Inflammation, a complex response to danger signals that is fundamental to host survival, has been implicated in the pathogenesis of many human diseases, including stroke.1 In this review, we discuss the impact of inflammation, including in the context of autoimmune conditions and infectious diseases, on ischemic stroke risk and outcomes (Figure). We complement a critical evaluation of epidemiological evidence with results of intervention studies.
Figure. Putative mechanisms of increased ischemic stroke risk in autoimmune disorders and infectious diseases.
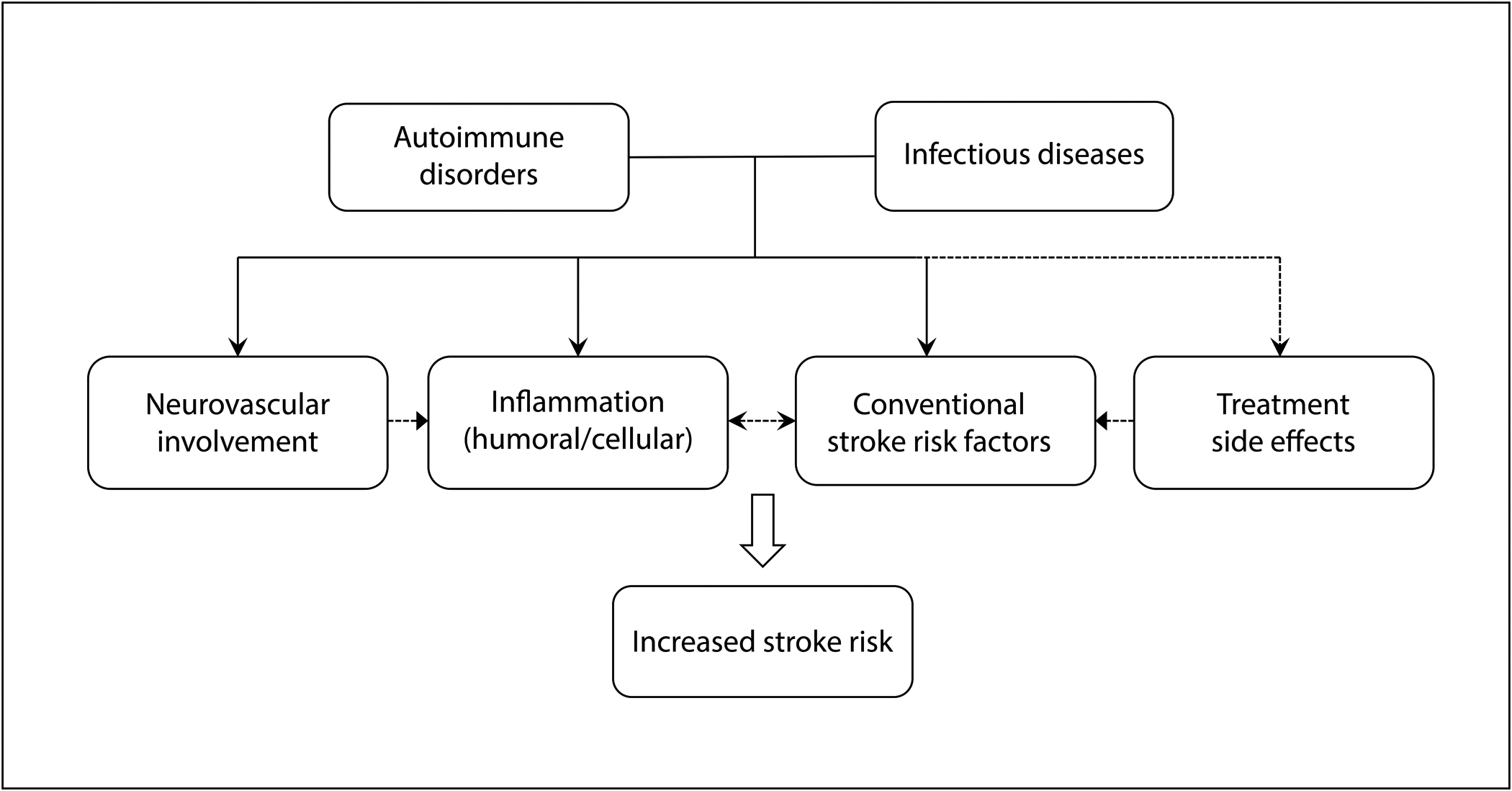
Autoimmune disorders and infectious diseases share overlapping mechanisms that may contribute to an increased risk of ischemic stroke. Apart from cerebral vasculitis, autoimmune disorders contribute to ischemic stroke risk through multiple mechanisms. Increased systemic inflammation may itself increase stroke risk and magnify the effect of conventional stroke risk factors, which are common comorbidities in individuals with autoimmune disorders such as rheumatoid arthritis. Treatment of autoimmune disorders often involves the use of glucocorticoids, which may worsen control of conventional stroke risk factors such as diabetes and hypertension. Similarly, some infectious diseases contribute to ischemic stroke through infectious cerebral vasculitis. Infectious diseases, especially chronic diseases, also contribute to systemic inflammation and are associated with a higher burden of conventional stroke risk factors, such as atherosclerosis in the case of human immunodeficiency virus.
Inflammation and Inflammatory Markers
Inflammation is a coordinated response to both extrinsic and intrinsic danger signals associated with innate and adaptive immunity.2 Foreign molecular complexes common to many infectious pathogens, termed pathogen-associated molecular patterns, are recognized by immune cell pattern recognition receptors, which, in turn, initiate innate and adaptive immune responses directed at neutralizing the threat. Similarly, host-derived danger-associated molecular patterns released from host cells during injury or stress engage pattern recognition receptors triggering immune responses. Immune cell activation leads to cascading effects on local and distant systems through the release of chemokines and cytokines. Co-activation of immune regulatory mechanisms leads to eventual resolution of inflammation. The molecular mechanisms that trigger, sustain, and resolve inflammation are multiple and complex, and are outside this scope of this review. Broadly, however, aberrations of these immune-regulatory processes can lead to under-active, over-active, non-resolving, or otherwise maladaptive inflammatory responses that can contribute to disease risk, pathology and tissue injury.
Inflammatory biomarkers, stroke risk, and outcomes
Inflammatory biomarkers include the cytokines, chemokines, and acute phase reactants that govern the inflammatory response. Elevations in inflammatory mediators interleukin-6 (IL-6), c-reactive protein (CRP), and lipoprotein-associated phospholipase A2 (Lp-PLA2) have been associated with increased stroke risk.3 An additional biomarker of increasing interest is soluble lectinlike oxidized low-density lipoprotein receptor-1, which is an inflammation-induced lipid receptor that contributes to atherogenesis and has been associated with stroke risk.4, 5 Elevated CRP was also associated with stroke recurrence6 in addition to recurrent vascular events, vascular death, and non-vascular death.7–10 IL-6 and Lp-PLA2 have similarly been linked to stroke recurrence, post-stroke myocardial infarction, and death.8, 11–16 The bulk of evidence supports an association between elevated inflammatory biomarkers and an increased risk of stroke, recurrent stroke, and post-stroke vascular events and mortality.
In addition to stroke recurrence, sources of post-stroke disability include physical disability, cognitive impairment, depression, and fatigue. Inflammatory biomarkers have been extensively studied as putative predictors of functional outcomes after ischemic stroke.17 Although the data are heterogeneous, several studies found elevated CRP to be independently associated with poor clinical outcomes after stroke.17, 18 IL-6, an upstream inflammatory cytokine, has more robustly been associated with poor functional outcomes in large meta-analysis.19 With respect to cognitive outcomes, limited data suggest that CRP,20 interleukin-12,21 and erythrocyte sedimentation rate,22 but not IL-6,20, 21 are associated with post-stroke cognitive impairment. Data regarding post-stroke depression are conflicting,23 with no evidence of an association between CRP levels and post-stroke depression in a recent multi-center prospective study.24 Evidence regarding post-stroke fatigue is similarly limited, with most studies including less than 50 subjects.25 CRP was also not consistently associated with fatigue across studies.26 In summary, inflammatory biomarkers at the time of stroke are fairly consistently associated with functional outcomes, but data regarding post-stroke cognitive impairment, depression, and fatigue are not definitive.
Insights from therapeutic trials
A causal association between inflammatory biomarkers and stroke risk can be inferred from clinical trials of immunomodulatory therapies. The Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER) seminally demonstrated that rosuvastatin for individuals with elevated hsCRP levels and with normal low-density lipoprotein C (LDL-C) levels reduced cardiovascular risk, including for the secondary outcome of stroke.27, 28 Rosuvastatin reduced stroke risk in subjects who either achieved LDL-C levels of < 70 mg/dL or hsCRP levels of <2 mg/L. Importantly, achieved LDL-C and hsCRP levels had poor correlation, suggesting that risk reductions could also be attributed to anti-inflammatory effects of rosuvastatin alone. This hypothesis was then specifically tested in the Canakinumab Antiinflammatory Thrombosis Outcome Study, which randomized patients with a history of myocardial infarction and elevated hsCRP level to canakinumab, a monoclonal antibody against interleukin-1beta, or placebo.29 Canakinumab reduced HsCRP levels in a dose-dependent fashion, without impact on LDL-C levels. There was a dose-dependent reduction in the primary cardiovascular disease (CVD) outcome, and stroke risk was nominally reduced in patients on the highest dose. In contrast, a related trial of methotrexate did not demonstrate reductions in inflammatory markers or CVD risk.30 Taken together, these data suggest that reduction of hsCRP, whether with statin therapy or canakinumab, results in a reduction of CVD risk, including stroke.
Trials of immunomodulatory therapy for the prevention of post-stroke unfavorable outcomes are few. The Japan Statin Treatment Against Recurrent Stroke trial randomized patients with prior stroke to pravastatin or placebo, and a secondary analysis evaluated whether changes in hsCRP were associated with recurrent event risk.31 Like in JUPITER, achieved LDL and achieved hsCRP levels did not correlate. Higher hsCRP levels during treatment were associated with a greater risk of recurrent stroke and vascular events. With regards to post-stroke functional outcomes, in an exploratory analysis of the Stroke Prevention by Aggressive Reduction in Cholesterol Levels trial, high dose atorvastatin was non-significantly associated with better functional outcomes compared to placebo (p=0.065).32 In a meta-analysis, statin therapy at the time of stroke was associated with less post-stroke disability.33 Last, two immunomodulatory multiple sclerosis therapies have been trialed in ischemic stroke. Natalizumab, a monoclonal antibody that reduces leukocyte infiltration, did not lower final infarct volumes but marginally improved functional outcomes.34 Fingolimod, an immunomodulator that reduces circulating lymphocytes, limited infarct volume and hemorrhagic transformation and improved functional outcomes when added to alteplase.35
Other stroke outcomes have been less studied. In the natalizumab trial mentioned above,34 exploratory analyses found non-significantly improved cognitive and mood outcomes with among those randomized to natalizumab. In the absence of trials of anti-inflammatory therapies for post-stroke cognitive impairment, depression, and fatigue, pharmacoepidemiology studies provide insights. A registry analysis found that anti-inflammatory treatment at the time of stroke was associated with a lower risk of depression within the first year.36 While data are conflicting,37 a prospective cohort study found that statins reduced the risk of post-stroke depression.38 Mechanistically, admission IL-6 levels have been associated with post-stroke depression at 1 year among statin non-users but not among statin-users.39 These data suggest that statins may ameliorate post-stroke inflammatory drivers of depression. Taken together, there is circumstantial evidence of a therapeutic effect of anti-inflammatory treatments on post-stroke outcomes. Trials that strategically target inflammation are needed.
Autoimmune disorders and ischemic stroke
Autoimmune disorders are frequently associated with stroke risk and provide insights into the impact of inflammation on cerebrovascular disease (Figure). Here, we focus on autoimmune disorders characterized by chronic systemic inflammation (Table 1). Cerebral vasculitides were reviewed elsewhere.40
Table 1.
Select autoimmune conditions and data* regarding associations with ischemic stroke risk
| Autoimmune condition | Association with ischemic stroke† | Dose-dependence‡ | Reduction with anti-inflammatories§ |
|---|---|---|---|
| Rheumatoid arthritis | +++ | ++ | + |
| Systemic lupus erythematous | + | − | − |
| Inflammatory bowel disease | ++ | + | − |
| Psoriasis | ++ | ++ | + |
| Ankylosing spondylitis | + | − | − |
| Polymyalgia rheumatica | + | − | + |
| Inflammatory myositis | + | − | − |
The strength of associations was judged based on the number, quality, and consistency of studies for each metric. Where meta-analyses were available, the totality of evidence was considered. The following categories of strength of evidence were used: +++, Compelling evidence; ++, Modest evidence; +, Limited evidence; −, No evidence.
Association with ischemic stroke was evaluated based on studies investigating the epidemiological association between individual conditions and stroke risk.
Dose-dependence was inferred from studies that investigated the association between severity or duration of each condition and ischemic stroke risk.
Results of pharmacoepidemiological studies investigating the association between treatment and stroke risk were evaluated.
See text for synthesis, summaries and results of individual studies, and references.
Rheumatoid arthritis
Rheumatoid arthritis (RA) is associated with an increased risk of CVD and has been included alongside conventional risk factors in a CVD risk score.41 Data specific to stroke are less abundant but consistent. A meta-analysis found that patients with RA had 1.6 times the odds of stroke as the general population, including when accounting for corticosteroid use.42–44 Although patients with RA have more vascular risk factors, this alone does not explain the increased risk of CVD since both conventional risk factors and markers of RA disease activity and severity, including elevated inflammatory markers, are associated with CVD risk.45–49 In administrative claims analyses, RA was associated an increased risk of recurrent stroke50 but not other stroke outcomes.51 Several pharmacoepidemiological studies, including a meta-analysis, provide mixed evidence that disease-modifying immunomodulatory therapies reduced CVD and stroke risk.52–54 In summary, RA is consistently associated with an increased stroke risk, and that risk can in part be attributed to inflammation, but whether targeting this inflammation reduces stroke risk remains unclear.
Systemic lupus erythematosus
In a recent meta-analysis, systemic lupus erythematosus (SLE) was associated with a two-fold increased odds of stroke compared to the general population.42 However, a subsequent large cohort study did not find an association.44 In an additional subsequent cohort study, SLE was not associated with stroke after accounting for steroid therapy as a confounder.55 Apart from inflammation, stroke mechanisms in SLE include cerebral arteritis, anti-phospholipid syndrome, and cardioembolism due to non-infectious endocarditis. Nephritis and related hypertension may also directly contribute to atherosclerosis and myocardial infarction risk.56, 57 Lastly, active steroid use is associated with CVD in patients with SLE.58 Therefore, there is insufficient evidence that SLE increases stroke risk through inflammation alone. Consistent with this notion, there is comparatively little data regarding disease modifying drugs and CVD risk in SLE.
Inflammatory bowel disease
Inflammatory bowel disease is associated with a modestly increased risk of stroke, with a meta-analysis reporting a hazard ratio of 1.3.59 Most studies reported an increased risk of stroke for both ulcerative colitis and Crohn’s disease, with the exception of two studies that found an association only for ulcerative colitis.60, 61 Several studies found the risk of stroke to be increased during times of greater disease activity.60, 62 Although the increased risk could also be attributable to treatment or detection bias, the evidence generally indicate that stroke is an inflammatory complication of these disorders.
Psoriasis
Psoriasis, with or without arthritis, is also associated with an increased stroke risk, with stroke risk paralleling disease severity.42, 63, 64 Disease duration was associated with vascular inflammation, assessed with 18F-fludeoxyglucose positron emission tomography, and ischemic stroke.65 A meta-analysis of observational studies found that systematic anti-inflammatory therapies were associated with a significantly decreased CVD risk compared to no systemic therapy or topical therapy.52 TNFα inhibition was associated with nearly an 50% reduction in stroke and transient ischemic attack, compared to methotrexate.66 Similarly, TNFα inhibition was associated with a lower CVD risk than phototherapy, despite phototherapy being used in milder cases.67 These data, in concert with the correspondence between psoriasis severity and duration and stroke, support a causal relationship between inflammation and stroke in this condition.
Other systemic inflammatory diseases
Other conditions preliminarily associated with stroke include ankylosing spondylitis,68 polymyalgia rheumatica,69 polymyositis, and dermatomyositis.70 In polymyalgia, cumulative exposure to glucocorticoids was associated with a tendency towards a decreased stroke and CVD risk.71 Because treatment with high dose steroids is typical in patients with more active disease, this finding raises the possibility that the anti-inflammatory effect of glucocorticoids mitigated the increased stroke and CVD risk.
To summarize, the autoimmune conditions reviewed here are associated with an increased stroke risk, with evidence of a dose-response relationship with disease duration and severity. Furthermore, targeting inflammation in some of these conditions reduces or mitigates stroke risk. These data provide clinical complement findings of inflammatory biomarker studies.
Chronic Infection and Ischemic Stroke
Inflammation is also seen in infectious diseases. Here we focus on select infectious conditions that may impact stroke risk and outcomes through inflammation (Table 2). Infectious diseases that cause stroke through other mechanisms were comprehensively reviewed elsewhere72 and are not discussed.
Table 2.
Select chronic infections and data* regarding associations with ischemic stroke risk
| Infection | Association with ischemic stroke | Dose-dependence | Reduction with infection control |
|---|---|---|---|
| Tuberculosis | +++ | ++ | ++ |
| Syphilis | ++ | − | ++ |
| Lyme disease | ++ | − | + |
| Human immunodeficiency virus | ++ | ++ | ++ |
| Varicella zoster virus | +++ | − | + |
| Cytomegalovirus | − | − | − |
| Hepatitis C virus | + | + | + |
| Helicobacter pylori | − | − | − |
| Chronic osteomyelitis | + | − | − |
| Periodontal Disease | ++ | + | + |
Please refer to Table 1 footnote for details.
Infectious Burden, Systemic Infections, and Stroke
Chronic infectious burden, the cumulative exposure to persistent or previous infections,73 may be associated with an increased stroke risk (Figure). Underlying mechanisms may include endothelial dysfunction and vascular inflammation promoting atherosclerosis,73, 74 and vascular injury from direct pathogenic invasion of the vessel wall.72–74 The chronic infectious burden construct includes Chlamydia pneumoniae, Mycoplasma Pneumoniae, Helicobacter pylori, Hemophilus influenza, Herpes simplex 1 and 2 (HSV), Cytomegalovirus (CMV), and Epstein Barr virus (EBV). The Northern Manhattan Study found an 1.4-fold increased risk of stroke among patients with high chronic infectious burden, defined by seropositivity.75 However, others have not replicated this finding, for example when adjusting for socioeconomic counfounders.76 Further studies are needed to understand whether chronic infectious burden confers an independently increased stroke risk.
Bacterial infections
Several chronic bacterial infections have been variably associated with stroke risk. Periodontitis, a polymicrobial destructive disease of the gums and supportive tissues, may increase stroke risk through systemic inflammation.77 For example, periodontal disease was associated with an increased risk of ischemic stroke in the Atherosclerosis Risk in Communities cohort.78 Periodontitis was independently associated with an increased stroke risk in a meta-analysis as well.79 Chronic osteomyelitis, another indolent infectious condition, was associated with an increased stroke risk in an administrative claims analysis.80 Finally, peptic ulcer, a common disease caused by Helicobacter pylori, was not associated with stroke in a recent, updated meta-analysis.81
Viral infections
Several chronic viral infections have also been implicated. CMV, a prevalent DNA herpesvirus, was observed in atherosclerotic plaque, raising the possibility that it contributes to atherosclerosis.82 CMV was not associated with stroke in the Framingham Heart Study,83 the Northern Manhattan Study,75 or in a meta-analysis.84 However, the Vascular effects of Infection in Pediatric Stroke study found that serological evidence of acute herpesvirus infections (a combination of HSV, CMV, EBV, and varicella zoster virus [VZV]) was associated with a two-fold odds of stroke.85 The lack of competing conventional risk factors may make infectious etiologies more relevant in children. In adults, hepatitis C virus (HCV) was associated with a two-fold increased risk of cerebrovascular death in a large cohort study, and a dose-response relationship with HCV RNA levels was seen.86 Furthermore, anti-viral treatment has been associated with a decreased ischemic stroke risk.87
Neurovascular Infections
Multiple infections lead to cerebral arterial inflammation through direct arterial wall invasion or immune complex deposition (Figure).88
Strokes can occur from tuberculosis meningitis, a progressive, necrotizing meningoencephalitis.89 It causes an exudative encasement and infiltration of vessels, leading to endarteritis.89 This, combined with mechanical stress from hydrocephalus,90 leads to stroke predominantly in the deep structures. Strokes occur in approximately 6% of cases of pulmonary tuberculosis and up to 20% of patients with tuberculous meningitis.91, 92 Stroke risk is also heightened among patients with pulmonary tuberculosis alone, compared to uninfected controls.91
Neurological involvement occurs in 10% of cases of untreated syphilis.93 Approximately 10% of patients with neurosyphilis, and 3% with any syphilis, develop stroke.93, 94 Patients with stroke are often young and with fewer conventional stroke risk factors, and may experience a prodrome of headache, malaise, and behavioral changes.95 The mechanism of stroke in syphilis is thought to be due to inflammation of the arterial wall secondary to an obliterative endarteritis of the medium to large arteries. Lyme disease, caused by borrelia burgdorferi, is another disease with protean cerebral manifestations including stroke.96 Stroke is thought to be due to an inflammatory vasculitic process of the cerebral vasculature.97
In a recent meta-analysis, HIV was associated with 1.8 times higher stroke risk compared to non-infected individuals.98 Lower CD4 counts and unsuppressed viral load were risk factors. HIV affects the central nervous system days after systemic infection and produces an inflammatory cascade within the cerebrospinal fluid and the brain parenchyma.99 On autopsy, arteries from HIV positive patients had more inflammation than arteries from HIV negative controls, though the association was attenuated after adjusting for possible confounders.100 Individuals with HIV have accelerated atherosclerosis, exemplified by higher carotid intima-media thickness and coronary artery calcium progression.101 Individuals with HIV also have evidence of arterial remodeling – leading to atherosclerosis and dolichoectasia – which may be a consequence of arterial inflammation.102
Following VZV (chickenpox) infection, VZV becomes latent in ganglionic neurons throughout the neuroaxis, and reactivation causes herpes zoster (shingles).103 VZV can also directly infect large and small cerebral arteries to cause a vasculopathy, resulting in both ischemic and hemorrhagic stroke.103, 104 In a meta-analysis, zoster was associated with a 1.5-fold increased stroke risk for four weeks after zoster.105 The risk of stroke was up to 4.5 times higher in the first 12 months after zoster ophthalmicus.106 The observation that antiviral treatment may mitigate stroke risk105 increases confidence in the causality of VZV’s association with stroke. Although the strength of associations and exact mechanisms vary, epidemiological data support the notion that several microbial infections are independent stroke risk factors, often acting through chronic inflammation.
Post-infectious stroke and post-stroke infection
There is a short-term increase in stroke risk after multiple acute infections. In a large, self-controlled case series analysis107 and in the Cardiovascular Health Study,108 short-term stroke risk markedly increased after respiratory and urinary infections. Influenza-like illnesses and sepsis also appear to increase short-term stroke risk.109, 110 The inflammatory response to the infection is thought to contribute to accelerated atherosclerosis, plaque rupture, and thrombosis.72, 111 Conversely, due in part to post-stroke immunosuppression,2 the rate of infection after stroke is as high as 30%.112 Infections, often pneumonia and urinary infections, are associated with poor outcomes and mortality.113, 114 However, a meta-analysis found that randomization to prophylactic antibiotics reduced infection risk without improving outcomes.115 Therefore, whether infection silently precedes stroke, is an epiphenomenon, or causally linked to outcomes remains unclear.
Conclusions
A rich body of literature demonstrates that inflammation is associated with increased stroke risk and may be an important determinant of outcomes. Data from pharmacological interventions and the study of autoimmune and infectious diseases provide additional support of a causal relationship between inflammation and increased stroke risk and severity. Currently, there is insufficient evidence to support the use of immunomodulatory therapies specifically to reduce stroke risk or improve outcomes. However, trials of newer immunomodulatory therapies, such as fingolimod and targeted inflammatory cytokine-neutralizing antibodies, combined with improved patient selection, may yield effective interventions to reduce the burden of stroke risk and improve stroke outcomes among the general population and in individuals with autoimmune and infectious diseases.
Sources of funding
NSP is supported by National Institute of Neurological Disorders and Stroke T32NS07153 (PI: Elkind), AEM by American Heart Association grant 18CDA34110419 and the Leon Levy Fellowship in Neuroscience, and CI by NIH/National Institute of Neurological Disorders and Stroke grants R01NS34179, R37-NS089323, R01-NS100447, 1R01-NS095441, and R01-NS/HL37853.
Footnotes
Disclosures
NSP: none.
AEM: none.
CI serves on the Strategic Advisory Board of Broadview Ventures.
References
- 1.Netea MG, Balkwill F, Chonchol M, Cominelli F, Donath MY, Giamarellos-Bourboulis EJ, et al. A guiding map for inflammation. Nat Immunol. 2017;18:826–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anrather J, Iadecola C. Inflammation and stroke: An overview. Neurotherapeutics. 2016;13:661–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Esenwa CC, Elkind MS. Inflammatory risk factors, biomarkers and associated therapy in ischaemic stroke. Nat Rev Neurol. 2016;12:594–604. [DOI] [PubMed] [Google Scholar]
- 4.Markstad H, Edsfeldt A, Yao Mattison I, Bengtsson E, Singh P, Cavalera M, et al. High levels of soluble lectinlike oxidized low-density lipoprotein receptor-1 are associated with carotid plaque inflammation and increased risk of ischemic stroke. J Am Heart Assoc. 2019;8:e009874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skarpengland T, Skjelland M, Kong XY, Skagen K, Holm S, Otterdal K, et al. Increased levels of lectin-like oxidized low-density lipoprotein receptor-1 in ischemic stroke and transient ischemic attack. J Am Heart Assoc. 2018;7: e006479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arenillas JF, Alvarez-Sabín J, Molina CA, Chacón P, Montaner J, Rovira A, et al. C-reactive protein predicts further ischemic events in first-ever transient ischemic attack or stroke patients with intracranial large-artery occlusive disease. Stroke. 2003;34:2463–2468. [DOI] [PubMed] [Google Scholar]
- 7.Whiteley W, Jackson C, Lewis S, Lowe G, Rumley A, Sandercock P, et al. Association of circulating inflammatory markers with recurrent vascular events after stroke: A prospective cohort study. Stroke. 2011;42:10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elkind MS, Tai W, Coates K, Paik MC, Sacco RL. High-sensitivity c-reactive protein, lipoprotein-associated phospholipase a2, and outcome after ischemic stroke. Arch Intern Med. 2006;166:2073–2080. [DOI] [PubMed] [Google Scholar]
- 9.Di Napoli M, Papa F, Bocola V. Prognostic influence of increased c-reactive protein and fibrinogen levels in ischemic stroke. Stroke. 2001;32:133–138. [DOI] [PubMed] [Google Scholar]
- 10.Di Napoli M, Papa F, Villa Pini Stroke Data Bank Investigators. Inflammation, hemostatic markers, and antithrombotic agents in relation to long-term risk of new cardiovascular events in first-ever ischemic stroke patients. Stroke. 2002;33:1763–1771. [DOI] [PubMed] [Google Scholar]
- 11.Welsh P, Lowe GD, Chalmers J, Campbell DJ, Rumley A, Neal BC, et al. Associations of proinflammatory cytokines with the risk of recurrent stroke. Stroke. 2008;39:2226–2230. [DOI] [PubMed] [Google Scholar]
- 12.Castillo J, Alvarez-Sabín J, Martínez-Vila E, Montaner J, Sobrino T, Vivancos J, et al. Inflammation markers and prediction of post-stroke vascular disease recurrence: The MITICO study. J Neurol. 2009;256:217–224. [DOI] [PubMed] [Google Scholar]
- 13.Wei L, Ke Z, Zhao Y, Cai Z. The elevated lipoprotein-associated phospholipase a2 activity is associated with the occurrence and recurrence of acute cerebral infarction. Neuroreport. 2017;28:325–330. [DOI] [PubMed] [Google Scholar]
- 14.Boehme AK, McClure LA, Zhang Y, Luna JM, Del Brutto OH, Benavente OR, et al. Inflammatory Markers and Outcomes After Lacunar Stroke: Levels of Inflammatory Markers in Treatment of Stroke Study. Stroke. 2016;47:659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Segal HC, Burgess AI, Poole DL, Mehta Z, Silver LE, Rothwell PM. Population-based study of blood biomarkers in prediction of subacute recurrent stroke. Stroke. 2014;45:2912–2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shenhar-Tsarfaty S, Ben Assayag E, Bova I, Shopin L, Fried M, Berliner S, et al. Interleukin-6 as an early predictor for one-year survival following an ischaemic stroke/transient ischaemic attack. Int J Stroke. 2010;5:16–20. [DOI] [PubMed] [Google Scholar]
- 17.Bustamante A, Simats A, Vilar-Bergua A, García-Berrocoso T, Montaner J. Blood/brain biomarkers of inflammation after stroke and their association with outcome: From c-reactive protein to damage-associated molecular patterns. Neurotherapeutics. 2016;13:671–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li J, Zhao X, Meng X, Lin J, Liu L, Wang C, et al. High-sensitive c-reactive protein predicts recurrent stroke and poor functional outcome: Subanalysis of the Clopidogrel in High-risk Patients with Acute Nondisabling Cerebrovascular Events Trial. Stroke. 2016;47:2025–2030. [DOI] [PubMed] [Google Scholar]
- 19.Bustamante A, Sobrino T, Giralt D, García-Berrocoso T, Llombart V, Ugarriza I, et al. Prognostic value of blood interleukin-6 in the prediction of functional outcome after stroke: A systematic review and meta-analysis. J Neuroimmunol. 2014;274:215–224. [DOI] [PubMed] [Google Scholar]
- 20.Rothenburg LS, Herrmann N, Swardfager W, Black SE, Tennen G, Kiss A, et al. The relationship between inflammatory markers and post stroke cognitive impairment. J Geriatr Psychiatry Neurol. 2010;23:199–205. [DOI] [PubMed] [Google Scholar]
- 21.Narasimhalu K, Lee J, Leong YL, Ma L, De Silva DA, Wong MC, et al. Inflammatory markers and their association with post stroke cognitive decline. Int J Stroke. 2015;10:513–518. [DOI] [PubMed] [Google Scholar]
- 22.Kliper E, Bashat DB, Bornstein NM, Shenhar-Tsarfaty S, Hallevi H, Auriel E, et al. Cognitive decline after stroke: Relation to inflammatory biomarkers and hippocampal volume. Stroke. 2013;44:1433–1435. [DOI] [PubMed] [Google Scholar]
- 23.Levada OA, Troyan AS. Poststroke depression biomarkers: A narrative review. Front Neurol. 2018;9:577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yin J, Zhong C, Zhu Z, Bu X, Xu T, Guo L, et al. Elevated circulating homocysteine and high-sensitivity c-reactive protein jointly predicts post-stroke depression among Chinese patients with acute ischemic stroke. Clin Chim Acta. 2018;479:132–137. [DOI] [PubMed] [Google Scholar]
- 25.Hinkle JL, Becker KJ, Kim JS, Choi-Kwon S, Saban KL, McNair N, et al. Poststroke fatigue: Emerging evidence and approaches to management: A scientific statement for healthcare professionals from the American Heart Association. Stroke. 2017;48:e159–e170. [DOI] [PubMed] [Google Scholar]
- 26.Wu S, Duncan F, Anderson NH, Kuppuswamy A, Macloed MR, Mead GE. Exploratory cohort study of associations between serum c - reactive protein and fatigue after stroke. PLoS One. 2015;10:e0143784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Kastelein JJ, et al. Rosuvastatin to prevent vascular events in men and women with elevated c-reactive protein. N Engl J Med. 2008;359:2195–2207. [DOI] [PubMed] [Google Scholar]
- 28.Everett BM, Glynn RJ, MacFadyen JG, Ridker PM. Rosuvastatin in the prevention of stroke among men and women with elevated levels of c-reactive protein: Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER). Circulation. 2010;121:143–150. [DOI] [PubMed] [Google Scholar]
- 29.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 30.Ridker PM, Everett BM, Pradhan A, MacFadyen JG, Solomon DH, Zaharris E, et al. Low-dose methotrexate for the prevention of atherosclerotic events. N Engl J Med. 2019;380:752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kitagawa K, Hosomi N, Nagai Y, Kagimura T, Ohtsuki T, Origasa H, et al. Reduction in high-sensitivity c-reactive protein levels in patients with ischemic stroke by statin treatment: Hs-CRP sub-study in J-STARS. J Atheroscler Thromb. 2017;24:1039–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldstein LB, Amarenco P, Zivin J, Messig M, Altafullah I, Callahan A, et al. Statin treatment and stroke outcome in the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial. Stroke. 2009;40:3526–3531. [DOI] [PubMed] [Google Scholar]
- 33.Ní Chróinín D, Asplund K, Åsberg S, Callaly E, Cuadrado-Godia E, Díez-Tejedor E, et al. Statin therapy and outcome after ischemic stroke: Systematic review and meta-analysis of observational studies and randomized trials. Stroke. 2013;44:448–456. [DOI] [PubMed] [Google Scholar]
- 34.Elkins J, Veltkamp R, Montaner J, Johnston SC, Singhal AB, Becker K, et al. Safety and efficacy of natalizumab in patients with acute ischaemic stroke (ACTION): A randomised, placebo-controlled, double-blind phase 2 trial. Lancet Neurol. 2017;16:217–226. [DOI] [PubMed] [Google Scholar]
- 35.Zhu Z, Fu Y, Tian D, Sun N, Han W, Chang G, et al. Combination of the immune modulator fingolimod with alteplase in acute ischemic stroke: A pilot trial. Circulation. 2015;132:1104–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wium-Andersen IK, Wium-Andersen MK, Jørgensen MB, Osler M. Anti-inflammatory treatment and risk for depression after first-time stroke in a cohort of 147 487 Danish patients. J Psychiatry Neurosci. 2017;42:320–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kang JH, Kao LT, Lin HC, Tsai MC, Chung SD. Statin use increases the risk of depressive disorder in stroke patients: A population-based study. J Neurol Sci. 2015;348:89–93. [DOI] [PubMed] [Google Scholar]
- 38.Kim JM, Stewart R, Kang HJ, Bae KY, Kim SW, Shin IS, et al. A prospective study of statin use and poststroke depression. J Clin Psychopharmacol. 2014;34:72–79. [DOI] [PubMed] [Google Scholar]
- 39.Kang HJ, Bae KY, Kim SW, Kim JT, Park MS, Cho KH, et al. Effects of interleukin-6, interleukin-18, and statin use, evaluated at acute stroke, on post-stroke depression during 1-year follow-up. Psychoneuroendocrinology. 2016;72:156–160. [DOI] [PubMed] [Google Scholar]
- 40.Byram K, Hajj-Ali RA, Calabrese L. CNS Vasculitis: An Approach to Differential Diagnosis and Management. Curr Rheumatol Rep. 2018;20:37. [DOI] [PubMed] [Google Scholar]
- 41.Hippisley-Cox J, Coupland C, Vinogradova Y, Robson J, Minhas R, Sheikh A, et al. Predicting cardiovascular risk in England and Wales: Prospective derivation and validation of QRISK2. BMJ. 2008;336:1475–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wiseman SJ, Ralston SH, Wardlaw JM. Cerebrovascular disease in rheumatic diseases: A systematic review and meta-analysis. Stroke. 2016;47:943–950. [DOI] [PubMed] [Google Scholar]
- 43.Lindhardsen J, Ahlehoff O, Gislason GH, Madsen OR, Olesen JB, Svendsen JH, et al. Risk of atrial fibrillation and stroke in rheumatoid arthritis: Danish nationwide cohort study. BMJ. 2012;344:e1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baena-Díez JM, Garcia-Gil M, Comas-Cufí M, Ramos R, Prieto-Alhambra D, Salvador-González B, et al. Association between chronic immune-mediated inflammatory diseases and cardiovascular risk. Heart. 2018;104:119–126. [DOI] [PubMed] [Google Scholar]
- 45.Crowson CS, Rollefstad S, Ikdahl E, Kitas GD, van Riel PLCM, Gabriel SE, et al. Impact of risk factors associated with cardiovascular outcomes in patients with rheumatoid arthritis. Ann Rheum Dis. 2018;77:48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Solomon DH, Kremer J, Curtis JR, Hochberg MC, Reed G, Tsao P, et al. Explaining the cardiovascular risk associated with rheumatoid arthritis: Traditional risk factors versus markers of rheumatoid arthritis severity. Ann Rheum Dis. 2010;69:1920–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Solomon DH, Reed GW, Kremer JM, Curtis JR, Farkouh ME, Harrold LR, et al. Disease activity in rheumatoid arthritis and the risk of cardiovascular events. Arthritis Rheumatol. 2015;67:1449–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Masuda H, Miyazaki T, Shimada K, Tamura N, Matsudaira R, Yoshihara T, et al. Disease duration and severity impacts on long-term cardiovascular events in Japanese patients with rheumatoid arthritis. J Cardiol. 2014;64:366–370. [DOI] [PubMed] [Google Scholar]
- 49.Zhang J, Chen L, Delzell E, Muntner P, Hillegass WB, Safford MM, et al. The association between inflammatory markers, serum lipids and the risk of cardiovascular events in patients with rheumatoid arthritis. Ann Rheum Dis. 2014;73:1301–1308. [DOI] [PubMed] [Google Scholar]
- 50.Chen YR, Hsieh FI, Lien LM, Hu CJ, Jeng JS, Peng GS, et al. Rheumatoid arthritis significantly increased recurrence risk after ischemic stroke/transient ischemic attack. J Neurol. 2018;265:1810–1818. [DOI] [PubMed] [Google Scholar]
- 51.Kang JH, Xirasagar S, Lin HC, Kao PF, Sung LC. Risk of adverse outcomes in patients with rheumatoid arthritis hospitalized for stroke-a cross-sectional study. Clin Rheumatol. 2018;37:2917–2926. [DOI] [PubMed] [Google Scholar]
- 52.Roubille C, Richer V, Starnino T, McCourt C, McFarlane A, Fleming P, et al. The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: A systematic review and meta-analysis. Ann Rheum Dis. 2015;74:480–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sharma TS, Wasko MC, Tang X, Vedamurthy D, Yan X, Cote J, et al. Hydroxychloroquine use is associated with decreased incident cardiovascular events in rheumatoid arthritis patients. J Am Heart Assoc. 2016;5: e002867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Solomon DH, Curtis JR, Saag KG, Lii J, Chen L, Harrold LR, et al. Cardiovascular risk in rheumatoid arthritis: Comparing TNF-α blockade with nonbiologic DMARDs. Am J Med. 2013;126:730.e9–730.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liou TH, Huang SW, Lin JW, Chang YS, Wu CW, Lin HW. Risk of stroke in patients with rheumatism: A nationwide longitudinal population-based study. Sci Rep. 2014;4:5110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wells DK, Ward MM. Nephritis and the risk of acute myocardial infarction in patients with systemic lupus erythematosus. Clin Exp Rheumatol. 2010;28:223–229. [PMC free article] [PubMed] [Google Scholar]
- 57.Gustafsson JT, Herlitz Lindberg M, Gunnarsson I, Pettersson S, Elvin K, Öhrvik J, et al. Excess atherosclerosis in systemic lupus erythematosus,-A matter of renal involvement: Case control study of 281 SLE patients and 281 individually matched population controls. PLoS One. 2017;12:e0174572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Magder LS, Petri M. Incidence of and risk factors for adverse cardiovascular events among patients with systemic lupus erythematosus. Am J Epidemiol. 2012;176:708–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xiao Z, Pei Z, Yuan M, Li X, Chen S, Xu L. Risk of stroke in patients with inflammatory bowel disease: A systematic review and meta-analysis. J Stroke Cerebrovasc Dis. 2015;24:2774–2780. [DOI] [PubMed] [Google Scholar]
- 60.Huang WS, Tseng CH, Chen PC, Tsai CH, Lin CL, Sung FC, et al. Inflammatory bowel diseases increase future ischemic stroke risk: A Taiwanese population-based retrospective cohort study. Eur J Intern Med. 2014;25:561–565. [DOI] [PubMed] [Google Scholar]
- 61.Dregan A, Chowienczyk P, Molokhia M. Cardiovascular and type 2 diabetes morbidity and all-cause mortality among diverse chronic inflammatory disorders. Heart. 2017;103:1867–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Le Gall G, Kirchgesner J, Bejaoui M, Landman C, Nion-Larmurier I, Bourrier A, et al. Clinical activity is an independent risk factor of ischemic heart and cerebrovascular arterial disease in patients with inflammatory bowel disease. PLoS One. 2018;13:e0201991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Armstrong EJ, Harskamp CT, Armstrong AW. Psoriasis and major adverse cardiovascular events: A systematic review and meta-analysis of observational studies. J Am Heart Assoc. 2013;2:e000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ahlehoff O, Gislason GH, Jørgensen CH, Lindhardsen J, Charlot M, Olesen JB, et al. Psoriasis and risk of atrial fibrillation and ischaemic stroke: A Danish nationwide cohort study. Eur Heart J. 2012;33:2054–2064. [DOI] [PubMed] [Google Scholar]
- 65.Egeberg A, Skov L, Joshi AA, Mallbris L, Gislason GH, Wu JJ, et al. The relationship between duration of psoriasis, vascular inflammation, and cardiovascular events. J Am Acad Dermatol. 2017;77:650–656.e653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu JJ, Guérin A, Sundaram M, Dea K, Cloutier M, Mulani P. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-α inhibitors versus methotrexate. J Am Acad Dermatol. 2017;76:81–90. [DOI] [PubMed] [Google Scholar]
- 67.Wu JJ, Sundaram M, Cloutier M, Gauthier-Loiselle M, Guérin A, Singh R, et al. The risk of cardiovascular events in psoriasis patients treated with tumor necrosis factor-α inhibitors versus phototherapy: An observational cohort study. J Am Acad Dermatol. 2018;79:60–68. [DOI] [PubMed] [Google Scholar]
- 68.Eriksson JK, Jacobsson L, Bengtsson K, Askling J. Is ankylosing spondylitis a risk factor for cardiovascular disease, and how do these risks compare with those in rheumatoid arthritis? Ann Rheum Dis. 2017;76:364–370. [DOI] [PubMed] [Google Scholar]
- 69.Kang JH, Sheu JJ, Lin HC. Polymyalgia rheumatica and the risk of stroke: A three-year follow-up study. Cerebrovasc Dis. 2011;32:497–503. [DOI] [PubMed] [Google Scholar]
- 70.Ungprasert P, Cheungpasitporn W, Wijarnpreecha K, Ahuja W, Ratanasrimetha P, Thongprayoon C. Risk of ischemic stroke in patients with polymyositis and dermatomyositis: A systematic review and meta-analysis. Rheumatol Int. 2015;35:905–909. [DOI] [PubMed] [Google Scholar]
- 71.Maradit Kremers H, Reinalda MS, Crowson CS, Davis JM, Hunder GG, Gabriel SE. Glucocorticoids and cardiovascular and cerebrovascular events in polymyalgia rheumatica. Arthritis Rheum. 2007;57:279–286. [DOI] [PubMed] [Google Scholar]
- 72.Fugate JE, Lyons JL, Thakur KT, Smith BR, Hedley-Whyte ET, Mateen FJ. Infectious causes of stroke. Lancet Infect Dis. 2014;14:869–880. [DOI] [PubMed] [Google Scholar]
- 73.Epstein SE, Zhou YF, Zhu J. Infection and atherosclerosis: Emerging mechanistic paradigms. Circulation. 1999;100:e20–28. [DOI] [PubMed] [Google Scholar]
- 74.Elkind MS. Infectious burden: A new risk factor and treatment target for atherosclerosis. Infect Disord Drug Targets. 2010;10:84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Elkind MS, Ramakrishnan P, Moon YP, Boden-Albala B, Liu KM, Spitalnik SL, et al. Infectious burden and risk of stroke: The Morthern Manhattan Study. Arch Neurol. 2010;67:33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Palm F, Pussinen PJ, Aigner A, Becher H, Buggle F, Bauer MF, et al. Association between infectious burden, socioeconomic status, and ischemic stroke. Atherosclerosis. 2016;254:117–123. [DOI] [PubMed] [Google Scholar]
- 77.Kinane DF, Stathopoulou PG, Papapanou PN. Periodontal diseases. Nat Rev Dis Primers. 2017;3:17038. [DOI] [PubMed] [Google Scholar]
- 78.Sen S, Giamberardino LD, Moss K, Morelli T, Rosamond WD, Gottesman RF, et al. Periodontal disease, regular dental care use, and incident ischemic stroke. Stroke. 2018;49:355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Leira Y, Seoane J, Blanco M, Rodríguez-Yáñez M, Takkouche B, Blanco J, et al. Association between periodontitis and ischemic stroke: A systematic review and meta-analysis. Eur J Epidemiol. 2017;32:43–53. [DOI] [PubMed] [Google Scholar]
- 80.Tseng CH, Chen JH, Muo CH, Chang YJ, Sung FC, Hsu CY. Increased risk of ischaemic stroke amongst patients with chronic osteomyelitis: A population-based cohort study in Taiwan. Eur J Neurol. 2015;22:633–639. [DOI] [PubMed] [Google Scholar]
- 81.Yu M, Zhang Y, Yang Z, Ding J, Xie C, Lu N. Association between helicobacter pylori infection and stroke: A meta-analysis of prospective observational studies. J Stroke Cerebrovasc Dis. 2014;23:2233–2239. [DOI] [PubMed] [Google Scholar]
- 82.Melnick JL, Petrie BL, Dreesman GR, Burek J, McCollum CH, DeBakey ME. Cytomegalovirus antigen within human arterial smooth muscle cells. Lancet. 1983;2:644–647. [DOI] [PubMed] [Google Scholar]
- 83.Haider AW, Wilson PW, Larson MG, Evans JC, Michelson EL, Wolf PA, et al. The association of seropositivity to helicobacter pylori, chlamydia pneumoniae, and cytomegalovirus with risk of cardiovascular disease: A prospective study. J Am Coll Cardiol. 2002;40:1408–1413. [DOI] [PubMed] [Google Scholar]
- 84.Wang H, Peng G, Bai J, He B, Huang K, Hu X, et al. Cytomegalovirus infection and relative risk of cardiovascular disease (ischemic heart disease, stroke, and cardiovascular death): A meta-analysis of prospective studies up to 2016. J Am Heart Assoc. 2017;6: e005025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Elkind MS, Hills NK, Glaser CA, Lo WD, Amlie-Lefond C, Dlamini N, et al. Herpesvirus infections and childhood arterial ischemic stroke: Results of the VIPS study. Circulation. 2016;133:732–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lee MH, Yang HI, Wang CH, Jen CL, Yeh SH, Liu CJ, et al. Hepatitis C virus infection and increased risk of cerebrovascular disease. Stroke. 2010;41:2894–2900. [DOI] [PubMed] [Google Scholar]
- 87.Hsu YC, Ho HJ, Huang YT, Wang HH, Wu MS, Lin JT, et al. Association between antiviral treatment and extrahepatic outcomes in patients with hepatitis C virus infection. Gut. 2015;64:495–503. [DOI] [PubMed] [Google Scholar]
- 88.Tarnacka B, Gromadzka G, Członkowska A. Increased circulating immune complexes in acute stroke: The triggering role of chlamydia pneumoniae and cytomegalovirus. Stroke. 2002;33:936–940. [DOI] [PubMed] [Google Scholar]
- 89.Lammie GA, Hewlett RH, Schoeman JF, Donald PR. Tuberculous cerebrovascular disease: A review. J Infect. 2009;59:156–166. [DOI] [PubMed] [Google Scholar]
- 90.Berenguer J, Moreno S, Laguna F, Vicente T, Adrados M, Ortega A, et al. Tuberculous meningitis in patients infected with the human immunodeficiency virus. N Engl J Med. 1992;326:668–672. [DOI] [PubMed] [Google Scholar]
- 91.Sheu JJ, Chiou HY, Kang JH, Chen YH, Lin HC. Tuberculosis and the risk of ischemic stroke: A 3-year follow-up study. Stroke. 2010;41:244–249. [DOI] [PubMed] [Google Scholar]
- 92.Merkler AE, Reynolds AS, Gialdini G, Morris NA, Murthy SB, Thakur K, et al. Neurological complications after tuberculous meningitis in a multi-state cohort in the United States. J Neurol Sci. 2017;375:460–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hooshmand H, Escobar MR, Kopf SW. Neurosyphilis. A study of 241 patients. JAMA. 1972;219:726–729. [DOI] [PubMed] [Google Scholar]
- 94.Singh AE, Romanowski B. Syphilis: Review with emphasis on clinical, epidemiologic, and some biologic features. Clin Microbiol Rev. 1999;12:187–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ahbeddou N, El Alaoui Taoussi K, Ibrahimi A, Ait Ben Haddou EH, Regragui W, Benomar A, et al. Stroke and syphilis: A retrospective study of 53 patients. Rev Neurol (Paris). 2018;174:313–318. [DOI] [PubMed] [Google Scholar]
- 96.Uldry PA, Regli F, Bogousslavsky J. Cerebral angiopathy and recurrent strokes following borrelia burgdorferi infection. J Neurol Neurosurg Psychiatry. 1987;50:1703–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Garkowski A, Zajkowska J, Zajkowska A, Kułakowska A, Zajkowska O, Kubas B, et al. Cerebrovascular manifestations of lyme neuroborreliosis-a systematic review of published cases. Front Neurol. 2017;8:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gutierrez J, Albuquerque ALA, Falzon L. HIV infection as vascular risk: A systematic review of the literature and meta-analysis. PLoS One. 2017;12:e0176686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Valcour V, Chalermchai T, Sailasuta N, Marovich M, Lerdlum S, Suttichom D, et al. Central nervous system viral invasion and inflammation during acute HIV infection. J Infect Dis. 2012;206:275–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gutierrez J, Menshawy K, Gonzalez M, Goldman J, Elkind MS, Marshall R, et al. Brain large artery inflammation associated with HIV and large artery remodeling. AIDS. 2016;30:415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hsue PY, Lo JC, Franklin A, Bolger AF, Martin JN, Deeks SG, et al. Progression of atherosclerosis as assessed by carotid intima-media thickness in patients with HIV infection. Circulation. 2004;109:1603–1608. [DOI] [PubMed] [Google Scholar]
- 102.Gutierrez J, Goldman J, Dwork AJ, Elkind MS, Marshall RS, Morgello S. Brain arterial remodeling contribution to nonembolic brain infarcts in patients with HIV. Neurology. 2015;85:1139–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nagel MA, Gilden D. The relationship between herpes zoster and stroke. Curr Neurol Neurosci Rep. 2015;15:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nagel MA, Cohrs RJ, Mahalingam R, Wellish MC, Forghani B, Schiller A, et al. The varicella zoster virus vasculopathies: Clinical, CSF, imaging, and virologic features. Neurology. 2008;70:853–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Forbes HJ, Williamson E, Benjamin L, Breuer J, Brown MM, Langan SM, et al. Association of herpesviruses and stroke: Systematic review and meta-analysis. PLoS One. 2018;13:e0206163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lin HC, Chien CW, Ho JD. Herpes zoster ophthalmicus and the risk of stroke: A population-based follow-up study. Neurology. 2010;74:792–797. [DOI] [PubMed] [Google Scholar]
- 107.Smeeth L, Thomas SL, Hall AJ, Hubbard R, Farrington P, Vallance P. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med. 2004;351:2611–2618. [DOI] [PubMed] [Google Scholar]
- 108.Elkind MS, Carty CL, O’Meara ES, Lumley T, Lefkowitz D, Kronmal RA, et al. Hospitalization for infection and risk of acute ischemic stroke: The Cardiovascular Health Study. Stroke. 2011;42:1851–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Boehme AK, Luna J, Kulick ER, Kamel H, Elkind MSV. Influenza-like illness as a trigger for ischemic stroke. Ann Clin Transl Neurol. 2018;5:456–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Boehme AK, Ranawat P, Luna J, Kamel H, Elkind MS. Risk of acute stroke after hospitalization for sepsis: A case-crossover study. Stroke. 2017;48:574–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lindsberg PJ, Grau AJ. Inflammation and infections as risk factors for ischemic stroke. Stroke. 2003;34:2518–2532. [DOI] [PubMed] [Google Scholar]
- 112.Westendorp WF, Nederkoorn PJ, Vermeij JD, Dijkgraaf MG, van de Beek D. Post-stroke infection: A systematic review and meta-analysis. BMC Neurol. 2011;11:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Finlayson O, Kapral M, Hall R, Asllani E, Selchen D, Saposnik G, et al. Risk factors, inpatient care, and outcomes of pneumonia after ischemic stroke. Neurology. 2011;77:1338–1345. [DOI] [PubMed] [Google Scholar]
- 114.Popović N, Stefanović-Budimkić M, Mitrović N, Urošević A, Milošević B, Pelemiš M, et al. The frequency of poststroke infections and their impact on early stroke outcome. J Stroke Cerebrovasc Dis. 2013;22:424–429. [DOI] [PubMed] [Google Scholar]
- 115.Zheng F, Spreckelsen NV, Zhang X, Stavrinou P, Timmer M, Dohmen C, et al. Should preventive antibiotics be used in patients with acute stroke? A systematic review and meta-analysis of randomized controlled trials. PLoS One. 2017;12:e0186607. [DOI] [PMC free article] [PubMed] [Google Scholar]


