [DOI] [PubMed] [Google Scholar]
- 53.Kruis MF, van de Kamer JB, Houweling AC, et al. PET motion compensation for radiation therapy using a CT-based mid-position motion model: methodology and clinical evaluation. Int J Radiat Oncol Biol Phys 2013;87:394–400. 10.1016/j.ijrobp.2013.06.007 [DOI] [PubMed] [Google Scholar]
- 54.van Griethuysen JJM, Fedorov A, Parmar C, et al. Computational Radiomics System to Decode the Radiographic Phenotype. Cancer Research 2017;77(21):e104–e107. 10.1158/0008-5472.CAN-17-0339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haralick RM, Shanmugam K, Dinstein I. Textural features for image classification. IEEE Trans Syst Man Cybern 1973;3:610–621. [Google Scholar]
- 56.Galloway MM. Texture analysis using gray level run lengths. Comput Graph Image Process 1975;4:172–179. [Google Scholar]
- 57.Thibault G, Angulo J, Meyer F. Advanced statistical matrices for texture characterization: application to cell classification. IEEE Trans Biomed Eng 2014;61:630–637. 10.1109/TBME.2013.2284600 [DOI] [PubMed] [Google Scholar]
- 58.Sun CJ, Wee WG. Neighboring gray level dependence matrix for texture classification. Comput Vision Graph Image Process 1983;23:341–52. [Google Scholar]
- 59.Amadasun M, King R. Textural features corresponding to textural properties. IEEE Trans Syst Man Cybern 1989;19:1264–1274. [Google Scholar]
- 60.Zwanenburg A, Leger S, Vallières M, et al. Image biomarker standardisation initiative—feature definitions. 2016. In eprint arXiv:1612.07003. [Google Scholar]
- 61.Bland JM, Altman DG. Statistical Methods for Assessing Agreement between Two Methods of Clinical Measurement. Lancet 1986;1(8476):307–10. [PubMed] [Google Scholar]
- 62.Shang J, Ling X, Zhang L, et al. Comparison of RECIST, EORTC criteria and PERCIST for evaluation of early response to chemotherapy in patients with non-small-cell lung cancer. Eur J Nucl Med Mol Imaging 2016;43:1945–53. 10.1007/s00259-016-3420-7 [DOI] [PubMed] [Google Scholar]
- 63.Zambelli AE. A data-driven approach to estimating the number of clusters in hierarchical clustering. F1000Res 2016; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Friedman J, Hastie T, Tibshirani R. Regularization Paths for Generalized Linear Models via Coordinate Descent. J Stat Soft 2010;33(1):1–22. [PMC free article] [PubMed] [Google Scholar]
- 65.Deist TM, Dankers FJWM, Valdes G, et al. Machine learning algorithms for outcome prediction in (chemo)radiotherapy: An empirical comparison of classifiers. Med Phys 2018;45(7):3449–3459. 10.1002/mp.12967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zou H, Hastie T. Regularization and variable selection via the elastic net. J Royal Stat Soc B 2005;67(2):301–320. [Google Scholar]
- 67.Larue RTHM, Van De Voorde L, van Timmeren JE, et al. 4DCT imaging to assess radiomics feature stability: an investigation for thoracic cancers. Radiother Oncol 2017;125(1):147–153. 10.1016/j.radonc.2017.07.023 [DOI] [PubMed] [Google Scholar]
- 68.Parmar C, Grossmann P, Bussink J, et al. Machine learning methods for quantitative radiomics biomarkers. Sci Rep. 2015;5:13087 10.1038/srep13087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Konert T, van de Kamer JB, Sonke JJ, et al. The developing role of FDG PET imaging for prognostication and radiotherapy target volume delineation in non-small cell lung cancer. J Thorac Dis 2018;10(21):2508–2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lovinfosse P, Janvary ZL, Coucke P, et al. FDG PET/CT texture analysis for predicting the outcome of lung cancer treated by stereotactic body radiation therapy. Eur J Nucl Med Mol Imaging 2016;43:1453–60. 10.1007/s00259-016-3314-8 [DOI] [PubMed] [Google Scholar]
- 71.Takeda K, Takanami K, Shirata Y, et al. Clinical utility of texture analysis of 18F-FDG PET/CT in patients with Stage I lung cancer treated with stereotactic body radiotherapy. J Radiat Res 2017;58(6):862–869. 10.1093/jrr/rrx050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wu J, Aguilera T, Shultz D, et al. Early-Stage Non-Small Cell Lung Cancer: Quantitative Imaging Characteristics of (18)F Fluorodeoxyglucose PET/CT Allow Prediction of Distant Metastasis. Radiology 2016;281(1):270–8. 10.1148/radiol.2016151829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yan J, Chu-Shern JL, Loi HY, et al. Impact of Image Reconstruction Settings on Texture Features in 18F-FDG PET. J Nucl Med 2015;56(11):1667–73. 10.2967/jnumed.115.156927 [DOI] [PubMed] [Google Scholar]
- 74.Orlhac F, Boughdad S, Philippe C, et al. A Postreconstruction Harmonization Method for Multicenter Radiomic Studies in PET. J Nucl Med. 2018. August;59(8):1321–1328. 10.2967/jnumed.117.199935 [DOI] [PubMed] [Google Scholar]
- 75.Pavlou M, Ambler G, Seaman S, et al. Review and evaluation of penalised regression methods for risk prediction in low-dimensional data with few events. Stat Med 2015;35:1159–1177. 10.1002/sim.6782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yip S, McCall K, Aristophanous M, et al. Comparison of texture features derived from static and respiratory-gated PET images in non-small cell lung cancer. PLoS One 2014;9:e115510 10.1371/journal.pone.0115510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yu K-H, Zhang C, Berry GJ, et al. Predicting non-small cell lung cancer prognosis by fully automated microscopic pathology image features. Nat. Commun.2016;7:12474 10.1038/ncomms12474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gillies RJ, Kinahan PE, Hricak H. Radiomics: images are more than pictures, they are data. Radiology 2016;278:563–577. 10.1148/radiol.2015151169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Parmar C, Leijenaar RT, Grossmann P, et al. Radiomic feature clusters and prognostic signatures specific for lung and head & neck cancer. Sci Rep 2015;5:11044 10.1038/srep11044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bertolaccini L, Solli P, Pardolesi A, et al. An overview of the use of artificial neural networks in lung cancer research. J Thorac Dis 2017;9:924–931. 10.21037/jtd.2017.03.157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hosny A, Parmar C, Quackenbush J, et al. Artificial intelligence in radiology. Nat Rev Cancer. 2018. August;18(8):500–510. 10.1038/s41568-018-0016-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Murphy A, Skalski M, Gaillard F. The utilisation of convolutional neural networks in detecting pulmonary nodules: a review. Br J Radiol 2018;91(1090):20180028 10.1259/bjr.20180028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vaidya M, Creach KM, Frye J, et al. Combined PET/CT image characteristics for radiotherapy tumour response in lung cancer. Radiother Oncol 2012;102(2):239–45. 10.1016/j.radonc.2011.10.014 [DOI] [PubMed] [Google Scholar]
- 84.Vallières M, Freeman CR, Skamene SR, et al. A radiomics model from joint FDG-PET and MRI texture features for the prediction of lung metastases in soft-tissue sarcomas of the extremities. Phys Med Biol 2015. July 21; 60(14):5471–96. 10.1088/0031-9155/60/14/5471 [DOI] [PubMed] [Google Scholar]