http://aasldpubs.onlinelibrary.wiley.com/hub/journal/10.1002/(ISSN)2046-2484/video/15-1-reading-klair a video presentation of this article
http://aasldpubs.onlinelibrary.wiley.com/hub/journal/10.1002/(ISSN)2046-2484/video/15-1-interview-murali the interview with the author
Abbreviations
- AASLD
American Association for the Study of Liver Diseases
- ALT
alanine aminotransferase
- APASL
Asian Pacific Association for the Study of the Liver
- AST
aspartate aminotransferase
- CHB
chronic hepatitis B
- CI
confidence interval
- CTL
cytotoxic T lymphocyte
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- IA‐CHB
immune active chronic hepatitis B
- IT
immune‐tolerant
- IT‐CHB
immune‐tolerant chronic hepatitis B
- SD
standard deviation
Immune‐tolerant (IT) chronic hepatitis B (IT‐CHB) phase is defined as the presence of hepatitis B surface antigen for ≥6 months, HBeAg positivity, high hepatitis B virus (HBV)‐DNA level (usually >1 million IU/mL per the American Association for the Study of Liver Diseases [AASLD], >20,000 IU/mL per the Asian Pacific Association for the Study of the Liver [APASL]), and normal alanine aminotransferase (ALT) and/or aspartate aminotransferase (AST) levels.1 Liver biopsy, if performed, should show no or minimal inflammation and/or fibrosis.1 AASLD guidelines do not recommend treatment for IT‐CHB based on four important assumptions as highlighted later. Recent evidence, however, suggests that these assumptions may not be true. We have discussed each of these assumptions in detail in this review.
Assumption 1
Patients with CHB with normal ALT level and high viral load have a healthy liver and do not have hepatic inflammation. This is not necessarily true because studies that have evaluated the presence of underlying hepatic inflammation in patients with CHB with high viral load and normal liver enzymes have shown varied results. Several studies have demonstrated that a significant proportion of patients with CHB (20%) with persistently normal ALT level have underlying hepatic inflammation (Table 1).2, 3, 4 These studies have clearly established the fact that persistently normal ALT level does not necessarily mean a normal healthy liver. A liver biopsy would thus be needed in patients with IT‐CHB to definitively rule out underlying hepatic inflammation or fibrosis, even in those with persistently normal liver enzymes. However, regular liver biopsies to determine the presence of hepatic inflammation is impractical, is not routinely performed in clinical practice in IT‐CHB, and also is not recommended to be performed routinely by the AASLD guidelines. Recent immunological studies also seem to support the notion that patients with IT‐CHB may not have an immune profile of T cell tolerance. Kennedy et al.5 in their study analyzed global T cell cytokine profiles, expression of T cell exhaustion markers, and HBV‐specific T cell responses in patients with CHB with varying age groups. They showed that there was no difference in the T cell cytokine profile between IT‐CHB and immune active CHB (IA‐CHB). In addition, they also showed that young adults had more HBV‐specific T cells capable of proliferating and producing cytokines as compared with their older counterparts. These results are not conclusive given the small sample size of the cohorts, but it does raise concerns that young adults may not be as tolerant to HBV antigens as previously thought. Thus, by not treating patients with IT‐CHB who have normal liver enzymes, we are likely missing out on treating a significant proportion of patients with underlying hepatic inflammation, thus maybe putting them at risk for the development of cirrhosis and hepatocellular carcinoma (HCC).
Table 1.
Underlying Inflammation and Fibrosis in Patients With CHB With Persistently Normal ALT Level
| Study Author (Year of Publication) | Mean Age, Years (95% CI or ±SD) | Baseline HBV DNA, log copies/mL (95% CI)* | Hepatic Inflammation (%) | Hepatic Fibrosis (%) |
|---|---|---|---|---|
| Seto et al.4 (2012) | 32 (17‐43) | 8.14 (4.83‐10.96) | 22.5 | 22.5 |
| Kumar et al.2 (2008) | 27.7 ± 15.3 | 5.23 (2.8‐9.3) | – | 39.7 |
| Lai et al.3 (2007) | 37 (33‐40) | 6.3 (5.9‐6.8) | 18 | 34 |
Studies performed in Asia define IT‐CHB as HBV viral load >20,000 IU/mL and normal ALT level as per APASL guidelines.
Assumption 2
Young patients with chronic HBV and normal liver enzymes are unlikely to have hepatic fibrosis. Studies that have evaluated the natural history of CHB have shown that young adults mostly convert from the IT stage to the immune active stage (IA‐CHB) in their third decade of life. In fact, the AASLD recommendation states that “adults >40 years” are to be considered for antiviral therapy despite a high viral load, and normal ALT, if biopsy shows necroinflammation or fibrosis.1 This may reduce the attention given to adults younger than 40 years with high viral load and normal liver enzymes who may have necroinflammation on liver histology. Not all young adults with CHB are IT. Studies have shown that a sizable proportion of young patients with CHB, high viral load, and normal liver enzymes may have greater than F2 fibrosis even before they reach the fourth decade of life (Table 1).2, 3, 4 In addition, high HBV DNA level such as that seen in IT‐CHB has been strongly associated with the development of cirrhosis in patients with CHB. These findings imply that the process of fibrogenesis may have begun at a young age when they are still in the IT phase. Worldwide, CHB has a low therapeutic coverage, and this is partly due to the restrictive treatment recommendations. Offering treatment early to patients with IT‐CHB will increase the overall treatment rate of patients with CHB and may decrease the risk of development of cirrhosis and cirrhosis‐related complications.
Assumption 3
IT‐CHB is a benign phase and does not contribute to the development of HCC. We believe that this is the most questionable assumption of all because evidence suggests that this may not be true. A large‐scale study performed in Taiwan in patients with CHB who acquired hepatitis B infection in the perinatal period showed that there was sharp increase in the incidence of HCC as early as age 30 years.6 It is well‐known that HBV infection acquired in the perinatal period is characterized by a prolonged immunotolerant phase. The fact that patients with CHB may develop HCC as early as age 30 years suggests that the process of oncogenesis of HCC begins in the IT‐CHB phase. This has also been supported by recent molecular studies. In patients with CHB, there is a decline in the fraction of hepatocytes infected by HBV over time, because these infected hepatocytes are killed by cytotoxic T lymphocytes (CTLs). This leads to clonal expansion of epigenetically altered hepatocytes that resist infection by HBV. These foci of altered hepatocytes also resist death by CTLs and thus can evolve into HCC (Fig. 1). Interestingly, Mason et al.7 demonstrated the presence of clones of epigenetically altered hepatocytes in patients with IT‐CHB. In addition, they also showed that the rates of HBV DNA integration and clonal hepatocyte expansion were similar in patients with IT‐CHB and patients with IA‐CHB, suggesting that IT‐CHB is not a benign phase and likely contributes to the development of HCC.
Figure 1.
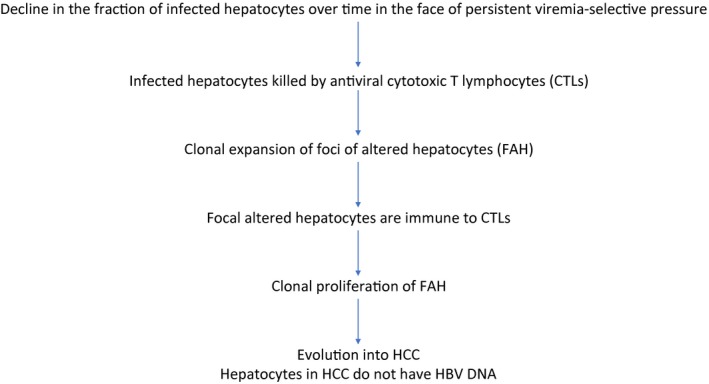
Summary of molecular and immunological features in patients with IT‐CHB.
Assumption 4
Treatment of patients with IT‐CHB may not be effective and may result in resistance to antiviral agents. In patients with IT‐CHB, rate of seroconversion of hepatitis e antigen with antiviral agents is low. However, antiviral therapy does result in effective suppression of HBV DNA level in patients with IT‐CHB.8, 9 HBV DNA level has been shown to be a significant predictor for the development of HCC, and thus may be used as a surrogate marker for development of HCC.10 In patients with IT‐CHB, antiviral therapy by decreasing the HBV viral load may decrease the risk for development of HCC. In addition, prior studies have demonstrated very low risk for resistance to antiviral therapy with both entecavir and tenofovir in patients with IT‐CHB.8, 9
Summary
Young adults with IT‐CHB (high viral load and normal liver enzymes) may have underlying hepatic inflammation, fibrosis, or both. Young adults with CHB are also at risk for development of HCC as early as age 30 years, suggesting that the process of development of HCC begins in the IT‐CHB phase. Patients with IT‐CHB seem to have a similar T cell cytokine profile, HBV DNA integration, and clonal expansion of altered hepatocytes as compared with IA‐CHB, suggesting that the IT‐CHB phase may not be a benign phase and may lead to the evolution of cirrhosis and HCC. Newer antiviral agents effectively suppress HBV DNA in patients with IT‐CHB with minimal drug resistance, and thus may decrease the development of cirrhosis and HCC.
In conclusion, patients with IT‐CHB should be considered for treatment with antiviral therapy. This is likely to improve the overall treatment rates for CHB worldwide and decrease the risk for development of cirrhosis and HCC. Treat IT‐HBV; fight against HBV‐HCC.
Acknowledgment
We thank the American Liver Foundation for providing us with the opportunity to participate in the clinical debate sponsored by the American Liver Foundation held in Chicago in March 2019.
Potential conflict of interest: Nothing to report.
References
- 1. Terrault NA, Lok ASF, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018;67:1560‐1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kumar M, Sarin SK, Hissar S, et al. Virologic and histologic features of chronic hepatitis B virus‐infected asymptomatic patients with persistently normal ALT. Gastroenterology 2008;134:1376‐1384. [DOI] [PubMed] [Google Scholar]
- 3. Lai M, Hyatt BJ, Nasser I, et al. The clinical significance of persistently normal ALT in chronic hepatitis B infection. J Hepatol 2007;47:760‐767. [DOI] [PubMed] [Google Scholar]
- 4. Seto WK, Lai CL, Ip PP, et al. A large population histology study showing the lack of association between ALT elevation and significant fibrosis in chronic hepatitis B. PLoS One 2012;7:e32622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kennedy PTF, Sandalova E, Jo J, et al. Preserved T‐cell function in children and young adults with immune‐tolerant chronic hepatitis B. Gastroenterology 2012;143:637‐645. [DOI] [PubMed] [Google Scholar]
- 6. Beasley RP, Hwang LY, Lin CC, et al. Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22 707 men in Taiwan. Lancet 1981;2:1129‐1133. [DOI] [PubMed] [Google Scholar]
- 7. Mason WS, Gill US, Litwin S, et al. HBV DNA integration and clonal hepatocyte expansion in chronic hepatitis B patients considered immune tolerant. Gastroenterology 2016;151:986‐998.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chang Y, Choe WH, Sinn DH, et al. Nucleos(t)ide analogue treatment for patients with hepatitis B virus (HBV) e antigen‐positive chronic HBV genotype C infection: a nationwide, multicenter, retrospective study. J Infect Dis 2017;216:1407‐1414. [DOI] [PubMed] [Google Scholar]
- 9. Kitrinos KM, Corsa A, Liu Y, et al. No detectable resistance to tenofovir disoproxil fumarate after 6 years of therapy in patients with chronic hepatitis B. Hepatology 2014;59:434‐442. [DOI] [PubMed] [Google Scholar]
- 10. Chen CJ, Yang HI, Su J, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA 2006;295:65‐73. [DOI] [PubMed] [Google Scholar]


