Abstract
An experiment was conducted to 1) test the hypothesis that a minimum adaptation period to diets used in ileal amino acid (AA) digestibility experiments with pigs is needed and 2) to test the null-hypothesis that ileal digestibility and basal endogenous losses of AA are not affected by the indigestible marker used. Eight ileal-cannulated barrows with an initial BW of 58.1 ± 4.3 kg were randomly allotted to a 2-period crossover design with 2 diets and 4 pigs per diet in each period. A soybean meal-based diet and an N-free diet were prepared. Both diets contained 0.4% chromium oxide, 0.4% titanium dioxide, and 0.4% Celite (a source of acid insoluble ash; AIA). Pigs were provided feed in a daily amount of 3 times the maintenance requirement for metabolizable energy, and 2 equal meals were provided each day. Ileal digesta samples were collected from 0800 to 2000 h on each day during the two 9-d collection periods. There was no period by diet interactions observed. Marker concentrations in ileal digesta were analyzed separately for each day, and the point where the concentration of each marker was stabilized in the digesta was determined using a linear broken-line analysis. For pigs fed the soybean meal diet, the breakpoints for Cr, Ti, and AIA in ileal digesta were 2.70, 2.45, and 3.77 d, respectively. In pigs fed the N-free diet, the breakpoints for Cr, Ti, and AIA in ileal digesta were 2.52, 2.39, and 2.29 d, respectively. Based on the pooled data, the basal endogenous losses of most AA calculated using Cr as an indigestible marker were less (P < 0.05) than the values calculated using Ti, but greater (P < 0.05) compared with values calculated based on AIA. The standardized ileal digestibility of most AA in soybean meal calculated using Cr or Ti as a digestibility marker were greater (P < 0.05) than the digestibility values calculated using AIA. In conclusion, 3 d of adaptation is required before markers are stabilized in the ileal outflow if Cr or Ti is used as an indigestible marker and 4 d of adaptation is required if AIA is the marker. Values for AA digestibility calculated using Cr or Ti as the marker are not different, but greater compared with values calculated using AIA as the marker.
Keywords: acid insoluble ash, chromium, digestibility, indigestible marker, pigs, titanium
Introduction
Values for digestibility of nutrients in diets or feed ingredients fed to pigs are determined by nutrient intake and nutrient excretion in feces or ileal digesta. Ileal nutrient digestibility is usually determined using an indigestible marker, which is also known as an index or an indicator because complete quantitative collection of ileal digesta is usually not possible (Adeola, 2001; Stein et al., 2007). As a consequence, an accurate analysis of marker concentration in ileal digesta is critical for accurate calculation of ileal digestibility values. Fundamental assumptions to use the markers include no interactions between markers and the body and, therefore, similar passage rate as digesta (Jagger et al., 1992; Adeola, 2001). A sufficient adaptation period before initiation of ileal digesta collection is also important to have a constant marker concentration in ileal digesta.
An adequate adaptation period is also necessary to make sure digesta from the previous diet has passed through the gastrointestinal tract before collection of digesta is initiated. For total tract digestibility experiments, an adaptation period of 3 to 7 d is needed (Adeola, 2001), but the recovery of Cr in feces from pigs was constant after 3 to 5.5 d of feeding (Clawson et al., 1955; Choi and Kim, 2019). However, in the case of ileal digesta, the period needed to obtain a constant recovery of the marker may differ from that for feces because passage time through the small intestine is shorter than through the total tract (Owusu-Asiedu et al., 2006). It is also possible that different types of indigestible markers may require different adaptation periods because the use of different markers may result in differences in calculated digestibility values (Jagger et al., 1992; Olukosi et al., 2012; Wang et al., 2017).
However, to our knowledge, no data for an adequate adaptation period for ileal digestibility experiments have been reported and it is not known if the needed adaptation period is different among different markers. Therefore, an experiment was conducted to test the hypothesis that a minimum adaptation period is needed in ileal digestibility experiments with pigs to have constant marker concentrations in ileal digesta. The second objective was to test the null-hypothesis that apparent ileal digestibility (AID) and standardized ileal digestibility (SID) of amino acid (AA) and the basal endogenous losses of AA are constant regardless of the indigestible marker used.
Materials and Methods
The protocol for the experiment was reviewed and approved by the Institutional Animal Care and Use Committee at the University of Illinois. All pigs used in the experiment were Landrace (3/4) by Large White (1/4) cross-bred barrows (Genetiporc, Alexandria, MN).
Animals and diets
Eight barrows with an initial BW of 58.1 ± 4.3 kg had a T-cannula installed in the distal ileum (Stein et al., 1998) and were randomly allotted to a 2-period crossover design with 2 diets and 4 pigs per diet in each period. Pigs were housed in individual pens (1.2 × 1.5 m), and each pen was equipped with a feeder and a nipple drinker and had fully slatted tribar floors and smooth sidewalls.
A soybean meal-based diet and an N-free diet were prepared (Tables 1 and 2). Both experimental diets contained 0.4% Cr2O3 (chromic oxide), 0.4% TiO2 (titanium dioxide), and 0.4% acid insoluble ash (AIA; Celite Corporation, World Minerals Co., Lompoc, CA) to provide indigestible markers. A washout diet with no exogenous indigestible marker was also prepared based on corn, distillers dried grains with solubles, and casein. Vitamins and minerals were included in all diets to meet or exceed nutrient requirement estimates (NRC, 1998).
Table 1.
Ingredient composition of experimental diets, as-fed basis
| Diet | |||
|---|---|---|---|
| Washout | Soybean meal | N-free | |
| Ground corn | 62.60 | — | — |
| Soybean meal, 48% CP | — | 35.45 | — |
| Distillers dried grains with solubles | 30.00 | — | — |
| Casein | 5.00 | — | — |
| Corn starch | — | 39.00 | 67.50 |
| Sucrose | — | 20.00 | 20.00 |
| Soybean oil | — | 2.00 | 4.00 |
| l-Lys∙HCl, 78% Lys | 0.30 | — | — |
| Solka floc1 | — | — | 4.00 |
| Ground limestone | 1.10 | 0.65 | 0.80 |
| Dicalcium phosphate | 0.30 | 1.00 | 1.30 |
| Potassium carbonate | — | — | 0.40 |
| Magnesium oxide | — | — | 0.10 |
| Salt | 0.40 | 0.40 | 0.40 |
| Vitamin–mineral premix2 | 0.30 | 0.30 | 0.30 |
| Chromic oxide | — | 0.40 | 0.40 |
| Titanium dioxide | — | 0.40 | 0.40 |
| Celite3 | — | 0.40 | 0.40 |
1Fiber Sales and Development Corp., Urbana, OH.
2Supplied per kg of complete diet: vitamin A, 11,128 IU; vitamin D3, 2,204 IU; vitamin E, 66 IU; vitamin K, 1.42 mg; thiamin, 0.24 mg; riboflavin, 6.58 mg; pyridoxine, 0.24 mg; vitamin B12, 0.03 mg; D-pantothenic acid, 23.5 mg; niacin, 44 mg; folic acid, 1.58 mg; biotin, 0.44 mg; Cu, 10 mg as copper sulfate; Fe, 125 mg as iron sulfate; I, 1.26 mg as potassium iodate; Mn, 60 mg as manganese sulfate; Se, 0.3 mg as sodium selenite; and Zn, 100 mg as zinc oxide.
3A source of acid insoluble ash; Celite Corporation, World Minerals Co., Lompoc, CA.
Table 2.
Analyzed nutrient composition of experimental diets, as-fed basis
| Diet | |||
|---|---|---|---|
| Composition, % | Washout | Soybean meal | N-free |
| DM | 89.9 | 92.8 | 92.3 |
| CP | 17.9 | 16.6 | 1.10 |
| Ash | 3.08 | 5.37 | 4.02 |
| Cr | — | 0.30 | 0.28 |
| Ti | — | 0.24 | 0.23 |
| Acid insoluble ash1 | — | 0.54 | 0.40 |
| Indispensable AA | |||
| Arg | 0.72 | 1.32 | 0.02 |
| His | 0.45 | 0.50 | 0.05 |
| Ile | 0.53 | 0.69 | 0.01 |
| Leu | 2.05 | 1.29 | 0.03 |
| Lys | 0.94 | 0.92 | 0.01 |
| Met | 0.39 | 0.29 | — |
| Phe | 0.91 | 0.89 | 0.01 |
| Thr | 0.83 | 0.75 | 0.01 |
| Trp | 0.20 | 0.23 | — |
| Val | 0.62 | 0.71 | 0.01 |
| Dispensable AA | |||
| Ala | 1.16 | 0.70 | 0.02 |
| Asp | 0.94 | 1.69 | 0.01 |
| Cys | 0.59 | 0.48 | 0.07 |
| Glu | 2.74 | 2.82 | 0.05 |
| Gly | 0.64 | 0.74 | 0.01 |
| Pro | 1.46 | 0.84 | 0.02 |
| Ser | 0.96 | 1.00 | 0.01 |
1Acid insoluble ash = analyzed acid insoluble ash − Cr − Ti.
Feeding and sample collection
The washout diet was provided for 7 d before each of the two 9-d experimental periods. During the washout periods, animals had free access to feed. On day 7 of each washout period, pigs were fasted from 1600 to 0800 hours the following day when experimental diets were provided. During the experimental periods, experimental diets were provided at daily levels of 3 times the estimated maintenance requirement for metabolizable energy (106 kcal/kg BW0.75; NRC, 1998), which is believed to be at least 85% of ad libitum intake. Equal meals were provided at 0800 and 2000 hours and the daily feed allowance was adjusted at the beginning of each experimental period when the BW of each pig was recorded. Animals had free access to water at all times through a nipple drinker.
Ileal digesta samples were collected from 0800 to 2000 hours on each day during the two 9-d collection periods. A 225-mL plastic bag was attached to the cannula barrel using a cable tie and digesta flowing into the bag were collected. Bags were removed whenever filled with digesta, or at least once every 30 min. Samples were kept separate for each animal and each collection day and stored at −20 °C.
Chemical analyses
At the conclusion of the experiment, ileal digesta samples were lyophilized. Diets and dried digesta samples were finely ground prior to chemical analysis. All diets and dried digesta samples were analyzed for Cr (Fenton and Fenton, 1979), Ti (Myers et al., 2004), AIA (van Keulen and Young, 1977), and dry matter (method 930.15; AOAC International, 2007). All diet samples were also analyzed for ash (method 942.05; AOAC International, 2007). The concentration of crude protein in diets was determined by the combustion procedure (Method 990.03; AOAC International, 2007) using an Elementar Rapid N-cube protein/nitrogen apparatus (Elementar Americas Inc., Mt. Laurel, NJ). Aspartic acid was used as a calibration standard and CP was calculated as N × 6.25. The concentration of AA in diet and ileal digesta samples were determined using a Beckman 6300 Amino Acid Analyzer (Beckman Instruments Corp., Palo Alto, CA) with ninhydrin for postcolumn derivatization and norleucine as the internal standard. Before AA analysis, samples were hydrolyzed for 24 h at 110 °C with 6 N HCl [method 982.30 E(a); AOAC International, 2007]. Methionine and Cys in hydrolyzates were determined as Met sulfone and cysteic acid, respectively, after an overnight cold performic acid-oxidation [method 982.30 E(b); AOAC International, 2007]. Tryptophan was determined after NaOH hydrolysis for 22 h at 110 °C [method 982.30 E(c); AOAC International, 2007].
Calculation and statistical analysis
Because Cr and Ti are insoluble in 4 N HCl and were included in the analyzed AIA in diets and ileal digesta samples, the AIA values were calculated by difference (i.e., AIA = analyzed AIA − Cr − Ti) based on the procedures used by McCarthy et al. (1974) and Kiarie and Nyachoti (2007). Marker concentrations in ileal digesta were converted to dry matter basis before all calculations. The basal endogenous losses of AA were calculated from pigs fed the N-free diet as previously described (Stein et al., 2007). The AID and the SID of AA in soybean meal were calculated from analyzed concentrations of AA and markers in diets and ileal digesta (Stein et al., 2007).
Any values that deviated from first or third quartiles by more than the interquartile range were considered outliers. The optimal adaptation period for each of the 3 markers was estimated by separate regression analyses for the 2 diets using the marker concentration in the ileal digesta from pigs fed the soybean meal diet or the N-free diet and sampling day. All analyses were completed using the NLIN procedure of SAS (SAS Inst. Inc., Cary, NC). The optimal adaptation period for each marker was determined by 1-slope broken-line analysis (Robbins et al., 2006) as the time it took to obtain a constant concentration of each marker in the digesta.
To compare values for the basal endogenous losses of AA, the AID of AA, and the SID of AA using the 3 different indigestible markers, pooled data from days 5 to 9 or days 6 and 7 were analyzed using MIXED procedures of SAS (SAS Inst. Inc., Cary, NC). Least squares means were calculated for each variable and mean separation was conducted by the PDIFF option with the Tukey’s adjustment. Data for days 5 to 9 were compared with data for days 6 and 7 using a contrast statement. The pig was the experimental unit. The statistical significance of the model was considered at P < 0.05 and the tendency was considered at 0.05 ≤ P < 0.10.
Results
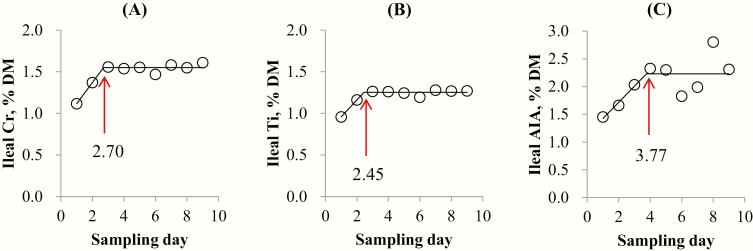
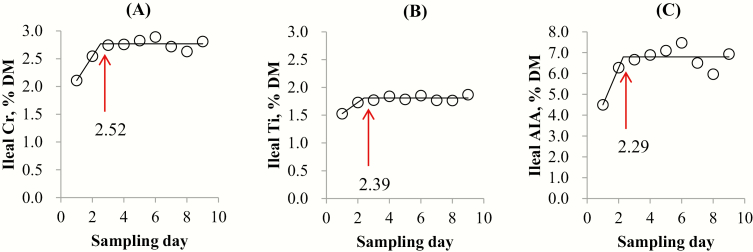
In pigs fed the soybean meal diet, the breakpoint for Cr, Ti, and AIA was 2.70 (SE = 0.64, P < 0.001), 2.45 (SE = 0.44, P < 0.001), and 3.77 d (SE = 1.81, P = 0.091), respectively (Figure 1). In pigs fed the N-free diet, the breakpoint for Cr, Ti, and AIA was 2.52 (SE = 0.60, P = 0.001), 2.39 (SE = 0.51, P = 0.002), and 2.29 d (SE = 0.58, P = 0.021), respectively (Figure 2).
Figure 1.
Data points represent means of 9 sampling days from 8 pigs (initial BW = 58.1 kg) fed a soybean meal-based diet (1.9 kg/d). Each regression model shows the marker concentrations in ileal digesta samples from pigs (Y) relative to sampling day (X). (A) The linear broken-line model for ileal digesta Cr concentration indicated that the breakpoint for ileal digesta Cr concentration was 2.70 d (SE = 0.64) based on the following equation: Y = 1.55 − 0.25 × (2.70 − X) where X is less than 2.70, with sum of squares of error = 3.69 and P < 0.001. (B) Linear broken-line model for ileal digesta Ti concentration indicated that the breakpoint for ileal Ti digesta concentration was 2.45 d (SE = 0.44) based on the following equation: Y = 1.25 − 0.20 × (2.45 − X) where X is less than 2.45, with sum of squares of error = 1.56 and P < 0.001. (C) The linear broken-line model for ileal digesta AIA concentration indicated that the breakpoint for ileal digesta AIA concentration was 3.77 d (SE = 1.81) based on the following equation: Y = 2.23 − 0.29 × (3.77 − X) where X is less than 3.77, with sum of squares of error = 64.94 and P = 0.091.
Figure 2.
Data points represent means of 9 sampling days from 8 pigs (initial BW = 58.1 kg) fed an N-free diet (1.9 kg/d). Each regression model shows the marker concentrations in ileal digesta samples from pigs (Y) relative to sampling day (X). (A) The linear broken-line model for ileal Cr concentration indicated that the breakpoint for ileal digesta Cr concentration was 2.52 d (SE = 0.60) based on the following equation: Y = 2.77 − 0.44 × (2.52 − X) where X is less than 2.52, with sum of squares of error = 12.86 and P = 0.001. (B) The linear broken-line model for ileal Ti concentration indicated that the breakpoint for ileal digesta Ti concentration was 2.39 d (SE = 0.51) based on the following equation: Y = 1.81 − 0.20 × (2.39 − X) where X is less than 2.39, with sum of squares of error = 2.48 and P = 0.002. (C) The linear broken-line model for ileal digesta acid insoluble ash (AIA) concentration indicated that the breakpoint for ileal digesta AIA concentration was 2.29 d (SE = 0.58) based on the following equation: Y = 6.80 − 1.78 × (2.29 − X) where X is less than 2.29, with sum of squares of error = 287.60 and P = 0.021.
Thus, data indicated that the minimum adaptation period needed before ileal digesta had a constant marker concentration was 3 to 4 d. Therefore, data for the digestibility of AA in soybean meal and for the basal endogenous losses of AA were pooled from days 5 to 9 to determine the influence of the marker on digestibility and basal endogenous losses of AA. Ileal digesta needed for ileal AA digestibility calculations are often collected on days 6 and 7. Therefore, data from days 6 and 7 were also pooled to determine the influence of the indigestible marker on digestibility and basal endogenous losses of AA during these days.
Based on the pooled data from days 5 to 9, the basal endogenous losses of all AA except Met, Phe, and Cys calculated using Cr as a digestibility marker were less (P < 0.05) than the basal endogenous losses calculated using Ti (Table 3). However, the basal endogenous losses of all AA calculated using Cr were greater (P < 0.05) compared with values calculated using AIA as the marker with the exception that no difference in the endogenous loss of Met, Phe, and Cys between Cr and AIA was observed. Basal endogenous losses of most AA calculated for days 6 and 7 were greatest (P < 0.05) if Ti was used as the marker and least if AIA was used with values obtained with Cr as the marker being intermediate. There were no differences in calculated endogenous losses between data for the days 5-to-9 period compared with data for the days 6-to-7 period.
Table 3.
Basal endogenous losses of AA (g/kg DMI) determined using different indigestible markers using the data collected from days 5 to 9 or from days 6 and 7 of pigs fed an N-free diet1
| Pooled data collected from days 5 to 92 | Pooled data collected from days 6 and 73 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Item, % | Cr | Ti | AIA4 | SEM | P | Cr | Ti | AIA | SEM | P |
| Indispensable AA | ||||||||||
| Arg | 0.71b | 0.86a | 0.49c | 0.11 | <0.001 | 0.61b | 0.76a | 0.39c | 0.09 | <0.001 |
| His | 0.17b | 0.20a | 0.12c | 0.03 | <0.001 | 0.16a | 0.19a | 0.10b | 0.02 | <0.001 |
| Ile | 0.23b | 0.28a | 0.17c | 0.04 | <0.001 | 0.23b | 0.28a | 0.15c | 0.02 | <0.001 |
| Leu | 0.41b | 0.49a | 0.30c | 0.07 | <0.001 | 0.40b | 0.50a | 0.26c | 0.05 | <0.001 |
| Lys | 0.25b | 0.31a | 0.19c | 0.04 | <0.001 | 0.24b | 0.29a | 0.15c | 0.03 | <0.001 |
| Met | 0.06ab | 0.07a | 0.05b | 0.02 | 0.001 | 0.06a | 0.07a | 0.04b | 0.01 | <0.001 |
| Phe | 0.15ab | 0.18a | 0.12b | 0.04 | 0.018 | 0.14b | 0.17a | 0.10c | 0.03 | <0.001 |
| Thr | 0.51b | 0.62a | 0.36c | 0.07 | <0.001 | 0.52b | 0.65a | 0.34c | 0.16 | <0.001 |
| Trp | 0.14b | 0.18a | 0.10c | 0.02 | <0.001 | 0.15b | 0.18a | 0.10c | 0.02 | <0.001 |
| Val | 0.33b | 0.40a | 0.24c | 0.05 | <0.001 | 0.33b | 0.41a | 0.21c | 0.03 | <0.001 |
| Total | 2.97b | 3.60a | 2.13c | 0.43 | <0.001 | 2.84b | 3.52a | 1.83c | 0.31 | <0.001 |
| Dispensable AA | ||||||||||
| Ala | 0.61b | 0.75a | 0.42c | 0.08 | <0.001 | 0.56b | 0.70a | 0.35c | 0.08 | <0.001 |
| Asp | 0.66b | 0.80a | 0.48c | 0.10 | <0.001 | 0.65b | 0.79a | 0.42c | 0.09 | <0.001 |
| Cys | 0.33ab | 0.40a | 0.24b | 0.08 | 0.001 | 0.35a | 0.45a | 0.21b | 0.07 | <0.001 |
| Glu | 0.98b | 1.19a | 0.69c | 0.13 | <0.001 | 0.93b | 1.15a | 0.59c | 0.09 | <0.001 |
| Gly | 1.91b | 2.33a | 1.27c | 0.23 | <0.001 | 1.76b | 2.19a | 1.10c | 0.25 | <0.001 |
| Pro | 6.96b | 8.51a | 4.55c | 1.06 | <0.001 | 6.06b | 7.52a | 3.67c | 1.09 | <0.001 |
| Ser | 0.59b | 0.72a | 0.42c | 0.08 | <0.001 | 0.59b | 0.72a | 0.38c | 0.07 | <0.001 |
| Total | 12.06b | 14.71a | 8.08c | 1.54 | <0.001 | 10.92b | 13.55a | 6.73c | 1.58 | <0.001 |
1Data for days 5 to 9 were compared with data for days 6 and 7, but no significant differences were observed.
2 n = 35.
3 n = 13.
4AIA, acid insoluble ash.
a–cWithin a row, mean within a common superscript differ (P < 0.05).
During the days 5-to-9 period, the AID and SID of all AA in soybean meal calculated using Cr or Ti as the digestibility marker were greater (P < 0.05) than the digestibility values calculated using AIA, but there were no differences between values calculated using Cr or Ti (Tables 4 and 5). The AID and SID of all AA in soybean meal calculated for days 6 and 7 using Cr or Ti as the digestibility marker were also greater (P < 0.05) than the digestibility values calculated using AIA, but there were no differences between values for AID and SID of AA in soybean meal calculated using Cr and Ti. Thus, the null-hypothesis that AID and SID values are independent of the marker used in the experiment was not confirmed.
Table 4.
Apparent ileal digestibility of AA in soybean meal determined using different indigestible markers using the data collected from days 5 to 9 or from days 6 and 7 of pigs fed a soybean meal-based diet1
| Pooled data collected from days 5 to 92 | Pooled data collected from days 6 and 73 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Item, % | Cr | Ti | AIA4 | SEM | P | Cr | Ti | AIA | SEM | P |
| Indispensable AA | ||||||||||
| Arg | 91.0a | 91.5a | 85.6b | 2.0 | 0.001 | 90.3a | 91.0a | 80.4b | 2.8 | 0.010 |
| His | 90.1a | 90.7a | 84.4b | 1.7 | 0.001 | 89.6a | 90.3a | 79.2b | 2.7 | 0.007 |
| Ile | 86.6a | 87.3a | 77.8b | 2.8 | 0.003 | 85.1a | 86.1a | 68.9b | 4.5 | 0.015 |
| Leu | 86.0a | 86.8a | 77.0b | 2.7 | 0.003 | 84.7a | 85.7a | 68.2b | 4.6 | 0.014 |
| Lys | 88.9a | 89.5a | 81.3b | 2.8 | 0.001 | 87.1a | 88.1a | 73.4b | 3.9 | 0.009 |
| Met | 90.1a | 90.7a | 83.4b | 2.3 | 0.004 | 88.6a | 89.4a | 76.1b | 3.6 | 0.021 |
| Phe | 89.7a | 90.3a | 83.4b | 2.0 | 0.004 | 88.6a | 89.3a | 77.1b | 3.1 | 0.022 |
| Thr | 80.6a | 81.7a | 68.2b | 3.7 | 0.003 | 79.2a | 80.6a | 56.3b | 6.5 | 0.015 |
| Trp | 82.0a | 83.0a | 70.6b | 3.5 | 0.003 | 81.7a | 82.9a | 60.8b | 6.2 | 0.016 |
| Val | 83.5a | 84.5a | 73.1b | 3.2 | 0.002 | 81.9a | 83.1a | 63.0b | 5.2 | 0.014 |
| Dispensable AA | ||||||||||
| Ala | 75.8a | 77.2a | 59.8b | 5.1 | 0.003 | 73.6a | 75.3a | 44.1b | 8.4 | 0.015 |
| Asp | 83.4a | 84.3a | 73.0b | 3.2 | 0.001 | 82.1a | 83.4a | 63.0b | 5.2 | 0.006 |
| Cys | 85.9a | 86.8a | 77.5b | 3.6 | 0.003 | 84.3a | 85.6a | 68.2b | 5.5 | 0.008 |
| Glu | 86.9a | 87.7a | 79.3b | 2.3 | 0.001 | 86.2a | 87.2a | 72.4b | 3.6 | 0.006 |
| Gly | 63.4a | 65.6a | 42.8b | 7.6 | 0.001 | 61.8a | 64.3a | 24.1b | 10.8 | 0.009 |
| Pro | 18.8a | 23.6a | -22.1b | 25.0 | 0.001 | 12.0a | 18.0a | −62.5b | 32.8 | 0.008 |
| Ser | 83.8a | 84.8a | 73.5b | 3.3 | 0.002 | 83.0a | 84.2a | 63.8b | 5.6 | 0.012 |
1Data for days 5 to 9 were compared with data for days 6 and 7. For Cr and Ti, days 5 to 9 values for Lys, Met, and Phe were greater (P < 0.05) than days 6 and 7 values. For AIA, days 5 to 9 values for Ile, Leu, Lys, Met, Phe, Val, Ala, and Cys were greater (P < 0.05) than days 6 and 7 values.
2 n = 32.
3 n = 14.
4AIA, acid insoluble ash.
a,bWithin a row, mean within a common superscript differ (P < 0.05).
Table 5.
Standardized ileal digestibility of AA in soybean meal determined using different indigestible markers using the data collected from days 5 to 9 or days 6 and 7 of pigs fed a soybean meal-based diet1,2
| Pooled data collected from days 5 to 93 | Pooled data collected from days 6 and 74 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Item, % | Cr | Ti | AIA5 | SEM | P | Cr | Ti | AIA | SEM | P |
| Indispensable AA | ||||||||||
| Arg | 95.8a | 97.3a | 89.2b | 2.0 | <0.001 | 95.1a | 96.7a | 83.9b | 2.8 | 0.002 |
| His | 93.1a | 94.4a | 86.8b | 1.7 | <0.001 | 92.6a | 94.0a | 81.6b | 2.7 | 0.003 |
| Ile | 89.6a | 91.2a | 80.3b | 2.8 | 0.001 | 88.1a | 90.0a | 71.4b | 4.5 | 0.009 |
| Leu | 88.8a | 90.5a | 79.4b | 2.7 | 0.001 | 87.5a | 89.5a | 70.6b | 4.6 | 0.009 |
| Lys | 91.3a | 92.7a | 83.3b | 2.8 | <0.001 | 89.6a | 91.2a | 75.4b | 3.9 | 0.005 |
| Met | 91.9a | 93.2a | 85.1b | 2.3 | 0.002 | 90.5a | 91.9a | 77.8b | 3.6 | 0.016 |
| Phe | 91.2a | 92.4a | 84.9b | 2.0 | 0.002 | 90.1a | 91.4a | 78.6b | 3.1 | 0.018 |
| Thr | 86.8a | 89.8a | 73.3b | 3.7 | <0.001 | 85.4a | 88.7a | 61.4b | 6.5 | 0.007 |
| Trp | 87.7a | 90.3a | 75.1b | 3.5 | <0.001 | 87.4a | 90.2a | 65.3b | 6.2 | 0.008 |
| Val | 87.7a | 89.9a | 76.6b | 3.2 | <0.001 | 86.1a | 88.6a | 66.5b | 5.2 | 0.008 |
| Dispensable AA | ||||||||||
| Ala | 83.6a | 86.6a | 65.7b | 5.1 | <0.001 | 81.4a | 84.8a | 50.0b | 8.4 | 0.007 |
| Asp | 86.9a | 89.0a | 76.0b | 3.2 | <0.001 | 85.7a | 88.1a | 65.9b | 5.2 | 0.004 |
| Cys | 92.0a | 94.4a | 82.0b | 3.6 | <0.001 | 90.5a | 93.2a | 72.8b | 5.5 | 0.002 |
| Glu | 90.1a | 91.6a | 81.7b | 2.3 | <0.001 | 89.4a | 91.1a | 74.8b | 3.6 | 0.003 |
| Gly | 86.8a | 95.3a | 61.3b | 7.6 | <0.001 | 85.2a | 94.1a | 42.6b | 10.8 | 0.001 |
| Pro | 88.9a | 106.3a | 28.7b | 25.0 | <0.001 | 82.0a | 100.7a | -11.7b | 32.8 | <0.001 |
| Ser | 89.3a | 91.8a | 77.9b | 3.3 | <0.001 | 88.4a | 91.2a | 68.1b | 5.6 | 0.006 |
1Values for standardized ileal digestibility were calculated by correcting the values for apparent ileal digestibility for basal ileal endogenous losses that were specific for Cr, Ti, and AIA, respectively.
2Data for days 5 to 9 were compared with data for days 6 and 7. For Cr and Ti, days 5 to 9 values for Lys, Met, and Phe were greater (P < 0.05) than days 6 and 7 values. For AIA, days 5 to 9 values for Ile, Leu, Lys, Met, Phe, Val, Ala, and Cys were greater (P < 0.05) than days 6 and 7 values.
3 n = 32.
4 n = 14.
5AIA, acid insoluble ash.
a,bMeans within a row without a common superscript letter differ (P < 0.05).
If Cr or Ti was used as a marker, values for AID and SID of Lys, Met, and Phe were greater (P < 0.05) for the days 5-to-9 period than for the days 6 and 7 period. However, if AIA was used as the marker, values for days 5 to 9 were greater (P < 0.05) than values for days 6 and 7 for the AID and SID of Ile, Leu, Lys, Met, Phe, Val, Ala, and Cys.
Discussion
Based on the total molecular mass of Cr2O3 and TiO2, calculated concentrations of Cr and Ti in diets were expected to be approximately 0.27% and 0.24%, respectively, and analyzed values were close to these estimates. Considering the inclusion rate and concentrations of AIA in the feed ingredients, the AIA concentrations in both the soybean meal diet and the N-free diet are also in agreement with calculated values (van Leeuwen et al., 1996; Sales and Janssens, 2003).
In most experiments to determine ileal digestibility of AA in feed ingredients, a 5-d adaptation period and a 2-d collection period are used (Baker and Stein, 2009; Liu et al., 2014), which is a consequence of a minimum of 5 d of adaptation is needed in experiments to determine total tract digestibility (Adeola, 2001). However, to our knowledge, there are no published data indicating that 5 d of adaption is needed to obtain a steady state of ileal digesta and we are also not aware of data demonstrating that a longer adaptation period may not be needed. Likewise, data to demonstrate the needed adaptation period for Cr, Ti, or AIA in ileal digesta to reach constant concentrations have not been reported. However, 3 to 4 d of adaptation is needed for Cr concentration in fecal samples to reach a plateau if grain-based diets are fed (Clawson et al., 1955), but 4 to 6 d may be needed to stabilize variation among pigs (Jang et al., 2014). Therefore, it was hypothesized that the time required to reach a stable marker concentration in ileal digesta is less than 4 to 6 d, and the data from the experiment, indicating that it took 2.3 to 3.8 d to reach a plateau for the markers, confirmed the hypothesis. It is not surprising that it takes fewer days to reach a plateau in ileal digesta than in feces because of the shorter time it takes for undigested feed to reach the distal ileum compared with the feces (Urriola and Stein, 2010; Navarro et al., 2018). Diet composition may influence the adaptation time needed to reach a constant concentration of digestibility markers because a minimum adaptation time of 3.5 d is needed to reach a constant concentration of marker in feces if a high-fiber diet is fed, whereas 5.5 d is needed if a low-fiber diet is used (Choi and Kim, 2019). However, it is not known if diet fiber concentration also influences the minimum adaptation time needed to reach a constant concentration of marker in ileal digesta and more research to investigate the impacts of different fiber levels on ileal digesta marker concentration is needed.
The present data demonstrated that breakpoints for Cr or Ti concentration in ileal digesta from pigs fed the soybean meal diet appeared to be close to the breakpoints for pigs fed the N-free diet, but that was not the case for pigs fed the diets using AIA as the marker. This observation indicates that AIA transit in the intestinal tract is more affected by diet composition than the transit of Cr and Ti. The rate of passage of digesta from the mouth to the distal ileum may be affected by the marker used (Imbeah et al., 1995). This may be due to finer particles leaving the stomach faster than coarser particles, but Cr appears to be unaffected by this, whereas other markers may be impacted by particle size (Imbeah et al., 1995).
The basal endogenous losses of AA determined regardless of the indigestible marker used and the values for SID of AA in soybean meal determined with Cr or Ti were within the range of values reported previously (NRC, 2012; Park et al., 2013; Adeola et al., 2016). However, the SID of AA was less compared with reported data (NRC, 2012) if AIA was used to calculate digestibility.
The AID of AA is influenced by endogenous AA in the ileal digesta if dietary CP is lower compared with diets containing greater CP (Fan et al., 1994; Fan and Sauer, 1997). However, if values for AID are corrected for the basal endogenous losses of AA to calculate SID values, the SID values are not affected by dietary CP (Stein et al., 2007). Therefore, it is important to determine basal endogenous losses of AA. The difference in SID values between calculations based on Cr or Ti and AIA may be explained by a different recovery of the markers (Kavanagh et al., 2001; Sales and Janssens, 2003). Recovery of markers in feces is possibly affected by dietary minerals (Cowieson and Bedford, 2009; Favero et al., 2014), dietary fiber (Wang et al., 2017; Choi and Kim, 2019), and uniformity of feces (Adeola, 2001). Ileal digestibility of AA and CP may be greater if Ti is used as an indigestible marker compared with Cr (Jagger et al., 1992; Olukosi et al., 2012; Wang et al., 2017), which may be a result of greater recovery of Ti in ileal digesta samples of pigs. However, in the present experiment, the SID of AA was not different between Cr and Ti indicating that different values are not always calculated from the 2 markers although the basal endogenous losses of AA were greater if Ti was used as an indigestible marker compared with values calculated with Cr. It, therefore, appears that the differences in basal endogenous losses of AA obtained between the 2 markers were not large enough to significantly affect the values for SID of AA in soybean meal.
The observation that SID of AA calculated based on AIA was less compared with values calculated with Cr or Ti is in agreement with reported data (Fan and Sauer, 2002; Brestenský et al., 2017; Wang et al., 2017). Thus, it appears that if AIA is used as the marker, SID values may be underestimated. This observation may be a result of analytical inaccuracies and variations in concentrations of AIA (Sales and Janssens, 2003).
A consequence of the markers being stable after 3 to 4 d is that the endogenous losses also are stabilized after this period of time, which is the reason there was no difference in endogenous losses calculated for the days 5-to-9 period compared with the days 6 and 7 period. The fact that 3 AA had greater AID and SID for the days 5-to-9 period compared with the days 6 and 7 period if Cr or Ti was used as the marker likely is of minor importance because the values, although significantly different for these 3 AA, only had small numerical differences. However, the observation that for AIA 8 AA had greater AID and SID values for days 5 to 9 compared with days 6 and 7 is a result of the greater variability in marker recovery during the last 4 d of the collection period. It is, therefore, likely that these differences were caused by the analytical difficulties associated with using AIA as a marker.
Conclusion
An adaptation period of at least 3 d is required before ileal digestibility markers are stabilized in the ileal outflow if Cr or Ti is used as an indigestible marker and at least 4 d of adaptation is required if AIA is the marker. Values for AA digestibility in soybean meal fed to growing pigs calculated using Cr or Ti were not different, but greater than values calculated based on AIA. Additional research is needed to determine why data for SID of AA are reduced if AIA is used as the marker. Based on results from the present experiment, it is recommended that Cr or Ti is used as indigestible marker in experiments to determine AID and SID of AA.
Glossary
Abbreviations
- AA
amino acids
- AIA
acid insoluble ash
- AID
apparent ileal digestibility
- SID
standardized ileal digestibility
Conflict of interest statement
The authors declare no real or perceived conflicts of interest.
Literature cited
- Adeola O. 2001. Digestion and balance techniques in pigs. In: Lewis A. J. and Southern L. L., editors, Swine nutrition. Washington (DC):CRC Press; p. 903–916. [Google Scholar]
- Adeola O., Xue P. C., Cowieson A. J., and Ajuwon K. M.. . 2016. Basal endogenous losses of amino acids in protein nutrition research for swine and poultry. Anim. Feed Sci. Technol. 221:274–283. doi: 10.1016/j.anifeedsci.2016.06.004 [DOI] [Google Scholar]
- AOAC International 2007. Official methods of analysis of AOAC int. 18th ed. Rev. 2nd ed. Gaithersburg (MD):AOAC International. [Google Scholar]
- Baker K. M., and Stein H. H.. . 2009. Amino acid digestibility and concentration of digestible and metabolizable energy in soybean meal produced from conventional, high-protein, or low-oligosaccharide varieties of soybeans and fed to growing pigs. J. Anim. Sci. 87:2282–2290. doi: 10.2527/jas.2008-1414 [DOI] [PubMed] [Google Scholar]
- Brestenský M., Nitrayová S., Heger J., and Patráš P.. . 2017. Chromic oxide and acid-insoluble ash as markers in digestibility studies with growing pigs and sows. J. Anim. Physiol. Anim. Nutr. 101:46–52. doi: 10.1111/jpn.12503 [DOI] [PubMed] [Google Scholar]
- Choi H., and Kim B. G.. . 2019. A low-fiber diet requires a longer adaptation period before collecting feces of pigs compared with a high-fiber diet in digestibility experiments using the inert marker method. Anim. Feed Sci. Technol. 256:114254. doi: 10.1016/j.anifeedsci.2019.114254 [DOI] [Google Scholar]
- Clawson A. J., Reid J. T., Sheffy B. E., and Willman J. P.. . 1955. Use of chromium oxide in digestion studies with swine. J. Anim. Sci. 14:700–709. doi: 10.1093/ansci/14.3.700 [DOI] [Google Scholar]
- Cowieson A. J., and Bedford M. R.. . 2009. The effect of phytase and carbohydrase on ileal amino acid digestibility in monogastric diets: complimentary mode of action? Worlds Poult. Sci. J. 65:609–624. doi: 10.1017/S0043933909000427 [DOI] [Google Scholar]
- Fan M. Z., Sauer W. C., Hardin R. T., and Lien K. A.. . 1994. Determination of apparent ileal amino acid digestibility in pigs: effect of dietary amino acid level. J. Anim. Sci. 72:2851–2859. doi: 10.2527/1994.72112851x [DOI] [PubMed] [Google Scholar]
- Fan M. Z., and Sauer W. C.. . 1997. Determination of true ileal amino acid digestibility in feedstuffs for pigs with the linear relationships between distal ileal outputs and dietary inputs of amino acids. J. Sci. Food Agric. 73:189–199. doi: [DOI] [Google Scholar]
- Fan M. Z., and Sauer W. C.. . 2002. Determination of true ileal amino acid digestibility and the endogenous amino acid outputs associated with barley samples for growing-finishing pigs by the regression analysis technique. J. Anim. Sci. 80:1593–1605. doi: 10.2527/2002.8061593x [DOI] [PubMed] [Google Scholar]
- Favero A., Ragland D., Vieira S. L., Owusu-Asiedu A., and Adeola. O.. 2014. Digestibility marker and ileal amino acid digestibility in phytase-supplemented soybean or canola meals for growing pigs. J. Anim. Sci. 92:5583–5592. doi: 10.2527/jas2014-7919 [DOI] [PubMed] [Google Scholar]
- Fenton T. W., and Fenton. M.. 1979. An improved procedure for the determination of chromic oxide in feed and feces. Can. J. Anim. Sci. 59:631–634. doi: 10.4141/cjas79-081 [DOI] [Google Scholar]
- Imbeah M., Sauer W. C., and Caine W. R.. . 1995. Comparison of the single dose and withdrawal method for measuring the rate of passage of two digestibility markers in digesta collected from the distal ileum and feces in growing pigs. Anim. Feed Sci. Technol. 52:41–50. doi: 10.1016/0377-8401(94)00708-H [DOI] [Google Scholar]
- Jagger S., Wiseman J., Cole D. J. A., and Craigon. J.. 1992. Evaluation of inert markers for the determination of ileal and faecal apparent digestibility values in the pig. Br. J. Nutr. 68:729–739. doi: 10.1079/Bjn19920129 [DOI] [PubMed] [Google Scholar]
- Jang Y. D., Lindemann M. D., Agudelo-Trujillo J. H., Escobar C. S., Kerr B. J., Inocencio N., and Cromwell G. L.. . 2014. Comparison of direct and indirect estimates of apparent total tract digestibility in swine with effort to reduce variation by pooling of multiple day fecal samples. J. Anim. Sci. 92:4566–4576. doi: 10.2527/jas2013-6570 [DOI] [PubMed] [Google Scholar]
- Kavanagh S., Lynch P. B., O’Mara F., and Caffrey P. J.. . 2001. A comparison of total collection and marker technique for the measurement of apparent digestibility of diets for growing pigs. Anim. Feed Sci. Technol. 89:49–58. doi: 10.1016/S0377-8401(00)00237-6 [DOI] [Google Scholar]
- Kiarie E., and Nyachoti C. M.. . 2007. Ileal digestibility of amino acids in co-extruded peas and full fat canola for growing pigs. Anim. Feed Sci. Technol. 139:40–51. doi: 10.1016/j.anifeedsci.2006.11.025 [DOI] [Google Scholar]
- Liu Y., Song M., Maison T., and Stein H. H.. . 2014. Effects of protein concentration and heat treatment on concentration of digestible and metabolizable energy and on amino acid digestibility in four sources of canola meal fed to growing pigs. J. Anim. Sci. 92:4466–4477. doi: 10.2527/jas.2013-7433 [DOI] [PubMed] [Google Scholar]
- McCarthy J. F., Aherne F. X., and Okai D. B.. . 1974. Use of HCl insoluble ash as an index material for determining apparent digestibility with pigs. Can. J. Anim. Sci. 54:107–109. doi: 10.4141/cjas74-016 [DOI] [Google Scholar]
- Myers W. D., Ludden P. A., Nayigihugu V., and Hess B. W.. . 2004. Technical note: a procedure for the preparation and quantitative analysis of samples for titanium dioxide. J. Anim. Sci. 82:179–183. doi: 10.2527/2004.821179x [DOI] [PubMed] [Google Scholar]
- Navarro D. M. D. L., Bruininx E. M. A. M., de Jong L., and Stein H. H.. . 2018. The contribution of digestible and metabolizable energy from high-fiber dietary ingredients is not affected by inclusion rate in mixed diets fed to growing pigs. J. Anim. Sci. 96:1860–1868. doi: 10.1093/jas/sky090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NRC 1998. Nutrient requirements of swine. 10th rev. ed. Washington (DC): The National Academic Press. [Google Scholar]
- NRC. 2012. Nutrient requirements of swine. 11th rev. ed. Washington (DC): The National Academic Press. [Google Scholar]
- Olukosi O. A., Bolarinwa O. A., Cowieson A. J., and Adeola O.. . 2012. Marker type but not concentration influenced apparent ileal amino acid digestibility in phytase-supplemented diets for broiler chickens and pigs. J. Anim. Sci. 90:4414–4420. doi: 10.2527/jas.2011-4801 [DOI] [PubMed] [Google Scholar]
- Owusu-Asiedu A., Patience J. F., Laarveld B., Van Kessel A. G., Simmins P. H., and Zijlstra R. T.. . 2006. Effects of guar gum and cellulose on digesta passage rate, ileal microbial populations, energy and protein digestibility, and performance of grower pigs. J. Anim. Sci. 84:843–852. doi: 10.2527/2006.844843x [DOI] [PubMed] [Google Scholar]
- Park C. S., Oh S. I., and Kim B. G.. . 2013. Prediction of basal endogenous losses of amino acids based on body weight and feed intake in pigs fed nitrogen-free diets. Rev. Colomb. Cienc. Pecu. 26:186–192. [Google Scholar]
- Robbins K. R., Saxton A. M., and Southern L. L.. . 2006. Estimation of nutrient requirements using broken-line regression analysis. J. Anim. Sci. 84 Suppl:E155–E165. doi: 10.2527/2006.8413_supple155x [DOI] [PubMed] [Google Scholar]
- Sales J., and Janssens. G. P. J.. 2003. Acid-insoluble ash as a marker in digestibility studies: a review. J. Anim. Feed Sci. 12:383–401. doi: 10.22358/jafs/67718/2003 [DOI] [Google Scholar]
- Stein H. H., Sève B., Fuller M. F., Moughan P. J., and de Lange C. F. M.. . 2007. Invited review: amino acid bioavailability and digestibility in pig feed ingredients: terminology and application. J. Anim. Sci. 85:172–180. doi: 10.2527/jas.2005-742 [DOI] [PubMed] [Google Scholar]
- Stein H. H., Shipley C. F., and Easter R. A.. . 1998. Technical note: a technique for inserting a T-cannula into the distal ileum of pregnant sows. J. Anim. Sci. 76:1433–1436. doi: 10.2527/1998.7651433x [DOI] [PubMed] [Google Scholar]
- Urriola P. E., and Stein H. H.. . 2010. Effects of distillers dried grains with solubles on amino acid, energy, and fiber digestibility and on hindgut fermentation of dietary fiber in a corn-soybean meal diet fed to growing pigs. J. Anim. Sci. 88:1454–1462. doi: 10.2527/jas.2009-2162 [DOI] [PubMed] [Google Scholar]
- van Keulen J., and Young B. A.. . 1977. Evaluation of acid-insoluble ash as a natural marker in ruminant digestibility studies. J. Anim. Sci. 44:282–287. doi: 10.2527/jas1977.442282x [DOI] [Google Scholar]
- van Leeuwen P., Veldman A., Boisen S., Deuring K., van Kempen G. J. M., Derksen G. B., Verstegen M. W. A., and Schaafsma. G.. 1996. Apparent ileal dry matter and crude protein digestibility of rations fed to pigs and determined with the use of chromic oxide (Cr2O3) and acid-insoluble ash as digestive markers. Br. J. Nutr. 76:551–562. doi: 10.1079/BJN19960062 [DOI] [PubMed] [Google Scholar]
- Wang T., Ragland D., and Adeola. O.. 2017. Combination of digestibility marker and fiber affect energy and nitrogen digestibility in growing pigs. Anim. Feed Sci. Technol. 230:23–29. doi: 10.1016/j.anifeedsci.2017.05.012 [DOI] [Google Scholar]