Abstract
Deficiency in retinoid acid receptor-related orphan receptor alpha (RORα) of staggerer mice results in extensive granule and Purkinje cell loss in the cerebellum as well as in learned motor deficits, cognition impairments and perseverative tendencies that are commonly observed in autistic spectrum disorder (ASD). The effects of RORα on brain lipid metabolism associated with cerebellar atrophy remain unexplored. The aim of this study is to examine the effects of RORα deficiency on brain phospholipid fatty acid concentrations and compositions. Staggerer mice (Rorasg/sg) and wildtype littermates (Rora+/+) were fed n-3 polyunsaturated fatty acids (PUFA) containing diets ad libitum. At 2 months and 7 or more months old, brain total phospholipids fatty acids were quantified by gas chromatography-flame ionization detection. In the cerebellum, all fatty acid concentrations were reduced in 2 months old mice. Since total fatty acid concentrations were significantly different at 2-month-old, we examined changes in fatty acid composition. The composition of ARA was not significantly different between genotypes; though DHA composition remained significantly lowered. Despite cerebellar atrophy at >7-months-old, cerebellar fatty acid concentrations had recovered comparably to wildtype control. Therefore, RORα may be necessary for fatty acid accretions during neurodevelopment. Specifically, the effects of RORα on PUFA metabolisms are region-specific and age-dependent.
Keywords: Cerebellum, Nuclear Receptor, Autistic Disorder, Phospholipids, Development
Introduction
Staggerer mice were first reported in 1962 as spontaneous mutant offspring of stock obese mice with ataxic gait, hypotonia, body imbalance and tremor associated with predominant cerebellar cortex degeneration [1]. The staggerer cerebellum exhibit folia with small fissures, indistinct lamination of cerebellar cortex, thin molecular layer and absence of granular layer [1–3]. Developmental atrophy of staggerer cerebellum begins at birth with accelerated degeneration of Purkinje and granule cells [4, 5]. Purkinje cells of staggerer cerebellum are small, scarce, and devoid of dendritic branchlet spines which contribute to a histologically ectopic scatter pattern as compared to a monolayer phenotype [1, 6, 7]. Staggerer mutation impairs early differentiation, maturation and survival of Purkinje cells, but does not affect neurogenesis [4, 8–12]. In contrast to earlier rates of Purkinje cell loss in developing cerebellum of 3-month-old male heterozygous staggerer mouse as compared to female [8], sex effects on Purkinje cell size and loss were not observed in homozygous staggerer mouse [13]. The intrinsic defect of staggerer Purkinje cell leads to deficits in innervation with granule cells; thereby reduces the rate of granule cell precursor proliferation leading to increased apoptosis and the consequent absence of granular layer [2, 3, 6, 14]. Similarly, in hippocampal dentate gyrus, it was reported that staggerer mutation affects neuron differentiation and maturation but not neurogenesis [15].
Staggerer mutation was traced by positional cloning to an intragenic deletion of retinoic acid-related orphan nuclear receptor alpha, RORα [16]. Comparative analyses had demonstrated similar abnormal cerebellar atrophy and Purkinje cell cytology between staggerer mutant mice and RORα knock-out mice; further providing evidence that loss of RORα function is the cause of staggerer characteristics [13, 17]. RORα expressions in murine and human tissues are ubiquitous [16, 18, 19]. In the brain, RORα expression is highest in cerebellum and thalamus followed by moderate-to-low expressions in olfactory bulbs and cortex, and undetectable expressions in striatum and hippocampus [15, 20, 21]. Specifically in the cerebellum, RORα is highly expressed in Purkinje cells followed by weaker expression in stellate and basket cells [16, 22, 23]; while granule cells do not express RORα [22]. In addition to neuronal expression of RORα, glial expression of RORα is restricted to astrocytes [24]. Genetic ablation of neuronal and astrocytic RORα expression induced cell-autonomous and non-cell-autonomous regression of dendritic arbor and loss of branchlet spines and functional synapses; thereby leading to impairment of motor coordination [25–27]. In contrast to Purkinje cells, RORα-expressing stellate and basket cells are unaffected by RORα deficiency [22]. RORα-expressing thalamic neuronal cells and retinal ganglion cells are also unaffected in staggerer mutants [22]. In olfactory bulbs, staggerer mutation reduces the number of neurons and interneurons in mitral cells as well as the astrocyte network in granular layer [28]. Therefore, degeneration of RORα-expressing cells are region-dependent and cell-restrictive.
RORα is implicated in neuropsychiatric disorders including autism spectrum disorders (ASD), bipolar disorder (BD) and major depressive disorder (MDD) [29–40]. Specifically, neuroanatomical abnormalities in patients with autism including loss of Purkinje cells, reduced cerebellar volume and interrupted cerebello-thalamo-cortical pathways resembles that of staggerer mice [41]. RORα expression and protein are reduced in lymphoblastoid cell lines and postmortem cerebellum of autistic patients, respectively [29, 42]. Furthermore, RORα transcriptionally regulates neuronal genes associated with ASD [30]. Even though the canonical functions of the cerebellum consist of balance control and motor coordination, emerging evidence supports additional cerebellar roles in emotional processing and cognition [43–47]. In addition to pronounced impairment in gait and balance, staggerer mice exhibits significant deficits in learned motor tasks [48]. Staggerer mice also exhibit deficiencies in cognitive behaviors. Staggerer mice commit significantly more errors in exploration and spatial learning tasks [49, 50]. While passive avoidance learning is unaffected in staggerer mice, deficits in active avoidance learning due to impairments in reversal training may explain the perseverative tendencies in staggerer mice [49, 51]. Therefore, staggerer may be a novel functionally relevant model of ASD.
Polyunsaturated fatty acids (PUFA), including omega-6 (n-6) and omega-3 (n-3) fatty acids, are critical for early brain development [52–55]. Particularly, the predominant species, arachidonic acid (ARA; n-6 PUFA) and docosahexaenoic acid (DHA; n-3 PUFA) [56, 57], are enriched for neuronal growth, synaptogenesis, neuronal survival and modulation of neurotransmitters [58–60]. Even though abnormal lipid metabolism in human autistic brains has not been investigated, emerging evidence has demonstrated abnormal lipid metabolism in plasma biomarkers [61, 62]. Nevertheless, PUFA interventions and modulations in brain phospholipid fatty acid composition may alleviate autistic-like cognitive and social behaviors [63–65]. Interestingly, staggerer mice exhibit aberrant lipid metabolism. Staggerer serum cholesterol and triglycerides are reduced due to lower expressions of apolipoprotein A-I and C-III, respectively [66, 67]. Moreover, RORα mediates the regulation of lipoprotein homeostasis [68]. Hepatic expressions of peroxisome proliferator-activated receptor-γ, coactivator 1 (PGC-1α) and lipin1 are increased in staggerer mice which suggests that RORα may regulate mitochondrial fatty acid oxidation [69]. RORα is essential in repressing proliferators-activated receptor-γ (PPARγ)-mediated lipogenic gene expressions in liver; thereby repressing diet-induced hepatic steatosis [70]. However, the effects of RORα deficiency on brain lipid metabolism are unknown. The aim of this study is to examine the effects of RORα deficiency on brain phospholipid fatty acid concentrations and compositions.
Methods
Animals and Diet
All procedures were approved and performed in accordance with the policies set by the Institutional Animal Care and Use Committee at the Boston Children’s Hospital and National Institute on Alcohol Abuse and Alcoholism (NIAAA). Rora heterozygous staggerer (Rora+/sg) mice were obtained from The Jackson Laboratory (stock number: 002651; Bar Harbor, ME, USA) and bred to generate homozygous RORα-deficient staggerer (Rorasg/sg) and wildtype littermates. No exclusion criteria were pre-determined. Mice were housed in pie-shaped cages with 2–5 companions. Wildtype mice were heavier in weight as compared to Rorasg/sg mice (2-month-old wildtype = 25g; 2-month-old Rorasg/sg = 17g; >7-month-old wildtype = 31; >7-month-old Rorasg/sg = 22g). Mice had ad libitum access to custom-designed diet (Diet 1: no EPA and DHA) from Dyets Inc. (Bethlehem, PA, USA) or Prolab Isopro RMH 3000 chow (Diet 2: with EPA and DHA) from LabDiet (St. Louis, MO) and water. The fat compositions of diet 1 and diet 2 were 16.5% and 14.3% (g of fat/kg of diet), respectively (Table 1). To investigate the effects of RORα mutation and sex on cerebellar phospholipid fatty acid concentrations and composition, a total of 23 three-month-old mice on diet 1 were selected for analysis (Male wildtype = 5; male Rorasg/sg = 7; female wildtype = 5; female Rorasg/sg = 5). To investigate the effects of age and brain region on RORα-mediated changes in brain phospholipid fatty acid concentration and composition, a total of 23 mice on diet 2 were selected for analysis (2-month-old wildtype = 6; 2-month-old Rorasg/sg = 7; >7-month-old wildtype = 5; >7-month-old Rorasg/sg = 5). On the days of euthanization, mice were anesthetized with intraperitoneal injection of ketamine/xylazine (100–120 mg/kg ketamine and 10 mg/kg xylazine) followed by cervical dislocation. Brains from both sexes were collected for primary endpoint of total phospholipids fatty acid analysis. Cerebellum of 3-month-old Rorasg/sg and wildtype littermate were collected; while 2-month-old and >7-month-old Rorasg/sg and wildtype littermate brains were dissected into prefrontal cortex, cortex, striatum, hippocampus and cerebellum. Serum was collected from 2-month-old mice.
Table 1.
Dietary fatty acid compositions (mean ± SD; n = 5)
| mg FA/g diet (mol% diet composition) |
||
|---|---|---|
| FA | Diet 1: No EPA and DHA | Diet 2: With EPA and DHA |
| 10:0 | 3 (2.5) ± 0.6 (0.1) | 0 (0) ± 0 (0) |
| 12:0 | 25 (21) ± 4.7 (0.3) | 0 (0) ± 0 (0) |
| 14:0 | 10 (8.4) ± 1.8 (0.08) | 0.73 (1.2) ± 0.14 (0.02) |
| 16:0 | 8.8 (7.4) ± 1.6 (0.06) | 11 (18) ± 0.11 (0.08) |
| 18:0 | 8.3 (6.9) ± 1.5 (0.1) | 4.4 (7.1) ± 0.048 (0.45) |
| 20:0 | 0.31 (0.26) ± 0.06 (0.005) | 0.19 (0.30) ± 0.006 (0.009) |
| 22:0 | 0.40 (0.33) ± 0.07 (0.007) | 0.13 (0.21) ± 0.004 (0.005) |
| 24:0 | 0.19 (0.16) ± 0.03 (0.006) | 0.10 (0.16) ± 0.006 (0.01) |
| 16:1n-7 | 0.062 (0.1) ± 0.009 (0.004) | 0.88 (1.4) ± 0.13 (0.016) |
| 18:1n-9 | 34 (28) ± 6.3 (0.3) | 18 (29) ± 0.13 (0.2) |
| 18:1n-7 | 0.51 (0.4) ± 0.08 (0.02) | 1.10 (1.8) ± 0.019 (0.025) |
| 20:1n-9 | 0.16 (0.13) ± 0.03 (0.003) | 0.32 (0.51) ± 0.007 (0.013) |
| 22:1n-9 | 0 (0) ± 0 (0) | 0.023 (0.04) ± 0.004 (0.007) |
| 24:1n-9 | 0.064 (0.05) ± 0.01 (0.003) | 0.06 (0.10) ± 0.004 (0.006) |
| 18:2n-6 | 28 (23) ± 5.1 (0.2) | 21 (33) ± 0.17 (0.31) |
| 18:3n-6 | 0 (0) ± 0 (0) | 0 (0) ± 0 (0) |
| 20:2n-6 | 0.015 (0.01) ± 0.001 (0.002) | 0.18 (0.28) ± 0.004 (0.006) |
| 20:3n-6 | 0 (0) ± 0 (0) | 0.04 (0.06) ± 0.005 (0.009) |
| 20:4n-6 | 0 (0) ± 0 (0) | 0.15 (0.25) ± 0.008 (0.012) |
| 22:4n-6 | 0.027 (0.02) ± 0.005 (0.004) | 0.05 (0.08) ± 0.005 (0.008) |
| 22:5n-6 | 0 (0) ± 0 (0) | 0.040 (0.06) ± 0.002 (0.003) |
| 18:3n-3 | 1.8 (1.5) ± 0.3 (0.01) | 2 (3.1) ± 0.02 (0.034) |
| 20:5n-3 | 0 (0) ± 0 (0) | 0.61 (1.0) ± 0.028 (0.043) |
| 22:5n-3 | 0 (0) ± 0 (0) | 0.13 (0.21) ± 0.007 (0.01) |
| 22:6n-3 | 0 (0) ± 0 (0) | 0.81 (1.3) ± 0.072 (0.11) |
| Total SFA | 56 (47) ± 10 (0.3) | 17 (27) ± 0.16 (0.12) |
| Total MUFA | 35 (29) ± 6 (0.3) | 21 (33) ± 0.14 (0.2) |
| Total n-6 PUFA | 28 (23) ± 5 (0.2) | 21 (34) ± 0.14 (0.3) |
| Total n-3 PUFA | 1.8 (1.5) ± 0.3 (0.01) | 4 (5.6) ± 0.094 (0.13) |
N denotes individual food pellet sampled for analysis.
Lipid Extraction
Brain and serum total lipids were extracted by chloroform:methanol:0.88% KCl (2:1:0.75 by vol.) developed by Folch, Lees and Sloane Stanley. Total phospholipids were isolated by thin-layer chromatography (TLC) using TLC G-plates (EMD Chemical, Gibbstown, NJ, purchase year: 2017) and authentic standards (Avanti, Alabaster, AL, purchase year: 2017). Mixture of heptane:diethyl ether:glacial acetic acid (60:40:2 by vol.) was used for separation of neutral lipids including total phospholipids. Band corresponding to total phospholipids was visualized under UV light after spraying with 0.1% 8-anilino-1-naphthalene sulfonic acid. Brain total phospholipid bands and serum total lipids were collected into test tubes with known quantities of internal standards, heptadecanoic acids (17:0) and n-3 docosatrienoic acid (22:3n-3). Fatty acids are converted into fatty acid methyl esters (FAME) with 14% boron trifluoride-methanol at 100°C for one hour. FAME were analyzed and quantified by gas chromatography-flame ionization detection (GC-FID).
Gas Chromatography-Flame Ionization Detection
FAME were analyzed using an Agilent 7890A gas chromatograph coupled with a flame ionization detector (Agilent Technologies, Inc., Santa Clara, CA). Highly efficient, 15-meter DB-FFAP capillary column was used for FAME separation (0.1 mm ID x 0.1 μm film thickness). Samples were injected with 60:1 split ratio. The injector and detector ports were set at 250°C. FAME were eluted using a temperature program set initially at 150°C for 0.25 minutes, followed by increases at 10°C/minute to 200°C for 5 minutes and finally at 10°C/minute to 245°C for 20 minutes to complete the run. The carrier gas was hydrogen with a constant flow rate of 40 ml/minute. Peaks were identified by retention times of authentic FAME standards (Nu-Chek-Prep., Elysian, MN). Area under the curve of each identified FAME peak are collected blindly by laboratory technician who were not provided with no information on the samples. The concentration of each fatty acid from brain total phospholipids and serum total lipids was quantified by peak comparison to the internal standards (17:0 and 22:3n-3) and expressed as nmol/g brain and nmol/ml, respectively, with no blinding. Fatty acid compositions were calculated as percentage of individual fatty acid concentrations to the total fatty acid concentrations of analyzed phospholipid class. Composition data were expressed as mol%. Total fatty acid concentrations were calculated by the sum of all quantified fatty acids.
Statistics
Data are expressed as means ± SD. No sample size calculation was performed a priori. Based on pilot data, a 50% difference between Rorasg/sg and wildtype littermate was estimated. Therefore, power analysis for two-sided test with α of 0.05 and desired power of 0.8 estimated sample size of three per group. The effect of genotype and sex on brain total fatty acid concentrations and compositions were determined by two-way ANOVA analysis (GraphPad Prism 8.0.1). Sex effect were not significant; thereby male and female samples were pooled to test the effect of genotype on brain phospholipid fatty acid concentrations and compositions at different age and brain regions. Means were compared by unpaired, two-tailed t-tests by Microsoft Excel 365 ProPlus. Statistical significance was set at P < 0.05.
Results
The effects of genotype and sex on cerebellar fatty acid concentrations
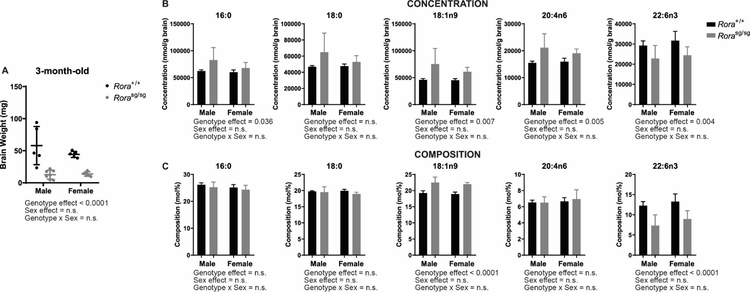
In accordance to literature, cerebellum atrophy was observed in Staggerer (Rorasg/sg) mice. At 3-month-old, there was a significant genotype effect on cerebellar weight (Figure 1A). Male Rorasg/sg cerebellum was 78 ± 12% lower as compared to wildtype littermate (Rora+/+). Female Rorasg/sg cerebellum was 70 ± 7.6% lower as compared to Rora+/+ mice. There was no significant effect of sex on cerebellar weights (Figure 1A).
Figure 1.
The effects of genotype and sex on total fatty acid concentrations and compositions of cerebellum from 3-month-old mice. A) Brain weights of dissected cerebellum for fatty acid quantification. B) Fatty acid concentrations of predominant species of saturated (16:0 and 18:0), monounsaturated (18:1n-9), n-6 (20:4n-6; AA) and n-3 polyunsaturated fatty acids (22:6n-3; DHA). C) Fatty acid compositions of predominant species of saturated (16:0 and 18:0), monounsaturated (18:1n-9), n-6 (20:4n-6) and n-3 polyunsaturated fatty acids (22:6n-3). Two-way ANOVA with post hoc Bonferroni multiple comparison test were performed. Statistical significance was set a p < 0.05. C)
Fatty acid concentrations of predominant species of saturated (SFA), monounsaturated (MUFA), omega-6 (n-6) and omega-3 (n-3) polyunsaturated fatty acids (PUFA) were affected by genotype but not by sex (Figure 1B). Since total fatty acid concentration was significantly different between genotype (Rora+/+ = 238410 ± 9480 nmol/g brain; Rorasg/sg = 308513 ± 85165 nmol/g brain; p = 0.02) (Supplementary Table 1), fatty acid compositions were calculated. After adjustment, significant main effect of genotype remained for oleic acid (18:1n-9) and docosahexaenoic acid (22:6n-3; DHA) (Figure 1C). Oleic acid composition in cerebellar total lipids was 14 ± 5.3% higher in Rorasg/sg as compared to Rora+/+ (Rorasg/sg = 22 ± 1.3 mol%; Rora+/+ = 19 ± 0.63 mol%). DHA composition in cerebellar total lipids was 37 ± 19% lower in Rorasg/sg as compared to Rora+/+ (Rorasg/sg = 8 ± 2.5 mol%; Rora+/+ = 13 ± 1.5 mol%).
Serum fatty acid concentration and brain weight
Fatty acid analysis of serum from 2-months-old mice demonstrated that majority of serum fatty acids did not exhibit significant changes between Rorasg/sg and Rora+/+. However, serum palmitate (16:0) and linoleic acid (18:2n-6) in Rorasg/sg were significantly higher in the serum as compared to Rora+/+ (Table 2).
Table 2.
Serum total fatty acids concentrations and compositions of 2-month-old staggerer mice (Rorasg/sg) and wildtype littermate (Rora+/+) (mean ± SD; Rorasg/sg n = 4, Rora+/+ n = 5)
| FA | Wildtype (Rora+/+) | Staggerer (Rorasg/sg) |
|---|---|---|
| nmol/ml (mol%) | nmol/ml (mol%) | |
| 12:0 | 1.0 (0.023) ± 0.21 (0.004) | 8.9 (0.17) ± 6.1 (0.10) |
| 14:0 | 23 (0.54) ± 5.3 (0.12) | 22 (0.43) ± 5.3 (0.062) |
| 16:0 | 1063 (25) ± 150 (1.1) | 1310 (26) ± 122* (0.62) |
| 18:0 | 462 (11) ± 57 (1.0) | 629 (12) ± 133 (1.5) |
| 20:0 | 7.5 (0.18) ± 2.4 (0.053) | 10 (0.20) ± 2.0 (0.056) |
| 22:0 | 5.8 (0.14) ± 3.1 (0.070) | 9.0 (0.18) ± 3.5 (0.085) |
| 24:0 | 4.0 (0.10) ± 0.7 (0.021) | 5.1 (0.10) ± 1.4 (0.035) |
| 16:1n7 | 87 (2.0) ± 20 (0.32) | 75 (1.5) ± 13 (0.16) |
| 18:1n9 | 597 (14) ± 92 (1.2) | 618 (12) ± 68 (0.28) |
| 18:1n7 | 79 (1.9) ± 11 (0.31) | 73 (1.4) ± 11 (0.090) |
| 20:1n9 | 15 (0.36) ± 3.5 (0.080) | 15 (0.30) ± 3 (0.091) |
| 22:1n9 | 3.5 (0.083) ± 0.56 (0.010) | 3.7 (0.072) ± 0.23 (0.0028) |
| 24:1n9 | 15 (0.35) ± 1.9 (0.0086) | 16 (0.31) ± 2.5 (0.024) |
| 18:2n6 | 1172 (28) ± 73 (1.6) | 1580 (31) ± 121** (1.9*) |
| 18:3n6 | 8.0 (0.19) ± 2.2 (0.036) | 7.8 (0.15) ± 1.3 (0.031) |
| 20:2n6 | 11 (0.25) ± 1.8 (0.047) | 11 (0.21) ± 2.6 (0.032) |
| 20:3n6 | 44 (1.0) ± 8.3 (0.11) | 38 (0.74) ± 4.9 (0.10) |
| 20:4n6 | 305 (7.1) ± 57 (0.68) | 305 (6.0) ± 29 (0.36) |
| 22:4n6 | 5.3 (0.13) ± 0.53 (0.012) | 5.4 (0.11) ± 1.8 (0.036) |
| 22:5n6 | 6.5 (0.15) ± 1.8 (0.048) | 7.6 (0.15) ± 2.8 (0.043) |
| 18:3n3 | 30 (0.72) ± 3.2 (0.12) | 37 (0.73) ± 3.9 (0.060) |
| 20:5n3 | 61 (1.4) ± 6.7 (0.050) | 80 (1.5) ± 14 (0.15) |
| 22:5n3 | 19 (0.45) ± 5.4 (0.10) | 20 (0.39) ± 3.5 (0.076) |
| 22:6n3 | 224 (5.2) ± 45 (0.66) | 227 (4.4) ± 24 (0.46) |
| Total FA | 4248 ± 468 | 5113 ± 492* |
Statistical significance:
p < 0.05
p < 0.01.
N denotes number of animals.
Even with addition of EPA and DHA in the diet, cerebellum atrophy was pronounced in Rorasg/sg mice. Rorasg/sg cerebellum weight was lower by 82 ± 5.2% at an earlier age of 2 months as compared to control. While cerebellum weight increased with age, at 7 months or older, Rorasg/sg cerebellum was still 55 ± 22% lower than Rora+/+ (Figure 2). No significant changes were detected between Rorasg/sg and Rora+/+ in other analyzed regions including prefrontal cortex, cortex, striatum and hippocampus (Figure 2).
Figure 2.
Brain weights of dissected brain regions for fatty acid quantification. 2-month-old n = 4-5; >7- month-old n = 4-7. * p < 0.01; ** p < 0.0001. Cereb, cerebellum; PFC, prefrontal cortex; Cor, cortex; Str, striatum; Hippo, hippocampus.
Cerebellum phospholipid fatty acid profile
At 2-months-old, cerebellar phospholipid fatty acid concentrations were lower in Rorasg/sg as compared to Rora+/+. Major species of SFA including palmitate (16:0) and stearate (18:0) were lower in Rorasg/sg mice by 75 ± 5.6% (Rorasg/sg, 7111 ± 1565 nmol/g brain; Rora+/+, 28054 ± 2584 nmol/g brain) and 73 ± 4.7% (Rorasg/sg, 6320 ± 1110 nmol/g brain; Rora+/+, 23744 ± 2270 nmol/g brain) as compared to age-control Rora+/+ (Table 3). Major species of MUFA, oleic acid, also followed the same trend with 63 ± 7% (Rorasg/sg, 8339 ± 1585 nmol/g brain; Rora+/+, 22636 ± 2669 nmol/g brain) lower in Rorasg/sg mice as compared to age-control Rora+/+ (Table 3). Predominant species of PUFA including ARA and DHA were both significantly lower in Rorasg/sg mice by 69 ± 9.3% (Rorasg/sg, 2193 ± 652 nmol/g brain; Rora+/+, 7000 ± 1024 nmol/g brain) and 86 ± 4.6% (Rorasg/sg, 2252 ± 732 nmol/g brain; Rora+/+, 16063 ± 2772 nmol/g brain) as compared to Rora+/+, respectively (Table 2). This translated to a collectively lower levels of SFA, MUFA, n-6 PUFA and n-3 PUFA in Rorasg/sg mice by 73 ± 5.2, 64 ± 7, 65 ± 12, and 85 ± 4.6% as compared to age-control wildtype littermate, respectively.
Table 3.
Cerebellum total phospholipid fatty acid concentration and composition at 2 months old and >7 months old (mean ± SD; 2 month-old n = 5/group, >7 month-old, n = 4-7/group).
| FA | Wildtype (Rora+/+) | Staggerer (Rorasg/sg) | Wildtype (Rora+/+) | Staggerer (Rorasg/sg) |
|---|---|---|---|---|
| nmol/g brain (mol%) | nmol/g brain (mol%) | nmol/g brain (mol%) | nmol/g brain (mol%) | |
| 2 month-old | 2 month-old | >7 month-old | >7 month-old | |
| 14:0 | 241 (0.2) ± 69 (0.05) | 98 (0.3) ± 43** (0.07) | 225 (0.2) ± 174 (0.1) | 170 (0.2) ± 111 (0.01) |
| 16:0 | 28054 (25) ± 2584 (0.6) | 7111 (22) ± 1565*** (1.4##) | 29390 (25) ± 8064 (1.5) | 26649 (26) ± 14357 (11) |
| 18:0 | 23744 (21) ± 2270 (0.4) | 6320 (20) ± 1110*** (0.7##) | 23420 (20) ± 6459 (0.9) | 26847 (27) ± 14482 (14) |
| 20:0 | 520 (0.5) ± 29 (0.05) | 266 (0.8) ± 73*** (0.1##) | 440 (0.4) ± 119 (0.1) | 1049 (1.0) ± 607 (0.6) |
| 22:0 | 354 (0.3) ± 35 (0.06) | 196 (0.6) ± 42*** (0.1##) | 343 (0.3) ± 74 (0.07) | 841 (0.8) ± 486 (0.5) |
| 24:0 | 494 (0.4) ± 69 (0.1) | 271 (0.9) ± 58*** (0.2##) | 426 (0.4) ± 165 (0.1) | 977 (1.0) ± 581 (0.5) |
| 16:1n-7 | 617 (0.5) ± 78 (0.07) | 246 (0.8) ± 64*** (0.1#) | 689 (0.6) ± 152 (0.07) | 617 (0.5) ± 616 (0.3) |
| 18:1n-9 | 22636 (20) ± 2669 (0.6) | 8339 (26) ± 1585*** (1.7###) | 23924 (21) ± 4551 (2.2) | 24894 (20) ± 24692 (13) |
| 18:1n-7 | 5989 (5.3) ± 618 (0.09) | 1904 (5.9) ± 411*** (0.5) | 5796 (5.1) ± 1073 (0.6) | 4941 (4.0) ± 4814 (2.5) |
| 20:1n-9 | 3145 (2.8) ± 423 (0.09) | 1050 (3.3) ± 333*** (0.9) | 3483 (3.1) ± 973 (0.9) | 4449 (3.5) ± 4459 (2.2) |
| 22:1n-9 | 239 (0.2) ± 11 (0.02) | 102 (0.3) ± 29*** (0.09#) | 194 (0.2) ± 53 (0.04) | 309 (0.3) ± 323 (0.2) |
| 24:1n-9 | 839 (0.7) ± 71 (0.1) | 410 (1.3) ± 122*** (0.4#) | 1258 (1.1) ± 374 (0.3) | 2255 (1.8) ± 1886 (0.4#) |
| 18:2n-6 | 905 (0.8) ± 87 (0.06) | 505 (1.6) ± 632 (2.0) | 773 (0.7) ± 148 (0.06) | 398 (0.4) ± 376 (0.2) |
| 18:3n-6 | 0 (0) ± 0 (0) | 0 (0) ± 0 (0) | 0 (0) ± 0 (0) | 0 (0) ± 0 (0) |
| 20:2n-6 | 309 (0.3) ± 22 (0.03) | 77 (0.2) ± 30*** (0.06) | 181 (0.2) ± 34 (0.03) | 189 (0.1) ± 214 (0.1) |
| 20:3n-6 | 516 (0.5) ± 37 (0.03) | 183 (0.6) ± 47*** (0.06#) | 450 (0.4) ± 93 (0.02) | 352 (0.3) ± 344 (0.2) |
| 20:4n-6 | 7000 (6.1) ± 1024 (0.3) | 2193 (6.7) ± 652*** (1.3) | 7552 (6.5) ± 2515 (0.8) | 5791 (4.8) ± 5565 (3.1) |
| 22:4n-6 | 1384 91.2) ± 234 (0.08) | 606 (1.9) ± 197*** (0.4#) | 1791 (1.5) ± 691 (0.3) | 2088 (1.7) ± 2006 (1.1) |
| 22:5n-6 | 82 (0.07) ± 23 (0.03) | 32 (0.1) ± 28* (0.09) | 75 (0.06) ± 75 (0.05) | 61 (0.06) ± 56 (0.04) |
| 18:3n-3 | 73 (0.06) ± 29 (0.02) | 29 (0.09) ± 5* (0.02) | 68 (0.06) ± 18 (0.01) | 115 (0.1) ± 74 (0.03) |
| 20:5n-3 | 79 (0.07) ± 10 (0.004) | 46 (0.1) ± 23* (0.07) | 62 (0.05) ± 15 (0.01) | 56 (0.07) ± 48 (0.07) |
| 22:5n-3 | 221 (0.2) ± 22 (0.02) | 97 (0.3) ± 20*** (0.04###) | 236 (0.2) ± 71 (0.01) | 233 (0.2) ± 253 (0.1) |
| 22:6n-3 | 16063 (14) ± 2772 (1.1) | 2252 (6.88) ± 732*** (1.5###) | 14393 (12) ± 4675 (1.5) | 7607 (6.1) ± 7324 (3.6#) |
| Total FA | 113503 ± 12518 | 32332 ± 6287*** | 115166 ± 27116 | 110888 ± 74438 |
Statistical significance for fatty acid concentration:
p < 0.05
p < 0.01
p < 0.001.
Statistical significance for fatty acid composition:
p < 0.05
p < 0.01
p < 0.001.
N denotes number of animals.
Since the size of cerebellum was significantly different between genotype, we calculated fatty acid composition to account for weight differences and to provide further insights to the relationships between fatty acids in phospholipid membrane. Composition analyses demonstrated that major species of SFA were significantly lower in Rorasg/sg mice as compared to Rora+/+; however, minor longer chain fatty acids (>20 carbon chain length) were significantly higher in Rorasg/sg mice as compared to Rora+/+ (Table 3). In contrast to the lowering of MUFA concentrations, MUFA compositions were significantly higher in Rorasg/sg mice as compared to Rora+/+ (Table 3). In the case of PUFA, the compositions of minor species of n-6 PUFA were significantly higher in Rorasg/sg mice as compared to Rora+/+. However, the composition of predominant species of n-6 PUFA, ARA, did not significantly change; even though concentration was significantly lower (Table 3). The compositions of minor species of n-3 PUFA were significantly higher in Rorasg/sg mice as compared to Rora+/+. In accordance to concentration analysis, the composition of the predominant species of n-3 PUFA, DHA, was 51 ± 10% (Rorasg/sg, 6.9 ± 1.4 mol%; Rora+/+, 14.1 ± 1.1 mol%) lower in Rorasg/sg mice as compared to Rora+/+ (Table 3).
In contrast to 2-months-old mice, fatty acid concentrations did not differ between >7-months-old Rorasg/sg and wildtype mice (Table 3). However, there were two significant changes in fatty acid composition in 24:1n-9 (Rorasg/sg, 1.8 ± 0.45 mol%; Rora+/+, 1.1 ± 0.31 mol%) and DHA (Rorasg/sg, 6.1 ± 3.6 mol%; Rora+/+, 12.3 ± 1.5 mol%). The consistent lowering of DHA composition in adolescent and adult mice may indicate a biologically essential role for RORα in the regulations of phospholipid DHA metabolism.
Cortex phospholipid fatty acid profiles
At 2-months-old and >7-months-old, cortical fatty acid concentrations of major species in four classes of fatty acids did not significantly differ between Rorasg/sg and wildtype mice. Lowering of minor species of SFA and MUFA were found in Rorasg/sg mice as compared to Rora+/+ littermates. At 2-months-old and >7-months-old, 20:0 was significantly lower; whereas 22:0 was lower at 2-months-old and 24:0 was lower at >7-months-old in Rorasg/sg mice as compared to Rora+/+ (Table 4). At 2-months-old, 20:1n-9 and 24:1n-9 were significantly lower in Rorasg/sg mice as compared to Rora+/+; while at >7-month-old, only 22:1n-9 exhibited significantly lowering in Rorasg/sg mice as compared to Rora+/+ (Table 4). In contrast to concentrations, fatty acid composition demonstrated that while absolute quantities of fatty acid did not differ, there were major changes to compositions of major species of fatty acid in the phospholipid membrane. This included a significantly higher composition of major SFA species, 16:0, in 2-month-old Rorasg/sg mice (31 ± 0.96 mol%) as compared Rora+/+ (29.6 ± 1.1 mol%). This observation continued in >7-months-old cortex. Similarly, a change in 20:0 concentration also translated to a significant but negligible lowering in 20:0 compositions at both age (Table 4). Lowering of MUFA composition was found to compensate for higher palmitate composition. 18:1n-9, 20:1n-9 and 24:1n-9 were significantly lower in 2-months-old Rorasg/sg mice as compared to Rora+/+; yet at >7-months-old only 18:1n-9 was significantly lower. PUFA concentration and composition were not affected in the cortex with an exception of 0.03 mol% lowering of 20:2n-6 composition at 2-months-old.
Table 4.
Cortex total phospholipid fatty acid concentration and composition at 2 months old and >7 months old (mean ± SD; 2 month-old n = 5-6/group, >7 month-old, n = 5-6/group).
| FA | Wildtype (Rora+/+) | Staggerer (Rorasg/sg) | Wildtype (Rora+/+) | Staggerer (Rorasg/sg) |
|---|---|---|---|---|
| nmol/g brain (mol%) | nmol/g brain (mol%) | nmol/g brain (mol%) | nmol/g brain (mol%) | |
| 2 month-old | 2 month-old | >7 month-old | >7 month-old | |
| 14:0 | 287 (0.2) ± 88 (0.03) | 319 (0.2) ± 64 (0.02) | 243 (0.1) ± 25 (0.01) | 244 (0.2) ± 56 (0.02) |
| 16:0 | 43203 (30) ± 9591 (1.1) | 47516 (31) ± 9561 (1.0#) | 45132 (27) ± 7236 (1) | 44841 (29) ± 7898 (0.6#) |
| 18:0 | 32960 (23) ± 6852 (0.6) | 34892 (23) ± 6549 (0.8) | 36768 (22) ± 5787 (0.1) | 33483 (21) ± 5571 (0.5) |
| 20:0 | 284 (0.2) ± 51 (0.03) | 222 (0.1) ± 38* (0.03#) | 330 (0.2) ± 43 (0.03) | 257 (0.2) ± 29** (0.01#) |
| 22:0 | 261 (0.2) ± 57 (0.06) | 193 (0.1) ± 23* (0.03) | 347 (0.2) ± 175 (0.1) | 231 (0.2) ± 90 (0.08) |
| 24:0 | 301 (0.2) ± 76 (0.08) | 236 (0.2) ± 54 (0.06) | 277 (0.2) ± 83 (0.05) | 167 (0.1) ± 46* (0.05) |
| 16:1n-7 | 944 (0.7) ± 163 (0.04) | 1164 (0.8) ± 287 (0.08#) | 1111 (0.7) ± 200 (0.05) | 991 (0.6) ± 162 (0.03) |
| 18:1n-9 | 23266 (16) ± 4108 (0.4) | 23276 (15) ± 4298 (0.3##) | 29139 (17) ± 4834 (0.4) | 26017 (17) ± 4309 (0.3##) |
| 18:1n-7 | 5534 (3.8) ± 958 (0.1) | 5580 (3.7) ± 1061 (0.2) | 6252 (3.7) ± 1006 (0.09 | 5835 (3.7) ± 981 (0.07) |
| 20:1n-9 | 1491 (1.0) ± 207 (0.2) | 992 (0.7) ± 159** (0.08##) | 1460 (0.8) ± 1165 (0.6) | 760 (0.5) ± 565 (0.5) |
| 22:1n-9 | 157 (0.1) ± 30 (0.03) | 138 (0.09) ± 39 (0.02) | 159 (0.1) ± 22 (0.01) | 133 (0.09) ± 12* (0.01) |
| 24:1n-9 | 550 (0.4) ± 100 (0.1) | 325 (0.2) ± 63** (0.05#) | 1180 (0.7) ± 444 (0.3) | 793 (0.5) ± 193 (0.2) |
| 18:2n-6 | 800 (0.6) ± 150 (0.06) | 880 (0.6) ± 173 (0.05) | 867 (0.5) ± 126 (0.05) | 743 (0.5) ± 150 (0.03) |
| 18:3n-6 | 28 (0.02) ± 37 (0.03) | 0 (0) ± 0 (0) | 0 (0) ± 0 (0) | 0 (0) ± 0 (0) |
| 20:2n-6 | 228 (0.2) ± 32 (0.02) | 200 (0.1) ± 46 (0.02#) | 180 (0.1) ± 8 (0.01) | 158 (0.1) ± 26 (0.01) |
| 20:3n-6 | 631 (0.4) ± 124 (0.03) | 645 (0.4) ± 119 (0.03) | 677 (0.4) ± 80 (0.03) | 632 (0.4) ± 134 (0.02) |
| 20:4n-6 | 12090 (8.3) ± 2303 (0.3) | 12543 (8.3) ± 2004 (0.3) | 15123 (9.0) ± 2395 (0.2) | 14058 (8.9) ± 2586 (0.3) |
| 22:4n-6 | 2768 (1.9) ± 532 (0.04) | 2864 (1.9) ± 452 (0.1) | 3831 (2.3) ± 578 (0.09) | 3619 (2.3) ± 681 (0.1) |
| 22:5n-6 | 254 (0.2) ± 53 (0.02) | 290 (0.2) ± 47 (0.01) | 267 (0.2) ± 95 (0.03) | 268 (0.2) ± 67 (0.02) |
| 18:3n-3 | 34 (0.02) ± 38 (0.02) | 44 (0.03) ± 19 (0.01) | 74 (0.04) ± 38 (0.01) | 63 (0.04) ± 28 (0.01) |
| 20:5n-3 | 52 (0.03) ± 30 (0.02) | 37 (0.03) ± 24 (0.02) | 58 (0.03) ± 12(0.004) | 68 (0.04) ± 20 (0.01) |
| 22:5n-3 | 234 (0.2) ± 39 (0.03) | 274 (0.2) ± 43 (0.02) | 313 (0.2) ± 64 (0.02) | 308 (0.2) ± 56 (0.02) |
| 22:6n-3 | 19092 (13) ± 3067 (0.8) | 19487 (13) ± 3537 (1.1) | 24406 (15) ± 3065 (0.6) | 23482 (15) ± 4842 (0.9) |
| Total FA | 145421 ± 27811 | 152117 ± 27698 | 168193 ± 25911 | 157152 ± 26668 |
Statistical significance for fatty acid concentration:
p < 0.05
p < 0.01
p < 0.001.
Statistical significance for fatty acid composition:
p < 0.05
p < 0.01
p < 0.001.
N denotes number of animals.
No significant difference was found in prefrontal cortex SFA concentrations at both ages. Nevertheless, composition of 16:0 was significantly higher in Rorasg/sg mice as compared to Rora+/+ at both ages (Table 5). Fatty acid concentrations of minor species of long-chain omega-9 MUFA (n-9) including 20:1n-9, 22:1n-9 and 24:1n-9 were significantly lower by 33 ± 8.9%, 35 ± 12% and 44 ± 20%, respectively, in 2-months-old Rorasg/sg mice as compared to Rora+/+ (Table 5). These lowering were translated in composition in addition to a significant lowering of 18:1n-9 composition (Table 5). In contrast, >7-months-old Rorasg/sg mice had significantly lower levels of 18:1n-9 (Rorasg/sg, 19837 ±1018 nmol/g brain; Rora+/+, 22194 ± 1327 nmol/g brain) and 20:1n-9 (Rorasg/sg, 1240 ± 165 nmol/g brain; Rora+/+, 1691 ± 187 nmol/g brain) as compared to Rora+/+ which directly translated to compositional differences between >7-months-old Rorasg/sg and Rora+/+ mice (Table 5). In contrast to cortex, prefrontal cortex exhibited significant changes to n-6 PUFA concentrations. At 2-months-old, 20:2n-6 concentration was significantly lower (Rorasg/sg, 181 ± 7 nmol/g brain; Rora+/+, 201 ± 6 nmol/g brain); while ARA (Rorasg/sg, 10468 ± 467 nmol/g brain; Rora+/+, 11754 ± 773 nmol/g brain) and 22:4n-6 (Rorasg/sg, 2656 ± 153 nmol/g brain; Rora+/+, 3109 ± 219 nmol/g brain) were significantly lower at >7-months-old Rorasg/sg mice as compared to Rora+/+ (Table 5). Even though n-6 PUFA concentrations were lowered, compositions of n-6 PUFA were not affected except for a statistically significant 0.18 mol% lowering of 22:4n-6 in >7-months-old Rorasg/sg mice.
Table 5.
Prefrontal cortex total phospholipid fatty acid concentration and composition at 2 months old and >7 months old (mean ± SD; 2 month-old n = 4- 5/group, >7 month-old, n = 4-5/group).
| FA | Wildtype (Rora+/+) | Staggerer (Rorasg/sg) | Wildtype (Rora+/+) | Staggerer (Rorasg/sg) |
|---|---|---|---|---|
| nmol/g brain (mol%) | nmol/g brain (mol%) | nmol/g brain (mol%) | nmol/g brain (mol%) | |
| 2 month-old | 2 month-old | >7 month-old | >7 month-old | |
| 14:0 | 234 (0.2) ± 20 (0.01) | 230 (0.2) ± 29 (0.02) | 138 (0.1) ± 46 (0.03) | 126 (0.1) ± 17 (0.02) |
| 16:0 | 37034 (27) ± 1677 (0.5) | 36268 (28) ± 1666 (0.4#) | 34753 (27) ± 2010 (0.5) | 34235 (29) ± 1047 (0.5##) |
| 18:0 | 29586 (22) ± 1337 (0.2) | 27736 (21) ± 1568 (0.07) | 28019 (22) ± 2411 (0.6) | 25208 (21) ± 1395 (0.2) |
| 20:0 | 302 (0.2) ± 46 (0.03) | 233 (0.2) ± 40 (0.02) | 246 (0.2) ± 38 (0.03) | 196 (0.2) ± 37 (0.02) |
| 22:0 | 417 (0.3) ± 69 (0.04) | 294 (0.2) ± 100 (0.07) | 220 (0.2) ± 123 (0.08) | 140 (0.1) ± 13 (0.01) |
| 24:0 | 385 (0.3) ± 76 (0.05) | 277 (0.2) ± 118 (0.09) | 181 (0.1) ± 76 (0.05) | 137 (0.1) ± 36 (0.02) |
| 16:1n-7 | 772 (0.6) ± 37 (0.02) | 793 (0.6) ± 91 (0.05) | 739 (0.6) ± 101 (0.05) | 722 (0.6) ± 86 (0.06) |
| 18:1n-9 | 21180 (15) ± 1347 (0.3) | 19273 (15) ± 1055 (0.2#) | 22194 (17) ± 1327 (0.4) | 19837 (17) ± 1018* (0.1#) |
| 18:1n-7 | 5094 (3.7) ± 192 (0.06) | 4834 (3.7) ± 190 (0.1) | 5091 (3.9) ± 267 (0.1) | 4796 (4) ± 157 (0.06) |
| 20:1n-9 | 1381 (1) ± 124 (0.05) | 933 (0.7) ± 124** (0.08###) | 1691 (1.3) ± 187 (0.09) | 1240 (1) ± 165** (0.09##) |
| 22:1n-9 | 127 (0.09) ± 16 (0.01) | 88 (0.07) ± 16** (0.01##) | 109 (0.08) ± 15 (0.01) | 106 (0.09) ± 30 (0.02) |
| 24:1n-9 | 728 (0.5) ± 107 (0.06) | 417 (0.3) ± 152** (0.1##) | 756 (0.6) ± 230 (0.1) | 515 (0.4) ± 95 (0.06) |
| 18:2n-6 | 822 (0.6) ± 53 (0.06) | 826 (0.6) ± 59 (0.03) | 663 (0.5) ± 51 (0.03) | 571 (0.5) ± 22* (0.01) |
| 18:3n-6 | 0 (0) ± 0 (0) | 0 (0) ± 0 (0) | 0 (0) ± 0 (0) | 0 (0) ± 0 (0) |
| 20:2n-6 | 201 (0.1) ± 6 (0.01) | 181 (0.1) ± 7** (0.01) | 232 (0.2) ± 40 (0.04) | 265 (0.2) ± 35 (0.02) |
| 20:3n-6 | 580 (0.4) ± 68 (0.04) | 553 (0.4) ± 70 (0.03) | 472 (0.4) ± 49 (0.02) | 451 (0.4) ± 26 (0.01) |
| 20:4n-6 | 12881 (9.4) ± 677 (0.2) | 12179 (9.4) ± 724 (0.2) | 11754 (9.1) ± 773 (0.3) | 10468 (8.8) ± 467* (0.1) |
| 22:4n-6 | 3111 (2.3) ± 273 (0.1) | 2838 (2.2) ± 204 (0.09) | 3109 (2.4) ± 219 (0.1) | 2656 (2.2) ± 153** (0.03#) |
| 22:5n-6 | 266 (0.2) ± 17 (0.02) | 272 (0.2) ± 24 (0.02) | 204 (0.2) ± 31 (0.02) | 186 (0.2) ± 18 (0.01) |
| 18:3n-3 | 56 (0.04) ± 6 (0.01) | 44 (0.03) ± 15 (0.01) | 30 (0.02) ± 11 (0.01) | 28 (0.02) ± 8 (0.01) |
| 20:5n-3 | 53 (0.04) ± 14 (0.01) | 51 (0.04) ± 8 (0.01) | 50 (0.04) ± 2 (0.003) | 52 (0.04) ± 9 (0.01) |
| 22:5n-3 | 248 (0.2) ± 27 (0.01) | 262 (0.2) ± 23 (0.01) | 241 (0.2) ± 42 (0.02) | 222 (0.2) ± 12 (0.01) |
| 22:6n-3 | 21371 (16) ± 772 (0.4) | 20704 (16) ± 1523 (0.3) | 18460 (14) ± 1431 (0.5) | 17232 (14) ± 876 (0.4) |
| Total FA | 136829 ± 6392 | 129286 ± 7450 | 129353 ± 8609 | 119390 ± 5422 |
Statistical significance for fatty acid concentration:
p < 0.05
p < 0.01
p < 0.001.
Statistical significance for fatty acid composition:
p < 0.05
p < 0.01
p < 0.001.
N denotes number of animals.
Striatum and hippocampus phospholipid fatty acid profiles
Striatal phospholipid fatty acid concentration did not differ between Rorasg/sg and Rora+/+ mice (Table 6). Palmitate (16:0) compositions were higher in Rorasg/sg mice as compared to Rora+/+ at both ages (Table 6). Stearate (18:0) composition was only significantly lower in >7-month-old Rorasg/sg mice as compared to Rora+/+ (Table 6). Slight lowering of 20:1n-9 in Rorasg/sg striatum as compared to Rora+/+ were observed at both ages (Table 6). PUFA compositions were largely unaffected. However, 20:2n-6 and 18:3n-3 (ALA) compositions were significantly lower in 2-months-old Rorasg/sg mice as compared to Rora+/+ (Table 6). Since these PUFA species are less accreted in brain phospholipids, the biological significance of these lowering may be inconsequential.
Table 6.
Striatum total phospholipid fatty acid concentration and composition at 2 months old and >7 months old (mean ± SD; 2 month-old n = 5/group, >7 month-old, n = 4/group).
| FA | Wildtype (Rora+/+) | Staggerer (Rorasg/sg) | Wildtype (Rora+/+) | Staggerer (Rorasg/sg) |
|---|---|---|---|---|
| nmol/g brain (mol%) | nmol/g brain (mol%) | nmol/g brain (mol%) | nmol/g brain (mol%) | |
| 2 month-old | 2 month-old | >7 month-old | >7 month-old | |
| 14:0 | 420 (0.2) ± 241 (0.02) | 543 (0.2) ± 230 (0.03) | 327 (0.1) ± 84 (0.04) | 319 (0.1) ± 45 (0.02) |
| 16:0 | 49559 (23) ± 25193 (0.8) | 57610 (25) ± 20717 (0.6#) | 67305 (22) ± 9427 (0.8) | 70536 (24) ± 4799 (0.3#) |
| 18:0 | 45251 (21) ± 23135 (0.3) | 49286 (21) ± 17590 (0.2) | 65504 (22) ± 11587 (0.3) | 60972 (21) ± 3805 (0.3##) |
| 20:0 | 847 (0.4) ± 509 (0.07) | 816 (0.3) ± 301 (0.03) | 1003 (0.3) ± 227 (0.02) | 875 (0.3) ± 93 (0.03) |
| 22:0 | 695 (0.4) ± 279 (0.2) | 749 (0.3) ± 198 (0.1) | 721 (0.2) ± 203 (0.03) | 672 (0.2) ± 33 (0.02) |
| 24:0 | 838 (0.4) ± 449 (0.1) | 915 (0.4) ± 337 (0.09) | 763 (0.3) ± 206 (0.03) | 629 (0.2) ± 57 (0.03) |
| 16:1n-7 | 1321 (0.6) ± 627 (0.07) | 1459 (0.6) ± 549 (0.08) | 1910 (0.6) ± 367 (0.06) | 1948 (0.7) ± 224 (0.06) |
| 18:1n-9 | 42965 (20) ± 22545 (0.9) | 44732 (19) ± 16612 (0.7) | 52549 (17) ± 28992 (8.6) | 63803 (22) ± 3963 (0.5) |
| 18:1n-7 | 9832 (4.6) ± 5036 (0.2) | 10253 (4.4) ± 3748 (0.2) | 26601 (8.8) ± 26489 (8.8) | 13389 (4.5) ± 848 (0.1) |
| 20:1n-9 | 4594 (2.1) ± 2733 (0.4) | 3902 (1.6) ± 1579 (0.2#) | 7732 (2.6) ± 1715 (0.1) | 6751 (2.3) ± 402 (0.1#) |
| 22:1n-9 | 535 (0.2) ± 410 (0.09) | 548 (0.2) ± 276 (0.08) | 562 (0.2) ± 103 (0.01) | 523 (0.2) ± 21 (0.01) |
| 24:1n-9 | 1744 (0.9) ± 788 (0.3) | 1608 (0.7) ± 500 (0.1) | 3136 (1) ± 841 (0.2) | 2679 (0.9) ± 233 (0.1) |
| 18:2n-6 | 1144 (0.5) ± 560 (0.04) | 1252 (0.5) ± 445 (0.05) | 1412 (0.5) ± 328 (0.03) | 1216 (0.4) ± 45 (0.04) |
| 18:3n-6 | 0 (0) ± 0 (0) | 0 (0) ± 0 (0) | 0 (0) ± 0 (0) | 0 (0) ± 0 (0) |
| 20:2n-6 | 320 (0.1) ± 169 (0.01) | 289 (0.1) ± 104 (0.02#) | 625 (0.2) ± 284 (0.07) | 773 (0.3) ± 64 (0.01) |
| 20:3n-6 | 1045 (0.5) ± 575 (0.04) | 1212 (0.5) ± 449 (0.02) | 1173 (0.4) ± 224 (0.02) | 1172 (0.4) ± 58 (0.02) |
| 20:4n-6 | 17106 (8.0) ± 8752 (0.2) | 19443 (8.4) ± 6771 (0.4) | 24249 (8.1) ± 3999 (0.2) | 24018 (8.1) ± 1400 (0.1) |
| 22:4n-6 | 5532 (2.6) ± 2975 (0.1) | 6043 (2.6) ± 2230 (0.1) | 7809 (2.6) ± 958 (0.2) | 7969 (2.7) ± 462 (0.09) |
| 22:5n-6 | 305 (0.1) ± 169 (0.02) | 330 (0.1) ± 109 (0.01) | 285 (0.1) ± 27 (0.01) | 293 (0.1) ± 25 (0.01) |
| 18:3n-3 | 144 (0.07) ± 73 (0.01) | 95 (0.04) ± 47 (0.01##) | 120 (0.04) ± 26 (0.005) | 147 (0.05) ± 65 (0.02) |
| 20:5n-3 | 144 (0.06) ± 91 (0.01) | 161 (0.07) ± 64 (0.01) | 177 (0.06) ± 38 (0.01) | 213 (0.07) ± 52 (0.02) |
| 22:5n-3 | 516 (0.2) ± 322 (0.04) | 643 (0.3) ± 272 (0.05) | 646 (0.2) ± 72 (0.04) | 657 (0.2) ± 39 (0.02) |
| 22:6n-3 | 28088 (13) ± 14133 (0.7) | 31264 (14) ± 10834 (0.4) | 35985 (12) ± 5308 (0.7) | 36030 (12) ± 3033 (0.5) |
| Total FA | 212946 ± 108699 | 233154 ± 83436 | 300596 ± 50877 | 295584 ± 17432 |
Statistical significance for fatty acid concentration:
p < 0.05
p < 0.01
p < 0.001.
Statistical significance for fatty acid composition:
p < 0.05
p < 0.01
p < 0.001.
N denotes number of animals.
Similarly, hippocampal phospholipid fatty acid concentration did not differ between Rorasg/sg and Rora+/+ mice (Table 7). Fatty acid composition of 2-months-old mice also did not significantly differ between genotype (Table 7). At >7-months-old mice, small but significantly lower compositions of 18:0 and 20:2n-6 were observed in Rorasg/sg mice as compared to Rora+/+. N-3 PUFA compositions were not significantly different between genotype at either age group (Table 7).
Table 7.
Hippocampus total phospholipid fatty acid concentration and composition at 2 months old and >7 months old (mean ± SD; 2 month-old n = 5/group, >7 month-old, n = 4-5/group).
| FA | Wildtype (Rora+/+) | Staggerer (Rorasg/sg) | Wildtype (Rora+/+) | Staggerer (Rorasg/sg) |
|---|---|---|---|---|
| nmol/g brain (mol%) | nmol/g brain (mol%) | nmol/g brain (mol%) | nmol/g brain (mol%) | |
| 2 month-old | 2 month-old | >7 month-old | >7 month-old | |
| 14:0 | 82 (0.2) ± 18 (0.05) | 76 (0.2) ± 8 (0.03) | 107 (0.2) ± 38 (0.06) | 100 (0.1) ± 13 (0.03) |
| 16:0 | 11387 (27) ± 4556 (1) | 8570 (28) ± 917 (0.9) | 18179 (26) ± 8273 (1.2) | 18625 (26) ± 3568 (1) |
| 18:0 | 9663 (23) ± 3738 (1.1) | 7085 (23) ± 754 (1.5) | 15746 (23) ± 6585 (0.5) | 15803 (22) ± 2838 (0.4#) |
| 20:0 | 101 (0.3) ± 24 (0.05) | 93 (0.3) ± 24 (0.09) | 150 (0.2) ± 36 (0.07) | 159 (0.2) ± 53 (0.05) |
| 22:0 | 93 (0.2) ± 34 (0.04) | 93 (0.3) ± 38 (0.2) | 137 (0.2) ± 64 (0.05) | 135 (0.2) ± 40 (0.04) |
| 24:0 | 109 (0.3) ± 33 (0.1) | 101 (0.3) ± 41 (0.2) | 127 (0.2) ± 46 (0.05) | 120 (0.2) ± 36 (0.05) |
| 16:1n-7 | 252 (0.6) ± 128 (0.09) | 184 (0.6) ± 40 (0.2) | 499 (0.7) ± 248 (0.1) | 425 (0.6) ± 76 (0.02) |
| 18:1n-9 | 6879 (17) ± 2645 (0.5) | 5312 (17) ± 836 (1.2) | 12591 (19) ± 5049 (1.6) | 12933 (18) ± 2564 (0.9) |
| 18:1n-7 | 1534 (3.6) ± 674 (0.1) | 1152 (3.7) ± 170 (0.09) | 2519 (3.7) ± 1050 (0.3) | 2648 (3.7) ± 491 (0.08) |
| 20:1n-9 | 462 (1.1) ± 146 (0.2) | 335 (1.1) ± 85 (0.2) | 1030 (1.6) ± 346 (0.5) | 988 (1.4) ± 341 (0.3) |
| 22:1n-9 | 55 (0.1) ± 20 (0.05) | 50 (0.2) ± 13 (0.04) | 77 (0.1) ± 30(0.04) | 131 (0.2) ± 46 (0.07) |
| 24:1n-9 | 177 (0.5) ± 28 (0.1) | 180 (0.6) ± 56 (0.2) | 486 (0.7) ± 222 (0.3) | 542 (0.8) ± 205 (0.2) |
| 18:2n-6 | 247 (0.6) ± 121 (0.05) | 204 (0.7) ± 28 (0.08) | 380 (0.6) ± 170 (0.07) | 355 (0.5) ± 86 (0.03) |
| 18:3n-6 | 0 (0) ± 0 (0) | 0 (0) ± 0 (0) | 0 (0) ± 0 (0) | 0 (0) ± 0 (0) |
| 20:2n-6 | 65 (0.2) ± 26 (0.01) | 46 (0.2) ± 7 (0.03) | 74 (0.1) ± 22 (0.02) | 60 (0.08) ± 9 (0.01#) |
| 20:3n-6 | 197 (0.5) ± 89 (0.06) | 150 (0.5) ± 38 (0.06) | 295 (0.4) ± 121 (0.09) | 254 (0.3) ± 80 (0.06) |
| 20:4n-6 | 3906 (9.3) ± 1630 (0.7) | 2813 (9) ± 547 (0.8) | 6415 (9.1) ± 3192 (1.2) | 6791 (9.4) ± 1449 (0.2) |
| 22:4n-6 | 867 (2.1) ± 352 (0.2) | 644 (2) ± 153 (0.3) | 1622 (2.3) ± 754 (0.3) | 1847 (2.6) ± 440 (0.2) |
| 22:5n-6 | 71 (0.2) ± 60 (0.09) | 60 (0.2) ± 14 (0.05) | 100 (0.2) ± 41 (0.05) | 133 (0.2) ± 9 (0.05) |
| 18:3n-3 | 15 (0.03) ± 21 (0.04) | 8 (0.02) ± 11 (0.03) | 13 (0.01) ± 18 (0.02) | 27 (0.03) ± 32 (0.04) |
| 20:5n-3 | 6 (0.01) ± 12 (0.03) | 5 (0.02) ± 11 (0.04) | 22 (0.03) ± 25 (0.03) | 29 (0.03) ± 37 (0.04) |
| 22:5n-3 | 93 (0.2) ± 27 (0.07) | 77 (0.2) ± 13 (0.02) | 143 (0.2) ± 64 (0.02) | 137 (0.2) ± 25 (0.02) |
| 22:6n-3 | 5495 (13) ± 2781 (1.7) | 3947 (13) ± 1110 (2.4) | 7949 (11) ±4018 (2) | 9447 (13) ± 1985 (0.7) |
| Total FA | 41756 ± 16998 | 31187 ± 4260 | 68662 ± 29678 | 71687 ± 13869 |
Statistical significance for fatty acid concentration:
p < 0.05
p < 0.01
p < 0.001.
Statistical significance for fatty acid composition:
p < 0.05
p < 0.01
p < 0.001.
N denotes number of animals.
Discussion
This study is the first to investigate the effects of RORα deficiency on fatty acid metabolism in the brain. Staggerer mice brain fatty acid metabolism was differentially affected among brain regions which may be dependent on regional expressions of RORα. Extensive atrophy of developing cerebellum was accompanied by extensive lowering of all fatty acid species concentrations. Despite lower levels of fatty acids in developing cerebellum of staggerer mutants, RORα deficiency does not appear to impede accretions of fatty acids in adulthood. By 7-month-old, fatty acid concentrations and compositions were indistinguishable between genotypes except for DHA, even though cerebellar weight at 7-month-old was still significantly smaller than wildtype. This implied that while all fatty acids may be initially low in developing staggerer cerebella, RORα may not affect the ability of cerebellum to compensate low concentrations via accelerated accretion of affected fatty acids between 2 months and 7 months of age. In fact, at 3 months old, staggerer cerebellum accreted significantly higher SFA, MUFA and PUFA in total phospholipids as compared to wildtype providing evidence that RORα deficiency delays fatty acid accretions during critical periods of development. The function of RORα on fatty acid metabolism may be associated with the temporal expressions of RORα during lifespan which warrants future investigations.
In contrast to other fatty acids, DHA concentrations remained significantly reduced in staggerer cerebellum as compared to wildtype at different ages and with the two different diets examined in this study. Moreover, the lowering of cerebellar DHA concentration in staggerer mice as compared to wildtype were statistically similar between sexes; even though RORα expression is differentially regulated by male and female hormones and may regulate the transcription of aromatase [31, 71]. DHA concentrations were reduced in staggerer cerebellar phospholipids of both sexes that were consuming diets without preformed EPA and DHA. This reduction in DHA concentration was not compensated by increasing levels of n-6 docosapentaenoic acid (n-6 DPA) but rather increases in other n-6 PUFA including ARA. This suggests an aberrant regulation of fatty acid accretions in cerebellum and that the lowering of DHA concentrations was not the result of n-3 PUFA deprivation. Furthermore, the reduction of DHA concentration in staggerer cerebellar phospholipids was not rescued by supplementation of EPA and DHA in the diet; thus, implying that RORα may have a direct or indirect effect on accretion of DHA to brain phospholipids and/or loss of DHA from brain phospholipids. PUFA metabolism was unaffected in regions with low and undetectable expression of RORα. While saturated fatty acids, MUFA, and minor species of n-6 PUFA concentrations and compositions were significantly affected in cortex and prefrontal cortex, the small effect sizes are likely biologically insignificant. Collectively, these findings suggest that RORα has a selective and targeted effect on DHA metabolism in cerebellum across ages. Therefore, future investigations should characterize phospholipid DHA metabolism via lipid kinetic study and profiles of DHA metabolism genes in staggerer mutants to better understand the findings of this study [72].
This research has implications for supplementing n-3 PUFA in humans throughout different stages of the lifespan. Fish oil is a common supplement used by families with individuals affected by ASD [73]. It is predominantly composed of two n-3 PUFA, eicosapentaenoic acid (EPA) and DHA. Dietary deficiency of n-3 PUFA during development had been shown to impair cognitive performance and prepulse inhibition (PPI) as well as increase in depressive-like and anxiety-like behaviors in rodents [74–79]. Furthermore, in environmental exposure models for ASD in rodents, n-3 PUFA or DHA supplementation were effective in reversing impairments in social interactions and elevation in social anxiety induced by maternal immune activation or exposure of food allergen during development [63, 80, 81]. Similarly, genetic models of ASD, including BTBR and serotonin transporter (SERT) knockout, demonstrated that pre- and postnatal n-3 PUFA or DHA supplementation may increase social interest and social preference for novel mice over objects as compared to mice fed n-3 PUFA deprived diets [82, 83]. The improvements in social behaviors were observed in conjunction with higher DHA composition in the brain [82–84]. Therefore, dietary DHA intervention shows promising results for the prevention of the core behaviors associated with ASD in different animal models.
Despite findings in animal models, the efficacy of DHA or n-3 PUFA supplementation for improvements of ASD symptoms in humans remain inconclusive. A meta-analysis of case control cohorts found that DHA and EPA levels in blood of ASD children aged 12 and under were selectively lower with no changes to total n-3 PUFA concentrations as compared to typically developing children, despite no differences in reported dietary intakes of n-3 PUFA between children with and without ASD [85]. The current randomized controlled trials are limited to small sample sizes, short duration of treatment, ambiguous biomarkers of fatty acid intake and recruitment of mostly ASD children with mild symptomology. However, it is also important to note that trials reporting increase in blood DHA after supplementation also failed to observe improvements of social behavior in children with ASD [86, 87]. Therefore, it is plausible that children with ASD may have dysfunction in fatty acid metabolism in the brain where increased level in blood may not be able to compensate for accelerated metabolisms of n-3 PUFA in autistic brain. Our study provide evidence in mice that a diet containing EPA and DHA may yield serum DHA level (4.4–5.2 mol% of total serum fatty acids) similarly to that of n-3 PUFA supplemented children (2.7–5.5%). Further, deficiency of RORα may selectively and negatively affect the metabolism of DHA in the brain either by slowing incorporation of DHA into brain phospholipids and/or accelerating loss of DHA from brain phospholipids; thereby resulting in an observed reduction of brain phospholipid DHA in half. RORα expression was reduced in a subset of ASD patients with severe language impairment [42]. Of the six RCT, the Mankad trial was conducted in nonverbal ASD children [88]. N-3 PUFA supplementation in this population did not alter plasma fatty acid composition like the staggerer mice and no improvement in social behaviors were observed; thus, providing additional evidence that gene deficiency may affect efficacy of dietary n-3 PUFA supplementation on aberrant social behaviour due to the role of gene on n-3 PUFA metabolism in the brain. Future studies should aim to investigate the role of RORα on social interaction behavior in rodent models. In addition, future studies should confirm cerebellar expressions of RORα in nonverbal autistic patients and associate expressions with brain phospholipid lipidome of postmortem autistic brains.
While the present findings support a role of RORα in fatty acid metabolisms, there are limitations to our study. The variability of brain fatty acid concentrations from older age mice was greater than that of younger age mice. This is most likely due to the variability in the age of the samples collected for >7-months-old group. Gait impairment and stunted growth affected the survival of staggerer mice to adulthood; thereby, sample size was reduced for staggerer mice of older age. Hence observations of older staggerer mice should be confirmed with a larger sample size. In addition, while this study examined fatty acid concentrations and compositions in total phospholipids, it is possible that there may be phospholipid class specific modulations that may be masked by total phospholipid analyses.
To our knowledge, this study is the first to examine the effects of an ASD associated gene on fatty acid metabolism in the brain. Deficiency in RORα may lower fatty acid concentration during neurodevelopment and delay fatty acid accretions during lifespan. Furthermore, RORα deficiency selectively lower DHA concentration in developing cerebellum across lifespan. The effect of RORα on fatty acid metabolism appears to be brain region-specific and age-dependent. The effects of ASD associated genes such as RORα on DHA metabolisms in the brain may explain the lack of efficacy of n-3 PUFA supplementation in children with ASD as compared to other psychiatric disorders. It is important to examine fatty acid metabolism in other genetic models for ASD to further understand the effects of gene-lipid interactions in the etiology and pathology.
Supplementary Material
Acknowledgements
This work is jointly supported by the Intramural Research Program of the National Institute on Alcohol Abuse and Alcoholism (National Institutes of Health, USA) and NIH R01 Grant to Dr. Jing Chen (EY024963). The views expressed are those of the author(s) and not necessarily those of the NIAAA and NIH.
List of abbreviations:
- RORα
retinoid acid-related orphan receptor alpha
- ALA
alpha-linolenic acid
- ASD
autism spectrum disorder
- DPA
docosapentaenoic acid
- EPA
eicosapentaenoic acid
Footnotes
All authors have declared no conflict of interests.
References
- [1].Sidman RL, Lane PW, Dickie MM, Staggerer, a new mutation in the mouse affecting the cerebellum, Science, 137 (1962) 610–612. [DOI] [PubMed] [Google Scholar]
- [2].Herrup K, Role of staggerer gene in determining cell number in cerebellar cortex. I. Granule cell death is an indirect consequence of staggerer gene action, Brain Res, 313 (1983) 267–274. [DOI] [PubMed] [Google Scholar]
- [3].Herrup K, Mullen RJ, Staggerer chimeras: intrinsic nature of Purkinje cell defects and implications for normal cerebellar development, Brain Res, 178 (1979) 443–457. [DOI] [PubMed] [Google Scholar]
- [4].Vogel MW, Sinclair M, Qiu D, Fan H, Purkinje cell fate in staggerer mutants: agenesis versus cell death, J Neurobiol, 42 (2000) 323–337. [DOI] [PubMed] [Google Scholar]
- [5].Yoon CH, Developmental mechanism for changes in cerebellum of “staggerer” mouse, a neurological mutant of genetic origin, Neurology, 22 (1972) 743–754. [DOI] [PubMed] [Google Scholar]
- [6].Landis DM, Sidman RL, Electron microscopic analysis of postnatal histogenesis in the cerebellar cortex of staggerer mutant mice, J Comp Neurol, 179 (1978) 831–863. [DOI] [PubMed] [Google Scholar]
- [7].Sotelo C, Changeux JP, Transsynaptic degeneration ‘en cascade’ in the cerebellar cortex of staggerer mutant mice, Brain Res, 67 (1974) 519–526. [DOI] [PubMed] [Google Scholar]
- [8].Doulazmi M, Frederic F, Lemaigre-Dubreuil Y, Hadj-Sahraoui N, Delhaye-Bouchaud N, Mariani J, Cerebellar Purkinje cell loss during life span of the heterozygous staggerer mouse (Rora(+)/Rora(sg)) is gender-related, J Comp Neurol, 411 (1999) 267–273. [PubMed] [Google Scholar]
- [9].Hadj-Sahraoui N, Frederic F, Zanjani H, Delhaye-Bouchaud N, Herrup K, Mariani J, Progressive atrophy of cerebellar Purkinje cell dendrites during aging of the heterozygous staggerer mouse (Rora(+/sg)), Brain Res Dev Brain Res, 126 (2001) 201–209. [DOI] [PubMed] [Google Scholar]
- [10].Zanjani HS, Mariani J, Delhaye-Bouchaud N, Herrup K, Neuronal cell loss in heterozygous staggerer mutant mice: a model for genetic contributions to the aging process, Brain Res Dev Brain Res, 67 (1992) 153–160. [DOI] [PubMed] [Google Scholar]
- [11].Boukhtouche F, Janmaat S, Vodjdani G, Gautheron V, Mallet J, Dusart I, Mariani J, Retinoid-related orphan receptor alpha controls the early steps of Purkinje cell dendritic differentiation, J Neurosci, 26 (2006) 1531–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Shirley LT, Messer A, Early postnatal Purkinje cells from staggerer mice undergo aberrant development in vitro with characteristic morphologic and gene expression abnormalities, Brain Res Dev Brain Res, 152 (2004) 153–157. [DOI] [PubMed] [Google Scholar]
- [13].Doulazmi M, Frederic F, Capone F, Becker-Andre M, Delhaye-Bouchaud N, Mariani J, A comparative study of Purkinje cells in two RORalpha gene mutant mice: staggerer and RORalpha(−/−), Brain Res Dev Brain Res, 127 (2001) 165–174. [DOI] [PubMed] [Google Scholar]
- [14].Herrup K, Mullen RJ, Role of the Staggerer gene in determining Purkinje cell number in the cerebellar cortex of mouse chimeras, Brain Res, 227 (1981) 475–485. [DOI] [PubMed] [Google Scholar]
- [15].Yi SS, Hwang IK, Shin JH, Baek SH, Yoon YS, Seong JK, Neuronal differentiation and developmental characteristics in the dentate gyrus of staggerer mutant mice, BMB Rep, 43 (2010) 122–126. [DOI] [PubMed] [Google Scholar]
- [16].Hamilton BA, Frankel WN, Kerrebrock AW, Hawkins TL, FitzHugh W, Kusumi K, Russell LB, Mueller KL, van Berkel V, Birren BW, Kruglyak L, Lander ES, Disruption of the nuclear hormone receptor RORalpha in staggerer mice, Nature, 379 (1996) 736–739. [DOI] [PubMed] [Google Scholar]
- [17].Dussault I, Fawcett D, Matthyssen A, Bader JA, Giguere V, Orphan nuclear receptor ROR alpha-deficient mice display the cerebellar defects of staggerer, Mech Dev, 70 (1998) 147–153. [DOI] [PubMed] [Google Scholar]
- [18].Uhlen M, Fagerberg L, Hallstrom BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson A, Kampf C, Sjostedt E, Asplund A, Olsson I, Edlund K, Lundberg E, Navani S, Szigyarto CA, Odeberg J, Djureinovic D, Takanen JO, Hober S, Alm T, Edqvist PH, Berling H, Tegel H, Mulder J, Rockberg J, Nilsson P, Schwenk JM, Hamsten M, von Feilitzen K, Forsberg M, Persson L, Johansson F, Zwahlen M, von Heijne G, Nielsen J, Ponten F, Proteomics. Tissue-based map of the human proteome, Science, 347 (2015) 1260419. [DOI] [PubMed] [Google Scholar]
- [19].Steinmayr M, Andre E, Conquet F, Rondi-Reig L, Delhaye-Bouchaud N, Auclair N, Daniel H, Crepel F, Mariani J, Sotelo C, Becker-Andre M, staggerer phenotype in retinoid-related orphan receptor alpha-deficient mice, Proc Natl Acad Sci U S A, 95 (1998) 3960–3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ikeda E, Matsunaga N, Kakimoto K, Hamamura K, Hayashi A, Koyanagi S, Ohdo S, Molecular mechanism regulating 24-hour rhythm of dopamine D3 receptor expression in mouse ventral striatum, Mol Pharmacol, 83 (2013) 959–967. [DOI] [PubMed] [Google Scholar]
- [21].Matsui T, Sashihara S, Oh Y, Waxman SG, An orphan nuclear receptor, mROR alpha, and its spatial expression in adult mouse brain, Brain Res Mol Brain Res, 33 (1995) 217–226. [DOI] [PubMed] [Google Scholar]
- [22].Ino H, Immunohistochemical characterization of the orphan nuclear receptor ROR alpha in the mouse nervous system, J Histochem Cytochem, 52 (2004) 311–323. [DOI] [PubMed] [Google Scholar]
- [23].Nakagawa S, Watanabe M, Inoue Y, Prominent expression of nuclear hormone receptor ROR alpha in Purkinje cells from early development, Neurosci Res, 28 (1997) 177–184. [DOI] [PubMed] [Google Scholar]
- [24].Journiac N, Jolly S, Jarvis C, Gautheron V, Rogard M, Trembleau A, Blondeau JP, Mariani J, Vernet-der Garabedian B, The nuclear receptor ROR(alpha) exerts a bi-directional regulation of IL-6 in resting and reactive astrocytes, Proc Natl Acad Sci U S A, 106 (2009) 21365–21370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Chen XR, Heck N, Lohof AM, Rochefort C, Morel MP, Wehrle R, Doulazmi M, Marty S, Cannaya V, Avci HX, Mariani J, Rondi-Reig L, Vodjdani G, Sherrard RM, Sotelo C, Dusart I, Mature Purkinje cells require the retinoic acid-related orphan receptor-alpha (RORalpha) to maintain climbing fiber mono-innervation and other adult characteristics, J Neurosci, 33 (2013) 9546–9562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Takeo YH, Kakegawa W, Miura E, Yuzaki M, RORalpha Regulates Multiple Aspects of Dendrite Development in Cerebellar Purkinje Cells In Vivo, J Neurosci, 35 (2015) 12518–12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Jolly S, Journiac N, Naudet F, Gautheron V, Mariani J, Vernet-der Garabedian B, Cell-autonomous and non-cell-autonomous neuroprotective functions of RORalpha in neurons and astrocytes during hypoxia, J Neurosci, 31 (2011) 14314–14323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Monnier Z, Bahjaoui-Bouhaddi M, Bride J, Bride M, Math F, Propper A, Structural and immunohistological modifications in olfactory bulb of the staggerer mutant mouse, Biol Cell, 91 (1999) 29–44. [PubMed] [Google Scholar]
- [29].Nguyen A, Rauch TA, Pfeifer GP, Hu VW, Global methylation profiling of lymphoblastoid cell lines reveals epigenetic contributions to autism spectrum disorders and a novel autism candidate gene, RORA, whose protein product is reduced in autistic brain, FASEB J, 24 (2010) 3036–3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sarachana T, Hu VW, Genome-wide identification of transcriptional targets of RORA reveals direct regulation of multiple genes associated with autism spectrum disorder, Mol Autism, 4 (2013) 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Sarachana T, Xu M, Wu RC, Hu VW, Sex hormones in autism: androgens and estrogens differentially and reciprocally regulate RORA, a novel candidate gene for autism, PLoS One, 6 (2011) e17116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Sayad A, Noroozi R, Omrani MD, Taheri M, Ghafouri-Fard S, Retinoic acid-related orphan receptor alpha (RORA) variants are associated with autism spectrum disorder, Metab Brain Dis, 32 (2017) 1595–1601. [DOI] [PubMed] [Google Scholar]
- [33].Geoffroy PA, Etain B, Lajnef M, Zerdazi EH, Brichant-Petitjean C, Heilbronner U, Hou L, Degenhardt F, Rietschel M, McMahon FJ, Schulze TG, Jamain S, Marie-Claire C, Bellivier F, Circadian genes and lithium response in bipolar disorders: associations with PPARGC1A (PGC-1alpha) and RORA, Genes Brain Behav, 15 (2016) 660–668. [DOI] [PubMed] [Google Scholar]
- [34].Geoffroy PA, Lajnef M, Bellivier F, Jamain S, Gard S, Kahn JP, Henry C, Leboyer M, Etain B, Genetic association study of circadian genes with seasonal pattern in bipolar disorders, Sci Rep, 5 (2015) 10232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Lai YC, Kao CF, Lu ML, Chen HC, Chen PY, Chen CH, Shen WW, Wu JY, Lu RB, Kuo PH, Investigation of associations between NR1D1, RORA and RORB genes and bipolar disorder, PLoS One, 10 (2015) e0121245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Etain B, Jamain S, Milhiet V, Lajnef M, Boudebesse C, Dumaine A, Mathieu F, Gombert A, Ledudal K, Gard S, Kahn JP, Henry C, Boland A, Zelenika D, Lechner D, Lathrop M, Leboyer M, Bellivier F, Association between circadian genes, bipolar disorders and chronotypes, Chronobiol Int, 31 (2014) 807–814. [DOI] [PubMed] [Google Scholar]
- [37].Ming Q, Wang X, Chai Q, Yi J, Yao S, Retinoid-related orphan receptor alpha (RORA) gene variation is associated with trait depression, Psychiatry Res, 229 (2015) 629–630. [DOI] [PubMed] [Google Scholar]
- [38].Maglione JE, Nievergelt CM, Parimi N, Evans DS, Ancoli-Israel S, Stone KL, Yaffe K, Redline S, Tranah GJ,W. Study of Osteoporotic Fractures in, G. Osteoporotic Fractures in Men Study Research, Associations of PER3 and RORA Circadian Gene Polymorphisms and Depressive Symptoms in Older Adults, Am J Geriatr Psychiatry, 23 (2015) 1075–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hennings JM, Uhr M, Klengel T, Weber P, Putz B, Touma C, Czamara D, Ising M, Holsboer F, Lucae S, RNA expression profiling in depressed patients suggests retinoid-related orphan receptor alpha as a biomarker for antidepressant response, Transl Psychiatry, 5 (2015) e538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Terracciano A, Tanaka T, Sutin AR, Sanna S, Deiana B, Lai S, Uda M, Schlessinger D, Abecasis GR, Ferrucci L, Costa PT Jr., Genome-wide association scan of trait depression, Biol Psychiatry, 68 (2010) 811–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Phillips JR, Hewedi DH, Eissa AM, Moustafa AA, The cerebellum and psychiatric disorders, Front Public Health, 3 (2015) 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hu VW, Sarachana T, Kim KS, Nguyen A, Kulkarni S, Steinberg ME, Luu T, Lai Y, Lee NH, Gene expression profiling differentiates autism case-controls and phenotypic variants of autism spectrum disorders: evidence for circadian rhythm dysfunction in severe autism, Autism Res, 2 (2009) 78–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Schmahmann JD, Weilburg JB, Sherman JC, The neuropsychiatry of the cerebellum - insights from the clinic, Cerebellum, 6 (2007) 254–267. [DOI] [PubMed] [Google Scholar]
- [44].Bugalho P, Correa B, Viana-Baptista M, [Role of the cerebellum in cognitive and behavioural control: scientific basis and investigation models], Acta Med Port, 19 (2006) 257–267. [PubMed] [Google Scholar]
- [45].Ravizza SM, McCormick CA, Schlerf JE, Justus T, Ivry RB, Fiez JA, Cerebellar damage produces selective deficits in verbal working memory, Brain, 129 (2006) 306–320. [DOI] [PubMed] [Google Scholar]
- [46].Konarski JZ, McIntyre RS, Grupp LA, Kennedy SH, Is the cerebellum relevant in the circuitry of neuropsychiatric disorders?, J Psychiatry Neurosci, 30 (2005) 178–186. [PMC free article] [PubMed] [Google Scholar]
- [47].Schmahmann JD, An emerging concept. The cerebellar contribution to higher function, Arch Neurol, 48 (1991) 1178–1187. [DOI] [PubMed] [Google Scholar]
- [48].Caston J, Delhaye-Bouchaud N, Mariani J, Motor behavior of heterozygous staggerer mutant (+/sg) versus normal (+/+) mice during aging, Behav Brain Res, 72 (1995) 97–102. [DOI] [PubMed] [Google Scholar]
- [49].Goldowitz D, Koch J, Performance of normal and neurological mutant mice on radial arm maze and active avoidance tasks, Behav Neural Biol, 46 (1986) 216–226. [DOI] [PubMed] [Google Scholar]
- [50].Lalonde R, Exploration and spatial learning in staggerer mutant mice, J Neurogenet, 4 (1987) 285–291. [PubMed] [Google Scholar]
- [51].Lalonde R, Strazielle C, Discrimination learning in Rora(sg) and Grid2(ho) mutant mice, Neurobiol Learn Mem, 90 (2008) 472–474. [DOI] [PubMed] [Google Scholar]
- [52].Crawford MA, Golfetto I, Ghebremeskel K, Min Y, Moodley T, Poston L, Phylactos A, Cunnane S, Schmidt W, The potential role for arachidonic and docosahexaenoic acids in protection against some central nervous system injuries in preterm infants, Lipids, 38 (2003) 303–315. [DOI] [PubMed] [Google Scholar]
- [53].Haag M, Essential fatty acids and the brain, Can J Psychiatry, 48 (2003) 195–203. [DOI] [PubMed] [Google Scholar]
- [54].Lauritzen L, Hansen HS, Jorgensen MH, Michaelsen KF, The essentiality of long chain n-3 fatty acids in relation to development and function of the brain and retina, Prog Lipid Res, 40 (2001) 1–94. [DOI] [PubMed] [Google Scholar]
- [55].Martinez M, Tissue levels of polyunsaturated fatty acids during early human development, J Pediatr, 120 (1992) S129–138. [DOI] [PubMed] [Google Scholar]
- [56].Brenna JT, Diau GY, The influence of dietary docosahexaenoic acid and arachidonic acid on central nervous system polyunsaturated fatty acid composition, Prostaglandins Leukot Essent Fatty Acids, 77 (2007) 247–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Diau GY, Hsieh AT, Sarkadi-Nagy EA, Wijendran V, Nathanielsz PW, Brenna JT, The influence of long chain polyunsaturate supplementation on docosahexaenoic acid and arachidonic acid in baboon neonate central nervous system, BMC Med, 3 (2005) 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Darios F, Davletov B, Omega-3 and omega-6 fatty acids stimulate cell membrane expansion by acting on syntaxin 3, Nature, 440 (2006) 813–817. [DOI] [PubMed] [Google Scholar]
- [59].Kim HY, Spector AA, N-Docosahexaenoylethanolamine: A neurotrophic and neuroprotective metabolite of docosahexaenoic acid, Mol Aspects Med, 64 (2018) 34–44. [DOI] [PubMed] [Google Scholar]
- [60].Kuperstein F, Eilam R, Yavin E, Altered expression of key dopaminergic regulatory proteins in the postnatal brain following perinatal n-3 fatty acid dietary deficiency, J Neurochem, 106 (2008) 662–671. [DOI] [PubMed] [Google Scholar]
- [61].Das UN, Autism as a disorder of deficiency of brain-derived neurotrophic factor and altered metabolism of polyunsaturated fatty acids, Nutrition, 29 (2013) 1175–1185. [DOI] [PubMed] [Google Scholar]
- [62].Tamiji J, Crawford DA, The neurobiology of lipid metabolism in autism spectrum disorders, Neurosignals, 18 (2010) 98–112. [DOI] [PubMed] [Google Scholar]
- [63].Weiser MJ, Mucha B, Denheyer H, Atkinson D, Schanz N, Vassiliou E, Benno RH, Dietary docosahexaenoic acid alleviates autistic-like behaviors resulting from maternal immune activation in mice, Prostaglandins Leukot Essent Fatty Acids, 106 (2016) 27–37. [DOI] [PubMed] [Google Scholar]
- [64].Shultz SR, MacFabe DF, Ossenkopp KP, Scratch S, Whelan J, Taylor R, Cain DP, Intracerebroventricular injection of propionic acid, an enteric bacterial metabolic end-product, impairs social behavior in the rat: implications for an animal model of autism, Neuropharmacology, 54 (2008) 901–911. [DOI] [PubMed] [Google Scholar]
- [65].Shultz SR, Macfabe DF, Martin S, Jackson J, Taylor R, Boon F, Ossenkopp KP, Cain DP, Intracerebroventricular injections of the enteric bacterial metabolic product propionic acid impair cognition and sensorimotor ability in the Long-Evans rat: further development of a rodent model of autism, Behav Brain Res, 200 (2009) 33–41. [DOI] [PubMed] [Google Scholar]
- [66].Raspe E, Duez H, Gervois P, Fievet C, Fruchart JC, Besnard S, Mariani J, Tedgui A, Staels B, Transcriptional regulation of apolipoprotein C-III gene expression by the orphan nuclear receptor RORalpha, J Biol Chem, 276 (2001) 2865–2871. [DOI] [PubMed] [Google Scholar]
- [67].Mamontova A, Seguret-Mace S, Esposito B, Chaniale C, Bouly M, Delhaye-Bouchaud N, Luc G, Staels B, Duverger N, Mariani J, Tedgui A, Severe atherosclerosis and hypoalphalipoproteinemia in the staggerer mouse, a mutant of the nuclear receptor RORalpha, Circulation, 98 (1998) 2738–2743. [DOI] [PubMed] [Google Scholar]
- [68].Vu-Dac N, Gervois P, Grotzinger T, De Vos P, Schoonjans K, Fruchart JC, Auwerx J, Mariani J, Tedgui A, Staels B, Transcriptional regulation of apolipoprotein A-I gene expression by the nuclear receptor RORalpha, J Biol Chem, 272 (1997) 22401–22404. [DOI] [PubMed] [Google Scholar]
- [69].Lau P, Fitzsimmons RL, Raichur S, Wang SC, Lechtken A, Muscat GE, The orphan nuclear receptor, RORalpha, regulates gene expression that controls lipid metabolism: staggerer (SG/SG) mice are resistant to diet-induced obesity, J Biol Chem, 283 (2008) 18411–18421. [DOI] [PubMed] [Google Scholar]
- [70].Kim K, Boo K, Yu YS, Oh SK, Kim H, Jeon Y, Bhin J, Hwang D, Kim KI, Lee JS, Im SS, Yoon SG, Kim IY, Seong JK, Lee H, Fang S, Baek SH, RORalpha controls hepatic lipid homeostasis via negative regulation of PPARgamma transcriptional network, Nat Commun, 8 (2017) 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Baron-Cohen S, Knickmeyer RC, Belmonte MK, Sex differences in the brain: implications for explaining autism, Science, 310 (2005) 819–823. [DOI] [PubMed] [Google Scholar]
- [72].Chen CT, Kitson AP, Hopperton KE, Domenichiello AF, Trepanier MO, Lin LE, Ermini L, Post M, Thies F, Bazinet RP, Plasma non-esterified docosahexaenoic acid is the major pool supplying the brain, Sci Rep, 5 (2015) 15791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Green VA, Pituch KA, Itchon J, Choi A, O’Reilly M, Sigafoos J, Internet survey of treatments used by parents of children with autism, Res Dev Disabil, 27 (2006) 70–84. [DOI] [PubMed] [Google Scholar]
- [74].Weiser MJ, Wynalda K, Salem N Jr., Butt CM, Dietary DHA during development affects depression-like behaviors and biomarkers that emerge after puberty in adolescent rats, J Lipid Res, 56 (2015) 151–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Bhatia HS, Agrawal R, Sharma S, Huo YX, Ying Z, Gomez-Pinilla F, Omega-3 fatty acid deficiency during brain maturation reduces neuronal and behavioral plasticity in adulthood, PLoS One, 6 (2011) e28451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Chen HF, Su HM, Exposure to a maternal n-3 fatty acid-deficient diet during brain development provokes excessive hypothalamic-pituitary-adrenal axis responses to stress and behavioral indices of depression and anxiety in male rat offspring later in life, J Nutr Biochem, 24 (2013) 70–80. [DOI] [PubMed] [Google Scholar]
- [77].Moriguchi T, Greiner RS, Salem N Jr., Behavioral deficits associated with dietary induction of decreased brain docosahexaenoic acid concentration, J Neurochem, 75 (2000) 2563–2573. [DOI] [PubMed] [Google Scholar]
- [78].Ng KF, Innis SM, Behavioral responses are altered in piglets with decreased frontal cortex docosahexaenoic acid, J Nutr, 133 (2003) 3222–3227. [DOI] [PubMed] [Google Scholar]
- [79].Fedorova I, Alvheim AR, Hussein N, Salem N Jr., Deficit in prepulse inhibition in mice caused by dietary n-3 fatty acid deficiency, Behav Neurosci, 123 (2009) 1218–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Fortunato JJ, da Rosa N, Martins Laurentino AO, Goulart M, Michalak C, Borges LP, da Cruz Cittadin Soares E, Reis PA, de Castro Faria Neto HC, Petronilho F, Effects of omega-3 fatty acids on stereotypical behavior and social interactions in Wistar rats prenatally exposed to lipopolysaccarides, Nutrition, 35 (2017) 119–127. [DOI] [PubMed] [Google Scholar]
- [81].de Theije CG, van den Elsen LW, Willemsen LE, Milosevic V, Korte-Bouws GA, Lopes da Silva S, Broersen LM, Korte SM, Olivier B, Garssen J, Kraneveld AD, Dietary long chain n-3 polyunsaturated fatty acids prevent impaired social behaviour and normalize brain dopamine levels in food allergic mice, Neuropharmacology, 90 (2015) 15–22. [DOI] [PubMed] [Google Scholar]
- [82].Matsui F, Hecht P, Yoshimoto K, Watanabe Y, Morimoto M, Fritsche K, Will M, Beversdorf D, DHA Mitigates Autistic Behaviors Accompanied by Dopaminergic Change in a Gene/Prenatal Stress Mouse Model, Neuroscience, 371 (2018) 407–419. [DOI] [PubMed] [Google Scholar]
- [83].van Elst K, Brouwers JF, Merkens JE, Broekhoven MH, Birtoli B, Helms JB, Kas MJH, Chronic dietary changes in n-6/n-3 polyunsaturated fatty acid ratios cause developmental delay and reduce social interest in mice, Eur Neuropsychopharmacol, 29 (2019) 16–31. [DOI] [PubMed] [Google Scholar]
- [84].Clouard C, Souza AS, Gerrits WJ, Hovenier R, Lammers A, Bolhuis JE, Maternal Fish Oil Supplementation Affects the Social Behavior, Brain Fatty Acid Profile, and Sickness Response of Piglets, J Nutr, 145 (2015) 2176–2184. [DOI] [PubMed] [Google Scholar]
- [85].Mazahery H, Stonehouse W, Delshad M, Kruger MC, Conlon CA, Beck KL, von Hurst PR, Relationship between Long Chain n-3 Polyunsaturated Fatty Acids and Autism Spectrum Disorder: Systematic Review and Meta-Analysis of Case-Control and Randomised Controlled Trials, Nutrients, 9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Bent S, Bertoglio K, Ashwood P, Bostrom A, Hendren RL, A pilot randomized controlled trial of omega-3 fatty acids for autism spectrum disorder, J Autism Dev Disord, 41 (2011) 545–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Voigt RG, Mellon MW, Katusic SK, Weaver AL, Matern D, Mellon B, Jensen CL, Barbaresi WJ, Dietary docosahexaenoic acid supplementation in children with autism, J Pediatr Gastroenterol Nutr, 58 (2014) 715–722. [DOI] [PubMed] [Google Scholar]
- [88].Mankad D, Dupuis A, Smile S, Roberts W, Brian J, Lui T, Genore L, Zaghloul D, Iaboni A, Marcon PM, Anagnostou E, A randomized, placebo controlled trial of omega-3 fatty acids in the treatment of young children with autism, Mol Autism, 6 (2015) 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.