Introduction
Acute hyperglycemia and contrast-induced nephropathy (CIN) are frequently observed in non-ST elevation myocardial infarction (NSTEMI) patients undergoing percutaneous coronary intervention (PCI), and both are associated with an increased mortality rate. We investigated the possible association between acute hyperglycemia and CIN in patients with NSTEMI undergoing PCI.
Materials and methods
We retrospectively enrolled 281(149, 53% men) NSTEMI patients undergoing PCI. For each patient, plasma glucose levels were secreened at hospital admission. Acute hyperglycemia was defined as glucose levels > 198 mg/dl. CIN was defined as an increase in serum creatinine 25% or 0.5 mg/dl from baseline in the first 48–72 hours.
Results
Overall, 44 (15.7%) patients had acute hyperglycemia. Patients with acute hyperglycemia had higher incidence of CIN than those without acute hyperglycemia (29.5 vs 5.1%, P < 0.001). Also, in-hospital mortality, length of hospital stay, major bleeding, requirement of mechanical ventilation and dialysis were observed significantly higher in patients with hyperglycemia. Patients were then reallocated to two groups according to the presence or absence of CIN. Overall, 25 cases (8.9%) of CIN were diagnosed. Diabetes mellitus, weight, age, glucose level and estimated glomerular filtration rate (eGFR) were detected as independent risk factors of CIN. Additionally, admission glucose levels were significantly correlated with creatinine levels after PCI, eGFR and contrast volume/eGFR ratio.
Conclusion
In NSTEMI patients undergoing primary PCI, acute hyperglycemia may be associated with an increased risk for CIN and in-hospital mortality and morbidity.
Keywords: hyperglycemia, myocardial infarction, nephropathy
Introduction
Percutaneous coronary intervention (PCI) in patients with non-ST elevation myocardial infarction (NSTEMI) reduces ischemic complications and improves survival. Patients undergoing PCI are at high risk for contrast-induced nephropathy (CIN) and this has been associated with renal dysfunction, longer hospital stay, increased cardiovascular events and mortality [1,2]. So many factors such as hypovolemia, contrast volume, and baseline glomerular filtration rate (GFR) may contribute to the development of CIN [3,4]. Because of this, identifying patients at the risk of CIN is important in patients undergoing PCI.
An increased glucose levels at hospital presentation (acute hyperglycemia) in patients with myocardial infarction causes increased motility and morbidity, even in those without established diabetes mellitus [5–7]. Several studies have demonstrated that acute hyperglycemia in miyocard infarction is associated with acute increase in inflammatory immune markers, impairment of epicardial coronary flow, increased left ventricular dysfunction, larger infarct size, higher congestive heart failure, cardiogenic shock and hospital mortality [3,4,8,9].
The association between diabetes mellitus and CIN risk has been previously recognized in patients undergoing elective PCI [10,11] and the relationship between acute hyperglycemia and CIN in STEMI patients treated with primary PCI has been showed before [12]. But there was no information in patients with NSTEMI. Pathophysiologic mechanisms of renal injury due to contrast administration were oxidative stress, endothelial dysfunction and apoptosis [13]. Also acute hyperglycemia activates all of these mechanisms [14]. The previous studies reported that fluctuations in glucose levels more harmful than chronically elevated glucose levels, and increases apoptosis and oxidative stress [14–16]. So, acute hyperglycemia may aggravate the negative effects of contrast exposure and increase the risk of CIN. Knowing this situation may help identification of potential risks for intervention and pharmacologic control of glucose levels might reduce CIN and improve patient outcome after PCI. Thus, we aim to retrospectively investigate the relationship between admission glucose levels and the risk of CIN and in-hospital major adverse events in patients with NSTEMI who undergo PCI.
Methods
We retrospectively observed 281 consecutive patients with NSTEMI undergoing PCI at the Avicenna Hospital Cardiology Department between January 2015 and January 2018. NSTEMI was defined according to the current guidelines [17]. We excluded patients with a severe valvular heart disease, severe or decompensated heart failure, intraaortic balloon pressure support requirement, severe renal failure and patients undergoing urgent cardiac surgery for revascularization. The study was approved by the Local Ethics Committee. In all patients, plasma glucose levels were secreened at hospital admission; and acute hyperglycemia was defined as glucose levels >198 mg/dl, regardless of the diabetic status [18]. Hypertension was defined as blood pressure more than 140/90 mmHg or being on treatment with antihypertensive medications. Also, diabetes mellitus was defined as fasting glucose levels >126 mg/dl and HbA1c > 6.5 or being on treatment with oral antidiabetic drugs or insulin. Finally, hyperlipidemia was defined by reference to current guidelines [19]. Serum creatinine concentration level was observed at hospital admission, every day for the following days and at hospital discharge. Estimated glomerular filtration rate (eGFR) was calculated using the modified formula of Levey et al. [20]. A nonionic, low-osmolality contrast agent (iomeprol) was used. After contrast exposure, isotonic (0.9%) saline was given intravenously at a rate of 1 ml/(kg h) [0.5 ml/(kg hour) in the case of left ventricular ejection fraction [LVEF] < 40% or heart failure] for 12 hours. CIN was defined as an increase in creatinine 25% or 0.5 mg/dl from the baseline value within the 48- to 72-hour period following PCI [21].
Statistical analysis
All analyses were performed using SPSS version 22 for Windows (SPSS Inc, Chicago, Illinois, USA). Numerical variables are presented as mean (SD) and nominals as percentages. All variables were subjected to Kolmogorov–Smirnov testing to determine whether they were normally distributed. The independent samples t test was used to compare the values of continuous variables between the two groups. Nonparametric values were compared using the Mann–Whitney U test. The Chi-square and Fisher’s Extract test were used to compare categorical data. To evaluate the effects of various factors on CIN development, we performed multivariate regression analyses using the backward logistic regression method. Variables for which the unadjusted P <0 0.05 was considered significant.
Results
A total of 281(149, 53% men) patients were included in this study and 79 (28.1%) of them had diabetes mellitus. 44 (15.7%) of patients had acute hyperglycemia and 237 (84.3%) of them had normal glucose levels. There was no significant difference between the two groups in terms of age and gender. Mean weight and eGFR were significantly higher in patients with hyperglycemia. General risk factors hyperlipidemia, diabetes mellitus, smoking and hypertension were same in both groups. Additionally, previous medications, Grace scores, HbA1c, peak troponin levels, LVEF, contrast volume, numbers of PCI and coronary artery bypass grafting (CABG) rates were not differed between two groups. The baseline clinical and procedural characteristics of patients were shown in Tables 1 and 2. Hyperglycemia patients had significantly higher incidence of CIN (13) 29.5% vs (12) 5.1%, respectively, P < 0.0001 (Fig. 1). Admission glucose levels were significantly correlated with creatinine levels after PCI, eGFR and contrast volume/eGFR ratio (r:0.239, P < 0.001, r:0.116, P:0.026, r:0.119, P:0.022, respectively). Hyperglycemia group also experienced a more complicated in-hospital clinical course and had higher in-hospital mortality rate (Table 3; Fig. 2). When patients with diabetes mellitus are excluded (202 patients, 71.9 %), 31 (15.3%) of patients had acute hyperglycemia and 171 (84.7 %) of them had normal glucose levels. Hyperglycemia patients had still significantly higher incidence of CIN [(9) 29.0% vs (5) 2.9%, respectively, P < 0.0001].
Table 1.
Main characteristics of patients with hyperglycemia and without hyperglycemia
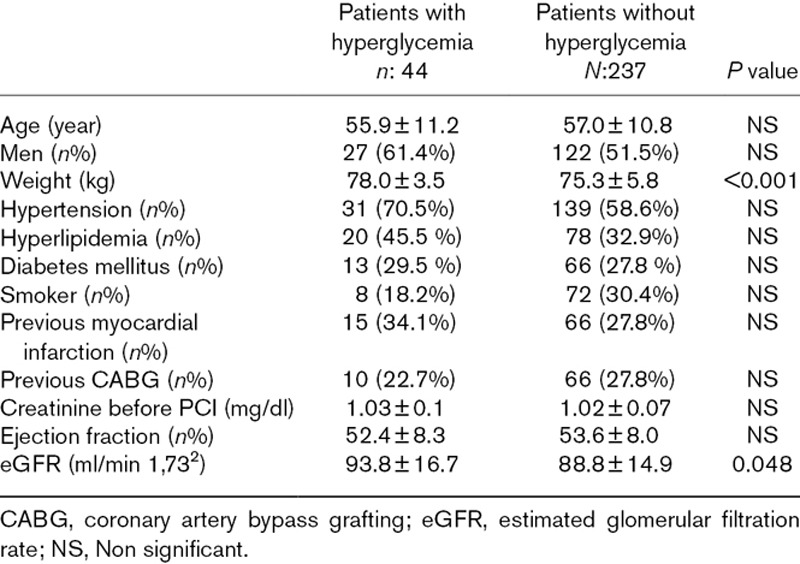
Table 2.
Medication characteristics of patients with hyperglycemia and without hyperglycemia
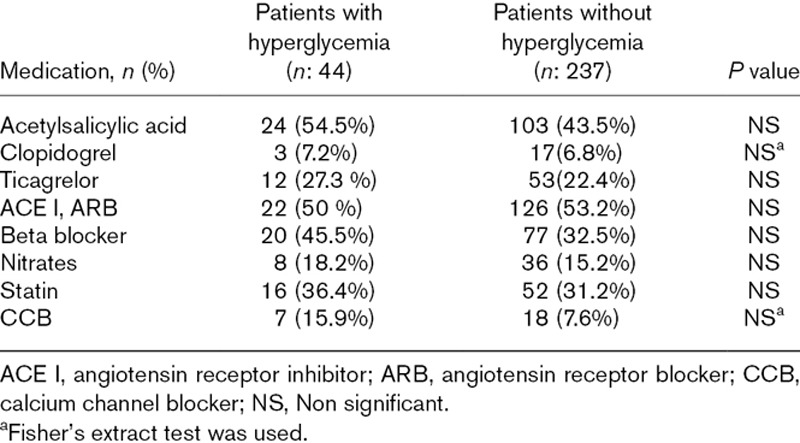
Fig. 1.
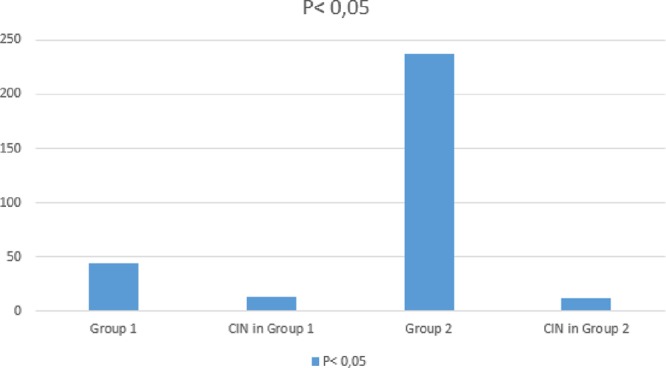
Patients with acute hyperglycemia had higher incidence of CIN than those without acute hyperglycemia (group 1: in patients with acute hyperglycemia, group 2: in patients with normoglycemia). CIN, contrast-induced nephropathy.
Table 3.
In-hospital clinical course of patients with hyperglycemia and without hyperglycemia
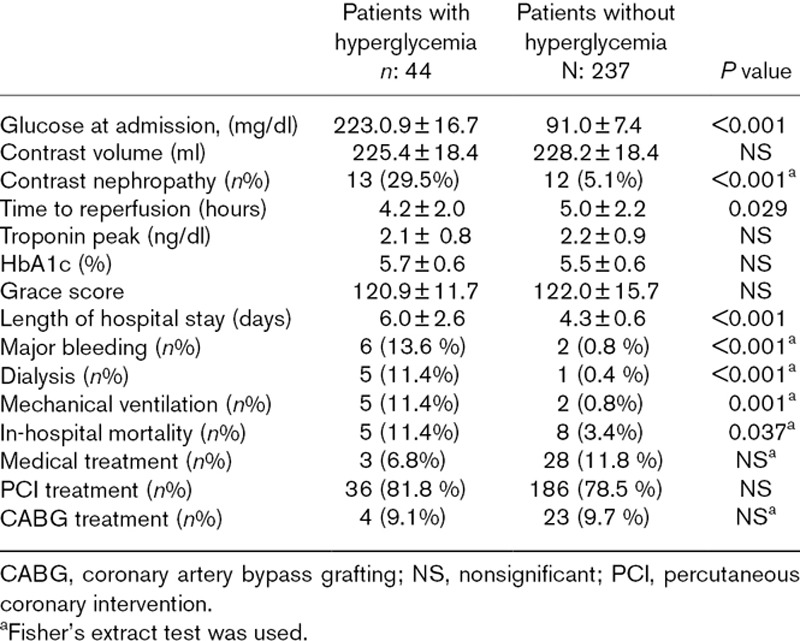
Fig. 2.
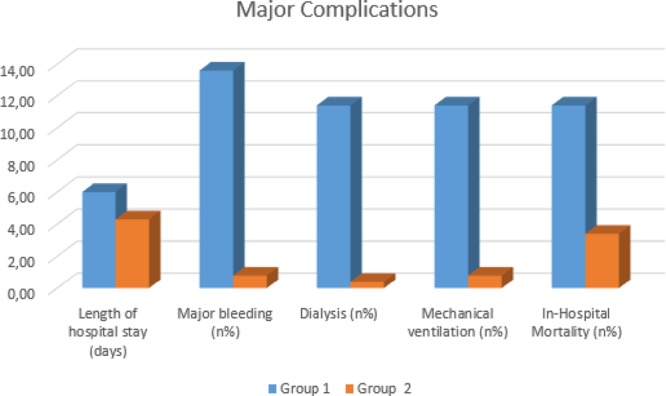
Group 1 experienced a more complicated in-hospital clinical course (group 1: in patients with acute hyperglycemia, group 2: in patients with normoglycemia, P < 0.05).
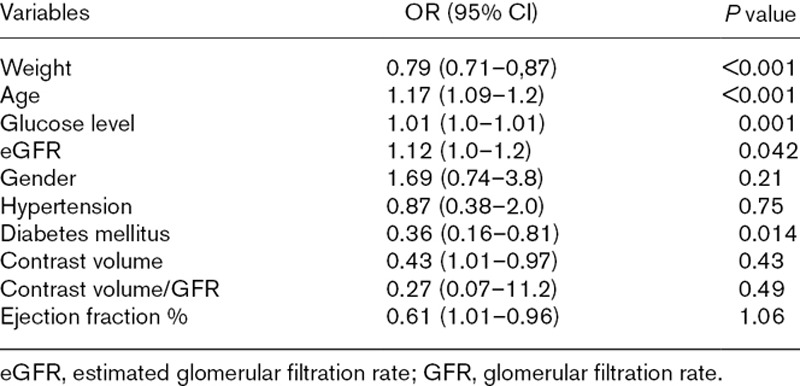
Patients were then reallocated to two groups according to the presence or absence of CIN. Overall, 25 cases (8.9%) of CIN were diagnosed. There was not also significant difference between the two groups in terms of age and gender. General risk factors that diabetes mellitus, smoking and hypertension were same in patients with CIN and without CIN. Additionally, previous medications, Grace scores, peak troponin levels, LVEF, contrast volume, numbers of PCI and CABG rates were not differed between two groups (Tables 4 and 5). Only admission glucose and HbA1c levels were significantly higher in patients with CIN (Table 5). Additionally, diabetes mellitus, weight, age, glucose level and eGFR were detected as independent risk factors of CIN in logistic regression analysis (Table 6).
Table 4.
Main characteristics of patients with contrast-induced nephropathy and without contrast-induced nephropathy
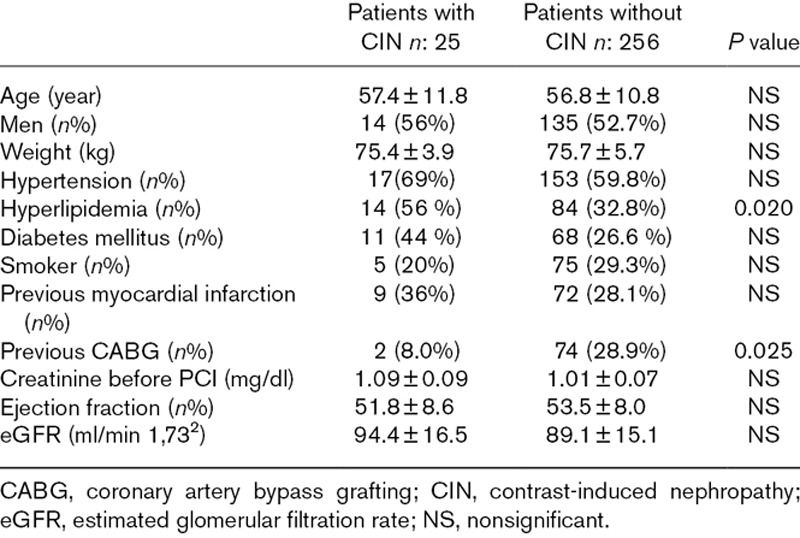
Table 5.
In-hospital clinical course of patients with contrast-induced nephropathy and without contrast-induced nephropathy
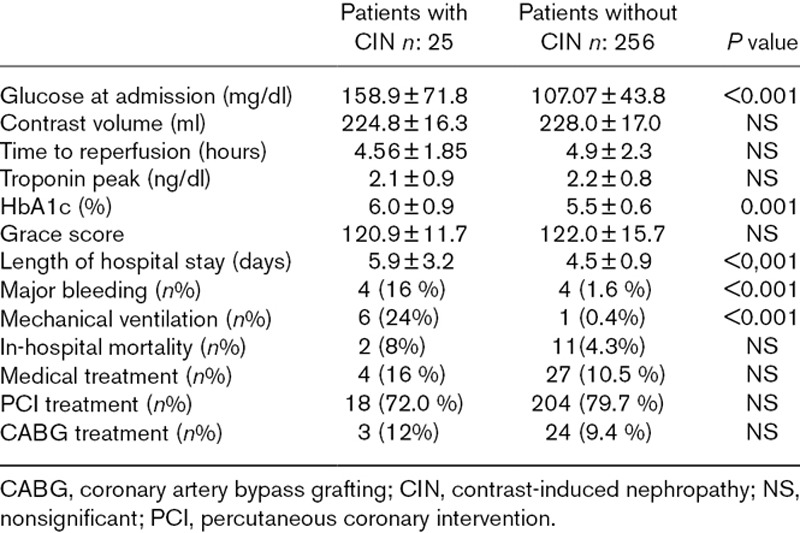
Table 6.
Independent risk factors of contrast-induced nephropathy in logistic regression analysis
Discussion
CIN is a frequent complication after invasive treatment of NSTEMI, even in patients with normal baseline renal function [22]. It is associated with increased in-hospital mortality and a prolonged hospitalization. Our results supported previous studies about CIN. In our study, we also demonstrated that NSTEMI patients undergoing PCI, admission hyperglycemia is an independent predictor of CIN and of poor in-hospital outcome. Additionally, admission glucose levels significantly correlated with creatinine levels after PCI.
The possible association between high glucose levels and acute kidney injury is further supported by previous studies [23,24]. Acute hyperglycemia is associated with increased production of oxygen-derived free radicals which cause vasoconstriction and induce osmotic diuresis, resulting in volume depletion, may impact negatively on kidney function and increase renal toxicity of contrast agents and CIN risk [25,26]. In addition, acute hyperglycemia is common in patients with NSTEMI, even in the absence of a history of type 2 diabetes mellitus due to stress response that is mediated by cortisol and catecholamines. Moreover, acute hyperglycemia associated with several adverse effects that endothelial dysfunction, increased cytokine activation, increased oxidative stress, impaired microcirculatory function and prothrombotic effects [27–29].
GRACE score is one of the most important indicators of mortality and morbidity in patients with NSTEMl [30]. Also it is a very useful identification of high-risk patients and determination of treatment strategy. In our study, there was no difference between the general risk factors and GRACE score in both groups, but in-hospital morbidity and mortality were found significantly higher in patients with hyperglycemia. Thus, tight control of hyperglycemia could be an useful strategy for improving prognosis in NSTEMI
Contrast volume is an important risk factor for CIN and dose minimization, on the background of a known baseline reduced renal function, may serve as an important strategy to limit the incidence of CIN [27]. However, we used a relatively small amount of contrast, and we did not find significant difference in terms of dose of contrast used in patients with and without CIN. We suggested that other factors, such as impaired renal function and diabetes mellitus, age and weight might contribute more to the development of CIN than contrast volume.
Our study may support relationship between high glucose levels and risk of acute renal dysfunction. Therefore, treatment of hyperglycemia could be an useful strategy for prevention CIN. In a recent study demonstrated that, acute hyperglycemia is associated with increased risk of CIN and poor outcome in STEMI patients as our study [12], and in an another study, glycemic control with insulin in patients with acute myocardial infarction who underwent coronary bypass surgery was associated with reduced oxidative stress, inflammation, apoptosis and remodeling [31].
These findings show the importance of control of acute hyperglycemia for prevention of CIN in NSTEMI patients undergoing PCI. In our study, we found that acute hyperglycemia was related with high CIN risk among patients with and without established diabetes mellitus. Acute hyperglycemia was associated with a significant increase of CIN risk among patients who did not have known diabetes mellitus. This observation emphasizes the importance of acute glucose level rise, as compared with its chronic elevation, as a predisposing factor for CIN. Whether this association is the effect of direct acute hyperglycemia and at multivariate analysis, acute hyperglycemia remained an independent predictor of CIN even after adjustment for major clinical variables.
Finally, we do not know whether early normalization of glycemic values is associated with a lower incidence of CIN and a better clinical outcome. These findings should be investigated in future studies.
Our study had some limitations. First, our study was a single-center study. Second, the study cohort was relatively small. Finally, some risk factors of CIN, such as proteinuria and other nephrotoxic agents, could not be fully assessed.
Conclusion
Acute hyperglycemia is closely associated with CIN risk, in-hospital morbidity and mortality in NSTEMI patients treated with PCI. Future investigations should be done about prophylactic strategies based on tight glycemic control to prevent CIN and improve the clinical outcome of patients with NSTEMI.
Acknowledgements
Conflicts of interest
There are no conflicts of interest.
References
- 1.Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet. 2003; 361:13–20 [DOI] [PubMed] [Google Scholar]
- 2.Marenzi G, Lauri G, Assanelli E, Campodonico J, De Metrio M, Marana I, et al. Contrast-induced nephropathy in patients undergoing primary angioplasty for acute myocardial infarction. J Am Coll Cardiol. 2004; 44:1780–1785 [DOI] [PubMed] [Google Scholar]
- 3.Ishihara M, Inoue I, Kawagoe T, Shimatani Y, Kurisu S, Nishioka K, et al. Impact of acute hyperglycemia on left ventricular function after reperfusion therapy in patients with a first anterior wall acute myocardial infarction. Am Heart J. 2003; 146:674–678 [DOI] [PubMed] [Google Scholar]
- 4.Nakamura T, Ako J, Kadowaki T, Funayama H, Sugawara Y, Kubo N, Momomura S. Impact of acute hyperglycemia during primary stent implantation in patients with ST-elevation myocardial infarction. J Cardiol. 2009; 53:272–277 [DOI] [PubMed] [Google Scholar]
- 5.Oswald GA, Smith CC, Betteridge DJ, Yudkin JS. Determinants and importance of stress hyperglycaemia in non-diabetic patients with myocardial infarction. Br Med J (Clin Res Ed). 1986; 293:917–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oswald GA, Yudkin JS. Hyperglycaemia following acute myocardial infarction: the contribution of undiagnosed diabetes. Diabet Med. 1987; 4:68–70 [DOI] [PubMed] [Google Scholar]
- 7.Capes SE, Hunt D, Malmberg K, Gerstein HC. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet. 2000; 355:773–778 [DOI] [PubMed] [Google Scholar]
- 8.Ishihara M, Kojima S, Sakamoto T, Asada Y, Tei C, Kimura K, et al. ; Japanese Acute Coronary Syndrome Study Investigators. Acute hyperglycemia is associated with adverse outcome after acute myocardial infarction in the coronary intervention era. Am Heart J. 2005; 150:814–820 [DOI] [PubMed] [Google Scholar]
- 9.Iwakura K, Ito H, Ikushima M, Kawano S, Okamura A, Asano K, et al. Association between hyperglycemia and the no-reflow phenomenon in patients with acute myocardial infarction. J Am Coll Cardiol. 2003; 41:1–7 [DOI] [PubMed] [Google Scholar]
- 10.Kosiborod M, Inzucchi SE, Krumholz HM, Masoudi FA, Goyal A, Xiao L, et al. Glucose normalization and outcomes in patients with acute myocardial infarction. Arch Intern Med. 2009; 169:438–446 [DOI] [PubMed] [Google Scholar]
- 11.Parfrey PS, Griffiths SM, Barrett BJ, Paul MD, Genge M, Withers J, et al. Contrast material-induced renal failure in patients with diabetes mellitus, renal insufficiency, or both. A prospective controlled study. N Engl J Med. 1989; 320:143–149 [DOI] [PubMed] [Google Scholar]
- 12.Marenzi G, De Metrio M, Rubino M, Lauri G, Cavallero A, Assanelli E, et al. Acute hyperglycemia and contrast-induced nephropathy in primary percutaneous coronary intervention. Am Heart J. 2010; 160:1170–1177 [DOI] [PubMed] [Google Scholar]
- 13.Persson PB, Tepel M. Contrast medium-induced nephropathy: the pathophysiology. Kidney Int. 2006; 69Suppl 100S8–S10 [DOI] [PubMed] [Google Scholar]
- 14.Ceriello A. Cardiovascular effects of acute hyperglycaemia: pathophysiological underpinnings. Diab Vasc Dis Res. 2008; 5:260–268 [DOI] [PubMed] [Google Scholar]
- 15.Piconi L, Quagliaro L, Assaloni R, Da Ros R, Maier A, Zuodar G, Ceriello A. Constant and intermittent high glucose enhances endothelial cell apoptosis through mitochondrial superoxide overproduction. Diabetes Metab Res Rev. 2006; 22:198–203 [DOI] [PubMed] [Google Scholar]
- 16.Quagliaro L, Piconi L, Assaloni R, Martinelli L, Motz E, Ceriello A. Intermittent high glucose enhances apoptosis related to oxidative stress in human umbilical vein endothelial cells: the role of protein kinase C and NAD(P)H-oxidase activation. Diabetes. 2003; 52:2795–2804 [DOI] [PubMed] [Google Scholar]
- 17.Hamm CW, Bassand JP, Agewall S, Bax J, Boersma E, Bueno H, et al. ; ESC Committee for Practice Guidelines. ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: the task force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2011; 32:2999–3054 [DOI] [PubMed] [Google Scholar]
- 18.Malmberg K, Rydén L, Wedel H, Birkeland K, Bootsma A, Dickstein K, et al. ; DIGAMI 2 Investigators. Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction (DIGAMI 2): effects on mortality and morbidity. Eur Heart J. 2005; 26:650–661 [DOI] [PubMed] [Google Scholar]
- 19.Expert Panel on Detection Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). JAMA. 2001; 285:2486–2497 [DOI] [PubMed] [Google Scholar]
- 20.Levey AS, Beto JA, Coronado BE, Eknoyan G, Foley RN, Kasiske BL, et al. Controlling the epidemic of cardiovascular disease in chronic renal disease: what do we know? What do we need to learn? Where do we go from here? National kidney foundation task force on cardiovascular disease. Am J Kidney Dis. 1998; 32:853–906 [DOI] [PubMed] [Google Scholar]
- 21.Thomsen HS. European Society of Urogenital Radiology (ESUR) guidelines on the safe use of iodinated contrast media. Eur J Radiol. 2006; 60:307–313 [DOI] [PubMed] [Google Scholar]
- 22.Tepel M, Aspelin P, Lameire N. Contrast-induced nephropathy: a clinical and evidence-based approach. Circulation. 2006; 113:1799–1806 [DOI] [PubMed] [Google Scholar]
- 23.van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001; 345:1359–1367 [DOI] [PubMed] [Google Scholar]
- 24.Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, et al. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006; 354:449–461 [DOI] [PubMed] [Google Scholar]
- 25.Kawano H, Motoyama T, Hirashima O, Hirai N, Miyao Y, Sakamoto T, et al. Hyperglycemia rapidly suppresses flow-mediated endothelium-dependent vasodilation of brachial artery. J Am Coll Cardiol. 1999; 34:146–154 [DOI] [PubMed] [Google Scholar]
- 26.Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, Colette C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006; 295:1681–1687 [DOI] [PubMed] [Google Scholar]
- 27.Yang Z, Laubach VE, French BA, Kron IL. Acute hyperglycemia enhances oxidative stress and exacerbates myocardial infarction by activating nicotinamide adenine dinucleotide phosphate oxidase during reperfusion. J Thorac Cardiovasc Surg. 2009; 137:723–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Undas A, Wiek I, Stêpien E, Zmudka K, Tracz W. Hyperglycemia is associated with enhanced thrombin formation, platelet activation, and fibrin clot resistance to lysis in patients with acute coronary syndrome. Diabetes Care. 2008; 31:1590–1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Worthley MI, Holmes AS, Willoughby SR, Kucia AM, Heresztyn T, Stewart S, et al. The deleterious effects of hyperglycemia on platelet function in diabetic patients with acute coronary syndromes mediation by superoxide production, resolution with intensive insulin administration. J Am Coll Cardiol. 2007; 49:304–310 [DOI] [PubMed] [Google Scholar]
- 30.Reaney PDW, Elliott HI, Noman A, Cooper JG. Risk stratifying chest pain patients in the emergency department using HEART, GRACE and TIMI scores, with a single contemporary troponin result, to predict major adverse cardiac events. Emerg Med J. 2018; 35:420–427 [DOI] [PubMed] [Google Scholar]
- 31.Marfella R, Di Filippo C, Portoghese M, Ferraraccio F, Rizzo MR, Siniscalchi M, et al. Tight glycemic control reduces heart inflammation and remodeling during acute myocardial infarction in hyperglycemic patients. J Am Coll Cardiol. 2009; 53:1425–1436 [DOI] [PubMed] [Google Scholar]


