Abstract
In human neonates, when the umbilical cord is kept intact postpartum, blood continues to flow to the neonate, but this procedure might be difficult in dogs owing to a shorter umbilical cord and several neonates in a litter. However, it might be possible to detach the placenta and keep the umbilical cord intact, allowing residual blood to flow to the puppies. This study compared the effects of clamping versus no clamping of the umbilical cord in dogs born by cesarean section on neonatal vitality. The puppies were assessed by Apgar and reflex scores. Fifty puppies delivered from 16 bitches were randomly allocated to receive immediate umbilical cord clamping (n=25) or no clamping for at least 3 min after the onset of breathing (n=25). The puppies were assessed during the first 5 min of life and 10 min after the first assessment. The no clamping group showed significantly higher Apgar scores (second assessment, P<0.01) and reflex scores (first and second assessments, P<0.05) than the clamping group, implying higher vitality in the no clamping group. The differences observed between the moments (first versus second assessment) of each group was significant (P<0.01), revealing higher vitality in the second assessment. The results suggest that keeping the umbilical cord intact for at least 3 min after the onset of breathing may contribute to increased vitality in puppies delivered by cesarean section without any negative consequences.
Keywords: Apgar score, cesarean section, dog, neonatal vitality, umbilical cord
In veterinary medicine, mortality rates are high during the neonatal period, ranging from 6.9% to 21.2% in dogs, increasing significantly during birth, immediately after birth, and during the first few days of life [6, 8]. About 90% of the deaths that happen before the second day of life are caused by extended periods of hypoxia during birth [12].
Dystocia is the primary cause of fetus injury by hypoxia, and several dogs presenting a profile of dystocia need to undergo cesarean section [4]. Neonatal dogs already present a physiological profile of hypoxemia due to the intermittent uterine contractions during birth [19], a condition that may be aggravated by dystocia or a cesarean section. Cesarean sections often lead to hypoxemia due to neonatal depression caused by the anesthetic agents administered to the bitches, which can hamper or delay the onset of breathing [2, 17].
Physiologically, venous blood is delivered to the placenta through the umbilical arteries, and the arterial blood is then perfused to the fetus through the umbilical vein. After birth, with the first breath and the increase in PaO2, the umbilical arteries contract within a 45-sec window, while the umbilical vein remains visible. The ductus venosus constricts, the oval foramen and the ductus arteriosus both close, and blood stops being circulated to the lungs [7, 24].
In human medicine, not clamping the umbilical cord, a procedure conducted both with cesarean sections and vaginal deliveries, stabilizes the neonate’s circulation, increases perfusion and reduces hypoxemia [3, 14]. Adequate blood flow is necessary for breathing to start after birth since the required cardiac output increases from 8–12% in the fetus to 40–50% in a human neonate [10]. The blood flow from the umbilical vein to the neonate is blocked abruptly by clamping, reducing the preload in the heart. At the same time, blockage of the umbilical arteries increases the afterload by increasing the peripheral vascular resistance, which results in a reduction in cardiac output [18].
When the umbilical cord is left intact, and the clamping is performed later, the blood flowing through the placenta and the umbilical cord at the time of birth is used for pulmonary circulation when breathing starts [18], which stabilizes circulation and minimizes changes to cardiac output and blood pressure in human neonates [3]. The recommended practice in human medicine is not to clamp the umbilical cord because of the benefits, such as a reduced number of babies requiring blood transfusions due to anemia, improved circulatory stability, reduced risk of an intraventricular brain hemorrhage, reduced risk of necrotizing enterocolitis, and reduced number of late sepsis cases [14].
In dogs, however, there are no known studies regarding the physiology or the benefits of not clamping the umbilical cord, which might be because the procedure is complicated by the number of puppies and short umbilical cords. However, even after removing the placenta from the uterus, keeping the umbilical cord intact allows the neonate to receive residual blood flow from the umbilical cord and placenta. Therefore, this study assesses the effect, on neonatal vitality, of keeping the umbilical cord intact (assessed with Apgar and reflex scores) in puppies born through cesarean section.
MATERIALS AND METHODS
This study was approved by the Ethics Committee of the College of Veterinary Medicine and Animal Sciences (FMVZ, Faculdade de Medicina Veterinária e Zootecnia, UNESP, Botucatu, Brazil).
The randomized study included 50 neonate dogs divided into two groups: those that underwent umbilical cord clamping (n=25), and those that did not (n=25). The neonate dogs were from 16 litters and the following breeds: four Miniature Pinschers, three French Bulldogs, three mixed-breeds, two Maltese, one Pug, one Shih Tzu, one Lhasa Apso, and one Australian Cattle Dog.
The inclusion criteria for the study were full-term bitches with the onset of birth signals that underwent therapeutic cesarean sections due to dystocia caused by uterine inertia, narrowing of the birth canal, or obstructive fetal position. The exclusion criteria were litters with fetuses presenting heart rates below 180 beats per min in an ultrasound Doppler examination before the cesarean section (the entire litter was excluded), single-fetus gestations, neonates with meconium in the amniotic fluid, neonates presenting congenital malformations, low blood glucose levels (<90 mg/dl) at the time of birth with collection through the jugular vein, or in need of emergency procedures, as well as those presenting placental pathologic alterations such as infarction or placentitis. One neonate was randomly excluded in litters with an odd number of neonates because the evaluation required an equal number of individuals in each group per litter.
A birth was defined as the removal of the neonates from the uterus. The animals were assigned to a group alternately upon birth. Not clamping the umbilical cord was defined as clamping the umbilical cord at least 3 min after the establishment of spontaneous pulmonary breathing. In this group, the placenta was removed from the uterus together with the neonate without severing the umbilical cord. Clamping was defined as clamping the umbilical cord 5–10 sec after removing the neonate from the uterus.
Anesthesia before the cesarean sections was induced with intravenous propofol, at a dose sufficient for loss of laryngotracheal reflex (dose effect). Lidocaine 2% was used for epidural anesthesia (0.25 ml per kg weight), and maintained with isoflurane diluted in oxygen. The procedure time between induction and removal of the neonates from the uterus was 15–20 min. Immediately after birth, neonates from each litter were alternately assigned to the clamping or no clamping groups. During breath stimulation, those assigned to the no clamping group were handled in the palm of the handler’s hand, in ventral decubitus, and a position with the head tilted downward, and the placenta elevated higher than the body. The placenta was removed carefully and without handling the umbilical cord in order to avoid compression. While one handler held the neonate, the second handler kept the placenta elevated in relation to the neonate due to the gravitational influence on blood transfusion. After spontaneous breathing began, the neonate was kept on a heated surface, with the placenta above its body. The umbilical cord in the early clamping group was clamped by Halstead hemostatic forceps 5 to 10 sec after the fetus was removed from the uterus.
After birth, the neonates were divided into two groups, kept on a heated surface (30 degrees Celsius), and identified with numbered bands on the thoracic limb. The airways were cleared with an aspirator and a chest rub performed to stimulate breathing. After neonatal breathing started, vitality was assessed with Apgar and reflex score assessment within the first 5 min and again 10 min after the first assessment. The umbilical cords in the no clamping group were connected to the placenta (elevated above the body) for at least 3 min after the onset of spontaneous pulmonary breathing; clamping was conducted immediately after the first assessment.
The Apgar score assessment included the color of the mucosae, heart rate, respiratory rate, muscle tone, and irritability reflex (Table 1). Heart rate was assessed with a stethoscope, and the respiratory rate was assessed through the observation of the thoracic movement. Irritability reflex was assessed through a painful stimulus in the footpads. Muscle tone was assessed with the neonate in supination, observing active movements and response to the limb’s passive moments. The color of the mucosae was assessed by visual examination of the oral mucosa. Each parameter received a score between zero and two, and the sum of all scores represented the neonatal vitality of each puppy. The overall score was classified as described by Veronesi et al. [23]: 0–3 low vitality, 4–6 moderate vitality, and 7–10 high vitality. Heart rate was also compared between the groups, separately from the Apgar score, to evaluate the influence of clamping umbilical cord on the neonatal circulatory pattern.
Table 1. Apgar score for dog neonates [24].
| Parameter/Score | 0 | 1 | 2 |
|---|---|---|---|
| Mucosae color | Cyanotic | Pale | Rosy |
| Heart rate (bpma)) | <180 | 180–220 | >220 |
| Respiratory rate (mpmb)) | Absent (<6) | Weak, Irregular (6–15) | Regular, Rhythmic (>15) |
| Muscle tone | Flaccid | Slight limb flexure | Flexure |
| Reflexive irritability | Absent | Some movement | Evident crying |
a) Beats per minute. b) Movements per minute, respiratory rate was assessed through the observation of thoracic movement.
The reflex score regarded the degree of neonatal depression, including suckling, rooting, and righting reflexes (Table 2) [22]. The suckling reflex was assessed by inserting the tip of the examiner’s little finger in the neonate’s mouth. The righting reflex was stimulated by placing the neonate in dorsal recumbency on a soft and heated surface. The expected response was the neonate righting its body, with a fast return to ventral recumbency. The rooting reflex was assessed by the examiner forming a circle with the thumb and index finger and placing the hand near the neonate’s face. The expected response was the neonate seeking to fit its face within the circle. The sum of the three scores was the reflex score of each puppy.
Table 2. Neonatal reflex score system [23].
| Reflex/Score | 0 | 1 | 2 |
|---|---|---|---|
| Suckle | Absent | Weak | Strong |
| Rooting | Absent | Slow fit of the nose in the circle | Immediate fit of the nose in the circle |
| Righting | Absent (remains in decubitus) | Slow repositioning | Immediate repositioning |
All data were assessed with the aid of the SAS System [15]. The differences between the treatments were analyzed through parametric and nonparametric tests according to the assumptions of residual normality (Gauss distribution) and homogeneity of variance. Differences between the groups and moments in the Apgar score and reflexes were analyzed using the Wilcoxon signed-rank test (nonparametric variables). The heart rate difference between the groups was analyzed using the Student’s t-test (parametric variables).
The answer variables also underwent Spearman’s correlation analysis, and the results were described as the mean and standard error. The significance level selected was less than 5% (P<0.05). The number of neonates per Apgar score (0 to 10) and reflex score (0 to 6) are given as percentages.
RESULTS
The study included 50 neonate dogs of varying breeds from 16 litters, with bitches between one and seven years old. Seven of the bitches were primiparous. The average number of neonates per litter was 4.7. The 16 litters had a total of 76 neonates, 50 of which were included in the study, and 26 of which were excluded by the pre-defined criteria (Table 3). Of the neonates, 29 were female, and 21 were male. The no clamping group comprised 12 females and 13 males, and the clamping group comprised 14 females and 11 males.
Table 3. Number of neonates per litter and per group.
| Litter | Total neonates | No clamping group | Clamping group | Neonates in the exclusion criteria |
|---|---|---|---|---|
| 1 | 6 | 2 | 2 | 2 |
| 2 | 4 | 2 | 2 | - |
| 3 | 5 | 2 | 2 | 1 |
| 4 | 2 | 1 | 1 | - |
| 5 | 6 | 1 | 1 | 4 |
| 6 | 5 | 2 | 2 | 1 |
| 7 | 8 | 2 | 2 | 4 |
| 8 | 3 | 1 | 1 | 1 |
| 9 | 7 | 2 | 2 | 3 |
| 10 | 6 | 2 | 2 | 2 |
| 11 | 4 | 2 | 2 | - |
| 12 | 4 | 1 | 1 | 2 |
| 13 | 3 | 1 | 1 | 1 |
| 14 | 5 | 1 | 1 | 3 |
| 15 | 5 | 2 | 2 | 1 |
| 16 | 3 | 1 | 1 | 1 |
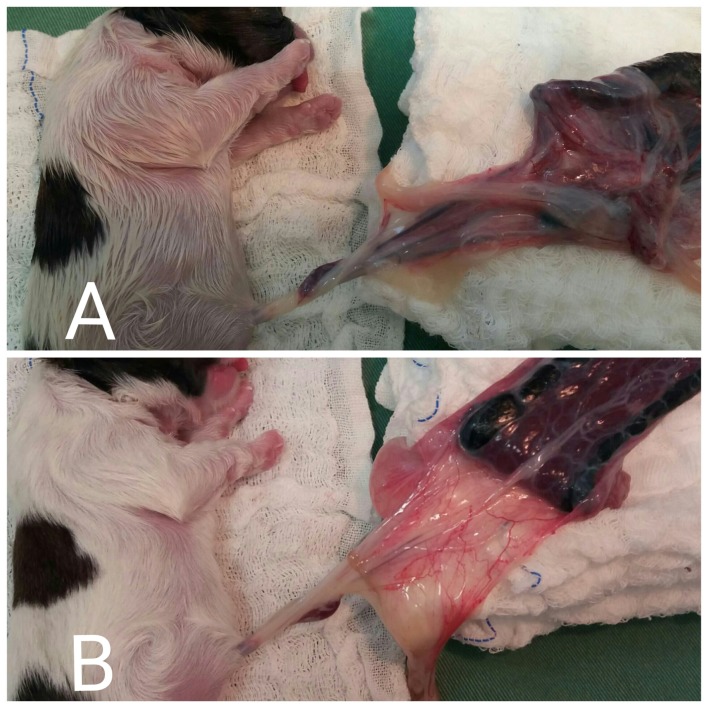
The frequency of animals with each Apgar score (0 to 10) and reflex score (0 to 6) are in Tables 4 and 5, respectively. In the no clamping group, after removing the neonates together with the umbilical cord and the placenta, we observed that the umbilical cords were full of blood at the time of birth and later became whitish and collapsed, demonstrating the blood transfer (Fig. 1).
Table 4. Number of neonates per Apgar score at the first and second moment within each group.
| Score | Apgar score | |||
|---|---|---|---|---|
| First moment | Second moment | |||
| No clamp | Clamp | No clamp | Clamp | |
| 0 | 0 | 0 | 0 | 0 |
| 1 | 1 (4%) | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 | 0 |
| 3 | 1 (4%) | 3 (12%) | 0 | 0 |
| 4 | 1 (4%) | 5 (20%) | 0 | 0 |
| 5 | 2 (8%) | 3 (12%) | 0 | 0 |
| 6 | 3 (12%) | 1 (4%) | 0 | 0 |
| 7 | 3 (12%) | 3 (12%) | 1 (4%) | 3 (12%) |
| 8 | 3 (12%) | 4 (16%) | 0 | 6 (24%) |
| 9 | 11 (44%) | 6 (24%) | 24 (96%) | 16 (64%) |
| 10 | 0 | 0 | 0 | 0 |
| Total | 25 (100%) | 25 (100%) | 25 (100%) | 25 (100%) |
Table 5. Number of neonates per reflex score at the first and second moment within each group.
| Score | Reflexes score | |||
|---|---|---|---|---|
| First moment | Second moment | |||
| No clamp | Clamp | No clamp | Clamp | |
| 0 | 6 (24%) | 8 (32%) | 0 | 0 |
| 1 | 2 (8%) | 4 (16%) | 0 | 0 |
| 2 | 3 (12%) | 3 (12%) | 0 | 0 |
| 3 | 4 (16%) | 6 (24%) | 0 | 5 (20%) |
| 4 | 3 (12%) | 4 (16%) | 3 (12%) | 3 (12%) |
| 5 | 4 (16%) | 0 | 3 (12%) | 5 (20%) |
| 6 | 3 (12%) | 0 | 19 (76%) | 12 (48%) |
| Total | 25 (100%) | 25 (100%) | 25 (100%) | 25 (100%) |
Fig. 1.
(A) After removing the neonate from the uterus, together with the umbilical cord and the placenta, we observed that the umbilical cord filled with blood at the time of birth. (B) Whitish umbilical cord 4 min after birth, demonstrating the transfer of blood.
No interactions were observed between the evaluations (first and second moment) and the groups (Clamping and No Clamping) (Table 6). The statistical differences in each group, and each moment are shown in Table 7. The Apgar score did not differ between the groups in the first moment (P=0.1) but was significantly higher (P<0.01) in the no clamping group than in the clamping group at the second moment. The reflex score was significantly (P<0.05) higher in the no clamping group than in the clamping group at both moments.
Table 6. Probability values for the main effect in the clamping vs. no clamping groups and the first vs. second moment, and the interaction for neonatal variables.
| Group | Moment | Group × Moment | |
|---|---|---|---|
| Apgar score (0–10) | 0.01 | <0.0001 | 0.38 |
| Reflexes score (0–6) | 0.003 | <0.0001 | 0.50 |
Table 7. Mean, standard error and probability values for the group effect (clamping vs. no clamping) and moment effect (first vs. second) by the Wilcoxon signed-rank test.
| Group | Moment | |||||||
|---|---|---|---|---|---|---|---|---|
| Clamp | No clamp | P-value | First | Second | P-value | |||
| Apgar score | ||||||||
| First | 6.3 ± 0.45 | 7.2 ± 0.44 | 0.1 | Clamp | 6.3 ± 0.45 | 8.5 ± 0.14 | <0.01 | |
| Second | 8.5 ± 0.4 | 8.9 ± 0.14 | <0.01 | No clamp | 7.2 ± 0.44 | 8.9 ± 0.14 | <0.01 | |
| Reflex score | ||||||||
| First | 1.7 ± 0.45 | 2.8 ± 0.14 | <0.05 | Clamp | 1.76 ± 0.45 | 4.96 ± 0.44 | <0.01 | |
| Second | 4.9 ± 0.44 | 5.6 ± 0.08 | <0.05 | No Clamp | 2.8 ± 0.14 | 5.6 ± 0.08 | <0.01 | |
When compared between moments, both Apgar and reflex scores were significantly (P<0.01) higher at the second movement than at first in both the groups. Both the no clamping and the clamping groups had a positive correlation between the reflex and Apgar scores (r=0.73, P<0.001; r=0.69, P<0.001, respectively).
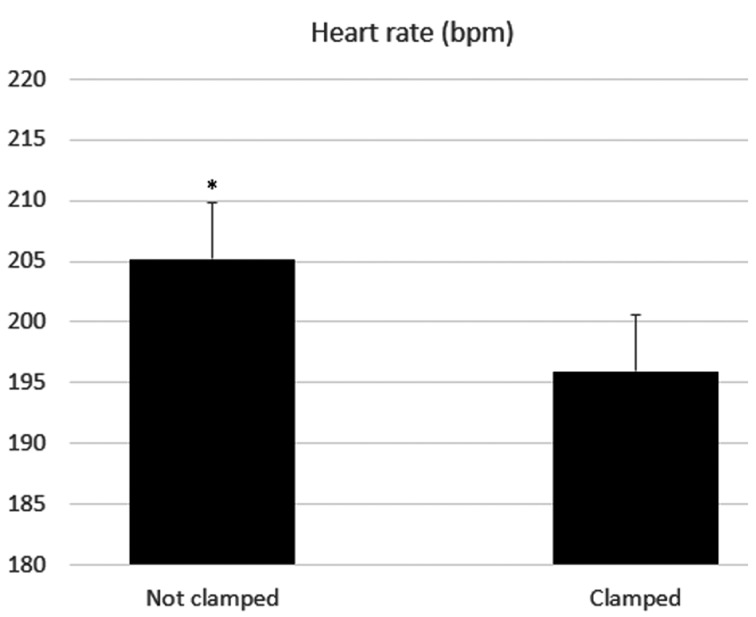
We also chose to combine all evaluations (first and second moment) in order to observe the effects of the groups over the heart rate. There was a significant difference (P<0.05) between the means of the clamped and not clamped groups (Fig. 2).
Fig. 2.
Mean and standard error for heart rate in neonates that underwent umbilical cord clamping and those that did not. bpm=beats per min. *indicates difference between the groups (clamping vs. no clamping) (P<0.05).
DISCUSSION
Conventionally, the standard procedure regarding neonate dogs and the placenta during delivery has been to clamp the umbilical cord immediately after birth [14]. Contemporarily, however, the benefits of not clamping the cord are well known in humans; yet, that is not an established practice in Veterinary Medicine, probably due little number research on the topic.
This study removed the placenta from the uterus together with the neonates, keeping the umbilical cord intact. Even in cases of placental abruption, the umbilical vein, which is a larger vessel than the umbilical arteries [11], retains a pulse for about 3 min, which aids residual placental blood flow to the neonates [7, 11]. The shorter umbilical cord in dogs may be helpful in this situation since the distance between the placenta and the neonate is shorter than in humans.
According to Khateria et al. [7], there is no urgency to sever the umbilical cord in human neonates, and it is safe to wait until the cord becomes flat and white, indicating that the neonate has received most of the placental blood. In this study with dogs, we observed that the umbilical cord also became white and flat.
During the extrauterine transfer, with the first breath and the increase in PaO2, the umbilical arteries contract within a 45-sec window, while the umbilical vein remains visible [7, 24]. During the procedure, we noticed that blood transfusion from the umbilical vein to the neonate remained active after the placenta was removed from the uterus, with the umbilical cord visibly filled with blood and transfused to the neonate from 2 to 4 min after birth (indicated by the whiteish coloration and eventual collapse). These findings suggest that there is a transfusion of the residual blood in the umbilical cord to the neonate dog.
In humans, blood flow from the placenta to the neonate is gravity-driven. Therefore, babies are kept at least 10 cm below the mother and the placenta [21]. In this study, the dog neonates were kept below the placenta for at least 3 min after breathing to aid blood flow.
In human neonates, blood can return to the placenta through the umbilical arteries, but a study has shown that blood flows from the neonate to the placenta for only 20–25 sec after birth, becoming negligible about 40–45 sec after birth [24]. On the other hand, the umbilical vein retains a pulse for about 3 min, allowing blood flow from the placenta to the neonate due to spasms in the umbilical arteries and the thicker umbilical vein [7, 11]. Therefore, we kept the umbilical cord intact in the no clamping group, without clamping, for at least 3 min after breathing. Clamping was postponed until after the establishment of pulmonary breathing because, due to the lack of uterine contractions, spontaneous breathing plays a significant role in enabling placental transfusion after the cesarean section [7]. According to Khateria et al. [7], spontaneous breathing and crying create negative pressure in the thoracic cavity and increase the gradient between placental vasculature and the fetal right atrium, improving blood flow from the placenta to the neonate.
A study conducted in lambs born through cesarean section showed that keeping the umbilical cord intact until the establishment of spontaneous breathing improves cardiovascular function and cerebral perfusion during the fetal-to-neonatal transition [3], corroborating the results observed in human babies [5, 13].
The Apgar and reflex scores (proposed by Veronesi et al. [23] and Vassalo et al. [22]) helped determine the clinical condition of the neonate at the time of birth and were used in this study since low scores are often observed in cases of hypoxia [21]. Physiologically, dog neonates are born with a natural degree of hypoxia [20] and are depressed by the anesthetic agents used in the caesarian section [2]. Therefore, the Apgar and reflex scores tend to be low at first, but vitality improves with time [21, 22], which was also observed in this study in both the clamping and no clamping groups, with significant differences between the two moments (P<0.01). In this study, evaluations conducted by a single evaluator are a limitation. However, a single evaluation helps reduce the subjectivity factor in the clinical examination, which might happen with multiple evaluators.
When comparing both groups, there was a significant difference in reflex score at the first and second evaluation; the scores were significantly higher in the no clamping group. This higher vitality may be linked to the improved perfusion and lower hypoxemia in the group. Apgar scores did not vary significantly between the groups in the first moment. However, there was a significant difference in the second moment. When the heart rate was evaluated separately, it was significantly higher in the no clamping group, suggesting that the residual blood flow from the placenta and the umbilical cord was sufficient to modify the neonatal circulatory pattern.
Studies comparing umbilical cord clamping versus no clamping in human neonates have shown no significant difference in Apgar score between the groups [1, 9], which is in line with the results observed with this study’s dog neonates, in the first moment. Although the procedure chosen for not clamping the cord is different from that employed in human medicine.
No significant difference between the groups for the Apgar score at the first evaluation may be related to the degree of anesthetic depression from the cesarean section, the physiological hypoxia during birth, or the adaptation towards extrauterine life [2, 16, 20]. With the physiological improvement observed in the first minutes of life, statistically significant differences were observed between the groups’ Apgar scores.
When assessing the number of neonates with each Apgar score (0 to 10) and reflexes score (0 to 6), the no clamping group at the second evaluation presented more animals with higher scores, than the clamped group, which is healthy vitality for animals born in favorable clinical conditions and is possibly related to improved neonatal viability and lower mortality risk [16, 21, 22].
Not clamping the umbilical cord is an option that should be considered for cesarean sections, since it may improve neonatal vitality. It is of utmost importance that the medical team is trained in neonatal procedures to improve survival rates. Not clamping the umbilical cord and removing the placenta from the bitch is not detrimental to the neonate dogs and could improve tissue perfusion due to residual blood flow from the placenta and umbilical cord, which might lead to improved neonatal vitality at birth.
Acknowledgments
We would like to thank both the Brazilian National Council for Scientific and Technological Development (CNPq, Conselho Nacional de Desenvolvimento Científico e Tecnológico), Brazil and the Small Animal Reproduction Service of the Department for Animal Reproduction and Veterinary Radiology at FMVZ-UNESP, Campus Botucatu, São Paulo, Brazil, for their financial and facilities support for this work.
REFERENCES
- 1.Andersson O., Domellöf M., Andersson D., Hellström-Westas L.2014. Effect of delayed vs early umbilical cord clamping on iron status and neurodevelopment at age 12 months: a randomized clinical trial. JAMA Pediatr. 168: 547–554. doi: 10.1001/jamapediatrics.2013.4639 [DOI] [PubMed] [Google Scholar]
- 2.Batista M., Moreno C., Vilar J., Golding M., Brito C., Santana M., Alamo D.2014. Neonatal viability evaluation by Apgar score in puppies delivered by cesarean section in two brachycephalic breeds (English and French bulldog). Anim. Reprod. Sci. 146: 218–226. doi: 10.1016/j.anireprosci.2014.03.003 [DOI] [PubMed] [Google Scholar]
- 3.Bhatt S., Alison B. J., Wallace E. M., Crossley K. J., Gill A. W., Kluckow M., te Pas A. B., Morley C. J., Polglase G. R., Hooper S. B.2013. Delaying cord clamping until ventilation onset improves cardiovascular function at birth in preterm lambs. J. Physiol. 591: 2113–2126. doi: 10.1113/jphysiol.2012.250084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doebeli A., Michel E., Bettschart R., Hartnack S., Reichler I. M.2013. Apgar score after induction of anesthesia for canine cesarean section with alfaxalone versus propofol. Theriogenology 80: 850–854. doi: 10.1016/j.theriogenology.2013.07.006 [DOI] [PubMed] [Google Scholar]
- 5.Ersdal H. L., Linde J., Mduma E., Auestad B., Perlman J.2014. Neonatal outcome following cord clamping after onset of spontaneous respiration. Pediatrics 134: 265–272. doi: 10.1542/peds.2014-0467 [DOI] [PubMed] [Google Scholar]
- 6.Indrebø A., Trangerud C., Moe L.2007. Canine neonatal mortality in four large breeds. Acta Vet. Scand. 49: S2.https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=17201915&dopt=Abstract [accessed on November 25, 2019]. doi: 10.1186/1751-0147-49-S1-S2 [DOI] [Google Scholar]
- 7.Katheria A. C., Lakshminrusimha S., Rabe H., McAdams R., Mercer J. S.2017. Placental transfusion: a review. J. Perinatol. 37: 105–111. doi: 10.1038/jp.2016.151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Konde A. M., Gitau G. K., Kiptoon J., Gakuya D.2015. Puppy morbidity and mortality among breeding kennels in Nairobi, Kenya. J. J. Vet. Sci. Res. 4: 1–7. [Google Scholar]
- 9.McDonald S. J., Middleton P., Dowswell T., Morris P. S.2014. Effect of timing of umbilical cord clamping of term infants on maternal and neonatal outcomes (Review). Evid. Based Child Health 9: 303–397. doi: 10.1002/ebch.1971 [DOI] [PubMed] [Google Scholar]
- 10.Mercer J. S., Skovgaard R. L.2002. Neonatal transitional physiology: a new paradigm. J. Perinat. Neonatal Nurs. 15: 56–75. doi: 10.1097/00005237-200203000-00007 [DOI] [PubMed] [Google Scholar]
- 11.Montenegro C. A. B., Filho J. R.2010. Obstetrícia, 11th ed., Guanabara Koogan, Rio de Janeiro.
- 12.Münnich A., Küchenmeister U.2014. Causes, diagnosis and therapy of common diseases in neonatal puppies in the first days of life: cornerstones of practical approach. Reprod. Domest. Anim. 49Suppl 2: 64–74. doi: 10.1111/rda.12329 [DOI] [PubMed] [Google Scholar]
- 13.Nevill E., Meyer M. P.2015. Effect of delayed cord clamping (DCC) on breathing and transition at birth in very preterm infants. Early Hum. Dev. 91: 407–411. doi: 10.1016/j.earlhumdev.2015.04.013 [DOI] [PubMed] [Google Scholar]
- 14.Rabe H., Diaz-Rossello J. L., Duley L., Dowswell T.2012. Effect of timing of umbilical cord clamping and other strategies to influence placental transfusion at preterm birth on maternal and infant outcomes. Cochrane Database Syst. Rev. 8: CD003248. [DOI] [PubMed] [Google Scholar]
- 15.SAS InstituteSAS/IML. 2011. 9.3 User’s Guide, SAS Institute Inc., Cary. [Google Scholar]
- 16.Silva L. C. G., Lúcio C. F., Veiga G. A. L., Rodrigues J. A., Vannucchi C. I.2009. Neonatal clinical evaluation, blood gas and radiographic assessment after normal birth, vaginal dystocia or caesarean section in dogs. Reprod. Domest. Anim. 44Suppl 2: 160–163. doi: 10.1111/j.1439-0531.2009.01392.x [DOI] [PubMed] [Google Scholar]
- 17.Traas A. M.2008. Resuscitation of canine and feline neonates. Theriogenology 70: 343–348. doi: 10.1016/j.theriogenology.2008.04.009 [DOI] [PubMed] [Google Scholar]
- 18.Uwins C., Hutchon D. J. R.2014. Delayed umbilical cord clamping after childbirth: potential benefits to baby’s health. Pediatric Health Med. Ther 5: 161–171. [Google Scholar]
- 19.Vannucchi C. I., Lourenço M. L. G.2015. Neonatologia. In: Routine Cases in Small Animal Veterinary Medicine, 3th ed. (Crivellenti, L. Z. and Borin-Crivellenti, S.), Medvet, São Paulo (in Portuguese). [Google Scholar]
- 20.Vannucchi C. I., Silva L. C. G., Lúcio C. F., Regazzi F. M., Veiga G. A. L., Angrimani D. S.2012. Prenatal and neonatal adaptations with a focus on the respiratory system. Reprod. Domest. Anim. 47Suppl 6: 177–181. doi: 10.1111/rda.12078 [DOI] [PubMed] [Google Scholar]
- 21.van Rheenen P. F., Gruschke S., Brabin B. J.2006. Delayed umbilical cord clamping for reducing anaemia in low birthweight infants: implications for developing countries. Ann. Trop. Paediatr. 26: 157–167. doi: 10.1179/146532806X120246 [DOI] [PubMed] [Google Scholar]
- 22.Vassalo F. G., Simões C. R. B., Sudano M. J., Prestes N. C., Lopes M. D., Chiacchio S. B., Lourenço M. L. G.2015. Topics in the routine assessment of newborn puppy viability. Top. Companion Anim. Med. 30: 16–21. [DOI] [PubMed] [Google Scholar]
- 23.Veronesi M. C., Panzani S., Faustini M., Rota A.2009. An Apgar scoring system for routine assessment of newborn puppy viability and short-term survival prognosis. Theriogenology 72: 401–407. doi: 10.1016/j.theriogenology.2009.03.010 [DOI] [PubMed] [Google Scholar]
- 24.Yao A. C., Lind J.1974. Blood flow in the umbilical vessels during the third stage of labor. Biol. Neonate 25: 186–193. doi: 10.1159/000240691 [DOI] [PubMed] [Google Scholar]