Abstract
Minimally invasive surgical procedures aiming to repair damaged maxillofacial tissues are hampered by its small, complex structures and difficult surgical access. Indeed, while arthroscopic procedures that deliver regenerative materials and/or cells are common in articulating joints such as the knee, there are currently no treatments that surgically place cells, regenerative factors or materials into maxillofacial tissues to foster bone, cartilage or muscle repair. Here, we developed hyaluronic acid (HA)-based hydrogels, which are suitable for use in minimally invasive procedures, that can adhere to the surrounding tissue, and deliver cells and potentially drugs. By modifying HA with both methacrylate (MA) and 3,4-dihydroxyphenylalanine (Dopa) groups using a completely aqueous synthesis route, we show that MA-HA-Dopa hydrogels can be applied under aqueous conditions, gel quickly using a standard surgical light, and adhere to tissue. Moreover, upon oxidation of the Dopa, human marrow stromal cells attach to hydrogels and survive when encapsulated within them. These observations show that when incorporated into HA-based hydrogels, Dopa moieties can foster cell and tissue interactions, ensuring surgical placement and potentially enabling delivery/recruitment of regenerative cells. Our findings suggest that MA-HA-Dopa hydrogels may find use in minimally invasive procedures to foster maxillofacial tissue repair.
Keywords: Hydrogel, adhesivity, biomaterial, tissue engineering, minimally invasive surgery
Degradable materials are used clinically to repair damaged musculoskeletal tissues, particularly cartilage in the knee, using procedures such as matrix-assisted autologous chondrocyte transplantation (MACT).[1] However, there are currently no similar procedures that surgically place degradable materials with cells or regenerative factors into maxillofacial tissues to foster the repair of muscle, bone, or cartilage. The lack of materials-based regenerative strategies for maxillofacial tissues stems from the strict design criteria that govern materials surgically placed in the head. Ideally, materials should be suitable for minimally invasive procedures, as they minimize the potential for nerve injury and infection.[2] However, limited surgical access and the complex and small anatomical structures in the head make standard arthroscopic MACT-like procedures unfeasible, particularly because in maxillofacial applications adhesion of the material to the damaged tissue is likely necessary. Therefore, a therapy that could deliver a material to stimulate repair, like MACT, but via a minimally invasive procedure suitable for maxillofacial applications, has the potential to transform the treatment of a range of degenerative and trauma-related maxillofacial disorders.
Regenerative therapies that aim to deliver a material to maxillofacial tissues are subject to numerous design limitations. First, for many applications, the material should bond to the tissue to facilitate integration. For example, unlike in the knee, where cartilaginous defects leave a reservoir in which a reparative solution/material can be placed, the fibrocartilage of the temporomandibular joint (TMJ) (Figure S2), which might similarly benefit from MACT, is only a few hundred microns thick.[3] Therefore, adhesivity is likely required to ensure correct placement. The material should also fully degrade, and importantly for maxillofacial applications which abut the brain, must be completely non-toxic. Moreover, the material should ideally be inexpensive, injectable, easily manufactured from materials already approved for clinical use in maxillofacial applications, and suitable for delivery using equipment available in standard surgical theatres. In addition, the material must be amenable to cross-linking under aqueous conditions (submerged in water), as minimally invasive maxillofacial procedures are often carried out with saline perfusion.
Hyaluronic acid (HA) is a hydrophilic polysaccharide composed of repeating disaccharide units of N-acetylglucosamine and D-glucuronic acid. HA possesses carboxyl and hydroxyl groups on its backbone suitable for chemical modification,[4, 5] allowing it to be cross-linked to form hydrogels. Moreover, HA can be safely degraded by hyaluronidases, which are ubiquitous in vivo. However, HA is non-adhesive, limiting surgical placement, and provides no sites for integrin-mediated interactions with cells, which may preclude invasion by endogenous cells. 3,4-dihydroxyphenylalanine (Dopa) contains catechol functional groups, the active component of the mussel foot protein, and is known to strongly adhere to inorganic and organic materials through both covalent and non-covalent interactions.[6] Moreover, its simple chemical structure allows it to be tethered to a range of synthetic and natural polymers.[6]
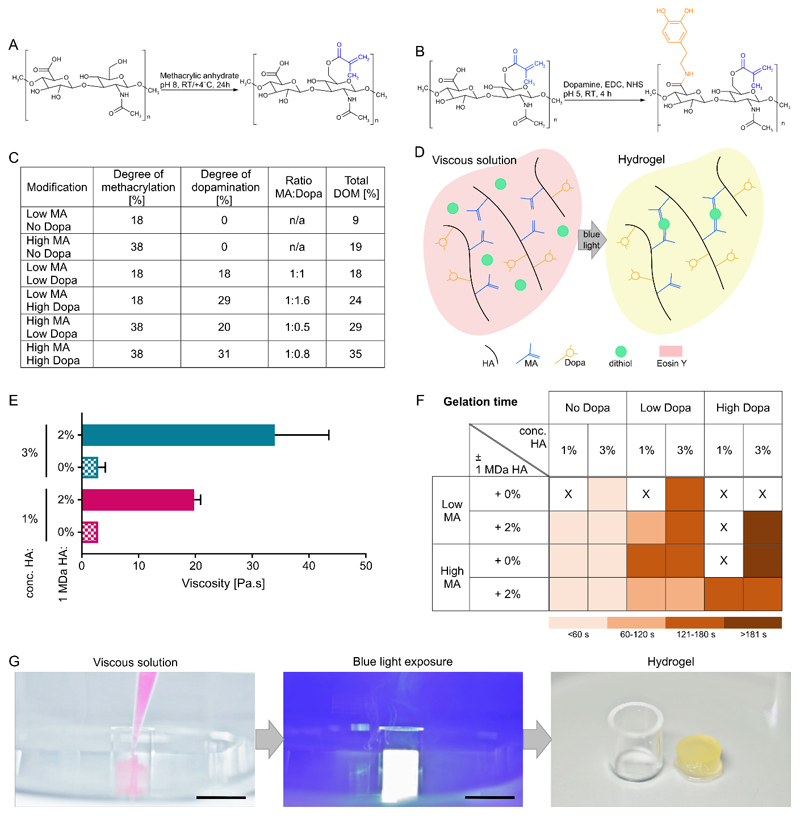
To create HA-based hydrogels that are potentially suitable for minimally invasive surgical procedures in maxillofacial applications, we carried out a double modification of 100-150 kDa HA to add both MA and Dopa groups using an aqueous synthesis route (Figure 1A and 1B). 1H NMR analysis of MA-HA-Dopa confirmed successful modification of the HA backbone (Figure S3 and S4), and demonstrated that by modifying the molar ratios of methacrylic anhydride and dopamine hydrochloride to HA, we could achieve both low and high degrees of methacrylation and dopamination with a total degree of modification of between 9 and 35% (Figure 1C). Stable hydrogels could then be formed in the presence of a cross-linker upon application of a standard clinical blue light (400-500 nm, 400-600 mW cm-2,[7] widely available in surgical theatres) (Figure 1D). Non-aqueous protocols for adding Dopa to synthetic and natural polymers have been reported;[8, 9] however, our aqueous synthesis avoids the use of potentially toxic solvents such as dichloromethane, which has been linked to facial nerve paralysis[10]. Indeed, avoiding potential toxins is particularly important in maxillofacial applications where biomaterial-mediated neurotoxicity has been reported.[11]
Figure 1. Monomer synthesis and hydrogel formation.
(A) Synthesis of MA-HA through reaction of HA with methacrylic anhydride; (B) Synthesis of MA-HA-Dopa by coupling MA-HA with dopamine; (C) Quantification of HA methacrylation and dopamination; (D) Reaction schematic demonstrating the formation of hydrogels. Upon blue light exposure, Eosin Y deprotonates di-thiol PEG, allowing it to react with methacrylate moieties on HA leading to a formation of kinetic chains. This process reduces Eosin Y to a colorless state; (E) Viscosity of 1 and 3% (w/v) precursor solutions with and without the addition of 2% (w/v) unmodified 1 MDa HA. Plot shows means and standard deviations, n=3; (F) Gelation times for different hydrogel compositions. Gelation times were grouped so that light color indicates fast gelation and darker colors show slower gelation; (G) Images showing in aqua application of viscous solution and gelation (see also Movie 1), Scale bar=10 mm.
1 and 3% solutions of 100-150 kDa HA have low viscosities (Figure 1E), rendering precise surgical application troublesome. As we aimed to create a material that could be injected, we first modified the viscosity of MA-HA-Dopa by adding 2% unmodified 1 MDa HA. The addition of unmodified HA increased the extensional viscosities of all formulations by approximately one order of magnitude placing them within the range of reported viscosities for clinically relevant materials that are similarly applied by syringe.[12] We then optimized hydrogel gelation by cross-linking the material’s MA groups with di-thiol linear poly(ethylene glycol) (PEG) (significantly less toxic than standard dithiothreitol, Figure S5), in the presence of non-toxic concentrations of the visible light photo-initiator Eosin Y (Figure S6). Upon irradiation, Eosin Y subtracts a hydrogen from the thiol group, leaving a thiyl-radical to undergo a thiol-ene reaction with a MA group[13].
The clinical application of in situ gelling materials requires the material to set quickly (~4 minutes). Therefore, we next used small amplitude oscillatory shear rheology as a function of time to monitor gelation (Figure 1F, Figure S7-S10, Table S1). We first observed that for all MA-HA and MA-HA-Dopa (low) formulations, the addition of 2% 1 MDa HA did not have an adverse effect on gelation time. However, while MA-HA and MA-HA-Dopa formulations with low degrees of Dopa (with the exception of 1%, without unmodified HA) gelled in less than 4 minutes (taken as the cross-over between G’ and G”), MA-HA-Dopa with high degrees of Dopa gelled more slowly (>4 minutes) or not at all. We expect our hydrogels to cross-link through thiol-ene radical addition reactions. However, catechols can scavenge free radicals, acting as polymerization inhibitors[14]. Here, catechol’s ability to scavenge free radicals appeared to inhibit thiol-ene type cross-links by reducing the number of thiyl radicals available to crosslink the hydrogels with increasing Dopa modification. Although Dopa can also react with free thiols upon oxidation to its quinone form, during photo-initiated cross-linking Dopa exists predominantly in its reduced form[15]. In short, likely by scavenging free radicals, hydrogels containing more Dopa form fewer crosslinks, an effect that has been previously described in catechol-functionalized thiol-ene polymer networks used as adhesives[16]. We then assessed MA-HA-Dopa’s ability to cross-link under aqueous conditions. We found that MA-HA-Dopa could be pipetted and cross-linked while fully immersed in buffer and still form stable hydrogels (Figure 1G, Movie 1). Taken together, these observations suggest that non-toxic MA-HA-Dopa hydrogels are suitable for injection and can cross-link in an appropriate time frame during minimally invasive procedures in which the joint space is perfused with buffer. Moreover, the material can potentially be placed while a patient is prone and molded many times, if necessary, prior to gelation.
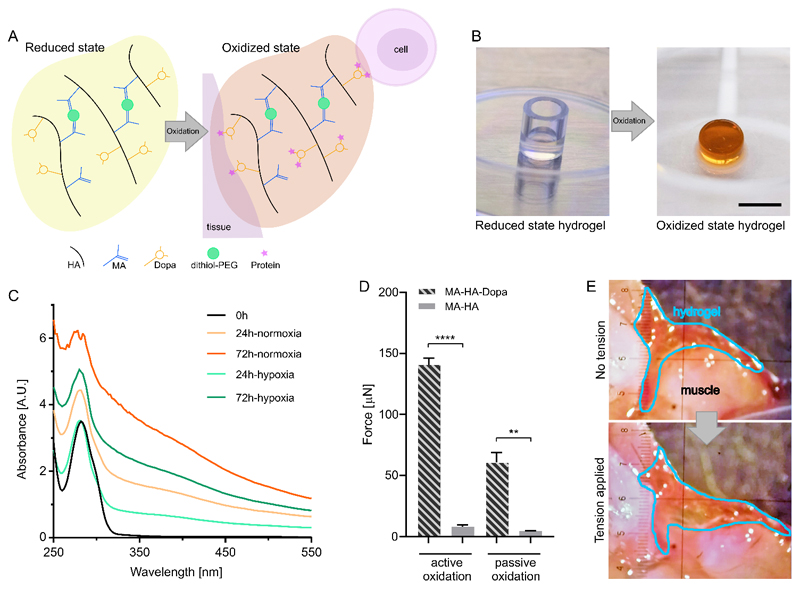
Dopa is recognized for its adhesive properties; however, stickiness requires the Dopa to be oxidized (Figure 2A).[6, 8] Oxidation can be achieved by adding sodium periodate or hydrogen peroxide,[8] but these can be biologically toxic. Therefore, we next aimed to determine if we could achieve Dopa oxidation under physiological conditions that would promote tissue interactions. After 24 hours in cell culture medium, MA-HA-Dopa hydrogels turned brown, indicating Dopa oxidation[8] (Figure 2B). This was confirmed by colorimetric measurements (Figure 2C), and absorption between 250-500 nm continued to increase over 72 hours, confirming that atmospheric oxygen was sufficient to oxidize the Dopa moiety. However, oxidation could be slowed by maintaining hydrogels under hypoxic conditions (5% O2), showing that lower, tissue-like levels of O2, could also foster the process. Next, to determine if the Dopa moiety allowed for tissue interactions, we formed MA-HA and MA-HA-Dopa hydrogels within a circular defect punched out of porcine articular cartilage. We then either actively oxidized the Dopa (NaIO4) or allowed it to undergo passive oxidation in cell culture media before measuring the force required to push the hydrogel out of the tissue. We found that both actively and passively oxidized Dopa-modified hydrogels required significantly higher push out forces than their MA-HA counterparts (Figure 2D). To further demonstrate hydrogel-tissue interactions, we then placed MA-HA and MA-HA-Dopa hydrogels along the cut surfaces of a mouse hind limb muscle (Figure 2E, Figure S11-S12). While MA-HA hydrogels fell away from the tissue upon minimally manipulation, MA-HA-Dopa hydrogels bonded to the tissue and remained adherent even as the muscle was physically manipulated (Movie 2), an effect that was maintained after 5 days in culture (Movie 3).
Figure 2. Dopa oxidation of hydrogels and tissue interactions.
(A) Diagram highlighting how Dopa oxidation impacts protein, cell and tissue interactions with hydrogels; (B) Images of hydrogels captured directly after gelation (left) or after 24 hours incubation in cell culture media (right) showing that the hydrogel becomes brown indicating oxidation of the Dopa; Scale bar=10 mm; (C) Colorimetric measurements of Dopa oxidation in hydrogels maintained in cell culture media under standard or hypoxic (5% O2) conditions; (D) Force required to push hydrogel (3%, high MA, low Dopa; high MA, no Dopa) out of circular defect created in porcine articular cartilage, either after being actively oxidized with NaIO4 or passively oxidized in cell culture media over 72 hours. Plots show means and standard deviations, n=3; unpaired t-test (two-tailed, **p<0.01, ****p<0.0001); (E) Images showing hydrogel (3%, high MA, low Dopa) adherent to muscle tissue (top). As the damaged muscle tissue is pulled apart, the hydrogel remains adherent to the tissue surfaces (bottom) (see also Movies 2&3).
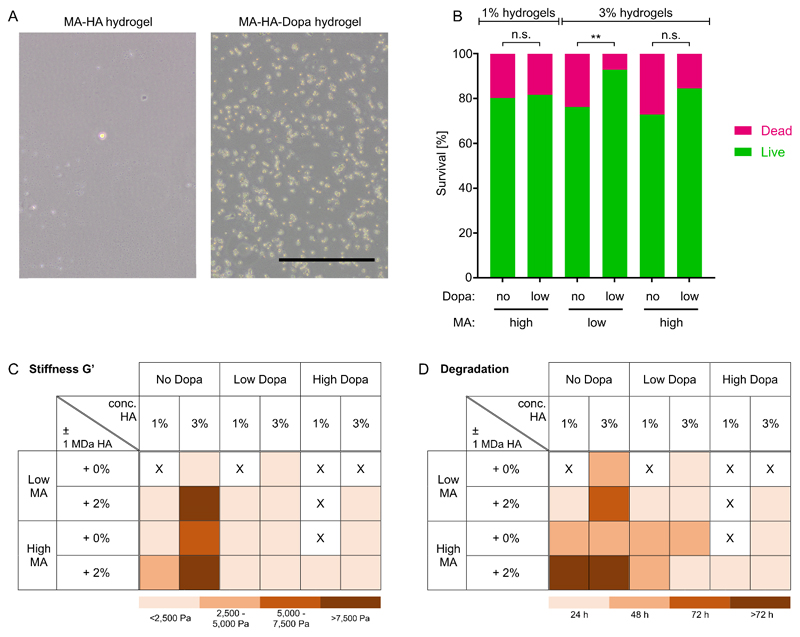
Materials-based strategies to regenerate cartilage, bone and muscle often call for the presence of cells that can secrete tissue-specific ECM.[17] Therefore, we next aimed to study cell interactions with MA-HA-Dopa hydrogels. We first seeded 17IA4 cells on the surface of hydrogels and examined their attachment. In line with previous observations,[5, 18] cells do not adhere to MA-HA hydrogels (Figure 3A); however, 16 hours after seeding, 17IA4 cells were adherent to MA-HA-Dopa hydrogels. HA has no sites for integrin-mediated interactions, so cells do not attach to it, even in the presence of serum proteins. Therefore, these observations suggest that Dopa fostered integrin-mediated interactions with the hydrogel, likely by binding proteins[6, 19] through which cells attached. Many tissue engineering strategies also aim to deliver cells within a material. Therefore, we also encapsulated primary human marrow stromal cells (hMSC) within hydrogels and found that the addition of Dopa significantly increased cell viability (Figure 3B and Figure S13). This confirmed previous reports that hMSC can survive within HA-based hydrogels lacking adhesive motifs,[20] but that the addition of Dopa could enhance cell survival. Taken together, these observations show that by providing sites for protein interaction and thus integrin-mediated interactions, MA-HA-Dopa hydrogels can potentially deliver viable cells or be infiltrated by endogenous cells made available using procedures such as microfracture.[21]
Figure 3. Hydrogel interactions with cells and physical characterization.
(A) Bright-field images of 17IA4 cells attached to MA-HA and MA-HA-Dopa hydrogels after 18 hours of culture. Very few cells were observed attached to MA-HA hydrogels. Scale bar=200 µm; (B) hMSC survival after 24 hours encapsulation within hydrogels. In 3% hydrogels, cells show significantly higher viability in MA-HA-Dopa hydrogels compared to MA-HA hydrogels. n=3 biological replicates, 300 cells per condition, Fisher’s exact test, **p<0.01; (C) Stiffness as determined by the elastic modulus G’ after 4 minutes light exposure. G’ values were grouped such that lighter colors indicate softer and darker colors show stiffer hydrogels; (D) Time for full enzymatic degradation of different hydrogel formulations. Lighter colors indicate fast degradation and darker colors show slower degradation. X indicates the absence of gel formation.
As hydrogel physical properties will influence both their performance in vivo and subsequent cell response[22], we next examined hydrogel stiffness (G’) and swelling behavior. 3% hydrogels tended to be stiffer and swell more than those formed with 1% (Figure 3C, Figure S14, Table S2-S4). Moreover, and in line with observations of gelling time, more extensive Dopa modification tended to result in lower values of G’. We then examined hydrogel degradation in the presence of hyaluronidase and confirmed that all compositions were degradable, but that degradation tended to be slower in compositions with higher levels of MA, lower levels of Dopa and in the presence of unmodified 1 MDa HA (Figure 3D, Figure S15), in line with the catechol-mediated negative impact on network formation. These observations were in line with previous reports of degradability in HA with similar degrees of modification.[23] The values of G’ we observed here are lower than those of many maxillofacial tissues. It is possible to make HA-based hydrogels stiffer;[18] however, this requires more extensive chemical modification, which can render the material non-degradable. However, hydrogels are suitable for infiltration within porous load-bearing materials, which have recently been used to resurface the articulating surfaces of the TMJ in an open procedure.[24] Therefore, combining the MA-HA-Dopa with a porous scaffold could provide mechanical support while retaining the benefits of the MA-HA-Dopa hydrogel for cell/drug delivery.
Finally, as the shelf life of viscous hydrogel precursors will impact their suitability for clinical applications, we next compared gelation after storage at room temperature (RT) and 4 °C for up to 8 days. While low MA hydrogel compositions stored for 1 or 8 days had stiffnesses that were significantly lower than those of freshly prepared hydrogels, suggesting that the functional cross-linking groups were lost during storage, material prepared with high MA modification, and particularly those with low Dopa, formed stable hydrogels with similar stiffnesses to freshly prepared material (Figure S16-S20). These observations not only show that MA-HA-Dopa precursor solutions can be stored for at least 1 week prior to use, but also suggest that our chemical strategy does not promote the spontaneous formation of Dopa-Dopa cross-links. Moreover, combined with our observation that high Dopa formulations either gelled more slowly than their low/No Dopa counterparts or not at all further suggests that our chemical strategy does not permit the Dopa moiety to participate in hydrogel cross-linking. Taken together, these data suggest that MA-HA-Dopa formulations with high degrees of methacrylation and low degrees of dopamination gel within a suitable time frame, can be stored prior to use, and have physical and adhesive properties appropriate for use in minimally invasive maxillofacial applications.
Maxillofacial tissues are amenable to regeneration. For example, Undt et al. showed that the cartilaginous surfaces of the TMJ could be regenerated upon surgical placement of appropriate cells and materials.[24] However, in a case series in 7 patients in which autologous chondrocytes combined with a collagen sponge were used to treat severe degeneration of the articular surfaces of the TMJ, two open surgical procedures were required. Here, we identified HA-based hydrogel compositions with mechanical, biological, adhesive and surgical handling properties suitable for delivery via a single minimally invasive procedure, with or without autologous cells. Adhesive materials for cartilage repair have been previously reported; however, 2-step procedures whereby an adhesive intermediate is “painted” onto the native tissue to foster interactions with the hydrogel[25] are unlikely to be amenable to minimally invasive procedures and the difficult surgical access characteristic of maxillofacial tissues. Others have reported the use of quinone-modified materials to foster tissue adhesion, but these often require oxidizing the material with harsh reagents.[8, 26] Moreover, in many strategies Dopa itself acts as a cross-linker, restricting potential tissue interactions. Our observations here suggest that MA-HA-Dopa hydrogels can foster tissue adhesion and cell attachment in the absence of harsh chemical treatments and complicated surgical procedures, potentially providing a first-of-its-kind therapy for damaged maxillofacial tissues.
Experimental Section
Details of the materials and experimental methods are available in the Supporting Information.
Supplementary Material
Acknowledgements
CS was supported by a Diane Trebble PhD Scholarship. EG acknowledges a Research Career Development Fellowship from the Wellcome Trust (WT093687) and a Philip Leverhulme Prize from the Leverhulme Trust. The authors are grateful for support from the Rosetrees Trust and the MRC-funded UK Regenerative Medicine Platform “Acellular/Smart Materials – 3D Architecture” Hub. RMPdS acknowledges the Wellcome Trust (Institutional Strategic Support Fund), King’s College London and the London Law Trust for a King’s Prize Fellowship. The authors wish to thank Dr RA Atkinson for assistance with NMR experiments and the NMR Facility of the Centre for Biomolecular Spectroscopy at King’s College London, which was established with awards from the Wellcome Trust, British Heart Foundation and King’s College London.
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest.
References
- [1].Knutsen G, Drogset JO, Engebretsen L, Grontvedt T, Isaksen V, Ludvigsen TC, Roberts S, Solheim E, Strand T, Johansen O. J Bone Joint Surg Am. 2007;89:2105. doi: 10.2106/JBJS.G.00003. [DOI] [PubMed] [Google Scholar]; Nehrer S, Dorotka R, Domayer S, Stelzeneder D, Kotz R. Am J Sports Med. 2009;37(Suppl 1):81S. doi: 10.1177/0363546509350704. [DOI] [PubMed] [Google Scholar]
- [2].Liu F, Giannakopoulos H, Quinn PD, Granquist EJ. Craniomaxillofac Trauma Reconstr. 2015;8:88. doi: 10.1055/s-0034-1393726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bibb CA, Pullinger AG, Baldioceda F. Arch Oral Biol. 1993;38:343. doi: 10.1016/0003-9969(93)90142-9. [DOI] [PubMed] [Google Scholar]
- [4].Smeds KA, Pfister-Serres A, Miki D, Dastgheib K, Inoue M, Hatchell DL, Grinstaff MW. J Biomed Mater Res. 2001;54:115. doi: 10.1002/1097-4636(200101)54:1<115::aid-jbm14>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]; Burdick JA, Chung C, Jia X, Randolph MA, Langer R. Biomacromolecules. 2005;6:386. doi: 10.1021/bm049508a. [DOI] [PMC free article] [PubMed] [Google Scholar]; Shu XZ, Liu Y, Luo Y, Roberts MC, Prestwich GD. Biomacromolecules. 2002;3:1304. doi: 10.1021/bm025603c. [DOI] [PubMed] [Google Scholar]
- [5].Ferreira SA, Motwani MS, Faull PA, Seymour AJ, Yu TTL, Enayati M, Taheem DK, Salzlechner C, Haghighi T, Kania EM, Oommen OP, et al. Nat Commun. 2018;9 doi: 10.1038/s41467-018-07843-1. 4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kord Forooshani P, Lee BP. J Polym Sci A Polym Chem. 2017;55:9. doi: 10.1002/pola.28368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Santini A, Gallegos IT, Felix CM. Prim Dent J. 2013;2:30. doi: 10.1308/205016814809859563. [DOI] [PubMed] [Google Scholar]; Dentsply Sirona QHL75 Curing Light. https://www.dentsplysirona.com/content/dam/dentsply/pim/manufacturer/Restorative/Accessories/Curing_Lights/Halogen_Lights/QHL75_Curing_Light/QHL75-2d9j1m0-en--1402.
- [8].Lee BP, Dalsin JL, Messersmith PB. Biomacromolecules. 2002;3:1038. doi: 10.1021/bm025546n. [DOI] [PubMed] [Google Scholar]
- [9].Guvendiren M, Messersmith PB, Shull KR. Biomacromolecules. 2008;9:122. doi: 10.1021/bm700886b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Jacubovich RM, Landau D, Bar Dayan Y, Zilberberg M, Goldstein L. Am J Ind Med. 2005;48:389. doi: 10.1002/ajim.20215. [DOI] [PubMed] [Google Scholar]
- [11].Renard JL, Felten D, Bequet D. Lancet. 1994;344:63. doi: 10.1016/s0140-6736(94)91089-8. [DOI] [PubMed] [Google Scholar]
- [12].Nie J, Yap A, Wang X. Operative Dentistry. 2018;43:656. doi: 10.2341/17-091-L. [DOI] [PubMed] [Google Scholar]; Al-Ahdal K, Silikas N, Watts DC. Dent Mater. 2014;30:517. doi: 10.1016/j.dental.2014.02.005. [DOI] [PubMed] [Google Scholar]
- [13].Hoyle CE, Bowman CN. Angewandte Chemie-International Edition. 2010;49:1540. doi: 10.1002/anie.200903924. [DOI] [PubMed] [Google Scholar]
- [14].Chen SA, Tsai LC. Makromolekulare Chemie-Macromolecular Chemistry and Physics. 1986;187:653. [Google Scholar]; Galano A, Perez-Gonzalez A. Theoretical Chemistry Accounts. 2012;131 [Google Scholar]
- [15].Forooshani PK, Lee BP. Journal of Polymer Science Part a-Polymer Chemistry. 2017;55:9. doi: 10.1002/pola.28368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Donovan BR, Cobb JS, Hoff EFT, Patton DL. Rsc Advances. 2014;4:61927. [Google Scholar]
- [17].Foyt DA, Norman MDA, Yu TTL, Gentleman E. Adv Healthc Mater. 2018;7:e1700939. doi: 10.1002/adhm.201700939. [DOI] [PMC free article] [PubMed] [Google Scholar]; Loaiza S, Ferreira SA, Chinn TM, Kirby A, Tsolaki E, Dondi C, Parzych K, Strange AP, Bozec L, Bertazzo S, Hedegaard MAB, et al. Biomaterials. 2018;183:102. doi: 10.1016/j.biomaterials.2018.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Guvendiren M, Burdick JA. Nat Commun. 2012;3 doi: 10.1038/ncomms1792. 792. [DOI] [PubMed] [Google Scholar]
- [19].Liu HY, Greene T, Lin TY, Dawes CS, Korc M, Lin CC. Acta Biomater. 2017;48:258. doi: 10.1016/j.actbio.2016.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bian L, Guvendiren M, Mauck RL, Burdick JA. Proc Natl Acad Sci U S A. 2013;110:10117. doi: 10.1073/pnas.1214100110. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lisignoli G, Cristino S, Piacentini A, Cavallo C, Caplan AI, Facchini A. J Cell Physiol. 2006;207:364. doi: 10.1002/jcp.20572. [DOI] [PubMed] [Google Scholar]; Zhu H, Mitsuhashi N, Klein A, Barsky LW, Weinberg K, Barr ML, Demetriou A, Wu GD. Stem Cells. 2006;24:928. doi: 10.1634/stemcells.2005-0186. [DOI] [PubMed] [Google Scholar]; Ferreira SA, Faull PA, Seymour AJ, Yu TTL, Loaiza S, Auner HW, Snijders AP, Gentleman E. Biomaterials. 2018;176:13. doi: 10.1016/j.biomaterials.2018.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Frehner F, Benthien JP. Cartilage. 2018;9:339. doi: 10.1177/1947603517700956. [DOI] [PMC free article] [PubMed] [Google Scholar]; Gottschalk O, Altenberger S, Baumbach S, Kriegelstein S, Dreyer F, Mehlhorn A, Horterer H, Topfer A, Roser A, Walther M. J Foot Ankle Surg. 2017;56:930. doi: 10.1053/j.jfas.2017.05.002. [DOI] [PubMed] [Google Scholar]; Sommerfeldt MF, Magnussen RA, Hewett TE, Kaeding CC, Flanigan DC. JBJS Rev. 2016;4 doi: 10.2106/JBJS.RVW.15.00005. [DOI] [PubMed] [Google Scholar]
- [22].Walters NJ, Gentleman E. Acta Biomater. 2015;11:3. doi: 10.1016/j.actbio.2014.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hahn SK, Park JK, Tomimatsu T, Shimoboji T. Int J Biol Macromol. 2007;40:374. doi: 10.1016/j.ijbiomac.2006.09.019. [DOI] [PubMed] [Google Scholar]; Park YD, Tirelli N, Hubbell JA. Biomaterials. 2003;24:893. doi: 10.1016/s0142-9612(02)00420-9. [DOI] [PubMed] [Google Scholar]
- [24].Undt G, Jahl M, Pohl S, Marlovits S, Moser D, Yoon HH, Frank J, Lang S, Czerny C, Klima G, Gentleman E, et al. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018;126:117. doi: 10.1016/j.oooo.2018.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].F S, Sharma B, Gibson M, Unterman S, Herzka DA, C J, Cascio B, Hui AY, Marcus N, E J, Gold GE. Tissue Eng. 2013 doi: 10.1126/scitranslmed.3004838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Fisher OZ, Larson BL, Hill PS, Graupner D, Nguyen-Kim MT, Kehr NS, De Cola L, Langer R, Anderson DG. Adv Mater. 2012;24:3032. doi: 10.1002/adma.201104437. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.