Abstract
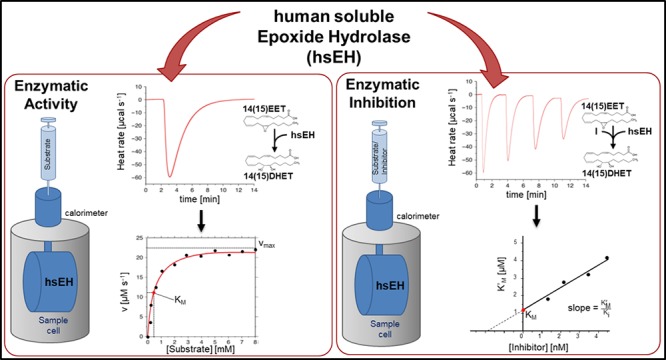
Isothermal titration calorimetry (ITC) is conventionally used to acquire thermodynamic data for biological interactions. In recent years, ITC has emerged as a powerful tool to characterize enzyme kinetics. In this study, we have adapted a single-injection method (SIM) to study the kinetics of human soluble epoxide hydrolase (hsEH), an enzyme involved in cardiovascular homeostasis, hypertension, nociception, and insulin sensitivity through the metabolism of epoxy-fatty acids (EpFAs). In the SIM method, the rate of reaction is determined by monitoring the thermal power, while the substrate is being depleted, overcoming the need for synthetic substrates and reducing postreaction processing. Our results show that ITC enables the detailed, rapid, and reproducible characterization of the hsEH-mediated hydrolysis of several natural EpFA substrates. Furthermore, we have applied a variant of the single-injection ITC method for the detailed description of enzyme inhibition, proving the power of this approach in the rapid screening and discovery of new hsEH inhibitors using the enzyme’s physiological substrates. The methods described herein will enable further studies on EpFAs’ metabolism and biology, as well as drug discovery investigations to identify and characterize hsEH inhibitors. This also promises to provide a general approach for the characterization of lipid catalysis, given the challenges that lipid metabolism studies pose to traditional spectroscopic techniques.
Human soluble epoxide hydrolase (hsEH, EC 3.3.2.10) is a bifunctional enzyme composed of two structurally and functionally independent domains.1,2 The C-terminal domain (CTD) is responsible for the hydrolysis of numerous epoxy-fatty acids (EpFAs), bioactive epoxidation products of mono- and polyunsaturated fatty acids with essential roles in cellular and organism homeostasis.2−4 hsEH CTD hydrolyzes EpFAs via an SN2 nucleophilic attack by D335 on the more accessible carbon of the epoxide ring, forming an alkyl-enzyme intermediate, which is then released by the assisted action of D496 and H524.1,2,5 The catalytic triad is located in the vertex of a large “L-shaped” active site and is surrounded by two hydrophobic surfaces dubbed the W334 niche and the F265 pocket, wherein the aliphatic chains of the EpFAs are accommodated.1,2,4−6
The best characterized EpFAs substrates of hsEH CTD are the epoxyeicosatrienoic acids (EETs), epoxy derivatives of arachidonic acid (ARA;7Figure S1A). Although four EET regioisomers, namely, 5(6)EET, 8(9)EET, 11(12)EET, and 14(15)EET, have been isolated in several organs,8 the latter two have been shown to be the predominant ARA epoxidation metabolites.9 EETs function primarily as endothelial-derived hyperpolarizing factors in the cardiovascular system and kidneys.7 They play a role in vasorelaxation and vascular homeostasis, exerting anti-inflammatory and pro-angiogenic actions.7 The bioavailability of EETs is reduced by hsEH-mediated hydrolysis of their epoxy ring to generate the corresponding vicinal diols, namely, dihydroxyeicosatrienoic acids (DHETs; Figure S1A), which possess a considerably reduced biological activity.7
In addition to EETs, hsEH hydrolyzes several bioactive epoxy derivatives of linoleic acid (LA) and α-linoleic acid (ALA), including α- and γ-epoxyoctadecadienoic acids (α/γ-EpODEs), epoxyeicosatetraenoic acids (EpETEs), epoxydocosapentaenoic acids (EpDPEs), and epoxyoctadecaenoic acids (EpOMEs;10,11Figure S1B). The physiological role of α- and γ-EpODEs is yet unknown, although their hydrolysis products, the γ-dihydroxy-octadecadienoic acids (γ-DiHODE), exhibit a moderate positive inotropic effect.12 EpETEs and EpDPEs show a similar breadth of activities to EETs.13 Vasodilation, antithrombotic, antiangiogenic, and anti-inflammatory effects have been ascribed to both EpETEs and EpDPEs, as well as diminished tumor growth and metastasis in murine models.10,14,15 Interestingly, the hsEH-mediated hydrolysis product of 19(20)EpDPE, namely, the 19(20)-dihydroxy-docosapentaenoic acid (19(20)DiHDPE), accumulates in the retinas and vitreous humor of diabetic retinopathy patients, as a result of increased expression levels of the enzyme, and aggravates disease severity by altering the localization of cholesterol-binding proteins in the cell membrane and leading to a breakdown of endothelial barrier function.16
Contrary to the largely beneficial physiological effects ascribed to other EpFAs, 9(10)- and 12(13)EpOMEs inhibit mitochondrial respiration in various tissues, leading to cardiotoxicity, renal failure, and adult respiratory distress syndrome,17,18 albeit cytotoxicity is significantly increased in their sEH-catalyzed products, the dihydroxy-octadecaenoic acids (DiHOMEs).17
Interestingly, a liquid chromatography tandem mass spectrometry (LC-MS/MS) study revealed that hsEH displays a different hydrolytic efficiency toward its various EpFA substrates.10 Although this work provided a first assessment of catalytic profiles for several epoxy fatty acids, potential drawbacks of this methodological approach include the following: (i) it is a discontinuous method, with potentially non-negligible experimental errors; (ii) it requires several sample manipulation steps that could lead to reproducibility issues; (iii) it is time-consuming, technically challenging, and expensive. Herein, we present an isothermal titration calorimetry (ITC)-based method for the systematic characterization of hsEH catalytic efficiency toward its EpFAs substrates. By measuring the intrinsic heat of hsEH-mediated hydrolysis of the epoxy-fatty acids in a continuous manner,19−23 our method circumvents the limiting issue of the lack of physicochemical properties of EpFAs substrates/products that can be monitored in real time in a continuous manner.19−23 This new ITC application shows promise in the complete and highly reproducible characterization of hsEH-mediated catalysis of epoxy-fatty acids, with relatively low sample amounts, low costs, and rapid acquisition times.
The second goal of our study was to establish an easy and versatile method to measure inhibition properties of sEH antagonists against natural substrates. Given that dihydroxy-fatty acids generated by hsEH exhibit either cytotoxic effects or reduced biological activity compared to their epoxy precursors, pharmacological inhibition of hsEH has emerged as an extremely appealing therapeutic strategy to increase EpFAs bioavailability and reap their beneficial properties.24−26 Currently, screening of hsEH inhibitors is mostly performed via a high-throughput spectrofluorimetric assay,27,28 a fast, economical and highly convenient method that nonetheless carries the main drawback of not employing physiological substrates. To address this, we have adapted an ITC method developed by Di Trani et al.21 Using a well-known hsEH antagonist as a model system, we demonstrate here that this technique holds promise for the quantitative screening of new hsEH inhibitors using endogenous EpFAs substrates.
Experimental Section
Enzyme, Substrate, and Inhibitor Sample Preparation
Recombinant hsEH CTD was obtained as described.29 This was shown to be necessary and sufficient for EpFA catalysis and to retain the same kinetic profile as full length protein.29 Protein, EpFAs substrates, and inhibitor AUDA (12-[[(tricyclo[3.3.1.13,7]dec-1-ylamino)carbonyl]amino]-dodecanoic acid) were prepared as reported in the Supporting Information.
Single-Injection ITC Kinetics Measurements
The theoretical basis of kinetic measurements by ITC has been described,19,22,30,31 and details are given in the Supporting Information. Briefly, in a single injection ITC experiment, the total heat measured is proportional to the apparent enthalpy (ΔHapp), and the number of moles of product generated. The reaction rate can be related to the amount of heat generated over time. From the derived Michaelis–Menten plots, the affinity for the substrate (KM), turnover rate (kcat), and catalytic efficiency (kcat/KM = Ksp) values can be obtained.
Experiments were performed on MicroCal PEAQ-ITC and MicroCal iTC200 calorimeters (Malvern). An hsEH CTD solution at 250 nM was placed in the sample cell, and a 0.5–1.5 mM substrate solution was loaded in the injection syringe. One single 38 μL injection was performed with a speed of 0.58–0.76 μL s–1. Controls were carried out as described in the Supporting Information. Apparent enthalpy of the reaction, heat rate (dQ/dt), and Michaelis–Menten plots were generated using MicroCal PEAQ-ITC Analysis Software (Malvern).
Progressive Inhibition ITC Enzyme Kinetics Measurements
A solution containing 0.5 mM of 14(15)EET and 67.37 nM of AUDA was injected into the cell containing 250 nM of hsEH CTD. Four 9.5 μL injections of 12.5 s each were performed at a speed of 0.76 μL s–1. Control experiments are described in the Supporting Information. Apparent reaction enthalpy, dQ/dt rates, and Michaelis–Menten parameters were generated using the MicroCal PEAQ-ITC Analysis Software (Malvern). Apparent KM values (KM′) were obtained,32 and data fitting in GraphPad provided the KM′/Ki ratio,20,21 where Ki is the inhibition constant.
Inhibitory Constant Measurements with a Spectrofluorometric Method
AUDA inhibitory potency was tested with a spectrofluorometric method,28 detailed in the Supporting Information.
Results
A Single-Injection ITC Method Characterizes the Kinetics of hsEH CTD-Mediated Hydrolysis of 14(15)EET
To probe the kinetics of EpFA hydrolysis catalyzed by hsEH CTD, we employed an ITC single-injection method (SIM).22,23 The substrate solution in the syringe of the calorimeter was injected in a single step into the sample cell containing the enzyme solution, producing a heat response which endured for as long as the reaction proceeds, returning to the baseline when the reaction reached completion and all the substrate had been transformed into product. Rapid catalyses give rise to narrow peaks, while slow catalyses generate broad peaks. Whereas the total area of the peak depends on the amount of substrate injected and the apparent enthalpy of the reaction, its shape is governed by enzyme concentration, Michaelis–Menten parameters, rate of substrate injection, and intrinsic calorimeter response.19,21
14(15)EET, the par excellence EpFA substrate of hsEH,8 was used as a test compound to demonstrate method applicability and to set up the experimental conditions for this study. Optimization included varying concentrations of substrate and enzyme, as well as reference power, injection speed, and spacing. The curve obtained by injecting 14(15)EET into a solution of hsEH CTD was negative, narrow, and deep, indicating a fast reaction (Figure 1A). The ΔHapp was calculated by integrating the area under the peak, and the Michaelis–Menten kinetics curve fitting was obtained by manually selecting the window between the end of the injection (maximum substrate concentration, corresponding to saturating enzyme conditions) and the end of the decaying portion of the injection (minimum substrate concentration, corresponding to the beginning of the kinetics curve; Figure 1B). The software fitting calculates {[S]i;vi} data points through eqs 2 and 3 (Supporting Information), building a Michaelis–Menten curve (Figure 1C), thereby providing kcat and KM values. The catalytic efficiency (Ksp) was manually calculated as the kcat/KM ratio. In the optimal conditions, these experiments gave a kcat of 6.64 ± 1.54 s–1 and a KM of 12.88 ± 1.94 μM for the hsEH-mediated hydrolysis of 14(15)EET (Table 1). To assess any product inhibition effect, the ΔHapp of two subsequent injections was compared: no significant variation was observed (Figure S2A and B), demonstrating an absence of product inhibition for this hsEH CTD-mediated hydrolysis reaction.
Figure 1.
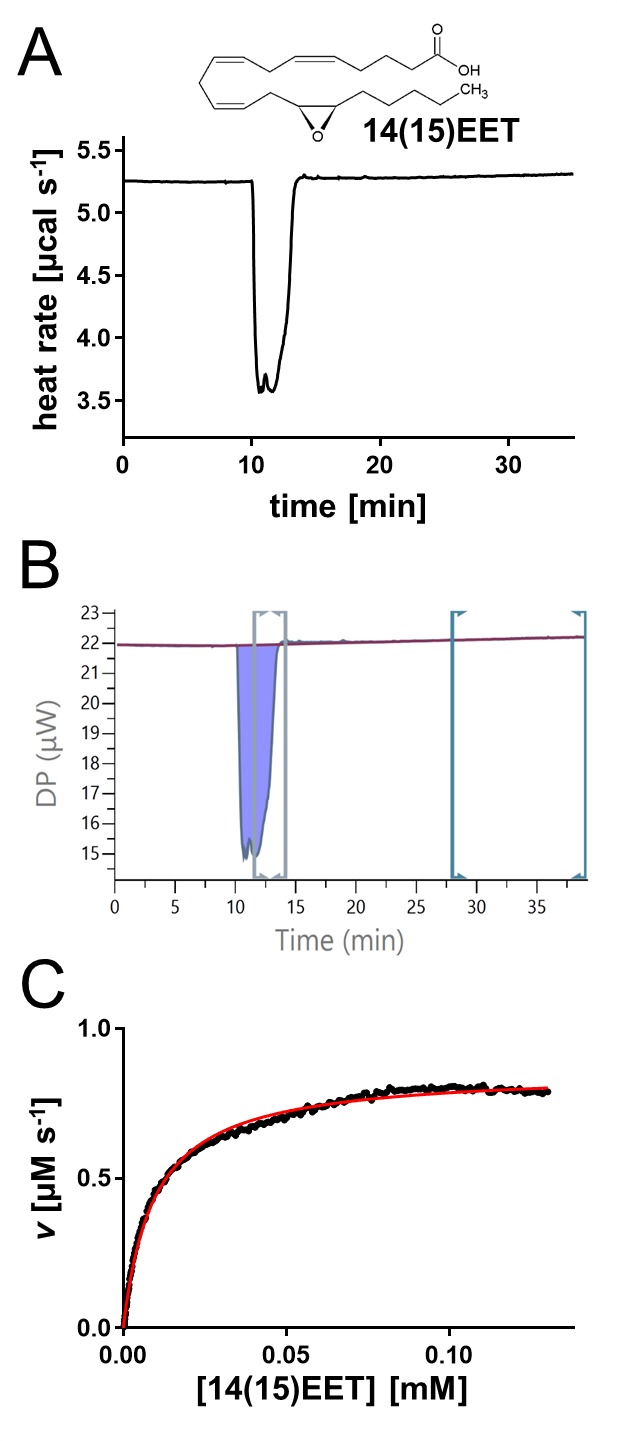
Quantitative characterization of hsEH-mediated hydrolysis of 14(15)EET. (A) Representative thermal profile of a single-injection of 14(15)EET into hsEH CTD. (B) Screenshot from the MicroCal PEAQ-ITC analysis software. The ΔHapp was calculated by integrating the area under the peak (violet), upon definition of the baseline through manual adjustments of the left markers workspace (green). The data points used for the Michaelis–Menten kinetics curve fitting were obtained by manual adjustments of the right markers workspace (gray), selecting the window between the end of the injection (maximum substrate concentration – saturating enzyme conditions) and the end of the decaying portion of the peak (minimum substrate concentration – beginning of the kinetics curve). Note that the first part of the curve corresponding to substrate injection was not included in the rate plot analysis. (C) Representative Michaelis–Menten fit of the {[S]i;vi} data point extrapolated from B using the MicroCal PEAQ-ITC analysis software analysis.
Table 1. Mean and Standard Error Values for the Apparent Enthalpy and Kinetics Parameters of hsEH CTD-Mediated Hydrolysis of the EpFAs Analyzed in This Studya.
| substrate | ΔHapp [kJ mol–1] | kcat [s–1] | KM [μM] | Ksp [s–1 μM–1] |
|---|---|---|---|---|
| 5(6)EET | –4.84 ± 0.65 | 0.35 ± 0.05 | 46.61 ± 10.98 | 0.01 ± 0.001 |
| 8(9)EET | –39.45 ± 3.88 | 1.28 ± 0.19 | 23.10 ± 2.39 | 0.054 ± 0.010 |
| 11(12)EET | –12.43 ± 0.62 | 4.31 ± 0.16 | 1.74 ± 0.20 | 2.53 ± 0.25 |
| 14(15)EET | –23.72 ± 3.46 | 6.64 ± 1.54 | 12.88 ± 1.94 | 0.527 ± 0.075 |
| 8(9)EpETE | –34.13 ± 0.93 | 1.61 ± 0.01 | 9.74 ± 0.13 | 0.173 ± 0.006 |
| 17(18)EpETE | –51.78 ± 1.73 | 0.64 ± 0.06 | 26.37 ± 4.05 | 0.025 ± 0.003 |
| 19(20)EpDPE | –23.73 ± 1.82 | 1.62 ± 0.08 | 26.60 ± 1.21 | 0.061 ± 0.003 |
| 12(13)EpOME | –5.67 ± 0.38 | 9.65 ± 0.19 | 2.98 ± 0.56 | 3.56 ± 0.86 |
The values were obtained as described in the methods.
Kinetic Characterization of hsEH CTD-Mediated Hydrolysis of All EETs
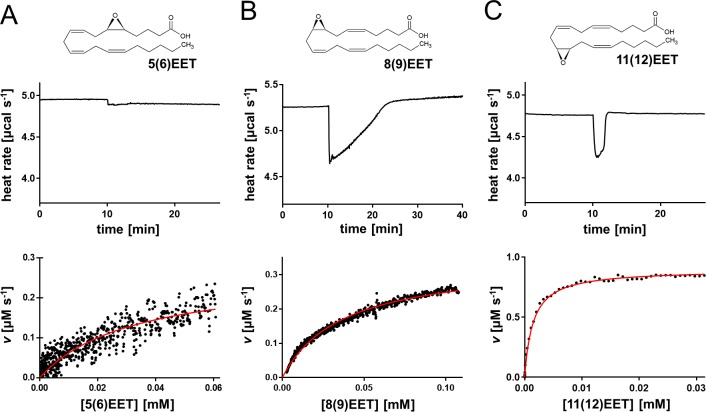
The experimental method and conditions optimized for 14(15)EET were applied to analyze the hsEH CTD-catalyzed hydrolysis of the other regioisomeric EETs. Thermal profiles and Michaelis–Menten parameters significantly differed (Table 1). The hydrolysis of 5(6)EET generated a very small and broad peak (Figure 2A), indicating a low ΔHapp. Although it was possible to extrapolate a kcat value of 0.35 ± 0.05 s–1 and a KM of 46.61 ± 10.98 μM, the error associated with this measurement was considerably greater than for the other EETs, due to the reduced magnitude of the heat response and the inability to reach enzyme saturation.23 Attempts to perform the experiments with higher substrate concentration in fact resulted in precipitation of the mixture. The experiment with 8(9)EET and 11(12)EET gave a kcat of 1.28 ± 0.19 s–1 and a KM of 23.10 ± 2.39 μM for the former and a kcat of 4.31 ± 0.16 s–1 and a KM of 1.74 ± 0.20 μM for the latter. Control experiments to check for substrate autohydrolysis and product inhibition are reported in Figures S2B and S3.
Figure 2.
Single-injection isotherms and data fitting for the hsEH-mediated hydrolysis of EETs. Experiments are shown for (A) 5(6)EET, (B) 8(9)EET, and (C) 11(12)EET. Each panel reports a representative thermal profile of a single-injection experiment (top plot) and the corresponding data fit using the Michaelis–Menten model (bottom plot). Note that the faster the reaction, the fewer data points will be available for fitting the plot.
Kinetic Characterization of hsEH CTD-Mediated Hydrolysis of n-3 and n-6 EpFAs
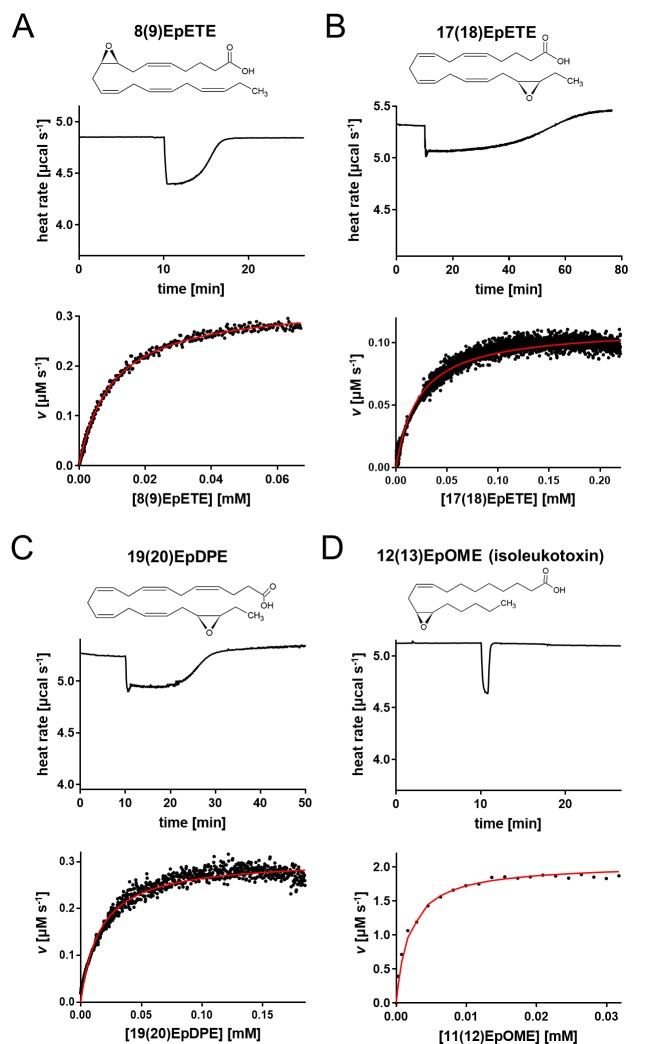
Beyond the EETs, the enzymatic activity of hsEH toward other EpFAs was measured by ITC. We selected two EpETEs, one EpDPE and one EpOME, to cover the chemical scaffold diversity of hsEH substrates. Though belonging to the same chemical class, 8(9)EpETE and 17(18)EpETE exhibited different enthalpy and kinetic values (Figure 3A and B, Table 1). The turnover rate for the 8(9)EpETE was 1.61 ± 0.01 s–1, significantly higher than for the 17(18)-regioisomer (0.64 ± 0.06 s–1), and its KM (9.74 ± 0.13 μM) was almost 3 times lower than 17(18)EpETE (26.37 ± 4.05 μM). A broad negative peak in the thermal profile was also obtained for 19(20)EpDPE, indicating a slow hsEH CTD-mediated hydrolysis (Figure 3C) described by a turnover rate of 1.62 ± 0.08 μM and a KM of 26.60 ± 1.21 μM (Table 1). The hydrolysis of 12(13)EpOME gave rise to a narrow heat flow profile (Figure 3D), similar to the ones observed for 11(12)- and 14(15)EET, with a kcat of 9.65 ± 0.19 s–1 and a KM of 2.98 ± 0.56 μM. As observed with the other substrates, negligible heat and product inhibition effects were observed in blank test injections (Figures S2B and S4).
Figure 3.
Single-injection isotherms and data fitting for the hsEH-mediated hydrolysis of EpFAs. Experiments are shown for (A) 8(9)EpETE, (B) 17(18)EpETE, (C) 19(20)EpDPE, and (D) 12(13)EpOME. Each panel reports a representative thermal profile of a single-injection experiment (top plot) and the corresponding data fit using the Michaelis–Menten model (bottom plot). Note that for faster reactions, fewer data points will be available for fitting the plot.
Characterization of hsEH CTD Inhibition Using ITC
We adapted a protocol that builds on SIM ITC enzyme kinetic measurements21 to set up a versatile and continuous method to measure the inhibitory potency of hsEH antagonists against natural substrates, as well as readily characterize their mode of inhibition. We evaluated the thermal power of 14(15)EET hydrolysis in the presence of the well-known hsEH inhibitor AUDA. The enzyme was placed in the calorimeter cell, while the syringe was loaded with a mixture of substrate and inhibitor. A series of injections was then performed (Figure 4A). In the experiment, AUDA accumulated in the sample cell with its concentration increasing 1-fold with each successive injection. This results in each injection producing a heat flow response that was progressively lower and broader than the preceding one, owing to increased hsEH CTD inhibition. Blank test injections showed little heat effects of substrate/inhibitor dilution into the buffer (Figure S5A). Each peak injection was analyzed individually to measure the ΔHapp and extract the Michaelis–Menten parameters. The kcat values derived from each peak were identical (8.89 ± 1.64 s–1) and in agreement with the value detected in the absence of inhibitor (Table 1). The apparent KM (KM′) for 14(15)EET increased at every successive injection with growing inhibitor concentration (Figure 4B). The kcat and KM′ values obtained by this analysis indicate a model of competitive inhibition,32 which is consistent with AUDA’s reported mode of action.26,33 As AUDA is a competitive inhibitor, the y-intercept of the KM′ vs AUDA concentration plot (Figure 4B) provides the true KM,32 measured as 11.91 ± 3.43 μM, in concurrence with the value for the 14(15)EET substrate obtained in the absence of inhibitor (Table 1). The analysis of the slope of the straight line fitted to the data points gives the KM′/Ki ratio,32 providing an average Ki for AUDA of 7.62 ± 2.81 nM. This is in excellent agreement with the value obtained from a spectrofluorimetric assay using the synthetic substrate PHOME28,29,34 (Figure S6A and B).
Figure 4.
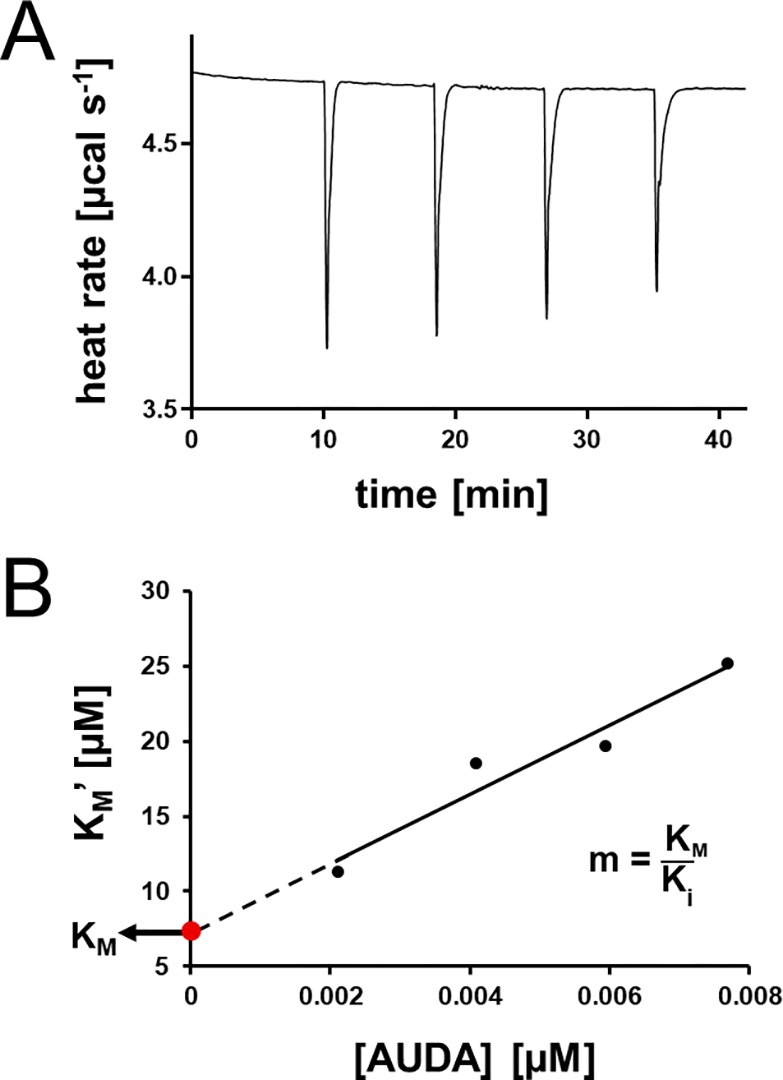
hsEH CTD inhibition studies. (A) Representative thermal power of 14(15)EET/AUDA injections into hsEH CTD. The magnitude of the peaks decreases with each successive injection due to the increased concentration of the inhibitor, while the slope of the recovery increased, suggesting a competitive mode of inhibition. (B) Linear KM′ vs AUDA concentration fitting from data shown in A.
Discussion
The study of EpFA catalysis mediated by hsEH is severely limited by the unavailability of fast, simple, and effective methods to study their kinetics of hydrolysis. The only technique available thus far is a LC-MS/MS-based methodology,10 which, although highly sensitive, is a postreaction ancillary technique, involving multiple steps of sample manipulation and analysis,32 and requiring highly specialized equipment and technical expertise. To develop a truly general, versatile, and rapid enzyme kinetics assay, we have developed a single-injection ITC (SIM) approach. By detecting the heat released or absorbed in real time during catalysis, this technique follows reactions of native substrates without the need of detectable changes in physicochemical properties to track the concentration of substrate or product over time. The single-injection ITC method yields both thermodynamic and kinetic parameters in a single experiment. Recent advances in instrumentation, faster response times, and analysis software (e.g. the new PEAQ-ITC analysis software) have allowed this approach to become more user-friendly and routine. Compared to the multi-injection ITC method counterpart, the SIM is significantly faster, requires less enzyme, and is less subject to errors linked to baseline drift, or other time-dependent effects, including enzyme aggregation and/or precipitation and substrate degradation,19 because of the reduced experimental time and faster data acquisition.19,30
The ITC single-injection application presented here enables the rapid characterization of kinetic parameters of various natural EpFA substrates of hsEH, such as EETs, EpETEs, EpDPEs, and EpOMEs, using small amounts of protein and substrate, with each experiment taking on an average 50 min (including washing and preparation of the instrument). To our knowledge, this is the first example of the ITC SIM applied to lipid catalysis, and it is the only method to date that allows a quantitative analysis of hsEH-mediated hydrolysis of EpFAs in a continuous manner.
Our investigations revealed that all EpFAs tested could be hydrolyzed by hsEH, albeit significant differences were observed in their kinetics profiles, in agreement with a previous study.10 It is noteworthy that although data fitted well to the Michaelis–Menten model, hsEH CTD performs a two-step catalysis, undergoing first the formation of a covalent enzyme–substrate alkyl intermediate followed by its hydrolysis.4,36 In these cases, KM values describe the concentration of the substrate for which the catalytic rate is half maximal, instead of providing an accurate measure of the substrate affinity for the enzyme,37 and kcat values represent mainly the hydrolysis rate of the covalent intermediate, given that this second step of the hsEH CTD-mediated catalysis is at least an order of magnitude slower than the first (formation of the alkyl covalent intermediate).4,36
Our results (Table 1) indicate that KM and kcat values vary significantly depending on the chemical scaffold and the epoxide position on the fatty acid structure. Whereas variations of KM values could not easily be correlated with substrate properties in this study, in general kcat values were the largest when the epoxide was central, on the carbon positions 11, 12, and 14 of the fatty acid chain. Comparison of catalytic efficiency (Ksp), a measurement of the overall rate of the reaction and specificity of an enzyme for a substrate,37 revealed the preferred EpFA targets for hsEH CTD, summarized in a heatmap (Figure 5). For the substrates tested in our study, the rank order of hsEH CTD catalytic preference is 12(13)EpOME > 11(12)EET > 14(15)EET > 8(9)EpETE > 19(20)EpDPE ≈ 8(9)EET > 17(18)EpETE > 5(6)EET. A trend is revealed here, correlating catalytic efficiency with the position of the epoxide function: hsEH CTD distinctly prefers epoxides located in the middle of the fatty acid chain, at positions 11, 12, and 14, with its activity steadily decreasing for substrates bearing the epoxide moiety closer to either the carboxyl acid group or to the methyl group at the end of the hydrocarbon chain. Having the epoxide close to the carboxyl function is the least preferred configuration, as indicated by the lowest efficiency for 5(6)EET. This appears consistent with the narrow “L-shaped” hsEH CTD active site, with the catalytic triad positioned deep in the vertex and surrounded by two large hydrophobic regions.1,6 Notably, the overall substrate preference for sEH revealed by ITC is largely in agreement with what was previously observed in Morisseau et al.10 by LC-MS/MS, although it is noteworthy that the discrete Michaelis–Menten parameters do differ in the two studies (especially kcat,).
Figure 5.
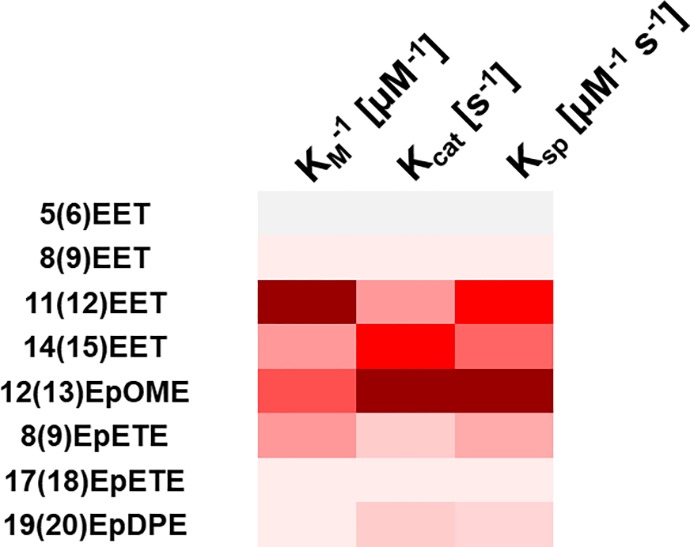
Heatmap of the kinetics parameters of the hsEH CTD-mediated hydrolysis of EpFAs showing Ksp inverse of KM (KM–1 [μM–1]) and kcat. Colors span from gray to dark red with increasing values. Each shade of gray-to-red indicates an increase of the 12.5th percentile of the total interval of values. A combination of dark red shades indicates high affinity, turnover rate, and efficiency.
Given that hsEH inhibition is a potential therapeutic approach in a number of pathological conditions, detailed knowledge of hsEH catalytic efficiency toward its various substrates is of great importance, as it will inform on how such an inhibition will affect the metabolism of several EpFAs, enabling the prediction of the expected outcome from their altered levels. As the relative abundance of each epoxy-fatty acid varies within the organism,10,13 a pharmacological intervention may have simultaneous assorted responses in different tissues and organs.
In addition to measuring the kinetics of catalysis for different EpFA targets of hsEH, we also devised an ITC method to screen new hsEH inhibitors using natural substrates. Our ITC application brings significant advantages. First, it evaluates inhibitor potency using hsEH physiological substrates, circumventing the problems associated with employing non-native fluorogenic compounds. This will have a particular bearing for the analysis of noncompetitive and mixed inhibitors, which bind respectively to the enzyme–substrate complex or to both the enzyme and substrate.32 Interestingly, new allosteric inhibitors of hsEH have started to emerge,34 increasing the timely relevance of this new methodology. Furthermore, as the efficacy of noncompetitive and mixed inhibitors may in principle differ from substrate to substrate, our ITC method offers a well-suited solution for a systematic characterization of inhibition versus a battery of EpFA substrates. As a second major benefit, the ITC method allows for a straightforward characterization of the mode of inhibition (i.e., competitive, uncompetitive or noncompetitive/mixed),32 which is critical to drug development as evaluating the inhibitory power, given that it reveals the nature of the inhibited state(s).
Conclusions
Despite the crucial biological roles of hsEH in a variety of physiological and pathological states, a comprehensive understanding of its catalytic activity against a compendium of natural substrates remains inadequate. Equally, the availability of assays that characterize and screen hsEH inhibitors using native substrates has been limited to date. We have presented a novel, versatile, expedient, and reliable ITC SIM application that has the potential to be adopted as the method of choice to perform such characterizations. Being not reliant on specific physicochemical and spectroscopic properties, our method is ideally posed to facilitate the discovery of new putative epoxy substrates of hsEH. Moreover, calorimetric measurements can be performed in mixtures and suspensions, e.g., in cellular and crude tissue extracts, as well as allowing measurements over a range of biologically relevant conditions19,38 (pH, redox conditions, salt concentration etc.), thereby having the potential to contribute to advances of hsEH biology in health and disease. As recent discoveries suggest a role for hsEH in redox regulatory systems,39,40,34 our newly developed ITC method can be used to assess the impact of the enzyme oxidative state on both catalysis and inhibition of EpFAs hydrolysis.
Taken together, our results show the first proof-of-concept for kinetic characterization and inhibitor screening of hsEH activities using ITC, an approach which is generally applicable to other enzymes involved in lipid metabolism, and should help in the search for novel inhibitors of this important class of enzymes.
Acknowledgments
G.A. was supported by a BHF interdisciplinary Ph.D. studentship and a pump priming award from the BHF Centre of Excellence, King’s College London. G.A. and M.R.C. thank Malvern Panalytical Ltd., particularly Maria Walton for the logistic support and Peter Gimeson for the help with the experimental setting and data analysis. The authors thank the Centre for Biomolecular Spectroscopy funded by the Wellcome Trust and British Heart Foundation (ref 202767/Z/16/Z and IG/16/2/32273, respectively).
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.analchem.9b01847.
Seven figures and one table reporting a schematic of the EpFAs metabolism, all the experimental controls, and the spectrofluorimetric analysis of hsEH CTD inhibition as well as full experimental procedures and protocols, as well as a full mathematical treatment of the theoretical basis of kinetic rates determination by ITC (PDF)
Author Present Address
∥ Division of Bioscience, Institute of Structural and Molecular Biology, University College London, London, WC1E 6BT, UK
Author Contributions
M.R.C., G.A., and R.P.-G. contributed to the planning and the design of the study. G.A. prepared the recombinant enzyme used in all the experiments and performed the spectrofluorometric analysis. G.A., R.P.-G., and M.R.C. optimized the experimental setup for the ITC kinetics analysis. G.A. and T.T.T.B. performed the ITC experiments for both kinetics and inhibition studies. M.R.C., G.A., and R.P.-G. participated in the data analysis and contributed to the writing of the manuscript. M.R.C. obtained funding for this work.
The authors declare no competing financial interest.
Supplementary Material
References
- Argiriadi M.; Morisseau C.; Hammock B. D.; Christianson D. W. Detoxification of environmental mutagens and carcinogens: structure, mechanism, and evolution of liver epoxide hydrolase. Proc. Natl. Acad. Sci. U. S. A. 1999, 96, 10637–10642. 10.1073/pnas.96.19.10637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez G. A.; Morisseau C.; Hammock B. D.; Christianson D. W. Biochemistry 2004, 43, 4716–4723. 10.1021/bi036189j. [DOI] [PubMed] [Google Scholar]
- Borhan B.; Jones D. A.; Pinot F.; Grant D. F.; Kurth M. J.; Hammock B. D. Mechanism of Soluble Epoxide Hydrolase. J. Biol. Chem. 1995, 270 (45), 26923–26930. 10.1074/jbc.270.45.26923. [DOI] [PubMed] [Google Scholar]
- Morisseau C.; Hammock B. D. Epoxide Hydrolases: mechanisms, inhibitor designs, and biological roles. Annu. Rev. Pharmacol. Toxicol. 2005, 45 (1), 311–333. 10.1146/annurev.pharmtox.45.120403.095920. [DOI] [PubMed] [Google Scholar]
- Argiriadi M. A.; Morisseau C.; Goodrow M. H.; Dowdy D. L.; Hammock B. D.; Christianson D. W. Binding of alkylurea inhibitors to epoxide hydrolase implicates active site tyrosines in substrate activation. J. Biol. Chem. 2000, 275 (20), 15265–15270. 10.1074/jbc.M000278200. [DOI] [PubMed] [Google Scholar]
- Gomez G. A.; Morisseau C.; Hammock B. D.; Christianson D. W. Human soluble epoxide hydrolase: Structural basis of inhibition by 4-(3-cyclohexylureido)-carboxylic acids. Protein Sci. 2006, 15, 58–64. 10.1110/ps.051720206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imig J. D. Epoxides and soluble Epoxide Hydrolase in cardiovascular physiology. Physiol. Rev. 2012, 92 (1), 101–130. 10.1152/physrev.00021.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector A. A.; Fang X.; Snyder G. D.; Weintraub N. L. Epoxyeicosatrienoic acids (EETs): Metabolism and biochemical function. Prog. Lipid Res. 2004, 43 (1), 55–90. 10.1016/S0163-7827(03)00049-3. [DOI] [PubMed] [Google Scholar]
- Capdevila J. H.; Falck J. R.; Harris R. C. Cytochrome P450 and arachidonic acid bioactivation. Molecular and functional properties of the arachidonate monooxygenase. J. Lipid Res. 2000, 41, 163–181. [PubMed] [Google Scholar]
- Morisseau C.; Inceoglu B.; Schmelzer K.; Tsai H.-J.; Jinks S. L.; Hegedus C. M.; Hammock B. D. Naturally occurring monoepoxides of eicosapentaenoic acid and docosahexaenoic acid are bioactive antihyperalgesic lipids. J. Lipid Res. 2010, 51 (12), 3481–3490. 10.1194/jlr.M006007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbs M.; Leng S.; Devassy J. G.; Monirujjaman M.; Aukema H. M. Advances in Our Understanding of Oxylipins Derived from Dietary PUFAs. Adv. Nutr. 2015, 6 (5), 513–540. 10.3945/an.114.007732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell L. A.; Grant D. F.; Melchert R. B.; Petty N. M.; Kennedy R. H. Linoleic acid metabolites act to increase contractility in isolated rat heart. Cardiovasc. Toxicol. 2002, 2 (3), 219–229. 10.1385/CT:2:3:219. [DOI] [PubMed] [Google Scholar]
- Gabbs M.; Leng S.; Devassy J. G.; Monirujjaman M.; Aukema H. M. Advances in Our Understanding of Oxylipins Derived from Dietary PUFAs. Adv. Nutr. 2015, 6 (5), 513–540. 10.3945/an.114.007732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G.; Panigrahy D.; Mahakian L. M.; Yang J.; Liu J.-Y.; Lee K. S. S.; Wettersten H. I.; Ulu A.; Hu X.; Tam S.; Hwang S. H.; Ingham E. S.; Kieran M. W.; Weiss R. H.; Ferrara K. W.; Hammock B. D. Epoxy metabolites of docosahexaenoic acid (DHA) inhibit angiogenesis, tumor growth, and metastasis. Proc. Natl. Acad. Sci. U. S. A. 2013, 110 (16), 6530–6535. 10.1073/pnas.1304321110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulu A.; Harris T. R.; Morisseau C.; Miyabe C.; Inoue H.; Schuster G.; Dong H.; Iosif A.-M.; Liu J.-Y.; Weiss R. H.; Chiamvimonvat N.; Imig J. D.; Hammock B. D. Anti-inflammatory effects of ω-3 polyunsaturated fatty acids and soluble epoxide hydrolase inhibitors in angiotensin-II-dependent hypertension. J. Cardiovasc. Pharmacol. 2013, 62 (3), 285–297. 10.1097/FJC.0b013e318298e460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J.; Dziumbla S.; Lin J.; Bibli S.; Zukunft S.; de Mos J.; Awwad K.; Frömel T.; Jungmann A.; Devraj K.; Cheng Z.; Wang L.; Fauser S.; Eberhart C. G.; Sodhi A.; Hammock B. D.; Liebner S.; Müller O. J.; Glaubitz C.; Hammes H. P.; Popp R.; Fleming I. Inhibition of soluble epoxide hydrolase prevents diabetic retinopathy. Nature 2017, 552, 248–252. 10.1038/nature25013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran J. H.; Weise R.; Schnellmann R. G.; Freeman J. P.; Grant D. F. Cytotoxicity of linoleic acid diols to renal proximal tubular cells. Toxicol. Appl. Pharmacol. 1997, 146 (1), 53–59. 10.1006/taap.1997.8197. [DOI] [PubMed] [Google Scholar]
- El-Sherbeni A. A.; El-Kadi A. O. S. The role of epoxide hydrolases in health and disease. Arch. Toxicol. 2014, 88 (11), 2013–2032. 10.1007/s00204-014-1371-y. [DOI] [PubMed] [Google Scholar]
- Mazzei L.; Ciurli S.; Zambelli B.. Isothermal Titration Calorimetry to Characterize Enzymatic Reactions; Elsevier Inc., 2016; vol. 1, p 567, 10.1016/bs.mie.2015.07.022. [DOI] [PubMed] [Google Scholar]
- Di Trani J. M.; Moitessier N.; Mittermaier A. K. Measuring Rapid Time-Scale Reaction Kinetics Using Isothermal Titration Calorimetry. Anal. Chem. 2017, 89 (13), 7022–7030. 10.1021/acs.analchem.7b00693. [DOI] [PubMed] [Google Scholar]
- Di Trani J. M.; Moitessier N.; Mittermaier A. K. Complete Kinetic Characterization of Enzyme Inhibition in a Single Isothermal Titration Calorimetric Experiment. Anal. Chem. 2018, 90 (14), 8430–8435. 10.1021/acs.analchem.8b00993. [DOI] [PubMed] [Google Scholar]
- Todd M. J.; Gomez J. Enzyme kinetics determined using calorimetry: a general assay for enzyme activity?. Anal. Biochem. 2001, 296 (2), 179–187. 10.1006/abio.2001.5218. [DOI] [PubMed] [Google Scholar]
- Transtrum M. K.; Hansen L. D.; Quinn C. Enzyme kinetics determined by single-injection isothermal titration calorimetry. Methods 2015, 76, 194–200. 10.1016/j.ymeth.2014.12.003. [DOI] [PubMed] [Google Scholar]
- Wagner K. M.; McReynolds C. B.; Schmidt W. K.; Hammock B. D. Soluble epoxide hydrolase as a therapeutic target for pain, inflammatory and neurodegenerative diseases. Pharmacol. Ther. 2017, 180, 62–76. 10.1016/j.pharmthera.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imig J.; Hammock B. Soluble epoxide hydrolase as a therapeutic target for cardiovascular diseases. Nat. Rev. Drug Discovery 2009, 8 (10), 794–805. 10.1038/nrd2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H. C.; Hammock B. D. Discovery of inhibitors of soluble Epoxide Hydrolase: A target with multiple potential therapeutic indications. J. Med. Chem. 2012, 55 (5), 1789–1808. 10.1021/jm201468j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisseau C.; Hammock B. D.. Measurement of soluble epoxide hydrolase (sEH) activity. Current Protocols in Toxicology; Wiley & Sons, Inc.: Hoboken, NJ, 2007; Chapter 4, pp 4.23.1–4.23.18, 10.1002/0471140856.tx0423s33 [DOI] [PubMed] [Google Scholar]
- Wolf N. M.; Morisseau C.; Jones P. D.; Hock B.; Hammock B. D. Development of a high-throughput screen for soluble epoxide hydrolase inhibition. Anal. Biochem. 2006, 355, 71–80. 10.1016/j.ab.2006.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abis G.; Charles R. L.; Eaton P.; Conte M. R. Expression, purification, and characterisation of human soluble Epoxide Hydrolase (hsEH) and of its functional C-terminal domain. Protein Expression Purif. 2019, 153, 105–113. 10.1016/j.pep.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen L. D.; Transtrum M. K.; Quinn C.; Demarse N. Enzyme-catalyzed and binding reaction kinetics determined by titration calorimetry. Biochim. Biophys. Acta, Gen. Subj. 2016, 1860 (5), 957–966. 10.1016/j.bbagen.2015.12.018. [DOI] [PubMed] [Google Scholar]
- Luo Q.; Chen D.; Boom R. M.; Janssen A. E. M. Revisiting the enzymatic kinetics of pepsin using isothermal titration calorimetry. Food Chem. 2018, 268 (June), 94–100. 10.1016/j.foodchem.2018.06.042. [DOI] [PubMed] [Google Scholar]
- Copeland R. A.Evaluation of Enzyme Inhibitors in Drug Discovery; Wiley, 2005. [PubMed] [Google Scholar]
- Kim I.; Morisseau C.; Watanabe T.; Hammock B. D. Design, synthesis, and biological activity of 1,3-disubstituted ureas as potent inhibitors of the soluble epoxide hydrolase of increased water solubility Solubility. J. Med. Chem. 2004, 47 (8), 2110–2122. 10.1021/jm030514j. [DOI] [PubMed] [Google Scholar]
- Abis G.; Charles R. L.; Kopec J.; Yue W. W.; Atkinson R. A.; Bui T. T. T.; Lynham S.; Popova S.; Sun Y.-B.; Fraternali F.; Eaton P.; Conte M. R. 15-deoxy-Δ12,14-Prostaglandin J2-mediated inhibition reveals two allosteric sites of the human soluble Epoxide Hydrolase. Commun. Biol. 2019, 2, 188. 10.1038/s42003-019-0426-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopmann K. H.; Himo F. Insights into the reaction mechanism of soluble epoxide hydrolase from theoretical active site mutants. J. Phys. Chem. B 2006, 110 (42), 21299–21310. 10.1021/jp063830t. [DOI] [PubMed] [Google Scholar]
- Price N. C.; Stevens L.. Fundamentals of Enzymology; Oxford University Press, 1999. [Google Scholar]
- Freyer M. W.; Lewis E. A. Isothermal Titration Calorimetry: Experimental Design, Data Analysis, and Probing Macromolecule/Ligand Binding and Kinetic Interactions. Methods Cell Biol. 2008, 84, 79–113. 10.1016/S0091-679X(07)84004-0. [DOI] [PubMed] [Google Scholar]
- Charles R. L.; Burgoyne J. R.; Mayr M.; Weldon S. M.; Hubner N.; Dong H.; Morisseau C.; Hammock B. D.; Landar A.; Eaton P. Redox regulation of soluble epoxide hydrolase by 15-Deoxy-delta-Prostaglandin J2 controls coronary hypoxic vasodilation. Circ. Res. 2011, 108 (3), 324–334. 10.1161/CIRCRESAHA.110.235879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles R. L.; Rudyk O.; Prysyazhna O.; Kamynina A.; Yang J.; Morisseau C.; Hammock B. D.; Freeman B. a; Eaton P. Protection from hypertension in mice by the Mediterranean diet is mediated by nitro fatty acid inhibition of soluble epoxide hydrolase. Proc. Natl. Acad. Sci. U. S. A. 2014, 111 (22), 8167–8172. 10.1073/pnas.1402965111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.