Abstract
Clostridium (reclassified as “ Clostridioides ”) difficile infection (CDI) occurs as a chronic or an acute illness with intensity varying from mild to severe. Although most cases of CDI can be managed with antibiotics and supportive care, when the patient presents with fulminant disease, the early decision to perform surgery is imperative for survival. The current standard of care is the subtotal colectomy. However, loop ileostomy with vancomycin enemas delivered into the colonic mucosa has been described as a viable option on selected patients.
Keywords: Clostridium difficile infection , surgery for complicated Clostridium difficile infection subtotal colectomy , loop ileostomy for complicated Clostridium difficile infection
Clostridium difficile a infection (CDI) has been present in the United States for several decades but the frequency and severity of cases has recently increased, likely from the emergence and spread of more virulent strains, resulting in increased costs and more importantly in increased morbidity and mortality for those patients affected with fulminant disease. 1 2 3
Clostridium difficile ( C. difficile ) is a gram-positive anaerobic bacteria, part of the natural flora of the intestines. In healthy patients, its presence is tolerated well, regulated by the presence of other bacteria. When this balance is perturbed, C. difficile is able to proliferate and result in inflammation and injury of the colon, leading to acute or chronic infections. 2
The reasons for this imbalance are multifactorial. Intake of antimicrobials, even a single dose, is most often sited. Other reasons for CDI are immunosuppression system from a preexisting infection or chronic medical problems and weakening of the colonic mucosa in tolerating infections. 4 Noted risk factors for CDI are age (patients over 60 years), previous hospitalizations; exposure to antimicrobials, inflammatory bowel disease, colon surgery, and acid-reducing medications 4 5
The mechanisms through which C. difficile causes inflammation and injury is believed to be through its production of toxins, although all strains don't produce toxins and there is no clear correlation between toxin production and severity. The toxins translocate into cells, disrupting their anatomy and subsequent death. This participate in the increased permeability of the intestinal barrier and release of proinflammatory agents, leading to systemic effects. As a result, patients will present clinically with diarrhea most often, although an ileus can also be present, dehydration from poor absorption and colitis with pseudo membrane. 6 The degree of clinical severity can vary from simple diarrhea to severe septic shock and multiorgan system failure. 7
Initial Management
The Clinical Practice Guidelines for C. difficile infection (CDI) in adults and children were recently updated by the Infectious Diseases Society of America (IDSA) and the Society for Healthcare Epidemiology of American (SHEA). 8 9 There are several significant changes in the new guidelines as follows.
These are the New Clinical Definitions
Nonsevere disease : leukocytosis with a white blood cell count of ≤15,000 cells/mL and a serum creatinine level <1.5 mg/dL.
Severe disease : leukocytosis with a white blood cell count of ≥15,000 cells/mL or a serum creatinine level >1.5 mg/dL.
Fulminant disease : previously referred to as severe, complicated CDI, characterized by hypotension or shock, ileus, or megacolon.
New Recommendations Regarding Treatment
Metronidazole (Flagyl) PO is no longer recommended as first-line therapy in adults.
Vancomycin or fidaxomicin PO are first-line recommended treatments. There is evidence to support that this treatment provides patients with the highest likelihood of sustained symptom resolution.
In patients with fulminant CDI, vancomycin PO 500 mg q6h with intravenous (IV) metronidazole is recommended, with vancomycin retention enema in patients with ileus.
New Recommendations Regarding the Surgical Treatment
Subtotal colectomy with preservation of the rectum is recommended as the gold-standard operation.
Diverting loop ileostomy with colonic lavage followed by antegrade vancomycin flushes is an alternative approach. This recommendation is a conditional recommendation. 9
It is important to emphasize that multidisciplinary collaboration and early surgical intervention can improve the outcome of patients with fulminant CDI. 7 9 10 11 Resuscitation to euvolemia, early aggressive treatment with the appropriate antibiotics, and early surgical intervention are pivotal factors for better outcomes.
Fecal Transplant
Fecal transplantation is a newer method to treat recurrent C. difficile infections. It consists in transferring fecal microbiota from a healthy donor into the colon of the patient. It can be done through pills ingested PO or through a colonoscopy. 12
This treatment should be reserved for the stable mild/moderate recurrent episode. 13 The use of fecal transplantation of fulminant CDI is still controversial. 14 Patients with fulminant CDI need aggressive resuscitation to euvolemia and prompt source control, as any other patient with septic shock. Delaying the initiation of appropriate antibiotics or source control can result in unnecessary mortality. 15 16 17
Surgical Intervention
When a patient presents with severe and severe complicated CDI, the decision for a surgical intervention need to be taken early to avoid mortality. Some patients with fulminant disease will present with an ileus, therefore the diagnosis of CDI via stool testing might not be possible. Ileus is an ominous sign of worsening disease and potentially bowel necrosis. Surgery in these cases becomes the life-saving intervention and should not be delayed. 7 17
Often, when surgical consultation is obtained, when the patient is already in multiorgan system failure with little reserved to tolerate a large operation or general anesthesia. It is pivotal to collaborate without medicine and infectious disease colleagues to create protocols or early aggressive treatment, as well as early surgical consultation, to help these patients in need.
Mortality increases exponentially when surgery is performed too late on patients already on vasopressors. 7 An obvious indication for surgical intervention is peritonitis; however, if this occurs secondary to colon necrosis and perforation, then the surgical intervention was performed too late. Other earlier signs are the development of respiratory distress, acute kidney injury, and lactic acidosis, as well as the lack of response to maximum treatment for 48 hours. 18
Since CDI is a disease of the mucosa, the serosa of the colon might appear completely intact upon exploration, this is why it is important that the surgical team understands that this is a disease of the entire colon. 1 2 6 7 9 17 19 20 21 Performing a partial colectomy or a surgical exploration without a procedure that can treat this infection has been associated with prohibitive mortality rates.
The standard of care is the subtotal colectomy. This operation leaves the patient with a permanent ostomy, and carries some blood loss and operative time; these factors can add to morbidity and mortality on a patient who is already physiologically compromised. 18 22 23 Other interventions, such as loop ileostomy with colonic lavage and antegrade enemas with vancomycin, have been advocated in certain instances. 19 22 This procedure allows for colonic preservation, and it is less invasive, leads to less blood loss, and physiologically can be better tolerated by patients in vasodilatory shock. However, this procedure is contraindicated in patients with distal strictures, necrotic or perforated colon, and abdominal compartments syndrome.
Since the timing for ostomy reversal after the infection subsided nor the recurrence rate after reanastomosis has been well studied, care must be taken when treating these patients after the initial septic shock has subsided.
Subtotal Colectomy
The standard of care or surgical intervention in the severe CDI patient is subtotal colectomy. Partial colectomy has not shown any improved outcomes and commits the surgeon having to return, often shortly thereafter, in a more hostile abdomen, prone to further complications. 9 18
As this procedure will most often be performed in the semiemergent setting, marking the patient for the end-ileostomy may not be possible, but having a general idea of the ostomy site prior to enter the abdomen may be helpful.
The patient can be placed either supine or in lithotomy position to allow for endoscopy intraoperatively as needed. In the case of CDI, the colon has the possibility of being significantly distended and good exposure is essential. Hence, a long–midline incision is recommended.
Once the abdominal cavity is entered, quickly examine all four quadrants for any purulence, feculence or gross perforation, run, and palpate the bowel for any lesions or injuries.
The colon is often so distended than mobilization is easier than with other colonic diseases. Since this is not an oncological resection, the operator can leave all the mesentery behind. Staying close to the colon can expedite this procedure and avoid further complications.
After the ileum and rectum are stapled off, the operator can decide to finalize the procedure with an ostomy.
If other risk factors, such as over resuscitation are placing the patient at risk of abdominal compartment syndrome, it is reasonable to perform damage control surgery and leave the bowel in discontinuity with an abdominal vacuum-assisted closure system and return to the operating room (OR) 24 to 48 hours later for further washout and reestablishment of continuity. On the other hand, if the patient is stable enough and the contamination was minimal, and end ileostomy can be performed with abdominal closure ( Fig. 1 ).
Fig. 1.
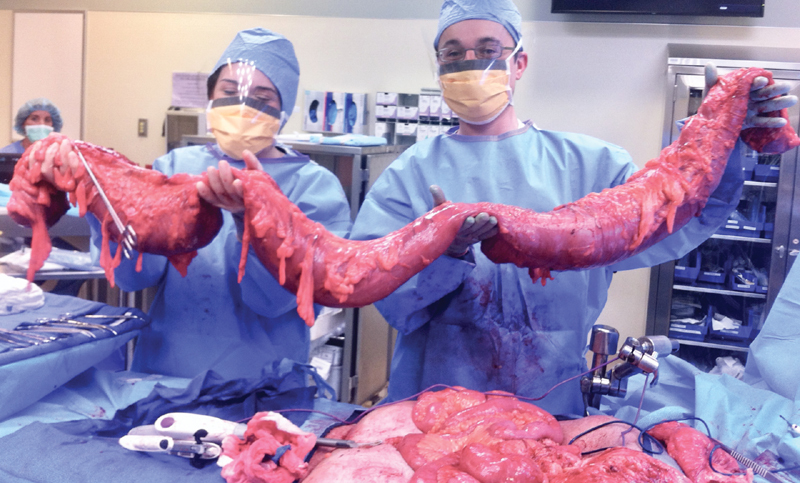
Fulminant CDI sp. total colectomy. In the picture Dr. Levi Procter. CDI, Clostridium difficile infection.
Loop Ileostomy and Colonic Lavage
As mentioned above, subtotal colectomy is a morbid procedure and alternative approach has emerged. One of those alternative approaches is the loop ileostomy with colonic lavage and high-dose vancomycin enemas in fulminant CDI. 19 22
Summary of the Literature Supporting the Use of Loop Ileostomy and Colonic Lavage
The first study advocating for loop ileostomy and washout for fulminant CDI was published by the University of Pittsburgh Acute Care Surgery group. 22 The authors described 42 patients prospectively treated with this technique. They placed a catheter through the loop ileostomy into the colon and lavage the colon with eight litters of polyethylene glycol 3,350/electrolyte solution. After the patients received vancomycin flushes of 500 mg in 500 mL of lactated ringers; q8 hours for a duration of 10 days directly into the colon, and in addition, patients were continued on IV metronidazole (500 mg q8 hours) for 10 days.
They successfully performed the procedure laparoscopically in 83% of the patients. They demonstrated that the procedure could be done in patients who were critically ill before the surgery, since the majority (90%) were in the intensive care unit before the procedure with a mean APACHE-II score of 29.7 at the time of surgical evaluation. They compared these results with matching number of patients treated with colectomy for the same condition before they started doing the LI and showed a decrease in mortality from 19 versus 50%(odds ratio = 0.24; p = 0.006).
After this single-center experience, there was an attempt for a randomized control trial, multicenter study. This study was not completed since we could not find enough participants (ClinicalTrials.gov Identifier: NCT01441271).
The Eastern Association for the Surgery of Trauma developed a multicenter retrospective trial. 19 The hypothesis of this study was the loop ileostomy and total colectomy carried a similar mortality rate.
We collected data from 10 centers and 98 patients with fulminant CDI. The overall mortality was 32 and 75% of the entire group had postoperative complications. It was difficult to compare the two procedures since only 21% of the patients underwent LI. However, we found that the groups of patients were similar in age and comorbidities. Patients who had a total colectomy also had a larger blood loss and operative time and a higher mortality rate (17.2 vs. 39.7%; p = 0.002).
Who Is Not a Candidate for This Approach?
Patients with fulminant CDI and perforation or colon necrosis are not a candidate for LI since the entire point of this surgery is to preserve the colon. The presence of transmural necrosis and/or perforation indicates the infectious process is too advanced to offer colon presentation. These patients have an extremely high mortality and should undergo a subtotal colectomy.
It is also important to ensure the patient does not have a distal obstruction, since this would not allow the vancomycin to reach the entire colon. Patients with intra-abdominal hypertension should undergo an exploratory laparotomy and total colectomy and are not candidates for colon preservation. 24 25
The approach can be either laparoscopic or open based on surgeon's preference and patient's capacity to tolerate insufflation. Hypovolemic patients or patients in shock can have further hypotension after the creation of pneumoperitoneum since the acute collapse of the inferior vena cava, decreased venous return, and acute decreased cardiac output. This can result in imminent cardiovascular deterioration. Each case needs to be considered separately.
Another anatomical consideration is the distention of the colon and the small bowel in severe cases. This can displace the location of the terminal ileum adding difficulty in doing the loop ileostomy via a small incision.
If colonic ischemia, obstruction, or perforation are noted, or if the patient's clinical status worsens, the procedure should be converted to a subtotal colectomy.
Technical Considerations
Create a loop ileostomy laparoscopically if the patient can tolerate this physiologically, or open if that is not the case. As mentioned previously take into consideration the colon and small bowel will be distended and, therefore, displaced from their usual positioning. A tube should be inserted into the colon, passed the ileocecal valve to perform an on table antegrade colonic lavage with 8L of warmed polyethylene glycol solution. 22 We have used Malecot's tubes for this purpose ( Fig. 2) . The tube should be left in place and postoperative antegrade vancomycin enema should be performed using it. It is critical that the vancomycin dose is high (500 mg) and that is diluted in enough fluid to reach the entire colon.
Fig. 2.

Drawing showing the loop ileostomy and direction of the vancomycin enemas (By Paula Ferrada).
If clinical improvement is not noted in the short postoperative period, the patient will need to return to the OR for subtotal colectomy. In some institutions, where surgeons are facile with this procedure, outcomes have been noted to have associated decreased morbidity in the patient population, although the reports are limited by the number of patients studied and institutions involved.
Failure of Loop Ileostomy
Several factors can be attributed to the failure of this procedure. Lack of adherence to the protocol or the prescience of a distal obstruction are two factors that can be corrected by the treating team. 26 27
Hypervirulent strains of Clostridium and resistance to the treatment are other considerations. 28
In our experience, when this procedure is performed patients start improving within 48 hours. If the patient is not responding to treatment considering an urgent total colectomy.
When to Reverse the Loop Ileostomy?
Ideally, the patient needs to be completely recovered from the initial physiological insult, tolerating regular diet and back to normal daily activities. It is important to discuss with the patient the risk of recurrence for fulminant CDI and, if this occurs, they could require a total colectomy. Some recurrences of CDI or reinfections can be seen when the continuity of the bowel is reestablished. 26 27 28 Fecal transplant could be a consideration before reanastomosis in these patients to help prevent recurrence. 29 30 31
Conclusion
The role of surgery in CDI is an early intervention in patients with severe and severe complicated disease as it improves outcomes significantly. Due to the level of acuity of patients with severe CDI, requiring surgery and the difficulty to assess colon involvement by examining the serosa, one surgical intervention should occur and remove the diseased colon. As a result, total colectomy remains the gold standard. Loop ileostomy with vancomycin enemas has emerged as a viable alternative in selected patients. Data regarding recurrence after reanastomosis with colon preservation surgery is lacking.
Conflict of Interest None declared.
The genus name “ Clostridium ” was reclassified as “ Clostridioides ” in 2016.
References
- 1.Khanna S, Pardi D S. The growing incidence and severity of Clostridium difficile infection in inpatient and outpatient settings . Expert Rev Gastroenterol Hepatol. 2010;4(04):409–416. doi: 10.1586/egh.10.48. [DOI] [PubMed] [Google Scholar]
- 2.Lessa F C, Mu Y, Bamberg W M et al. Burden of Clostridium difficile infection in the United States . N Engl J Med. 2015;372(09):825–834. doi: 10.1056/NEJMoa1408913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vindigni S M, Surawicz C M. C. difficile infection: changing epidemiology and management paradigms . Clin Transl Gastroenterol. 2015;6:e99. doi: 10.1038/ctg.2015.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodemann J F, Dubberke E R, Reske K A, Seo D H, Stone C D. Incidence of Clostridium difficile infection in inflammatory bowel disease . Clin Gastroenterol Hepatol. 2007;5(03):339–344. doi: 10.1016/j.cgh.2006.12.027. [DOI] [PubMed] [Google Scholar]
- 5.Vesteinsdottir I, Gudlaugsdottir S, Einarsdottir R, Kalaitzakis E, Sigurdardottir O, Bjornsson E S. Risk factors for Clostridium difficile toxin-positive diarrhea: a population-based prospective case-control study . Eur J Clin Microbiol Infect Dis. 2012;31(10):2601–2610. doi: 10.1007/s10096-012-1603-0. [DOI] [PubMed] [Google Scholar]
- 6.Voth D E, Ballard J D. Clostridium difficile toxins: mechanism of action and role in disease . Clin Microbiol Rev. 2005;18(02):247–263. doi: 10.1128/CMR.18.2.247-263.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrada P, Velopulos C G, Sultan S et al. Timing and type of surgical treatment of Clostridium difficile -associated disease: a practice management guideline from the Eastern Association for the Surgery of Trauma . J Trauma Acute Care Surg. 2014;76(06):1484–1493. doi: 10.1097/TA.0000000000000232. [DOI] [PubMed] [Google Scholar]
- 8.Cohen S H, Gerding D N, Johnson S et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA) Infect Control Hosp Epidemiol. 2010;31(05):431–455. doi: 10.1086/651706. [DOI] [PubMed] [Google Scholar]
- 9.McDonald L C, Gerding D N, Johnson S et al. Clinical Practice Guidelines for Clostridium difficile infection in adults and children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA) . Clin Infect Dis. 2018;66(07):987–994. doi: 10.1093/cid/ciy149. [DOI] [PubMed] [Google Scholar]
- 10.Bagdasarian N, Rao K, Malani P N. Diagnosis and treatment of Clostridium difficile in adults: a systematic review . JAMA. 2015;313(04):398–408. doi: 10.1001/jama.2014.17103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Louie T J, Miller M A, Mullane K M et al. Fidaxomicin versus vancomycin for Clostridium difficile infection . N Engl J Med. 2011;364(05):422–431. doi: 10.1056/NEJMoa0910812. [DOI] [PubMed] [Google Scholar]
- 12.Guo B, Harstall C, Louie T, Veldhuyzen van Zanten S, Dieleman L A. Systematic review: faecal transplantation for the treatment of Clostridium difficile -associated disease . Aliment Pharmacol Ther. 2012;35(08):865–875. doi: 10.1111/j.1365-2036.2012.05033.x. [DOI] [PubMed] [Google Scholar]
- 13.Hota S S, Poutanen S M. Fecal microbiota transplantation for recurrent Clostridium difficile infection . CMAJ. 2018;190(24):E746. doi: 10.1503/cmaj.171454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duarte-Chavez R, Wojda T R, Zanders T B, Geme B, Fioravanti G, Stawicki S P. Early results of fecal microbial transplantation protocol implementation at a community-based university hospital. J Glob Infect Dis. 2018;10(02):47–57. doi: 10.4103/jgid.jgid_145_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Plevin R, Callcut R. Update in sepsis guidelines: what is really new? Trauma Surg Acute Care Open. 2017;2(01):e000088. doi: 10.1136/tsaco-2017-000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weisberg A, Park P, Cherry-Bukowiec J R. Early goal-directed therapy: the history and ongoing impact on management of severe sepsis and septic shock. Surg Infect (Larchmt) 2018;19(02):142–146. doi: 10.1089/sur.2017.221. [DOI] [PubMed] [Google Scholar]
- 17.Kulaylat A S, Kassam Z, Hollenbeak C S, Stewart D B., Sr A surgical Clostridium -associated risk of death score predicts mortality after colectomy for Clostridium difficile. Dis Colon Rectum. 2017;60(12):1285–1290. doi: 10.1097/DCR.0000000000000920. [DOI] [PubMed] [Google Scholar]
- 18.Lamontagne F, Labbé A C, Haeck O et al. Impact of emergency colectomy on survival of patients with fulminant Clostridium difficile colitis during an epidemic caused by a hypervirulent strain . Ann Surg. 2007;245(02):267–272. doi: 10.1097/01.sla.0000236628.79550.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferrada P, Callcut R, Zielinski M Det al. Loop ileostomy vs. total colectomy as surgical treatment for Clostridium difficile associated disease: an eastern association for the surgery of trauma multicenter trial J Trauma Acute Care Surg2017 [DOI] [PMC free article] [PubMed]
- 20.Pépin J, Valiquette L, Alary M E et al. Clostridium difficile -associated diarrhea in a region of Quebec from 1991 to 2003: a changing pattern of disease severity . CMAJ. 2004;171(05):466–472. doi: 10.1503/cmaj.1041104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Wilden G M, Chang Y, Cropano C et al. Fulminant Clostridium difficile colitis: prospective development of a risk scoring system . J Trauma Acute Care Surg. 2014;76(02):424–430. doi: 10.1097/TA.0000000000000105. [DOI] [PubMed] [Google Scholar]
- 22.Neal M D, Alverdy J C, Hall D E, Simmons R L, Zuckerbraun B S. Diverting loop ileostomy and colonic lavage: an alternative to total abdominal colectomy for the treatment of severe, complicated Clostridium difficile associated disease Ann Surg 201125403423–427., discussion 427–429 [DOI] [PubMed] [Google Scholar]
- 23.Sailhamer E A, Carson K, Chang Yet al. Fulminant Clostridium difficile colitis: patterns of care and predictors of mortality Arch Surg 200914405433–439., discussion 439–440 [DOI] [PubMed] [Google Scholar]
- 24.Rezende-Neto J B, Rotstein O D. Abdominal catastrophes in the intensive care unit setting. Crit Care Clin. 2013;29(04):1017–1044. doi: 10.1016/j.ccc.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 25.Thai H, Guerron A D, Bencsath K P, Liu X, Loor M. Fulminant Clostridium difficile enteritis causing abdominal compartment syndrome . Surg Infect (Larchmt) 2014;15(06):821–825. doi: 10.1089/sur.2013.026. [DOI] [PubMed] [Google Scholar]
- 26.Fashandi A Z, Ellis S R, Smith P W, Hallowell P T. Overwhelming recurrent clostridium difficile infection after reversal of diverting loop ileostomy created for prior fulminant C. difficile colitis . Am Surg. 2016;82(08):e194–e195. [PMC free article] [PubMed] [Google Scholar]
- 27.Fashandi A Z, Martin A N, Wang P T et al. An institutional comparison of total abdominal colectomy and diverting loop ileostomy and colonic lavage in the treatment of severe, complicated Clostridium difficile infections . Am J Surg. 2017;213(03):507–511. doi: 10.1016/j.amjsurg.2016.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stewart D B. Loop ileostomy for Clostridium difficile infection: know thy enemy . J Trauma Acute Care Surg. 2017;83(06):1214–1215. doi: 10.1097/TA.0000000000001688. [DOI] [PubMed] [Google Scholar]
- 29.Eliakim-Raz N, Bishara J. Prevention and treatment of Clostridium difficile associated diarrhea by reconstitution of the microbiota . Hum Vaccin Immunother. 2019;15(06):1453–1456. doi: 10.1080/21645515.2018.1472184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Juul F E, Garborg K, Bretthauer M et al. Fecal microbiota transplantation for primary Clostridium difficile infection . N Engl J Med. 2018;378(26):2535–2536. doi: 10.1056/NEJMc1803103. [DOI] [PubMed] [Google Scholar]
- 31.Messias B A, Franchi B F, Pontes P H, Barbosa DÁAM, Viana C AS. Fecal microbiota transplantation in the treatment of Clostridium difficile infection: state of the art and literature review. Rev Col Bras Cir. 2018;45(02):e1609. doi: 10.1590/0100-6991e-20181609. [DOI] [PubMed] [Google Scholar]


