Abstract
Intraoral administration of canine interferon alpha (CaIFN-α) has been shown to reduce gingivitis in dogs, but has not been confirmed in cats. Therefore, in this study, a CaIFN-α preparation was used for feline gingivitis, and the degree and duration of its effects were examined. Cats were divided into two groups: one was administered CaIFN-α, and the other was not. They were observed up to 12 months. It was suggested that CaIFN-α have a reducing effect on gingivitis and halitosis for a certain period although may not on plaque or calculus. In addition, the duration of the CaIFN-α gingivitis-reducing effect was suggested to be about three months. The CaIFN-α preparation is considered to be a useful treatment for oral hygiene control.
Keywords: canine interferon alpha, cat, gingivitis
Gingivitis is a reversible inflammation of the gingiva caused by oral bacteria in dental plaque. It is the earliest sign of periodontal disease, the most common oral disease seen in cats [5]. Individuals with untreated gingivitis may develop periodontitis that causes irreversible destruction of the periodontium, including alveolar bone [5]. Therefore, early treatment or prevention of gingivitis is required in order to avoid periodontitis.
In dogs, it has been shown experimentally that low dose oral administration of canine interferon alpha (CaIFN-α) subtype 4 reduced gingivitis [9]. Furthermore, it has been reported that CaIFN-α is useful clinically in dogs for oral hygiene control after dental treatment, or from a young age, due to its gingivitis-reducing effect and long-term action [16]. However, the efficacy of CaIFN-α in the treatment of feline gingivitis has not been confirmed.
CaIFN-α is a type I interferon that exhibits immunostimulating, antibacterial, and anti-inflammatory activities in vivo [3, 6, 10]. In dogs, it was reported that the number of Porphyromonas spp. considered to be closely involved in periodontal disease was reduced by intraoral administration of CaIFN-α [7, 9, 14]. In cats, Porphyromonas spp. is also frequently isolated from the oral cavity [13]. Based on these findings, it was considered that gingivitis may be reduced by intraoral administration of CaIFN-α not only in dogs, but also in cats.
This study was performed to clarify the clinical usefulness of intraoral administration CaIFN-α in cats. The degree and duration of its effects against feline gingivitis were examined by observing cats administered a CaIFN-α preparation over a long period.
We randomly selected 13 cats with mild gingivitis visiting our veterinary clinic (Table 1). They had not received plaque/calculus removal treatment such as scaling within the previous three months. The cases were divided into two groups: a CaIFN-α administered group (group A: nine cats) and an untreated group (group B: four cats). In addition, we prohibited any diet changes, use of antibiotics or other interferon preparations in these cats, in order to avoid any other influence on the intraoral environment after starting observation.
Table 1. Details of cats observed in this study.
| Group | No. | Age (years) | Breed | Sex | Weight (kg) | Remarks |
|---|---|---|---|---|---|---|
| A | 1 | 10.0 | DSH | FS | 4.9 | |
| 2 | 8.0 | DSH | FS | 3.5 | Missing upper left canine tooth | |
| 3 | 5.0 | DSH | FS | 4.0 | ||
| 4 | 2.3 | DSH | FS | 4.6 | Observed up to 9 months | |
| 5 | 8.4 | DSH | MC | 5.4 | Observed up to 6 months, FCV (+) | |
| 6 | 4.1 | DSH | MC | 4.8 | Observed up to 9 months, FeLV (+) | |
| 7 | 4.0 | Maine Coon | MC | 4.2 | ||
| 8 | 1.0 | DSH | FS | 2.7 | ||
| 9 | 2.7 | DSH | FS | 3.1 | FIV (+) | |
| B | 1 | 3.0 | DSH | MC | 4.5 | |
| 2 | 0.8 | DSH | FS | 4.3 | ||
| 3 | 6.8 | DSH | FS | 4.5 | ||
| 4 | 4.8 | DSH | FS | 3.2 | ||
Each cat visited our hospital on routine examinations and was diagnosed mild gingivitis. Then, they were classified into canine interferon alpha administered group A and untreated group B and were observed the progress. DSH: domestic shorthair (the crossbreed cat); FS: female, spayed; MC: male, castrated; FCV: feline calicivirus; FeLV: feline leukemia virus; FIV: feline immunodeficiency virus.
In group A, InterBerry α® (Hokusan Co., Ltd., Hokkaido, Japan/DS Pharma Animal Health Co., Ltd., Osaka, Japan) which is a lyophilized strawberry powder expressing CaIFN-α subtype 4 by gene recombination was used as the CaIFN-α preparation. For each administration, 0.25 g of a powder preparation containing 250 Laboratory Units of CaIFN-α was suspended in 0.1 ml of water and applied to the entire gingiva with a finger. This treatment was administered once every three days, for a total of 10 times (30 days). This was regarded as one course of treatment. The method of administration and dosage in this study followed those of InterBerry α®.
Observations were performed at the start of CaIFN-α administration (zero months) and at 1, 2, 3, 6, 9 and 12 months after the start of administration. At each observation time point, the degree of gingivitis, plaque/calculus deposition and halitosis were evaluated and scored based on criteria (Table 2, Fig. 1) modified from the previously reported evaluation method [4, 11, 15]. Regarding the degree of gingivitis and plaque/calculus deposition, the buccal sides of the left, right, upper and lower canines and carnassials (upper fourth premolar and lower first molar) were examined. Halitosis was organoleptically evaluated by putting the observer’s nose to within 10 cm of each cat’s open mouth.
Table 2. Evaluation criteria and score for each item.
| Score | Evaluation criteria | ||
|---|---|---|---|
| Gingivitis | Plaque/Calculus | Halitosis | |
| 0 | No gingival inflammation | No plaque or calculus deposition | Odorless |
| 0.5 | Slight gingival inflammation (slight change in color) | − | − |
| 1 | Mild gingival inflammation (clear redness and edema; no bleeding on probing) | 1/3 or less of the crown buccal surface covered | Smell, but no malodor |
| 2 | Moderate gingival inflammation (strong redness and edema; bleeding on probing) | 1/3–2/3 of the crown buccal surface covered | Faint malodor |
| 3 | Severe gingival inflammation (marked redness and edema; ulceration; tendency to spontaneously bleed) | 2/3 or more of the crown buccal surface covered | Definite malodor |
| 4 | − | − | Bearable, strong malodor |
| 5 | − | − | Unbearable, intensive malodor |
Fig. 1.
Gingival appearance for each gingivitis score evaluation criteria. All images show the buccal gingival margin of the upper right canine. Scores were determined by gingival color, edema, bleeding, etc.
The total score for gingivitis and plaque/calculus for each cat were obtained by adding the scores for all eight investigated teeth. Since A-2 lacked one tooth at the starting observation, the total score of this case was expressed as 8/7 times. Cases A-4/A-6 and A-5 were only observed up to six and nine months, respectively, as they were no longer available for observation. For each item, the difference between the scores at zero months and at each observation time point was expressed as the difference value, and statistical analysis was performed for the median of the difference value. Significant differences between at zero months and at each observation time point were determined by Wilcoxon’s signed rank sum test. Significant difference between groups A and B at each observation time point was determined by the Mann-Whitney U test. P values of <0.05 were considered significant.
The changes in the gingivitis score were compared in groups A and B (Fig. 2). In group A, the scores decreased after starting CaIFN-α administration and were significantly lower at one to three months than at zero months. Thereafter, the scores tended to increase, becoming similar to the score at zero months at nine months. In group B, the scores tended to increase, and were higher through all observation time points than the score at zero months. Furthermore, the difference values at one to three months in group A were significantly lower than those in group B.
Fig. 2.
Boxplots showing differences at each observation time point from zero months regarding the total gingivitis score in groups A and B. Group A (nine cats) was administered canine interferon alpha (CaIFN-α). Group B (four cats) was not administered CaIFN-α. The line with a point within the box represents the median, and the lower and upper lines of the box represent the 25th and 75th percentiles (interquartile range, IQR). Whiskers represent the maximum and minimum values within 1.5 times the IQR. The point outside the whiskers is an outlier. *indicates significant difference at each observation point from zero months (P<0.05). **indicates significant difference between groups A and B (P<0.05).
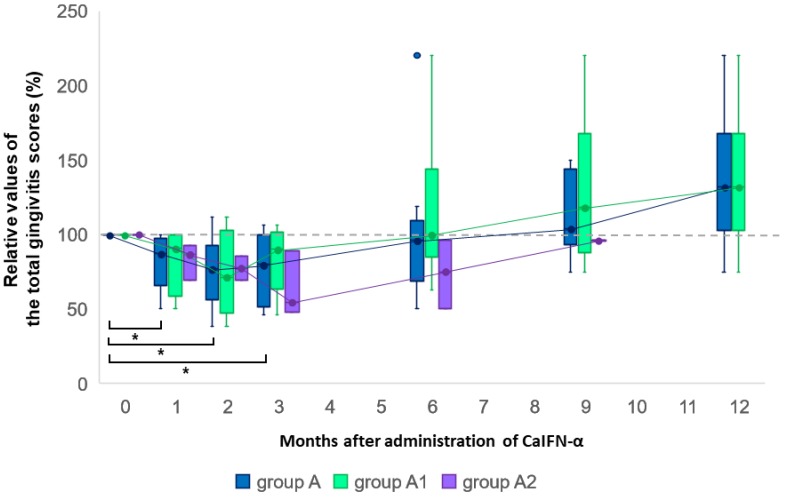
In addition, cats in group A were divided into two groups according to their total gingivitis score at zero months: One group with scores lower than 10 (group A1; six cats) and the other group with scores higher than 10 (group A2; three cats). The percent change in the total gingivitis score at each observation time point after zero months was expressed as a relative value, and compared among groups A, A1 and A2 (Fig. 3). Significant differences among groups A, A1 and A2 at each observation time point were determined by the Kruskal-Wallis test. Although the score in group A2 decreased more than in the other groups, both groups A1 and A2 showed the lowest values at two or three months, and the transition was similar to that in group A.
Fig. 3.
Boxplots showing percent changes at each observation time point from zero months regarding the total gingivitis score in groups A, A1 and A2. Group A (nine cats) was administered canine interferon alpha (CaIFN-α). Cats in group A were divided into two groups according to their total gingivitis score at zero months: group A1 (six cats) with a score lower than 10 and group A2 (three cats) with a score higher than 10. The line with a point within the box represents the median, and the lower and upper lines of the box represent the 25th and 75th percentiles (interquartile range, IQR). Whiskers represent the maximum and minimum values within 1.5 times the IQR. The point outside the whiskers is an outlier. *indicates significant difference at each observation point from zero months (P<0.05). No significant difference among groups A, A1 and A2 at all observation points (P<0.05).
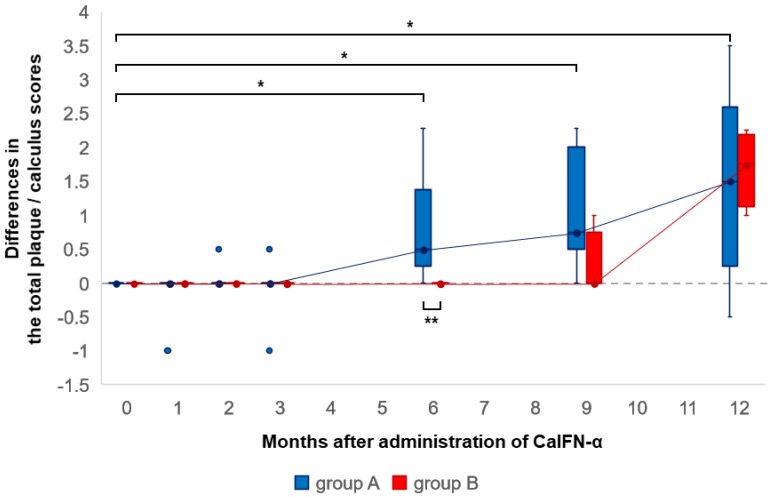
The changes in the plaque/calculus score were compared in groups A and B (Fig. 4). The scores did not decrease in either group A or B. In group A, the scores were significantly higher at six to 12 months than the score at zero months. In group B, the scores increased at 12 months without significant difference. Furthermore, the difference value at six months in group A was significantly higher than that in group B.
Fig. 4.
Boxplots showing differences at each observation time point from zero months regarding the total plaque/calculus score in groups A and B. Group A (nine cats) was administered canine interferon alpha (CaIFN-α). Group B (four cats) was not administered CaIFN-α. The line with a point within the box represents the median, and the lower and upper lines of the box represent the 25th and 75th percentiles (interquartile range, IQR). Whiskers represent the maximum and minimum values within 1.5 times the IQR. The point outside the whiskers is an outlier. *indicates significant difference at each observation point from zero months (P<0.05). **indicates significant difference between groups A and B (P<0.05).
The changes in the halitosis score were compared in groups A and B (Fig. 5). In group A, the scores were significantly lower at one to two months than the score at zero months. Thereafter, the scores became similar to the score at zero months. In group B, the score tended to increase without decreasing. There was no significant difference between groups A and B.
Fig. 5.
Boxplots showing differences at each observation time point from zero months regarding the halitosis score in groups A and B. Group A (nine cats) was administered canine interferon alpha (CaIFN-α). Group B (four cats) was not administered CaIFN-α. The line with a point within the box represents the median, and the lower and upper lines of the box represent the 25th and 75th percentiles (interquartile range, IQR). Whiskers represent the maximum and minimum values within 1.5 times the IQR. *indicates significant difference at each observation point from zero months (P<0.05). No significant difference between groups A and B at all observation points (P<0.05).
In this study, gingivitis and halitosis scores decreased only in the CaIFN-α-administered group. Feline gingivitis is caused by a proliferation of bacteria (mainly Porphyromonas spp.) in the gingival sulcus [13]. Also, halitosis is caused by volatile sulfur compounds produced by these periodontal pathogenic bacteria [2]. It was speculated that the reduction in gingivitis and halitosis in these cats was caused by a reduction in Porphyromonas spp. due to the action of CaIFN-α, as reported in dogs.
It was reported that the gingivitis-reducing effect of CaIFN-α in dogs was maintained up to nine months [16]. However, the duration of the CaIFN-α gingivitis-reducing effect in cats was suggested to be about three months and shorter than that in dogs.
From a comparison within group A, it was found that administration of the CaIFN-α preparation reduced gingivitis at a constant rate, regardless of the degree. In other words, CaIFN-α was at least partially effective against severe gingivitis. However, this also indicates that using CaIFN-α alone is not an adequate treatment for severe gingivitis. For example, it is thought gingivitis with a score of three could not be reduced to a score of zero using CaIFN-α alone. Therefore, it is necessary that the CaIFN-α preparation be administered after basic treatment for periodontal disease, such as scaling, if cats have severe gingivitis.
Plaque is a biofilm mainly composed of aggregates of bacteria, and calculus is calcified plaque [5]. Therefore, it was speculated that plaque and calculus are also suppressed by the antibacterial action of CaIFN-α. However, this study showed CaIFN-α may not have a reducing or preventive effect on plaque or calculus. Even so, when the same numbers of specimens with almost the same control score were selected from groups A and B and compared, inhibition of deposition of plaque/calculus by CaIFN-α was suggested. Regarding these effects, further investigation is necessary.
In 1999, Amimoto et al. [1] reported that periodontal disease symptoms, including gingivitis, were present in 58.2% of cats even in the first year of life, at which point calculus deposition is low, and that its degree worsened with age. Also, according to the AAHA Dental Care Guidelines in 2013 [8], periodontal diseases may accompany various abnormalities in the oral cavity from birth to two years of age in cats. Furthermore, the WSAVA Global Dental Guidelines in 2017 [12], reported that 70% of cats have some form of periodontal disease by two years of age. Recently, to control oral hygiene before periodontal disease becomes severe or after dental treatment such as dental calculus removal, as well as efforts to prevent periodontal disease, have been regarded as important. Although tooth brushing is the most recommended approach to oral hygiene control, it is difficult to perform in cats. Therefore, a CaIFN-α preparation that can be administered intraorally with relative ease, may useful for oral hygiene control in young cats or after dental treatment.
REFERENCES
- 1.Amimoto A., Noguchi M., Hachimura H., Suzuki T., Nakano M.1999. Survey of the incidence of calculus deposition and periodontal disease in cats. J. Anim. Clin. Med. 8: 99–102. [Google Scholar]
- 2.Culham N., Rawlings J. M.1998. Oral malodor and its relevance to periodontal disease in the dog. J. Vet. Dent. 15: 165–168. doi: 10.1177/089875649801500401 [DOI] [PubMed] [Google Scholar]
- 3.Dec M., Puchalski A.2008. Use of oromucosally administered IFN-α in the prevention and treatment of animal diseases. Pol. J. Vet. Sci. 11: 171–182. [PubMed] [Google Scholar]
- 4.Eubanks D. L.2010. Periodontal disease assessment. J. Vet. Dent. 27: 58–60. doi: 10.1177/089875641002700112 [DOI] [PubMed] [Google Scholar]
- 5.Gorrel C.2004. Periodontal disease. pp. 87–110. In: Veterinary Dentistry for the General Practitioner, Elsevier Health Science, London. [Google Scholar]
- 6.Guarda G., Braun M., Staehli F., Tardivel A., Mattmann C., Förster I., Farlik M., Decker T., Du Pasquier R. A., Romero P., Tschopp J.2011. Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity 34: 213–223. doi: 10.1016/j.immuni.2011.02.006 [DOI] [PubMed] [Google Scholar]
- 7.Hardham J., Dreier K., Wong J., Sfintescu C., Evans R. T.2005. Pigmented-anaerobic bacteria associated with canine periodontitis. Vet. Microbiol. 106: 119–128. doi: 10.1016/j.vetmic.2004.12.018 [DOI] [PubMed] [Google Scholar]
- 8.Holmstrom S. E., Bellows J., Juriga S., Knutson K., Niemiec B. A., Perrone J., American Veterinary Dental College2013. 2013 AAHA dental care guidelines for dogs and cats. J. Am. Anim. Hosp. Assoc. 49: 75–82. doi: 10.5326/JAAHA-MS-4013 [DOI] [PubMed] [Google Scholar]
- 9.Ito A., Isogai E., Yoshioka K., Sato K., Himeno N., Gotanda T.2010. Ability of orally administered IFN-α4 to inhibit naturally occurring gingival inflammation in dogs. J. Vet. Med. Sci. 72: 1145–1151. doi: 10.1292/jvms.09-0201 [DOI] [PubMed] [Google Scholar]
- 10.Malireddi R. K., Kanneganti T. D.2013. Role of type I interferons in inflammasome activation, cell death, and disease during microbial infection. Front. Cell. Infect. Microbiol. 3: 77. doi: 10.3389/fcimb.2013.00077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miyazaki H., Arao M., Okamura K., Kawaguchi Y., Toyofuku A., Hoshi K., Yaegaki K.1999. Tentative classification for halitosis patients and its treatment needs. Niigata. Dent. J. 29: 11–15. [Google Scholar]
- 12.Niemiec B. A., Gawor J., Nemec A., Clarke D., Tutt D., Gioso M., Stegall P., Chandler M., Morgenegg G., Jouppi R., Stewart K.2017. World Small Animal Veterinary Association Global Dental Guidelines, https://www.wsava.org/WSAVA/media/Documents/Guidelines/Dental-Guidleines-for-endorsement_0.pdf [accessed on January 5, 2019].
- 13.Pérez-Salcedo L., Herrera D., Esteban-Saltiveri D., León R., Jeusette I., Torre C., O’Connor A., González I., González I.2013. Isolation and identification of Porphyromonas spp. and other putative pathogens from cats with periodontal disease. J. Vet. Dent. 30: 208–213. doi: 10.1177/089875641303000402 [DOI] [PubMed] [Google Scholar]
- 14.Senhorinho G. N. A., Nakano V., Liu C., Song Y., Finegold S. M., Avila-Campos M. J.2011. Detection of Porphyromonas gulae from subgingival biofilms of dogs with and without periodontitis. Anaerobe 17: 257–258. doi: 10.1016/j.anaerobe.2011.06.002 [DOI] [PubMed] [Google Scholar]
- 15.Ueda I.1990. Ekigaku. pp. 59–88. In: Koukuueiseigaku (Nakao, S., Iizuka, K., Ueda, I. and Konishi, K. eds.), Quintessence Publishing, Tokyo (in Japanse). [Google Scholar]
- 16.Yamaki S., Hachimura H., Wada S., Oonari A., Ogawa M., Kanegae S., Amimoto A.2017. Long-term follow-up study after the administration of canine interferon-α preparation for gingivitis in dogs. J. Jpn. Med. Assoc. 70: 589–593. doi: 10.12935/jvma.70.589 [DOI] [Google Scholar]