Abstract
Despite the recent rigorous studies towards a possible cure, cancer still remains as one of the most daunting problems faced by the humanity. Currently utilized two-dimensional cancer models are known to have various insuperable limitations such as insufficient biomimicry of the heterogeneous conditions of tumors and their three-dimensional structures. Discrepancies between the laboratory models and the actual tumor environment significantly impair a thorough comprehension of the carcinogenesis process and development of successful remedies against cancer. Modeling tumor microenvironments through bioprinting poses strong potential to minimize the effects of the aforementioned issues thanks to its freeform nature, adaptability, customizability, scalability and diversity. Numerous research studies involving three-dimensional modeling of various cancer types using bioprinting technologies have been reported, recently. In this review, we provide a broad summary of these studies to help better represent their potential and analyze their contribution to cancer research.
Keywords: Bioprinting, Cancer Modelling, 3D Printing, 3D Microenvironment, Cancer
1. Introduction
Cancer is one of the deadliest diseases resulting from the unregulated cell growth leading to the spread or invasion other parts of the body. One of the biggest challenges against cancer treatment is the large variety of cancer types with different gene signatures which makes the development of a generic cure extremely difficult and expensive [1]. In addition, cancer initiation and progression are highly dependent upon the complex, three-dimensional, in vivo tumor microenvironments. Most of the in vitro tumor modelling studies, however, are based upon planar, 2D geometries which are far from mimicking the actual tumor initiation and progression sites. In addition, oversimplified cancer models suffer from the lack of accurate exposition of cancer microenvironment with cell and matrix interactions, which they would normally experience in vivo. These limitations seriously impair the efficiency of cancer research studies. In order to tackle such limitations of the current cancer investigations and broaden the perspective of cancer modeling, bioprinting poses a suitable alternative in creating a more realistic, 3-dimensional cancer microenvironment and the controlled distribution of the tumor cells within this microenvironment [2].
It has been proven by several researchers that the replacement of 2D cancer models with 3D alternatives can lead to significant modifications on gene and protein expressions, gradient profiling of proteins, cell signaling, morphology and viability [3-7]. These observations have evidenced the importance of replicating 3D cancer initiation and progression using the 3D bioprinting process in vitro.
There have been excellent reviews concerning the use of 3D printing technologies for biomedical applications, their benefits and limitations [8, 9]. In this review, however, we focus on specific applications of these technologies for the fabrication of 3-dimensional tumor microenvironments to understand cancer development process. In addition, limitations and the benefits of the most commonly used bioprinting techniques are outlined in this review to guide the cancer researchers in selection of the suitable bioprinting methodology. Reviewing the recent studies in this emerging field will guide cancer researchers to comprehend the capabilities of 3D printing technology and broaden the extents of cancer research by utilizing and further developing these unique technologies.
2. Bioprinting Methods of Cancer Environment
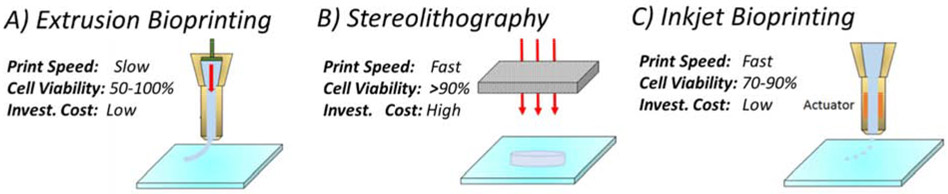
Cancer bioprinting can be defined as the additive manufacturing of a bioink material which includes cancer cells, cancer tissues or other biomaterials to mimic cancer initiation and progression. Numerous studies have investigated 3D printing of cancer cells or the environment surrounding these cells to better understand cancer progression and drug resistance in these 3D printed systems. Existing 3D printing technologies which can be used for 3D cancer modelling can be classified into 3 major categories: extrusion, stereolithography and inkjet printing as shown in Figure1. Unique advantages and limitations of each technique and current applications in cancer research are described in the next section.
Figure 1:
Bioprinting technologies, A) Extrusion bioprinting, B) Stereolithography bioprinting, C) Inkjet bioprinting
2.1. Extrusion Bioprinted Cancer Environments
In extrusion bioprinting, bioink is extruded through the nozzle of the extrusion system by a pneumatic or piston-controlled pressure system and deposited onto a platform bed layer by layer forming a 3D structure (Figure 1A). To obtain the structural integrity of the extruded material, the bioink must have high viscosity which can be achieved by crosslinking the hydrogel bioink by applying external stimulation such as heat application or light projection.
Extrusion process is by far the most commonly used bioprinting technique in cancer research, currently. This is mainly because of ots simplicity, low investment cost and the capability of printing highly viscous bioinks loaded with high density of cancer cells. Multi-material printing is also possible using multiple extrusion channels integrated on the same bioprinting setup. As a result of these benefits, extrusion have been used by numerous researchers investigating different cancer types, including breast, ovarian, breast, oral and cervical cancers.
An initial study in extrusion-based cancer bioprinting was performed by Xu et al in 2010 where human ovarian cancer cells (OVCAR-5) and normal fibroblasts were extruded on a Matrigel substrate in different patterns [10]. Using extrusion bioprinting in this study instead of manual ejection using micropipettes allowed the researchers to enhance the spatial control of cancer cell positioning and the repeatability in test results. Cell viability in this automated, high-throughput cancer cell bioprinting system was more than 90% during printing and continued to proliferate upon patterning, suggesting that the process did not impact the cell viability. The process parameters such as the duration of culturing and droplet deposition speed were demonstrated to affect the resultant cell viability.
In addition to this pioneering study, several proof of concept studies have been introduced evidencing the importance of extrusion bioprinting in cancer research. Lee et al. utilized an extrusion-based bioprinting method to fabricate a type of brain tumor glioblastoma multiforme (GBM) [11]. In this study, 3-dimensional GBM-vascular niche model was bioprinted to analyze the cell-cell interaction where a patient-derived GBM cell cluster was positioned by fluidic vessel. Similarly, Almela et al. bioprinted 3-D multi-layered oral cancer model as a representative tool to engineer and study oral cancer at different anatomical levels [12]. Scaffold-free extrusion printing of glioma stem cells was studied by Van Pel et al. through the use of a customized technology that comprises a spheroid dispensing printer with 9×9 array of needles [13]. This platform allowed examining the invasion human glioma cells into different neural progenitor cell-derived spheroids, thus mimicking differences that might be observed in patient brain tissue. Extrusion bioprinting was also integrated with the magnetic levitation to form tumor spheres where printed cancer cells were magnetically levitated to form 3-D tumor structures (tumor sphere) mimicking those in vivo [14]. This unique bioprinting platform allowed the fabrication of tumor spheres with defined cellular composition and density, as quickly as less than 24 hours. In vitro cervical tumor modeling was conducted by Pang et al. by extrusion of HeLa cells composed of gelatin/alginate/Matrigel bioink with more than 95% viability of HeLa cells. In this research, epithelial-to-mesenchymal transition, which is an important stage of dissemination of carcinoma leading to metastatic tumors was studied. HeLa cells rapidly proliferated into spheroids and exhibited tumorigenic characteristics in the 3D-printed structure [15].
Aforementioned extrusion-based fabrication platforms have provided realistic tumor microenvironments to better understand the tumor initiation and progression mechanisms and develop cancer drugs with higher efficacies. Zhao et al. used extrusion-based bioprinting to prepare an in vitro cervical tumor model for drug testing with more than 90% cell viability [16]. In this study, utilization of bioprinting significantly enhanced the proliferation rate, matrix metallopeptidases (MMP) protein expression and chemoresistance compared to 2D culturing. In another research involving 3D modeling of glioma, Dai et al. demonstrated the extrusion printing of these cells for in vitro brain tumor modeling and their applications on drug susceptibility. Using modified porous gelatin / alginate / fibrinogen bioink mimicking the extracellular matrix, high cell viability of 87% was achieved in this study [17]. Drug-sensitivity results showed that 3D printed tumor model was more resistant to temozolomide drug than 2D monolayer model which showed the importance of using the 3D bioprinted model for studying gliomagenesis, glioma stem cell biology, drug resistance, and anticancer drug susceptibility in vitro. Lastly, in an inspiring study, Heinrich et al. investigated bioprinting of mini-brain tissues consisting of glioblastoma cells and macrophages to examine the interactions of these two cell types and effect of therapeutics that target this particular interaction [18]. They concluded that, the glioblastoma cells effectively recruited glioblastoma-associated macrophages (GAMs) and polarized them into a GAM-specific phenotype. As demonstrated in this study, bioprinting creates the controlled microenvironment (cell density, cell distance and extracellular matrix) in complex 3D geometry which would not be possible with the conventional cancer test platforms in 2D.
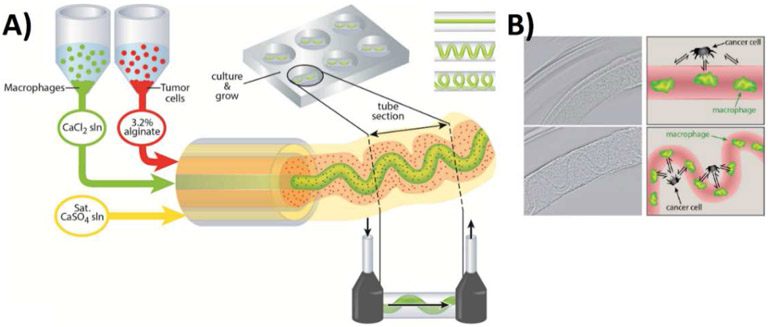
Extrusion bioprinting has also been preferred by cancer researchers since it is the only bioprinting technique which makes the fabricating core-shell type of biomaterial structures possible. Fabricating multiple biomaterials in a core-shell configuration allows investigation of specific cell-cell and cell-extracellular matrix interactions. This process is also known as coaxial extrusion. Grolman et al., used this technique to form a microenvironment composed of multiple cell types, human breast adenocarcinoma (MDA-MB-231) and mouse macrophage (RAW 264.7) to demonstrate the interactions between the two cell lines as shown in Figure 2 [19]. Peptide conjugated alginate fibers along with macrophages in the core and tumor shells were deposited in a core-shell geometry. Using different printing parameters, a range of geometric architectures (straight, serpentine and helically packed) were obtained and multiple cell types were analyzed in these complex 3D microenvironments. The migration of segregated tumor cells and macrophages is explored using drugs that inhibit heterotypic interactions.
Figure 2:
A-coextrusion system for bioprinting of MDA-MB231 breast cancer cells and RAW 264.7 macrophages. B- Illustration of formation of straight and patterned hollow alginate structures and comparison of how the arrangement of cells may affect their signaling in naturally occurring architectures and model systems. Adapted with permission from [19].
Similarly, high heterogeneity in glioma tumor microenvironments may be mimicked through coaxial extrusion, which was used by Wang et al. to model and examine the drug resistance of glioma cells in vitro [20]. The coaxial extrusion system in this study comprised glioma stem cells (GSC23) in the shell and glioma cell line (U118) in the core as alginate bioink which resulted in high cell viability. It was reported that, U118 cells proliferated into fiberlike cell aggregates that resulted in enhanced cell-cell and cell-extracellular matrix (ECM) interactions in the presence of Temozolomide.
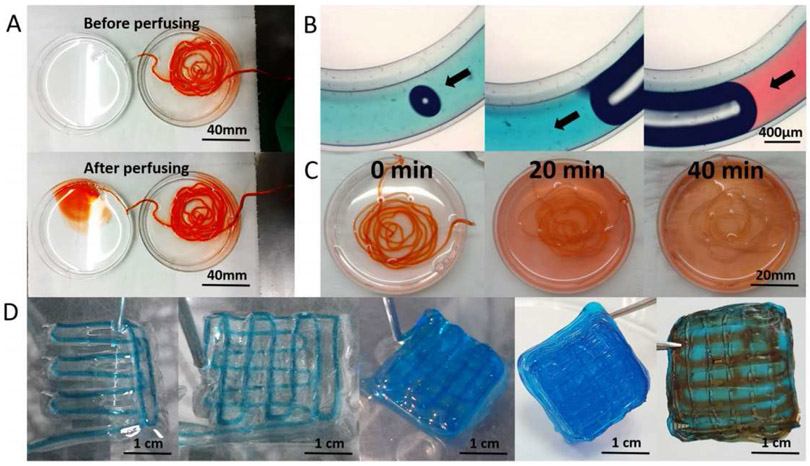
Another modified extrusion-based bioprinting method with coaxial nozzle was demonstrated by Dai et al. to fabricate self-assembled multicellular heterogeneous brain tumor fibers in vitro [21]. This custom-made coaxial extrusion bioprinting system allowed high cell viability, proliferative activity and efficient tumor-stromal interactions as shown in Figure 3. It was reported in this study that tumor-stroma cells interacted with each other and fused together. Tumor-stroma cell interaction was higher in 3D printed model than that of 2D culture model as quantified by the transcription of the fluorescence labeled genes. These results evidence that coaxial 3D bioprinted tumor tissue-like fibers provided preferable 3D models for studying tumor microenvironment in vitro, especially for tumor-stromal interactions.
Figure 3:
A,B) Flow behavior of the extruded fiber structures before and after perfusing, C) Permeability and diffusion of the dye in 40 minutes, D) 90° orientation of the layers during bioprinting and subsequent perfusion of the dyes. Uniform diffusion of the red dye evidenced the high penetrability of the printed alginate-gelatin shell system, allowing high cell migration levels. Adapted with permission from [21].
Extrusion is a simple and cost-effective bioprinting process. High customizability of this technique renders it feasible to implement innovative modifications on the printing system, such as co-extrusion and multi-material printing. However, the shear stress induced during the extrusion process may cause cell deformation and damage. Therefore, cell viability may be lowered if the process parameters such as biomaterial concentration, nozzle pressure, and nozzle diameter are not successfully optimized.
2.2. Stereolithography Bioprinted Cancer Environments
Stereolithography bioprinting is based on the concept of photopolymerization of a bioink which consists of light-sensitive polymers (Figure 1B). The pattern of interest is selectively cured layer by layer to create 3D biomaterial. Light can be applied on the biomaterial in terms of raster scanning of the laser or 2D projection to make the desired pattern. Projecting the 2D image leads to a significant increase in printing speed compared to the line-by-line raster scanning. Stereolithography has higher resolutiuon compared to extrusion bioprinting. UV light source is preferred due to its high energy to cure the photopolymer at high speed. Stereolithography bioprinters provide high cell viability (>85%). Unlike extrusion process, selective crosslinking of bioink by light does not result in any shear stress on cells in stereolithography bioprinting.
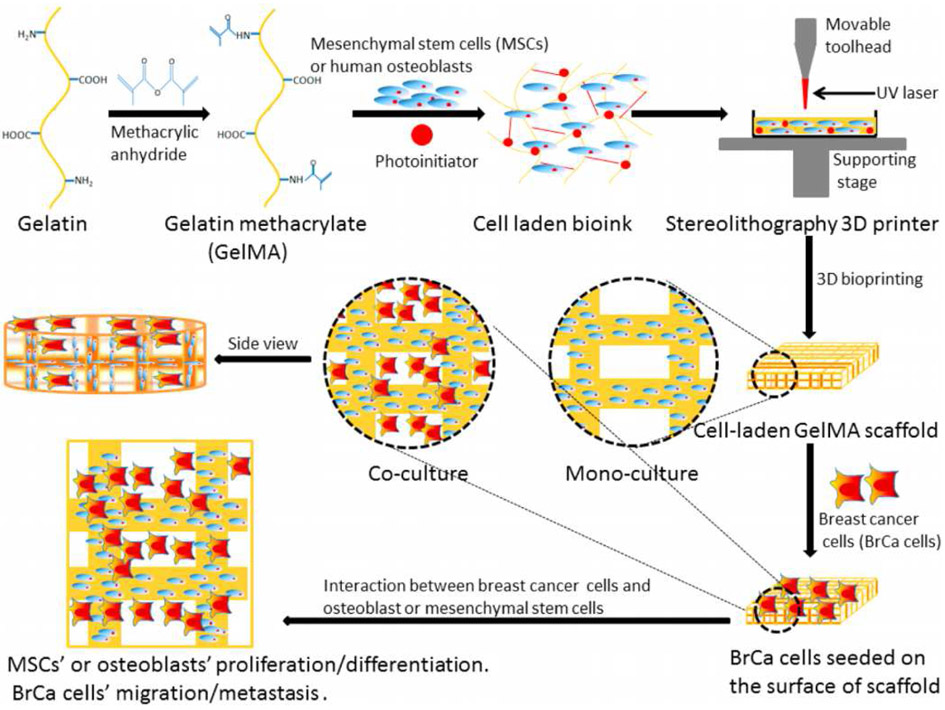
Such advantages make stereolithography an important technique for fabricating cancer bioenvironment at high speed and high resolution. In this regard, Zhou et al. [22] utilized a small-scale, stereolithography bioprinter to produce in vitro biomimetic bone biostructure and investigated the effect of breast cancer (BrCa) on bone stromal cells, as illustrated in Figure 4. It was reported in this study that, the growth of BrCa cells increased in the presence of osteoblasts or mesenchymal stem cells (MSCs) and the proliferation of the latter was negatively impacted by the BrCa cells. They also compared the vascular endothelial growth factor (VEGF) secretion between BrCa cells that were monocultured and co-cultured with MSCs or osteoblasts and reported an increase of VEGF along with a reduction in the alkaline phosphatase activity in the latter. Therefore, biomimetic bone structure fabricated via stereolithography bioprinting created a bonelike microenvironment and allowed cross-talk between different cell types in 3D similar to that observed in vivo.
Figure 4:
Direct bioprinting of cell-laden bone matrix and subsequent invasion of seeded BrCa cells thereon for biomimetic model for BrCa metastasis by Zhou et al. Adapted with permission from[22].
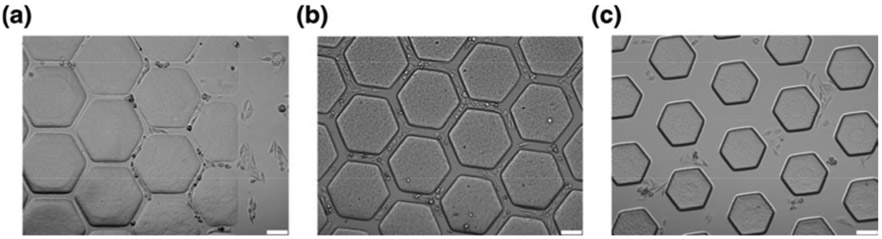
Another stereolithography based approach was utilized by Huang et al. to investigate the migration of cancerous HeLa cells and non-cancerous fibroblasts on complex 3D surfaces similar to those in vivo [23]. Unlike raster scanning the UV laser to create 2D pattern, a UV projection system was used in this study which enhanced the bioprinting speed significantly. Honeycomb microchips were printed with stereolithography using polyethyleneglycol diacrylate (PEGDA) bioink due to its high-water content, biocompatibility, and tunable mechanical properties. Honeycomb hydrogel consisted of a microvascular system with channel widths of 25, 45, and 120 μm to reflect effect of blood vessel diameters as shown in Figure 5. Normal and cancerous cells were then seeded in these microchannels to investigate their migration properties. This study concluded that, channel width was not significantly effective on 10T1/2 cell migration, whereas HeLa cancer cell migration exhibited inverse relation with channel width. This study also evidenced the importance of using stereolithography bioprinting in cancer research since it allows the formation of cancer microenvironments at high resolution (< 25 μm) which cannot be achieved with extrusion bioprinting.
Figure 5:
Optical microscopy of the bioprinted PEGDA microstructures with various values of width: a) 25 μm, b) 45 μm, c) 120 μm. In this research, 3D vascularization was succeeded through printing of a scaffold that was obtained using tomographic scan of rat vessels. Each width was printed to test cell migration behavior through vessels with different diameters. Adapted with permission from [23].
To explore cancer migration in bone-like microenvironment, Zhu et al. 3D-printed hydrogels filled with hydroxyapatite nanoparticles [24]. Bone marrow mesenchymal stem cells were stereolithography bioprinted and co-cultured with tumor cells after which spheroid clusters were observed. Different geometries were fabricated such as square and hexagon lattice structures and cell-cell interactions as well as drug response of cancer cells in these complex microenvironments were measured and compared against the conventional models where cells are seeded in 2D. It was shown that, breast cancer cells growing on the 3D scaffold exhibited a greater migration capacity compared with conventional 2D cell culture. In addition, 3D printed matrix showed greater drug resistivity compared to 2D models. Therefore, 3D printed scaffolds provided biomimetic microenvironments for breast cancer cell growth and may be used to study the behavior of breast cancer invasion and the evaluation of new therapies.
Stereolithography bioprinting has great potential for cancer research due to its high resolution and high speed (especially when light projection is used). However, bioink must be transparent in stereolithography bioprinting to allow light to pass the material and crosslink the photopolymer without significant scattering. To minimize light scattering and homogenous crosslinking, cancer cell density is usually kept at low level which is another limiting factor for this technique. Stereolithography printing process is more complex compared to the extrusion which increases the cost of the printer instrument and the difficulty of its customization.
2.3. Inkjet Bioprinted Cancer Environments
Inkjet bioprinting is another bioprinting methodology where the bioink is sprayed (or jetted) onto a surface in a drop-on-demand fashion. In this bioprinting technique, bioink deposition can be regulated by thermal or piezoelectric actuation as shown in Figure 1C. In thermal actuation, heating element near the nozzle increases the bioink temperature forming a bubble. This bubble forces the bioink to move out of the nozzle orifice. In piezoelectric actuation however, inkjet printer utilizes piezoelectric element to generate pressure pulse which leads to dispensing of liquid through the nozzle orifice. Similar to the extrusion bioprinters, inkjet bioprinters are relatively cheap and simple manufacturing systems. However, the speed of inkjet printing is much faster since multiple printheads can work in parallel (rather than in series in extrusion bioprinting) which allows deposition of multiple cell types at high speed. Due to the precise control of the drop-wise bioink deposition, inkjet printing has high resolution (~30 μm) [25].
Regarding breast cancer, Ling et al. demonstrated a customized inkjet bioprinting system that uses cell-embedded hydrogel arrays deposited using drop-on-demand strategy onto polyethylene glycoldimethacrylate chips to study cell-cell interaction in breast cancer modeling [26]. The biocompatible gelatin arrays with human breast cancer cells (MCF-7) were used to produce Polyethylene Glycol – Dimethacrylate (PEG-DMA) wells. The utilized systematic fabrication of concave-wells followed by in situ cell seeding is as shown in Figure 6 below.
Figure 6:
Fabrication of hydrogel concave cells with printed gelatin arrays for breast cancer modeling by Ling et al. A-C) Printed gelatin arrays with various sizes on petri dish, D-F) Formation of wells on the PEG-DMA substrate upon molding. Reused with permission from [26].
This inkjet bioprinting system allowed fabrication of cellular breast cancer spheroids in a high-throughput, flexible, and controlled manner. Fabricating uniform spheroids on microchips at high speeds holds great potential for the pathological studies and the screening of cancer drugs since these spheroids serve as 3-dimensional cancer models mimicking the cancer microenvironments in vivo.
In addition to high-throuhput and high resolution benefits, inkjet bioprinting results in relatively high cell viability for especially piezoelectric actuation systems. The main limitation of the inkjet printing is the low viscosity requirement of bioink (~0.1 Pa.s) [27]. This requirement makes the printing of viscous hydrogels and extracellular matrix rather difficult. Cell aggregation and nozzle clogging are also commonly observed issues when high cell densities are used. Therefore, the printing is usually operated with low cell density bioinks to assure printability.
3. Summary of the Previously Reported Cancer Bioprinting Studies
The versatility and boosted freedom of design offered by 3D printing techniques have opened a great range of opportunities especially for in vitro monitoring of cancer progression with increased control on the extracellular matrix. Bioprinting has significantly improved biomimicry of cancer modeling compared to conventional tissue engineering, thanks to the enhanced accuracy and composition of tumor environment coupled with the availability of improved vascularization [28, 29]. Existing studies utilizing cancer bioprinting are summarized in the following table where the bioink composition, investigated cancer type and the cell viability in these studies are described briefly.
4. Conclusion and Future Outlook
In this review, we explored cancer bioprinting technologies which are used to mimic tumor microenviroments in vivo. The major advantage of bioprinting is that it allows more realistic, accurate and facile 3D tumor modeling compared to the well-established 2D techniques. As a result, bioprinting technologies have attracted growing interest by the cancer research commmunity to further illuminate the cancer progession, in vivo cell interactions, drug efficiency and treatment methods against different cancer types.
Existing bioprinting methods used in cancer research are limited to extrusion, stereolithography and inkjet printing. Each of these methods have certain advantages and limitations. The unsophisticated, inexpensive nature and high cell viability yield of extrusion printing has made it a preferred method for 3D modeling of tumors, especially breast cancer. In addition, coaxial extrusion modification has been proven to create unique cancer microenvironments for more accurate modeling of cancer cell interactions with the surrounding cells and the extracellular matrix. On the other hand, stereolithography bioprinting is known to offer high dimensional accuracy and speed to fabricate complex 3D microenvironments. However, these advantages come along with a compromise in cell viability due to the harmful effects of UV light source on DNA of cancer cells. Instead of using UV light, a visible light could be used as an alternative light source in future cancer bioprinting research to avoid the cell damage and enhance viability. High investment cost is also another drawback of stereolithography methods. Inkjet printing offers high throuput cancer bioprinting by jetting biomaterials with multiple nozzles simultaneously. Low cell density is the major requirement of this technology.
The cell viability, which is a crucial parameter for cancer modeling is in the constant scope of cancer bioprinting. Highly flexible or compliant hydrogel bioinks may effectively mimic the in vivo environment, allow growth and migrataion of cells, and hence maximize the cell viability. However, printing of these bioinks is rather difficult since the printed biomaterial must overcome the gravitaional forces and retain its shape after printing. New natural and synthetic hydrogels with optimized mechanical properties and enhanced high viability are being developed currently and these novel bioinks can significantly enhance the accuracy of 3D bioprinted cancer models.
Another shortcoming in the existing cancer bioprinting is the lack of resemblance of the bioink-extracellular matrix encapsulating the cancer cells to those in vivo. The extracellular matrix is a 3-dimensional network of extracellular proteins (collagen, fibrinogen, glycoproteins, enzymes etc.) and it involves numerous biological functions including the cellular growth, tissue repair and remodeling. Current bioinks used in cancer bioprinting includes one or a mix of these proteins to support the cellular functions during and after the printing process. These bioinks however can hardly mimic the native extracellular properties due to the complexity of the native ECM structure. Decellularized extracellular matrix (dECM) is a promising candidate as a bioink precursor since it has the right structure and inductive cues to drive cellular growth and differentiation. This novel bioink material can be synthesized from the patients’ own tissues and used in bioprinting to better mimic the cancer microenvironment in vivo.
As an efficient tumor modeling system in the stage of infancy, bioprinting has been applied to a limited variety of cancers: breast, brain, cervical, oral, ovarian and glioblastoma. Further development of bioink preparation and bioprinting process can significantly improve the cancer drug efficacy and explore tumor cell interaction during initiation and progression steps. Rapidly advancing pace of bioprinting technology is expected to cover a wider range of cancer types in the future and make a greater impact in biomedical science and engineering.
Table 1:
Summary of recent research studies on cancer bioprinting
| Bioink Composition | Viability | Cancer Type | Method | Ref |
|---|---|---|---|---|
| Ovarian Cancer Cells / Fibroblasts + Matrigel | >95% | Ovarian | Extrusion | [10] |
| Endothelial Cells + Glioma Stem Cells + Collagen/Laminin | N/A | Brain | Extrusion | [11] |
| β-Tricalciumphosphate | N/A | Oral | Extrusion | [12] |
| U118 glioma + Pluripotent Stem Cell Derived Neural Organoid | N/A | Brain | Extrusion | [13] |
| MDA-MB-231/IMR-90 MCTS | High | Breast | Extrusion | [14] |
| HeLa + Gelatin/Alginate/Fibrinogen | >95% | Cervical | Extrusion | [15] |
| HeLa + Gelatin / Alginate / Fibrinogen | >90% | Cervical | Extrusion | [16] |
| Human Glioma Stem Cells+Gelatin/Alginate/Fibrinogen | 87% | Brain | Extrusion | [17] |
| GAM + GBM+Gelatin Methacryloyl /Gelatin | High | Brain | Extrusion | [18] |
| Breast Adenocarcinoma + Mouse Macrophage+ Sodium alginate | >90% | Breast | Coaxial Extrusion | [19] |
| GSC123+U118+ Sodium alginate | >90% | Brain | Coaxial Extrusion | [20] |
| GSC23 +HMSCs+Sodium Alginate/Gelatin | >90% | Brain | Coaxial Extrusion | [21] |
| Mesenchymal Stem Cells + Gelatin Methacrylate | 30% - 50% | Breast | Stereolithography | [22] |
| HeLa + 10 T1/2+PEGDA | N/A | Cervical | Stereolithography | [23] |
| hBMSCs+ MDA-MB-231/MCF-7 | 53% - 80% | Breast | Stereolithography | [24] |
| MCF-7+PEG | >90% | Breast | Inkjet | [26] |
6. Acknowledgements
Dr. Leblanc appreciates the financial support by National Science Foundation under Grant 011298 and National Institute of Health under Grant 012394.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare no conflict of interest.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
7. References
- 1.Palanisamy N, et al. , Rearrangements of the RAF kinase pathway in prostate cancer, gastric cancer and melanoma. 2010. 16(7): p. 793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knowlton S, et al. , Bioprinting for cancer research. 2015. 33(9): p. 504–513. [DOI] [PubMed] [Google Scholar]
- 3.Ridky TW, et al. , Invasive three-dimensional organotypic neoplasia from multiple normal human epithelia. 2010. 16(12): p. 1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghosh S, et al. , Three-dimensional culture of melanoma cells profoundly affects gene expression profile: A high density oligonucleotide array study. 2005. 204(2): p. 522–531. [DOI] [PubMed] [Google Scholar]
- 5.Kim BJ, et al. , Cooperative roles of SDF-1α and EGF gradients on tumor cell migration revealed by a robust 3D microfluidic model. 2013. 8(7): p. e68422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zervantonakis IK, et al. , Three-dimensional microfluidic model for tumor cell intravasation and endothelial barrier function. 2012. 109(34): p. 13515–13520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li C-L, et al. , Survival advantages of multicellular spheroids vs. monolayers of HepG2 cells in vitro. 2008. 20(6): p. 1465–1471. [PubMed] [Google Scholar]
- 8.Ventola CL, Medical Applications for 3D Printing: Current and Projected Uses. P T, 2014. 39(10): p. 704–11. [PMC free article] [PubMed] [Google Scholar]
- 9.Ahangar P, et al. , Current Biomedical Applications of 3D Printing and Additive Manufacturing. Applied Sciences-Basel, 2019. 9(8). [Google Scholar]
- 10.Xu F, et al. , A three-dimensional in vitro ovarian cancer coculture model using a high-throughput cell patterning platform. Biotechnology Journal, 2011. 6(2): p. 204–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee VK, et al. , Generation of 3-D Glioblastoma-Vascular Niche using 3-D Bioprinting. 2015 41st Annual Northeast Biomedical Engineering Conference (Nebec), 2015. [Google Scholar]
- 12.Almela T, et al. , 3D printed tissue engineered model for bone invasion of oral cancer. Tissue Cell, 2018. 52: p. 71–77. [DOI] [PubMed] [Google Scholar]
- 13.van Pel DM, et al. , Modelling glioma invasion using 3D bioprinting and scaffold-free 3D culture. Journal of Cell Communication and Signaling, 2018. 12(4): p. 723–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leonard F and Godin B, 3D In Vitro Model for Breast Cancer Research Using Magnetic Levitation and Bioprinting Method. Methods Mol Biol, 2016. 1406: p. 239–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pang Y, et al. , TGF-beta induced epithelial-mesenchymal transition in an advanced cervical tumor model by 3D printing. Biofabrication, 2018. 10(4): p. 044102. [DOI] [PubMed] [Google Scholar]
- 16.Zhao Y, et al. , Three-dimensional printing of Hela cells for cervical tumor model in vitro. Biofabrication, 2014. 6(3): p. 035001. [DOI] [PubMed] [Google Scholar]
- 17.Dai X, et al. , 3D bioprinted glioma stem cells for brain tumor model and applications of drug susceptibility. Biofabrication, 2016. 8(4): p. 045005. [DOI] [PubMed] [Google Scholar]
- 18.Heinrich MA, et al. , 3D-Bioprinted Mini-Brain: A Glioblastoma Model to Study Cellular Interactions and Therapeutics. Adv Mater, 2019. 31(14): p. e1806590. [DOI] [PubMed] [Google Scholar]
- 19.Grolman JM, et al. , Rapid 3D Extrusion of Synthetic Tumor Microenvironments. Adv Mater, 2015. 27(37): p. 5512–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X, et al. , Coaxial extrusion bioprinted shell-core hydrogel microfibers mimic glioma microenvironment and enhance the drug resistance of cancer cells. Colloids Surf B Biointerfaces, 2018. 171: p. 291–299. [DOI] [PubMed] [Google Scholar]
- 21.Dai X, et al. , Coaxial 3D bioprinting of self-assembled multicellular heterogeneous tumor fibers. Sci Rep, 2017. 7(1): p. 1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou X, et al. , 3D Bioprinting a Cell-Laden Bone Matrix for Breast Cancer Metastasis Study. ACS Appl Mater Interfaces, 2016. 8(44): p. 30017–30026. [DOI] [PubMed] [Google Scholar]
- 23.Huang TQ, et al. , 3D printing of biomimetic microstructures for cancer cell migration. Biomed Microdevices, 2014. 16(1): p. 127–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu W, et al. , 3D printed nanocomposite matrix for the study of breast cancer bone metastasis. Nanomedicine, 2016. 12(1): p. 69–79. [DOI] [PubMed] [Google Scholar]
- 25.Kim YK, et al. , Drop-on-demand inkjet-based cell printing with 30-mum nozzle diameter for cell-level accuracy. Biomicrofluidics, 2016. 10(6): p. 064110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ling K, et al. , Bioprinting-Based High-Throughput Fabrication of Three-Dimensional MCF-7 Human Breast Cancer Cellular Spheroids. Engineering, 2015. 1(2): p. 269–274. [Google Scholar]
- 27.Yi HG, Lee H, and Cho DW, 3D Printing of Organs-On-Chips. Bioengineering (Basel), 2017. 4(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang B, et al. , 3D bioprinting: an emerging technology full of opportunities and challenges. Bio-Design and Manufacturing, 2018. 1(1): p. 2–13. [Google Scholar]
- 29.Mehrotra S, et al. , 3D printing/bioprinting based tailoring of in vitro tissue models: Recent advances and challenges. ACS Applied Bio Materials, 2019. [DOI] [PubMed] [Google Scholar]