Abstract
The goal of our research has been to investigate the neuronal integration that coordinates feeding movements in the marine toad (genus Bufo). Using injections of fluorescein dextran amines, combined with activity-dependent uptake of sulforhodamine 101, peripheral hypoglossal and trigeminal nerves involved with tongue and jaw movements were labeled. We identified the rostrocaudal distribution of hypoglossal and trigeminal motor nuclei, and their sensory projections. We also identified the extent of neuronal networks for the medial reticular formation, the raphe nucleus, the glossopharyngeal nuclei, and the Purkinje cell layer of the cerebellum. The sensory fibers of hypoglossal and trigeminal nerves were found projecting to the Purkinje cell layer of cerebellum and the trigeminal motor nuclei. The activity dependent sulforhodamine 101 uptake after the trigeminal and hypoglossal nerves stimulation labeled the bilateral hypoglossal motor nuclei, the trigeminal motor nuclei, the medial reticular formation nuclei, the raphe nuclei, the glossopharyngeal nuclei, and the Purkinje cell layer of cerebellum, suggesting that all these neurons have the potential to be the components of feeding pathways. Taken together, these data are important for understanding the neuronal integration of extremely rapid jaw-tongue coordination during feeding in the marine toad.
Keywords: anuran amphibian, trigeminal, hypoglossal, reticular formation, cerebellar cortex
INTRODUCTION
Among the various nerves involved in the initiation of feeding movements in anurans, the primary cranial nerves involved are the hypoglossal, trigeminal and glossopharyngeal, as these nerves directly innervate the tongue and jaw muscles. The hypoglossal nerve innervates the tongue protractor muscle, the m. genioglossus and the tongue retractor muscle, the m. hyoglossus. The trigeminal nerve innervates the jaw levator muscles and the m. submentalis which is a transversely orientated muscle at the mandibular tip. The basic neural circuits that mediate rapid tongue protrusion are reasonably well characterized in the leopard frog, Rana pipiens (Anderson, 2001; Clark et al., submitted). Although the basic mechanism for jaw-tongue coordination appears similar in Rana and Bufo, long protrusible tongues have independently evolved multiple times in these anurans (Nishikawa, 2000). Evolutionary shifts in morphology can modify structural and functional aspects of an organism (Lauder, 1981; Lauder and Liem, 1989; Roth and Wake, 1989). In fact some biomechanical differences have already been demonstrated between Rana and Bufo. Thus it is important to investigate the neuroanatomical differences in these different anurans’ genera in order to understand apparently simple but highly complex neuronal pathways for extremely rapid jaw-tongue coordination during feeding motor programs.
Previous work has focused on identifying the descending pathways involved in the generation of amphibian feeding motor patterns (Matesz, 1979; Marín et al., 1997; Dicke et al., 1998; Ewert et al., 1999; Roth et al., 1999; Sanchez-Camacho et al., 2001). Our focus has been on the ascending neuronal pathways. While the existence of muscle spindles in the tongue has been shown to help regulate tongue movements in mammals, studies have additionally demonstrated the presence of a sensory afferent component of the peripheral hypoglossal nerve that is used in regulating tongue and jaw movements; for example in mammals (Tarkhan and Abou-El-Naga, 1947; Tarkhan and El-Malek, 1950; Zimny et al., 1970), in birds (Wild, 1981, 1990), and in amphibians (Downman, 1939; Stuesse et al., 1983; Nishikawa and Gans, 1992; Anderson and Nishikawa, 1993, 1996, 1997). Recently published data has shown the presence of a sensory pathway in the leopard frog, Rana pipiens, that originates from the tongue epithelium (Harwood and Anderson, 2000) and is carried in the hypoglossal nerve (Anderson and Nishikawa, 1996, 1997). This feedback pathway coordinates the timing of the tongue with the jaw so that the muscular system can properly time these movements during feeding (Anderson and Nishikawa, 1993).
The hypoglossal nerve does not innervate the mandibular depressors and levators, thus theoretically denervation of this nerve should not affect the jaw closing or opening. But the electrophysiological and biomechanical studies show that this is not the case. Following bilateral peripheral hypoglossal nerve transection in toads, the jaw fails to open due to simultaneous activation of both the mandibular depressors and levators (Nishikawa and Gans, 1992). This suggests further the physiological role of sensory feedback in jaw-tongue coordination that is carried by the hypoglossal nerve. Recent studies in our lab in Bufo have shown that following bilateral mandibular nerve transection just prior to innervation of the submentalis muscle, the jaw opens but the tongue fails to protract initially to its full length (Anderson, personal communication). The submentalis muscle is hypothesized to be involved with jaw-tongue coordination through sensory feedback as it has no mechanical role in jaw opening (Nishikawa, 2000; Wolff et al., 2004). Thus, there is reciprocal organization for sensory control mechanism in that hypoglossal afferents affect jaw movements and trigeminal (mandibular branch) afferents affect tongue movements. The integration of feedback information from both of these nerves is likely important for coordinating jaw-tongue motor output in toads. Electrophysiological studies have shown that the trigeminal sensory afferents integrate within the brainstem for coordinated tongue movement in several animals (Morimoto et al., 1978; Morimoto et al., 1989; Tolu et al., 1994; Ishiwata et al., 2000; Luo et al., 2001, 2006; Rácz et al., 2008), however, there is minimal data to verify these physiological findings anatomically and the pattern of sensory integration within the brainstem of toads.
We began with single labeling the distal branches of the hypoglossal nerve and the mandibular branch of the trigeminal nerve to identify the exact distribution of motor nuclei of these nerves. There are minimal studies on toads regarding the distribution of the hypoglossal and trigeminal nuclei. Oka et al. (1987) described dorsomedial and ventrolateral subnuclei of hypoglossal nerve, and other cranial nerves in the Japanese toad but did not describe any other subnuclei of the hypoglossal. Once we identified the distribution of the hypoglossal and trigeminal nerve nuclei, we used several labeling techniques to investigate the convergence of these nerve afferents in the brainstem.
Using bath-applied SR 101 to a semi-intact brainstem, we stimulated the peripheral hypoglossal nerve to identify the location and distribution of pre-motor and motor nuclei. This resulted in the labeling of the ventrolateral, intermediate, dorsomedial and previously undescribed medial group of subnuclei of the hypoglossal nerve. Additionally, the raphe nuclei, the trigeminal motor nuclei, the medial reticular formation and the Purkinje cell layer of the cerebellar cortex were labeled. The activity dependent SR 101 uptake after the peripheral trigeminal stimulation resulted in the labeling of the same groups of nuclei as mentioned above for the hypoglossal stimulation. These data suggest that all these nuclei are components of the neural pathway of interest and that there is a reciprocal loop among the trigeminal motor nuclei, the medial reticular formation and the hypoglossal nuclei.
Anatomical data in Rana has shown that sensory neurons from the hypoglossal nerve and other nerves project to a specific portion of the medulla, the medial reticular formation, and here it is hypothesized that the integration of sensory and motor commands occurs before the muscle commands are sent out to the various skeletal muscles (Weerasuriya, 1989; Anderson and Nishikawa, 1997; Anderson, 2001; Rácz et al., 2008). In addition, hypoglossal afferents have been shown to project to the cerebellum (Anderson and Nishikawa, 1997; Anderson, 2001), a pathway likely involved in coordinating the proper timing of various postural and locomotor movements during feeding. We investigated in more detail the anatomical connections between motor neurons innervating the tongue and jaw musculatures and their sensory afferents projecting to the brainstem and cerebellum. We analyzed further if the data in Bufo support the hypothesis for the medial reticular formation as the location of sensory-motor integration.
METHODS AND MATERIALS
Bufo marinus (n=28), weighing 25–40 gms were obtained from a commercial supplier (Nature Coast Exotics, Florida, USA) and maintained in captivity. Neuronal labeling was done initially in an intact animal (peripheral hypoglossal and trigeminal nerve labeling), followed by double labeling with FDA (Fluorescein dextran amine) and SR101. In n=20 cases a semi-intact cranial preparation was used in conjunction with bath-applied SR101 to investigate activity-dependent dye uptake following hypoglossal and trigeminal nerves stimulation.
To label the afferent projections traveling in the peripheral hypoglossal and trigeminal nerves, as well as, their motor neurons, the toads were anesthetized by immersion in 0.02% tricaine methane sulfonate (MS-222) for 15–30 minutes. They were then wrapped in damp paper draping and placed under a dissecting microscope for surgery. The hypoglossal nerve was exposed by making a small incision through the skin of the lower jaw and deflecting the m. intermandibularis. The hypoglossal nerve was then dissected distal to the branch that innervates the geniohyoideus muscle to ensure that the afferent fibers labeled were only those that originated from the tongue and the retrogradely labeled motor neurons were those providing innervation to the muscle of the tongue. Retrograde neuronal tracers, 3,000 MW FDA, 3000 MW and 10,000 MW Alexa Fluor 488 (Molecular Probes, Eugene, Ore. USA) were used. For the injection, 2 mg of the neuronal tracer was dissolved in 50 μl of nanopure water and refrigerated. Using a microsyringe pump controller (Micro-1, WPI instruments) and a fire-pulled glass micropipette, 5 nanoliters of tracer was pressure injected into the intact nerve over a period of 5 minutes. The injection was repeated 4–5 times and about 1–2 mm away from initial site for the total of 20–25 nanoliters of tracer. The nerve was then rinsed with distilled water and the incision closed with veterinary grade cyanoacrylate surgical glue.
Initial transport time for the labeling of the hypoglossal nerve ranged from 4–6 hours and up to 20 hours for the 10,000 molecular weight Alexa Fluor. Following transport, the toads were deeply anesthetized using 0.02% MS-222, decapitated, and the brains placed in 3 % paraformaldehyde overnight. The next day the brain was removed, mounted in gelatin, and sectioned at 50 μm on a sliding microtome with a freezing platform (Leica SM2000R). The brainstem and spinal cord sections were mounted in phosphate-buffered saline, air-dried, and mounted with SlowFade Gold antifade reagent (Invitrogen, Eugene, Oregon, USA).
SR101 (Molecular Probes) is a small, sulfonated, charged fluorescent molecule that is taken up by endocytosis in synaptically activated neurons (Lichtman et al., 1985; Keifer et al., 1992; Teng et al., 1999; Anderson, 2001). The preparation of the tissue is similar to the dextran retrograde labeling, however, the application involves using a semi-intact in vitro preparation. With SR 101, the pathway(s) of interest must be isolated and spontaneous activation of the brain minimized. After deep anesthesia with 0.02 % MS-222, the brain was exposed and the lower jaw was dissected to expose the trigeminal nerve or the hypoglossal nerve. Following this, the toad was decapitated and the lower jaw was separated from the cranium on one side. The lower jaw was then rotated 180°, keeping nervous input to and from the lower jaw intact on one side. That allowed both the exposed brain surface and the lower jaw to be accessed at the same time. Soon after decapitation, the brainstem preparation was bathed in the cold oxygenated Ringer’s solution. Then the already exposed nerve of interest was placed on a stimulating electrode wire. The peripheral nerve was then stimulated while SR101 dye was bath-applied to the brainstem surface. The nerve was stimulated at 2x the voltage necessary to evoke a visible muscle contraction (range 2–4 V) at 0.1 pulses/s for 2 h. Following stimulation, the entire preparation was washed in phosphate-buffered saline for approximately 30 min and immersion-fixed in 3 % paraformaldehyde overnight. The brain was removed, mounted in gelatin, and sectioned (at brainstem and spinal cord levels) at 50 μm on a sliding microtome, coverslipped in antifade reagent, and viewed under fluorescent light microscopy. All the images were taken with Leica DMRA microscope using IPLab 4.0 imaging software; brightness, contrast, sharpness, gamma correction were adjusted and pseudocolor was applied according to the filter used.
For double labeling with FDA or Alexa Fluor and SR101, the toads were anesthetized by immersion in 0.02% MS-222 for 15–30 minutes and then the retrograde tracer was injected into the nerve. The toads were maintained in captivity and allowed the transport time of 4–6 hours. The toads were re-anesthetized by immersion in 0.02% MS-222 for 15–30 minutes for SR101 labeling. The brain was exposed, the trigeminal nerve dissected, and the toad was decapitated. The rest of the procedures for labeling and tissue preparation were similar as described above for SR101.
For the controls, the toads (n=2) were deeply anesthetized by immersion in 0.02% MS-222 for 30–45 minutes. No dye was applied to any peripheral nerves. They were decapitated and put in 3% paraformaldehyde overnight. The next day the brain was removed, mounted in gelatin, and sectioned at 50 μm on a sliding microtome with a freezing platform. The sections were mounted in phosphate-buffered saline, air-dried, mounted with antifade reagent and viewed with fluorescent microscopy.
The original research reported herein was performed under guidelines established and approved by the Idaho State University Institutional Animal Care and Use Committee.
RESULTS
Because different techniques of labeling were utilized in this study, the results are presented by the type and tracer experiment performed.
Injection of tracer into the hypoglossal nerve
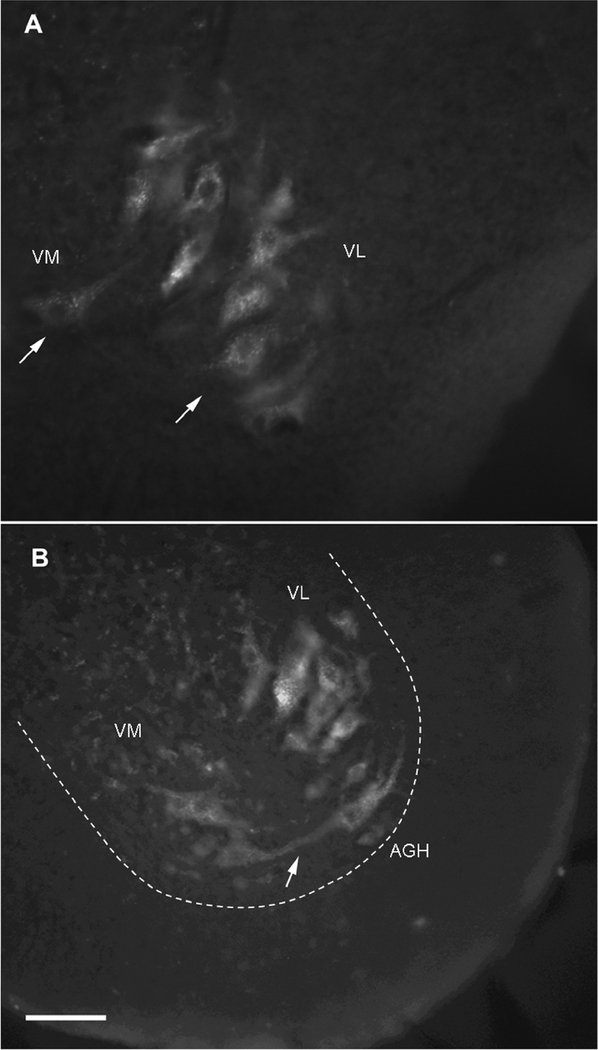
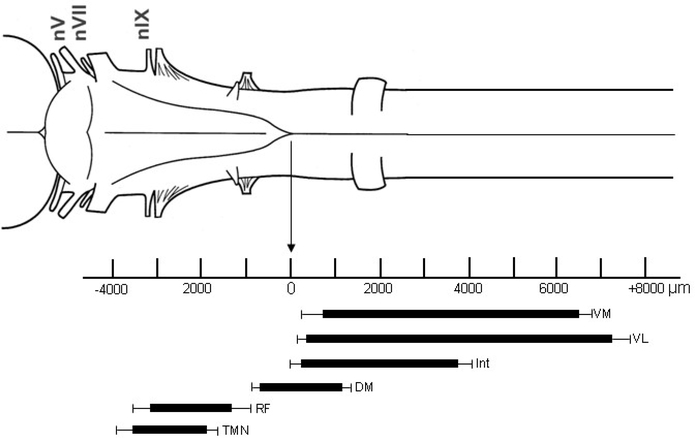
For the injections into the peripheral hypoglossal nerve, numerous labeled nuclei were identified in the spinal cord and the brainstem. Two groups of subnuclei were identified in the ventral horn of the spinal cord (Figs. 1A, B). The lateral subnucleus appeared homologous to the ventrolateral subnucleus in Rana, but the medial group has not been previously described. We termed this medial subnucleus as the ventromedial subnucleus. This additional ventromedial group of neurons in the ventral horn of the spinal cord was fewer in number compared to ventrolateral neurons. The rostrocaudal extent of ventromedial subnucleus was 797 μm (standard error, SE=102 μm) to 6517 μm (SE=292 μm) caudal to the obex (Fig. 7). The cell processes between ventromedial and ventrolateral nuclei were also observed in a few sections (Fig. 1B). The ventrolateral subnucleus was found in the ventral horn of the spinal cord (Figs. 1A, B) and it extended from 263 μm (SE=119 μm) to 7299 μm (SE=262μm) caudal to the obex (Fig. 7).
Fig. 1.
Cross sections through the spinal cord of Bufo showing labeling of the hypoglossal motor nuclei. Fluorescein dextran amine (FDA) of 3,000 MW was injected into the hypoglossal nerve near the base of the tongue. A: Ventromedial (left arrow) and ventrolateral (right arrow) hypoglossal motor subnuclei. B: Cell process (arrow) between ventromedial and ventrolateral nuclei. (VM Ventromedial subnucleus, VL Ventrolateral subnucleus, AGH Anterior gray horn). Scale bar 100 μm.
Fig. 7.
Schematic drawing illustrating the rostrocaudal distribution of the hypoglossal nerve subnuclei, the trigeminal motor nuclei and the reticular formation within the brainstem of Bufo. The thinner bars represent the standard error of mean for the distribution of nuclei independently for rostral and caudal ends.(VL, ventromedial subnuclei; VL, ventrolateral subnuclei; Int, intermediate subnuclei; DM, dorsomedial subnuclei; RF, medial reticular formation; TMN trigeminal motor nuclei).
The intermediate hypoglossal subnucleus was found dorsolateral to the ventral group of subnuclei and comprised of more neurons than ventrolateral group (Figs. 2A, B, C). The intermediate subnuclei extended in the spinal cord from 136 μm (SE=110 μm) to 3860 μm (SE=323μm) caudal to the obex (Fig. 7).
Fig. 2.
Cross sections through the spinal cord and brainstem of Bufo showing the specific populations of hypoglossal nuclei as we sectioned more rostrally to the level of Fig.1. Fluorescein dextran amine (FDA) of 3,000 MW was injected into the hypoglossal nerve near the base of the tongue. A: Ventrolateral (lower arrow) and intermediate (upper arrow) hypoglossal subnuclei. B: Ventromedial, ventrolateral and intermediate hypoglossal subnuclei. C: Dorsomedial subnuclei and few intermediate hypoglossal neurons. (CC Central canal, INT Intermediate subnucleus, VL Ventrolateral subnucleus, VM Ventromedial subnucleus, DM Dorsomedial subnucleus). Scale bar 100 μm.
The dorsomedial subnucleus was found in the basal caudal portion of the medulla and it extended from 707 μm (SE=128 μm) rostral to 1212 μm (SE=171μm) caudal to the obex (Figs. 2C, 7).
If only a single branch of the hypoglossal nerve was injected, neural cell bodies were labeled bilaterally demonstrating a bilateral projection of the hypoglossal nuclei. These data are consistent with Anderson (2001). With the controls we observed that hypoglossal motor nuclei were slightly auto-fluorescent occasionally with the FITC filter but not with the Rhodamine filter (data not shown).
Activity dependent uptake of SR 101 after hypoglossal stimulation
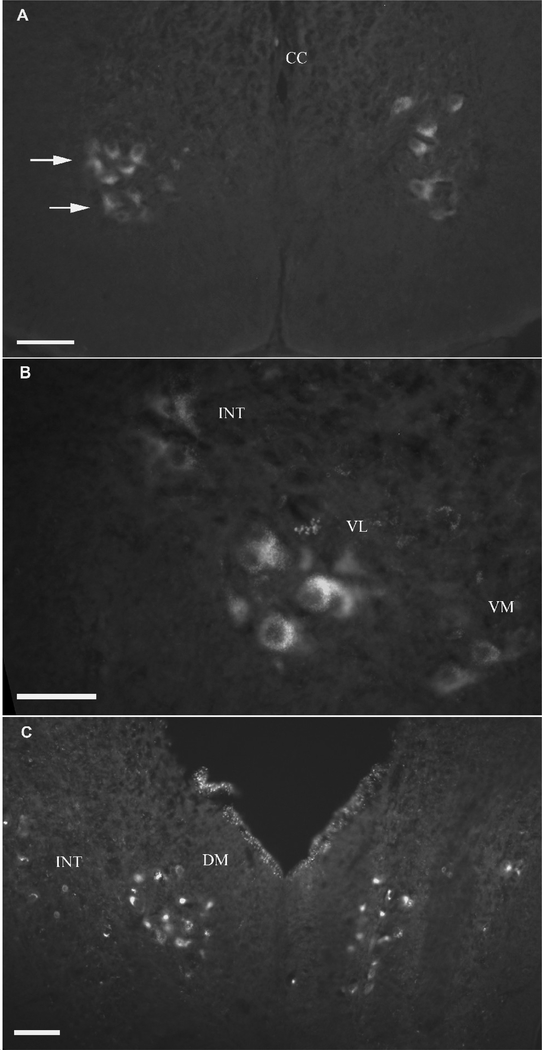
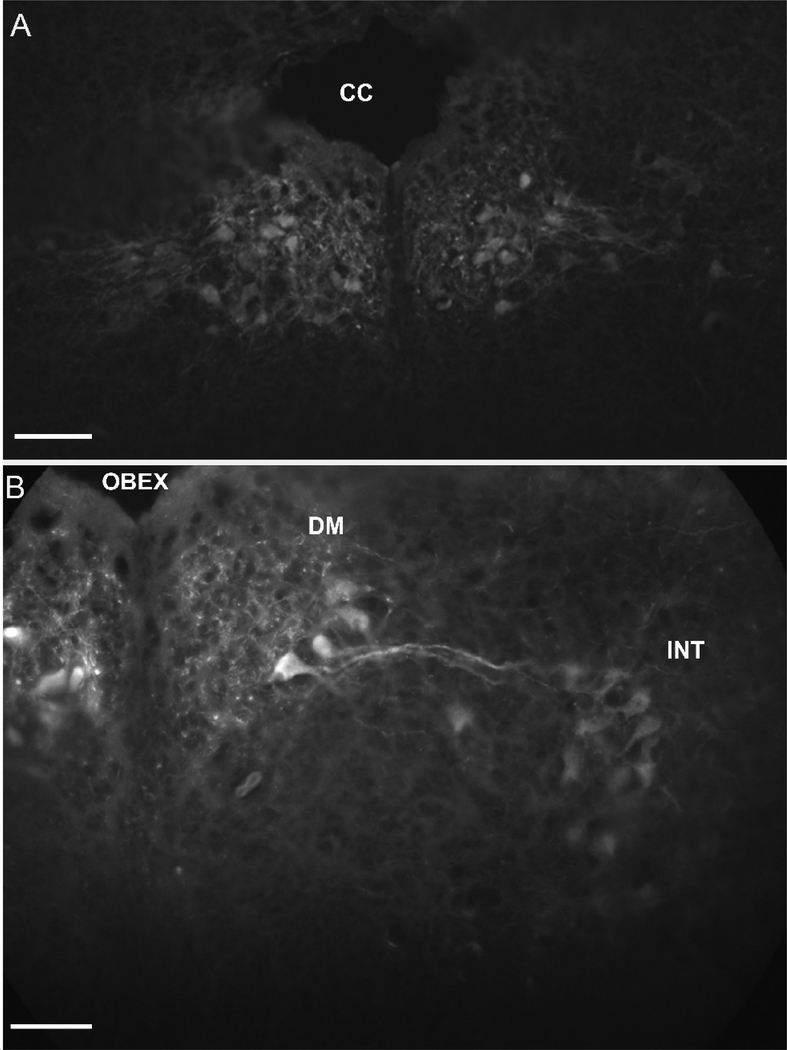
Stimulating the isolated, peripheral hypoglossal nerve (where the nerve enters the base of the tongue) resulted in clear labeling in the ventromedial (Figs. 3A, B), ventrolateral (Figs. 3A, B), intermediate (Figs. 3B, C) and dorsomedial (Fig. 3C) hypoglossal motor subnuclei. The trigeminal motor nuclei (Fig. 3D), the medial reticular formation nuclei (Fig. 3E), the raphe nuclei, the glossopharyngeal nuclei and the Purkinje cell layer of the cerebellar cortex (Fig. 3F) were also labeled. The function of this glossopharyngeal nucleus is not known. All nuclei were labeled bilaterally.
Fig. 3.
Results from bath applied sulforhodamine 101 to a semi-intact brainstem preparation while hypoglossal nerve was stimulated. A: Photomicrograph of the ventromedial and ventrolateral hypoglossal subnuclei. B: The ventromedial, ventrolateral and intermediate hypoglossal subnuclei. C: The dorsomedial hypoglossal subnuclei. D: The trigeminal motor neurons. E: Neurons in the medial reticular formation. F: Purkinje cell layer of the cerebellum. (VENT Fourth ventricle, PCL Purkinje cell layer). Scale bar 100 μm.
Activity dependent uptake of SR 101 after trigeminal stimulation
Stimulating the isolated, lateral branch of the peripheral trigeminal nerve just prior to the innervation of the submentalis muscle resulted in clear labeling in the ventromedial, ventrolateral, intermediate (Fig. 4B) and dorsomedial (Figs. 4A, B) hypoglossal subnuclei; the medial reticular formation nuclei, the trigeminal motor nuclei, the raphe nuclei, and the Purkinje cell layer of the cerebellar cortex. From the combined results of activity dependent uptake of SR 101 after the hypoglossal and the trigeminal nerves stimulation, the extent of the trigeminal motor neurons and the medial reticular formation were identified. The trigeminal motor neurons extended from 1905 μm (SE=193 μm) to 3562μm (SE=380μm) rostral to the obex (Fig. 7). The reticular formation extended from 1214 μm (SE=269μm) to 3371μm (SE=413μm) rostral to the obex (Fig. 7). The dorsomedial subnuclei were heavily labeled with this technique (Fig. 4A). Besides labeling of the intermediate and the dorsomedial hypoglossal motor subnuclei with this experiment, the dendritic branches extending between these two subnuclei were also identified (Fig. 4B).
Fig. 4.
Results from bath applied sulforhodamine 101 to a semi-intact brain preparation while the trigeminal nerve was stimulated. A: Dorsomedial hypoglossal motor subnuclei anterior to the central canal. B: Dendritic extensions of the dorsomedial and intermediate hypoglossal motor neurons. Scale bar 100 μm.
Double labeling with injection of FDA or Alexa Fluor 488 to the hypoglossal nerve and activity dependent uptake of SR 101 after the trigeminal nerve stimulation
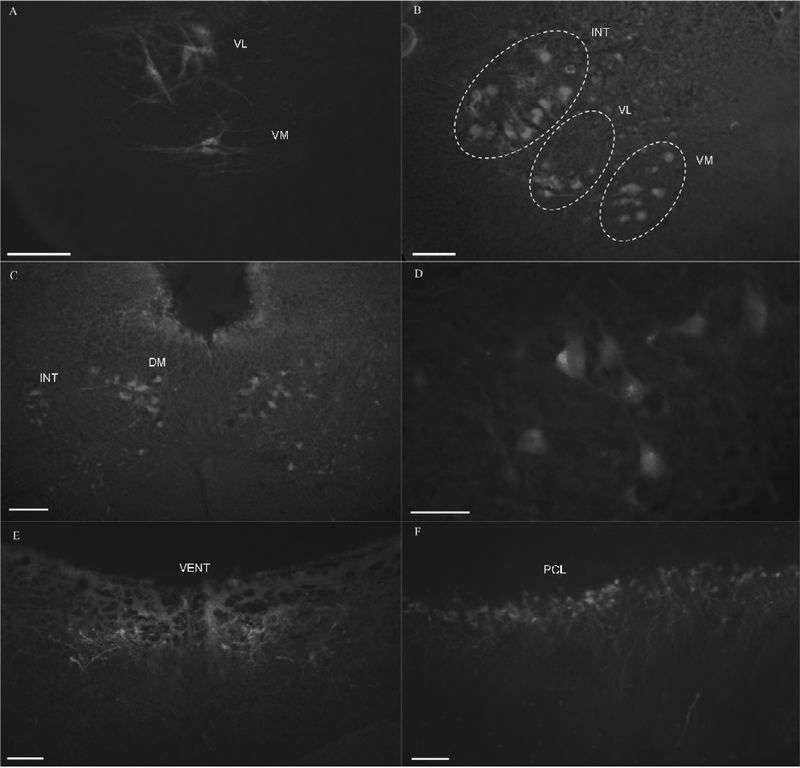
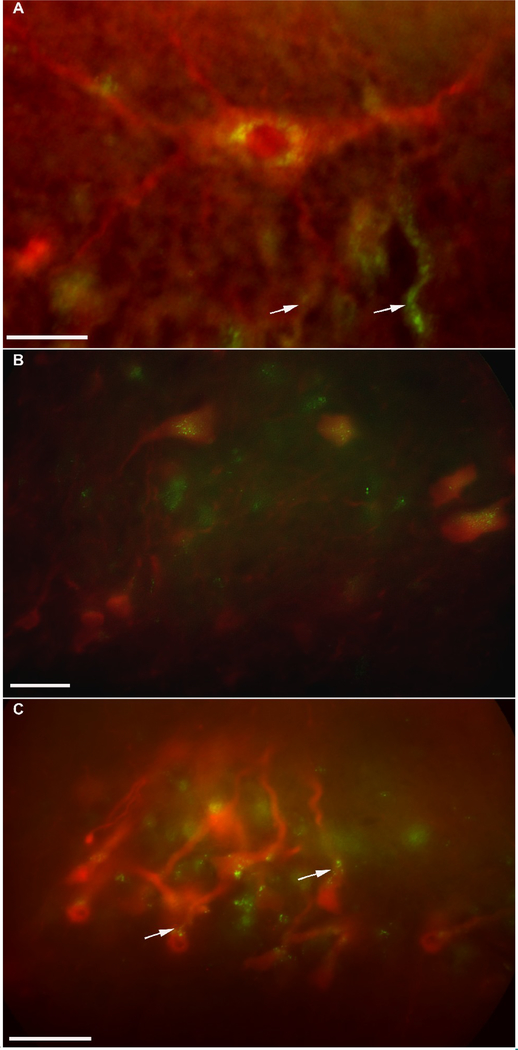
Stimulating the isolated, lateral branch of peripheral mandibular nerve resulted in clear labeling in the ventromedial, ventrolateral (Fig. 5A), intermediate and dorsomedial hypoglossal motor subnuclei; the medial reticular formation nuclei, the trigeminal motor nuclei, the raphe nuclei, and the Purkinje cell layer of the cerebellar cortex. Using the FITC filter for FDA or Alexa Fluor and the Rhodamine filter to identify SR 101 labeling, the photomicrographs were taken and merged using IPLab software. The merged photomicrographs revealed the terminals from hypoglossal nerve projecting to the trigeminal motor nuclei (Fig.5B) and the Purkinje cell layer of the cerebellum (Fig. 5C).
Fig. 5.
Merged photomicrographs from double labeling with the injection of Alexa Fluor 488 or FDA into the peripheral hypoglossal nerve and activity dependent uptake of SR 101 after the trigeminal nerve stimulation. For a single focal plane, the FITC filter was used to take an image and identify labeling with Alexa Fluor or FDA and the Rhodamine filter for SR101. Two images were merged using IPLab software to obtain the single image. A: Merged photomicrograph of a ventrolateral hypoglossal neuron labeled with SR101 and peri-nuclear filling (green fluorescence) with Alexa Fluor. Few neuronal networks were seen being filled with Alexa Fluor (arrows). B: hypoglossal sensory terminals (green dots) closely apposing the somata of the trigeminal motor neurons. C: The hypoglossal sensory terminals (green dots) projecting to the somata and axon hillock (arrows) of the Purkinje cells of the cerebellum. Scale bar 50 μm.
Single labeling and DIC microscopy
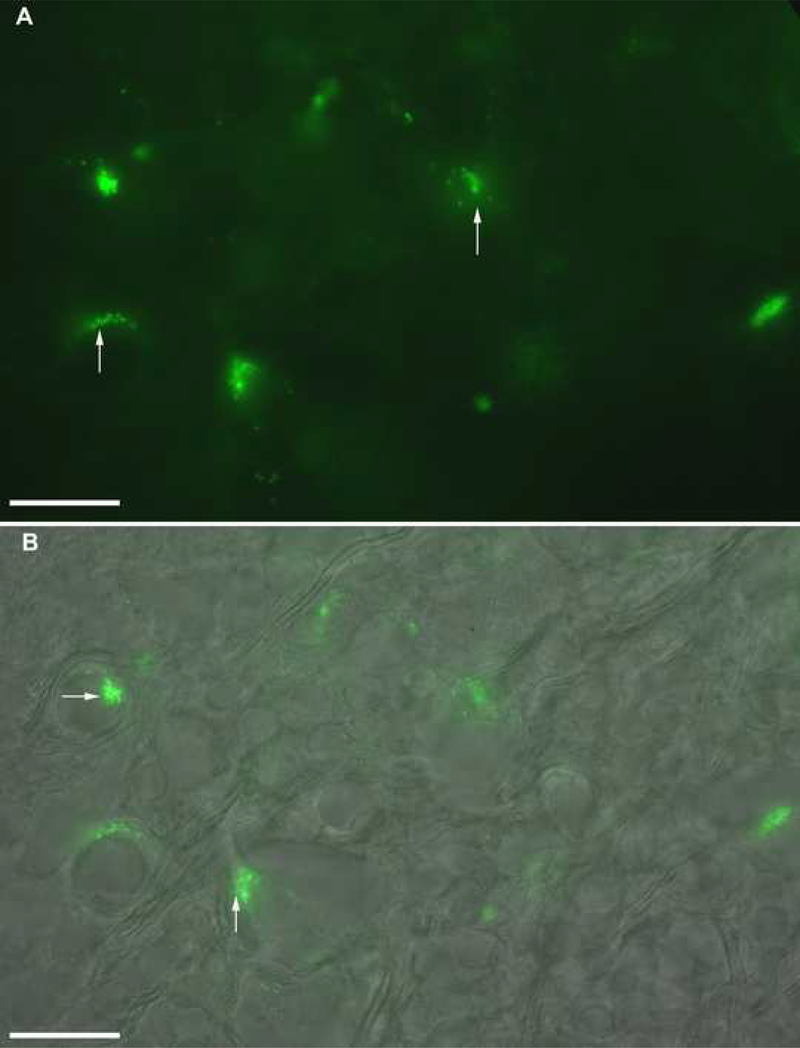
With single labeling of the hypoglossal nerve with FDA or Alexa 488 and use of fluorescence microscopy, axon terminals were identified in the Purkinje cell layer of the cerebellum (Fig. 6A). Using differential interference contrast microscopy (DIC), photomicrographs of the somata of the Purkinje cells of cerebellum were taken. The two photomicrographs were merged to obtain a single image which elucidated hypoglossal sensory terminals (green dots) projecting onto the somata, axon hillock and a few terminals closely apposing somata of the Purkinje cells of the cerebellum (Fig. 6B). Similar findings were obtained with labeling of the mandibular branch of the trigeminal nerve (data not shown). Few boutons were non fluorescent suggesting afferent terminals project only to the specific population of the Purkinje cell layer of the cerebellum.
Fig. 6.
Cross sections through the brainstem of Bufo showing labeling of the Purkinje cell layer of the cerebellum. Fluorescein dextran amine (FDA) or Alexa Fluor 488 was injected into bilateral peripheral hypoglossal nerves near the base of the tongue. A: Purkinje cell layer of the cerebellum viewed with FITC filter; sensory terminals as green fluorescent dots (arrows). B: Merged photomicrographs showing sensory terminals (green dots) projecting to the somata (horizontal white arrow) and axon hillock (vertical arrow) of the Purkinje cells of the cerebellum. The first image was differential interference contrast (DIC) image and the second image was fluorescent image with FITC filter. Two images were merged using IPLab software to obtain the single image. Scale bar 20 μm.
DISCUSSION
Using fluorescent labeled nuclei in the brainstem and the spinal cord, we describe the rostrocaudal distribution of hypoglossal and trigeminal nuclei in Bufo. We have identified the neural substrates for jaw-tongue coordination that has not been well described in the previous studies. This finding is in concordance with several biomechanical studies that have shown a precise coordination of jaw-tongue muscles during extremely rapid feeding movements in toads.
We have identified four different types of hypoglossal subnuclei; three of them i.e. ventrolateral, intermediate and ventromedial subnuclei extending into the spinal cord, and the dorsomedial subnucleus that extended rostral to the obex in the medulla. We made serial sections of the retrogradely labeled hypoglossal motor neurons and identified the ventromedial subnucleus in the ventral horn of the spinal cord at each level. In the experiments that we performed, the tracer was applied beyond the branches projecting to the hyoid muscles. Anterograde labeling by injecting tracer into the cerebellum or brainstem will not be able to distinguish between these different populations of hypoglossal nuclei. Therefore, the only hypoglossal cell bodies labeled were those innervating the tongue musculature. The four groups of hypoglossal motor nuclei identified in Bufo should therefore, only innervate the tongue musculature. The previous studies in Rana have described only three different populations of hypoglossal motor neurons that innervate the protractor or retractor muscles of the tongue (Matesz et al., 1999; Anderson, 2001). The ventromedial (VM) has an almost overlapping rostrocaudal extent with the ventrolateral subnuclei. This suggests that this additional subnucleus may have been isolated from the ventrolateral subnuclei during the independent evolution of a long protrusible tongue in anurans. While most of the hypoglossal nuclei have been shown to demonstrate musculotopic organization, we suggest that this ventromedial subnucleus also innervates the tongue. However, its functional significance is still under investigation.
When SR 101 was bath applied while the peripheral hypoglossal nerve was stimulated, it labeled the hypoglossal nuclei, the medial reticular formation, raphe nuclei, the Purkinje cell layer of the cerebellum, the trigeminal motor nuclei and the glossopharyngeal nuclei. Similar nuclei were labeled when the peripheral trigeminal nerve was stimulated and SR 101 was bath applied. This suggests that all these nuclei have potential to be the components of feeding pathways and that there are reciprocal pathways among the hypoglossal sensory, the medial reticular formation, hypoglossal and trigeminal motor neurons.
When SR 101 was bath applied during the peripheral trigeminal stimulation, we identified the dendritic extension between dorsomedial and intermediate hypoglossal subnuclei. The trigeminal motor nuclei coordinate the motor output of the m. adductor mandibulae, the primary jaw-closing muscle. In Rana and Bufo, the dorsomedial and intermediate subnuclei innervate tongue protractor and retractor muscles (Sokoloff, 1991; Matesz, 1999; Nishikawa, 2000). Studies in other animals suggest that jaw levators and tongue retractors are activated simultaneously in rabbits (Ariyashinge et al., 2004), and that the genioglossus (tongue protractor muscle) activity is inhibited during jaw closing phase (Liu et al., 1993). The anatomical pathway for how the genioglossus activity is inhibited during jaw closing has not been described. It is possible that this inter-nuclei dendritic overlapping interacts for inhibition of tongue protractors during jaw closing phase.
Several electrophysiological studies have justified the importance of feedback pathways originating from the peripheral musculature that coordinate the mouth opening with tongue protraction. For examples, a failure of the jaw to open after hypoglossal transection demonstrated by Nishikawa and Gans (1992), and the failure of the tongue to protract to its full extent after trigeminal transection in toads (Anderson, personal communication). This evidence was shown by electrophysiological data and biomechanical studies. However, neuroanatomical data was lacking. We have demonstrated the direct sensory projections of the hypoglossal nerve onto the Purkinje cell layer of the cerebellum, as well as to the trigeminal motor neuron by double labeling technique using SR 101 and FDA or Alexa Fluor. We also demonstrated this by a single labeling of the hypoglossal nerve with FDA or Alexa 488 and use of fluorescence and DIC microscopy. Below we describe at least two possible explanations for the significance of these feedback pathways.
During feeding in the marine toad, both the mandibular depressors and levators are activated simultaneously and are preloaded (Nishikawa and Gans, 1992). When the toad properly orients to the prey and is ready to open the mouth, sudden inhibition of the mandibular levator muscles occur. The anatomical data suggest that it is the hypoglossal sensory afferents that relay the inhibitory signal by synaptic modulation to the trigeminal motor nuclei, thereby suppressing mandibular levators and allowing the jaw to open.
The Purkinje cells of the cerebellum probably influence the timing of activation of the tongue and jaw muscles after receiving information through the sensory hypoglossal and trigeminal projections. An interesting caveat is that the exact role of the cerebellum during feeding motor output in Rana is unclear as cerebellar cortex lesions failed to produce quantifiable deficits in the timing or coordination of feeding movements (Longmore and Anderson, in progress). This will be tested in Bufo in future experiments.
The hypoglossal nerve has been shown to project to the medial reticular formation, a pathway previously described in Rana (Anderson and Nishikawa, 1997; Anderson, 2001; Rácz et al., 2008). In Bufo, we have demonstrated that the hypoglossal and trigeminal nerves do communicate with the medial reticular formation in the brainstem. We found that the medial reticular formation was labeled with activity dependent uptake of SR101 in separate experiments in which stimulation of the hypoglossal and trigeminal nerves were performed independently. This also labeled raphe nucleus. These data suggest the sensory feedback pathways that relay information from the hypoglossal and trigeminal nerves to the medial reticular formation, the hypoglossal motor nuclei, the trigeminal motor nuclei and the Purkinje cell layer of the cerebellum presumably influence the synchronous activity of tongue and jaw muscles. In order to execute the jaw-tongue coordination, the two groups of motor nuclei, the trigeminal and hypoglossal, have to share common inputs for their movement trajectories. In Rana, the medial reticular formation has been hypothesized to be the motor pattern-generating neurons in the brainstem which signals the hypoglossal motor neurons to fire (Anderson, 2001). Taken the SR101 uptake data for the hypoglossal and the trigeminal nerves together, it is possible that these cranial nerves share common premotor neuronal pools in the medial reticular formation.
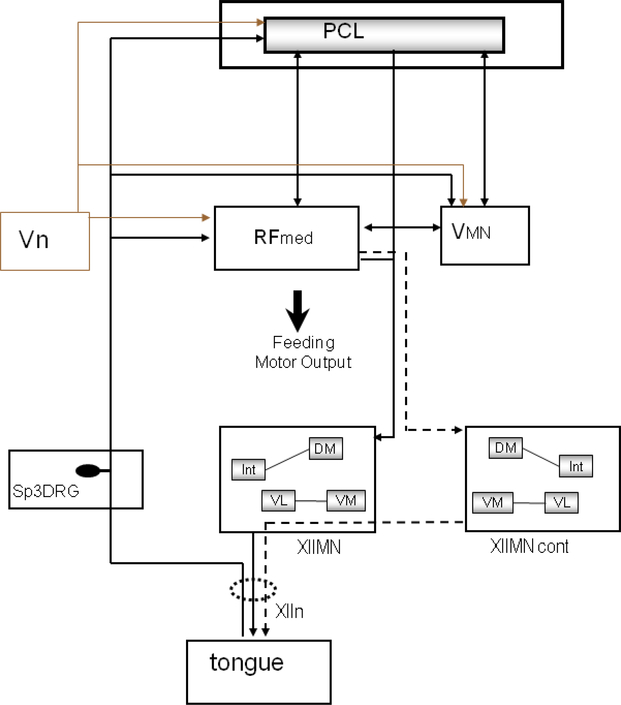
Figure 8 illustrates our current understanding of the brainstem circuits that mediate coordinated jaw-tongue activity during feeding in Bufo. This figure does not account for incoming, feed-forward sensory information (i.e., visual, olfactory, vestibulocochlear) that elicits a feeding movement, nor does it account for forebrain or midbrain modulating circuits believed to be involved in the sensorimotor processing of information before the feeding behavior is initiated. In other words, any projections rostral to the cerebellum and into the telencephalon were not investigated in this study. One component of the feeding motor output is to excite hypoglossal motor neurons controlling tongue protraction. This movement initiates activity in afferents originating in the tongue and traveling back to the brain in the hypoglossal nerve, ultimately projecting to the trigeminal nuclei, the reticular formation and the Purkinje cell layer of the cerebellum. We hypothesize that this feedback information influences inhibition of the jaw levator muscles, thus coordinating the activity in the tongue with the opening of the mouth. The Purkinje cell layer of the cerebellum probably influences overall timing of jaw-tongue coordination through the efferent output to the medial reticular formation, the trigeminal motor nuclei and to the hypoglossal motor nuclei. Evidence suggests the medial reticular formation as the common pre-motor region for the hypoglossal and the trigeminal motor nuclei. The inter-nuclei dendritic extension between the dorsomedial and intermediate subnuclei is probably a component of secondary pathways to coordinate tongue retraction during jaw closing. Overall we have demonstrated the anatomical substrate in the brainstem and the spinal cord for a set of feedback mechanisms that coordinate the rapid synchronization and timing of muscle activity during feeding. Further studies are investigating sensory inputs from other cranial nerves onto the reticular formation.
Fig. 8.
Diagrammatic representation of our current knowledge of the brainstem pathways potentially involved in coordinating feeding motor output in toads. This figure summarizes the anatomical basis for feeding reflex modulation once the medulla receives the signal to initiate motor output. The solid lines indicate the direct projection based on the dextran labeling. The dotted lines indicate an inferred projection based on the SR101 or single labeling data. (PCL Purkinje cell layer, RFmed Medial reticular formation, Vn peripheral trigeminal nerve, VMN trigeminal motor nuclei, XIIMN hypoglossal motor nuclei, XIIMNcont contralateral hypoglossal motor nuclei, XIIn peripheral hypoglossal nerve, SP3 DRG dorsal root ganglion of the third spinal nerve).
Acknowledgements
The work reported here was supported by NSF Grant # 0623791 to CWA. Partially supported by NIH Grant # P20RR016454 from INBRE. A portion of this work was presented at the Society of Integrative and Comparative Biology conference, San Antonio (2008).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LITERATURE CITED
- Anderson CW, Nishikawa KC, 1993. A prey-type dependent hypoglossal feedback system in the frog, Rana pipiens. Brain Behav. Evol. 42, 189–196. [DOI] [PubMed] [Google Scholar]
- Anderson CW, Nishikawa KC, 1996. The role of sensory information during motor program choice in frogs. J. Comp. Physiol 176, 753–762. [DOI] [PubMed] [Google Scholar]
- Anderson CW, Nishikawa KC, 1997. The functional anatomy and evolution of hypoglossal afferents in the leopard frog, Rana pipiens. Brain Res. 771, 285–291. [DOI] [PubMed] [Google Scholar]
- Anderson CW, 2001. Anatomical evidence for brainstem circuits mediating feeding motor programs in the leopard frog, Rana pipiens. Exp. Brain Res 140, 12–19. [DOI] [PubMed] [Google Scholar]
- Ariyashinghe S, Inoue M, Yamamura K, Harasawa Y, Kurose M, Yamada K, 2004. Coordination of jaw and extrinsic tongue muscle activity during rhythmic jaw movements in anesthetized rabbits. Brain Res. 1016, 201–216. [DOI] [PubMed] [Google Scholar]
- Dicke U, Roth G, Matsushima T, 1998. Neural substrate for motor control of feeding in amphibians. Acta Anat. 163, 127–143. [DOI] [PubMed] [Google Scholar]
- Downman CB, 1939. Afferent fibres of the hypoglossal nerve. J. Anat 73, 387–95. [PMC free article] [PubMed] [Google Scholar]
- Ewert JP, Buxbaum-Conradi H, Glagow M, Rottgen A, Schurg-Pfeiffer E, Schwippert WW, 1999. Forebrain and midbrain structures involved in prey-catching behaviour of toads: Stimulus-response mediating circuits and their modulating loops. Eur. J. Morphol 37, 172–176. [DOI] [PubMed] [Google Scholar]
- Harwood DV, Anderson CW, 2000. Evidence for the anatomical origins of hypoglossal afferents in the tongue of the leopard frog, Rana pipiens. Brain Res. 862, 288–291. [DOI] [PubMed] [Google Scholar]
- Ishiwata Y, Ono T, Kuroda T, Nakamura Y, 2000. Jaw-tongue reflex: Afferents, central pathways, and synaptic potentials in hypoglossal motoneurons in the cat. J. Dent. Res 79, 1626–1634. [DOI] [PubMed] [Google Scholar]
- Keifer J, Vyas D, Houk JC, 1992. Sulforhodamine labeling of neural circuits engaged in motor pattern generation in the in vitro turtle brainstem-cerebellum. J. Neurosci 12, 3187–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauder GV, 1981. Form and function: structural analysis in evolutionary morphology. Paleobiol. 7, 430–442. [Google Scholar]
- Lauder GV, Liem K, 1989. The role of historical factors in the evolution of complex organismal functions In Complex organismal functions: integration and evolution in vertebrates, (ed. by Wake DB and Roth G) Wiley, Boston. [Google Scholar]
- Lichtman JW, Wilkinson RS, Rich MM, 1985. Multiple innervation of tonic endplates revealed by activity-dependent uptake of fluorescent probes. Nature. 314, 357–9. [DOI] [PubMed] [Google Scholar]
- Liu ZJ, Masuda Y, Inoue T, Fuchihata H, Sumida A, Takada K, Morimoto T, 1993. Coordination of cortically induced rhythmic jaw and tongue movements in the rabbit. J. Neurophysiol 69, 569–584. [DOI] [PubMed] [Google Scholar]
- Luo PF, Dessem D, Zhang JD, 2001. Axonal projections and synapses from the supratrigeminal region to hypoglossal motoneurons in the rat. Brain Res. 890, 314–329. [DOI] [PubMed] [Google Scholar]
- Luo PF, Zhang JD, Yang R, Pendlebury W, 2006. Neuronal circuitry and synaptic organization of trigeminal proprioceptive afferents mediating tongue movement and jaw-tongue coordination via hypoglossal premotor neurons. Eur. J. Neurosci 23, 3269–3283. [DOI] [PubMed] [Google Scholar]
- Marin O, Gonzalez A, Smeets W, 1997. Basal ganglia organization in amphibians: Efferent connections of the striatum and the nucleus accumbens. J. Comp. Neurol 380, 23–50. [PubMed] [Google Scholar]
- Matesz C, 1979. Central projection of the VIIIth cranial nerve in the frog. Neuroscience. 4, 2061–71. [DOI] [PubMed] [Google Scholar]
- Matesz C, Schmidt I, Szabo L, Birinyi A, Szekely G, 1999. Organization of the motor centres for the innervation of different muscles of the tongue: A neuromorphological study in the frog. Eur. J. Morphol 37, 190–194. [DOI] [PubMed] [Google Scholar]
- Morimoto T, Takebe H, Sakan I, Kawamura Y, 1978. Reflex activation of extrinsic tongue muscles by jaw closing muscle proprioceptors. Jpn. J. Physiol 28, 461–71. [DOI] [PubMed] [Google Scholar]
- Morimoto T, Inoue T, Masuda Y, Nagashima T, 1989. Sensory components facilitating jaw-closing muscle activities in the rabbit. Exp. Brain Res 76, 424–40. [DOI] [PubMed] [Google Scholar]
- Nishikawa KC, Gans C, 1992. The role of hypoglossal sensory feedback during feeding in the marine toad, Bufo marinus. J. Exp. Zool 264, 245–52. [DOI] [PubMed] [Google Scholar]
- Nishikawa KC, 2000. Feeding in frogs In: Schwenk K (ed) Feeding in tetrapod vertebrates: Form, function and evolution. in tetrapod vertebrates. Academic, New York. [Google Scholar]
- Oka Y, Takeuchi H, Satou M, Ueda K, 1987. Cobaltic lysine study of the morphology and distribution of the cranial nerve efferent neurons (motoneurons and preganglionic parasympathetic neurons) and rostral spinal motoneurons in the Japanese toad. J. Comp. Neurol 259, 400–23. [DOI] [PubMed] [Google Scholar]
- Racz E, Bacskai T, Szabo G, Szekely G, Matesz C, 2008. Organization of last-order premotor interneurons related to the protraction of tongue in the frog, Rana esculenta. Brain Res. 1187, 111–115. [DOI] [PubMed] [Google Scholar]
- Roth G, Wake DB, 1989. Conservatism and innovation in the evolution of feeding in vertebrates In Complex organismal functions: integration and evolution in vertebrates (ed. by Roth G, Wake DB) Wiley, Boston. [Google Scholar]
- Roth G, Dicke U, Grunwald W, 1999. Morphology, axonal projection pattern, and response types of tectal neurons in plethodontid salamanders. II: Intracellular recording and labeling experiments. J. Comp. Neurol 404, 489–504. [DOI] [PubMed] [Google Scholar]
- Sanchez-Camacho C, Marin O, Ten Donkelaar HJ, Gonzalez A, 2001. Descending supraspinal pathways in amphibians. I. A dextran amine tracing study of their cells of origin. J. Comp. Neurol 434, 186–208. [DOI] [PubMed] [Google Scholar]
- Sokoloff AJ, 1991. Musculotopic organization of the hypoglossal nucleus in the grass frog, Rana pipiens. J. Comp. Neurol 308, 505–12. [DOI] [PubMed] [Google Scholar]
- Stuesse SL, Cruce WL, Powell KS, 1983. Afferent and efferent components of the hypoglossal nerve in the grass frog, Rana pipiens. J. Comp. Neurol 217, 432–9. [DOI] [PubMed] [Google Scholar]
- Tarkhan AA, Abou-El-Naga I, 1947. Sensory fibres in the hypoglossal nerve. J. Anat 81, 23–32 1. [PubMed] [Google Scholar]
- Tarkhan AA, El-Malek SA, 1950. On the presence of sensory nerve cells on the hypoglossal nerve. J. Comp. Neurol 93, 219–28. [DOI] [PubMed] [Google Scholar]
- Tolu E, Caria MA, Simula ME, Lacana P, 1994. Muscle spindle and periodontal trigeminal afferents modulate the hypoglossal motoneuronal activity. Arch. Ital. Biol 132, 93–104. [PubMed] [Google Scholar]
- Weerasuriya A, 1989. In search of the motor pattern generator for snapping in toads In:Ewert J-P, Arbib MA (eds) Visuomotor coordination: amphibians, comparisons, models, and robots. Plenum; New York, pp. 589–614. [Google Scholar]
- Wild JM, 1981. Identification and localization of the motor nuclei and sensory projections of the glossopharyngeal, vagus, and hypoglossal nerves of the cockatoo (Cacatua roseicapilla), Cacatuidae. J. Comp. Neurol 203, 351–77. [DOI] [PubMed] [Google Scholar]
- Wild JM, 1990. Peripheral and central terminations of hypoglossal afferents innervating lingual tactile mechanoreceptor complexes in Fringillidae. J. Comp. Neurol 298, 157–71. [DOI] [PubMed] [Google Scholar]
- Wolff JB, Lee MJ, Anderson CW, 2004. Contribution of the submentalis muscle to feeding mechanics in the leopard frog, Rana pipiens. J. Exp. Zool. Part a-Comparative Experimental Biology 301A, 666–673. [DOI] [PubMed] [Google Scholar]
- Zimny R, Sobusiak T, Matlosz Z, 1970. The afferent components of the hypoglossal nerve. An experimental study with toluidine blue and sliver impregnation methods. J. Hirnforsch 12, 83–100. [PubMed] [Google Scholar]