Summary
Objective
Left ventricular mass (LVM) is a clinical prognostic indicator of cardiovascular disease. Left ventricular mass is associated with body size (body mass index [BMI], weight, and body surface area [BSA]). This study examined if the association between body size (weight, BMI, and BSA) and LVM is influenced by body composition and cardiorespiratory fitness in adults who are overweight or obese.
Methods
This study included cross‐sectional baseline data from a randomized clinical trial. Participants included 379 adults (age, 45.6 ± 7.9 y) who were overweight or obese (BMI, 32.4 ± 3.8 kg·m−2). Measures included weight, height, BMI, BSA, body composition, cardiorespiratory fitness, and LVM by cardiac magnetic resonance imaging (CMR).
Results
Left ventricular mass was positively associated with weight, BMI, BSA, and fitness (P < .0001) and inversely associated with percent body fat (P < .0001). Stepwise multiple regression models showed that body fatness was inversely associated and cardiorespiratory fitness was positively associated with LVM even after considering weight, BMI, or BSA in the analyses.
Conclusions
These cross‐sectional findings support that in adults who are overweight or obese but otherwise relatively healthy, LVM is associated with both body composition and cardiorespiratory fitness. This may indicate the need to reduce body fatness and improve fitness for patients with obesity to enhance cardiovascular structure and function.
Keywords: Cardiac magnetic resonance imaging (CMR), exercise, fitness, obesity
1. INTRODUCTION
In adults over the age of 20 years, the prevalence of obesity (body mass index [BMI] ≥ 30 kg·m−2) in the United States has increased from 30.5% in 1999‐2000 to 39.6% in 2016 on the basis of National Health and Nutrition Examination Survey data.1 These data also show that the prevalence of obesity was 37.9% for men and 41.1% for women. Excess body weight is of public health concern because of its association with chronic diseases, with a major concern being the association with cardiovascular disease (CVD).2, 3
Left ventricular mass (LVM) of the heart is a clinically important prognostic indicator of CVD, with early data reported from the Framingham Heart Study supporting this conclusion.4, 5 Larger body size, as measured BMI, is associated with greater LVM measured with echocardiography.6 Echocardiography employs assumptions to estimate LVM, and cardiac magnetic resonance imaging (CMR) is considered by some as the clinical gold standard for LVM measurement. Studies using CMR have shown larger LVM at higher levels of BMI.7 Given that BMI is a clinical indicator of overweight or obesity, these findings may suggest that a pathway by which overweight and obesity contribute to CVD is through a maladaptive larger LVM. However, many of these studies have not included measures of body composition to quantify body fatness, which may be a more sensitive measure of obesity than BMI alone, and may further contribute to the risk of CVD beyond what is observed with BMI or other measures of body size alone (eg, body weight and body surface area [BSA]).
Studies have shown that cardiorespiratory fitness is an important physiological indicator of CVD risk.8, 9, 10 Additional evidence suggests that even in the presence of overweight and obesity, higher levels of cardiorespiratory fitness may provide protection from CVD.11, 12, 13, 14, 15, 16, 17 However, studies that have examined the association between indices of body size (eg, BMI, body weight, and BSA) and LVM have not considered the contribution of cardiovascular fitness as an important factor that may also contribute to LVM. Athletes have greater LVM than less active individuals,18 and individuals who are more active have a lower CVD risk,19, 20, 21, 22, 23 possibly suggesting that the higher LVM found in more active and fit individuals represents a favourable adaptive response. The CARDIA Study, a prospective observational study, has examined cardiorespiratory fitness as a predictor of LVM while also considering other lifestyle factors such as BMI, and it showed that LVM measured by echocardiography was not associated with cardiorespiratory fitness.24 However, there are few additional studies that have examined these relationships, with few examining the combined contributions of body composition and fitness on LVM in adults clinically defined as overweight or obese.
Baseline assessments from the Heart Health Study included measures of LVM using CMR, body composition using dual‐energy X‐ray absorptiometry (DXA), and cardiorespiratory fitness using a graded exercise test in adults who are overweight or obese. With the use of these data, analyses were conducted to examine whether the association between body size (weight, BMI, and BSA) and LVM is influenced by both body composition and cardiorespiratory fitness in adults who are overweight or obese.
2. MATERIALS AND METHODS
2.1. Subjects
Subjects (N = 383) were recruited for participation into the Heart Health Study, which is a randomized clinical trial to examine the effects of weight loss through diet and physical activity on measures of CMR and other CVD risk factors. Data presented in this manuscript reflect measures assessed at baseline and prior to engagement in the weight loss intervention. Subjects were considered eligible if their age was 18 to 55 years and BMI was 25 to <40 kg·m−2 at the baseline assessment when eligibility was determined. Ineligibility criteria included (a) self‐reporting ≥ 60 min/wk of structured moderate‐to‐vigorous intensity physical activity; (b) weight loss of ≥5% within the prior 6 months or a history of bariatric surgery; (c) history of cardiometabolic disease, diabetes mellitus, or cancer; (d) taking medication that could affect heart rate or blood pressure; (e) taking medication that could influence body weight; (f) treatment for psychological conditions that included medication or counselling; (g) currently pregnant, pregnant within the prior 6 months, or planning a pregnancy within the next 12 months; (h) planning on geographical relocation outside of the region within 12 months; (i) inability to comply with the components of the interventions; or (j) had a contraindication that would prohibit magnetic resonance imaging (MRI) scanning. Participants provided written informed consent and medical clearance from their physician prior to engaging in this study. All procedures were approved by the University of Pittsburgh's Institutional Review Board and Human Research Protection Office.
2.2. Measures
2.2.1. Descriptive characteristics
Information on gender, race, and ethnicity was collected via questionnaire. Age was confirmed from the birth date contained on a government issued identification card (eg, driver's license and passport).
2.2.2. Weight, height, BMI, and BSA
Weight and height were assessed with the subject clothed in a lightweight hospital gown with shoes removed. Weight was assessed using a calibrated digital scale to the nearest 0.1 kg with duplicate measures differing by ≤0.5 kg. Height was assessed using a wall‐mounted stadiometer to the nearest 0.1 cm with duplicate measures differing by ≤0.5 cm. Weight and height were used to compute BMI (kg·m−2). Body surface area was computed as BSA = 0.0235 × Height in cm0.42246 × Weight in kg0.51456.
2.2.3. CMR measures
Participants were scanned by dedicated CMR technologists on a 1.5‐tesla Siemens Magnetom Espree scanner (Erlangen, Germany) with a 32‐channel phased array cardiovascular coil. Standard long axis cines were acquired in the two‐, three‐, and four‐chamber orientations. Measurements without geometric assumptions from manual end‐diastolic and end‐systolic endocardial and epicardial traces of short axis stacks of cines acquired with 6‐mm slice thickness using commercially available software were taken for LVM. There was no inter‐slice gap to improve spatial resolution in the long axis direction and accurately measure left ventricular myocardium at the base of the heart. Papillary muscles were excluded from LVM measures. Typical parameters were as follows: field of view 380 × 340 cm, matrix 256 × 144, 1.5 × 2.4‐mm pixels, flip angle (FA) 50°, temporal resolution 30 to 45 milliseconds, 30 frames per cardiac cycle, TR/TE = 2.9/1.2 milliseconds, pixel bandwidth = 930 Hz, and parallel imaging factor 2 or 3 (GRAPPA).
2.2.4. Body composition
Total body composition (fat mass, lean mass, percent body fat, bone mineral content, and bone mineral density) was measured from a total body scan using DXA (GE Lunar iDXA, Madison, WI). Girth measures of the waist and hip were taken using a Gulick anthropometric measuring tape, with measures taken to the nearest 0.1 cm with duplicate measures differing by ≤1.0 cm. Waist circumference was measured horizontally at both the umbilicus and the iliac crest. Hip circumference was measured at the widest visual protrusion of the buttocks.
2.2.5. Cardiorespiratory fitness
Submaximal cardiorespiratory fitness was assessed with a submaximal graded exercise test performed on a motorized treadmill as previously described.25 The treadmill speed was maintained at 80.4 m·min−1. The incline of the treadmill was initiated at 0% and increased by 1% at 1‐minute intervals. The test was terminated when the participant first achieved or exceeded 85% of their age‐predicted maximal heart rate.25 Oxygen consumption (L·min−1 and mL·kg−1·min−1) was measured with indirect calorimetry using a metabolic cart (Carefusion Vmax Encore, Yorba Linda, CA), with submaximal fitness represented by oxygen consumption achieved during the final 20 seconds prior to test termination.
2.2.6. Resting blood pressure, mean arterial pressure, and heart rate
Following initial circumference measures to determine appropriate cuff size, resting blood pressure and heart rate measurements were obtained using an automated blood pressure cuff (DINAMAP V100, GE Medical System Technologies; Milwaukee, WI) following a 5‐minute seated rest period. Blood pressure was expressed as the mean of duplicate measures, with systolic blood pressure (SBP) measures differing by ≤10 mmHg and diastolic blood pressure (DBP) measures differing by ≤6 mmHg. If these criteria were not achieved, a third measurement of SBP and DBP was taken. Mean arterial pressure (MAP) was computed from the mean values of SBP and DBP using the following formula: MAP = DBP + ([SBP – DBP]/3). The mean of the heart rate measures obtained with the blood pressure measures was used to represent resting heart rate.
2.3. Statistical analysis
Analyses were performed using SAS version 9.4 (SAS Institute, Cary NC). Descriptive statistics, mean ± standard deviation, or median (interquartile range) for continuous variables and frequency (percentage) for categorical variables was presented. Spearman correlation coefficient was used to examine bivariate correlation between LVM and continuous variables of interest, eg, age, weight, BMI, BSA, MAP, and fitness measures. Also presented are the Spearman partial correlation coefficient controlling for sex, age, and race. For the bivariate relationship between LVM and categorical variables of sex and race, the Wilcoxon rank sum test and the Kruskal‐Wallis test were used, respectively. Linear regression models were fit to the outcome of LVM. Stepwise regression analyses were performed to select a set of significant predictors from the variables showing significant bivariate correlations, after forcing age, gender, race, MAP, and height in the models. Analyses were repeated with height removed from the analyses. The type I error rate was fixed at 5%.
3. RESULTS
The flow of participants into this study is illustrated in Figure 1. Descriptive characteristics of the participants are shown in Table 1, and LVM by sex and race is shown in Table 2. Data for LVM, weight, height, BMI, BSA, percent body fat, and termination time on the exercise test were available for 383 participants. Data from four participants were not available, with two missing CMR data and two missing oxygen consumption (L·min−1 and mL·kg−1·min−1). Thus, complete data are available for 379 participants.
Figure 1.
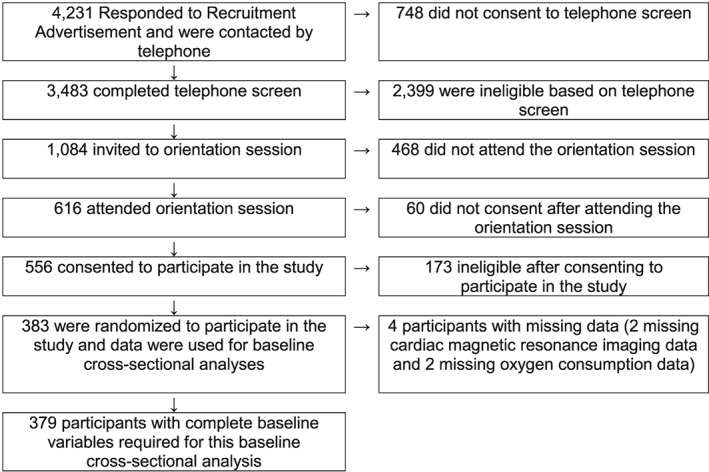
Consort diagram for participant recruitment
Table 1.
Descriptive characteristics and bivariate associations with left ventricular mass
| N | M ± SD | Spearman Correlation Coefficient (ρ) | P Value | |
|---|---|---|---|---|
| Age (y) | 379 | 45.6 ± 7.9 | −.027 | .5954 |
| Mean arterial pressure | 379 | 88.1 ± 9.0 | .360 | <.0001 |
| Height (cm) | 379 | 167.4 ± 8.0 | .552 | <.0001 |
| Weight (kg) | 379 | 90.8 ± 13.7 | .629 | <.0001 |
| BMI (kg·m−2) | 379 | 32.4 ± 3.8 | .355 | <.0001 |
| % body fat | 379 | 43.3 ± 5.5 | −.282 | <.0001 |
| Body surface area | 379 | 2.1 ± 0.2 | .657 | <.0001 |
| Cardiorespiratory fitness (oxygen consumption, L·min−1)a | 379 | 2.1 ± 0.5 | .613 | <.0001 |
| Cardiorespiratory fitness (oxygen consumption, mL·kg·min−1)a | 379 | 22.6 ± 4.4 | .291 | <.0001 |
| Cardiorespiratory fitness (termination time)a | 379 | 7.7 ± 3.0 | .281 | <.0001 |
Abbreviation: BMI, body mass index.
Evaluation of cardiorespiratory fitness was assessed with a submaximal graded exercise test terminated at 85% of age‐predicted maximal heart rate.
Table 2.
Left ventricular mass by sex and race
| Descriptive Category | N (% Total Sample) | M ± SD (Median [25th, 75th Percentile]) | P Value | |
|---|---|---|---|---|
| Total sample | 379 | |||
| Sex | Female | 303 (79.95%) | 81.92 ± 15.09 (81.20 [72.00, 91.20]) | <.0001* |
| Male | 76 (20.05%) | 116.08 ± 18.44 (112.75 [103.00, 127.61]) | ||
| Race | African American/Black | 86 (22.69) | 88.80 ± 18.59 (86.35 [77.10, 97.70]) | .1435** |
| Asian | 11 (2.90) | 73.78 ± 17.07 (75.32 [59.40, 88.25]) | ||
| Caucasian/white | 271 (71.50) | 89.47 ± 21.60 (84.51 [73.49, 102.50]) | ||
| Other | 4 (1.06) | 93.31 ± 20.16 (88.02 [78.50, 108.12]) | ||
| Mixed race | 7 (1.85) | 82.52 ± 21.73 (69.75 [67.10, 107.55]) | ||
P value based on Wilcoxon sum rank test.
P value based on Kruskal‐Wallis test.
Bivariate analyses were conducted to examine the association between the descriptive characteristics and LVM (Table 1). Left ventricular mass was positively associated with weight, height, BMI, BSA, and all measures of fitness (P < .0001) and inversely associated with percent body fat (P < .0001). Median LVM was significantly different when analysed by sex (P < .0001) but not by race (P < .1435) (Table 2).
Results of stepwise multivariate analyses examining the association between measures of fitness and body size (weight, BMI, and BSA) are shown in Tables 3, 4 and 5. In these analyses, body weight was positively associated with LVM (P < .0001); however, percent body fat was inversely associated with LVM (P ≤ .0001) (Table 3). In the same models, cardiorespiratory fitness expressed as oxygen consumption (L·min−1) (P = .0098) was positively associated with a larger LVM. This model reflected an r 2 of.6672. When additional analyses were performed with cardiorespiratory fitness expressed as mL·kg−1·min−1 or test termination time, similar patterns of results were observed, with an r 2 of.6662 and.6659, respectively. Analyses were repeated with height removed as a variable; and the pattern of results for weight, percent body fat, and cardiorespiratory fitness was similar to the findings presented in Table 3 (data not shown).
Table 3.
Predictors (weight, percent body fat, and cardiorespiratory fitness) of left ventricular mass measured by cardiac magnetic resonance imaging using stepwise regression analysis
| Dependent Variable | Variable | Step | β | SE | P Value | Partial r 2 | Model r 2 |
|---|---|---|---|---|---|---|---|
| Left ventricular mass | .6676 | ||||||
| Intercept | 43.53 | 23.706 | .0671 | ||||
| Age (years)a | 0.092 | 0.084 | .2734 | ||||
| Gender (female)a | −10.711 | 2.915 | .0003 | ||||
| Race (white)a | −2.684 | 1.473 | .0692 | ||||
| Height (cm)a | −0.149 | 0.132 | .2601 | ||||
| Mean arterial pressurea | 0.353 | 0.076 | <.0001 | ||||
| Weight (kg) | 1 | 0.787 | 0.095 | <.0001 | .1075 | ||
| % body fat | 2 | −0.866 | 0.213 | <.0001 | .0263 | ||
| Fitness (oxygen consumption, L·min−1) | 3 | 5.563 | 2.143 | .0098 | .0061 | ||
| Left ventricular mass | .6662 | ||||||
| Intercept | 35.775 | 25.336 | .1588 | ||||
| Age (years)a | 0.087 | 0.084 | .3001 | ||||
| Gender (female)a | −11.062 | 2.907 | .0002 | ||||
| Race (white)a | −2.546 | 1.472 | .0845 | ||||
| Height (cm)a | −0.153 | 0.133 | .2500 | ||||
| Mean arterial pressurea | 0.352 | 0.076 | <.0001 | ||||
| Weight (kg) | 1 | 0.915 | 0.078 | <.0001 | .1075 | ||
| % body fat | 2 | −0.897 | 0.212 | <.0001 | .0263 | ||
| Fitness (oxygen consumption, mL·kg−1·min−1) | 3 | 0.438 | 0.193 | .0235 | .0047 | ||
| Left ventricular mass | .6659 | ||||||
| Intercept | 48.441 | 23.501 | .040 | ||||
| Age (years)a | 0.080 | 0.084 | .340 | ||||
| Gender (female)a | −11.622 | 2.872 | <.0001 | ||||
| Race (white)a | −2.551 | 1.475 | .0845 | ||||
| Height (cm)a | −0.187 | 0.132 | .1573 | ||||
| Mean arterial pressurea | 0.347 | 0.076 | <.0001 | ||||
| Weight (kg) | 1 | 0.922 | 0.078 | <.0001 | .1075 | ||
| % body fat | 2 | −0.918 | 0.210 | <.0001 | .0263 | ||
| Fitness (termination time) | 3 | 0.590 | 0.269 | .0293 | .0043 |
These variables were forced in the model during the stepwise regression analysis.
Table 4.
Predictors (body mass index, percent body fat, and cardiorespiratory fitness) of left ventricular mass measured by cardiac magnetic resonance imaging using stepwise regression analysis
| Dependent Variable | Variable | Step | β | SE | P Value | Partial r 2 | Model r 2 |
|---|---|---|---|---|---|---|---|
| Left ventricular mass | .6666 | ||||||
| Intercept | −98.252 | 20.503 | <.0001 | ||||
| Age (years)a | 0.097 | 0.084 | .2489 | ||||
| Gender (female)a | −10.686 | 2.921 | .0003 | ||||
| Race (white)a | −2.753 | 1.474 | .0626 | ||||
| Height (cm)a | 0.696 | 0.113 | <.0001 | ||||
| Mean arterial pressurea | 0.359 | 0.076 | <.0001 | ||||
| Body mass index (kg·m−2) | 1 | 2.189 | 0.267 | <.0001 | .1053 | ||
| % body fat | 2 | −0.876 | 0.215 | <.0001 | .0268 | ||
| Fitness (oxygen consumption, L·min−1) | 3 | 5.819 | 2.135 | .0067 | .0067 | ||
| Left ventricular mass | .6646 | ||||||
| Intercept | −129.750 | 22.738 | <.0001 | ||||
| Age (years)a | 0.091 | 0.084 | .2812 | ||||
| Gender (female)a | −11.122 | 2.915 | .0002 | ||||
| Race (white)a | −2.593 | 1.475 | .0796 | ||||
| Height (cm)a | 0.837 | 0.105 | <.0001 | ||||
| Mean arterial pressurea | 0.359 | 0.076 | <.0001 | ||||
| Body mass index (kg·m−2) | 1 | 2.565 | 0.222 | <.0001 | .1053 | ||
| % body fat | 2 | −0.916 | 0.214 | <.0001 | .0268 | ||
| Fitness (oxygen consumption, mL·kg−1·min−1) | 3 | 0.441 | 0.193 | .0228 | .0047 | ||
| Left ventricular mass | .6643 | ||||||
| Intercept | −118.193 | 21.028 | <.0001 | ||||
| Age (years)a | 0.084 | 0.084 | .3189 | ||||
| Gender (female)a | −11.684 | 2.880 | <.0001 | ||||
| Race (white)a | −2.599 | 1.478 | .0794 | ||||
| Height (cm)a | 0.810 | 0.105 | <.0001 | ||||
| Mean arterial pressurea | 0.354 | 0.076 | <.0001 | ||||
| Body mass index (kg·m−2) | 1 | 2.584 | 0.222 | <.0001 | .1053 | ||
| % body fat | 2 | −0.938 | 0.212 | <.0001 | .0268 | ||
| Fitness (termination time) | 3 | 0.595 | 0.270 | .0281 | .0044 |
These variables were forced in the model during the stepwise regression analysis.
Table 5.
Predictors (body surface area, percent body fat, and cardiorespiratory fitness) of left ventricular mass measured by cardiac magnetic resonance imaging using stepwise regression analysis
| Dependent Variable | Variable | Step | β | SE | P Value | Partial r 2 | Model r 2 |
|---|---|---|---|---|---|---|---|
| Left ventricular mass | .6677 | ||||||
| Intercept | 35.238 | 23.175 | .1292 | ||||
| Age (years)a | 0.092 | 0.084 | .2733 | ||||
| Gender (female)a | −10.552 | 2.918 | .0003 | ||||
| Race (white)a | −2.637 | 1.473 | .0743 | ||||
| Height (cm)a | −0.502 | 0.160 | .0018 | ||||
| Mean arterial pressurea | 0.352 | 0.076 | <.0001 | ||||
| Body surface area | 1 | 67.052 | 8.101 | <.0001 | .1071 | ||
| % body fat | 2 | −0.880 | 0.214 | <.0001 | .0268 | ||
| Fitness (oxygen consumption, L·min−1) | 3 | 5.555 | 2.143 | .0099 | .0060 | ||
| Left ventricular mass | .6663 | ||||||
| Intercept | 26.118 | 24.963 | .2691 | ||||
| Age (years)a | 0.087 | 0.084 | .2999 | ||||
| Gender (female)a | −10.877 | 2.913 | .0002 | ||||
| Race (white)a | −2.492 | 1.472 | .0913 | ||||
| Height (cm)a | −0.563 | 0.157 | .0004 | ||||
| Mean arterial pressurea | 0.352 | 0.076 | <.0001 | ||||
| Body surface area | 1 | 78.029 | 6.686 | <.0001 | .1071 | ||
| % body fat | 2 | −0.913 | 0.213 | <.0001 | .0268 | ||
| Fitness (oxygen consumption, mL·kg−1·min−1) | 3 | 0.437 | 0.194 | .0237 | .0047 | ||
| Left ventricular mass | .6659 | ||||||
| Intercept | 38.783 | 23.126 | .0944 | ||||
| Age (years)a | 0.079 | 0.084 | .3420 | ||||
| Gender (female)a | −11.447 | 2.878 | <.0001 | ||||
| Race (white)a | −2.491 | 1.475 | .0921 | ||||
| Height (cm)a | −0.599 | 0.156 | .0001 | ||||
| Mean arterial pressurea | 0.347 | 0.076 | <.0001 | ||||
| Body surface area | 1 | 78.557 | 6.679 | <.0001 | .1071 | ||
| % body fat | 2 | −0.936 | 0.211 | <.0001 | .0268 | ||
| Fitness (termination time) | 3 | 0.583 | 0.269 | .0311 | .0042 |
These variables were controlled for in the stepwise regression analysis.
Additional analyses were performed replacing body weight with BMI (Table 4). In these stepwise regression models, BMI was positively associated with LVM (P < .0001), percent body fat inversely associated with LVM, and fitness positively associated with LVM. The r 2 in these three models ranged from.6643 to.6666. Similar patterns of results were observed when the analyses were performed with BSA rather than BMI (Table 5). Again, analyses were repeated with height removed as a variable; and the pattern of results for BMI, BSA, percent body fat, and cardiorespiratory fitness was similar to the findings presented in Tables 4 and 5 (data not shown).
4. DISCUSSION
Higher levels of body fatness may be associated with a lower LVM measured by CMR, which may reflect a maladaptive response, whereas higher levels of cardiorespiratory fitness may be associated with a favourable adaptive response to LVM. Prior studies have shown that body size, defined by BMI or BSA, is associated with a larger LVM.6, 7 The results of this study support these findings with bivariate analyses showing associations between LVM and measures of body size that included weight, BMI, and BSA (see Tables 3, 4, and 5). However, when considered in multivariate analyses, body weight was not associated with LVM measured by CMR; however, both BMI and BSA remained significantly associated with a greater LVM (see Tables 3, 4, and 5). Moreover, in all analyses, percent body fat, measured by DXA, was inversely associated with LVM. This study also showed that cardiorespiratory fitness was positively associated with LVM after accounting for body size and body fatness.
The results of this study are of clinical importance given the association between obesity and CVD morbidity and mortality,26, 27 along with the inverse association between cardiorespiratory fitness and CVD morbidity and mortality.8, 9, 10, 12 This has resulted in debate regarding whether it is the excess body weight or low cardiorespiratory fitness that contributes to all‐cause mortality and CVD. There have been reports to support both sides of this debate with some studies supporting that fitness is more highly associated with CVD,11, 12, 13, 14 while other studies support the position that excess weight is an important contributor to CVD.17, 28 The results of this current study in adults who are overweight or obese, engaging in low levels of physical activity, and without known CVD suggest that both adiposity and fitness are associated with LVM. This may suggest that both prevention and treatment of excess levels of adiposity, combined with emphasis on engaging in behaviours that influence cardiorespiratory fitness, such as physical activity, may be important clinical targets that impact LVM. Moreover, while dietary change may reduce weight and adiposity, it will not have a direct influence on cardiorespiratory fitness. However, moderate‐to‐vigorous physical activity will result in reduced weight and adiposity along with improved cardiorespiratory fitness, with both of these factors shown to impact LVM in this current study.
The finding that measures of body size (BMI and BSA) were positively associated with LVM, whereas percent body fat was inversely associated with LVM, is an interesting observation. This may suggest that body size and total mass are associated with LVM, but when a higher percent of the mass is fat, this may reduce the quality of the myocardium. This reduced quality of the myocardium may provide partial explanation for how excess body fat may be associated with cardiac structure and potential downstream health risks. This observation warrants confirmation and further examination to potential health‐related implications.
The association between cardiorespiratory fitness and LVM should be interpreted with caution, given that the direction of this association is not able to be determined from this cross‐sectional analysis. For example, it is possible that targeting behaviours, such as physical activity, that result in improved cardiorespiratory fitness, may contribute to an increase in LVM. Conversely, it is possible that an adult with a larger LVM may have enhanced cardiorespiratory fitness, which may allow for greater engagement in physical activity. Thus, this may indicate the need for a properly designed prospective and intervention studies to examine the magnitude of change in LVM that occurs as cardiorespiratory fitness either increases or decreases, and when these changes in cardiorespiratory fitness are accompanied by changes in body size and body fatness.
This study has numerous strengths. These strengths include the use of CMR to assess LVM, cardiorespiratory fitness assessed from a graded exercise test, and body fatness assessed using DXA. However, there are limitations that are important to recognize. This study included participants with a BMI range from 25 to <40 kg·m−2, which limited the ability to examine whether the associations observed would remain with the inclusion of participants at the lower or higher ranges of BMI. Participants with known CVD were also excluded, which may have also excluded individuals with clinically relevant left ventricular hypertrophy, and therefore, it will be important to consider how the factors observed in this study would impact LVM in that clinical population. This study also included participants who self‐reported low participation in regular moderate‐to‐vigorous physical activity, which limited the range of fitness considered in the analyses. Moreover, the sample of participants examined, aside from being overweight or obese, and engaging in low amounts of physical activity, was otherwise relatively healthy. Therefore, whether similar findings would be observed in participants who may have other risks factors or known chronic health conditions is unable to be determined. In addition, the study is unable to examine if similar findings would be observed in an older population (>55 y of age) or in participants with different demographic characteristics than those examined.
Another key consideration is that this study was initiated prior to the emergence of extracellular volume (ECV) measures. Patient vulnerability may relate more to myocardial “quality” (ie, extent of excess interstitial expansion, usually from myocardial fibrosis) than quantity (LVM).29, 30, 31 Extracellular volume measures32, 33 the volume percent of the interstitial compartment, which represents a well‐validated surrogate of myocardial fibrosis in most settings.34
5. CONCLUSION
These cross‐sectional findings support that LVM in adults who are relatively healthy, overweight, or obese and engaging in low amounts of physical activity is associated with body size (BMI and BSA), body fatness, and cardiorespiratory fitness. Given that LVM is as an important clinical indicator of CVD, these findings may provide relevant information regarding interventions that may influence LVM, such as interventions to prevent gain of weight and fat mass, to promote weight loss, and to promote improvements in cardiorespiratory fitness. Thus, future studies should investigate whether changes in weight, body composition, and fitness have differential influences on LVM. Moreover, these findings build on prior studies that have shown that body size is associated with LVM, with these findings indicating that evaluation of LVM should also consider potential associations with body composition and cardiorespiratory fitness.
CONFLICT OF INTEREST STATEMENT
No conflict of interest was declared.
FUNDING
This study was supported by grant R01 HL096770 from the National Institutes of Health and the National Heart, Lung, and Blood Institute and by the University of Pittsburgh Clinical and Translational Science Institute (CTSI) (UL1 TR001857) that is supported by the National Institutes of Health. The funding source (National Institutes of Health) was invited to have a representative present during Data and Safety Monitoring Board meetings but otherwise did not have a role in the study design, conduct of the study, data analysis, or interpretation of the study findings.
DISCLOSURES
Dr. Rogers was the principal investigator on a grant awarded to the University of Pittsburgh by Weight Watchers International. Dr. Schelbert accepted contrast material from Bracco Diagnostics for research purposes and has served on advisory boards for Merck and Bayer. Dr. Jakicic received an honorarium for serving on the Scientific Advisory Board for Weight Watchers International and was a coinvestigator on a grant awarded to the University of Pittsburgh by Weight Watchers International.
DATA SHARING
Data sharing for this cross‐sectional analysis of baseline data will not be made available. Investigators interested in data from the parent study should contact Dr. Jakicic (jjakicic@pitt.edu), who is the principal investigator, for data sharing policies and procedures after publication of the primary outcomes of this study.
AUTHOR CONTRIBUTIONS
J.M.J., E.B.S., and W.L. conceived the study design. R.J.R., J.M.J., E.B.S., and Y.F. were responsible for data collection. W.L., N.Y., J.M.J., and E.B.S. were responsible for data analysis. R.J.R. and J.M.J. were involved in writing the paper, and all authors had final approval of the submitted and published versions.
ACKNOWLEDGEMENTS
The authors recognize the contribution of the staff and graduate students at the Physical Activity and Weight Management Research Center at the University of Pittsburgh who received salary support on this project or contributed in other aspects of this project.
Rogers RJ, Schelbert EB, Lang W, Fridman Y, Yuan N, Jakicic JM. Association of fitness and body fatness with left ventricular mass: The Heart Health Study . Obes Sci Pract. 2020;6:19–27. 10.1002/osp4.380
Clinical Trial Registration: http://ClinicalTrials.gov NCT01500356
REFERENCES
- 1. Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of Obesity Among Adults and Youth: United States, 2015‐2016. Hyattsville, MD: National Center for Health Statistics; 2017. [Google Scholar]
- 2. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol. 2014;63:2985‐3023. [DOI] [PubMed] [Google Scholar]
- 3. National Institutes of Health National Heart Lung and Blood Institute . Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults—the evidence report. Obes Res. 1998;6:67S‐82S. [PubMed] [Google Scholar]
- 4. Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Left ventricular mass and incidence of coronary heart disease in an elderly cohort. The Framingham Heart Study. Ann Intern Med. 1989;110:101‐107. [DOI] [PubMed] [Google Scholar]
- 5. Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561‐1566. [DOI] [PubMed] [Google Scholar]
- 6. Kishi S, Armstrong AC, Gidding SS, et al. Association between obesity in early adulthood and middle age with incipient left ventricular dysfunction and structural remodeling: the CARDIA study (Coronary Artery Risk Development in Young Adults). JACC Heart Fail. 2014;2:500‐508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Turkbey EB, McClelland RL, Kronmal RA, et al. The impact of obesity on the left ventricle. J Am Coll Cardiol Img. 2010;3:266‐274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Blair SN, Kampert JB, Kohl H III, et al. Influence of cardiorespiratory fitness and other precursors on cardiovascular disease and all‐cause mortality in men and women. JAMA. 1996;276:205‐210. [PubMed] [Google Scholar]
- 9. Blair SN, Kohl H III, Barlow CE, Paffenbarger RS, Gibbons LW, Macera CA. Changes in physical fitness and all‐cause mortality: a prospective study of healthy and unhealthy men. JAMA. 1995;273:1093‐1098. [PubMed] [Google Scholar]
- 10. Blair SN, Kohl H III, Paffenbarger RS, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all‐cause mortality. A prospective study of healthy men and women. JAMA. 1989;262:2395‐2401. [DOI] [PubMed] [Google Scholar]
- 11. Barlow CE, Kohl HW, Gibbons LW, Blair SN. Physical activity, mortality, and obesity. Int J Obes. 1995;19:S41‐S44. [PubMed] [Google Scholar]
- 12. Church TS, LaMonte MJ, Barlow CE, Blair SN. Cardiorespiratory fitness and body mass index as predictors of cardiovascular disease mortality among men with diabetes. Arch Intern Med. 2005;165:2114‐2120. [DOI] [PubMed] [Google Scholar]
- 13. Farrell SW, Braun L, Barlow CE, Cheng YJ, Blair SN. The relation of body mass index, cardiorespiratory fitness, and all‐cause mortality in women. Obes Res. 2002;10:417‐423. [DOI] [PubMed] [Google Scholar]
- 14. Lee CD, Blair SN, Jackson AS. Cardiorespiratory fitness, body composition, and all‐cause and cardiovascular disease mortality in men. Am J Clin Nutr. 1999;69:373‐380. [DOI] [PubMed] [Google Scholar]
- 15. Lee DC, Sui X, Blair SN. Does physical activity ameliorate the health hazards of obesity? Br J Sports Med. 2009;43:49‐51. [DOI] [PubMed] [Google Scholar]
- 16. The Look AHEAD Research Group . Association of the magnitude of weight loss and changes in physical fitness with long‐term cardiovascular disease outcomes in overweight or obese people with type 2 diabetes: a post‐hoc analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol. 2016;4:913‐921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wing RR, Jakicic J, Neiberg R, et al. Fitness, fatness, and cardiovascular risk factors in type 2 diabetes: Look AHEAD Study. Med Sci Sports Exerc. 2007;39:2107‐2116. [DOI] [PubMed] [Google Scholar]
- 18. Whalley GA, Doughty RN, Gamble GD, et al. Association of fat‐free mass and training status with left ventricular size and mass in endurance‐trained athletes. J Am Coll Cardiol. 2004;44:892‐896. [DOI] [PubMed] [Google Scholar]
- 19. Lee I‐M, Paffenbarger R. Associations of light, moderate, and vigorous intensity physical activity with longevity: The Harvard Alumni Health Study. American Journal of Epidemiology. 2000;151:293‐299. [DOI] [PubMed] [Google Scholar]
- 20. Lee I‐M, Rexrode KM, Cook NR, Manson JE, Buring JE. Physical activity and coronary heart disease in women. JAMA. 2001;285:1447‐1454. [DOI] [PubMed] [Google Scholar]
- 21. Paffenbarger RS, Blair SN, Lee I‐M, Hyde RT. Measurement of physical activity to assess health effects in free‐living populations. Med Sci Sports Exerc. 1993;25:60‐70. [DOI] [PubMed] [Google Scholar]
- 22. Paffenbarger RS, Hyde RT, Wing AL, Hsieh CC. Physical activity, all‐cause mortality, and longevity of college alumni. N Engl J Med. 1986;314:605‐613. [DOI] [PubMed] [Google Scholar]
- 23. Paffenbarger RS, Hyde RT, Wing AL, Lee I‐M, Jung DL, Kampert JB. The association of changes in physical‐activity level among other lifestyle characteristics with mortality among men. N Engl J Med. 1993;328:538‐545. [DOI] [PubMed] [Google Scholar]
- 24. Gidding SS, Carnethon MR, Daniels S, et al. Low cardiovascular risk is associated with favorable left ventricular mass, left ventricular wall thickness, and left atrial size: The CARDIA Study. J Am Soc Echocardiogr. 2010;23:816‐822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jakicic JM, Davis KK, Rogers RJ, et al. Effect of wearable technology combined with a lifestyle intervention on long‐term weight loss in the IDEA Study: a randomized clinical trial. JAMA. 2016;316:1161‐1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bastien M, Poirier P, Lemieux I, Despres JP. Overview of epidemiology and contribution of obesity to cardiovascular disease. Progress in cardiovascular diseases. 2014;56:369‐381. [DOI] [PubMed] [Google Scholar]
- 27. Borrell LN, Samuel L. Body mass index categories and mortality risk in US adults: the effect of overweight and obesity on advancing health. Am J Public Health. 2014;104:512‐519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Look AHEAD Research Group . Association of the magnitude of weight loss and physical fitness change with long‐term cardiovascular disease outcomes in overweight and obese people with type 2 diabetes: a post‐hoc analysis of the Look AHEAD randomized clinical trial. The lancet Diabetes & endocrinology. 2016;4:913‐921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moon JC, Treibel TA, Schelbert EB. Fibrosis in hypertensive heart failure: does quality rather than quantity matter? J Am Coll Cardiol. 2016;67:261‐263. [DOI] [PubMed] [Google Scholar]
- 30. Schelbert EB, Fridman Y, Wong TC, H. AD, Piehler KM, Kadakkal A, et al. Temporal relation between myocardial fibrosis and heart failure with preserved ejection fraction: association with baseline disease severity and subsequent outcome. JAMA Cardiol. 2017;2:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schelbert EB, Piehler KM, Zareba KM, et al. Myocardial fibrosis quantified by extracellular volume is associated with subsequent hospitalization for heart failure, death, or both across the spectrum of ejection fraction and heart failure stage. J Am Heart Assoc. 2015;4:e002613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Moon JC, Messroghli DR, Kellman P, et al. Myocardial T1 mapping and extracellular volume quantification: a Society for Cardiovascular Magnetic Resonance (SCMR) and CMR Working Group of the European Society of Cardiology consensus statement. J Cardiovasc Magn Reson. 2013;15:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Messroghli DR, Moon JC, Ferreira VM, et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: a consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J Cardiovasc Magn Reson. 2017;19:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schelbert EB, Sabbah HN, Butler J, Gheorghiade M. Employing extracellular volume cardiovascular magnetic resonance measures of myocardial fibrosis to foster novel therapeutics. Circ Cardiovasc Imaging. 2017;10:e005619. [DOI] [PubMed] [Google Scholar]


